Abstract
IgE-mediated release of proinflammatory mediators and cytokines from basophils and mast cells is a central event in allergic disorders. Several groups of investigators have demonstrated the presence of autoantibodies against IgE and/or FcεRI in patients with chronic spontaneous urticaria. By contrast, the prevalence and functional activity of anti-IgE autoantibodies in atopic dermatitis (AD) are largely unknown. We evaluated the ability of IgG anti-IgE from patients with AD to induce the in vitro IgE-dependent activation of human basophils and skin and lung mast cells. Different preparations of IgG anti-IgE purified from patients with AD and rabbit IgG anti-IgE were compared for their triggering effects on the in vitro release of histamine and type 2 cytokines (IL-4, IL-13) from basophils and of histamine and lipid mediators (prostaglandin D2 and cysteinyl leukotriene C4) from human skin and lung mast cells. One preparation of human IgG anti-IgE out of six patients with AD induced histamine release from basophils, skin and lung mast cells. This preparation of human IgG anti-IgE induced the secretion of cytokines and eicosanoids from basophils and mast cells, respectively. Human monoclonal IgE was a competitive antagonist of both human and rabbit IgG anti-IgE. Human anti-IgE was more potent than rabbit anti-IgE for IL-4 and IL-13 production by basophils and histamine, prostaglandin D2 and leukotriene C4 release from mast cells. Functional anti-IgE autoantibodies rarely occur in patients with AD. When present, they induce the release of proinflammatory mediators and cytokines from basophils and mast cells, thereby possibly contributing to sustained IgE-dependent inflammation in at least a subset of patients with this disorder.
Keywords: allergy, anti-IgE, atopic dermatitis, basophils, IL-4, IL-13, mast cells
Introduction
Mast cells and basophils are important cells of the immune system (1–3) and play critical roles in several allergic (4–9) and autoimmune disorders (10–12), infections (13, 14), cardiovascular diseases (15–17), immunodeficiencies (18), and cancer (19–22). The secretion of preformed mediators (e.g. histamine) and de novo synthesis of lipid mediators (e.g. leukotriene C4, prostaglandin D2) and various cytokines following FcεRI cross-linkage plays key roles in diverse IgE-mediated allergic conditions, including atopic dermatitis (AD) (23), chronic spontaneous urticaria (CSU) (24, 25), asthma (5, 26, 27), allergic rhinitis (28), food allergies (29), and anaphylaxis (30–32).
Human mast cells and basophils express a complete (αβγ2), high-affinity receptor for IgE (FcεRI) (33). The interaction of IgE with its receptor is characterized by a very slow dissociation rate (Koff < 10-5/s), accounting for its uniquely high affinity, the highest reported for a human immunoglobulin (Ig) to any of its receptors (34, 35). Aggregation of FcεRI bound to IgE by multivalent antigens, anti-IgE antibodies generated in rabbit or goat (36, 37), or superantigens (38–41) leads to mast cell and basophil activation and mediator release.
Several studies have reported the presence of spontaneously occurring autoantibodies to IgE (36, 42–45), FcεRI (46–49), or both in diverse allergic (36, 42–46, 48, 50–52) and autoimmune disorders (47, 53). Most of these studies have focused on the ability of anti-IgE/FcεRI autoantibodies isolated from patients with CSU to activate peripheral blood basophils (36, 42, 46–48). However, most anti-IgE/FcεRI antibodies isolated from patients with CSU (36), asthma (50), or AD (44) are ineffective basophil secretagogues, which might explain some of the controversies in the field (50, 54). These controversial findings do not necessarily rule out the ability of some of these autoantibodies to activate human tissue mast cells. In any instance, the recent documentation of IgE autoantibodies against eosinophil peroxidase and eosinophil cationic protein in some patients with CSU and AD further reinforce the notion that shared, dysregulated immune functions may differentially contribute to the pathogenesis of these conditions (55).
Even though basophils account for approximately 1% of circulating peripheral blood leukocytes, analysis of basophil activation in vitro has become a mainstay of research in allergy and immunology for some compelling reasons. First, these cells can play critical roles in the activation of type 2 immune responses through the production of such Th2-like cytokines as IL-4 and IL-13 (38, 39, 56–62); second, basophils have the propensity to migrate into the sites of allergic inflammation (63–65); last, but not least, these cells are much more readily available for analysis than human tissue-resident mast cells.
The purpose of this study was four-fold. First, we examined the presence of functional IgG anti-IgE autoantibodies in patients with AD and compared their functions to rabbit IgG anti-IgE and to human polyclonal IgG. Second, we evaluated the effects of functional IgG anti-IgE on the release of Th2-like cytokines (IL-4 and IL-13) from human basophils. Third, we investigated whether human monoclonal IgE is a competitive antagonist of human and rabbit IgG anti-IgE. Finally, we examined the ability of functional human IgG anti-IgE to activate human primary skin and lung mast cells.
Materials and Methods
Reagents and Buffers
Bovine serum albumin, human serum albumin, piperazine-N,N’-bis (2-ethanesulfonic acid) (Pipes), L-glutamine, antibiotic-antimycotic solution (10,000 IU penicillin, 10 mg/mL streptomycin, and 25 µg/mL amphotericin B), collagenase (Worthington Biochemical Corp., Lakewood, NJ, USA), Hanks’ balanced salt solution, fetal calf serum (FCS) (Thermo-Fisher, Grand Island, NY, USA), pronase (Merck Millipore, Burlington, CA, USA), RPMI 1640 with 25 mM HEPES buffer, Eagle’s minimum essential medium (Fuji Film, Research Triangle Park, NC, USA), Percoll (Pharmacia Fine Chemicals, Uppsala, Sweden), CD117 MicroBeads (Miltenyi Biotech, Bologna, Italy), Iscove modified Dulbecco Medium (IMDM) (Fuji Film, Research Triangle Park, NC, USA), HClO4 (Baker Chemical Co., Deventer, Netherlands), hyaluronidase, chymopapain, elastase type I, cysteinyl leukotriene C4 (LTC4), and prostaglandin D2 (PGD2) (Sigma Chemical Co., St. Louis, MO), deoxyribonuclease I (Merck Millipore, Burlington, CA, USA), (3H)-LTC4 and (3H)-PGD2 (New England Nuclear, Boston, MA) were commercially purchased. Rabbit IgG anti-IgE antibody, produced by rabbit immunization with the Fc fragment of a human IgE myeloma (patient PS) and then absorbed with the IgE Fab as previously described (37), was kindly donated by Drs. Kimishige and Teruko Ishizaka (La Jolla Institute for Allergy and Immunology, La Jolla, CA). Rabbit anti-LTC4 and anti-PGD2 antibodies were a gift of Dr. Lawrence M. Lichtenstein (The Johns Hopkins University, Baltimore, MD). The Pipes buffer used in these experiments was a mixture of 25 mM Pipes, 110 mM NaCl, 5 mM KCl, pH 7.37, referred to as P. P2CG contains, in addition to P, 2 mM CaCl2 and 1 g/L dextrose (66); pH was titrated to 7.4 with NaHCO3.
Atopic Dermatitis Patients
The study was approved by the Ethics Committee of the University of Naples Federico II, School of Medicine (Prot. 198/18), and informed consent was obtained from all participants prior to collection of blood according to recommendations from the Declaration of Helsinki. Serum samples from six patients with AD (aged 5 to 17 years) and six normal donors (aged 6 to 22 years) were collected and stored at -20°C. Patients with AD had similar clinical pictures, characterized by a chronic, pruritic skin eruption marked by erythema, papules, or lichenification of flexural areas of the extremities, face and neck (67). Serum samples were obtained from these patients after not taking any drug for at least one week.
Purification of Human Monoclonal IgE
IgE myeloma protein was purified from a myeloma patient (68) by gel filtration on Sepharose G-200 followed by elution through a Sepharose CL-4B column. Analysis by sodium dodecyl-sulfate polyacrylamide gel electrophoresis of purified human monoclonal IgE proteins demonstrated a single protein with a m.w. of 180,000-200,000 D. Analysis by radioimmunoassay showed no IgG, IgM, or IgA contamination (38, 69, 70).
Purification of Human Polyclonal IgG
Human IgG were purified by precipitation of human serum with 50% saturated NH4SO4 followed by chromatography on a DEAE-cellulose column equilibrated with 0.01 M phosphate buffer (pH 7.9), as previously described (70, 71).
Purification of Human IgG Anti-IgE Antibody
Comparable levels of IgG anti-IgE antibodies were detected in serum samples from the six AD patients studied, which averaged 1,020 ng/ml (± 135 ng/ml), much higher than in nonatopic controls (< 50 ng/ml) (45). For affinity purification of these autoantibodies, sera (3 ml for each run) were passed through an immunosorbent Sepharose 4B column (1.2 x 5 cm) coated with IgE purified from ADZ (45). Immunosorbent-bound Ig with anti-IgE activity were eluted with glycine HCl buffer 0.2 M (pH 2.8), and the pH was rapidly readjusted by the addition of 2 M NaOH. The total content of immunoglobulins of the eluted fraction was measured by radioimmunoassay. Anti-IgE activity belonged to the IgG isotype. IgE content was less than 0.05 U/ml. The specificity and activity of IgG anti-IgE were tested as described elsewhere (45).
Purification of Human Basophils
Basophils were purified from peripheral blood of healthy volunteers, aged 19-45 years, undergoing hemapheresis within the Immunohematology Unit at the University of Naples Federico II. Buffy coats were subjected to double-Percoll density centrifugation, which produced basophil-depleted and basophil-enriched cell suspensions (72). Basophils were purified from the basophil-enriched cell suspensions using the Basophil Isolation Kit II (Miltenyi, Biotec, Bologna, Italy). Basophils, with purity ≥ 95%, assessed by Alcian blue staining, were incubated in IMDM in the presence of activating stimuli for 4 hours (IL-4 secretion) or 18 hours (IL-13 secretion) at 37°C (38). At the end of these incubations, the cell-free supernatants were stored at -20°C for subsequent assay of IL-4 and IL-13.
Isolation of Human Skin Mast Cells
The study was approved by the Ethics Committee of the University of Naples Federico II (Protocol: Human MC No. 7/19) and informed consent was obtained from all donors. Skin obtained from patients undergoing either mastectomy for breast cancer or elective cosmetic surgery was separated from the subcutaneous fat by blunt dissection. The tissue was finely cut into 1- to 2-mm fragments and dispersed into single-cell suspension as previously described (73). Yields with this technique ranged between 0.1 and 0.9 × 106 mast cells/g of wet tissue, and purity was between 5 and 10%. Human skin mast cells (HSMCs) were further purified using a CD117 MicroBead kit cell sorting system (Miltenyi Biotec, Bologna, Italy) according to the manufacturer’s instructions. Mast cell purity using this technique ranged from 36 to 71% as assessed by Alcian blue staining.
Isolation of Human Lung Mast Cells
Human lung mast cells (HLMCs) were purified from macroscopically normal lung tissue obtained from patients [hepatitis C virus (HCV−), hepatitis B surface Ag (HBsAg−), HIV−] affected by lung adenocarcinoma undergoing thoracic surgery (74, 75). Freshly resected lung tissue was obtained intraoperatively and was minced finely with scissors and washed extensively with Pipes buffer over Nytex cloth (120-μm pore size) (Tetko, Elmsford, NY, USA). The cells were suspended (106 cells/mL) in RPMI 1640 with 5% FCS, 2 mM L-glutamine, and 1% antibiotic-antimycotic solution and incubated in 24-well plates (Falcon, Becton Dickinson, Milan, Italy). The enzymatic tissue dispersion yielded ≈5 × 105 mast cells/gram of lung tissue and purity ranged from 4% to 19% (40). HLMCs were further purified using a CD117 MicroBead kit cell sorting system (Miltenyi Biotec, Bologna, Italy) according to the manufacturer’s instructions (40). Mast cell purity using this technique ranged from 58% to 82% as assessed by Alcian blue staining.
Histamine Release From Human Basophils
Whole blood samples were processed immediately after collection to obtain leukocyte-enriched preparations (76, 77). Duplicate leukocyte aliquots were incubated (45 minutes at 37°C) in P2CG buffer with increasing concentrations of rabbit IgG anti-human IgE myeloma (patient PS; anti-IgE) or human IgG anti-IgE. Cell-free supernatants were collected and stored at −20°C for subsequent assay of histamine content using an automated fluorometric technique (78). Histamine release (HR) was expressed as percent of the total content assessed in parallel samples lysed by addition of 2% HClO4, minus the basal, or spontaneous release (77). Percent HR values were the means of duplicate determinations, differing by <5%. Basophil reactivity, that is, the maximal percent histamine release (HRMAX), and threshold sensitivity (HRSENS), that is, 100x the inverse of the secretagogue concentration inducing half-maximal HR (EC50), were calculated as described (76, 79–81).
Histamine Release From Mast Cells
HSMCs or HLMCs (≈3 × 104 mast cells per tube) were resuspended in P2CG. 0.3 mL of the cell suspensions were placed in 12 × 75 mm polyethylene tubes. 0.2 mL of each prewarmed releasing stimulus was added, and incubation was continued at 37°C for 45 min (40, 41). At the end of incubation, cells were centrifuged (1000× g, 4°C, 5 min) and supernatants were stored at –20°C for subsequent assay of histamine content. Histamine was measured in duplicate determinations with an automated fluorometric technique (78).
IL-4 and IL-13 ELISA
IL-4 and IL-13 were assessed in duplicate samples using ELISA kits according to manifacturer’s instructions (Quantikine ELISA Kit) (R&D Systems, Minneapolis, MN, USA). The ELISA detection range was 31-2,000 pg/ml (IL-4) and 125-4,000 pg/ml (IL-13).
Immunoassay of LTC4 and PGD2
LTC4 and PGD2 were measured in duplicate samples by radioimmunoassay (40, 82). The anti-LTC4 and anti-PGD2 antibodies are highly selective, with less than 1% cross-reactivity to other eicosanoids (82, 83).
Statistical Analysis
Data were analyzed with the GraphPad Prism 8 software package (GraphPad Software, La Jolla, CA, USA). Values were expressed as mean ± SEM (standard error of the mean). Statistical analysis was performed using Student’s t-test or one-way analysis of variance. Values were considered significant when the probability was below the 5% confidence level (p < 0.05).
Results
Effects of Human and Rabbit IgG Anti-IgE on Histamine Release From Human Basophils
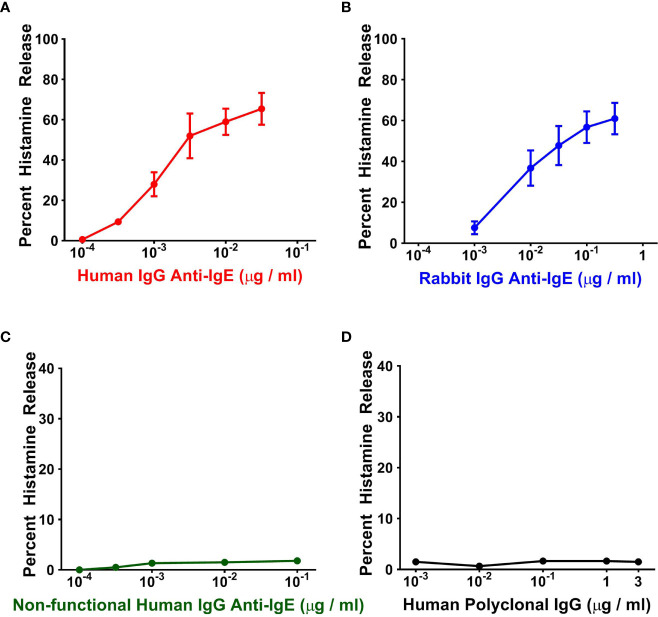
In a first group of experiments, we compared the effects of increasing concentrations of human IgG anti-IgE purified from the sera of six patients with AD, rabbit IgG anti-IgE and human polyclonal IgG on HR from human basophils. Figure 1A shows that increasing concentrations (10-4 to 3 x 10-2 μg/ml) of human IgG anti-IgE isolated from only one out of six AD patients, as previously described (44), induced the release of substantial amounts of histamine from basophils isolated from six different normal donors. Shown for comparison is the concentration-dependent release of histamine induced by higher concentrations of rabbit IgG anti-IgE (10-3 to 3 x 10-1 μg/ml) in parallel experiments with the same basophil preparations ( Figure 1B ). Similarly, in the same experiments, non-functional human IgG anti-IgE purified from the other five AD patients did not induce HR from basophils ( Figure 1C ). In these experiments, human polyclonal IgG (10-3 to 3 μg/ml) purified from six healthy donors failed to induce mediator release from basophils ( Figure 1D ). Basophil reactivity, that is the maximal percent HR (HRMAX) in response to human IgG anti-IgE (70.0% ± 3.80%), was similar to basophil reactivity to rabbit IgG anti-IgE (65.8% ± 3.68%). By contrast, the secretagogue concentration inducing half-maximal histamine release (EC50) induced by the functionally active human anti-IgE preparation (2.4 x 10-3 ± 5 x 10-4 μg/ml) was significantly lower than the corresponding concentration of rabbit anti-IgE (4 x 10-2 ± 1 x 10-2 μg/ml), hence resulting in significantly higher HRSENS (p < 0.05). These results indicate that one preparation of the human IgG anti-IgE preparations tested was active on human basophils. This preparation of human IgG anti-IgE is from now on referred to as “human anti-IgE”.
Figure 1.
Effects of increasing concentrations of human IgG anti-IgE (A) and rabbit IgG anti-IgE (B) on HR from basophils obtained from six normal donors. Neither non-functional human IgG anti-IgE obtained from the other five atopic dermatitis donors (C) nor human polyclonal, pooled from six nonatopic donors, IgG induced mediator release from basophils (D). Each point represents the mean ± SEM percent HR in six different preparations of basophils. Error bars are not shown when graphically too small.
Effects of Human and Rabbit Anti-IgE on Cytokine Production by Human Basophils
IgE cross-linking induced by rabbit or goat anti-IgE (57, 58, 60, 61, 72, 84–87) or superantigens (38, 39, 59) can induce the production of IL-4 and IL-13 from human basophils. In a series of parallel experiments, we compared the effects of human and rabbit anti-IgE on the release of IL-4 and IL-13 from peripheral blood basophils purified (> 95%) from healthy donors. Figure 2 shows the results of five independent experiments in which we examined the effects of increasing concentrations (10-3 to 10-1 μg/ml) of human and rabbit anti-IgE. In these experiments, basophils were incubated 4 hours at 37°C to evaluate IL-4 release, whereas they were incubated 18 hours at 37°C to examine IL-13 production, as previously reported (38, 39, 60, 72). Both preparations of anti-IgE induced a concentration-dependent release of IL-4 ( Figure 2A ) and IL-13 ( Figure 2B ). However, human anti-IgE, at all tested concentrations, was more effective than the corresponding concentrations of rabbit anti-IgE in inducing the release of both IL-4 and IL-13 from basophils. IgG with anti-IgE activity obtained from the other five AD patients did not cause IL-4 and IL-13 release from human basophils (data not shown). Similarly, human polyclonal IgG obtained from six normal donors did not induce cytokine release from basophils (data not shown).
Figure 2.
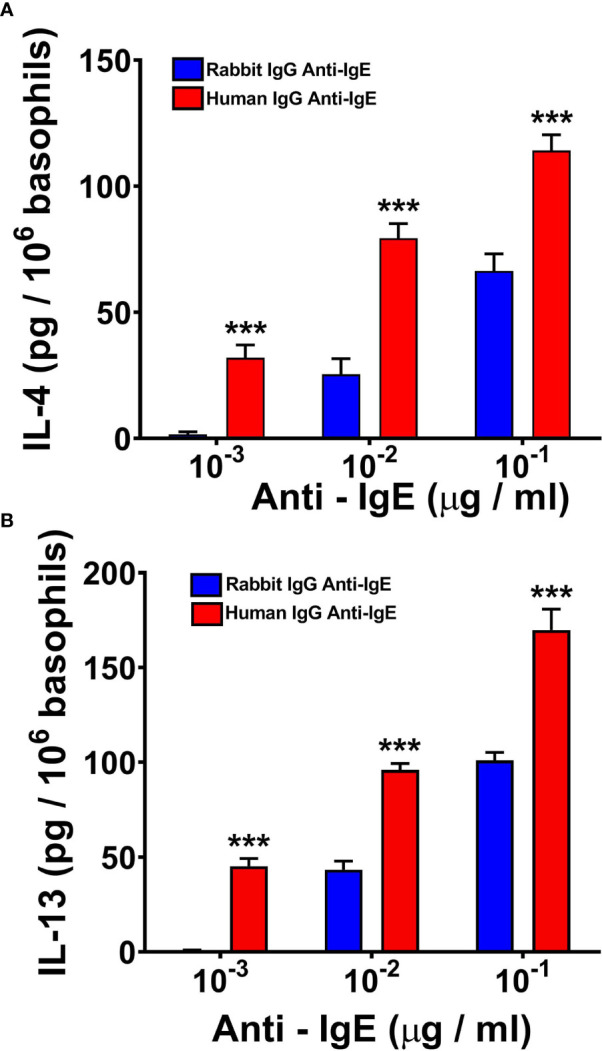
Effects of increasing concentrations of human IgG anti-IgE (red bars) and rabbit IgG anti-IgE (blue bars) on IL-4 (A) and IL-13 (B) release from human basophils obtained from five donors. Basophils were incubated with secretagogues for 4 hours (IL-4) or 18 hours (IL-13) at 37°C. Each bar represents the mean ± SEM in five parallel experiments. Error bars are not shown when graphically too small. ***p < 0.001 when compared to the corresponding value obtained with rabbit IgG anti-IgE.
Effects of Human Monoclonal IgE on Human or Rabbit Anti-IgE-Induced Mediator Release From Human Basophils
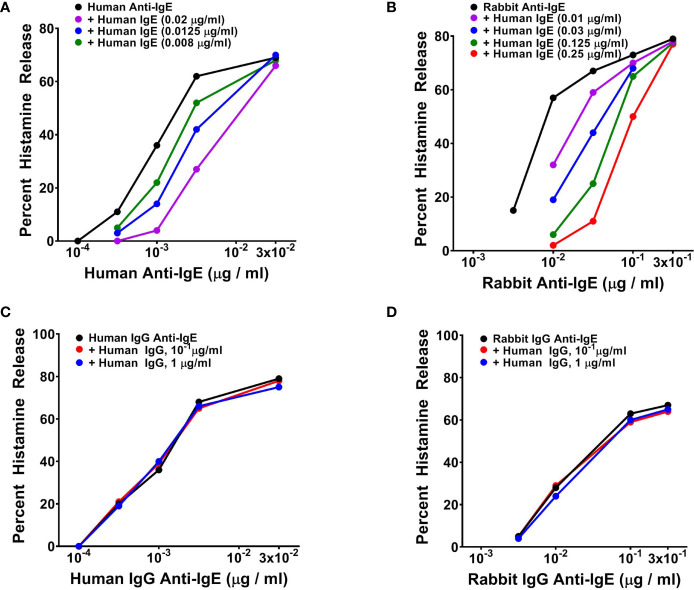
The ability of human and rabbit anti-IgE to trigger basophil mediator release suggested that it might interact with basophil-bound IgE. To test this hypothesis we conducted experiments to verify whether soluble human monoclonal IgE purified from a myeloma patient (68) (70) might inhibit the mediator response to human and rabbit anti-IgE. To this end, basophils were preincubated (10 min at 37°C) with increasing concentrations of human IgE and the cells were incubated for an additional 30 min at 37°C in the presence of increasing concentrations of human or rabbit anti-IgE. Figure 3 illustrates the results of typical experiments showing that preincubation with increasing concentrations of human monoclonal IgE concentration-dependently shifted to the right effects on basophil HR of both human ( Figure 3A ) and rabbit anti-IgE ( Figure 3B ). Preincubation (10 min at 37°C) of human basophils with tenfold higher concentrations of human polyclonal IgG did not interfere with either human ( Figure 3C ) or rabbit anti-IgE effects ( Figure 3D ). Similar results were obtained in three additional experiments. The parallel shift to the right of the HR curve caused by increasing concentrations of human monoclonal IgE on both human and rabbit anti-IgE, without changes in maximal efficacy, suggested that it might act as a competitive inhibitor.
Figure 3.
(A) Effects of increasing concentrations of human monoclonal IgE on human IgG anti-IgE-induced HR from human basophils. Cells were preincubated (10 minutes, 37°C) with the indicated concentrations of IgE and then challenged with the indicated concentrations of human IgG anti-IgE for an additional 30 minutes at 37°C. Each value is the mean of duplicate determinations in a typical experiment out of three similar experiments. (B) Effects of increasing concentrations of human monoclonal IgE on rabbit IgG anti-IgE-induced HR from human basophils. Cells were preincubated (10 minutes, 37°C) with increasing concentrations of IgE and then challenged with the indicated concentrations of rabbit IgG anti-IgE for an additional 30 minutes at 37°C. Each value is the mean of duplicate determinations in a typical experiment out of four. (C) Effect of increasing concentrations of human polyclonal IgG purified from a healthy donor on human IgG anti-IgE-induced HR from human basophils. Cells were preincubated (10 minutes, 37°C) with increasing concentrations of human polyclonal IgG and then challenged with the indicated concentrations of human IgG anti-IgE for an additional 30 minutes at 37°C. (D) Effect of increasing concentrations of human polyclonal IgG purified from a healthy donor on rabbit IgG anti-IgE-induced HR from human basophils. Cells were preincubated (10 minutes, 37°C) with increasing concentrations of human polyclonal IgG and then challenged with the indicated concentrations of rabbit IgG anti-IgE for an additional 30 minutes at 37°C. Each value is the mean of duplicate determinations in a typical experiment out of four.
Effects of Human and Rabbit Anti-IgE on Histamine Release and De Novo Synthesis of PGD2 From Human Skin Mast Cells
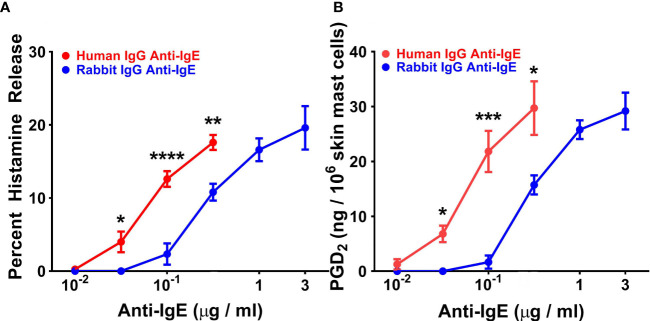
In five parallel experiments, we compared the activating properties of human and rabbit anti-IgE on HR ( Figure 4A ) and de novo synthesis of PGD2 by HSMCs ( Figure 4B ). The maximal percent HR caused by human anti-IgE (17.8 ± 0.91%) was similar to that induced by rabbit anti-IgE (20.2 ± 2.8%). Similarly, the maximal production of PGD2 induced by human anti-IgE (31.1 ± 3.7 ng/106 cells) was comparable to that caused by rabbit anti-IgE (30.5 ± 2.6 ng/106 cells). By contrast, the secretagogue concentration inducing half-maximal histamine release (EC50) for histamine release was significantly lower (5 x 10-2 ± 1 x 10-2 μg/ml) for human anti-IgE compared to rabbit anti-IgE (2.5 x 10-1 ± 6 x 10-2 μg/ml) (p < 0.05), indicating a comparably higher HRSENS. Similarly, the EC50 for PGD2 production caused by human anti-IgE (7.2 x 10-2 ± 2.1 x 10-3 μg/ml) was significantly lower than that of rabbit anti-IgE (2.9 x 10-1 ± 3 x 10-2 μg/ml) (p < 0.05).
Figure 4.
Effects of increasing concentrations of human IgG anti-IgE and rabbit IgG anti-IgE on HR (A) and the de novo synthesis of PGD2 (B) from HSMCs obtained from five different donors. HSMCs were incubated (45 min at 37°C) in the presence of the indicated concentrations of human IgG anti-IgE or rabbit IgG anti-IgE. Each point shows the mean ± SEM. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05 when compared to the corresponding value. Error bars are not shown when graphically too small.
Effects of Human and Rabbit Anti-IgE on Histamine Release and De Novo Synthesis of Lipid Mediators From Human Lung Mast Cells
In five experiments, we compared the effects of increasing concentrations of human and rabbit anti-IgE on HR and de novo synthesis of LTC4 and PGD2 from HLMCs. Increasing concentrations (10-2 to 3x10-1 μg/ml) of human or rabbit anti-IgE (10-1 to 3 μg/ml) caused a concentration-dependent release of histamine from HLMCs ( Figure 5A ). The maximal percent HR in response to human anti-IgE (18.4% ± 1.8%) was similar to HLMC reactivity to rabbit anti-IgE (20.2% ± 1.2%). By contrast, the EC50 was significantly lower (4.6 x 10-2 ± 4 x 10-3 μg/ml) for human compared to rabbit anti-IgE (3.4 x 10-1 ± 8 x 10-2 μg/ml) (p < 0.01). In these experiments, we also compared the effects of human and rabbit anti-IgE on the de novo synthesis of LTC4 and PGD2 from HLMCs. Figure 5B shows that the maximal production of LTC4 by HLMCs exposed to human anti-IgE (40.9 ± 2.2 ng/106 cells) was similar to that caused by rabbit anti-IgE (42.5 ± 2.0 ng/106 cells). By contrast, the concentration of human anti-IgE inducing half-maximal LTC4 release was significantly lower (4.0 x 10-2 ± 4 x 10-3 μg/ml) than the EC50 for rabbit anti-IgE (2.5 x 10-1 ± 6 x 10-2 μg/ml) (p < 0.05). Similarly, HLMC reactivity to human anti-IgE (31.4 ± 2.6 ng/106 cells) was similar to rabbit anti-IgE (38.9 ± 3.0 ng/106 cells) with respect to PGD2 production ( Figure 5C ). The EC50 for PGD2 production caused by human anti-IgE (4.2 x 10-2 ± 1 x 10-3 μg/ml) was significantly lower than that of rabbit anti-IgE (2.8 x 10-1 ± 8 x 10-2 μg/ml) (p < 0.05).
Figure 5.
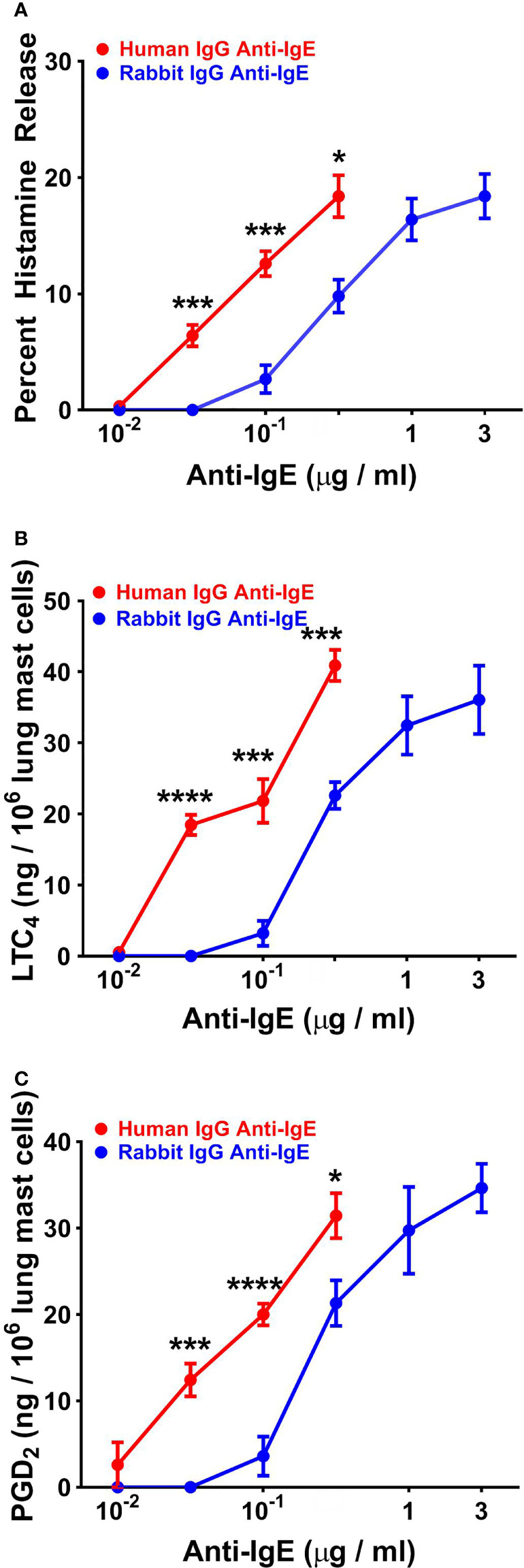
Effects of increasing concentrations of human IgG anti-IgE and rabbit IgG anti-IgE on HR (A) and the de novo synthesis of LTC4 (B) and PGD2 (C) from HLMCs obtained from five different donors. HLMCs were incubated (45 min at 37°C) in the presence of the indicated concentrations of human IgG anti-IgE or rabbit IgG anti-IgE. Each point shows the mean ± SEM. **** p < 0.0001, *** p < 0.001, * p < 0.05 when compared to the corresponding value. Error bars are not shown when graphically too small.
Discussion
Our results indicate that although autoantibodies against IgE can be found in some patients with AD, these can rarely induce the activation of human basophils and mast cells. We have detected functional IgG anti-IgE in one out of six patients with AD and characterized its ability to trigger mediator release from human basophils and mast cells. This human IgG anti-IgE is a more potent secretagogue than rabbit IgG anti-IgE, and human monoclonal IgE appears to act as a competitive antagonist of either antibody. A novel finding emerging from this study is the ability of human anti-IgE from AD to induce the release of IL-4 and IL-13 from human basophils. Another novel aspect is the observation that human anti-IgE activates not only human basophils, but also skin and lung mast cells to release histamine and arachidonic acid metabolites.
The role of naturally occurring anti-IgE/FcεRI autoantibodies in allergic and non allergic disorders is still a fascinating and unsettled issue, as recently discussed by Galli (54). Several investigators have found these autoantibodies in CSU (42, 46–49, 88–91) and in asthma (43, 50, 92). By contrast, anti-IgE autoantibodies have been inconsistently found in AD patients (43–45, 47, 52). Anti-IgE/FcεRI autoantibodies of the IgG class have been found in most of these studies (43–48, 88, 90, 91, 93, 94), while IgM (42, 49), and/or IgA autoantibodies have been only documented in rare instances (49). In most cases the autoantibodies found in patients with CSU or AD lacked the capacity to activate human basophils in vitro (36, 44, 47). While in some studies human IgE-specific IgG autoantibodies were able to activate human basophils (44, 47), in others they even inhibited basophil activation (36, 50).
A limitation in most of these functional studies was that they only examined the potential effects of autoantibodies to IgE or FcεRI on HR from human peripheral blood basophils (36, 42, 46–48, 88, 90, 91). The above results, while contrasting, do not necessarily rule out the hypothesis that these naturally occurring autoantibodies can activate human basophils to release cytokines (e.g., IL-4, IL-13) or tissue mast cells to produce arachidonic acid metabolites.
In this study, we found that only one preparation of human IgG anti-IgE out of six patients with AD had the ability to activate peripheral blood basophils purified from normal donors and mast cells isolated from human skin or lung tissue. Although the sample size examined in this study is too small to conclusively estimate the prevalence of functional anti-IgE autoantibodies in AD patients, these results allow to raise a few points. The apparent low prevalence of functional autoantibodies to IgE might explain, at least in part, the controversial results on the presence of functional such autoantibodies in AD patients (43–45, 47, 52). Moreover, our findings are in line with the systematic, aptly controlled observations by MacGlashan demonstrating that the autoantibodies to IgE and/or FcεRI from the vast majority of patients with CSU lacked the capacity to activate human basophil mediator release (36).
Our results provide some information on the functional potency of the IgG anti-IgE isolated from a patient with AD. Although basophil reactivity, that is the maximal HR in response to human anti-IgE, was similar to that induced by rabbit anti-IgE, the potency of human anti-IgE was significantly higher than that of rabbit anti-IgE. Similar results were obtained when comparing the reactivity and threshold sensitivity of human skin and lung mast cells to human and rabbit anti-IgE in experiments looking not only at the HR but also the de novo synthesis of lipid mediators (i.e., PGD2, and LTC4). Collectively, these results indicate that human anti-IgE, when it is functionally present, can be significantly more potent than rabbit anti-IgE preparations commonly used in experimental or diagnostic in vitro protocols for IgE-dependent activation of human FcεRI+ cells.
We also provide some clues on the immunologic mechanism of activation of human basophils by human IgG anti-IgE. We found that preincubation of human basophils with increasing concentrations of human monoclonal IgE purified from a myeloma patient (68, 70) concentration-dependently interfered with the activating properties of both human and rabbit anti-IgE. The specificity of this response was confirmed by the observation that preincubation of basophils with tenfold higher concentrations of human polyclonal IgG did not antagonize the ability of both human and rabbit to trigger mediator release anti-IgE.
A novel finding of this study is the ability of human IgG anti-IgE to induce the release of Th2-like cytokines (e.g., IL-4, IL-13) from human basophils. The vast majority of studies exploring the functional activity of human anti-IgE and anti-FcεRI have evaluated the ability of these autoantibodies to induce HR from human basophils (36, 42, 47, 48, 50, 88, 90, 91). To the best of our knowledge, we provide the first evidence that a functional preparation of human IgG anti-IgE can also induce the release of IL-4 and IL-13 from human basophils. Also in this case, we observed that only IgG anti-IgE obtained from one out of six AD donors could cause cytokine release from basophils.
Our findings may have some translational relevance. AD is characterized by robust Th2-mediated immune responses to numerous environmental stimuli (95). The Th2 cytokines IL-4 and IL-13 are believed to play pivotal roles in the pathogenesis of AD (96, 97). Consistent with these findings, dual IL-4 and IL-13 blockade with the IL-4Rα antagonist, dupilumab showed unprecedented efficacy in adult AD patients (98, 99). Moreover, recent evidence indicates that LTC4 plays a role in a mouse model of AD (100). The observation that human IgG anti-IgE is a potent stimulus for the production of IL-4/IL-13 from basophils and LTC4 from mast cells suggests that these autoantibodies may play a role in the onset and progression of at least a subset of AD patients.
Human basophils and mast cells are key contributors to allergic disorders (1, 13, 26), including AD (67). A closer understanding of their roles in allergies has been marked by the considerable heterogeneity of these cells, whereby distinct morphologic and functional properties can not only be appreciated between mast cells and basophils (26) but also between cells located in different tissues and districts (40, 101–104). In this study, we demonstrated that human IgG anti-IgE is a potent stimulus for the production of Th2-like cytokines, hinting at a possible role in the upstream control of allergic responses, including IgE synthesis. Further, the agonist effects on prostanoids secretion from skin mast cells, mediators found at substantial levels in AD lesions (105), might have important clinical implications in AD.
In conclusion, our results extend previous findings (36, 44) indicating that only a minority of IgG anti-IgE isolated from patients with AD activates human FcεRI+ cells. Our data show that when functional autoantibodies to IgE are present, these can be more potent than rabbit IgG anti-IgE in inducing the release of histamine, cytokines (IL-4, IL-13) and lipid mediators (PGD2, and LTC4) from human basophils and/or mast cells. Further studies in larger cohorts of patients with different phenotypes of AD are needed to more conclusively assess the prevalence of functional autoantibodies to IgE or FcεRI and their possible contribution to disease pathogenesis and the response to current and prospective therapeutic strategies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the University of Naples Federico II, School of Medicine (Prot. 198/18), and informed consent was obtained from all participants prior to collection of blood according to recommendations from the Declaration of Helsinki. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
RP, IQ, GM, VC, and GV designed the research. RP, IQ, AP, and VC did the experiment. RP, GM, MT, VC, and GV analyzed the data and wrote the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported in part by grants from the CISI-Lab Project (University of Naples Federico II) and TIMING Project and Campania Bioscience (Regione Campania).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Dr. Gjada Criscuolo for her excellent managerial assistance in preparing it and the administrative staff (Dr. Roberto Bifulco, Dr. Anna Ferraro and Dr. Maria Cristina Fucci), without whom it would not be possible to work as a team.
References
- 1. Galli SJ, Gaudenzio N, Tsai M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu Rev Immunol (2020) 38:49–77. doi: 10.1146/annurev-immunol-071719-094903 [DOI] [PubMed] [Google Scholar]
- 2. Hamey FK, Lau WWY, Kucinski I, Wang X, Diamanti E, Wilson NK, et al. Single-Cell Molecular Profiling Provides a High-Resolution Map of Basophil and Mast Cell Development. Allergy (2021) 76:1731–42. doi: 10.1111/all.14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miyake K, Shibata S, Yoshikawa S, Karasuyama H. Basophils and Their Effector Molecules in Allergic Disorders. Allergy (2021) 76:1693–706. doi: 10.1111/all.14662 [DOI] [PubMed] [Google Scholar]
- 4. Schroeder JT. Basophils: Emerging Roles in the Pathogenesis of Allergic Disease. Immunol Rev (2011) 242:144–60. doi: 10.1111/j.1600-065X.2011.01023.x [DOI] [PubMed] [Google Scholar]
- 5. Marone G, Borriello F, Varricchi G, Genovese A, Granata F. Basophils: Historical Reflections and Perspectives. Chem Immunol Allergy (2014) 100:172–92. doi: 10.1159/000358734 [DOI] [PubMed] [Google Scholar]
- 6. Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP Promotes Interleukin-3-Independent Basophil Haematopoiesis and Type 2 Inflammation. Nature (2011) 477:229–33. doi: 10.1038/nature10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siracusa MC, Wojno ED, Artis D. Functional Heterogeneity in the Basophil Cell Lineage. Adv Immunol (2012) 115:141–59. doi: 10.1016/B978-0-12-394299-9.00005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bradding P, Arthur G. Mast Cells in Asthma–State of the Art. Clin Exp Allergy (2016) 46:194–263. doi: 10.1111/cea.12675 [DOI] [PubMed] [Google Scholar]
- 9. Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast Cells as "Tunable" Effector and Immunoregulatory Cells: Recent Advances. Annu Rev Immunol (2005) 23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025 [DOI] [PubMed] [Google Scholar]
- 10. Rivellese F, Nerviani A, Rossi FW, Marone G, Matucci-Cerinic M, de Paulis A, et al. Mast Cells in Rheumatoid Arthritis: Friends or Foes? Autoimmun Rev (2017) 16:557–63. doi: 10.1016/j.autrev.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 11. Maurer M, Altrichter S, Schmetzer O, Scheffel J, Church MK, Metz M. Immunoglobulin E-Mediated Autoimmunity. Front Immunol (2018) 9:689. doi: 10.3389/fimmu.2018.00689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamri Y, Vibhushan S, Pacreau E, Boedec E, Saidoune F, Mailleux A, et al. Basophils and IgE Contribute to Mixed Connective Tissue Disease Development. J Allergy Clin Immunol (2021) 147:1478–89.e1411. doi: 10.1016/j.jaci.2020.12.622 [DOI] [PubMed] [Google Scholar]
- 13. Eberle JU, Voehringer D. Role of Basophils in Protective Immunity to Parasitic Infections. Semin Immunopathol (2016) 38:605–13. doi: 10.1007/s00281-016-0563-3 [DOI] [PubMed] [Google Scholar]
- 14. Yamanishi Y, Miyake K, Iki M, Tsutsui H, Karasuyama H. Recent Advances in Understanding Basophil-Mediated Th2 Immune Responses. Immunol Rev (2017) 278:237–45. doi: 10.1111/imr.12548 [DOI] [PubMed] [Google Scholar]
- 15. Shi GP, Bot I, Kovanen PT. Mast Cells in Human and Experimental Cardiometabolic Diseases. Nat Rev Cardiol (2015) 12:643–58. doi: 10.1038/nrcardio.2015.117 [DOI] [PubMed] [Google Scholar]
- 16. Varricchi G, Marone G, Kovanen PT. Cardiac Mast Cells: Underappreciated Immune Cells in Cardiovascular Homeostasis and Disease. Trends Immunol (2020) 41:734–46. doi: 10.1016/j.it.2020.06.006 [DOI] [PubMed] [Google Scholar]
- 17. Sicklinger F, Meyer IS, Li X, Radtke D, Dicks S, Kornadt MP, et al. Basophils Balance Healing After Myocardial Infarction via IL-4/IL-13. J Clin Invest (2021) 131:e136778. doi: 10.1172/JCI136778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marone G, Varricchi G, Loffredo S, Galdiero MR, Rivellese F, de Paulis A. Are Basophils and Mast Cells Masters in HIV Infection? Int Arch Allergy Immunol (2016) 171:158–65. doi: 10.1159/000452889 [DOI] [PubMed] [Google Scholar]
- 19. Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Granata F. Are Mast Cells MASTers in Cancer? Front Immunol (2017) 8:424. doi: 10.3389/fimmu.2017.00424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sektioglu IM, Carretero R, Bulbuc N, Bald T, Tuting T, Rudensky AY, et al. Basophils Promote Tumor Rejection via Chemotaxis and Infiltration of CD8+ T Cells. Cancer Res (2017) 77:291–302. doi: 10.1158/0008-5472.CAN-16-0993 [DOI] [PubMed] [Google Scholar]
- 21. Hayes MD, Ward S, Crawford G, Seoane RC, Jackson WD, Kipling D, et al. Inflammation-Induced IgE Promotes Epithelial Hyperplasia and Tumour Growth. Elife (2020) 9:e51862. doi: 10.7554/eLife.51862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Monte L, Wormann S, Brunetto E, Heltai S, Magliacane G, Reni M, et al. Basophil Recruitment Into Tumor-Draining Lymph Nodes Correlates With Th2 Inflammation and Reduced Survival in Pancreatic Cancer Patients. Cancer Res (2016) 76:1792–803. doi: 10.1158/0008-5472.CAN-15-1801-T [DOI] [PubMed] [Google Scholar]
- 23. Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, et al. House Dust Mites Activate Nociceptor-Mast Cell Clusters to Drive Type 2 Skin Inflammation. Nat Immunol (2019) 20:1435–43. doi: 10.1038/s41590-019-0493-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rauber MM, Pickert J, Holiangu L, Mobs C, Pfutzner W. Functional and Phenotypic Analysis of Basophils Allows Determining Distinct Subtypes in Patients With Chronic Urticaria. Allergy (2017) 72:1904–11. doi: 10.1111/all.13215 [DOI] [PubMed] [Google Scholar]
- 25. Vasagar K, Vonakis BM, Gober LM, Viksman A, Gibbons SP, Jr., Saini SS. Evidence of In Vivo Basophil Activation in Chronic Idiopathic Urticaria. Clin Exp Allergy (2006) 36:770–6. doi: 10.1111/j.1365-2222.2006.02494.x [DOI] [PubMed] [Google Scholar]
- 26. Varricchi G, Raap U, Rivellese F, Marone G, Gibbs BF. Human Mast Cells and Basophils-How are They Similar How are They Different? Immunol Rev (2018) 282:8–34. doi: 10.1111/imr.12627 [DOI] [PubMed] [Google Scholar]
- 27. Galli SJ, Tsai M. IgE and Mast Cells in Allergic Disease. Nat Med (2012) 18:693–704. doi: 10.1038/nm.2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhai GT, Wang H, Li JX, Cao PP, Jiang WX, Song J, et al. IgD-Activated Mast Cells Induce IgE Synthesis in B Cells in Nasal Polyps. J Allergy Clin Immunol (2018) 142:1489–1499.e1423. doi: 10.1016/j.jaci.2018.07.025 [DOI] [PubMed] [Google Scholar]
- 29. Macchia D, Melioli G, Pravettoni V, Nucera E, Piantanida M, Caminati M, et al. Guidelines for the Use and Interpretation of Diagnostic Methods in Adult Food Allergy. Clin Mol Allergy (2015) 13:27. doi: 10.1186/s12948-015-0033-933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leyva-Castillo JM, Galand C, Kam C, Burton O, Gurish M, Musser MA, et al. Mechanical Skin Injury Promotes Food Anaphylaxis by Driving Intestinal Mast Cell Expansion. Immunity (2019) 50:1262–75.e1264. doi: 10.1016/j.immuni.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choi HW, Suwanpradid J, Kim IH, Staats HF, Haniffa M, MacLeod AS, et al. Perivascular Dendritic Cells Elicit Anaphylaxis by Relaying Allergens to Mast Cells via Microvesicles. Science (2018) 362:eaao0666. doi: 10.1126/science.aao0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dispenza MC, Krier-Burris RA, Chhiba KD, Undem BJ, Robida PA, Bochner BS. Bruton's Tyrosine Kinase Inhibition Effectively Protects Against Human IgE-Mediated Anaphylaxis. J Clin Invest (2020) 130:4759–70. doi: 10.1172/JCI138448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blank U, Huang H, Kawakami T. The High Affinity IgE Receptor: A Signaling Update. Curr Opin Immunol (2021) 72:51–8. doi: 10.1016/j.coi.2021.03.015 [DOI] [PubMed] [Google Scholar]
- 34. Garman SC, Wurzburg BA, Tarchevskaya SS, Kinet JP, Jardetzky TS. Structure of the Fc Fragment of Human IgE Bound to its High-Affinity Receptor Fc epsilonRI Alpha. Nature (2000) 406:259–66. doi: 10.1038/35018500 [DOI] [PubMed] [Google Scholar]
- 35. Kulczycki A, Jr., Metzger H. The Interaction of IgE With Rat Basophilic Leukemia Cells. II. Quantitative Aspects of the Binding Reaction. J Exp Med (1974) 140:1676–95. doi: 10.1084/jem.140.6.1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. MacGlashan D. Autoantibodies to IgE and FcepsilonRI and the Natural Variability of Spleen Tyrosine Kinase Expression in Basophils. J Allergy Clin Immunol (2019) 143:1100–1107.e1111. doi: 10.1016/j.jaci.2018.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ishizaka T, Ishizaka K, Johansson SG, Bennich H. Histamine Release From Human Leukocytes by Anti-Gamma E Antibodies. J Immunol (1969) 102:884–92. [PubMed] [Google Scholar]
- 38. Genovese A, Borgia G, Bjorck L, Petraroli A, de Paulis A, Piazza M, et al. Immunoglobulin Superantigen Protein L Induces IL-4 and IL-13 Secretion From Human Fc Epsilon RI+ Cells Through Interaction With the Kappa Light Chains of IgE. J Immunol (2003) 170:1854–61. doi: 10.4049/jimmunol.170.4.1854 [DOI] [PubMed] [Google Scholar]
- 39. Patella V, Florio G, Petraroli A, Marone G. HIV-1 Gp120 Induces IL-4 and IL-13 Release From Human Fc Epsilon RI+ Cells Through Interaction With the VH3 Region of IgE. J Immunol (2000) 164:589–95. doi: 10.4049/jimmunol.164.2.589 [DOI] [PubMed] [Google Scholar]
- 40. Cristinziano L, Poto R, Criscuolo G, Ferrara AL, Galdiero MR, Modestino L, et al. IL-33 and Superantigenic Activation of Human Lung Mast Cells Induce the Release of Angiogenic and Lymphangiogenic Factors. Cells (2021) 10:145. doi: 10.3390/cells10010145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marone G, Rossi FW, Pecoraro A, Pucino V, Criscuolo G, Paulis A, et al. HIV Gp120 Induces the Release of Proinflammatory, Angiogenic, and Lymphangiogenic Factors From Human Lung Mast Cells. Vaccines (Basel) (2020) 8:208. doi: 10.3390/vaccines8020208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gruber BL, Baeza ML, Marchese MJ, Agnello V, Kaplan AP. Prevalence and Functional Role of Anti-IgE Autoantibodies in Urticarial Syndromes. J Invest Dermatol (1988) 90:213–7. doi: 10.1111/1523-1747.ep12462239 [DOI] [PubMed] [Google Scholar]
- 43. Nawata Y, Koike T, Hosokawa H, Tomioka H, Yoshida S. Anti-IgE Autoantibody in Patients With Atopic Dermatitis. J Immunol (1985) 135:478–82. [PubMed] [Google Scholar]
- 44. Marone G, Casolaro V, Paganelli R, Quinti I. IgG Anti-IgE From Atopic Dermatitis Induces Mediator Release From Basophils and Mast Cells. J Invest Dermatol (1989) 93:246–52. doi: 10.1111/1523-1747.ep12277582 [DOI] [PubMed] [Google Scholar]
- 45. Quinti I, Brozek C, Wood N, Geha RS, Leung DY. Circulating IgG Autoantibodies to IgE in Atopic Syndromes. J Allergy Clin Immunol (1986) 77:586–94. doi: 10.1016/0091-6749(86)90350-7 [DOI] [PubMed] [Google Scholar]
- 46. Niimi N, Francis DM, Kermani F, O'Donnell BF, Hide M, Kobza-Black A, et al. Dermal Mast Cell Activation by Autoantibodies Against the High Affinity IgE Receptor in Chronic Urticaria. J Invest Dermatol (1996) 106:1001–6. doi: 10.1111/1523-1747.ep12338544 [DOI] [PubMed] [Google Scholar]
- 47. Fiebiger E, Hammerschmid F, Stingl G, Maurer D. Anti-FcepsilonRIalpha Autoantibodies in Autoimmune-Mediated Disorders. Identification of a Structure-Function Relationship. J Clin Invest (1998) 101:243–51. doi: 10.1172/JCI511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hide M, Francis DM, Grattan CE, Hakimi J, Kochan JP, Greaves MW. Autoantibodies Against the High-Affinity IgE Receptor as a Cause of Histamine Release in Chronic Urticaria. N Engl J Med (1993) 328:1599–604. doi: 10.1056/NEJM199306033282204 [DOI] [PubMed] [Google Scholar]
- 49. Altrichter S, Zampeli V, Ellrich A, Zhang K, Church MK, Maurer M. IgM and IgA in Addition to IgG Autoantibodies Against FcvarepsilonRIalpha are Frequent and Associated With Disease Markers of Chronic Spontaneous Urticaria. Allergy (2020) 75:3208–15. doi: 10.1111/all.14412 [DOI] [PubMed] [Google Scholar]
- 50. Chan YC, Ramadani F, Santos AF, Pillai P, Ohm-Laursen L, Harper CE, et al. "Auto-Anti-IgE": Naturally Occurring IgG Anti-IgE Antibodies may Inhibit Allergen-Induced Basophil Activation. J Allergy Clin Immunol (2014) 134:1394–1401 e1394. doi: 10.1016/j.jaci.2014.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nawata Y, Koike T, Yanagisawa T, Iwamoto I, Itaya T, Yoshida S, et al. Anti-IgE Autoantibody in Patients With Bronchial Asthma. Clin Exp Immunol (1984) 58:348–56. [PMC free article] [PubMed] [Google Scholar]
- 52. Czech W, Stadler BM, Schopf E, Kapp A. IgE Autoantibodies in Atopic Dermatitis–Occurrence of Different Antibodies Against the CH3 and the CH4 Epitopes of IgE. Allergy (1995) 50:243–8. doi: 10.1111/j.1398-9995.1995.tb01141.x [DOI] [PubMed] [Google Scholar]
- 53. Gruber BL, Kaufman LD, Marchese MJ, Roth W, Kaplan AP. Anti-IgE Autoantibodies in Systemic Lupus Erythematosus. Prevalence and Biologic Activity. Arthritis Rheum (1988) 31:1000–6. doi: 10.1002/art.1780310810 [DOI] [PubMed] [Google Scholar]
- 54. Galli SJ. Complexities in Analyzing Human Basophil Responses to Autoantibodies to IgE or FcepsilonRI. J Allergy Clin Immunol (2019) 143:932–4. doi: 10.1016/j.jaci.2018.12.998 [DOI] [PubMed] [Google Scholar]
- 55. Sanchez J, Sanchez A, Munera M, Garcia E, Lopez JF, Velasquez-Lopera M, et al. Presence of IgE Autoantibodies Against Eosinophil Peroxidase and Eosinophil Cationic Protein in Severe Chronic Spontaneous Urticaria and Atopic Dermatitis. Allergy Asthma Immunol Res (2021) 13:746–61. doi: 10.4168/aair.2021.13.5.746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Galeotti C, Stephen-Victor E, Karnam A, Das M, Gilardin L, Maddur MS, et al. Intravenous Immunoglobulin Induces IL-4 in Human Basophils by Signaling Through Surface-Bound IgE. J Allergy Clin Immunol (2019) 144:524–535.e528. doi: 10.1016/j.jaci.2018.10.064 [DOI] [PubMed] [Google Scholar]
- 57. Rivellese F, Suurmond J, de Paulis A, Marone G, Huizinga TW, Toes RE. IgE and IL-33-Mediated Triggering of Human Basophils Inhibits TLR4-Induced Monocyte Activation. Eur J Immunol (2014) 44:3045–55. doi: 10.1002/eji.201444731 [DOI] [PubMed] [Google Scholar]
- 58. Silver MR, Margulis A, Wood N, Goldman SJ, Kasaian M, Chaudhary D. IL-33 Synergizes With IgE-Dependent and IgE-Independent Agents to Promote Mast Cell and Basophil Activation. Inflammation Res (2010) 59:207–18. doi: 10.1007/s00011-009-0088-5 [DOI] [PubMed] [Google Scholar]
- 59. Patella V, Giuliano A, Bouvet JP, Marone G. Endogenous Superallergen Protein Fv Induces IL-4 Secretion From Human Fc Epsilon RI+ Cells Through Interaction With the VH3 Region of IgE. J Immunol (1998) 161:5647–55. [PubMed] [Google Scholar]
- 60. Redrup AC, Howard BP, MacGlashan DW, Jr., Kagey-Sobotka A, Lichtenstein LM, Schroeder JT. Differential Regulation of IL-4 and IL-13 Secretion by Human Basophils: Their Relationship to Histamine Release in Mixed Leukocyte Cultures. J Immunol (1998) 160:1957–64. [PubMed] [Google Scholar]
- 61. Ochensberger B, Daepp GC, Rihs S, Dahinden CA. Human Blood Basophils Produce Interleukin-13 in Response to IgE-Receptor-Dependent and -Independent Activation. Blood (1996) 88:3028–37. [PubMed] [Google Scholar]
- 62. MacGlashan D, Jr., White JM, Huang SK, Ono SJ, Schroeder JT, Lichtenstein LM. Secretion of IL-4 From Human Basophils. The Relationship Between IL-4 mRNA and Protein in Resting and Stimulated Basophils. J Immunol (1994) 152:3006–16. [PubMed] [Google Scholar]
- 63. Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil Recruitment and Activation in Inflammatory Skin Diseases. Allergy (2011) 66:1107–13. doi: 10.1111/j.1398-9995.2011.02570.x [DOI] [PubMed] [Google Scholar]
- 64. Guo CB, Liu MC, Galli SJ, Bochner BS, Kagey-Sobotka A, Lichtenstein LM. Identification of IgE-Bearing Cells in the Late-Phase Response to Antigen in the Lung as Basophils. Am J Respir Cell Mol Biol (1994) 10:384–90. doi: 10.1165/ajrcmb.10.4.7510984 [DOI] [PubMed] [Google Scholar]
- 65. Irani AM, Huang C, Xia HZ, Kepley C, Nafie A, Fouda ED, et al. Immunohistochemical Detection of Human Basophils in Late-Phase Skin Reactions. J Allergy Clin Immunol (1998) 101:354–62. doi: 10.1016/S0091-6749(98)70248-9 [DOI] [PubMed] [Google Scholar]
- 66. Patella V, de Crescenzo G, Marino I, Genovese A, Adt M, Gleich GJ, et al. Eosinophil Granule Proteins Activate Human Heart Mast Cells. J Immunol (1996) 157:1219–25. [PubMed] [Google Scholar]
- 67. Nomura T, Kabashima K. Advances in Atopic Dermatitis in 2019-2020: Endotypes From Skin Barrier, Ethnicity, Properties of Antigen, Cytokine Profiles, Microbiome, and Engagement of Immune Cells. J Allergy Clin Immunol (2021) 148:1451–62. doi: 10.1016/j.jaci.2021.10.022 [DOI] [PubMed] [Google Scholar]
- 68. Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a Natural Antagonist for Tie2 That Disrupts In Vivo Angiogenesis. Science (1997) 277:55–60. doi: 10.1126/science.277.5322.55 [DOI] [PubMed] [Google Scholar]
- 69. Romagnani S, Damiani G, Giudizi MG, Biagiotti R, Almerigogna F, Delprete GF, et al. In Vitro Production of IgE by Human Peripheral Blood Mononuclear Cells. III. Demonstration of a Circulating IgE-Bearing Cell Involved in the Spontaneous IgE Biosynthesis. Clin Exp Immunol (1982) 49:176–84. [PMC free article] [PubMed] [Google Scholar]
- 70. Marone G, Tamburini M, Giudizi MG, Biagiotti R, Almerigogna F, Romagnani S. Mechanism of Activation of Human Basophils by Staphylococcus Aureus Cowan 1. . Infect Immun (1987) 55:803–9. doi: 10.1128/iai.55.3.803-809.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Romagnani S, Giudizi MG, del Prete G, Maggi E, Biagiotti R, Almerigogna F, et al. Demonstration on Protein A of Two Distinct Immunoglobulin-Binding Sites and Their Role in the Mitogenic Activity of Staphylococcus Aureus Cowan I on Human B Cells. J Immunol (1982) 129:596–602. [PubMed] [Google Scholar]
- 72. Schroeder JT, Bieneman AP. Activation of Human Basophils by A549 Lung Epithelial Cells Reveals a Novel IgE-Dependent Response Independent of Allergen. J Immunol (2017) 199:855–65. doi: 10.4049/jimmunol.1700055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. de Paulis A, Stellato C, Cirillo R, Ciccarelli A, Oriente A, Marone G. Anti-Inflammatory Effect of FK-506 on Human Skin Mast Cells. J Invest Dermatol (1992) 99:723–8. doi: 10.1111/1523-1747.ep12614216 [DOI] [PubMed] [Google Scholar]
- 74. Staiano RI, Loffredo S, Borriello F, Iannotti FA, Piscitelli F, Orlando P, et al. Human Lung-Resident Macrophages Express CB1 and CB2 Receptors Whose Activation Inhibits the Release of Angiogenic and Lymphangiogenic Factors. J Leukoc Biol (2016) 99:531–40. doi: 10.1189/jlb.3HI1214-584R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ferrara AL, Galdiero MR, Fiorelli A, Cristinziano L, Granata F, Marone G, et al. Macrophage-Polarizing Stimuli Differentially Modulate the Inflammatory Profile Induced by the Secreted Phospholipase A2 Group IA in Human Lung Macrophages. Cytokine (2021) 138:155378. doi: 10.1016/j.cyto.2020.155378 [DOI] [PubMed] [Google Scholar]
- 76. Casolaro V, Spadaro G, Marone G. Human Basophil Releasability. VI. Changes in Basophil Releasability in Patients With Allergic Rhinitis or Bronchial Asthma. Am Rev Respir Dis (1990) 142:1108–11. doi: 10.1164/ajrccm/142.5.1108 [DOI] [PubMed] [Google Scholar]
- 77. Spadaro G, Giurato G, Stellato C, Marone G, Casolaro V. Basophil Degranulation in Response to IgE Ligation is Controlled by a Distinctive Circadian Clock in Asthma. Allergy (2020) 75:158–68. doi: 10.1111/all.14002 [DOI] [PubMed] [Google Scholar]
- 78. Siraganian RP. An Automated Continuous-Flow System for the Extraction and Fluorometric Analysis of Histamine. Anal Biochem (1974) 57:383–94. doi: 10.1016/0003-2697(74)90093-1 [DOI] [PubMed] [Google Scholar]
- 79. MacGlashan DW, Jr. Basophil Activation Testing. J Allergy Clin Immunol (2013) 132:777–87. doi: 10.1016/j.jaci.2013.06.038 [DOI] [PubMed] [Google Scholar]
- 80. Dahlen B, Nopp A, Johansson SG, Eduards M, Skedinger M, Adedoyin J. Basophil Allergen Threshold Sensitivity, CD-Sens, is a Measure of Allergen Sensitivity in Asthma. Clin Exp Allergy (2011) 41:1091–7. doi: 10.1111/j.1365-2222.2011.03763.x [DOI] [PubMed] [Google Scholar]
- 81. Glaumann S, Nopp A, Johansson SG, Rudengren M, Borres MP, Nilsson C. Basophil Allergen Threshold Sensitivity, CD-Sens, IgE-Sensitization and DBPCFC in Peanut-Sensitized Children. Allergy (2012) 67:242–7. doi: 10.1111/j.1398-9995.2011.02754.x [DOI] [PubMed] [Google Scholar]
- 82. de Paulis A, Cirillo R, Ciccarelli A, de Crescenzo G, Oriente A, Marone G. Characterization of the Anti-Inflammatory Effect of FK-506 on Human Mast Cells. J Immunol (1991) 147:4278–85. [PubMed] [Google Scholar]
- 83. Patella V, Casolaro V, Bjorck L, Marone G. Protein L. A Bacterial Ig-Binding Protein That Activates Human Basophils and Mast Cells. J Immunol (1990) 145:3054–61. [PubMed] [Google Scholar]
- 84. Gibbs BF, Haas H, Falcone FH, Albrecht C, Vollrath IB, Noll T, et al. Purified Human Peripheral Blood Basophils Release Interleukin-13 and Preformed Interleukin-4 Following Immunological Activation. Eur J Immunol (1996) 26:2493–8. doi: 10.1002/eji.1830261033 [DOI] [PubMed] [Google Scholar]
- 85. Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 Amplifies Both Th1- and Th2-Type Responses Through its Activity on Human Basophils, Allergen-Reactive Th2 Cells, iNKT and NK Cells. Int Immunol (2008) 20:1019–30. doi: 10.1093/intimm/dxn060 [DOI] [PubMed] [Google Scholar]
- 86. Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, et al. Immunoglobulin D Enhances Immune Surveillance by Activating Antimicrobial, Proinflammatory and B Cell-Stimulating Programs in Basophils. Nat Immunol (2009) 10:889–98. doi: 10.1038/ni.1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sharma M, Das M, Stephen-Victor E, Galeotti C, Karnam A, Maddur MS, et al. Regulatory T Cells Induce Activation Rather Than Suppression of Human Basophils. Sci Immunol (2018) 3:eaan0829. doi: 10.1126/sciimmunol.aan0829 [DOI] [PubMed] [Google Scholar]
- 88. Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil Phenotypes in Chronic Idiopathic Urticaria in Relation to Disease Activity and Autoantibodies. J Invest Dermatol (2008) 128:1956–63. doi: 10.1038/jid.2008.55 [DOI] [PubMed] [Google Scholar]
- 89. Sabroe RA, Francis DM, Barr RM, Black AK, Greaves MW. Anti-Fc(episilon)RI Auto Antibodies and Basophil Histamine Releasability in Chronic Idiopathic Urticaria. J Allergy Clin Immunol (1998) 102:651–8. doi: 10.1016/s0091-6749(98)70283-0 [DOI] [PubMed] [Google Scholar]
- 90. Grattan CE, Francis DM, Hide M, Greaves MW. Detection of Circulating Histamine Releasing Autoantibodies With Functional Properties of Anti-IgE in Chronic Urticaria. Clin Exp Allergy (1991) 21:695–704. doi: 10.1111/j.1365-2222.1991.tb03198.x [DOI] [PubMed] [Google Scholar]
- 91. Soundararajan S, Kikuchi Y, Joseph K, Kaplan AP. Functional Assessment of Pathogenic IgG Subclasses in Chronic Autoimmune Urticaria. J Allergy Clin Immunol (2005) 115:815–21. doi: 10.1016/j.jaci.2004.12.1120 [DOI] [PubMed] [Google Scholar]
- 92. Ritter C, Battig M, Kraemer R, Stadler BM. IgE Hidden in Immune Complexes With Anti-IgE Autoantibodies in Children With Asthma. J Allergy Clin Immunol (1991) 88:793–801. doi: 10.1016/0091-6749(91)90187-s [DOI] [PubMed] [Google Scholar]
- 93. Marone G, Spadaro G, Palumbo C, Condorelli G. The Anti-IgE/anti-FcepsilonRIalpha Autoantibody Network in Allergic and Autoimmune Diseases. Clin Exp Allergy (1999) 29:17–27. doi: 10.1046/j.1365-2222.1999.00441.x [DOI] [PubMed] [Google Scholar]
- 94. Paganelli R, Quinti I, D'Offizi GP, Papetti C, Nisini R, Aiuti F. Studies on the In Vitro Effects of Auto-Anti-IgE. Inhibition of Total and Specific Serum IgE Detection by a Human IgG Autoantibody to IgE. J Clin Lab Immunol (1988) 26:153–7. [PubMed] [Google Scholar]
- 95. Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive Activation of T(H)2/T(H)22 Cytokines and Selective Epidermal Proteins Characterizes Acute and Chronic Atopic Dermatitis. J Allergy Clin Immunol (2012) 130:1344–54. doi: 10.1016/j.jaci.2012.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of Care for the Management of Atopic Dermatitis: Section 1. Diagnosis and Assessment of Atopic Dermatitis. J Am Acad Dermatol (2014) 70:338–51. doi: 10.1016/j.jaad.2013.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory Neurons Co-Opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell (2017) 171:217–228.e213. doi: 10.1016/j.cell.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab Treatment in Adults With Moderate-to-Severe Atopic Dermatitis. N Engl J Med (2014) 371:130–9. doi: 10.1056/NEJMoa1314768 [DOI] [PubMed] [Google Scholar]
- 99. Thaci D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, et al. Efficacy and Safety of Dupilumab in Adults With Moderate-to-Severe Atopic Dermatitis Inadequately Controlled by Topical Treatments: A Randomised, Placebo-Controlled, Dose-Ranging Phase 2b Trial. Lancet (2016) 387:40–52. doi: 10.1016/S0140-6736(15)00388-8 [DOI] [PubMed] [Google Scholar]
- 100. Voisin T, Perner C, Messou MA, Shiers S, Ualiyeva S, Kanaoka Y, et al. The CysLT2R Receptor Mediates Leukotriene C4-Driven Acute and Chronic Itch. Proc Natl Acad Sci USA (2021) 118:1031-44.e18. doi: 10.1073/pnas.20220871182022087118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cohen M, Giladi A, Gorki AD, Solodkin DG, Zada M, Hladik A, et al. Lung Single-Cell Signaling Interaction Map Reveals Basophil Role in Macrophage Imprinting. Cell (2018) 175:1031–1044 e1018. doi: 10.1016/j.cell.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 102. Pellefigues C, Mehta P, Chappell S, Yumnam B, Old S, Camberis M, et al. Diverse Innate Stimuli Activate Basophils Through Pathways Involving Syk and IkappaB Kinases. Proc Natl Acad Sci USA (2021) 118:e2019524118. doi: 10.1073/pnas.2019524118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Varricchi G, Loffredo S, Borriello F, Pecoraro A, Rivellese F, Genovese A, et al. Superantigenic Activation of Human Cardiac Mast Cells. Int J Mol Sci (2019) 20:1828. doi: 10.3390/ijms20081828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Vivanco Gonzalez N, Oliveria JP, Tebaykin D, Ivison GT, Mukai K, Tsai MM, et al. Mass Cytometry Phenotyping of Human Granulocytes Reveals Novel Basophil Functional Heterogeneity. iScience (2020) 23:101724. doi: 10.1016/j.isci.2020.101724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sawada Y, Honda T, Nakamizo S, Nakajima S, Nonomura Y, Otsuka A, et al. Prostaglandin E2 (PGE2)-EP2 Signaling Negatively Regulates Murine Atopic Dermatitis-Like Skin Inflammation by Suppressing Thymic Stromal Lymphopoietin Expression. J Allergy Clin Immunol (2019) 144:1265–1273 e1269. doi: 10.1016/j.jaci.2019.06.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.