Abstract
Laboratory experiments have definitively shown that exopolymer-producing bacteria have the potential to modify the flow of fluids in oil reservoirs to enhance oil production. Once injected into the reservoir, they will be subjected to a wide range of pH values and to starvation resulting from nutrient depletion. For successful field implementation it is necessary to have a fundamental understanding of these effects on the viability of bacteria. This paper addresses the effects of pH and trace minerals on cell viability of Leuconostoc mesenteroides during carbon source depletion. Two different carbon sources were used to grow cells before transferring the cells to starvation conditions: sucrose and a combination of glucose and fructose. These substrates were chosen because L. mesenteroides produces a significant amount of water-insoluble exopolymers (dextran) under sucrose-fed conditions, which may enhance cell survival under harsh conditions. The effects of dextran on the cell viability were tested at different pH values with and without trace minerals. The rate of cell death followed an exponential-decay law for different values of the solution pH. The optimal solution pH for survival was pH 5, whereas cells died rapidly at pH 3 and below and at pH 13 and above. The sucrose-fed cells showed a greater viability than cells fed glucose and fructose for all pH ranges tested. The results indicated that water-insoluble exopolymers help cells survive for longer periods of time under starvation conditions. The effects of trace minerals on cell culturability were tested at two pH values, 4.5 and 7. For both cases, cells showed a greater culturability (smaller decay rate constant) in the presence of trace minerals than without trace minerals. It was also found that the effects of trace minerals on cell culturability were greater for glucose-fructose-fed cells than for sucrose-fed cells. The Michaelis pH function theory was used for comparing the relationships between the cell decay rate and pH.
Microorganisms are used beneficially in many subsurface engineering operations including microbially enhanced oil recovery, biobarrier establishment, and in situ bioremediation. In the oil industry, exopolymer-producing bacteria have shown great promise to enhance oil recovery by bacterial profile modification (BPM). Here bacteria are injected into the reservoir, are fed for a period of time in order to grow, and form a plug which blocks undesirable flow channels. As a result, the injected water flows through the more-desirable channels containing the oil to push it out a production well. Because of economic and operation considerations, the nutrient supply can only be provided for a finite period of time, and it is important to understand if the bacterial plug will remain effective. After the nutrient supply is terminated, the bacteria will undergo starvation and will be exposed to a wide range of pH values ranging from 1 to 14. The lower pH values occur during acidification processes, while the high pH values occur during caustic flooding conditions. In order to carry out bacterial profile modification in an efficient and cost-effective manner, a fundamental understanding of the effect of solution pH on bacterial viability is required. This paper describes the results of a study on the number of culturable cells (Leuconostoc mesenteroides NRRL B-523) under starvation conditions at different pH values.
Under the proper nutrient conditions, cells form biofilms on solid surfaces, and most of the biological activities are carried out within the biofilm (14, 38). The biofilm is composed of microbial cells and metabolic by-products including extracellular polymers (exopolymers). Biofilm growth in porous media can lead to a reduction in permeability, which results in a decrease in the nutrient supply downstream of the area where the biological operation is being carried out (36). Also, cells near the surface of a porous wall will experience a lack of nutrients owing to mass transfer limitations as the biofilm thickness increases (3). In BPM for oil recovery and biobarrier technology, a permeability zone is selectively plugged to divert the fluid flow or to mitigate the diffusion of hazardous chemicals. In BPM, further nutrient supply must be discontinued after a desired permeability is attained (24). In this case, a lack of nutrients will cause the cells to undergo starvation.
Bacterial starvation affects the performance of the biological activities of cells in in situ bioremediation and oil recovery. Cell death or deactivation caused by starvation will slow down or stop the biological processes. Cell death can also affect cell adhesion and biofilm attachment (2, 16), which may jeopardize the stability of a biofilm. However, microbial cells have always found various ways to survive in adverse environments by forming spores, by cell miniaturization, by cannibalism, etc. (27). Bacterial cell viability is affected by the conditions of the environment during starvation. Parameters that can affect bacterial viability during starvation in natural or engineering applications include temperature, trace minerals, solution pH, metabolic by-products, and chemical biocides (1, 9, 35, 43).
Among the complicated subsurface conditions, the effects of pH and mineral ions on the bacterial starvation were investigated, because solution pH and some trace minerals have been known to play an important role in bacterial metabolism (8, 11, 15). The solution pH can be easily changed due to metabolic by-products and other chemicals present in or injected into the subsurface. Trace minerals are also ubiquitous in the subsurface. While many starvation survival studies have been carried out, most of them have focused on spores, marine bacteria, and gram-negative cells (8, 30, 32). Furthermore, few studies have examined the effects of pH and trace minerals on cell culturability during starvation conditions (37, 42). In this study, the combined effects of pH and trace minerals on cell culturability during starvation were investigated. Various pH values ranging from 3 to 13 were examined in batch reactors with and without trace amounts of metal ions of Mg, Fe, and Mn. These ions have been shown to affect not only cell growth but also cell survival during starvation as shown for Aerobacter aerogenes under starvation conditions (42).
L. mesenteroides NRRL B-523 was used as a model bacterium because of its rapid growth and exopolymer production and its potential use in BPM. The species L. mesenteroides is classified as a member of the lactobacilli, which are gram positive. It is spherical (0.5 to 1.2 μm in diameter), forms long chains during growth, is an oxygen-tolerant anaerobe, and it is known to produce extracellular polysaccharides when fed sucrose (20, 22, 25). The sucrose-induced enzyme dextransucrase breaks down sucrose into glucose and fructose and links the glucose molecules into dextran. The cell growth on sucrose was reported to be virtually the same as the cell growth on the stoichiometrically equivalent mixture of glucose and fructose (25). The kinetics of cell growth and enzyme and exopolymer production have been well studied in our laboratory and can be found elsewhere (19, 25).
MATERIALS AND METHODS
Cell growth and starvation media.
Batch experiments were carried out to determine how the bacteria respond to carbon source depletion in different pH ranges with and without trace minerals. For the pH effects, different pH values (from 3 to 13) were tested using two separate sets of batch reactors in the presence of trace minerals, one with water-insoluble exopolymers and one without exopolymers. The effects of trace minerals were tested at two different pH values, 7 and 4.5, with and without exopolymers. The trace minerals examined were a combination of Mg, Mn, and Fe. The trace minerals were prepared by individually dissolving MgSO4 · 7H2O (12.3 g/ml), FeSO4 · 7H2O (1.39 g/ml), and MnSO4 (0.85 g/ml) in deionized water to make stock solutions. Each stock solution (2 ml) was added to 1 dm3 of phosphate-buffered saline solution. The final concentrations of trace minerals in the growth and starvation media were 12.7 μM for MnSO4, 10 μM for FeSO4 · 7H2O, and 100 μM for MgSO4 · 7H2O. The medium used to grow the inoculum was a combination of yeast extract (1% by weight), NaCl (0.07 M), NH4Cl (0.06 M), CH3COONa (0.06 M), ascorbic acid (0.5 g/dm3), trace minerals, and a carbon source (sucrose or combination of glucose and fructose; 0.1 M) in phosphate-buffered saline solution. The experiments were performed at a constant temperature of 25°C. Batch reactors were shaken slowly (∼60 rpm) for 1 min every 4 h during growth and once a day during starvation.
Two different carbon sources were used to elucidate the effect of insoluble dextran on cell viability: sucrose and the combination of glucose and fructose. The sugar concentration of the growth medium was 0.1 M. L. mesenteroides NRRL B-523 (ATCC 14935) produces insoluble dextran polymerized by dextransucrase, which is induced by sucrose (28). Cell growth on sucrose has been reported to be the same as cell growth on the mixture of glucose and fructose for stoichiometrically equivalent concentrations of glucose and fructose. In the batch experiments, stock solutions of 15 g of sucrose/dm3 and the mixture of 7.9 g of fructose and 7.9 g of fructose were prepared and added to growth media. Due to the different exopolymer production depending on the carbon sources, i.e., insoluble dextran production on sucrose feed and no dextran production on glucose and fructose, L. mesenteroides shows distinct differences in the plugging of porous media (26).
Procedures.
Two separate sets of experiments were carried out: one for the effects of pH and the other one for the effects of trace minerals.
(i) Experiments for pH effects with water-insoluble exopolymers.
Cells were inoculated into 1,000 ml of growth medium which contained sucrose as a carbon source. At the end of the exponential growth (20 h after inoculation), 500 ml of the suspension was taken and centrifuged (6,000 × g) for 20 min to separate cells and water-insoluble exopolymers from suspension. Cells and water-insoluble exopolymers were collected, washed twice with phosphate-buffered saline solution, and transferred to a 500-ml solution at the predetermined pH value to initiate starvation; pH values used were 3, 4, 5, 6, 7, 8, 10, and 12. Samples (1 ml for culturable-cell measurement and 4 ml for exopolymer analysis) were taken every 4 to 6 h to count culturable cells (CFU) during the first week of starvation, once a day for the second week, and every other day during the remainder of the experiments. The spread plate method was used to measure the number of culturable cells. After successive dilutions of a 1-ml sample in phosphate-buffered saline solution (10 ml), 100 μl of diluted suspension was transferred onto an MRS agar plate. The colonies were counted after 1.5 days of incubation at 30°C. A counter (model ZF; Coulter Electronics Inc.) was also used to determine the total number of cells. All experiments were done in triplicate. The solution pH was monitored every 4 h during the first 2 days of starvation and once in 2 or 3 days for the rest of the experiments. Adjustment of the solution pH was done using 1 N NaOH for basic conditions and 1 N acetic acid for acidic conditions.
(ii) Experiments for pH effects without water-insoluble exopolymers.
Cells were inoculated into 1,000 ml of growth medium which contained a combination of glucose and fructose as a carbon source. The rest of the experiments were carried out in the same manner as in the previous paragraph.
(iii) Experiments for trace mineral effects.
In order to better observe the effects of trace minerals, a separate set of experiments were carried out at two pH values: 4.5 and 7. Cells were inoculated in two separate growth media; one contained sucrose for insoluble exopolymer production, and one contained a mixture of glucose and fructose. Cells were separated after the growth phase, washed with the buffer solution, transferred to starvation solutions, and measured in the same manner as stated above. All experiments were done in triplicate.
Exopolymer measurements.
The extracellular polysaccharide (exopolymer) concentration was measured during both the growth and starvation experiments. Samples (4 ml) were taken from original cultures every 2 h during the exponential-growth phase. During starvation, the samples were taken at the same frequency as stated in the above procedure. The samples were then centrifuged (6,000 × g) for 20 min to separate insoluble materials (cells and insoluble exopolymers) from soluble materials (soluble exopolymers and metabolic by-products). To separate the cells from the insoluble exopolymer, a 10% potassium hydroxide solution was added to dissolve the exopolymer, while keeping the cells intact. The cells and dissolved exopolymer were separated by centrifugation, and the supernatant was saved for insoluble exopolymer precipitation. To precipitate the exopolymers, the supernatants were treated with an excess of 100% ethanol. The precipitate was filtered with a 0.2-μm-pore-size nylon filter (Cole-Parmer), and the filter paper was rinsed with 100% ethanol. Precipitated exopolymers were treated in the following manner. Hydrochloric acid (2 M) was added to the samples to a pH of 1 to 2 in 20-ml borosilicate vials with tightly sealed caps, and the samples were boiled for 3 h in a water bath to break down exopolymers into monomers (glucose molecules). After the boiling, the pH of the sample was adjusted to 6.5 with 10% KOH. This solution was then assayed for glucose using the phenol-sulfuric acid assay (12). Detailed procedures for exopolymer separation and analysis are well described by Jeanes (21).
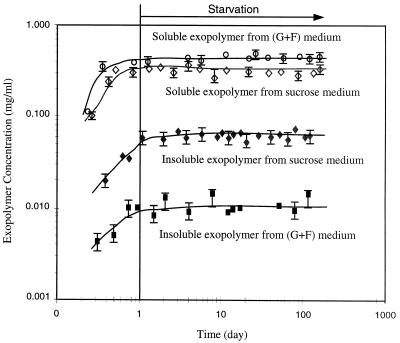
Exopolymer measurements from the sucrose-fed and glucose-fructose-fed cells at pH 5 were used to plot the exopolymer concentration during starvation (Fig. 5).
FIG. 5.
Concentrations of exopolymers during growth and starvation. G+F, glucose-fructose.
Dextranase measurements.
In addition to the dextran analysis, dextranase analysis was carried out to verify that no dextranase was induced by dextran from L. mesenteroides NRRL B-523 during starvation. Measurements for dextranase were carried out in the following manner. Cells were inoculated with insoluble dextran into a phosphate-buffered saline solution (starvation medium; 500 ml). Samples (1 ml) were taken once in 2 or 3 days for 93 days. Samples were centrifuged, and the supernatants were processed using the method described by Somogyi (40).
RESULTS
Effects of pH.
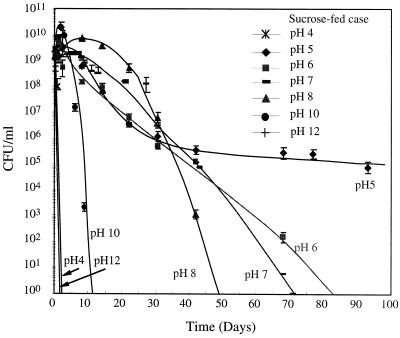
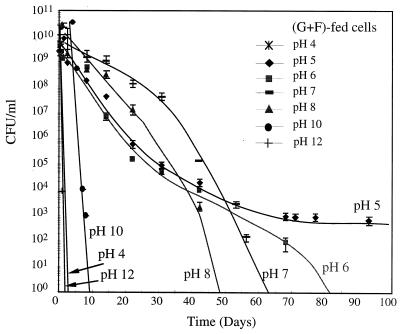
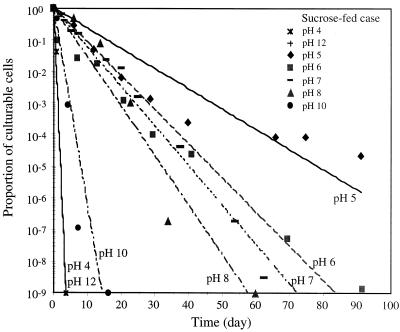
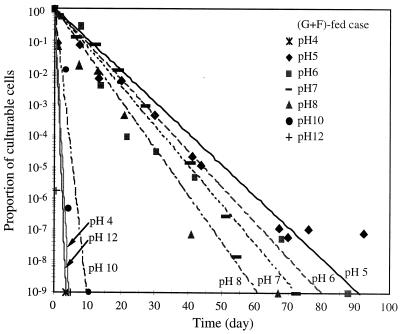
Figure 1 shows CFU numbers at different pH values for the sucrose-fed cells. Cells showed the highest viability at pH 5, surviving more than 93 days. An average CFU value below 1 at a specific day indicates no culturable cells. For all of the tested pH values except for pH 5, the CFU values decreased until they became nondetectable during the experiment. The data points for CFU values of 0 are not shown in Fig. 1 and 2 due to the log scale of the y axis. As shown in Fig. 2, cells transferred from the glucose-fructose-fed system showed a trend similar to that for the sucrose-fed system. At high pH values (12 and 13) and at low pH values (3 and 4), cells rapidly became nonculturable, i.e., within 2 days of starvation. Trace minerals were present for all cases.
FIG. 1.
pH effects on culturable cell number for sucrose-fed cells.
FIG. 2.
pH effects on culturable cell number for glucose-fructose (G+F)-fed cells.
Effects of trace minerals.
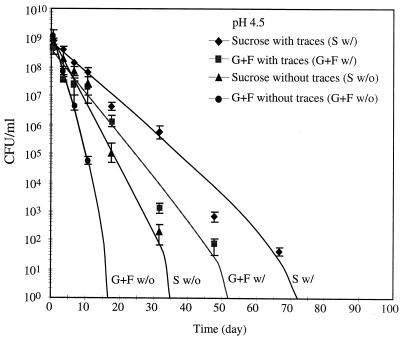
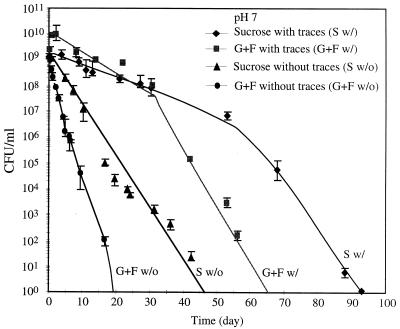
The culturable cell number (CFU) was determined based on the spread plate method, and the results are shown in Fig. 3 and 4. At pH 4.5, cells became nonculturable within 70 days whether the trace minerals were present or not (Fig. 3). Cells with trace minerals showed a greater ability to remain culturable than those without trace minerals. In contrast to the results at pH 4.5, cells undergoing starvation at pH 7 survived up to 95 days as shown in Fig. 4. Cells from the sucrose-fed system survived better than those from the glucose-fructose-fed system in all cases.
FIG. 3.
Trace mineral (traces) effects on culturable cell number at pH 4.5. G+F, glucose-fructose.
FIG. 4.
Trace mineral (traces) effects on culturable cell number at pH 7. G+F, glucose-fructose.
Exopolymer analysis.
Soluble and insoluble exopolymer concentrations were measured during cell growth and during starvation to examine the effects of exopolymers on cell culturability under different pH conditions. The total amounts of exopolymers (both soluble and insoluble) produced in sucrose-fed and glucose-fructose-fed reactors were 0.37 (±0.02) and 0.46 mg/ml (±0.02), respectively. The exopolymer analysis results are summarized in Table 1. Approximately 80 to 98% of soluble exopolymers were produced in both sucrose- and glucose-fructose-fed cases. About sevenfold more insoluble exopolymer was produced in sucrose-fed cultures than in glucose-fructose-fed cultures. The concentration of insoluble exopolymers in glucose-fructose-fed batches was considered below detection, because the averaged value of the measured concentration was within the experimental error range. As shown in Fig. 5, no change in exopolymer concentration was detected throughout the starvation experiments, which indicates that exopolymer was not consumed by the starving bacteria. Furthermore, no dextranase was detected within the error range during the 93 days of starvation in the presence of insoluble dextran, which supports the lack of dextran degradation by starving bacteria.
TABLE 1.
Comparison of exopolymer production levels between the sucrose-fed and glucose-fructose-fed batches
| Carbon source | Exopolymer productiona (mg/ml)
|
% Soluble | % Insoluble | ||
|---|---|---|---|---|---|
| Soluble | Insoluble | Total | |||
| Sucrose | 0.3 | 0.07 | 0.37 | 81 | 19 |
| Glucose-fructose | 0.45 | BDb | 0.46 | 98 | 2 |
Numerical values are approximate. Error is ±0.02 mg/ml.
BD, below detection.
DISCUSSION
The numbers of culturable cells, at various pH values with and without trace minerals, were measured during bacterial starvation. Cells became nonculturable at different rates depending on pH, the presence of trace minerals, and the presence of exopolymers. The relationship between pH and cell decay rate during starvation was explained using the Michaelis pH function.
The culturability of cells grown on a complex medium supplemented with sucrose was a focus of this study. The medium used for the culturable cell count contained the same components as the cell growth medium. Because dextran production is a major concern, bacterial growth and resuscitation were carried out using sucrose-supplemented media. However, it is possible that L. mesenteroides can enter the viable but nonculturable (VBNC) state under starvation conditions, and as a result, no culturable cells were detected with the resuscitation medium we have used (39). The VBNC phenomenon has been described for over 30 bacterial species (33, 34, 44). Although the VBNC state is still controversial, more studies are necessary to understand the viability of L. mesenteroides under starvation conditions (5, 17, 33).
Effects of pH.
The cells were transferred to pH-adjusted phosphate-buffered saline solutions which contained trace minerals. To analyze the cell decay kinetics at different pH values, a first-order decay model was used to describe the culturable population (CFU) measurements: n = n0 exp(−kdt) where n is the number of CFU per milliliter, n0 is the initial number of CFU per milliliter, kd is the decay rate constant (in inverse days), and t is the starvation time (in days). kd values in Fig. 6 and 7 were determined using linear regression of a plot of the ratio of culturable cells to the maximum number of culturable cells at the beginning of starvation. In the experiment at solution pHs 5 and 6, there were negative deviations from the fitted equation at early time periods and positive deviations at later time periods, which suggests that some fraction of the population is more resistant to starvation at pHs 5 and 6. Some cells were still culturable after long-term starvation periods at pH 5. The kd values for different pH conditions are summarized in Table 2.
FIG. 6.
Proportion of culturable cells during starvation and data fitting with an exponential function for sucrose-fed cases.
FIG. 7.
Proportion of culturable cells during starvation and data fitting with an exponential function for glucose-fructose (G+F)-fed cases.
TABLE 2.
Death rate constants determined by data fitting with exponential function using linear regression
| pH | Data for cells fed with:
|
|||
|---|---|---|---|---|
| Sucrose
|
Glucose-fructose
|
|||
| kd | R2 | kd | R2 | |
| 4 | 5.68 | 0.91 | 6.03 | 0.56 |
| 5 | 0.15 | 0.62 | 0.16 | 0.85 |
| 6 | 0.18 | 0.95 | 0.21 | 0.93 |
| 7 | 0.29 | 0.97 | 0.40 | 0.93 |
| 8 | 0.71 | 0.94 | 0.96 | 0.94 |
| 10 | 1.85 | 0.88 | 2.13 | 0.87 |
| 12 | 5.75 | 0.79 | 5.80 | 0.99 |
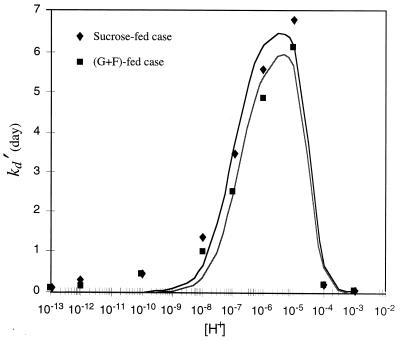
In order to elucidate the relation between kd and the solution pH, kd′, the inverse of kd, was plotted as a function of hydrogen ion concentration ([H+]). The inverse of the decay rate constant may be interpreted as the mean life span of the organism (4). The dependence of kd on pH was demonstrated by plotting kd as a function of [H+]. The model equation that can fit the relationship between kd′ and [H+] is suggested as follows (10):
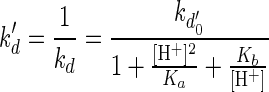 |
where kd0′ is the inverse of the maximum decay rate constant in days, ka is the parameter for the acidic side (in moles squared per square liter) and Kb is the parameter for the basic side (in moles per liter). The method of least squares was used to determine the parameters Ka and Kb. For the sucrose-fed case, Ka = 6.15 × 10−10 and Kb = 9.41 × 10−8, and for the glucose-fructose-fed case, Ka = 3.40 × 10−10 and Kb = 2.16 × 10−7. The parameters Ka and Kb represent ionization constants of the complex which affect the acid sides and the alkaline sides of the pH curves, respectively. As shown in Fig. 8, the model equation is consistent with the cell decay data for different pH values. It is interesting that the effect of pH on cell decay kinetics produces a curve shape similar to that for enzyme deactivation kinetics as a function of pH (10, 18). From the analogy between the effects of pH on cell decay kinetics and enzyme deactivation, a mechanism of cell decay at high and low pHs can be explained based on enzyme deactivation at different pH values (15, 29) and the Michaelis pH function theory (23, 31).
FIG. 8.
Inverse of death rate constant (kd′) and model equation as a function of [H+] for sucrose-fed and for glucose-fructose (G+F)-fed cells.
In summary, the rapid cell decay at high and low pH values can be modeled using the Michaelis pH function theory. Enzymatic activity has been reported to be related to cell viability during starvation (6, 7, 41), and ionization of the active sites of enzymes or enzyme-substrate complexes is known to deactivate enzymatic activity, which leads to cell death. Therefore, the rapid cell decay at high and low pH values under starvation conditions can be related to the ionization of the enzyme-substrate complex. The optimum condition at pH 5 is believed to provide the preferred proton concentration gradient for ATPase and enzyme-substrate complexes, helping them utilize stored nutrient to survive longer. However, it should be emphasized that a further study is necessary to determine the mechanism for a rapid cell decay at high and low pH values under starvation conditions.
Effects of exopolymers.
In the starvation experiments, only the insoluble exopolymers were present with cells, because the growth medium suspension was removed by centrifugation at the end of growth phase, with only the insoluble material (cells and insoluble exopolymers) being transferred to starvation reactors. As shown in Table 1, insoluble exopolymer was a factor of 7 greater in the sucrose-fed batches than in the glucose-fructose-fed batches. As a result, cells transferred from the sucrose-fed culture solutions had more insoluble exopolymers than cells from glucose-fructose-fed batches. The only difference between cells from sucrose-fed and glucose-fructose-fed batches is the presence of insoluble exopolymer, and cells with insoluble exopolymers (i.e., cells from the sucrose-fed reactor) showed better culturability than cells with negligible exopolymer concentration. Consequently, the insoluble exopolymer appears to contribute to the greater culturability of cells during starvation. Exopolymer has been known to help cells store nutrients and to protect cells from a harsh environment (13). As shown in Fig. 6 and 7, in the presence of a high concentration of insoluble exopolymers (sucrose-fed cases), there were a greater number of culturable L. mesenteroides cells in the tested pH range than in the presence of a negligible amount of insoluble exopolymers (glucose-fructose-fed cases). No changes in the exopolymer concentration were observed, indicating that the exopolymer matrix is not degraded by the bacteria undergoing starvation, and this result is promising for BPM to maintain biofilm plugging for long periods of time under starvation conditions.
Effects of trace minerals.
The number of culturable cells was normalized based on the maximum number that appeared after starvation began. To determine the decay rate constants, the proportion of culturable cells was fitted with a first-order decay model. The kd values are summarized in Table 3. At each solution pH, the effect of exopolymers during starvation was also examined. As shown in Table 3, kd is larger for glucose-fructose-fed cells for both pH values of 4.5 and 7 than for sucrose-fed cells.
TABLE 3.
Death rate constants for trace effects at pHs 4.5 and 7 determined using linear regression
| pH |
kd (R2) for cultures:
|
|||
|---|---|---|---|---|
| With trace minerals
|
Without trace minerals
|
|||
| Sucrose-fed | Glucose-fructose-fed | Sucrose-fed | Glucose-fructose-fed | |
| 4.5 | 0.27 (0.98) | 0.34 (0.96) | 0.49 (0.99) | 1.17 (0.94) |
| 7 | 0.25 (0.95) | 0.29 (0.94) | 0.44 (0.98) | 1.04 (0.98) |
From Table 3, it is clear that trace minerals help cells survive better. For both sucrose-fed and glucose-fructose fed cases, kd is smaller in the presence of trace minerals, which means that cells became nonculturable slower with trace minerals. Furthermore, the effect of trace minerals on the cell decay rate is greater for glucose-fructose-fed cells than for sucrose-fed cells. For both pH values of 4.5 and 7, the kd values for sucrose-fed cells are approximately twofold larger without trace minerals than with trace minerals, whereas glucose-fructose-fed cells showed a three- to fourfold-larger decay rate in the absence of trace minerals than in their presence. Therefore, the absence of trace minerals has a more significant effect on cell viability when there is a low concentration of water-insoluble exopolymer surrounding the cells. Overall, cells with insoluble exopolymers survive longer than those without insoluble exopolymers do, regardless of the presence of trace minerals which can enhance cell viability. The effect of trace minerals was observed more clearly for glucose-fructose-fed cases, as the difference in kd values with and without trace minerals was larger than that for the sucrose-fed case.
Very low concentrations of metal ions are known to stimulate growth, even though higher concentrations inhibit growth and can destroy the organisms in nutrient media. At very low concentrations, metal elements such as calcium, iron, and magnesium serve as enzyme cofactors and as important building blocks of various cell constituents. It has been reported that these inorganic ions play an important role in bacterial survival. For example, Mg2+, Ca2+, and Fe3+ were reported to delay the death of Aerobacter aerogenes in starvation condition (42). A threshold ion concentration beyond which a solution is toxic to the starved bacteria has been reported (43). In addition to the previous observations of trace mineral effects on cell viability, trace mineral effects on cell viability were observed to be influenced by the presence of exopolymers in this study.
ACKNOWLEDGMENT
We thank Jeremy Samrau at the Civil and Environmental Engineering Department, University of Michigan, Ann Arbor, for reviewing this paper.
REFERENCES
- 1.Alvarez P J J, Cronkhite L A, Hunt C S. Use of benzoate to establish reactive buffer zone for enhanced attenuation of BTX migration: aquifer column experiments. Environ Sci Technol. 1998;32:509–515. [Google Scholar]
- 2.Applegate D H, Bryers J D. Effects of carbon and oxygen limitations and calcium concentrations on biofilm removal processes. Biotechnol Bioeng. 1991;37:17–25. doi: 10.1002/bit.260370105. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson B, How S Y. The overall rate of substrate uptake (reaction) by microbial films. Trans Inst Chem Eng. 1974;52:260–268. [Google Scholar]
- 4.Bailey J E, Ollis D F. Biochemical engineering fundamentals. 2nd ed. New York, N.Y: McGraw-Hill, Inc.; 1986. [Google Scholar]
- 5.Bogosian G. Viable but nonculturable, or dead? ASM News. 1998;64:547. [Google Scholar]
- 6.Boylen C W, Ensign J C. Intracellular substrates for endogenous metabolism during long-term starvation of rod and spherical cells of Arthrobacter crystallopoietes. J Bacteriol. 1970;103:578–587. doi: 10.1128/jb.103.3.578-587.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boylen C W, Mulks M H. The survival of coryneform bacteria during periods of prolonged nutrient starvation. J Gen Microbiol. 1978;105:323–334. [Google Scholar]
- 8.Brown D E, McAvoy A. A pH-controlled fed-batch process for dextransucrase production. J Chem Technol Biotechnol. 1990;48:405–414. [Google Scholar]
- 9.Burton H, Jayne-Williams D. Sterilized milk. In: Hawthorn J, Leitch J M, editors. Recent advances in food science. Vol. 2. London, United Kingdom: Butterworths & Co.; 1962. p. 107. [Google Scholar]
- 10.Carbonell R G, Kostin M D. Enzyme kinetics and engineering. AICHE J. 1972;18:1–12. [Google Scholar]
- 11.Champagne C P, Gardner N, Doyon G. Production of Leuconostoc oenos biomass under pH control. Appl Environ Microbiol. 1989;55:2488–2492. doi: 10.1128/aem.55.10.2488-2492.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaplin M F, Kennedy J F. Carbohydrate analysis. 2nd ed. London, United Kingdom: Oxford University Press; 1994. [Google Scholar]
- 13.Costerton J W, Geesey G G, Cheng K. How bacteria stick. Sci Am. 1978;238:86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- 14.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 15.Dawes E A. Growth and survival of bacteria. In: Poindexter J S, Leadbetter E R, editors. Bacteria in nature. Vol. 3. New York, N.Y: Plenum Press; 1985. pp. 67–187. [Google Scholar]
- 16.Dawson M P, Humphrey B A, Marshall K C. Adhesion: a tactic in the survival strategy of a marine vibrio during starvation. Curr Microbiol. 1981;6:195–199. [Google Scholar]
- 17.Dixon B. Viable but nonculturable: is the concept of “viable but nonculturable” still viable itself, or does it call for reassessment? ASM News. 1998;64:372–373. [Google Scholar]
- 18.Dixon M, Webb E C. Enzymes. 3rd ed. New York, N.Y: Academic Press; 1979. [Google Scholar]
- 19.Doles M, Remaud-Simeon M, Willemot R M, Demuth B, Jordening H J, Buchholz K, Monsan P. Kinetic modeling of oligosaccharide synthesis catalyzed by Leuconostoc mesenteroides NRRL B-1299 dextransucrase. Biotechnol Bioeng. 1999;63:308–315. doi: 10.1002/(sici)1097-0290(19990505)63:3<308::aid-bit7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Holzapfel W H, Schillinger U. The genus Leuconostoc. In: Starr M P, editor. The prokaryotes: a handbook on habitats, isolation, and identification of bacteria. Berlin, Germany: Springer-Verlag; 1981. pp. 1508–1534. [Google Scholar]
- 21.Jeanes A. Preparation of dextrans from growing Leuconostoc cultures. In: Whistler R L, BeMiller J N, Wolfrom M L, editors. Methods in carbohydrate chemistry. New York, N.Y: Academic Press; 1965. pp. 118–127. [Google Scholar]
- 22.Jeanes A, Haynes W C, Wilham C A, Rankin J C, Melvin E H, Austin M J, Cluskey J E, Fisher B E, Tsuchiya H M, Rist C E. Characterization and classification of dextran from ninety-six strains of bacteria. J Am Chem Soc. 1954;76:5041–5052. [Google Scholar]
- 23.Kim D S. Ph.D. thesis. Ann Arbor: University of Michigan; 1999. [Google Scholar]
- 24.Lappan R E, Scott Fogler H. Reduction of porous media permeability from in situ Leuconostoc mesenteroides growth and dextran production. Biotechnol Bioeng. 1996;50:6–15. doi: 10.1002/(SICI)1097-0290(19960405)50:1<6::AID-BIT2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Lappan R E, Scott Fogler H. Leuconostoc mesenteroides growth kinetics with application to bacterial profile modification. Biotechnol Bioeng. 1994;434:865–873. doi: 10.1002/bit.260430905. [DOI] [PubMed] [Google Scholar]
- 26.Lappan R E, Scott Fogler H. Effect of bacterial polysaccharide production on formation damage. Soc Petrol Eng J. 1992;7:167–171. [Google Scholar]
- 27.Lappin-Scott H M, Costerton J W. Starvation and penetration of bacteria in soils and rocks. Experientia. 1990;46:807–812. [Google Scholar]
- 28.Lawford G R, Klingerman A, Williams T. Dextran biosynthesis and dextransucrase production by continuous culture of Leuconostoc mesenteroides. Biotechnol Bioeng. 1979;21:1121–1131. doi: 10.1002/bit.260210704. [DOI] [PubMed] [Google Scholar]
- 29.Lodish H, Baltimore D, Berk A, Zipursky S L, Matsudaira P, Darnell J. Molecular cell biology. 3rd ed. New York, N.Y: Scientific American Books, Inc.; 1995. [Google Scholar]
- 30.Meganathan R, Ensign J C. Stability of enzymes in starving Arthrobacter crystallopoietes. J Gen Microbiol. 1976;94:90–96. doi: 10.1099/00221287-94-1-90. [DOI] [PubMed] [Google Scholar]
- 31.Michaelis L. Die Wasserstoffionenkonzentration. Berlin, Germany: Springer-Verlag; 1922. [Google Scholar]
- 32.Morita R Y. Bacteria in oligotrophic environments. New York, N.Y: Chapman & Hall; 1997. [Google Scholar]
- 33.Oliver J D. Viable but nonculturable—alive? ASM News. 1999;65:185–186. [Google Scholar]
- 34.Oliver J D, Hite F, McDougald D, Andon N L, Simpson L M. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Appl Environ Microbiol. 1995;61:2624–2630. doi: 10.1128/aem.61.7.2624-2630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pampulha M E, Loureiro-Dias M C. Activity of glycolytic enzymes of Saccharomyces cerevisiae in the presence of acetic acid. Appl Microbiol Biotechnol. 1990;34:375–380. [Google Scholar]
- 36.Peyton B M. Improved biomass distribution using pulsed injections of electron donor and acceptor. Water Res. 1996;30:756–758. [Google Scholar]
- 37.Postgate J R, Hunter J R. The survival of starved bacteria. J Gen Microbiol. 1962;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- 38.Potera C. Biofilms invade microbiology. Environ Sci Technol. 1996;273:1795–1797. doi: 10.1126/science.273.5283.1795. [DOI] [PubMed] [Google Scholar]
- 39.Roszak D B, Colwell R R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987;51:365–378. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somogyi M. A reagent for the copper-iodometric determination of very small amounts of sugar. J Biol Chem. 1937;117:771–776. [Google Scholar]
- 41.Strange R E. Stability of β-galactosidase in starved Escherichia coli. Nature. 1966;209:428–429. doi: 10.1038/209428a0. [DOI] [PubMed] [Google Scholar]
- 42.Trulear M G, Characklis W G. Dynamics of biofilm processes. J Water Pollut Control Fed. 1982;54:1288–1301. [Google Scholar]
- 43.Winslow C-E A, Falk I S. Studies on salt action. VIII. The influence of calcium and sodium salts at various hydrogen ion concentrations upon the viability of Bacterium coli. J Bacteriol. 1923;8:215–236. doi: 10.1128/jb.8.3.215-236.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu H-S, Roberts N, Singleton F L, Attwell R W, Grimes D J, Colwell R R. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol. 1982;8:313–323. doi: 10.1007/BF02010671. [DOI] [PubMed] [Google Scholar]