Abstract
Study suggested that dysbiosis of the gut microbiota may affect the etiology of woody breast (WB). In the current study, the cecal microbiota and WB in chickens fed three different diets were investigated. A total of 504 male chicks were used in a randomized complete block design with a 3 (Diet) × 2 (Challenge) factorial arrangement of treatments with 6 replicates per treatment, 6 treatments per block, and 14 birds per treatment. The experimental diets were a control diet (corn-soybean meal basal diet), an antibiotic diet (basal diet + 6.075 mg bacitracin/kg feed), and a probiotic diet (basal diet + 2.2 × 108 CFU Bacillus subtilis PB6/kg feed). On d 14, birds that were assigned to the challenge treatment received a 20 × live cocci vaccine. On d 41, breast muscle hardness in live birds was palpated and grouped into normal (NB) and WB phenotypes. Cecal contents were collected and their bacterial compositions were analyzed and compared. The genomic DNA of the cecal contents was extracted and the V3 and V4 regions of 16S rRNA gene were amplified and sequenced via an Illumina MiSeq platform. There were no differences (P > 0.05) in Shannon and Chao 1 indexes between the challenges, diets, and phenotypes (NB vs. WB). However, there was a difference (P = 0.001) in the beta diversity of the samples between the challenged and nonchallenged groups. Relative bacterial abundance differed (false discovery rate, FDR < 0.05) between the challenge treatments, but there were no significant differences (FDR > 0.05) among the three diets or two phenotypes. Predicted energy metabolism, nucleotide metabolism, and amino acid and coenzyme biosynthesis activities only differed (q-value < 0.05) between challenged and nonchallenged groups. The cocci challenge altered the gut microbial composition on Butyricicoccus pullicaecorum, Sporobacter termitidis, and Subdoligranulum variabile, but the dietary antibiotic and probiotic treatments did not impact gut microbial composition. No strong association was found between WB myopathy and gut microbial composition in this study.
Key words: broiler chicken, woody breast, gut microbiome, Eimeria, probiotics
INTRODUCTION
Woody breast (WB) is a myopathy that occurs in commercial broilers that is associated with rapid broiler growth and high breast muscle yield (Petracci et al., 2015). WB first became common in the broiler industry between 2011 and 2013 (Petracci et al., 2019) and was characterized by the pathological description of the lesions associated with WB meat (Sihvo et al., 2014), and the post mortem physical characteristics of WB muscle (Soglia et al., 2016). The multifactorial etiology of WB includes hypoxia, inflammation, oxidative stress, and metabolic disorder (Emami et al., 2021; Xing et al., 2021).
The gut microbiota is the community of microorganisms (bacteria, archaea, viruses, and eukaryotes) that colonize the digestive tract and responsible for maintaining animal health. Gut microbiota dysbiosis speeds up the development of oxidative stress and inflammation that contribute to reduced gut integrity and functional gastrointestinal disorders (Marchesi et al., 2016; Dumitrescu et al., 2018; Gao et al., 2018). Throughout broiler GI tract, the cecum have the most diverse and abundant gut microbiota (Gong et al., 2007) and is an organ involved in cellulose digestion, fermentation, nutrient utilization, and absorption (Clench and Mathias, 1995; Gong et al., 2007). The microbiota in the ceca is responsible for oligosaccharide degradation and the fermentation of short-chain fatty acids (Sergeant et al., 2014), which positively affect broiler growth performance and gut health (Angelakis, 2017; Broom and Kogut, 2018; Clavijo and Flórez, 2018; Diaz Carrasco et al., 2019). Recently, the role of gut microbiota compositional changes and their relationship with WB has been reported (Zhang et al., 2021b).
The gut microbiota and metabolic pathway differences were observed in broilers with WB. It was reported that broilers with WB contained a decreased abundance of beneficial bacteria (Butyricicoccus pullicaecorum and Lactobacillus hamster) and decreased microbial diversity. Also, broilers with WB predicted decreased glycolysis and urea cycling metabolic pathways. The results suggest that ceca microbiota may modulate the profile of metabolic pathways, which have been linked to differences between normal and WB meat (Zhang et al., 2021b). Dietary treatments and coccidiosis infections are two main factors that shaped the gut microbiota composition and abundance. Antibiotics were commonly used as growth promotor in broiler farm in the past decades because of the function of improving feed efficiency and inhibiting pathogen proliferation (Engberg et al., 2000; Stub and Vestergaard, 2001). Bacitracin has an impact on gut microbiota, specifically, changed the composition and increased the diversity of ceca microbiota and increased feed efficiency (Crisol-Martinez et al., 2017). In recent years, different strains of Bacillus subtilis (B. subtilis) have been used as antibiotic alternatives to promote growth, modulate broiler gut microbiota, and improve the overall gut health (Wang et al., 2019b). Our former research reported that dietary antibiotic (Bacitracin) and a probiotic (B. subtilis), and cocci challenge treatments increased WB incidence in commercial broilers (Jia et al., 2022). However, minimal research has been reported on the gut microbiota composition of broilers that yield WB regarding the association between specific dietary treatment and disease challenge and the phenotypic expression of WB meat. Further scientific investigations are necessary to identify the components of the gut microflora associated with the development of WB. The identification of microbial composition is a method by which to understand gut conditions and nutrition uptake, which may relate to WB development.
We hypothesized that dietary additives (antibiotic and probiotic) and cocci challenge treatments may have affected gut microbiota composition and the manipulated gut microbiota may further related to the WB development in broilers. Therefore, the modulation of gut microbiota under different dietary and cocci challenge conditions were evaluated. In addition, this study evaluated the cecal microbial composition of the birds with WB in comparison to the birds with normal breasts within different treatment combinations.
MATERIALS AND METHODS
Experimental Design and Birds Management
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of Mississippi State University (IACUC-16-542). One-day-old male Ross 708 chicks were obtained from a commercial hatchery. The house was divided into 6 blocks with 6 pens per block. Within each block, 504 male chicks were randomly assigned to 6 pens (14 birds/pen/block). A 3 (diet) × 2 (cocci challenge) factorial arrangement of treatments was used in this study. Birds in each pen within each block were randomly assigned to one of the above 6 treatments. The birds were fed with control diet (corn-soybean meal basal diet), antibiotic diet (basal diet + 6.075 mg bacitracin/kg feed), or probiotic diet (basal diet + 2.2 × 108 CFU Bacillus subtilis PB6/kg feed) in starter (d 0–14). Grower (d 14–28), and finisher (d 28–41) phases which former described (Jia et al., 2022). Under each dietary treatments, birds were either challenged with 1 mL 20 × live cocci vaccine (COCCIVAC-B52, containing E. acerivulina, E. maxima, E. maxima MFP, E. mivati, and E. tenella) or same amount of distilled water on d 14.
Sample Collection
At d 41, all live birds were evaluated for WB myopathy by manual palpation (Zhang et al., 2021a). Birds with WB score 0 was recorded as normal breast (NB) and birds with WB score 1, 2, or 3 were recorded as WB. A total of 2 birds/pen (one with WB and one with NB) was randomly selected for necropsy per each experimental treatment (n = 12). Birds were then humanely euthanized by CO2 inhalation and the WB score of breast meat was confirmed according to Tijare et al. (2016). The cecal contents were squeezed out and washed twice with 0.1 M PBS buffer that contained 0.1% tween 20 and 1% β-mercaptoethanol (Zhang et al., 2021b). The cecal samples were then stored at −80°C until further analysis.
Microbial Community DNA Isolation and DNA Amplification
The QIAamp PowerFecal DNA Kit (Qiagen, Germantown, MD) was used to extract DNA from cecal content according to the manufacturer's instructions. The quality and RNA contamination of the extracted DNA was examined using 0.8% agarose gel electrophoresis, and the purity and concentrations were determined using a NanoDrop One spectrophotometer (Thermo Scientific, Wilmington, DE). Before PCR amplification, an aliquot of the extracts was adjusted to 20 ng/µL using Tris-EDTA buffer. The universal primers (Forward primer [5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′] and reverse primer [5’-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGG-TATCTAATCC-3’]) were selected to amplify the V3-V4 regions of 16S rRNA gene from each DNA sample (Klindworth et al., 2013). The applied 25 μL reaction was composed of 12.5 μL 2x KAPA HiFi HotStart ReadyMix (KAPA Biosystems, Wilmington, MA), 0.5 μL forward and 0.5 μL reverse primers (10 μM), 10.5 μL nuclease-free water, and 1 μL of microbiota DNA template (20 ng/μL). PCR was performed using an Eppendorf Mastercycler ep gradient thermocycler (Eppendorf, Enfield, CT) with the following conditions: the initial denaturation step was carried out at 95°C for 3 min and 35 cycles were carried out at 95°C for 30 s, 55°C for 30 s, 72°C for 30 s; and a final extension of 72°C for 5 min. The PCR products (∼550 bp) were confirmed by gel electrophoresis using 1% agarose gel that was stained with SYBR Safe DNA Gel Stain (Invitrogen, Carlsbad, CA). Each PCR product was purified with Agencourt AMPure XP beads (Beckman Coulter, Brea, CA) according to the manufacturer's protocol.
16S rRNA Gene Libraries Construction and Sequencing
The 16S rRNA gene libraries were generated using the Nextera XT Index Kit (Illumina, San Diego, CA) according to the 16S Metagenomics Sequencing Library Preparation guide (Illumina, San Diego, CA, Part #15044223 Rev. B). The index PCR was carried out in a 50 μL reaction system containing 25 μL 2x KAPA HiFi HotStart ReadyMix (KAPA Biosystems, Wilmington, MA), 5 μL [each] Nextera XT Index Primer, 5 μL DNA and 10 μL nuclease-free water. The PCR reactions were performed by initial denaturation at 95°C for 3 min, followed by 8 cycles of denaturation at 95°C for 30 s, primer annealing at 55°C for 30 s, and extension at 72°C for 30s, with a final elongation step at 72°C for 5 min. PCR clean-up was performed using a 1:1 ratio of Agencourt AMPure XP beads to PCR reaction products following the Nextera protocol. The 16S rRNA gene libraries were quantified with Qubit dsDNA HS Assay Kit using Qubit 2.0 fluorometer (Life Technologies, Carlsbad, CA). The size profiles of the libraries were determined using a 2100 Bioanalyzer with a DNA 1000 chip (Agilent Technologies, Inc., Santa Clara, CA), with an expected size of the final library as ∼630 bp. The individual DNA library was normalized to 45 nM according to the Qubit reading and bioanalyzer size profiling, then 72 libraries were pooled together with equal molar. The pooled library was desaturated with NaOH and diluted to equal concentration (6 pM) before being mixed to achieve a PhiX concentration of 5% of the total volume. The denatured libraries were loaded onto the reagent cartridge and sequenced on an Illumina MiSeq Next Generation Sequencer (Illumina, San Diego, CA) to generate paired-end 2 × 300 reads.
Data Processing and Statistical Analysis
The sequence data was processed as previously described with some modifications (Zhang et al., 2021b). Briefly, sequences were quality filtered, denoised, and analyzed using Quantitative Insights Into Microbial Ecology 2 software (QIIME 2 version 2020.2). The number of reads in the feature table/operational taxonomic unit (OTU) table was standardized using the total sum approach to generate relative abundance. Alpha diversity (Chao1 index and Shannon index) and beta diversity (Jaccard index) measurements were calculated using QIIME. Beta diversity was visualized using Principal Coordinate Analysis (PCoA) plots. The “qiime diversity beta-group-significance” plugin and the “qiime emperor plot” plugin were used to perform group significance tests. The “qiime feature-classifier” plugin was used to examine the taxonomic composition of the samples using the greengenes (16S rRNA) reference database (2019.10). The bacterial community's relative abundance was estimated, and the graph was created with R version 4.0.5 (R Core Team, 2021). PICRUSt2 (phylogenetic study of the community by reconstruction of unobserved States) was used to predict functional profiling of microbial communities (Douglas et al., 2020). Group analyses were performed using the Kruskal-Wallis test in STAMP (Statistical Analysis of Metagenomic Profiles) (Parks et al., 2014).
The relative abundance differences were examined using the 2-sided Welch's t-test (P < 0.05) with an adaptive FDR correction of 0.05 (Benjamini and Hochberg, 1995). The Kruskal-Wallis test was used to compare the alpha diversity of each group. Permutational multivariate analysis of variance (PERMANOVA) tests were utilized to examine beta diversity between each interaction and main effects. To establish the statistical significance of predicted functions and pathways, a 2-sided Welch's t-test was performed, followed by an FDR correction of 0.05 to calculate the q-value (Storey, 2002). All associations with P or q < 0.05 were considered significant.
RESULTS
Diversity of Cecal Microbiota
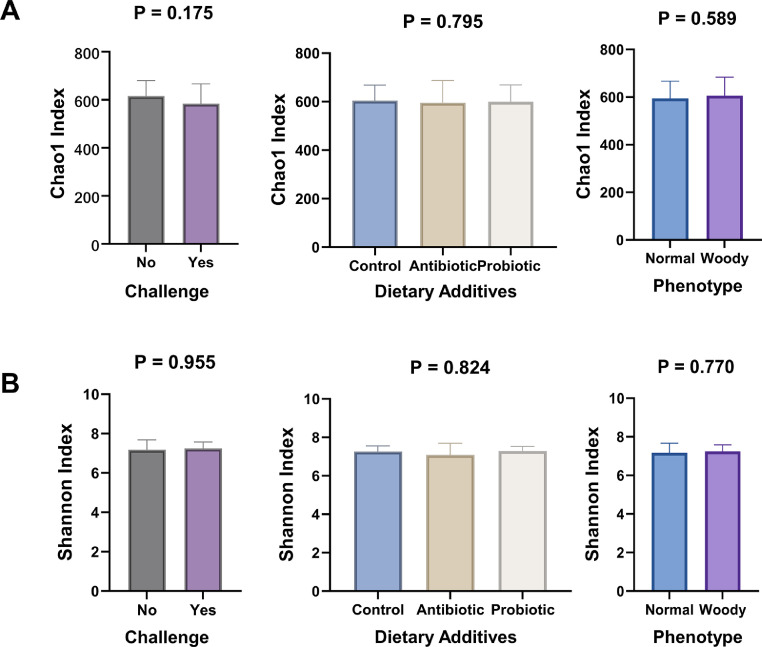
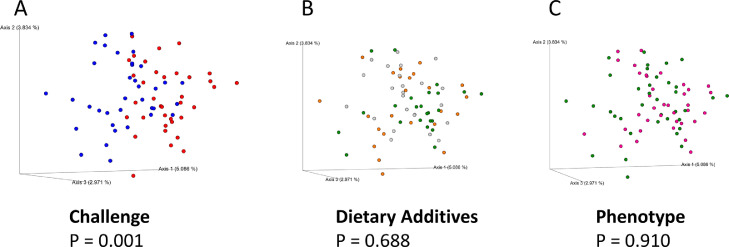
The richness and evenness of the species (alpha diversity) in each sample were reported by Chao 1 and Shannon indexes (Figure 1). Accounting for both indexes, there were no differences (P > 0.05) in the richness and evenness of cecal microbiota between the 2 coccidiosis conditions (challenge vs. nonchallenge), the 3 diets (control, antibiotic, and probiotic), or 2 phenotypes (NB vs. WB). Based on the PERMANOVA test, the beta diversity was different in samples between cocci challenges (P = 0.001, Figure 2) but not different in samples among diets or between phenotypes (P > 0.05). In addition, no difference in beta diversity was observed between NB and WB across dietary treatments and cocci challenges (Table 1).
Figure 1.
Barplots for Alpha diversity based on (A) richness (Chao1 index) and (B) evenness (Shannon index). Barplots for Alpha diversity based on (A) richness (Chao1 index) and (B) evenness (Shannon index) of pooled cecal samples from broilers with normal (NB) and woody breasts (WB) (phenotype), broilers fed with a control (control), antibiotic, and probiotic diets, and cocci challenged (Yes).and non-challenged (No). P-values were calculated using the pairwise Kruskal-Wallis test.
Figure 2.
Principal coordinate analysis (PCoA) plots with Jaccard index for Beta diversity. Principal coordinate analysis (PCoA) plots with Jaccard index for Beta diversity of (A) Pooled cecal samples from broilers challenged (blue) and non-challenged (red); (B) Pooled cecal samples from broilers fed with a control (gray), an antibiotic (orange), and a probiotic diet (green); (C) Pooled cecal samples from broilers with normal (green) and woody breasts (pink). P-values were calculated using PERMANOVA test.
Table 1.
P and Q values from the PERMANOVA test comparing phenotypes (NB vs. WB) at different dietary treatments and cocci challenges.
| Diet1 | Challenge2 | P-value | Q-value |
|---|---|---|---|
| Control | Challenge | 0.935 | 0.950 |
| Control | Nonchallenge | 0.696 | 0.820 |
| Antibiotic | Challenge | 0.442 | 0.634 |
| Antibiotic | Nonchallenge | 0.881 | 0.933 |
| Probiotic | Challenge | 0.741 | 0.843 |
| Probiotic | Nonchallenge | 0.685 | 0.820 |
Experiment diets included a control diet (corn and soybean-meal basal diet), an antibiotic (basal diet + 6.075 mg bacitracin /kg feed), and a probiotic diet (basal diet + 2.2 × 108 CFU Bacillus subtilis PB6 /kg feed).
The birds were either challenged with 1 mL 20 × cocci vaccine (COCCIVAC-B52, containing E. acerivulina, E. maxima, E. maxima MFP, E. mivati, and E. tenella) or gavaged the same amount of distilled water on d 14.
Relative Abundance of Cecal Microflora
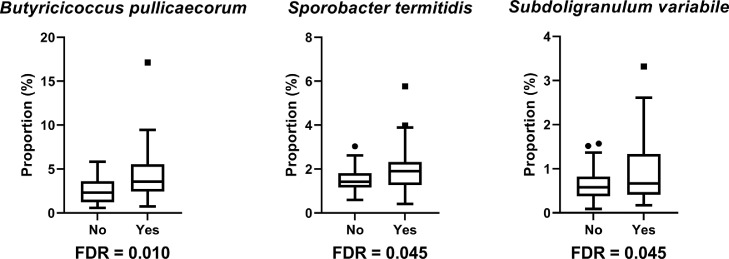
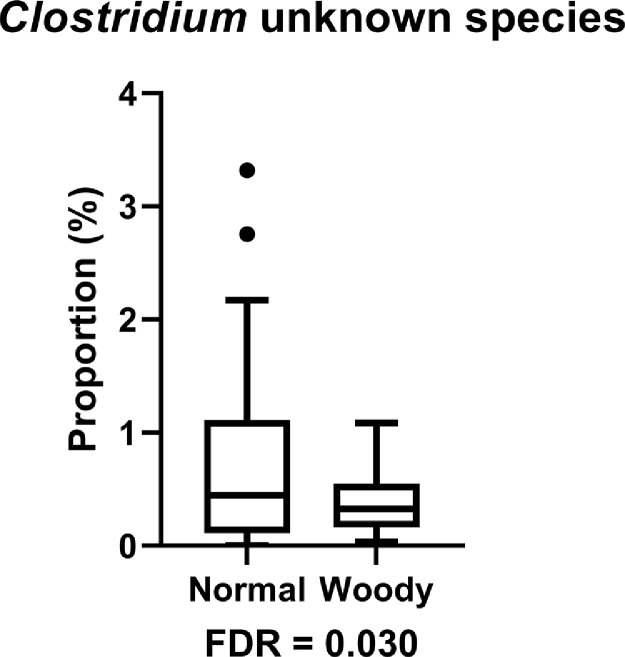
There was no significant difference on relative abundance of cecal microflora due to dietary additives and phenotype effects. The effect of cocci challenge on the bacterial taxonomy of broilers is shown (Table 2). Cocci challenge did not affect the relative abundance at the phylum, class, and order levels. Comparative analysis of species level shows cocci challenge and nonchallenge samples detected three bacteria that were more abundant in the cocci challenge group: Butyricicoccus pullicaecorum (FDR = 0.010), Sporobacter termitidis (FDR = 0.045), and Subdoligranulum variabile (FDR = 0.045) (Figure 3). At the species level, an unknown species belongs to Clostridiaceae genus and Clostridiales family have lower abundance in birds with WB (FDR = 0.030) (Figure 4).
Table 2.
Bacterial taxonomy within the cecal digesta of broilers.
| Item | Challenge1 | Nonchallenge2 | SEM | P-value |
|---|---|---|---|---|
| Phylum | ||||
| Firmicutes | 97.8 | 97.8 | 0.437 | 0.958 |
| Cyanobacteria | 0.51 | 0.78 | 0.261 | 0.303 |
| Lentisphaerae | 0.00 | 0.08 | 0.049 | 0.137 |
| Class | ||||
| Clostridia | 97.1 | 97.1 | 0.477 | 0.992 |
| Lentisphaeria | 0.00 | 0.08 | 0.049 | 0.137 |
| Synergistia | 0.00 | 0.05 | 0.025 | 0.055 |
| Order | ||||
| Clostridiales | 97.0 | 97.0 | 0.485 | 0.871 |
| Victivallales | 0.00 | 0.08 | 0.049 | 0.137 |
The birds were either challenged with 1 mL 20 × cocci vaccine (COCCIVAC-B52, containing E. acerivulina, E. maxima, E. maxima MFP, E. mivati, and E. tenella) in challenge group or gavaged the same amount of distilled water in non-challenge group.
Figure 3.
The relative abundance of bacteria population at the species level in cecal microbiota of broilers that were challenged (Yes) and not challenged (No) (FDR < 0.05). The centre line denotes the median value (50th percentile), while the box contains the 25th to 75th percentiles of dataset. The whiskers mark the 5th and 95th percentiles, and values beyond these upper and lower bounds are considered outliers, marked with circle and square dots.
Figure 4.
The relative abundance of bacteria population at the species level in cecal microbiota of broilers with normal breast (Normal) and woody breast (Woody) (FDR < 0.05). The centre line denotes the median value (50th percentile), while the box contains the 25th to 75th percentiles of dataset. The whiskers mark the 5th and 95th percentiles, and values beyond these upper and lower bounds are considered outliers, marked with circle and square dots.
Functional Metagenome Prediction
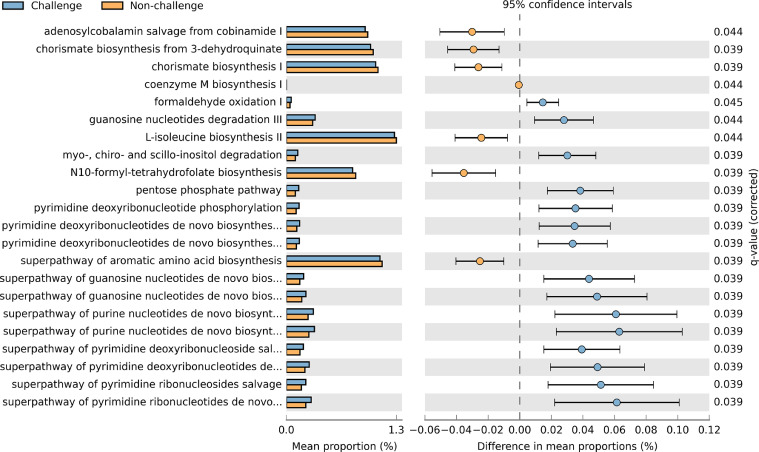
To show the metabolic function of the cecal microbial population in broilers, PICRUSt2 was used to predict the functional profile from 16S rRNA sequences. At KEGG level 2, 22 microbial metabolic activities in the ceca were different (P < 0.05) between challenge and nonchallenge broilers (Figure 5), but none were different (P > 0.05) between broilers with different phenotypes or diets. The pairwise analysis of the abundance of microbial pathways revealed that the changed pathways included energy metabolism pathways, nucleotide metabolic pathways, and amino acid and coenzyme biosynthesis. Some energy metabolism pathways, such as the pentose phosphate pathway and formaldehyde oxidation I were enhanced (q < 0.05) in the microflora from the challenge group. For the broilers from the cocci challenge group, pathways including myo-, chiro-, and scyllo-inositol degradation were elevated (q < 0.05). Purine, pyrimidine, and derivative nucleotide metabolic pathways were increased (q < 0.05) in broilers that were challenged with cocci. Chorismate, aromatic amino acid, L-isoleucine, and coenzyme M biosynthesis pathways were all downregulated (q < 0.05) in broilers that were exposed to cocci. In total, 15 out of 22 pathways were upregulated (q < 0.05) and 7 were downregulated (q <0.05) in broilers that were treated with the cocci challenge.
Figure 5.
Predicted metabolic pathways in cecal microbiota from broilers with cocci challenge (Challenge) and without cocci challenge (Non-challenge) based on Welch's t-test followed by a Storey FDR correction.
DISCUSSION
The current study investigated the effect of dietary treatment and Eimeria spp. challenge on broiler gut microbiome composition and cecal bacterial community profiles in chickens exhibiting the WB myopathy. Furthermore, the nutrient metabolism, as well as a comparative prediction analysis of the functional metagenome in the broiler chickens were evaluated.
The relative abundance of species Butyricicoccus pullicaecorum, Sporobacter termitidis, and Subdoligranulum variabile were increased in the cocci challenge group (Figure 3). It has been shown that Butyricicoccus pullicaecorum (Ruminococcaceae genus) and Subdoligranulum variabile are butyrate-producing strains (Holmstrøm et al., 2004; Eeckhaut et al., 2008). Short-chain fatty acid butyrate, an essential byproduct of anaerobic bacterial carbohydrate fermentation, has been shown to reduce proinflammatory cytokine in broilers and to promote production of antimicrobial components in the chicken gut (Zhang et al., 2011). Butyrate inhibits Salmonella invasion and colonization while stimulating intestinal epithelial cell development (Van Immerseel et al., 2005; Kien et al., 2007). In the cocci challenge group, the increased relative abundance of Butyricicoccus pullicaecorum and Subdoligranulum variabile may produce more butyrate in the chicken gut, thus improving broiler health and increasing growth performance. One of our previous studies indicated that body weight gain of challenged broilers was increased in the late growth phase (d 29–43) (Jia et al., 2022). This finding may be related to the increase in butyrate producers in the chicken gut can improve gut health conditions and promote broiler growth. The abundance of Sporobacter termitidis was greater in the cocci challenge group. Higher abundance of Sporobacter has been linked to pathological conditions such as constipation in mice (Wang et al., 2019a) and E. coli infection in weaned pigs (Zhang et al., 2017). Similarly, Venardou et al. (2021) reported that Sporobacter might behave as a pathogen in the host. Sporobacter abundance was reduced following supplementation with a mixture of probiotic Bifidobacterium strains (Wang et al., 2019a). Our study found that cocci challenge increased the relative abundance of Sporobacter, which suggests that cocci challenge has a negative effect on gut environment.
We observed different metabolic pathways between cocci challenged and non-challenged birds (Figure 5). The cocci challenge group exhibited an increase in the pentose phosphate pathway (PPP) in the microbial population. The activation of PPP can increase the production of NADPH, which is an electron donor in all organisms (Ramos-Martinez, 2017). It was reported that cells under oxidative stress can activate PPP to increase NADPH, thus improving the cells’ ability to tolerate oxidative stress (Hayes et al., 2020). Another product of PPP is ribose-5-phosphate sugar, which is used to make DNA and RNA (Ramos-Martinez, 2017). Deoxyribonucleotides and nucleotides biosynthesis pathways were upregulated in the microbes of cocci challenged broilers, which may be regulated by the DNA and RNA produced from PPP. Folate transformations III related to vitamin B9 were enriched in nonchallenge birds. Folate is required to form many compounds, including glycine, methionine, formylmethionine, thymidylate, pantothenate, and purine nucleotides (Scaglione and Panzavolta, 2014). The changed folate metabolism in the birds with cocci challenge indicates a different vitamin need for the cecal bacteria, and thus altered biological metabolism in activities. The enhanced super pathway of aromatic amino acid biosynthesis and L-isoleucine biosynthesis II shows that nonchallenged birds may have higher levels of L-tryptophan and L-isoleucine.
B. subtilis was not detected from the cecal content sample in this study, indicating that the B. subtilis strain used in this study did not colonize in the ceca. Ma et al. (2018) reported that supplementation with B. subtilis improved the growth performance and ileum structure of broilers. However, these authors didn't observe the composition difference of B. subtilis species in cecal microbiota. Similarly, Wang et al. (2019b) found that B. subtilis promoted broiler growth performance via modulating microbial community. However, there was no data reported pertaining to for the B. subtilis population in the ceca content. It has been indicated that in the ceca of broilers Clostridia are the predominate organisms (Ballou et al., 2016), B. subtilis may be more active and have a large population in the ileum, which is dominated by Lactobacillus (Ranjitkar et al., 2016; Glendinning et al., 2019). The fermentation product of the proliferation of B. subtilis may travel into ceca and thus modulate the cecal microbiota. The colonization of B. subtilis in other gut parts was not investigated in this study. The ileum microbiota is worthy of investigation in future studies for a better understanding of the function of B. subtilis in the broiler GI tract.
Bacitracin is a nonribosomal polypeptide combination that inhibits bacterial cell wall production by blocking dephosphorylation of the carrier for N-acetylmuramyl pentapeptide intermediates (Butaye et al., 2003). Bacitracin is one of the most widely utilized antibiotic growth promoters (AGPs) that are used to increase poultry performance (Huyghebaert et al., 2011). Studies have indicated that supplementation with bacitracin altered the intestinal bacterial population of broiler chickens (Lu et al., 2008; Torok et al., 2011). Lu et al. (2008) reported a decrease in the diversity of the gut microbiome when bacitracin was administered. However, conflicting results have been reported that suggests that bacitracin and other AGPs do not significantly impair microbial diversity (Gong et al., 2008; Neumann and Suen, 2015; Fasina et al., 2016). Our previous research showed an increase in body weight gain in the 0 to 14 day growth phase, but no growth stimulating impact due to antibiotic additive after d 14 (Jia et al., 2022). One possible explanation for this finding is that antibiotics could not change the composition of gut microbiota in the later growth phase on d 41.
We found that there was no difference in gut microbiota composition between broilers with or without WB within each treatment combination group (Table 1). This result indicates that gut microbiota is not related to WB on d 41. The result is inconsistent with Zhang et al. (2021b), who reported that broilers with WB have higher relative abundance of Bacteroides plebeius and Selenomonas bovis at 8 wk of age. In our study, we sampled birds at d 41 (around 6 wk of age). The different sampling time may cause this inconsistence because age is one of the factors that affects WB (Maharjan et al., 2021). Zhang et al. (2021b) reported that in the data analysis procedure, they grouped WB score higher or equals than 2 on d 56 as WB, unlike our study grouped scores 1 to 3 as WB. The different standard for describing normal breast and WB may cause the different microbiota composition results. At the family level, Clostridiales in the WB group were reduced (Figure 4). The reasons for the decline in these genera are currently unclear.
The present study confirmed gut microbiome differences among broilers that have been cocci challenged, but dietary supplement of the antibiotic or probiotic did not affect the composition of the gut microbiota. Birds that were treated with the cocci challenge contained an increased abundance of Butyricicoccus pullicaecorum, and Subdoligranulum variabile, which are beneficial bacteria, which contribute to butyrate production. In cocci challenged birds, the ceca were characterized by decreased amino acid, vitamin, and coenzyme biosynthesis, increased energy production, and deoxyribonucleotide and nucleotide biosynthesis. However, there was no difference for the beta diversity of the microbiota from broilers with or without WB in any diet treatments and cocci challenges, which indicate that gut microbiota was not related to WB on d 41.
In conclusion, the cocci challenge manipulated the gut microbiota composition, but the dietary antibiotic and probiotic did not impact the gut microbiota composition on d 41 in this study. In addition, WB formation was not related to gut microbiota composition on d 41. Future research should focus on possible mechanisms that could explain the reason why dietary additive and cocci challenge increased WB incidence and if there are differences in microbial composition in the guts among birds that are raised to 41 and 56 d, in the same study.
Acknowledgments
ACKNOWLEDGMENTS
This publication is a contribution of the Mississippi Agricultural and Forestry Experiment Station. This material is based upon work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project under accession numbers of MIS-329250/NE-1442 and MIS-322370.
DISCLOSURES
The authors have declared no conflict of interest.
REFERENCES
- Angelakis E. Weight gain by gut microbiota manipulation in productive animals. Microb. Pathog. 2017;106:162–170. doi: 10.1016/j.micpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Ballou A.L., Ali R.A., Mendoza M.A., Ellis J.C., Hassan H.M., Croom W.J., Koci M.D. Development of the chick microbiome: how early exposure influences future microbial diversity. Front. Vet. Sci. 2016;3:2. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. B. 1995;57:289–300. [Google Scholar]
- Broom L.J., Kogut M.H. The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 2018;204:44–51. doi: 10.1016/j.vetimm.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Butaye P., Devriese L.A., Haesebrouck F. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 2003;16:175–188. doi: 10.1128/CMR.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo V., Flórez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clench M.H., Mathias J.R. The avian cecum: a review. Wilson Bull. 1995:93–121. [Google Scholar]
- Crisol-Martinez E., Stanley D., Geier M.S., Hughes R.J., Moore R.J. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: linking gut microbiota and growth performance in chickens. Appl. Microbiol. Biotechnol. 2017;101:4547–4559. doi: 10.1007/s00253-017-8193-9. [DOI] [PubMed] [Google Scholar]
- Diaz Carrasco J.M., Casanova N.A., Fernández Miyakawa M.E. Microbiota, gut health and chicken productivity: what is the connection? Microorganisms. 2019;7:374. doi: 10.3390/microorganisms7100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas G.M., Maffei V.J., Zaneveld J.R., Yurgel S.N., Brown J.R., Taylor C.M., Huttenhower C., Langille M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020;38:685–688. doi: 10.1038/s41587-020-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu L., Popescu-Olaru I., Cozma L., Tulbă D., Hinescu M.E., Ceafalan L.C., Gherghiceanu M., Popescu B.O. Oxidative stress and the microbiota-gut-brain axis. Oxidative Med. Cell. Longevity. 2018;2018 doi: 10.1155/2018/2406594. -2406594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhaut V., Van Immerseel F., Teirlynck E., Pasmans F., Fievez V., Snauwaert C., Haesebrouck F., Ducatelle R., Louis P., Vandamme P. Butyricicoccus pullicaecorum gen. nov., sp. nov., an anaerobic, butyrate-producing bacterium isolated from the caecal content of a broiler chicken. Int. J. Syst. Evol. Microbiol. 2008;58:2799–2802. doi: 10.1099/ijs.0.65730-0. [DOI] [PubMed] [Google Scholar]
- Emami N.K., Cauble R.N., Dhamad A.E., Greene E.S., Coy C.S., Velleman S.G., Orlowski S., Anthony N., Bedford M., Dridi S. Hypoxia further exacerbates woody breast myopathy in broilers via alteration of satellite cell fate. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg R.M., Hedemann M.S., Leser T.D., Jensen B.B. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult. Sci. 2000;79:1311–1319. doi: 10.1093/ps/79.9.1311. [DOI] [PubMed] [Google Scholar]
- Fasina Y.O., Newman M.M., Stough J.M., Liles M.R. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult. Sci. 2016;95:247–260. doi: 10.3382/ps/pev329. [DOI] [PubMed] [Google Scholar]
- Gao J., Xu K., Liu H., Liu G., Bai M., Peng C., Li T., Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell Infect. Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. -13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning L., Watson K.A., Watson M. Development of the duodenal, ileal, jejunal and caecal microbiota in chickens. Anim. Microbiome. 2019;1:17. doi: 10.1186/s42523-019-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Si W., Forster R.J., Huang R., Yu H., Yin Y., Yang C., Han Y. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: from crops to ceca. FEMS Microbiol. Ecol. 2007;59:147–157. doi: 10.1111/j.1574-6941.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- Gong J., Yu H., Liu T., Gill J.J., Chambers J.R., Wheatcroft R., Sabour P.M. Effects of zinc bacitracin, bird age and access to range on bacterial microbiota in the ileum and caeca of broiler chickens. J. Appl. Microbiol. 2008;104:1372–1382. doi: 10.1111/j.1365-2672.2007.03699.x. [DOI] [PubMed] [Google Scholar]
- Hayes J.D., Dinkova-Kostova A.T., Tew K.D. Oxidative stress in cancer. Cancer Cell. 2020;38:167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrøm K., Collins M.D., Møller T., Falsen E., Lawson P.A. Subdoligranulum variabile gen. nov., sp. nov. from human feces. Anaerobe. 2004;10:197–203. doi: 10.1016/j.anaerobe.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Jia L., Zhang X., Li X., Schilling W., David Peebles E., Kiess A.S., Zhai W., Zhang L. Bacitracin, Bacillus subtilis, and Eimeria spp. challenge exacerbates woody breast incidence and severity in broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kien C.L., Blauwiekel R., Bunn J.Y., Jetton T.L., Frankel W.L., Holst J.J. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J Nutr. 2007;137:916–922. doi: 10.1093/jn/137.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Gl€ockner F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Hofacre C., Smith F., Lee M.D. Effects of feed additives on the development on the ileal bacterial community of the broiler chicken. Animal. 2008;2:669–676. doi: 10.1017/S1751731108001894. [DOI] [PubMed] [Google Scholar]
- Ma Y., Wang W., Zhang H., Wang J., Zhang W., Gao J., Wu S., Qi G. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 2018;8:15358. doi: 10.1038/s41598-018-33762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharjan P., Beitia A., Weil J., Suesuttajit N., Hilton K., Caldas J., Umberson C., Martinez D., Kong B., Owens C.M., Coon C. Woody breast myopathy broiler show age-dependent adaptive differential gene expression in Pectoralis major and altered in-vivo triglyceride kinetics in adipogenic tissues. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi J.R., Adams D.H., Fava F., Hermes G.D., Hirschfield G.M., Hold G., Quraishi M.N., Kinross J., Smidt H., Tuohy K.M. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann A.P., Suen G. Differences in major bacterial populations in the intestines of mature broilers after feeding virginiamycin or bacitracin methylene disalicylate. J. Appl. Microbiol. 2015;119:1515–1526. doi: 10.1111/jam.12960. [DOI] [PubMed] [Google Scholar]
- Parks D.H., Tyson G.W., Hugenholtz P., Beiko R.G. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. Worlds Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Petracci M., Soglia F., Madruga M., Carvalho L., Ida E., Estévez M. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 2019;18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2021. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Ramos-Martinez J.I. The regulation of the pentose phosphate pathway: remember Krebs. Arch. Biochem. Biophys. 2017;614:50–52. doi: 10.1016/j.abb.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Ranjitkar S., Lawley B., Tannock G., Engberg R.M. Bacterial succession in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2016;82:2399–2410. doi: 10.1128/AEM.02549-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglione F., Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44:480–488. doi: 10.3109/00498254.2013.845705. [DOI] [PubMed] [Google Scholar]
- Sergeant M.J., Constantinidou C., Cogan T.A., Bedford M.R., Penn C.W., Pallen M.J. Extensive microbial and functional diversity within the chicken cecal microbiome. PloS One. 2014;9:e91941. doi: 10.1371/journal.pone.0091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvo H.K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Soglia F., Mudalal S., Babini E., Di Nunzio M., Mazzoni M., Sirri F., Cavani C., Petracci M. Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2016;95:651–659. doi: 10.3382/ps/pev353. [DOI] [PubMed] [Google Scholar]
- Storey J.D. A direct approach to false discovery rates. J. R. Stat. Soc.: Ser. B (Stat. Method.) 2002;64:479–498. [Google Scholar]
- Stub C., Vestergaard K.S. Influence of zinc bacitracin, light regimen and dustbathing on the health and welfare of broiler chickens. Br. Poult. Sci. 2001;42:564–568. doi: 10.1080/00071660120088344. [DOI] [PubMed] [Google Scholar]
- Tijare V.V., Yang F.L., Kuttappan V.A., Alvarado C.Z., Coon C.N., Owens C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Torok V.A., Allison G.E., Percy N.J., Ophel-Keller K., Hughes R.J. Influence of antimicrobial feed additives on broiler commensal posthatch gut microbiota development and performance. Appl. Environ. Microbiol. 2011;77:3380–3390. doi: 10.1128/AEM.02300-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F., Boyen F., Gantois I., Timbermont L., Bohez L., Pasmans F., Haesebrouck F., Ducatelle R. Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poult. Sci. 2005;84:1851–1856. doi: 10.1093/ps/84.12.1851. [DOI] [PubMed] [Google Scholar]
- Venardou B., O'Doherty J.V., Vigors S., O'Shea C.J., Burton E.J., Ryan M.T., Sweeney T. Effects of dietary supplementation with a laminarin-rich extract on the growth performance and gastrointestinal health in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Chen C., Cui S., Lee Y.K., Wang G., Zhao J., Zhang H., Chen W. Adhesive Bifidobacterium induced changes in cecal microbiome alleviated constipation in mice. Front. Microbiol. 2019;10:1721. doi: 10.3389/fmicb.2019.01721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Farnell Y.Z., Kiess A.S., Peebles E.D., Wamsley K.G.S., Zhai W. Effects of Bacillus subtilis and coccidial vaccination on cecal microbial diversity and composition of Eimeria-challenged male broilers. Poult. Sci. 2019;98:3839–3849. doi: 10.3382/ps/pez096. [DOI] [PubMed] [Google Scholar]
- Xing T., Pan X., Zhang L., Gao F. Hepatic oxidative stress, apoptosis, and inflammation in broiler chickens with wooden breast myopathy. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.659777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhang X., Schilling M.W., Li X., Tabler G.T., Peebles E.D., Zhai W. Effects of broiler genetic strain and dietary amino acid reduction on meat yield and quality (part II) Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhu Y.H., Zhou D., Wu Q., Song D., Dicksved J., Wang J.F. Oral administration of a select mixture of Bacillus probiotics affects the gut microbiota and goblet cell function following Escherichia coli challenge in newly weaned pigs of genotype MUC4 that are supposed to be enterotoxigenic E. coli f4ab/ac receptor negative. Appl. Environ. Microbiol. 2017;83:e02747–16. doi: 10.1128/AEM.02747-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.H., Jiang Y., Zhu Q.F., Gao F., Dai S.F., Chen J., Zhou G.H. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br. Poult. Sci. 2011;52:292–301. doi: 10.1080/00071668.2011.578121. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang L., Li X., White S., Campbell Y.L., Ruiz A.A., To K.V., Zhai W., Schilling M.W. Cecal microbiota contribute to the development of woody breast myopathy. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101124. [DOI] [PMC free article] [PubMed] [Google Scholar]