Abstract
The aim of this study was to investigate the protective effect of trans-anethole (TA) on lipopolysaccharide-induced acute liver inflammation model of chickens by determining the levels of inflammatory mediators in serum and liver, relative mRNA expression and protein expression of inflammation-related genes in NF-κB signaling pathway. A total of 160 one-day-old male chickens (Arbor Acres) were assigned into 4 treatments with 8 replicates of 5 birds each. On d 20, the control group was intraperitoneally injected with sterile saline and the other groups were injected with lipopolysaccharide (LPS; 5 mg/kg body weight). There were no significant differences in average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR) among groups. However, compared with the control group, the LPS group significantly increased (P < 0.01) the serum levels of interleukin-6 (IL-6), interleukin-1beta (IL-1β), tumor necrosis factor-alpha (TNF-α), alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and decreased (P < 0.01) the interleukin-10 (IL-10) level. TA attenuated (P < 0.01) these increases in IL-1β, TNF-α, ALT, and AST levels and improved (P < 0.01) the IL-10 level. In liver, the groups fed with TA had lower (P < 0.01) concentrations of IL-6 and TNF-α as well as higher (P < 0.05) concentration of IL-10. Furthermore, TA downregulated (P < 0.05) the mRNA expression levels of nuclear factor kappa B p65 (NF-κB p65) and TNF-α, also upregulated (P < 0.05) IL-10 and inhibitor of NF-κB alpha (IκBα) upon LPS challenge. In protein level, supplementation of 600 mg/kg of TA downregulated (P < 0.05) and upregulated (P < 0.05) the protein expression of NF-κB p65 and IκBα, respectively. The present findings suggest that TA could alleviate the acute liver inflammation induced by LPS via blocking the activation of NF-κB and the 600 mg/kg of TA plays more fruitful role in protecting broilers against LPS stimulus.
Key words: trans-anethole, acute liver inflammation, lipopolysaccharide, broiler, NF-κB signaling pathway
INTRODUCTION
The acute inflammatory response plays a pivotal role in innate immune reaction occurred when the body is exposed to a variety of stimuli, which is manifested as amplified vascular permeability and release of proinflammatory cytokines including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and interleukin-1beta (IL-1β; Varela et al., 2018). The acute inflammation is a short process generally lasting from hours to a few days until the endogenous stimuli are scavenged totally, however, if not, this procedure will turn to chronic inflammation, which can lead to mortal diseases, such as autoimmune disorder, pulmonary sepsis, and so forth (Arulselvan et al., 2016). Many studies have identified universally that animal acute inflammation models are well-established in seeking and screening the anti-inflammatory activity of phytopharmaceuticals (Shen et al., 2010; Dickson et al., 2016; Patil and Patil, 2017; Patil et al., 2019). In the acute inflammation models, lipopolysaccharide (LPS) is the primary stimulus consisting of lipid A and a polysaccharide chain, which derives from the outermost membrane of Gram-negative bacteria (Rossol et al., 2011). In addition, during LPS infection process, it can provoke different extent of damage in organs including paw edema (Vajja et al., 2004), acute lung injury (ALI) (Wang and Xiao, 2019), acute liver failure (ALF; Li et al., 2021), and pleurisy (Sampaio et al., 2004). Researches concerning LPS-induced animal models have suggested that the liver is the target for activating acute inflammatory response (Savio et al., 2017; Wilde and Katsounas, 2019; Li et al., 2021).
Herbal plants are the major sources for healing many common symptoms of diseases like flatulence and ulceration with few side effects in Chinese traditional medicine (Liu et al., 2015). Trans-anethole (TA) is an alkenylbenzene compound, as the main bioactive component of the volatile oil which is isolated from the seeds of anise and fennel responsible for its multiple bioactivities including antioxidative, antiulcer, anticonvulsant, insecticidal, and anti-inflammatory activity. (Mimica-Dukić et al., 2003; Ghosh et al., 2012; Yu et al., 2020a). Furthermore, TA is easy to be deteriorated when exposed to light and high temperature. It has been recognized as safe by the Food and Drug Administration (FDA), and mainly applied in food, cosmetic, perfume, and medical industries (Aprotosoaie et al., 2016). Numerous studies shed light on the anti-inflammatory activity of TA in intestinal inflammation, pulmonary inflammation, hepatic inflammation, and so forth (Yea et al., 2006; Sung et al., 2012; Ritter et al., 2013; Wisniewski-Rebecca et al., 2015). Moreover, TA is reported to inhibit the activation of nuclear factor kappa B (NF-κB) signaling pathway in LPS-stimulated inflammatory response (Kang et al., 2013). Nonetheless, few researches was done to investigate the anti-inflammatory effect of TA on chickens under the challenge of LPS. Thus, we hypothesized that TA may have protective effect on the acute liver inflammation by suppressing the NF-κB signaling pathway.
MATERIALS AND METHODS
Preparation of Trans-anethole and Lipopolysaccharide
TA was obtained from Nanjing Dilger Medical Technology Co., Ltd, the purity of which was 98.35%. The TA was kept in glass bottles avoiding light at 4°C until use. LPS was purchased from Sigma-Aldrich Chemical Co. (O55:B5; #L2880; St. Louis, MO), which was dissolved in 0.86 % (w/v) sterile saline prepared to 1 mg/mL concentration solution before used for administration.
Animals and Experimental Design
All of the experimental procedures in this study were approved by the Institution of Animal Care and Use Committee of Nanjing Agriculture University (Nanjing, China). A total of 160 one-day-old male broiler chickens (Arbor Acres) were purchased from a commercial hatchery (Yantai Land Animal Husbandry Co., Ltd). All chicks were randomly assigned to four treatment groups with eight replicates of 5 birds. Birds were divided into 4 groups: 1) broilers fed with basal diet (CON); 2) LPS-stimulated broilers fed with basal diet (LPS); 3) LPS-stimulated broilers fed basal diet with 400 mg/kg TA (LPS + TA400); 4) LPS-stimulated broilers fed basal diet with 600 mg/kg TA (LPS + TA600). The basal diet was formulated to meet nutrient requirements of broilers according to the Feeding Standard of chicken of the People's Republic of China (NY/T 33-2004). The ingredients and nutrient levels of basal diet were shown in Table 1. At the 20 d of age, LPS-challenged broilers were intraperitoneally injected with a dose of 5 mg/kg body weight (BW) LPS solution and broilers in control group were treated with an equal amount of sterile saline. The dosage of LPS was referred to earlier studies (Tavakoli et al., 2020; Zhang et al., 2020; Chen and Yu, 2021). The experiment period lasted for 21 d when birds were housed in battery cages that supplied water and feed for ad libitum amount. These cages were placed in a room where the temperature was kept at 35°C in first week and then reduced gradually by 0.5°C per day to a range of 21°C to 26°C.
Table 1.
Ingredients and nutrient composition of the basal diet1 (%, as fed-basis).
| Ingredient | % | Nutrient levels1 | % |
|---|---|---|---|
| Corn | 55.60 | Metabolizable energy, Mcal/kg | 2.87 |
| Expanded soybean meal | 29.00 | Crude protein | 20.95 |
| Cottonseed meal | 2.50 | Total calcium | 0.96 |
| Wheat flour | 4.00 | Total phosphorus | 0.66 |
| Hydrolyzed feather meal | 1.50 | Total lysine | 1.11 |
| Soybean oil | 2.00 | Total methionine | 0.35 |
| Dicalcium phosphate | 0.90 | Total threonine | 0.82 |
| Limestone | 1.50 | ||
| Bentonite | 1.00 | ||
| Premix2 | 2.00 | ||
| Total | 100.00 |
All nutrient levels were analyzed values, except metabolizable energy.
Supplied per kilogram of diet: vitamin A, 11,500 IU; cholecalciferol, 3,500 IU; vitamin E, 30 mg; vitamin K3, 5 mg; thiamin, 3.38 mg; riboflavin, 9.0 mg; pyridoxine, 8.96 mg; vitamin B12, 0.025 mg; choline chloride, 800 mg; calcium pantothenate, 13 mg; niacin, 45 mg; biotin, 0.15 mg; folic acid, 1.20 mg; Mn, 60 mg; Fe, 66.5 mg; Zn, 88 mg; Cu, 8.8 mg; I, 0.70 mg; Se, 0.288 mg.
Growth Performance
On d 20, the BW and feed intake of birds in each replicate were recorded to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR). Meanwhile, the values were adjusted based on the mortality of birds.
Sample Collection
On d 21, eight birds were selected randomly from each treatment group. About 3 mL of blood samples were collected from the wing vein into the coagulation-promoting tubes. The blood samples were incubated at 37°C for 2 h and centrifuged at 3,000 g for 10 min at 4 °C. After that, the supernatant was transferred into 1.5 mL centrifugal tube, then stored at −20°C until analysis. A total 32 birds were sacrificed by cervical dislocation, livers of which were collected as fast as into sterile tubes and stored in liquid nitrogen for further analyses.
Determination of Inflammatory Cytokines
The concentration of IL-6, interleukin-10 (IL-10), IL-1β, and TNF-α in the serum and liver were determined by ELISA kits (Jiangsu Meimian Industry Co., Ltd, China) following the manufacturer's instructions. The levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, China).
RNA Extraction and Fluorescence Quantitative Real-Time PCR
Total RNA from liver samples was isolated with the Trizol reagent (Nanjing Vazyme Biotech Co., Ltd, China) according to the manufacturer's instructions. After measured the purity and concentration using a spectrophotometer (NanoDrop Products, Wilmington, DE), 500 ng of total RNA was performed reverse transcription reaction to product cDNA using EasyScript All-in-one First-Strand cDNA Synthesis SuperMix for quantitative real-time qPCR products (TransGen Biotech, Beijing, China) following the manufacturer's instructions. The cDNA was used to amplify with PerfectStart Green qPCR SuperMix (TransGen Biotech) for assessing the expression level of genes following the manufacturer's instructions based on Applied Biosystems QuantStudio 7 Flex apparatus. The primer sequences were presented in Table 2 and β-Actin was used as an internal reference. The mRNA expression of target genes relative to β-Actin was calculated by 2−△△CT method.
Table 2.
Gene-specific primers sequences for quantitative real-time PCR.
| Gene name1 | GenBank2 | Primer sequence3 (5′→3′) | Length |
|---|---|---|---|
| IL-6 | AB302327.1 | AACAACCTCAACCTGCCCAA | 112 |
| AGGTCTGAAAGGCGAACAGG | |||
| IL-8 | DQ393272.2 | CCTCCTCCTGGTTTCAGCTG | 136 |
| TGGCGTCAGCTTCACATCTT | |||
| IL-10 | NM_012854.2 | CAGACCAGCACCAGTCATCA | 96 |
| TCCCGTTCTCATCCATCTTCTC | |||
| NF-κB | NM_001012887.2 | AAGATCTGGTGGTGTGCCTG | 137 |
| AGTGGAACCTTTCGCGGATT | |||
| IκBα | NM_001001472.2 | CAGCACTACACTTGGCCGTA | 101 |
| GGAGTAGCCCTGGTAGGTCA | |||
| TNF-α | HQ739087.1 | GAACCCTCCGCAGTACTCAG | 116 |
| AACTCATCTGAACTGGGCGG | |||
| TLR4 | KP410249.1 | CGGCTCCGCATCTTGGATAT | 148 |
| GGGCTTGGAGTGGCTTGTAT | |||
| IFN-γ | NM_205149.1 | TGTAGCTGACGGTGGACCTA | 134 |
| GCGGCTTTGACTTGTCAGTG | |||
| β-Actin | NM_205518.1 | ACCGGACTGTTACCAACACC | 116 |
| CCTGAGTCAAGCGCCAAAAG |
Abbreviations: IL, interleukin; IκBα, inhibitor of NF-κB alpha; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; TLR4, toll-like receptor 4.
GenBank Accession Number.
Shown as the forward primer then the reverse primer.
Western Blot Analysis
Western blot was conducted for determining the liver protein expression level related to NF-κB signaling pathway. Four replicates per treatment were randomly selected, which was referred to previous studies (Hollemans et al., 2021; Xing et al., 2021). Cytoplasmic protein and nuclear protein were obtained using the Nuclear and Cytoplasmic Protein Extraction kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer's instructions. Subsequently, the bicinchoninic acid (BCA) Protein Assay kit (Beyotime Biotechnology) was used for the determination of protein concentration. Preparing the protein gel using the 12.5% PAGE Gel FAST Preparation Kit (Epizyme Biomedical Technology Co., Ltd, Shanghai, China), after that, separating the protein samples on a protein gel and transferring the target gel to the polyvinylidene fluoride (PVDF) membrane. After closed in blocking buffer for 1.5 h, the membranes were incubated with the primary antibodies against NF-κB p65 (Proteintech Group, Inc., Wuhan, China), inhibitor of NF-κB alpha (IκBα; Proteintech Group, Inc.), β-Actin (Affinity Bioscicences Co., Ltd, Jiangsu, China), Lamin B1 (Proteintech Group, Inc) overnight at 4°C, β-Actin and Lamin B1 were used as cytoplasmic and nuclear internal reference, respectively, then, the bands were washed using Tris-buffered saline (TBS) containing 0.1% Tween 20 (TBST) buffer for 3 times and incubated with secondary antibody for 1.5 h. The expression of target proteins was detected using ChemiDoc MP Imaging System (Bio-Rad Laboratories, Inc., Hercules, CA) with enhanced chemiluminescence (ECL) reagent (Nanjing Vazyme Biotech Co., Ltd).
Statistical Analysis
All data were analyzed using one-way ANOVA of SPSS software (ver. 21.0; IBM-SPSS, Inc., Chicago, IL). The values were expressed as the mean ± SEM and the significant differences among groups were assessed by Tukey's HSD test. P < 0.05 and P < 0.01 were considered as statistically significant and highly significant, respectively.
RESULTS
Growth Performance
The effects of TA on the growth performance of broilers before LPS challenge are shown in Table 3. All of the birds were healthy and no mortality appeared. Birds supplemented with 600 mg/kg of TA had a higher (P < 0.01) ADFI than other groups. There were no significant differences in ADG and FCR of broilers among groups from d 0 to d 20.
Table 3.
Effects of trans-anethole on the growth performance of broilers before LPS challenge1.
| Items2 | CON | LPS | LPS+TA400 | LPS+TA600 | P-value |
|---|---|---|---|---|---|
| ADG, g/d | 54.53 ± 0.70 | 54.41 ± 0.56 | 55.01 ± 1.41 | 59.57 ± 1.27 | 0.223 |
| ADFI, g/d | 40.79 ± 1.37b | 41.5 ± 0.59b | 41.68 ± 1.16b | 44.17 ± 1.33a | 0.008 |
| FCR, g/g | 1.31 ± 0.02 | 1.31 ± 0.01 | 1.32 ± 0.01 | 1.35 ± 0.01 | 0.243 |
The values in the same row with different superscripts means significantly different (P < 0.05).
The values are represented as mean ± SEM, n = 8.
Abbreviatios: ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio. CON, control group fed with basal diet and treated with saline; LPS, group fed with basal diet and treated with lipopolysaccharide; LPS+TA400, group fed with 400 mg/kg trans-anethole and treated with lipopolysaccharide; LPS+TA600, group fed with 600 mg/kg trans-anethole and treated with lipopolysaccharide.
Levels of ALT and AST in the Serum
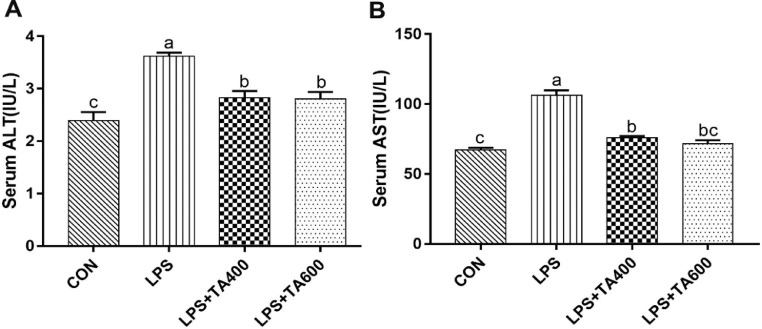
As shown in Figure 1, compared with the control group, the LPS group significantly increased (P < 0.01) the levels of serum ALT and AST. These increases were attenuated (P < 0.01) by dietary supplementation of TA.
Figure 1.
Effects of trans-anethole on the serum levels of ALT and AST in lipopolysaccharide-challenged broilers. The values are represented as mean (n = 8) with their standard errors. Bars with unlike letters means significantly different. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase. CON, control group fed with basal diet and treated with saline; LPS, group fed with basal diet and treated with lipopolysaccharide; LPS+TA400, group fed with 400 mg/kg trans-anethole and treated with lipopolysaccharide; LPS+TA600, group fed with 600 mg/kg trans-anethole and treated with lipopolysaccharide.
Inflammatory Cytokines Levels in the Serum
As shown in Table 4, LPS challenge increased (P < 0.01) the serum concentrations of IL-6, IL-1β, TNF-α but decreased (P < 0.01) the IL-10 concentration compared with control group. However, birds supplemented with TA had lower (P < 0.01) serum concentrations of IL-1β, TNF-α, as well as higher (P < 0.01) concentration of IL-10.
Table 4.
Effects of trans-anethole on the serum inflammatory cytokine levels of LPS-challenged broilers1.
| Items2 | CON | LPS | LPS+TA400 | LPS+TA600 | P-value |
|---|---|---|---|---|---|
| IL-6, pg/mL | 3.23 ± 0.08b | 3.66 ± 0.06a | 3.57 ± 0.05a | 3.56 ± 0.06a | <0.001 |
| IL-10, pg/mL | 8.72 ± 0.07c | 8.37 ± 0.07b | 9.57 ± 0.14a | 9.84 ± 0.15a | <0.001 |
| IL-1β, pg/mL | 77.54 ± 1.33c | 85.75 ± 0.68a | 83.17 ± 0.35ab | 81.80 ± 0.91b | <0.001 |
| TNF-α, pg/mL | 11.55 ± 0.12b | 12.85 ± 0.13a | 11.72 ± 0.16b | 11.61 ± 0.16b | <0.001 |
The values in the same row with different superscripts means significantly different (P < 0.05).
The values are represented as mean ± SEM, n = 8.
Abbreviations: IL-6, interleukin-6; IL-10, interleukin-10; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α. CON, control group fed with basal diet and treated with saline; LPS, group fed with basal diet and treated with lipopolysaccharide; LPS+TA400, group fed with 400 mg/kg trans-anethole and treated with lipopolysaccharide; LPS+TA600, group fed with 600 mg/kg trans-anethole and treated with lipopolysaccharide.
Inflammatory Cytokines Levels in the Liver
As shown in Table 5, although LPS enhanced (P < 0.05) the levels of IL-6, IL-1β, and TNF-α and declined (P < 0.05) the IL-10 level, the levels of IL-6 and TNF-α in the liver were decreased (P < 0.01) by dietary TA supplementation as compared with LPS group. However, compared with the LPS group, the IL-10 concentration in the liver was increased (P < 0.05) by the administration of 600 mg/kg of TA.
Table 5.
Effects of trans-anethole on the liver inflammatory cytokine levels of LPS-challenged broilers1.
| Items2 | CON | LPS | LPS+TA400 | LPS+TA600 | P-value |
|---|---|---|---|---|---|
| IL-6, ng/g protein | 22.83 ± 0.51c | 36.21 ± 1.22a | 30.90 ± 1.21b | 28.22 ± 0.59b | <0.001 |
| IL-10, ng/g protein | 38.37 ± 1.87a | 33.31 ± 1.12b | 35.31 ± 1.35ab | 39.33 ± 0.41a | 0.023 |
| IL-1β, ng/g protein | 49.30 ± 1.40b | 78.15 ± 0.96a | 74.51 ± 4.64a | 70.72 ± 1.72a | <0.001 |
| TNF-α, ng/g protein | 51.13 ± 1.99c | 85.48 ± 3.26a | 69.69 ± 3.28b | 67.22 ± 1.02b | <0.001 |
The values in the same row with different superscripts means significantly different (P < 0.05).
The values are represented as mean ± SEM, n = 8.
Abbreviations: IL-6, interleukin-6; IL-10, interleukin-10; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α. CON, control group fed with basal diet and treated with saline; LPS, group fed with basal diet and treated with lipopolysaccharide; LPS+TA400, group fed with 400 mg/kg trans-anethole and treated with lipopolysaccharide; LPS+TA600, group fed with 600 mg/kg trans-anethole and treated with lipopolysaccharide.
Expression Levels of Inflammation-Related Genes in the Liver
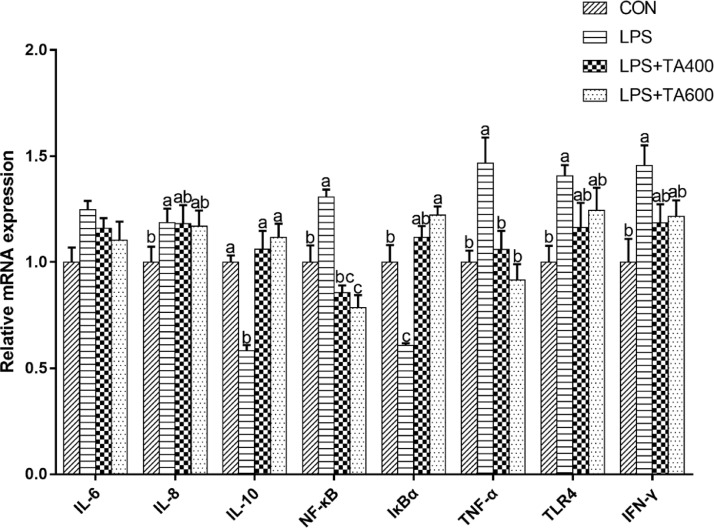
The expression levels of inflammation-related genes in the liver of birds are shown in Figure 2. Challenging birds with LPS enhanced (P < 0.05) the liver mRNA expression of interleukin-8 (IL-8), NF-κB p65, TNF-α, toll-like receptor 4 (TLR4), and interferon-gamma (IFN-γ), but downregulated (P < 0.05) the mRNA expression of IL-10 and IκBα as compared with unchallenged birds. Dietary supplementation of TA decreased (P < 0.05) the mRNA expression levels of NF-κB p65 and TNF-α, also enhanced (P < 0.05) IL-10 and IκBα in LPS-challenged birds. The results indicated that LPS challenge activated NF-κB signaling pathway in the liver and supplementation of TA exerted inhibitory effect on that.
Figure 2.
Effects of trans-anethole on the relative mRNA expression of genes related to inflammation in the liver of LPS-challenged broilers. The values are represented as mean (n = 8) with their standard errors. Bars with unlike letters means significantly different. Abbreviations: IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; NF-κB, nuclear factor kappa B; IκBα, inhibitor of NF-κB alpha; TNF-α, Tumor necrosis factor-α; TLR4, Toll-like receptor 4; IFN-γ, Interferon-γ. CON, control group fed with basal diet and treated with saline; LPS, group fed with basal diet and treated with lipopolysaccharide; LPS+TA400, group fed with 400 mg/kg trans-anethole and treated with lipopolysaccharide; LPS+TA600, group fed with 600 mg/kg trans-anethole and treated with lipopolysaccharide.
Relative Protein Expression Levels in the Liver
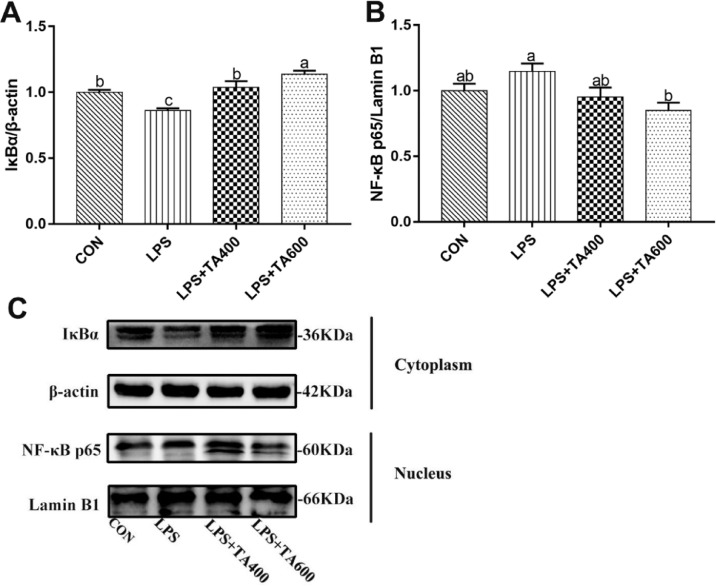
As shown in Figure 3, LPS challenge downregulated (P < 0.01) the relative protein expression of IκBα in the liver of birds in comparison with control group. Supplementation of 600 mg/kg of TA suppressed (P < 0.05) the activation of NF-κB signaling pathway induced by LPS challenge.
Figure 3.
Effects of trans-anethole on the protein expression of NF-κB pathway in the liver of LPS-challenged broilers. The values are represented as mean (n = 4) with their standard errors. Bars with unlike letters means significantly different. Abbreviations: NF-κB p65, nuclear factor kappa B p65; IκBα, inhibitor of NF-κB alpha. CON, control group fed with basal diet and treated with saline; LPS, group fed with basal diet and treated with lipopolysaccharide; LPS+TA400, group fed with 400 mg/kg trans-anethole and treated with lipopolysaccharide; LPS+TA600, group fed with 600 mg/kg trans-anethole and treated with lipopolysaccharide.
DISCUSSION
It is well established that LPS, as a constituent of Gram-negative bacterial outer membrane, can recognize and bind with the receptor proteins on the surface of immune-related cells leading to the subsequent commence of innate immune response in host (Fenton and Golenbock, 1998). Moreover, LPS is also known as endotoxins that exist in gut microbiota with their high abundance (Verhaar et al., 2020). When the host is infected by exogenous LPS or the endogenous LPS in gut translocates into blood circulation, which can in turn contribute to pulmonary sepsis, hepatic failure, intestinal barrier damage, and even death (Seeley and Ghosh, 2017). Therefore, LPS has been extensively used for acute inflammation modeling in animals, which simultaneously may pave a way for developing and assessing therapeutic agents to control the endotoxins. To the best of our knowledge, much scientific work has been published about the anti-inflammatory effect of TA on mammals in vivo and in vitro (Cavalcanti et al., 2012; Estevao-Silva et al., 2014; da Rocha et al., 2017) but little on poultry. Meanwhile, in the context of intensive production system, chickens are very susceptible to bacterial diseases which can cause widespread death. Thus, this study aimed to investigate the protective effect of TA on chickens under the challenge of LPS via determining the levels of inflammatory mediators in serum and liver, thereafter, to further explore the protective mechanism of TA by detecting the mRNA expression of inflammation-related genes and protein expression of key proteins involved NF-κB signaling pathway.
The serum ALT and AST activities, as biomarkers for liver injury, are commonly used in many studies (Hao et al., 2015; Ren et al., 2021). A former study exhibited that the mean levels of the AST and ALT activities were increased in the endotoxin shock model of mice challenged with LPS (Motobu et al., 2006). In consistency with that, the serum levels of ALT and AST were obviously increased by LPS stimulation in the current study, which implied that the administration of LPS caused hepatic lesion in broilers. However, dietary supplementation of TA decreased the high levels of serum ALT and AST. It is in accordance with the results of Cho et al. (2013), who showed that pretreatment with TA attenuated the increased level of serum ALT in mice after hepatic ischemia/reperfusion (I/R) and the increased level of ALT is correlated with the production of proinflammatory cytokines.
It is well known that inflammatory cytokines are critical to participating innate immune response processes, which are categorized as proinflammatory and anti-inflammatory (Rossol et al., 2011). During immune response processes activated by bacterial stimuli, the macrophages and monocytes are in charge of secreting these inflammatory cytokines (Cavaillon, 2018). Among these cytokines, TNF-α is the prototype of proinflammatory cytokines contributing to many pathogenic processes, such as periodontitis and acute lung injury (ALI) (Jiao et al., 2021). Similarly, IL-1β is the most studied member of IL-1 family released from macrophages and IL-6 is one of the major regulators in acute phase reaction (Iannarelli et al., 2018). In contrast, IL-10 is involved in antagonizing inflammation response by inhibiting the production of proinflammatory cytokines (Cavaillon, 2018). Hence, we determined the levels of the 4 representative cytokines in the serum and liver. The results of this study exhibited that the concentrations of serum and liver IL-1β, IL-6 and TNF-α were elevated, but the serum and liver levels of IL-10 were decreased after LPS challenge. Nevertheless, birds supplemented with TA had not only reduced serum concentration of IL-1β and TNF-α, but also increased concentration of IL-10. The similar changes of these cytokines were observed in liver, except there is a significantly declined level of IL-6 instead of IL-1β. We noted that there were no differences among groups in the gene expression of IL-6. This may be explained by the post-translational modification of mRNA, which could cause the discrepancy between mRNA and protein levels (Leutert et al., 2021). Numerous reports showed that LPS is a potent stimulus to elicit the release of inflammatory cytokines (Wu et al., 2017; Ramires et al., 2021). It has been documented that anethole was capable of controlling the periodontitis through suppressing the serum IL-1β and TNF-α levels (Moradi et al., 2014). Furthermore, Zhang et al. (2018) elucidated that TA increased the gene expression of IL-10 in acute lung injury mice. Intriguingly, a study based on LPS-induced inflammation model of mice revealed that TA had no effect on the production of IL-6 (Kang et al., 2013). In the model of pleurisy rats, the level of IL-1β was not significantly altered by treatment with TA (Domiciano et al., 2013). These evidences validate the results with respect to the variations of inflammatory cytokines in the present study, which may partially attribute to the animal species (Klasing, 1994).
The association between LPS and NF-κB has been illustrated most clearly due to the broad function of NF-κB pathway acting to the inflammatory response, which is a complex process characterized by a cascade of the expression of both proinflammatory and anti-inflammatory mediators like IL-1β, TNF-α, IL-6, and IL-10 (Lawrence, 2009). NF-κB is an inducible nuclear transcription factor, the whole pathway of which comprises of a plenty of positive and negative modulatory elements (Dolcet et al., 2005). One of the basic core components is NF-κB dimers responsible for multifunctional regulation of downstream events including binding to DNA and inducing the transcription of proinflammatory cytokines (Hayden and Ghosh, 2008). Among the dimers of NF-κB, the most canonical heterodimers consisted by p65 and p50 are largely bound to IκBα protein, these bounding forms of which constitute inactive compounds in the cytoplasm (Newton and Dixit, 2012). A diversity of stimuli was reported to activate NF-κB pathway as a result of the release of NF-κB dimers after the degradation of IκBα, which further cause the translocation of NF-κB dimers into the nucleus initiating the inflammatory response (Poveda et al., 2017; Akhter et al., 2018; Yu et al., 2020b). An earlier study demonstrated that the LPS toxin triggered the activation of NF-κB pathway in the model of acute lung injury rats (Feng et al., 2019). Thereby, this study investigated whether TA could suppress NF-κB signaling pathway in LPS-induced inflammation in broilers. The results showed that LPS enhanced the mRNA expression of NF-κB p65 and downregulated the mRNA and protein expression of IκBα in the liver of broilers. Whereas, TA supplementation downregulated the mRNA and protein expression of NF-κB p65, meanwhile, up-regulated the mRNA and protein expression of IκBα. This is in accordance with the findings of Rezayat et al. (2018), who revealed that Foeniculum vulgare essential oil protected rat from acetic acid-induced colitis by inhibiting NF-κB signaling pathway. Moreover, Chainy et al. (2000) indicated that TA blocked IκBα phosphorylation by acting on IκBα kinase. Taken together, TA may ameliorate the liver inflammatory response upon LPS challenge through repressing the activation of NF-κB signaling pathway and inhibiting the production of proinflammatory cytokines in broilers.
In the present study, it is worthwhile to mention that administration of 600 mg/kg of TA is more effective on protecting broilers from liver damage after LPS stimulation than 400 mg/kg of TA. We also found that 600 mg/kg of TA supplementation significantly improved the ADFI of broilers before LPS challenge. It is similar with the results of Yu et al. (2021), who revealed that the inclusion of TA significantly increased the ADFI of broilers from d 1 to d 42 and it may be due to the sweet odor of TA that further stimulates appetite. This study also reported that 800 mg/kg of TA had adverse effect on intestinal barrier function of broilers, which provided a reference for choosing the efficient concentrations of TA for ameliorating acute inflammation in the present study. As a matter of fact, Kang et al. (2013) also revealed that anethole (62.5, 125, 250, and 500 mg/kg) reduced cell numbers of leukocyte in a dose-dependent manner, which may support this finding that 600 mg/kg of TA plays more fruitful role in protecting broilers against LPS stimulus. In conclusion, dietary supplementation of TA at 600 mg/kg could show the promising effect on LPS-induced acute liver inflammation by inhibiting the activation of NF-κB signaling pathway and improving the hepatic inflammatory response in broilers. Although the studies on TA made a great progress in recent years, much remains to be done to investigate the anti-inflammatory mechanisms of TA more specifically, for instance, except from the perspective of nutrition, if TA could cause the mutation of amino acid residues from IκBα protein in order to preventing its ubiquitination and degradation in genomics level.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (No. 2018YFD0501101).
DISCLOSURES
The authors declare that there is no conflict of interest.
REFERENCES
- Akhter N., Hasan A., Shenouda S., Wilson A., Kochumon S., Ali S., Tuomilehto J., Sindhu S., Ahmad R. TLR4/MyD88-mediated CCL2 production by lipopolysaccharide (endotoxin): implications for metabolic inflammation. J. Diabetes. Metab. Disord. 2018;17:77–84. doi: 10.1007/s40200-018-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprotosoaie A.C., Costache I.-I., Miron A. Anethole and its role in chronic diseases. Adv. Exp. Med. Biol. 2016;929:247–267. doi: 10.1007/978-3-319-41342-6_11. [DOI] [PubMed] [Google Scholar]
- Arulselvan P., Fard M.T., Tan W.S., Gothai S., Fakurazi S., Norhaizan M.E., Kumar S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J.M. Exotoxins and endotoxins: inducers of inflammatory cytokines. Toxicon. 2018;149:45–53. doi: 10.1016/j.toxicon.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Cavalcanti J.M., Leal-Cardoso J.H., Diniz L.R., Portella V.G., Costa C.O., Linard C.F., Alves K., Rocha M.V., Lima C.C., Cecatto V.M., Coelho-de-Souza A.N. The essential oil of Croton zehntneri and trans-anethole improves cutaneous wound healing. J. Ethnopharmacol. 2012;144:240–247. doi: 10.1016/j.jep.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Chainy G.B., Manna S.K., Chaturvedi M.M., Aggarwal B.B. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene. 2000;19:2943–2950. doi: 10.1038/sj.onc.1203614. [DOI] [PubMed] [Google Scholar]
- Chen J.Y., Yu Y.H. Bacillus subtilis-fermented products ameliorate the growth performance and alter cecal microbiota community in broilers under lipopolysaccharide challenge. Poult. Sci. 2021;100:875–886. doi: 10.1016/j.psj.2020.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.I., Kim K.M., Kwak J.H., Lee S.K., Lee S.M. Protective mechanism of anethole on hepatic ischemia/reperfusion injury in mice. J. Nat. Prod. 2013;76:1717–1723. doi: 10.1021/np4004323. [DOI] [PubMed] [Google Scholar]
- da Rocha B.A., Ritter A.M., Ames F.Q., Goncalves O.H., Leimann F.V., Bracht L., Natali M.R., Cuman R.K., Bersani-Amado C.A. Acetaminophen-induced hepatotoxicity: preventive effect of trans anethole. Biomed. Pharmacother. 2017;86:213–220. doi: 10.1016/j.biopha.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Dickson R.P., Singer B.H., Newstead M.W., Falkowski N.R., Erb-Downward J.R., Standiford T.J., Huffnagle G.B. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat. Microbiol. 2016;1:16113. doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcet X., Llobet D., Pallares J., Matias-Guiu X. NF-κB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- Domiciano T.P., Dalalio M.M., Silva E.L., Ritter A.M., Estevao-Silva C.F., Ramos F.S., Caparroz-Assef S.M., Cuman R.K., Bersani-Amado C.A. Inhibitory effect of anethole in nonimmune acute inflammation. Naunyn Schmiedebergs Arch. Pharmacol. 2013;386:331–338. doi: 10.1007/s00210-012-0820-5. [DOI] [PubMed] [Google Scholar]
- Estevao-Silva C.F., Kummer R., Fachini-Queiroz F.C., Grespan R., Nogueira de Melo G.A., Baroni S., Cuman R.K., Bersani-Amado C.A. Anethole and eugenol reduce in vitro and in vivo leukocyte migration induced by fMLP, LTB4, and carrageenan. J. Nat. Med. 2014;68:567–575. doi: 10.1007/s11418-014-0839-7. [DOI] [PubMed] [Google Scholar]
- Feng T., Zhou L., Gai S., Zhai Y., Gou N., Wang X., Zhang X., Cui M., Wang L., Wang S. Acacia catechu (L.f.) Willd and Scutellaria baicalensis Georgi extracts suppress LPS-induced pro-inflammatory responses through NF-κB, MAPK, and PI3K-Akt signaling pathways in alveolar epithelial type II cells. Phytother. Res. 2019;33:3251–3260. doi: 10.1002/ptr.6499. [DOI] [PubMed] [Google Scholar]
- Fenton M.S., Golenbock D.T. LPS-binding proteins and receptors. J. Leukoc. Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Chisti Y., Banerjee U.C. Production of shikimic acid. Biotechnol. Adv. 2012;30:1425–1431. doi: 10.1016/j.biotechadv.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Hao L., Xie Y., Wu G., Cheng A., Liu X., Zheng R., Huo H., Zhang J. Protective effect of hericium erinaceus on alcohol induced hepatotoxicity in mice. Evid. Based. Complement. Alternat. Med. 2015;2015 doi: 10.1155/2015/418023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden M.S., Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hollemans M.S., de Vries Reilingh G., de Vries S., Parmentier H.K., Lammers A. Effects of early nutrition and sanitary conditions on antibody levels in early and later life of broiler chickens. Dev. Comp. Immunol. 2021;117 doi: 10.1016/j.dci.2020.103954. [DOI] [PubMed] [Google Scholar]
- Iannarelli R., Marinelli O., Morelli M.B., Santoni G., Amantini C., Nabissi M., Maggi F. Aniseed (Pimpinella anisum L.) essential oil reduces pro-inflammatory cytokines and stimulates mucus secretion in primary airway bronchial and tracheal epithelial cell lines. Ind. Crops Prod. 2018;114:81–86. [Google Scholar]
- Jiao Y., Zhang T., Zhang C., Ji H., Tong X., Xia R., Wang W., Ma Z., Shi X. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit. Care. 2021;25:356. doi: 10.1186/s13054-021-03775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P., Kim K.Y., Lee H.S., Min S.S., Seol G.H. Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci. 2013;93:955–961. [PubMed] [Google Scholar]
- Klasing K.C. Avian leukocytic cytokines. Poult. Sci. 1994;73:1035–1043. doi: 10.3382/ps.0731035. [DOI] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1 doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutert M., Entwisle S.W., Villen J. Decoding post-translational modification crosstalk with proteomics. Mol. Cell Proteomics. 2021;20 doi: 10.1016/j.mcpro.2021.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Tan Y., Chen S., Xiao X., Zhang M., Wu Q., Dong M. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling. J. Recept. Signal Transduct. Res. 2021;41:294–303. doi: 10.1080/10799893.2020.1808675. [DOI] [PubMed] [Google Scholar]
- Liu S.H., Chuang W.C., Lam W., Jiang Z., Cheng Y.C. Safety surveillance of traditional Chinese medicine: current and future. Drug Saf. 2015;38:117–128. doi: 10.1007/s40264-014-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimica-Dukić N., Kujundžić S., Soković M., Couladis M. Essential oil composition and antifungal activity of Foeniculum vulgare Mill obtained by different distillation conditions. Phytother. Res. 2003;17:368–371. doi: 10.1002/ptr.1159. [DOI] [PubMed] [Google Scholar]
- Moradi J., Abbasipour F., Zaringhalam J., Maleki B., Ziaee N., Khodadoustan A., Janahmadi M. Anethole, a medicinal plant compound, decreases the production of pro-inflammatory TNF-α and IL-1β in a rat model of LPS-induced periodontitis. Iran. J. Pharm. Res. 2014;13:1319–1325. [PMC free article] [PubMed] [Google Scholar]
- Motobu M., Amer S., Koyama Y., Hikosaka K., Sameshima T., Yamada M., Nakamura K., Koge K., Kang C.B., Hayasidani H., Hirota Y. Protective effects of sugar cane extract on endotoxic shock in mice. Phytother. Res. 2006;20:359–363. doi: 10.1002/ptr.1860. [DOI] [PubMed] [Google Scholar]
- Newton K., Dixit V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil K.R., Mahajan U.B., Unger B.S., Goyal S.N., Belemkar S., Surana S.J., Ojha S., Patil C.R. Animal models of inflammation for screening of anti-inflammatory drugs: implications for the discovery and development of phytopharmaceuticals. Int. J. Mol. Sci. 2019;20:4367. doi: 10.3390/ijms20184367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil K.R., Patil C.R. Anti-inflammatory activity of bartogenic acid containing fraction of fruits of Barringtonia racemosa Roxb. in acute and chronic animal models of inflammation. J. Tradit. Complement. Med. 2017;7:86–93. doi: 10.1016/j.jtcme.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poveda J., Sanz A.B., Carrasco S., Ruiz-Ortega M., Cannata-Ortiz P., Sanchez-Nino M.D., Ortiz A. Bcl3: a regulator of NF-κB inducible by TWEAK in acute kidney injury with anti-inflammatory and antiapoptotic properties in tubular cells. Exp. Mol. Med. 2017;49:e352. doi: 10.1038/emm.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramires C.C., Balbinot D.T., Cidral-Filho F.J., Dias D.V., Dos Santos A.R., da Silva M.D. Acupuncture reduces peripheral and brainstem cytokines in rats subjected to lipopolysaccharide-induced inflammation. Acupunct. Med. 2021;39:376–384. doi: 10.1177/0964528420938379. [DOI] [PubMed] [Google Scholar]
- Ren Z., Deng H., Deng Y., Tang W., Wu Q., Zuo Z., Cui H., Hu Y., Yu S., Xu S.Y., Deng J. Effects of selenium on arsenic-induced liver lesions in broilers. Biol. Trace Elem. Res. 2021;199:1080–1089. doi: 10.1007/s12011-020-02222-8. [DOI] [PubMed] [Google Scholar]
- Rezayat S.M., Dehpour A.R., Motamed S.M., Yazdanparast M., Chamanara M., Sahebgharani M., Rashidian A. Foeniculum vulgare essential oil ameliorates acetic acid-induced colitis in rats through the inhibition of NF-κB pathway. Inflammopharmacology. 2018;26:851–859. doi: 10.1007/s10787-017-0409-1. [DOI] [PubMed] [Google Scholar]
- Ritter A.M., Domiciano T.P., Verri W.A., Jr., Zarpelon A.C., da Silva L.G., Barbosa C.P., Natali M.R., Cuman R.K., Bersani-Amado C.A. Antihypernociceptive activity of anethole in experimental inflammatory pain. Inflammopharmacology. 2013;21:187–197. doi: 10.1007/s10787-012-0152-6. [DOI] [PubMed] [Google Scholar]
- Rossol M., Heine H., Meusch U., Quandt D., Klein C., Sweet M.J., Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011;31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- Sampaio A.L., Rae G.A., Henriques M. Effects of endothelin ETA receptor antagonism on granulocyte and lymphocyte accumulation in LPS-induced inflammation. J. Leukoc. Biol. 2004;76:210–216. doi: 10.1189/jlb.1003504. [DOI] [PubMed] [Google Scholar]
- Savio L.E.B., de Andrade Mello P., Figliuolo V.R., de Avelar Almeida T.F., Santana P.T., Oliveira S.D.S., Silva C.L.M., Feldbrugge L., Csizmadia E., Minshall R.D., Longhi M.S., Wu Y., Robson S.C., Coutinho-Silva R. CD39 limits P2 × 7 receptor inflammatory signaling and attenuates sepsis-induced liver injury. J. Hepatol. 2017;67:716–726. doi: 10.1016/j.jhep.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley J.J., Ghosh S. Molecular mechanisms of innate memory and tolerance to LPS. J. Leukoc. Biol. 2017;101:107–119. doi: 10.1189/jlb.3MR0316-118RR. [DOI] [PubMed] [Google Scholar]
- Shen Y.B., Piao X.S., Kim S.W., Wang L., Liu P. The effects of berberine on the magnitude of the acute inflammatory response induced by Escherichia coli lipopolysaccharide in broiler chickens. Poult. Sci. 2010;89:13–19. doi: 10.3382/ps.2009-00243. [DOI] [PubMed] [Google Scholar]
- Sung Y.Y., Kim Y.S., Kim H.K. Illicium verum extract inhibits TNF-α- and IFN-γ-induced expression of chemokines and cytokines in human keratinocytes. J. Ethnopharmacol. 2012;144:182–189. doi: 10.1016/j.jep.2012.08.049. [DOI] [PubMed] [Google Scholar]
- Tavakoli M., Abdi-Hachesoo M.B., Nazifi S., Mosleh N., Hosseinian S.A., Nakhaee P. Comparative effects of dexamethasone and meloxicam on magnitude of the acute inflammatory response induced by escherichia coli lipopolysaccharide in broiler chickens. J. Inflamm. Res. 2020;13:487–495. doi: 10.2147/JIR.S258328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajja B.N., Juluri S., Kumari M., Kole L., Chakrabarti R., Joshi V.D. Lipopolysaccharide-induced paw edema model for detection of cytokine modulating anti-inflammatory agents. Int. Immunopharmacol. 2004;4:901–909. doi: 10.1016/j.intimp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Varela M.L., Mogildea M., Moreno I., Lopes A. Acute inflammation and metabolism. Inflammation. 2018;41:1115–1127. doi: 10.1007/s10753-018-0739-1. [DOI] [PubMed] [Google Scholar]
- Verhaar B.J.H., Prodan A., Nieuwdorp M., Muller M. Gut microbiota in hypertension and atherosclerosis: a review. Nutrients. 2020;12:2982. doi: 10.3390/nu12102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Xiao L. Isochlorogenic acid A attenuates acute lung injury induced by LPS via NF-κB/NLRP3 signaling pathway. Am. J. Transl. Res. 2019;11:7018–7026. [PMC free article] [PubMed] [Google Scholar]
- Wilde B., Katsounas A. Immune dysfunction and albumin-related immunity in liver cirrhosis. Mediators Inflamm. 2019;2019 doi: 10.1155/2019/7537649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski-Rebecca E.S., Rocha B.A., Wiirzler L.A., Cuman R.K., Velazquez-Martinez C.A., Bersani-Amado C.A. Synergistic effects of anethole and ibuprofen in acute inflammatory response. Chem. Biol. Interact. 2015;242:247–253. doi: 10.1016/j.cbi.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Wu Q.J., Wang Y.Q., Qi Y.X. Influence of procyanidin supplementation on the immune responses of broilers challenged with lipopolysaccharide. Anim. Sci. J. 2017;88:983–990. doi: 10.1111/asj.12729. [DOI] [PubMed] [Google Scholar]
- Xing Y.Y., Zheng Y.K., Yang S., Zhang L.H., Guo S.W., Shi L.L., Xu Y.Q., Jin X., Yan S.M., Shi B.L. Artemisia ordosica polysaccharide alleviated lipopolysaccharide-induced oxidative stress of broilers via Nrf2/keap1 and TLR4/NF-κB pathway. Ecotoxicol. Environ. Saf. 2021;223 doi: 10.1016/j.ecoenv.2021.112566. [DOI] [PubMed] [Google Scholar]
- Yea S.S., Jeong H.S., Choi C.Y., Park K.R., Oh S., Shin J.G., Yun C.H. Inhibitory effect of anethole on T-lymphocyte proliferation and interleukin-2 production through down-regulation of the NF-AT and AP-1. Toxicol. In Vitro. 2006;20:1098–1105. doi: 10.1016/j.tiv.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Yu C., Zhang J., Wang T. Star anise essential oil:chemical compounds, antifungal and antioxidant activities: a review. J. Essen. Oil. Res. 2020;33:1–22. [Google Scholar]
- Yu C., Zhang J., Zhang H., Chen Y., Wang C., Zhang L., Ding L., Wang T., Yang Z. Influence of Trans-anethole on the nutrient digestibility and intestinal barrier function in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Lin L., Zhang Z., Zhang H., Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct. Target. Ther. 2020;5:209. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chen Y., Chen Y., Li Y., Jia P., Ji S., Zhou Y., Wang T. Dietary pterostilbene supplementation attenuates intestinal damage and immunological stress of broiler chickens challenged with lipopolysaccharide. J. Anim. Sci. 2020;98:1–9. doi: 10.1093/jas/skz373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Chen X., Devshilt I., Yun Q., Huang C., An L., Dorjbat S., He X. Fennel main constituent, trans-anethole treatment against LPS-induced acute lung injury by regulation of Th17/Treg function. Mol. Med. Rep. 2018;18:1369–1376. doi: 10.3892/mmr.2018.9149. [DOI] [PMC free article] [PubMed] [Google Scholar]