Summary
Alveolar type 2 cells (AT2s) serve as stem cells of the alveoli and restore cell numbers after injury. Here, we describe a detailed protocol for the isolation, purification, and culture of murine and human AT2s. We have developed chemically defined and stroma-free culture conditions that enable expansion and maintenance of AT2s. The culture conditions are scalable and compatible with high-throughput chemical and genetic screenings and can potentially be used to generate large AT2 numbers for cell-based therapies.
For complete details on the use and execution of this protocol, please refer to Katsura et al. (2020).
Subject areas: Cell Biology, Cell culture, Cell isolation, Stem Cells, Organoids
Graphical abstract
Highlights
-
•
Dissociation of murine and human lung into single-cell suspension
-
•
Isolation and 3D culture of murine and human primary alveolar type 2 cells (AT2s)
-
•
Chemically defined and stroma-free culture conditions for AT2s expansion and maintenance
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Alveolar type 2 cells (AT2s) serve as stem cells of the alveoli and restore cell numbers after injury. Here, we describe a detailed protocol for the isolation, purification, and culture of murine and human AT2s. We have developed chemically defined and stroma-free culture conditions that enable expansion and maintenance of AT2s. The culture conditions are scalable and compatible with high-throughput chemical and genetic screenings and can potentially be used to generate large AT2 numbers for cell-based therapies.
Before you begin
Before using the methods described in this protocol, it is essential that the users obtain the necessary permissions from the local regulatory agency.
Institutional permissions
All laboratory mice were maintained under standard husbandry and housing conditions approved by the Duke University Institutional Animal Care and Use Committee (IACUC). All animals were handled in accordance with the NIH and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines for humane care and use of laboratory animals.
Human lung tissues from donors without pre-existing chronic lung diseases were obtained from the Biorepository and Precision Pathology Center (BRPC) at Duke University, under protocols approved by the Duke University Institutional Review Board and the Marsico Lung Institute at the University of North Carolina at Chapel Hill.
Matrigel aliquoting
Timing: 1 day
-
1.Matrigel.
-
a.Thaw matrigel in a 4°C refrigerator for 15–18 h.
-
b.Pre-chill 1 mL pipette tips and 1.5 mL tubes on ice.
-
c.Put matrigel bottle on ice, and place in a tissue culture hood.
-
d.Mix the matrigel by swirling the bottle.
-
e.Aliquot 500 μL matrigel in individual pre-chilled tubes and store at −20°C.
-
a.
CRITICAL: It is crucial that pipette tips and 1.5 mL tubes be pre-chilled. Matrigel will quickly solidify and adhere to any item that is above 10°C. During aliquoting discard any clogged pipette tip and use a new chilled pipette tip. Keep the aliquots on ice until they are placed at −20°C.
Preparation of enzyme stock solutions
Timing: 1 day
-
2.
4500 units/mL Collagenase I in PBS.
-
3.
330 units/mL DNase I in PBS.
Note: The activity (U/mg) of Collagenase I and DNase I vary from lot to lot. Therefore, weigh the amount according to U/mg indicated on each vial. (E.g., if Collagenase I vial indicates 360 Units/mg, dissolve 125 mg of Collagenase I powder in 10 mL of PBS).
Note: Sterile filter prepared enzyme stocks with 0.22 μm syringe filter. Aliquot and store at −20°C.
-
4.50 units/mL Dispase I.
-
a.Thaw a bottle of Dispase I in a 4°C refrigerator for 15–18 h.
-
b.Mix Dispase I well, make aliquots and store at −20°C until expiration date.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit Polyclonal anti-Prosurfactant protein C (1:500) | Millipore | Cat # ab3786, RRID: AB_91588 |
| Rat Monoclonal anti-RAGE/AGER (1:500) | R&D systems | Cat # MAB1179, RRID: AB_2289349 |
| Goat Polyclonal anti-hRAGE/AGER (1:500) | R&D systems | Cat # AF1145, RRID: AB_354628 |
| Mouse Monoclonal anti-HTII-280 (1:50) | Terrace Biotech | Cat # TB-27AHT2-280 RRID: AB 2832931 |
| Rabbit Polyclonal anti-SFTPB (1:500) | Thermo Fisher Scientific | Cat # PA5-42000 RRID: AB_2609628 |
| Alexa Fluor 488 goat anti-mouse IgM | Thermo Fisher Scientific | Cat # 10680 RRID: AB_10892893 |
| Alexa Fluor 488 donkey anti-rabbit IgG | Thermo Fisher Scientific | Cat # A21206 RRID: AB_141708 |
| Alexa Fluor 594 goat anti-mouse IgG1 | Thermo Fisher Scientific | Cat # A21125, RRID: AB_2535767 |
| Alexa Fluor 647 donkey anti-goat IgG | Thermo Fisher Scientific | Cat # A21447 RRID: AB_141844 |
| Alexa Fluor 647 donkey anti-rat | Thermo Fisher Scientific | Cat # A48272 RRID: AB_2893138 |
| Rat Monoclonal Brilliant Violet 711(TM) anti-mouse CD326 (EpCAM) (1:200) | BioLegend | Cat #118233 RRID: AB_2632775 |
| Rat Monoclonal PE anti-mouse CD140a (1:200) | BioLegend | Cat # 135905 RRID: AB_1953268 |
| Rat Monoclonal eFluor 450 anti-mouse CD31 (PECAM-1) (1:200) | Thermo Fisher Scientific | Cat # 48-0311-82 RRID: AB_10598807 |
| Rat Monoclonal eFluor 450 anti-mouse CD45 (1:200) | Thermo Fisher Scientific | Cat # 48-0451-82 RRID: AB_1518806 |
| Biological samples | ||
| Human lung tissue collected from males and females donors ranging from 1-70 years old | N/A | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| SB431542 | Abcam | Cat # Ab120163 |
| CHIR99021 | Tocris Bioscience | Cat # 4423 |
| BIRB796 | Tocris Bioscience | Cat # 5989 |
| Heparin | Sigma-Aldrich | Cat # H3149 |
| N-Acetyl-L-cysteine (NAC) | Sigma-Aldrich | Cat # A9165 |
| hEGF | Gibco | Cat # PHG0313 |
| Y27632 2HCl | Selleckchem | Cat # S1049 |
| B-27 Supplement (50×) | Thermo Fisher Scientific | Cat # 17504044 |
| N-2 Supplement (100×) | Thermo Fisher Scientific | Cat # 17502048 |
| Insulin-Transferrin-Selenium (ITS) (100×) | Thermo Fisher Scientific | Cat # 41400-045 |
| Glutamax | Thermo Fisher Scientific | Cat # 35050061 |
| HEPES (1 M) | Thermo Fisher Scientific | Cat # 15630080 |
| Antibiotic-Antimycotic (Anti-Anti) (100×) | Thermo Fisher Scientific | Cat # A5955-100ML |
| hFGF10 | BioLegend | Cat # 559304 |
| mIL-1β | BioLegend | Cat # 575104 |
| hIL-1β | BioLegend | Cat # 579404 |
| mNoggin | PeproTech | Cat # 250-38 |
| mFGF10 | R&D systems | Cat # 751004 |
| Tamoxifen | Sigma-Aldrich | Cat # T5648 |
| Dispase I | Corning | Cat # 354235 |
| DNase I | Sigma-Aldrich | Cat # 10104159001 |
| Collagenase type I | Gibco | Cat # 17100-017 |
| Collagenase type I | Worthington | Cat # LS004197 |
| Matrigel | Corning | Cat # 354230 |
| Lysotracker (1:10000) | Thermo Fisher Scientific | Cat # L7526 |
| Red Blood Cell Lysis Buffer | Sigma-Aldrich | Cat # 11814389001 |
| Citrate Buffer, pH 6.0 (10×) | Sigma-Aldrich | Cat # C9999 |
| Fluoromount-G, with DAPI | Thermo Fisher Scientific | Cat # 00-4959 |
| PBS (1×) | Gibco | Cat # 20012027 |
| Paraformaldehyde | Sigma-Aldrich | Cat # P6148 |
| TrypLE™ Select Enzyme | Thermo Fisher Scientific | Cat # 12563029 |
| O.C.T. Compound | Fisher Scientific | Cat # 23-730-571 |
| Advanced DMEM/F-12 | Thermo Fisher Scientific | Cat # 12634028 |
| Trypan Blue Solution, 0.4% | Thermo Fisher Scientific | Cat # 15250061 |
| ACK Lysing Buffer | Thermo Fisher Scientific | Cat # A1049201 |
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | Cat # A7906 |
| Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA) | Sigma-Aldrich | Cat # E5134 |
| Human TruStain FcX™ (Fc Receptor Blocking Solution) | BioLegend | Cat # 422302 |
| Anti-Mouse IgM MicroBeads | Miltenyi Biotec | Cat # 130-047-302 |
| Experimental models: Organisms/strains | ||
| C57BL/6J (males and females, 8–14 week old) | The Jackson Laboratory | Jax # 000664 RRID: IMSR_JAX:000664 |
| Software and algorithms | ||
| FIJI | National Institutes of Health | https://fiji.sc |
| Other | ||
| Cell culture incubator | Thermo Fisher Scientific | Cat # 51033557 |
| Heraeus Megafuge 8R Compact Centrifuge | Thermo Fisher Scientific | Cat # 75007213 |
| Biosafety cabinet level 2 (tissue culture hood) | N/A | N/A |
| Hemocytometer | VWR | Cat # 470019-796 |
| Incubated Tube Rotator - Hybaid Maxi 14 Rotisserie Hybridization Workstation | Thermo Fisher Scientific | Cat # 6246 |
| Labquake™ Tube Shaker/Rotators | Fisher Scientific | Cat #13-687-12Q |
| Water Bath | Thermo Fisher Scientific | Cat # TSGP15D |
| Razor Blade (no.9) | VWR | Cat # 55411-050 |
| Advanced Cell Strainers, 40 μm | Genesee Scientific | Cat # 25-375 |
| Advanced Cell Strainers, 100 μm | Genesee Scientific | Cat # 25-375 |
| Reducing Adapters for Cell Strainers | Genesee Scientific | Cat # 25-278 |
| Tissue culture plate 24 well | SARSTEDT INC | Cat # 83.3922 |
| Tissue culture plate 6 well | SARSTEDT INC | Cat # 83.3920 |
| Syringe filter, PES, 0.22 μm | Genesee Scientific | Cat #25-243 |
| BD 10 mL Syringe | BD | Cat # 302995 |
| 3-0 Silk Suture Spool, Black Braid | LOOK | MFIDSP117 |
| 22G needle | BD | Cat # 305155 |
| 15 mL Conical Sterile Polypropylene Centrifuge Tubes | Thermo Fisher Scientific | Cat # 339650 |
| 50 mL Conical Sterile Polypropylene Centrifuge Tubes | Thermo Fisher Scientific | Cat # 339652 |
| MACS MultiStand | Miltenyi Biotec | Cat # 130-042-303 |
| MidiMACS Separator | Miltenyi Biotec | Cat # 130-042-302 |
| LS Column | Miltenyi Biotec | Cat # 130-042-401 |
| Cell Lifter, Double End Flat Blade & J-Hock | CELLTREAT Scientific Products | Cat # 229306 |
| Falcon® 5 mL Round Bottom Polystyrene Test Tube, with Cell Strainer Snap Cap | Corning | Cat # 352235 |
| 500 mL vacuum Filter/Storage System, PES, 0.22 μm | Corning | Cat # 431097 |
| 250 mL vacuum Filter/Storage System, PES, 0.22 μm | Corning | Cat # 431096 |
| Cryomold 10 mm × 10 mm × 5 mm | VWR | Cat # 25608-922 |
| Cryomold 15 mm × 15 mm × 5 mm | VWR | Cat # 25608-924 |
| 1.7 mL tubes | Genesee Scientific | Cat # 24-282 |
Materials and equipment
Digestion solution for murine lung
| Reagent | Final concentration | Amount |
|---|---|---|
| Collagenase type I (4500 units/mL) | 450 units/mL | 400 μL |
| Dispase I (50 units/mL) | 5 units/mL | 400 μL |
| DNase I (330 units/mL) | 0.33 units/mL | 4 μL |
| DMEM/F12 with 1× Anti-Anti | n/a | 3196 μL |
| Total | n/a | 4 mL |
Note: Make solution immediately before use.
Digestion solution for human lung
| Reagent | Final concentration | Amount |
|---|---|---|
| Collagenase type I (4500 units/mL) | 450 units/mL | 1.5 mL |
| Dispase I (50 units/mL) | 5 units/mL | 1.5 mL |
| DNase I (330 units/mL) | 10 units/mL | 454.5 μL |
| DMEM/F12 with 1× Anti-Anti | n/a | 11.545 mL |
| Total | n/a | 15 mL |
Note: Make solution immediately before use.
MACS (Magnet activated cell sorting) buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| BSA | 1% | 0.5 g |
| EDTA (500 mM) | 2 mM | 200 μL |
| PBS (1×) | 1× | 49.3 mL |
| Antibiotic antimycotic (Anti-Anti) (100×) | 1× | 500 μL |
| Total | n/a | 50 mL |
Preparation of medium components
Timing: 1 day
-
•100 mM SB431542.
-
•E.g., Dissolve 25 mg of SB431542 (MW 384.4) in 650.4 μL of DMSO.
-
•Aliquot and store at −20°C for up to 1 month.
-
•
-
•10 mM CHIR99021.
-
•E.g., Dissolve 25 mg of CHIR99021 (MW 465.3) in 5.373 mL of DMSO.
-
•Aliquot and store at −20°C for up to 1 month.
-
•
-
•10 mM BIRB796.
-
•E.g., Dissolve 25 mg of BIRB796 (MW 527.7) in 4.738 mL of DMSO.
-
•Aliquot and store at −20°C for several months.
-
•
-
•50 mg/mL Heparin.
-
•E.g., Dissolve 50 mg of heparin in 1 mL of sterile PBS.
-
•Filter-sterile with 0.22 μm syringe filter.
-
•Aliquot and store at 4°C for up to 1 year.
-
•
-
•1 mg/mL EGF.
-
•E.g., Dissolve 1 mg of lyophilized EGF in 1 mL of sterile PBS.
-
•Aliquot and store at −20°C for up to 12 months.
-
•
-
•500 mM N-Acetylcysteine (NAC).
-
•E.g., Dissolve 816.0 mg of NAC (MW 163.19) in 10 mL of sterile PBS.
-
•Filter-sterile with 0.22 μm syringe filter.
-
•Aliquot and store at −20°C for 1 month.
-
•
-
•10 mM Y27632 2HCl.
-
•E.g., Dissolve 10 mg of lyophilized Y-27632 2HCl (MW 320.26) in 3.12 mL of DMSO.
-
•Aliquot and store at −20°C for up to 12 months. Protect from light.
-
•
-
•200 μg/mL hFGF10.
-
•E.g., Dissolve 25 μg of lyophilized hFGF10 in 125 μL of filter-sterilized 0.1%BSA in PBS to make a 200 μg/mL stock solution.
-
•Aliquot hFGF10 and store at −20°C for up to 3 months.
-
•To make working stock solution of 10 μg/mL, add 190 μL of PBS to 10 μL of 200 μg/mL hFGF10 stock. Store at 4°C for 1–2 weeks.
-
•
Note: To make stocks of 200 μg/mL mFGF10, 200 μg/mL hIL1ß, 200 μg/mL mIL1ß, 200 μg/mL mNoggin follow instructions as described in point 8 (hFGF10).
AT2 Maintenance Medium (AMM) for murine culture
| Reagent | Final concentration | Amount |
|---|---|---|
| SB431542 (100 mM) | 10 μM | 5 μL |
| CHIR99021 (10 mM) | 3 μM | 15 μL |
| BIRB796 (10 mM) | 1 μM | 5 μL |
| Insulin-Transferrin-Selenium (ITS) (100×) | 1× | 500 μL |
| HEPES (1 M) | 15 mM | 750 μL |
| Antibiotic antimycotic (Anti-Anti) (100×) | 1× | 500 μL |
| Glutamax (100×) | 1× | 500 μL |
| hEGF (1 mg/mL) | 50 ng/mL | 2.5 μL |
| Heparin (50 mg/mL) | 5 μg/mL | 5 μL |
| mNoggin (10 μg/mL) | 10 ng/mL | 50 μL |
| B27 supplement (50×) | 1× | 1 mL |
| N2 supplement (100×) | 1× | 500 μL |
| N-Acetyl Cysteine (500 mM) | 1.25 mM | 125 μL |
| Y27632 (10 mM) | 10 μM | 50 μL |
| mFGF10 (10 μg/mL) | 10 ng/mL | 50 μL |
| mIL-1β (10 μg/mL) | 10 ng/mL | 50 μL |
| Advanced DMEM/F12 | n/a | 45.89 mL |
| Total | n/a | 50 mL |
Serum-Free, Feeder-Free (SFFF) medium for human culture
| Reagent | Final concentration | Amount |
|---|---|---|
| SB431542 (100 mM) | 10 μM | 5 μL |
| CHIR99021 (10 mM) | 3 μM | 15 μL |
| BIRB796 (10 mM) | 1 μM | 5 μL |
| Insulin-Transferrin-Selenium (ITS) (100×) | 1× | 500 μL |
| HEPES (1 M) | 15 mM | 750 μL |
| Glutamax (100×) | 1× | 500 μL |
| Anti-biotic anti-mycotic (Anti-Anti) (100×) | 1× | 500 μL |
| hEGF (1 mg/mL) | 50 ng/mL | 2.5 μL |
| Heparin (50 mg/mL) | 5 μg/mL | 5 μL |
| B27 (50×) | 1× | 1 mL |
| N2 (100×) | 1× | 500 μL |
| N-Acetyl Cysteine (500 mM) | 1.25 mM | 125 μL |
| Y27632 (10 mM) | 10 μM | 50 μL |
| hFGF10 (10 μg/mL) | 10 ng/mL | 50 μL |
| hIL-1β (10 μg/mL check Note below) | 10 ng/mL | 50 μL |
| Advanced DMEM/F12 | n/a | 45.942 mL |
| Total | n/a | 50 mL |
Note: Y27632 (ROCK inhibitor) is added to the medium at the time of culture setup.
Note: Although IL-1β is not required for growth of human alveolospheres, addition of hIL-1β between day 0–4 increases colony formation efficiency and alveolosphere size. However, we recommend removal of hIL-1β from the medium after day 4. Of note, although hIL-1ß (first 4 days) enhances the growth of human AT2s, they can grow without IL-1ß. Prolonged treatment with hIL-1ß might lead to changes in morphology (saccular to rounded) of human alveolospheres and loss of AT2 cell markers (SFTPC). In addition, eliminating hIL-1ß from the medium is suggested in studies involving viral infections and pharmacological screens.
Step-by-step method details
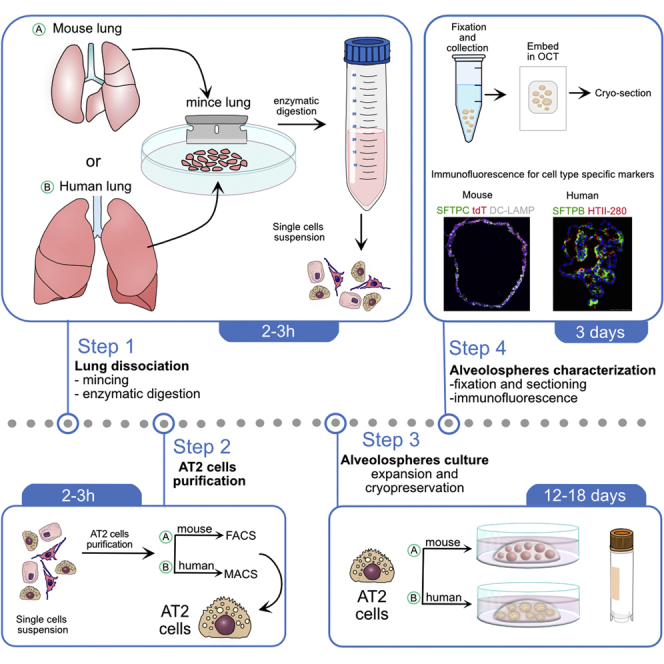
Isolation and purification of murine alveolar epithelial type 2 cells (AT2s)
Timing: 30 min for lung harvesting and mincing, 2 h for dissociation
In this step, harvest murine lung followed by cell dissociation to make a single-cell suspension (Figure 1A). Next, purify murine alveolar epithelial type 2 cells using flow cytometry (FACS).
-
1.
Prepare the digestion solution for murine lung (Collagenase type I: 450 units/mL, Dispase: 5 units/mL, DNase I: 0.33 units/mL) as described above. Incubate at 37°C prior to use.
-
2.Harvest murine lung.
-
a.Sacrifice the mouse using method approved by the local Institutional Animal Care and Use Committee (IACUC) (e.g., carbon dioxide (CO2) inhalation in chamber).
-
b.Place the mouse front side up and generously spray the exposed side of mouse with 70% ethanol.
-
c.Using clean scissors make an incision in the skin from the abdomen to neck, and tear skin with forceps to expose the subcutaneous tissue from the neck to the abdomen.
-
d.Cut the abdominal muscle to expose the liver and move liver and intestine to expose the diaphragm.
-
e.Carefully cut the diaphragm, cut through the lateral extreme of the left and right rib cage and remove the sternum and the rib cages to expose the heart and lungs.
-
f.Cut through the renal artery.
-
g.Perfuse the lung using a 10 mL syringe with a 25G needle to inject sterile PBS, through the right ventricle of the heart until the lung turn pale (Figure 1B). Generally, 5 mL of PBS is sufficient for perfusion.
-
h.Remove the heart tissue.
-
i.Cut away salivary glands covering trachea. Then carefully cut thin muscle lining the trachea to expose the trachea.
-
j.Use forceps to put a ∼ five cm-long nylon string under the trachea and keep the string ready to tie.
-
k.Insert a 22G needle into the trachea and tie a knot with a string to secure needle and trachea together firmly.
-
l.Slowly inflate the lung by injecting 1 mL of the digestion solution with 1 mL syringe (Figure 1B). Make sure to inflate to the cardiac lobe.
-
m.Remove the needle from the trachea and tie a knot with string around the trachea.
-
n.Remove the lung from the mouse and transfer to a 10 cm dish. Separate each lobe of the lung and remove connective tissue.
-
a.
-
3.Dissociation of murine lung.
-
a.Mince the lung lobes with a single edge blade (No.9) into 1–2 mm3 pieces (Figure 1C).
-
b.Cut the tip of P1000 pipet and transfer lung pieces to a 15 mL tube with 2 mL of warm digestion solution.
-
c.Rinse the dish with 1 mL of digestion solution to collect any residual pieces of tissue, and combine them with the tissue collected from step b.
-
d.Incubate the sample at 37°C for 20 min with constant and horizontal rotation at approximately 10 rpm.
-
e.Vigorously mix samples with a P1000 pipette approximately 30 times in a tissue culture hood.
-
f.Return the sample to 37°C incubator and continue incubation for an additional 15 min with constant rotation.
-
g.Vigorously mix samples with a P1000 pipette approximately 30 times in a tissue culture hood.Note: Total digestion time of the lung pieces is 35 min. Vigorous pipetting during and after dissociation helps obtain better single cells suspension and yields higher cell numbers (Figure 1D).
-
h.Connect a 100 μm cell strainer to a reducing adapter and place on a 15 mL tube. Wet the cell strainer with 1 mL of 10% FBS in DMEM/F12 containing anti-anti and transfer cell suspension to strain and allow cells to drain into the tube.
-
i.Rinse the cell strainer with 10 mL of 10% FBS in DMEM/F12 to stop the enzymatic reaction.
-
j.Collect any residual cell suspension from the bottom side of the cell strainer using a P1000.
-
k.Centrifuge the tube at 400 g for 5 min at 4°C.
-
l.Aspirate the supernatant.
-
m.Add 2 mL of Red Blood Cell (RBC) lysis buffer, resuspend the pellet (Figure 1E) by pipetting and incubate for 2 min at 20°C–25°C.
-
n.Stop RBC lysis with 10 mL of 10% FBS in DMEM/F12 containing anti-anti.
-
o.Place a 40 μm cell strainer on a 50 mL tube and pass the cell suspension through the strainer and wash cell strainer with 10 mL of 10% FBS in DMEM/F12.
-
p.Centrifuge the tube at 400 g for 5 min at 4°C.
-
q.Aspirate the supernatant.
-
r.Resuspend the cell pellet in 1 mL with 2% FBS in DMEM/F12 and transfer to 1.5 mL tube.Note: If Sftpc-creER-R26R-tdTomato mouse (Madisen et al., 2010; Rock et al., 2011) induced with tamoxifen was used for lung dissociation, proceed to fluorescence activated cell sorting (FACS) to collect tdTomato+ cells. Otherwise see below for staining cells for FACS.
-
a.
-
4.Staining of single-cell suspension for FACS.
-
a.Centrifuge the tube from the previous step (r) at 400 g for 5 min at 4°C.
-
b.Dilute the antibodies (see below) in 200 μL of DMEM/F12 containing 2% FBS.EpCAM/CD326-Brilliant Violet 711 [1:200].Lysotracker-Green DND-26 [1:10000].CD140a-PE [1:200].CD31/CD45-eFluor 450 [1:200].
-
c.Use the antibodies in the dilution ratio listed above.
-
d.Resuspend cell pellet with diluted antibodies in 1.5 mL tube.
-
e.Cover the tube with aluminum foil and incubate for 30 min at 4°C with constant horizontal rotation at 8 rpm.
-
f.Centrifuge the tube at 400 g at 4°C for 5 min.
-
g.Aspirate the supernatant and wash cells twice with 1 mL of 2% FBS in DMEM/F12. After each wash centrifuge the tube at 400 g at 4°C for 5 min.
-
h.Resuspend the cell pellet with 1 mL of 2% FBS in DMEM/F12.
-
i.Transfer the single-cell suspension to a 35 μm filter cap of FACS cell collection tube.
 CRITICAL: Before adding the single-cell suspension it is important to equilibrate the 35 μm filter of FACS cell collection tube with 0.5 mL of 2%FBS in DMEM/F12.
CRITICAL: Before adding the single-cell suspension it is important to equilibrate the 35 μm filter of FACS cell collection tube with 0.5 mL of 2%FBS in DMEM/F12. -
j.Wash the filter with 500 μL of 2% FBS in DMEM/F12 and place the tube on ice.
-
k.Sort cells on a FACS machine using the gating strategy shown in (Figure 2).
-
l.Collect the sorted AT2 cells in 2% FBS in DMEM/F12.Note: The estimated number of AT2 cells obtained from one mouse is around 120–180K. The purity of AT2 cells after sorting is approximately 99% (Figure 2).
-
a.
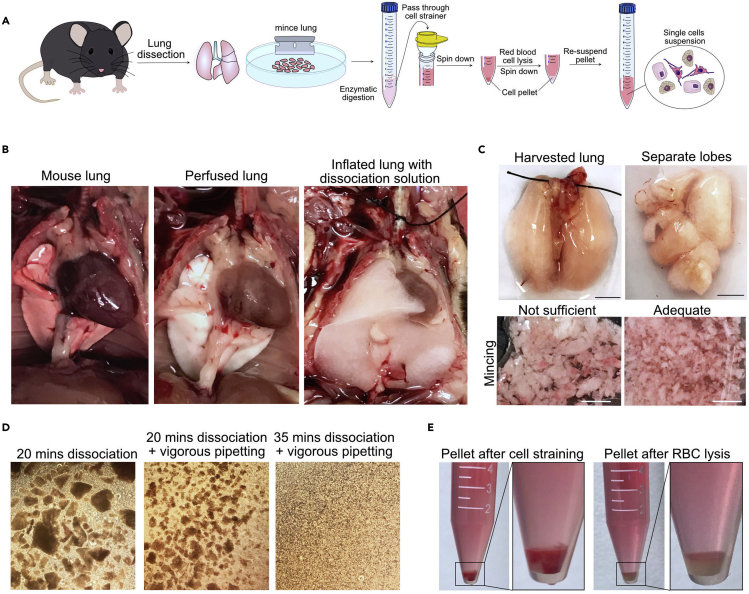
Figure 1.
Dissection and dissociation of murine lung
(A) Schematic representation of crucial steps of murine lung dissection and dissociation to single cells suspension.
(B) Images illustrate murine lung before and after perfusion and inflation with digestion solution.
(C) Images illustrate steps after lung removal, separated lobes and lung mincing. Scale bar: 5 mm.
(D) Examples of lung dissociation at indicated incubation time with digestion solution.
(E) Visualization of cell pellet before and after red blood lysis buffer.
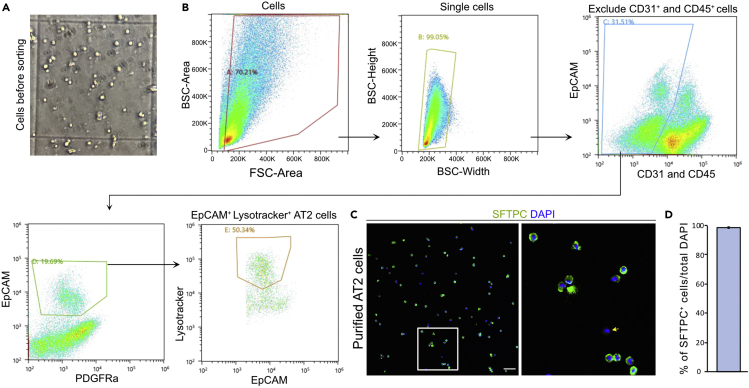
Figure 2.
Single-cell suspension and FACS sorting of murine AT2c
(A) Visualization of lung single-cell suspension.
(B) Gates for FACS sorting of AT2 cells.
(C) Validation of AT2s purity using cytospin preparations of sorted cells and staining with SFTPC (green). The yellow arrow indicates SFTPC negative cell. Scale bar:50 μm.
(D) Quantification of SFTPC+ in total DAPI+ cells. Data are presented as mean ± SEM. n=3.
Isolation and purification of human alveolar epithelial type 2 cells (AT2s)
Timing: 15–20 min for removing pleura, airways, large veins, and arteries and mincing;h for dissociation; and 3hforMACS sorting
In this step, mince human lung followed by cell dissociation to make a single-cell suspension (Figures 3A–3C). Next, purify human alveolar epithelial type 2 cells using magnet activated cell sorting (MACS).
-
5.
Prepare the digestion solution (Collagenase type I: 450 units/mL, Dispase: 5 units/mL, DNase I: 10 units/mL) as described above. Incubate at 37°C prior to use.
-
6.Mincing and dissociation of the human lung.
-
a.Take a piece of lung and remove the pleura by pulling with forceps.
-
b.Cut approximately 3 g of human lung tissue and move to 10 cm petri dish.
-
c.With forceps dissect out and discard airways, large veins, and arteries.
-
d.Mince the lung chunks with a single edge blade (No.9) into small pieces.
 CRITICAL: Make sure that lung tissue is minced into small (1–2 mm3) pieces, as shown in Figure 3. Large lung chunks will not yield high cell numbers.
CRITICAL: Make sure that lung tissue is minced into small (1–2 mm3) pieces, as shown in Figure 3. Large lung chunks will not yield high cell numbers. -
e.Cut the tip of P1000 pipet and transfer the lung pieces to a 50 mL tube with 14 mL of warm digestion solution.
-
f.Rinse the dish with 1 mL of digestion solution to collect any residual pieces of the tissue, and combine them with the tissue collected from step e.
-
g.Incubate sample at 37°C for 1 h with constant horizontal rotation at approximately 10 rpm.
 CRITICAL: Rigorously mix the samples with P1000 pipette about 30 times under the tissue culture hood. Repeat this every 15 min. Vigorous pipetting between and after dissociation helps to get better single cells suspension and yield higher cell numbers.
CRITICAL: Rigorously mix the samples with P1000 pipette about 30 times under the tissue culture hood. Repeat this every 15 min. Vigorous pipetting between and after dissociation helps to get better single cells suspension and yield higher cell numbers. -
h.Set up two 100 μm cell strainers on 50 mL tubes. Wet the cells strainer with 5 mL of 10% FBS in DMEM/F12 containing anti-anti.
-
i.Pipette approximately 7.5 mL of cell suspension on each cell strainer and rinse the sample with additional 20 mL of 10% FBS in DMEM/F12 containing anti-anti.
-
j.Centrifuge the tubes at 450 g for 10 min at 4°C.
-
k.Collect the supernatant to fresh tubes and repeat centrifugation.Note: Additional centrifugation of supernatant is not necessary, but it will increase cell yield.
-
l.Resuspend the pellet from step j in 2 mL Red blood cell lysis buffer and incubate for 2 min at 20°C–25°C.Note: If the starting lung tissue has a high number of blood cells, extend Red blood cell lysis buffer incubation to 5 min.
-
m.Stop RBC lysis with 10 mL of 10% FBS in DMEM/F12 containing anti-anti.
-
n.Perform the red blood cell lysis on pellet from step k and stop the lysis with 10 mL of 10% FBS in DMEM/F12 containing anti-anti.
-
o.Filter the sample through 40 μm cell strainer.Note: Combine samples from step m and n.
-
p.Centrifuge cell suspension at 450 g for 10 min at 4°C.
-
q.Aspirate the supernatant.
-
r.Resuspend the cell pellet in 1 mL of 2% FBS in DMEM/F12 and transfer to 1.5 mL tube.Note: Perform magnet activated cell sorting (MACS) as described previously (Katsura et al., 2020) and according to the manufacturer’s instructions (Midi MACS separator protocol https://www.miltenyibiotec.com/_Resources/Persistent/6f925763fa5a75c4bac43db9a0aab1bbc654961e/DS_MidiMACS%20Separator.pdf).
-
s.Centrifuge and resuspend the cell pellet in 500 μL of MACS buffer (1% BSA, 2 mM EDTA in PBS containing 1% anti-anti - Sterile filter with 0.22 μm filter and store at 4°C for 3–4 weeks).Note: These steps describe the purification of approximately 5–10 million total dissociated human lung cells.
-
t.Add 10 μL of blocking reagent TruStain FcX and incubate at 4°C for 15 min.
-
u.Add 500 μL of MACS buffer and centrifuge at 450 g for 5 min at 4°C.
-
v.Wash the pellet with 1 mL of MACS buffer and centrifuge at 450 g for 5 min at 4°C.
-
w.Add 5 μL of HTII-280 mouse IgM antibody and resuspend the pellet in 300 μL of MACS buffer followed by incubation at 4°C for 1 h with horizontal rotation at 8 rpm.
-
x.Add 1 mL of MACS buffer and centrifuge at 450 g for 5 min at 4°C.
-
y.Wash the pellet with 1 mL of MACS buffer and centrifuge at 450 g for 5 min at 4°C.
-
z.Discard the supernatant.
-
aa.Dilute secondary anti-mouse IgM-magnetic beads (1:10) in 300 μL of MACS buffer and use this to resuspend cell pellet followed by incubation at 4°C for 30 min with horizontal rotation at 8 rpm.
-
bb.Add 1 mL of MACS buffer and centrifuge at 450 g for 5 min at 4°C.
-
cc.Wash the cell pellet with 1 mL of MACS buffer and centrifuge at 450 g for 5 min at 4°C.
-
dd.Resuspend the cell pellet in 500 μL MACS buffer.
-
ee.Set MACS separator with LS column in tissue culture hood, equilibrate LS column with 3 mL of MACS buffer and discard flow-through.
-
ff.Apply the sample to LS column and discard the flow-through.
-
gg.Wash LS column three times with 3 mL of MACS buffer.
-
hh.Add 5 mL of MACS buffer, plunge immediately and collect flow-through in 15 mL conical tube.
-
ii.Centrifuge flow-through at 450 g for 5 min at 4°C.
-
jj.Resuspend the pellet in 500 μL of SFFF medium and count the cell number.Note: The yield of AT2 cells is approximately 1%–2% of the total cells used for purification.
-
a.
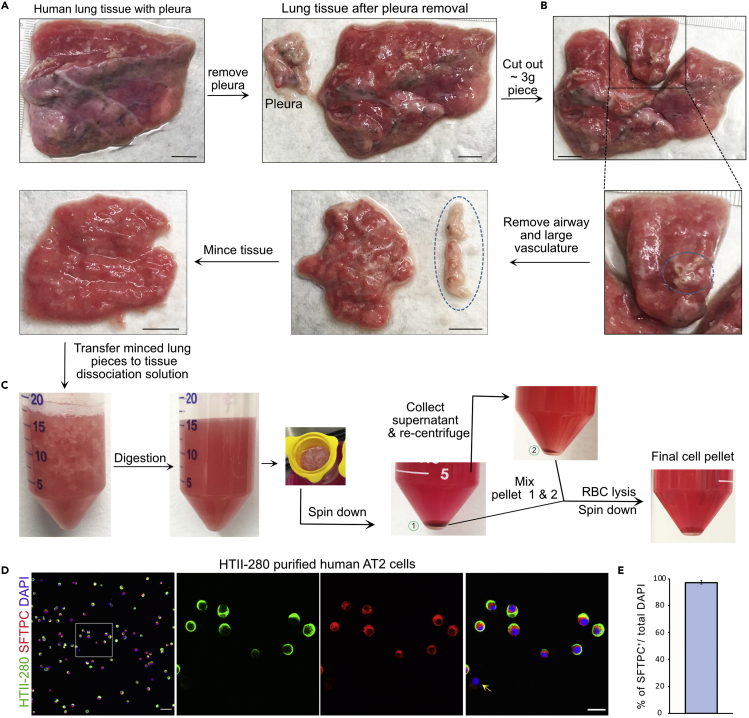
Figure 3.
Mincing and dissociation of human lung
(A) Visualization of human lung before and after pleura removal. Scale bar: 10 mm.
(B) Illustration of lung piece cut out for dissection. Blue circle depicted airway and vasculature that were dissected before lung mincing. Scale bar: 10 mm.
(C) Images taken at different steps of lung dissociation.
(D) Validation of human AT2s purity using cytospin preparations of HTII-280+ sorted cells and staining with HTII-280 (green) and SFTPC (red). The yellow arrow points out on SFTPC negative cell. Scale bar:50 μm.
(E) Quantification of SFTPC+ in total DAPI+ cells. Data are presented as mean ± SEM. n=3.
3D culture of murine and human isolated alveolar epithelial type 2 cells
Timing: 40–50 min to set up 3D cultures
This step describes set up and culture of isolated alveolar epithelial type 2 cells (AT2s) in matrigel droplet format.
Note: Thaw matrigel aliquots at 4°C approximately 5–7 h prior to use.
-
7.Count cell number.
-
a.Centrifuge the cell suspension (from FACS or MACS) at 4°C, 450 g for 10 min.
-
b.Aspirate the supernatant and suspend the cell pellet in 500 μL of advanced DMEM/F12.
-
c.Count cells using a hemocytometer or automated cell counter.
-
i.Take 10 μL of cells suspension and mix with 10 μL of 0.4% trypan blue solution.
-
ii.Place coverslip on a hemocytometer and transfer 10 μL of trypan blue/cell suspension solution between coverslip and hemocytometer base. Count all cells in four 1 mm2 corner squares. Nonviable cells stain in blue. Calculate the number of viable cells.
-
i.
-
a.
-
8.Plating AT2 cells in 3-dimensional matrix to generate alveolospheres (Figure 4A).
-
a.For each drop take 2000–3000 cells in a volume of 25 μL of culture medium (mouse AT2-AMM medium, human AT2 cells – SFFF).Note: SFFF and AMM media keep sterile and store at 4°C for 3–4 weeks.
 CRITICAL: Since matrigel solidifies quickly above 10°C, always keep matrigel on ice.
CRITICAL: Since matrigel solidifies quickly above 10°C, always keep matrigel on ice. -
b.Add the same amount of matrigel (25 μL for each droplet) to the cell suspension and mix gently by pipetting.
 CRITICAL: Care must be taken while pipetting matrigel/cell suspension to avoid air bubbles.EXAMPLE: For setting up four drops, take 8000–12000 cells in a final volume of 100 μL of culture medium and mix with 100 μL of matrigel (Figure 4).
CRITICAL: Care must be taken while pipetting matrigel/cell suspension to avoid air bubbles.EXAMPLE: For setting up four drops, take 8000–12000 cells in a final volume of 100 μL of culture medium and mix with 100 μL of matrigel (Figure 4). -
c.Pipette 50 μL of matrigel and cell suspension to the bottom of the culture plate. 3–5 bubbles can be placed in one well of a 6-well plate.
 CRITICAL: The presence of air bubbles can disrupt matrigel droplet stability during the culture. Therefore, care must be taken while pipetting matrigel/cell suspension to avoid air bubbles.
CRITICAL: The presence of air bubbles can disrupt matrigel droplet stability during the culture. Therefore, care must be taken while pipetting matrigel/cell suspension to avoid air bubbles. -
d.Immediately place the plate with droplets in a 37°C incubator and wait 20 min to solidify the matrigel.
-
e.Add 2 mL of SFFF (human AT2 cells) or AMM (mouse AT2 cells) in each well. (Figure 4B).
-
f.Change medium every 2–3 days.
-
a.
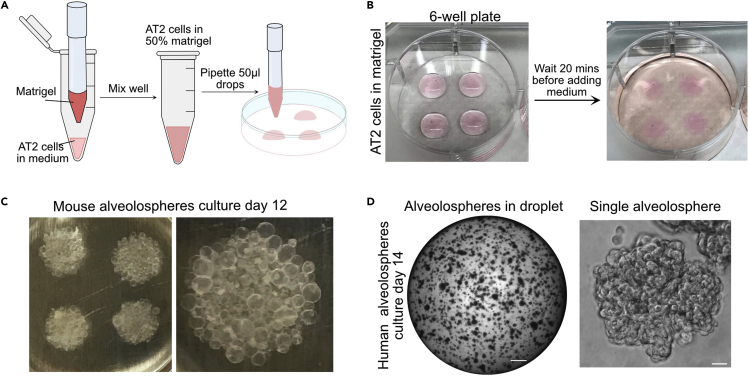
Figure 4.
Alveolospheres plating and expansion
(A) Schematic illustration of alveolar culture preparation and plating.
(B) Example of matrigel droplets containing AT2 cells.
(C) Mouse alveolospheres cultured for 10–12 days.
(D) Human alveolospheres cultured for 12–14 days. Scale bar: 500 μm and 50 μm.
Passaging and cryopreservation of alveolar epithelial type 2 cells
Timing: 40–50 min
This step describes protocol to passage 3-D alveolospheres cultures.
-
9.Passaging of murine and human AT2 alveolospheres.Note: Mouse alveolospheres need to be passaged and re-plated in matrigel between day 12–14 of culture (Figure 4C). One drop of mouse alveolospheres contain approximately 100,000 cells. Human alveolospheres needs passaging every 14–18 days (Figure 4D). Around 80,000–120,000 cells can be expected from one drop.Note: Thaw matrigel aliquots at 4°C approximately 5–7 h prior to use.
-
a.Aspirate the culture medium without disruption of matrigel droplets using a vacuum aspirator.
-
b.Add 1 mL of TrypLE select to one well of 6 well plate.
-
c.Disrupt the matrigel droplets using a cell lifter.
-
d.Pipette several times with P1000 and transfer the suspension to a 15 mL tube.
-
e.Incubate at 37°C for 5–7 min followed by vigorous mixing by pipetting.
-
f.Add 10 mL of 10%FBS in DMEM/F12 to stop enzymatic digestion.
-
g.Centrifuge the tube at 450 g for 5 min at 4°C.
-
h.Aspirate the supernatant, resuspend the cell pellet in 500 μL of culture medium and transfer cells suspension into a fresh 1.5 mL tube.
-
i.Count the cells and set up droplets with cells described above (See: 2. Plating AT2 cells).
-
a.
-
10.Cryopreservation of mouse and human AT2 cells.
-
a.Prepare freezing medium containing 10% Dimethyl Sulfoxide (DMSO), 20% FBS in DMEM/F12.Optional: Commercial freezing medium can also be used for AT2 cells cryopreservation.
-
b.Dissociate mouse or human alveolospheres as described above in passaging section.
-
c.Resuspend 200,000–1 million cells in 1 mL of freezing medium.
-
d.Aliquot the cell suspension into cryogenic vials.
-
e.Place the cryogenic vials in an isopropanol-containing cryo-freezing container or an isopropanol-free container and store them for 15–18 h in a −80°C freezer.
-
f.Transfer cryogenic vials to a liquid nitrogen tank for long term storage.
-
a.
Characterization of murine and human alveolospheres
Timing: 2 h for fixation and preparation of cryo-blocks; 5–6 h for immunofluorescence staining for AT2 and AT1 cell specific markers
This step describes fixation and preparation of cryo-blocks of murine and human alveolospheres for cryo-sectioning as well as immunofluorescence staining.
-
11.Alveolospheres fixation.
-
a.Carefully aspirate the medium in the culture well and wash with 1 mL of PBS.
-
b.Remove PBS carefully and add 1–1.5 mL of 4%PFA in PBS.
-
c.Incubate the plate for 1 h at 20°C–25°C.
-
d.Remove the 4%PFA and wash the well with PBS.
-
e.Proceed to OCT embedding steps.
-
a.
Optional: Plates with fixed alveolospheres can be stored at 4°C for two weeks in PBS.
-
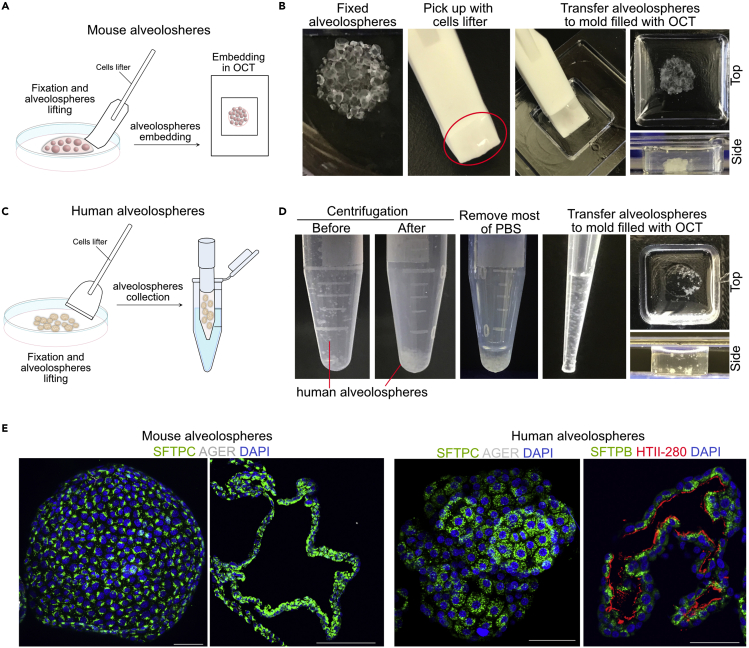
12.Embedding of murine alveolospheres for cryosectioning (Figures 5A and 5B).
-
a.Use J-Hock side of the cell filter to gently move the matrigel droplet with alveolospheres from the bottom of the plate.
-
b.Carefully scoop the alveolospheres with matrigel.
-
c.Move alveolospheres to the cryo mold filled with OCT.
-
d.Adjust the position of alveolospheres with the pipette tip. Move the alveolospheres near the bottom of the mold (Figures 5A and 5B).
-
a.
-
13.Embedding of human alveolospheres for cryosectioning (Figures 5C and 5D).
-
a.Use cell lifter to scrape alveolospheres from the plate. Tilt the plate (∼45 degrees) and collect the organoids (together with PBS) using a P1000 pipette and transfer them to 1.5 mL tubes.Note: If some alveolospheres are left in the plate, then add 200 μL PBS and collect them into the same tube.
-
b.Spin down sample at 300 g for 2 min at 4°C. Most alveolospheres will settle at the bottom of the tube.
-
c.Carefully remove medium with P1000 pipette leaving around 50 μL of solution inside the tube. Make sure not to remove alveolospheres.
-
d.Use P200 pipette to gently move alveolospheres to cryomold filled with OCT.
-
e.Place cryomold on dry ice and wait until it solidifies (turns white) then transfer the block to −80°C for long term storage.
 CRITICAL: Make sure to touch the bottom of the cryomold before pipetting the alveolospheres out of the tip. Care must be taken to avoid introducing air bubbles while pipetting.
CRITICAL: Make sure to touch the bottom of the cryomold before pipetting the alveolospheres out of the tip. Care must be taken to avoid introducing air bubbles while pipetting. -
f.Adjust the position of alveolospheres with pipette tip.
 CRITICAL: Incubation of human alveolospheres with 4% PFA results in dissolution of matrigel, which leads to release of alveolospheres into suspension. Care must be taken to avoid loss of alveolospheres in the subsequent steps. Collect alveolospheres carefully using a wide-bore P1000 tip and pipette to the cryomold.
CRITICAL: Incubation of human alveolospheres with 4% PFA results in dissolution of matrigel, which leads to release of alveolospheres into suspension. Care must be taken to avoid loss of alveolospheres in the subsequent steps. Collect alveolospheres carefully using a wide-bore P1000 tip and pipette to the cryomold.
-
a.
-
14.Cryosectioning of alveolospheres.
-
a.Cryosection the frozen sample at 8–10 μm thickness.
-
b.Collect sections on Superfrost plus slides, dry at 20°C–25°C and store at −80°C.
-
a.
-
15.Immunofluorescence staining for AT2 and AT1 cell specific markers.
-
a.Bring OCT sections to 20°C–25°C and wash with PBS.
-
b.Perform antigen retrieval using 10 mM sodium citrate buffer in water bath (95°C for 15 min).
-
c.Wash sections twice with PBS each 5 min followed by permeabilization using PBST (0.1% Triton X-100 in PBS) for 10 min.
-
d.Incubate with 1% BSA in PBST for 30 min at 20°C–25°C.
-
e.Prepare primary antibodies dilutions in blocking buffer. Examples of antibodies that could be used for validation of alveolospheres are listed below (Figure 5E):
-
i.Prosurfactant protein C (SFTPC) (1:500).
-
ii.Prosurfactant protein B (SFTPB) (1:500).
-
iii.RAGE/AGER (1:500).
-
iv.HTII-280 (1:250).
-
i.
-
f.Incubate with primary antibodies for 2 h at 20°C–25°C or 15–18 h at 4°C.
-
g.Wash slides 3 times with PBST.
-
h.Secondary antibodies (1:500) diluted in blocking buffer.
-
i.Incubate sections with secondary antibodies for 1 h at 20°C–25°C.
-
j.Wash slides 3 times with PBST and mount the slides using Fluoromount G reagent with DAPI.
-
a.
Figure 5.
Validation of alveolospheres culture
(A) Schematic representation of fixation and embedding of mouse alveolospheres.
(B) Images showing indicated step of mouse alveolospheres embedding for cryosection.
(C) Schematic illustration of human alveolospheres collection following fixation.
(D) Images showing steps for human alveolospheres embedding.
(E) Immunostaining with AT2 cell marker -SFTPC, SFTPC and HTII-280 and AT1 cell marker - AGER (gray) on sections collected from mouse (left) and human (right) alveolospheres. Scale bar: 100 and 30 μm.
Expected outcomes
This step-by-step protocol provides detailed information for the isolation and purification of primary alveolar type 2 cells and their culture in 3-dimensional alveolospheres using serum-free and feeder-free medium. It is expected that murine AT2s will generate spherical alveolospheres in 10–12 days (Figure 4C). Murine and human alveolospheres can be expanded and passaged for several rounds (mouse – up to 5 passages and human 10–15 passages) without losing AT2 cell characteristics. Details of the passaging and colony formation efficiency can be found in our previous publication (Katsura et al., 2020). The cellular composition and molecular characterization of alveolospheres can be determined by immunofluorescence analysis as described. Human and mouse alveolospheres cultured in above-described medium conditions are expected to express AT2 cells marker (SFTPC) but not AT1 cells marker (AGER) (Figure 5E).
Limitations
This culture method can be applied to primary alveolar epithelial cells from human and mouse lungs. However, in some cases, we observed differences in the growth kinetics and cellular composition of human lung alveolospheres. This might be due to intrinsic variability (age, prior health status, and influence of lifestyle etc.). Additionally, some human AT2 cells may show limited growth after several rounds of passages. Therefore, we advise that quality control measures be taken once cells reach about 10–12 passages. In the case of mouse alveolospheres, although they continue to grow, the AT2 cells tend to show spontaneous differentiation into AT1 cells after about five passages.
Troubleshooting
Problem 1
After pipetting the AT2s in the droplet format, cells might go to the bottom of the well-plate and adhere to the plastic bottom surface (related to step 8).
Potential solution
To avoid AT2s moving down and touching well bottom, immediately move the plate to the incubator.
Problem 2
During alveolospheres culture, either entire or parts of droplets may lift-off and float in the culture medium (related to step 8).
Potential solution
To avoid droplet lifting, care must be taken during medium changes. If this occurs, do not use suction-based aspirator to remove medium during routine medium changes. Instead, use P1000 pipette to gently remove or add fresh medium.
Problem 3
Alveolosphere colony formation efficiency is low from cryopreserved cells (related to step 8).
Potential solution
Due to several variables, such as cryofreezing conditions, freezing cell densities, etc., freshly thawed cells may yield low colony forming efficiency. We recommend using excess cells (4000 cells) in each droplet during set up.
Problem 4
Loss of cells during dissociation of alveolospheres (related to step 9).
Potential solution
Insufficient matrigel dissolution during alveolospheres passaging may lead to significant loss of cells. It is important to check cell suspension under microscope to ensure complete dissolution of matrigel. If necessary, gently mix the cell suspension using a P1000 pipette. Alternatively, extend the time of TrypLE incubation, if necessary.
If this problem persists, add an additional digestion step with accutase to enhance the removal of matrigel and thereby increase the number of cells. See below for step-by-step protocol for accutase digestion protocol.
-
•
Aspirate the culture medium without disruption of bubbles and add 1 mL of pre-warmed accutase to one well of 6-well plate
-
•
Use a cell lifter to scrape the drops to detach from plate, pipette several times with a P1000, collect the cell suspension to 1.5 mL tube.
-
•
Incubate at 37°C for 20 min with horizontal rotation.
-
•
Centrifuge the tube at 450 g for 5 min at 4°C and aspirate the supernatant.
-
•
Add 500 μL of TrypLE select to the tube, pipette several times, and incubate at 37°C followed by vigorous mixing by pipetting.
-
•
Add 10 mL of DMEM/F12 containing 10% FBS to stop digestion and centrifuge the tube at 450 g for 5 min at 4°C.
-
•
Aspirate the supernatant, resuspend the cell pellet in 500 μL of culture medium and transfer cell suspension to a fresh 1.5 mL tube.
Problem 5
Matrigel related issues causing a decline in colony numbers and growth rate.
Potential solution
Matrigel quality and protein concentration can vary from lot to lot. We recommend procuring Matrigel containing 10 mg/mL or above protein content.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Purushothama Rao Tata, purushothamarao.tata@duke.edu.
Materials availability
This study did not generate unique reagents.
Acknowledgments
We thank Brigid Hogan and members of Tata lab for fruitful discussions. We thank Randell lab members (University of North Carolina at Chapel Hill) and Duke BioRepository and Precision Pathology Center (BRPC) for providing human lung tissue. BRPC received support from P30 CA014236 and UM1 CA239755. We acknowledge support from Duke Cancer Institute Flow Cytometry Shared Resource for cell sorting. S.K. is a fellow of the Japan Society for the Promotion of Science Overseas Research. This work was supported by NHLBI/NIH (R00HL127181, R01HL146557, R01HL153375) and funds from Regeneration NeXT and Kaganov-MEDx Pulmonary Research Initiative at Duke University to P.R.T. This work was partially supported by a grant from the United Therapeutics Corporation (to P.R.T.). P.R.T. is a Whitehead Scholar at Duke University.
Author contributions
S.K. performed the experiments, analyzed the data, and co-wrote the manuscript. A.T. co-designed, conceived and performed the experiments, analyzed the data, and co-wrote the manuscript. P.R.T. co-designed, conceived and supervised the work, and co-wrote the manuscript. All authors reviewed and edited the manuscript.
Declaration of interests
A patent application (PCT/US20/53158) related to this work has been filed. P.R.T. is listed as a coinventor on this application. P.R.T. serves as acting CEO of Iolux Inc. P.R.T. serves as a consultant for Surrozen Inc., Cellarity Inc., and Celldom Inc. on work not related to the contents of this manuscript.
Contributor Information
Aleksandra Tata, Email: aleksandra.tata@duke.edu.
Purushothama Rao Tata, Email: purushothamarao.tata@duke.edu.
Data and code availability
This study did not generate datasets/code.
References
- Katsura H., Sontake V., Tata A., Kobayashi Y., Edwards C.E., Heaton B.E., Konkimalla A., Asakura T., Mikami Y., Fritch E.J., et al. Human lung stem cell-based alveolospheres provide insights into SARS-CoV-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell. 2020;27:890–904.e8. doi: 10.1016/j.stem.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Barkauskas C.E., Cronce M.J., Xue Y., Harris J.R., Liang J., Noble P.W., Hogan B.L.M. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl. Acad. Sci. U.S.A. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate datasets/code.