Abstract
Background
Despite the large number of hospitalized patients affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, few data are available about risk factors and mortality in subjects with nosocomially acquired respiratory infection of Coronavirus Disease 2019 (COVID-19).
Methods
We retrospectively evaluated in a multicentric study -during the pre-vaccination era-all patients admitted with confirmed diagnosis of nosocomial COVID-19 (NC). Patients were classified according to provenance: hospital-acquired NC or long-term care (LTC) facilities.
Results
Among overall 1047 patients evaluated with COVID-19, 137 had a confirmed diagnosis of NC (13%). 78 (56.9%) patients had hospital-acquired NC and 59 (43%) had LTC NC. Overall mortality was 35.8%, in hospital-acquired NC 24.4%, in LTC NC 50.8% (p < 0.001) (Log Rank test: p = 0.001). Timing of diagnosis was significantly different between hospital acquired and LTC NC (3.5 vs 10 days, p < 0.001). In multivariate analysis age, intensive-care unit admission, LTC provenance and sepsis were significant predictors of mortality in patients with NC infection.
Conclusion
Patients with NC are at higher risk of mortality (especially for LTC NC) and required preventive strategies, early diagnosis, and treatment to avoid COVID-19 cluster.
Keywords: COVID-19, SARS-CoV-2, Hospital infection, Nosocomial, Mortality, Long-term care
Introduction
The novel coronavirus-19 disease (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a pandemic illness associated with high mortality and morbidity due to several complications such as interstitial pneumonia, acute respiratory distress syndrome (ARDS) and multiorgan failure [1]. Due to the incubation period, the appropriate diagnosis of nosocomial COVID-19 (NC) according to the European Centre for Disease Prevention and Control (ECDC) definitions [2] maybe delayed and troublesome. The main difficulties include a delayed diagnosis in patients without clinical evidence of typical symptoms and on the other side the need to limit as much as possible the spread of infection inside the hospitals. The average prevalence of nosocomial bloodstream, urinary or lung infection before the COVID-19 pandemic was 8.7% [3], while in SARS-CoV-1 and Middle East respiratory syndrome (MERS) was previously reported as 36% and 56%, respectively. The prevalence of NC is widely different among available data: in the first study conducted by Wang et al. at Wuhan NC prevalence was 41% [4], in the study by Carter et al. 12.5% [5], and in the study by Rhee et al. 1.7% [6]. These differences can be largely related to heterogeneous characteristics of these studies such as the time of infection, healthcare management and number of the included patients.
In this paper we analyzed the prevalence and the risk factors for mortality related to NC in a multicentric cohort of patients admitted with COVID-19 disease, according to their provenance, severity of illness, pre-existing comorbidities, hospital division and antiviral or supportive therapies administered against SARS-CoV-2 infection.
Methods
Data were extracted from the CORACLE Registry [7], a multicenter regional register of hospitalized COVID-19 patients in Piedmont (Italy). Included center were: “Saint Andrea Hospital”, Vercelli; “Cardinal Massaia Hospital”, Asti and “Città della Salute e della Scienza”, Turin, Italy. In each of these were present a long-term hospital ward, a medium-intensity ward, and an intensive-care-unit (ICU). All consecutive patients admitted from March to October 2020 -during the pre-vaccination era-with confirmed diagnosis of NC were included in this analysis. The diagnosis of NC was defined as: SARS-CoV-2 infection documented by nasopharyngeal RT-PCR test in patients admitted to the hospital for other reason than COVID-19 infection, or in patients with a previous RT-PCR test negative at the time of admission and a following test positive during the hospital stay. Demographics, clinical, biological, and therapeutic data were collected. We reported the medical comorbidities, the time after symptoms onset and the RT-PCR test positive, days of hospitalization, clinical department of provenance and clinical outcomes.
Statistical analysis
Patient’ characteristics and laboratory examinations were reported as mean and standard deviation (SD) (continuous variables) or frequencies and percentages (categorical variables); differences in non-Gaussian distributions were determined using the Mann-Whitney U-test, and normal distributions with Student's t test. Survival data analyzed with the Kaplan-Meier plots and log-rank test. Multivariate analysis was used to assess the risk of mortality using the Cox regression model adjusted for age, sex and comorbidities. The values are reported as odds ratio (OR) with 95% confidence interval (CI) and the p value < 0.05 was considered significant.
Results
Among the overall 1047 patients evaluated within the CORACLE cohort, we included 137 subjects with a confirmed diagnosis of NC. The rate of NC in our cohort was 13%. Overall mortality in the CORACLE registry was 27% with median age 83 years. The most common variants of SARS-CoV-2 detected in the study period were naïve (alpha) and delta; however, this test was not performed routinely, and some data were referred to the national epidemiology of most prevalent variants.
Baseline characteristics of the study population are reported in Table 1 . Median age was 77 years; 71 patients were male (51.8%), median BMI was 25.5. Median days from the symptom onset to PCR-RT test positive for SARS-CoV-2 was 7.5 days; median time of hospitalization was 12.5 days. With regard to clinical presentation 90 patients (65.7%) had interstitial pneumonia, 41 (29.9%) required non-invasive ventilation (NIV), 28 (20.4%) required admission in intensive care unit (ICU); antiviral treatment was given in 32 subjects (23.4%), corticosteroid treatment in 56 (40.9%); 29 patients presented with sepsis (21.2%) and the overall mortality was 35.8% (49/137). Several patients with diagnosis of NC were from long-term care (LTC) facilities (n = 59, 43.1%) with higher prevalence of geriatric or neurological chronic diseases; other patients had a positive SARS-Cov-2 test (n = 78, 56.9%) acquired during hospitalization in other hospital wards: internal medicine (n = 15, 10.9%), general surgery (n = 5, 3.6%), orthopedics (n = 12, 8.8%), urology (n = 1, 0.7%), nephrology/dialysis (n = 3, 2.2%), cardiology (n = 3, 2.2%), ICU (n = 5, 3.6%), psychiatry (n = 2, 1.5%), neurology (n = 4, 2.9%), pediatrics (n = 1, 0.7%), hematology (n = 1, 0.7%); in 26 patients (19%) the provenance was not available.
Table 1.
Baseline characteristics of the study population.
| Characteristics | Overall patients (n = 137) |
|---|---|
| Age (median, IQR) | 77 [65–85.5] |
| Male sex (n, %) | 71 (51.8) |
| Body mass index (median, IQR) | 25.5 [23.4–27.8] |
| Comorbidity (n, %) | |
| Coronary artery disease | 41 (29.9) |
| Diabetes | 43 (31.4) |
| Hypertension | 68 (49.6) |
| Neurological disease | 41 (29.9) |
| Psychiatric disease | 16 (11.7) |
| Immunological disease | 11 (8) |
| COPDa | 19 (13.9) |
| Kidney disease | 10 (7.3) |
| Liver disease | 4 (2.9) |
| Malignancies | 4 (2.9) |
| Days from the symptoms to PCR diagnosis (median, IQR) | 7.5 [3–12.5] |
| Provenance (n, %) | |
| -long-term care | 59 (43.1) |
| -internal medicine | 15 (10.9) |
| -general surgery | 5 (3.6) |
| -Orthopedics | 12 (8.8) |
| -Urology | 1 (0.7) |
| -nephrology/dialysis | 3 (2.2) |
| -Cardiology | 3 (2.2) |
| -ICUb | 5 (3.6) |
| -Psychiatry | 2 (1.5) |
| -Neurology | 4 (2.9) |
| -Pediatrics | 1 (0.7) |
| -Hematology | 1 (0.7) |
| -not reported | 26 (19) |
| Interstitial pneumonia (n, %) | 90 (65.7) |
| NIVc (n, %) | 41 (29.9) |
| ICU admission (n, %) | 28 (20.4) |
| WBCd (109/L) | 7336 [4414–10384] |
| Platelets (109/L) | 223 [142–288] |
| eGFRe (mL/min) | 66.7 [41.4–84.5] |
| CRPf (mg/L) | 9.7 [3.4–14.3] |
| Ferritin (ng/mL) | 560.5 [269.5–1319.5] |
| D-dimer (ng/mL) | 500 [210–1250] |
| P/Fg (median, IQR) | 262 [217–329] |
| Days of hospitalization (median, IQR) | 12.5 [7–19.5] |
| Antiviral treatment (n, %) | 32 (23.4) |
| Corticosteroid treatment (n, %) | 56 (40.9) |
| Sepsis (n, %) | 29 (21.2) |
| Death (n, %) | 49(35.8) |
Chronic obstructive pulmonary disease.
Intensive-care unit.
Non-invasive ventilation.
White blood cells.
Estimated glomerular filtration rate.
C-reactive protein.
PaO2/FiO2.
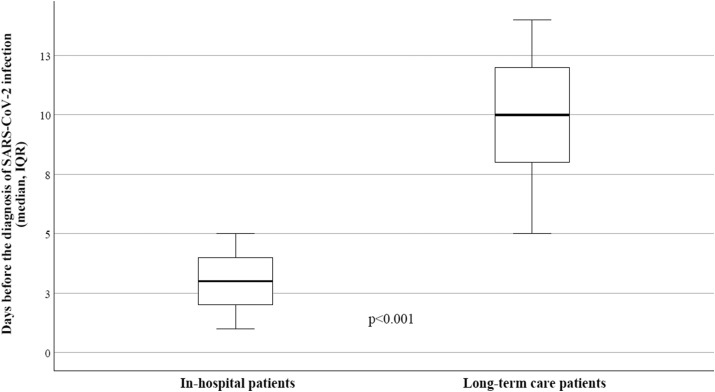
Table 2 describes the different baseline characteristics between patients with hospital-acquired NC and LTC NC. In the group of patients coming from LTC facilities were higher: the median age (82.4 vs 73.4 years), the time between symptoms’ onset and PCR diagnosis (10 vs 3.5 days) (Fig. 1 ) and the hospitalization time (13.8 vs 9.6 days); we observed higher prevalence of neurological diseases (47.4%), higher risk of sepsis and mortality (33.8% and 50.8%, respectively).
Table 2.
Different baseline characteristics and mortality in the study population by patients’ provenance.
| Hospital-acquired n = 78 | Long-term care n = 59 | P value | |
|---|---|---|---|
| Age (median, IQR) | 73.4 [61.6–82.7] | 82.4 [72.5–89.4] | 0.004 |
| Neurological disease | 13 (16.6) | 28 (47.4) | <0.001 |
| Days from the symptoms to PCR diagnosis (median, IQR) | 3.5 [1.5–4.5] | 10 [6.5–12.5] | <0.001 |
| Days of hospitalization (median. IQR) | 9.6 [7.1–13.5] | 13.8 [9.6–22.5] | 0.005 |
| Interstitial pneumonia (n, %) | 43 (59.8) | 47 (79.7) | <0.001 |
| P/Fa (median, IQR) | 271 [244–316] | 184 [81–271] | <0.001 |
| eGFRb (mL/min) | 68.5 [55–84.5] | 51.4 [39.5–66.7] | 0.003 |
| CRPc (mg/L) | 7.4 [3.6–11.8] | 10.9 [7.8–23.8] | <0.001 |
| Ferritin (ng/mL) | 433 [320–1226] | 694 [491–3389] | <0.001 |
| Sepsis (n, %) | 9 (11.5) | 20 (33.8) | 0.004 |
| Death (n, %) | 19 (24.4) | 30 (50.8) | <0.001 |
PaO2/FiO2.
Estimated glomerular filtration rate.
C-reactive protein.
Fig. 1.
Time before the diagnosis of SARS-CoV-2 infection by RT-PCR in hospitalized patients and LTC patients.
The univariate analysis considering the in-hospital mortality as outcome was performed including the following clinical variables: age, sex, BMI, comorbidities, time for PCR diagnosis, presence of interstitial pneumonia, pO2/FiO2 (P/F), CRP, ferritin, NIV, ICU admission, LTC vs hospital NC, sepsis, antiviral therapy and corticosteroids (Table 3 ). CRP and ferritin levels as baseline were associated with severe disease, P/F was the most accurate indicator in respiratory failure.
Table 3.
Univariate and multivariate logistic regression considering the mortality in the study population.
|
Factors |
OR, 95% CI, p |
|
Univariate analysis | |
| Age | 1.045 (1.015–1.076) p = 0.003 |
| Sex | 1.080 (0.537–2.174) p = 0.829 |
| BMI | 1.787 (0.850–1.078) p = 0.473 |
| Comorbidities | 1.641 (0.775–3.476) p = 0.477 |
| Days from the symptoms to PCR diagnosis | 1.139 (1.029–1.261) p = 0.001 |
| Interstitial pneumonia | 3.867 (1.462–10.225) p = 0.006 |
| P/Fa | 0.988 (0.981–0.994) p = 0.005 |
| CRPbat baseline | 1.087 (1.018–1.150) p < 0.001 |
| Ferritin at baseline | 1.032 (0.894–2.226) p = 0.256 |
| NIVc | 2.520 (1.167–5.442) p = 0.019 |
| ICUdadmission | 2.582 (1.046–6.374) p = 0.002 |
| Long term-care vs hospital acquired | 3.212 (1.553–6.643) p < 0.001 |
| Sepsis | 10.256 (3.797–27.704) p < 0.001 |
| Antiviral therapy | 0.525 (0.187–1.477) p = 0.420 |
| Corticosteroid therapy | 0.388 (0.430–2.272) p = 0.977 |
| Multivariate analysis | |
| Age | 1.108 (1.028–1.194) p = 0.008 |
| Days from the symptoms to PCR diagnosis | 1.717 (0.917–4.552) p = 0.467 |
| Interstitial pneumonia | 7.221 (0.839–62.139) p = 0.072 |
| P/Fa | 0.912 (0.826–2.224) p = 0.081 |
| CRPb at baseline | 1.739 (0.920–11.551) p = 0.225 |
| NIVc | 0.847 (0.184–3.900) p = 0.832 |
| ICUdadmission | 8.140 (1.015–65.301) p = 0.048 |
| Long term-care vs hospital acquired | 9.421 (1.891–46.934) p = 0.006 |
| Sepsis | 12.488 (2.585–60.341) p = 0.002 |
Bold specifies "statistically significant" value.
PaO2/FiO2.
C-reactive protein.
Non-invasive ventilation.
Intensive-care unit.
In the multivariate analysis resulted significantly predictive of in-hospital mortality: age (OR = 1.108; 1.028–1.194; p = 0.008); ICU admission (OR = 8.140; 1.015–65.301; p = 0.006); LTC NC vs hospital NC (OR = 9.421; 1.891–46.934; p = 0.002); sepsis (OR = 12.488; 2.585–60.341; p = 0.002) (Table 3).
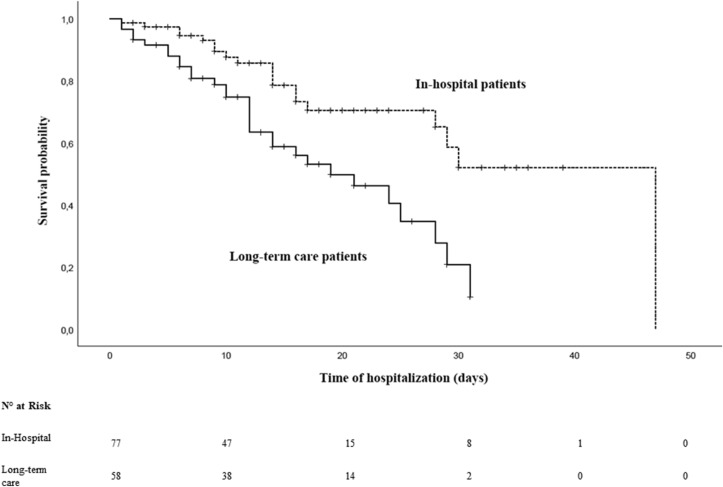
The in-hospital survival was significantly lower in patients with LTC NC than NC (Log Rank test: p = 0.001) (Fig. 2 ).
Fig. 2.
Survival analysis in patients with hospital-acquired NC and LTC NC.
Discussion
In our study the observed rate of nosocomial acquisition in COVID-19 patients was 13% with a mortality in this group of 35.8%. Patients with NC were classified according to the setting of the infection: inside the hospital (hospital-acquired NC, n = 78; 56.9%) or in LTC facilities (LTC NC, n = 59; 43%). In hospital-acquired NC the mortality was 24.4%, while in LTC patients was 50.8%. These data are similar to those reported in the study by Carter et al. [5]; the prevalence of nosocomial COVID-19 infection was lower than the reported of 41% by Wang et al. [4], but higher than the 1.7% described by Rhee et al. [6]. The reason of these differences can be explained through the characteristics of the study populations, the infection control policies in the involved countries and the timing of COVID-19 spread. In our country a great impact on the overall mortality in hospitalized COVID-19 patients was due to the high number or subjects come from the LTC facilities [8] and this aspect was also reported in other populations [9]. We know that the COVID-19 infection had a more serious course in older age, comorbidities and without an early and appropriate intervention [10]; in this group of patients major comorbidities are neurological chronic diseases (dementia, Alzheimer disease, Parkinson's disease) or other geriatric conditions such as diabetes, vasculopathy, kidney or liver disease. The median time from the symptom's onset and the PCR test for SARS-CoV-2 was significantly higher in LTC patients (10 days) than in hospitalized NC (3.5 days); this condition led to a delayed access to hospitalization and the start of treatment. Several reasons are related to this delayed diagnosis of SARS-CoV-2 infection in LTC patients: first, the symptoms onset in older patients are typically different, with more difficult diagnosis [11] due to atypical presentation and other serious comorbidities. Second, the role of asymptomatic carriers of viral infection both in visitors and in healthcare workers contributed in a short time to the spread of infection in clusters with a consequent quick increase of illness patients and obviously the difficult in clinical management. Third, the LTC facilities evidenced some important limitations in the advanced clinical management especially due to infectious diseases: sub-optimal education of healthcare workers in the infectious disease control with lack of droplet precaution, hand hygiene, disinfection of surfaces, social/working distancing and isolation; diagnostic test with RT-PCR for SARS-CoV-2 were unavailable in the first weeks of pandemic and this made a quick and certain diagnosis impossible; on the other hand, patients with lung involvement not received the optimal care due to unavailability of adequate supportive therapy with oxygen or diagnostic tool as chest radiography. All these aspects conditioned the delayed hospital admission with consequent frequent critical condition and higher mortality. On the other side, patients with hospital-acquired NC are younger, with different comorbidities, less serious clinical condition, and a faster time of SARS-CoV-2 diagnosis by PCR. These aspects are related to the need to minimize the nosocomial spread of COVID-19 infection with procedure of early diagnosis, patient's isolation and early treatment (if needed) with antiviral or oxygen support in presence of lung involvement. In most cases the hospital infections derived from asymptomatic healthcare workers because all patients were tested for SARS-CoV-2 before the admission to exclude a pre-clinical viral infection. Therefore, the NC is more frequent in the wards with higher number of patients and health workers: internal medicine, general surgery and orthopedics. This study has some strength point: first, we reported the NC diagnosis in a large and multicentric cohort in the Nord-West Italy at the time of deep impact of pandemic; second, we reported the provenance of patients according to different wards or LTC facilities. Major limitations are: retrospective design, some patients with not reported provenance, lack of longitudinal analysis during the different phases of pandemic, mortality rate referred to a pre-vaccination cohort. In conclusion, as observed in the multivariate analysis, age, ICU admission, sepsis and LTC provenance are the predictive factors for mortality in NC; older patients with comorbidities come from LTC facilities should therefore receive early diagnosis and treatment and quick hospital admission before the clinical worsening.
Ethics
Approved by local Ethic Committee (N. Prot. CE 0031285 24 March 2020, n.0000381 31/03/2020).
Authorship statement
F.D.R, L.B. and S.C. were responsible for the organization and coordination of the study. L.B. and G.C. were chief investigators and responsible for the data analysis. T.L., S.S., S.M.P., I.D.B., N.S. and T.R. developed the study design. All authors contributed to the writing of the final manuscript. All members of the CORACLE registry contributed to the management of the study.
Conflict of interest
All authors have no conflicts of interest to declare.
Funding
None.
Provenance and peer review
Not commissioned; externally peer reviewed.
Availability of data and material
Available on affordable request.
References
- 1.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. apr. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Case definition for coronavirus disease 2019 (COVID-19), as of 3 December 2020. European Centre for Disease Prevention and Control; 2020. https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition (accessed in the April 2022) [Google Scholar]
- 3.Zhou Q., Gao Y., Wang X., Liu R., Du P., Wang X., et al. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med. 2020;8(10):629. doi: 10.21037/atm-20-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter B., Collins J.T., Barlow-Pay F., Rickard F., Bruce E., Verduri A., et al. Nosocomial COVID-19 infection: examining the risk of mortality. The COPE-Nosocomial Study (COVID in Older PEople) J Hosp Infect. 2020;106(2):376–384. doi: 10.1016/j.jhin.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhee C., Baker M., Vaidya V., Tucker R., Resnick A., Morris C.A., et al. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open. 2020;3(9):e2020498. doi: 10.1001/jamanetworkopen2020.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Rosa F.G., Palazzo A., Rosso T., Shbaklo N., Mussa M., Boglione L., et al. Risk factors for mortality in COVID-19 hospitalized patients in Piedmont, Italy: results from the multicenter, regional, CORACLE registry. J Clin Med. 2021;10(9) doi: 10.3390/jcm10091951. Art. n. 9, gen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EpiCentro A survey on COVID-19 infection in long-stay residential care homes. 2021. https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-survey-rsa (consultato 4 giugno 2021)
- 9.Iritani O., Okuno T., Hama D., Kane A., Kodera K., Morigaki K., et al. Clusters of COVID-19 in long-term care hospitals and facilities in Japan from 16 January to 9 May 2020. Geriatr Gerontol Int. 2020;20(7):715–719. doi: 10.1111/ggi.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellan M., Patti G., Hayden E., Azzolina D., Pirisi M., Acquaviva A., et al. Fatality rate and predictors of mortality in an Italian cohort of hospitalized COVID-19 patients. Sci Rep. 2020;10(1):20731. doi: 10.1038/s41598-020-77698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Adamo H., Yoshikawa T., Ouslander J.G. Coronavirus disease 2019 in geriatrics and long-term care: the ABCDs of COVID-19. J Am Geriatr Soc. 2020;68(5):912–917. doi: 10.1111/jgs.16445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available on affordable request.