Abstract
Borrelia sp. prevalence in ticks on migratory birds was surveyed in central Japan. In autumn, a total of 1,733 birds representing 40 species were examined for ticks. A total of 361 ticks were obtained from 173 birds of 15 species, and these ticks were immature Haemaphysalis flava (94.4%), Haemaphysalis longicornis, Ixodes columnae, Ixodes persulcatus, Ixodes turdus, and an unidentified Ixodes species. Of these, 27 juveniles of H. flava on Turdus pallidus, Turdus cardis, or Emberiza spodocephala, 2 juveniles of I. persulcatus on T. pallidus, and 1 female H. flava molted from a T. pallidus-derived nymph were positive for the presence of Borrelia by Barbour-Stoenner-Kelly culture passages. In spring, a total of 16 ticks obtained from 102 birds of 21 species were negative for the spirochete. Isolates from 15 ticks were characterized by 5S-23S rRNA intergenic spacer restriction fragment length polymorphism analysis; all isolates were identified as Borrelia garinii with pattern B/B′ based on the previous patterning. According to the intergenic spacer sequences, 2 of 15 isolates, strains Fi14f and Fi24f, were highly similar to B. garinii strains 935T of Korea and ChY13p of Inner Mongolia, China, respectively. These findings indicate that Lyme disease-causing B. garinii may have been introduced to Japan by migratory birds from northeastern China via Korea. Additionally, a case of transstadial transmission of B. garinii from nymph to adult H. flava suggests that the infected H. flava may transmit Borrelia to large animals.
Lyme disease is primarily caused by three genomic species, Borrelia burgdorferi sensu stricto, Borrelia garinii, and Borrelia afzelii (2, 4). B. garinii and B. afzelii are widely distributed from Europe to the Far East including Japan, while B. burgdorferi sensu stricto is prevalent in North America and has been confirmed in part of Europe. B. burgdorferi sensu lato is mainly transmitted by some tick species of the Ixodes ricinus complex, and these ticks infest both mammals and birds (1, 9, 10, 13, 19, 25).
Concerning the prevalence of Borrelia in ticks feeding on birds, B. garinii has been isolated from Ixodes persulcatus on Emberiza spodocephala and Turdus chrysolaus in Hokkaido, Japan (20, 24), and B. burgdorferi sensu stricto, B. garinii, B. afzelii, and Borrelia andersonii have been isolated from Ixodes dentatus, I. ricinus, Ixodes scapularis, Ixodes uriae, and other ticks, which infest a large number of bird species in Europe and North America (6, 13, 15, 27, 28, 30). Furthermore, Haemaphysalis leporispalustris, detected on some bird species, was reported to be a reservoir of B. burgdorferi sensu stricto in North America (13, 26).
B. afzelii is transmitted between I. persulcatus and field rodents, and B. garinii is transmitted between I. persulcatus and migratory birds or rodents, in Hokkaido, Japan (24, 25). However, there has not been a survey of Borrelia in migratory birds which travel directly between the Asiatic continent and Japan. In this survey, we examined the Borrelia prevalence in juvenile ticks removed from birds captured on the Japanese mainland.
MATERIALS AND METHODS
Survey site.
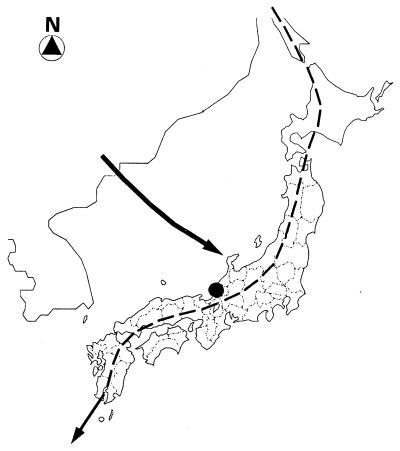
From September to November 1995 to 1997, surveys were carried out at the Bird Banding Otayama Station, located in the mountainous area (maximum elevation, about 600 m above sea level) in Fukui Prefecture of central Japan along the coast of the Sea of Japan (Fig. 1). Additional surveys were carried out at the same station from late April to early May 1996 to 1997.
FIG. 1.
Survey site (solid circle) in central Japan and migratory routes of T. pallidus (solid line) and E. spodocephala (dashed line) around the Japanese mainland during autumn.
Tick collection.
We took part in the bird banding performed by the Yamashina Institute for Ornithology and Fukui Branch of the Wild Bird Society of Japan. Migratory birds were captured using about 40 Japanese mist nets (12 m in length, 36-mm mesh) at ground level. The species of all birds were identified, and, if possible, their sexes and ages were determined. Prior to banding and release, each bird was closely examined, particularly around the head and neck. Ticks were removed with forceps and placed in separate glass vials containing moist sanitary cotton. The stage and species of each tick were identified as described by Takada (34).
Spirochete isolation.
The internal organs of live ticks that were insufficiently engorged were immediately removed for spirochete isolation and placed in Barbour-Stoenner-Kelly II (BSK) II medium as described previously (3, 7). Healthy specimens of fully engorged larvae and nymphs of ticks were kept in glass vials containing moist sanitary cotton in a thermostatic chamber at 25°C and then dissected to isolate spirochetes as soon as the ticks molted to the later stages. All cultures were incubated at 32°C and examined for spirochetes by phase-difference microscopy once weekly for 5 weeks. Dead ticks and the birds themselves were not examined for spirochetes.
PCR and RFLP analysis.
Spirochete isolates were identified by 5S-23S rRNA intergenic spacer restriction fragment length polymorphism (RFLP) analysis. Primers corresponding to the 3′ end of the rRNA (rrf [5′-CTGCGAGTTCGCGGGAGA-3′]) and the 5′ end of the 23S rRNA (rrl [5′-TCCTAGGCATTCACCATA-3′]) as described previously (29) were synthesized using b-cyanoethyl phosphoramidite by a custom oligonucleotide synthesis service (Bex Co., Tokyo, Japan). Two-milliliter aliquots of culture were washed, and the cells were resuspended in 100 ml of water. The resultant cell suspensions were boiled at 100°C for 10 min. PCR was performed by a method previously described (18, 29). The amplicon obtained after PCR was digested with MseI and DraI according to the manufacturer's recommendations (New England Biolabs, Beverly, Mass.), and the digested DNA was electrophoresed through a 16% polyacrylamide gel and subsequently stained with ethidium bromide. Marker 10 purchased from Nippon Gene Co. (Toyama, Japan) was used as a molecular weight marker.
Sequencing of amplified products.
Each PCR product was cloned into the pCR II plasmid vector, and the recombinant plasmids were transformed into Escherichia coli INV-α F′ using a TA cloning kit (Invitrogen Co., San Diego, Calif.) according to the manufacturer's instructions. The recombinant plasmids were extracted from E. coli cultures in Luria-Bertani broth using the Wizard 373 DNA purification system (Promega Co., Madison, Wis.) and sequenced by a dideoxy chain termination method using the dye terminator Taq cycle sequencing kit and a model 373A DNA sequencer (Applied Biosystems Inc., Foster City, Calif.). At least two clones were sequenced for determination of each strain.
Nucleotide sequence accession numbers.
The intergenic spacer sequences were assigned the following accession numbers: strain Fi14f, AB015911; strain Fi24f, AB015912.
RESULTS
Tick collection.
A total of 1,733 birds representing 40 species were examined for ticks in autumn, and 361 ticks were removed from 173 birds of 15 species. All ticks removed were juvenile ticks from six species of two genera: Haemaphysalis flava, 341; Haemaphysalis longicornis, 4; Ixodes columnae, 1; I. persulcatus, 9; Ixodes turdus, 4; unidentified species (related to I. persulcatus with a few morphological differences), 2. Of these, H. flava ticks were detected on 145 birds of 13 species and constituted 94.4% of all tick specimens. The tick prevalence rates on Turdus pallidus and Turdus cardis were 31.7 and 35.1%, respectively. The rate on E. spodocephala was 3.0% (Table 1). I. persulcatus was detected on nine birds of three species. The prevalence rate of I. persulcatus was 2.6% (7 of 271) on T. pallidus (Table 2). In additional surveys in spring, only 16 juvenile ticks (2 of H. flava, 2 of H. longicornis, 1 of Haemaphysalis phasiana, and 11 of I. turdus) were removed from 102 birds of 21 species (not shown).
TABLE 1.
Tick prevalence in migratory birds in autumn in central Japan and Borrelia prevalence in larval and nymphal H. flava removed from them
| Species | No. of birds with ticks/total no. of birds examined | Larvae
|
Nymphs
|
||||
|---|---|---|---|---|---|---|---|
| Total no. removed | No. positive/no. tested for ticks that were:
|
Total no. removed | No. positive/no. tested for ticks that were:
|
||||
| Unmolted | Molted to nymphs | Unmolted | Molted to adults | ||||
| Anthus hodgsoni | 1/5 | 2 | 0/1 | 0 | |||
| Cettia diphone | 1/73 | 1 | 0/1 | 0 | |||
| Emberiza spodocephala | 20/668 | 19 | 1/14 | 10 | 0/8 | 0/2 | |
| Emberiza variabillis | 3/30 | 4 | 0/4 | 0 | |||
| Emberiza cioides | 1/5 | 1 | NTb | 0 | |||
| Ficedula narcissina | 1/27 | 0 | 1 | 0/1 | |||
| Otus bakkamoena | 2/5 | 1 | 0/1 | 5 | 0/5 | ||
| Otus scops | 1/1 | 1 | 0/1 | 2 | 0/2 | ||
| Parus varius | 1/10 | 2 | 0/1 | 0 | |||
| Phasianus soemmerringii | 1/1 | 3 | 0/3 | 0 | |||
| Turdus cardis | 34/97 | 42 | 1/32 | 0/2 | 31 | 0/26 | |
| Turdus obscurus | 4/67 | 1 | 0/1 | 3 | 0/3 | ||
| Turdus pallidus | 86/271 | 100 | 10/34 | 0/46 | 112 | 15/61 | 1/38 |
| Othersa | 0/473 | ||||||
Includes 27 species of 21 genera (Accipiter, Aegithalos, Carpodacus, Cettia, Cyanoptila, Erithacus, Emberiza, Ficedula, Fringilla, Garrulus, Hypsipetes, Lanius, Muscicapa, Parus, Phoenicurus, Phylloscopus, Picus, Pyrrhula, Tarsiger, Turdus, and Zosteops).
NT, not tested.
TABLE 2.
Tick prevalence in migratory birds in autumn in central Japan and Borrelia prevalence in larval and nymphal H. longicornis and some Ixodes species removed from thema
| Species | No. of birds with ticks/total no. of birds examined |
Borrelia prevalenceb (no. positive/no. tested) inb:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
H. longicornis
|
I. columnae
|
I. persulcatus
|
I. turdus
|
Ixodes sp.c
|
|||||
| L | N | L | L | N | L | N | L | ||
| Cettia diphone | 1/73 | 0/1 | |||||||
| Erithacus calliope | 1/8 | 0/1 | |||||||
| Emberiza spodocephala | 7/668 | 0/3 | 0/1 | 0/1 | 0/2 | ||||
| Garrulus glandarius | 1/10 | 0/1 | |||||||
| Turdus cardis | 2/97 | 0/1 | 0/1 | ||||||
| Turdus pallidus | 8/271 | 1/1 | 1/6 | 0/1 | |||||
Bird samples are the same as those used for Table 1.
L, larva; N, nymph.
Unidentified Ixodes species.
Isolation of spirochetes from unmolted ticks.
In autumn, 10 of 34 larvae and 15 of 61 nymphs of H. flava feeding on T. pallidus, 1 of 32 larvae of H. flava on T. cardis, and 1 of 14 larvae of H. flava on E. spodocephala were positive for spirochetes (Table 1), and also 1 larva and 1 of 6 nymphs of I. persulcatus on T. pallidus were positive for spirochetes (Table 2). All other tick species were negative for spirochetes. All of the 16 ticks obtained in spring were negative for the presence of spirochetes.
Isolation of spirochetes from molted ticks.
One of 16 H. flava females molted from nymphs that fed on T. pallidus in autumn was positive for spirochetes, while spirochetes were not detected in 46 nymphs molted from larvae or 22 males molted from nymphs feeding on T. pallidus (Table 1). Some ticks on T. cardis and E. spodocephala were negative for spirochetes.
Identification of spirochete isolations.
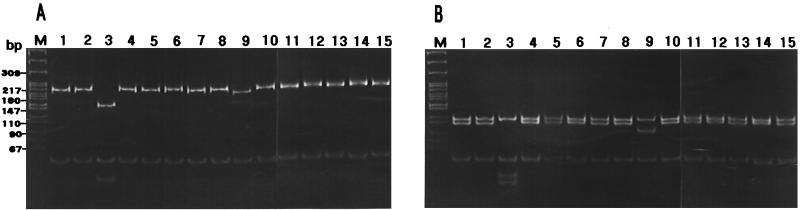
Of a total of 30 isolates, 15 strains were used for 5S-23S intergenic spacer RFLP: 10 of 25 from unmolted juveniles of H. flava that fed on T. pallidus (15 isolates from juvenile ticks that fed on the same bird were not used), one each from larval H. flava that fed on T. cardis and E. spodocephala, 1 from molted adults of H. flava, and 2 from unmolted juveniles of I. persulcatus. Figure 2 shows RFLP patterns observed among the isolates. Thirteen of 15 isolates analyzed showed pattern B and pattern B′ by MseI and DraI digestion, respectively, according to the patterning in a previous report (29) and were consequently identified as B. garinii of pattern B/B′. However, two isolates, Fi14f and Fi24f, were designated variants (Rv1 and Rv2) of pattern R, because their patterns resembled those of strains 935T (accession no. L39081) from Korea and ChY13p (accession no. AB007450) from China, which had been classified as pattern R (14) (Table 3). To confirm the uniqueness of strains Fi14f and Fi24f, their intergenic spacer sequences were compared with those of some known strains (Table 4). The sequences of strains Fi14f and Fi24f were highly similar to that of strain 935T (99.6%) from Korea and that of strain ChY13p (97.9%) from Inner Mongolia, China (14, 34), respectively. Since strains belonging to the same species usually showed over 95% similarity values for 5S-23S rRNA intergenic spacer sequences in previous experiments (18, 29) and also since strains 935T and ChY13p had been clustered into the B. garinii group based on 16S rRNA sequences (11, 14, 17), strains Fi14f and Fi24f were identified as B. garinii.
FIG. 2.
Representative RFLP patterns of the 5S-23S rRNA intergenic spacer observed among Borrelia isolates from bird-feeding ticks. The PCR products were digested by DraI (A) or MseI (B). DNA was electrophoresed on a 16% polyacrylamide gel and stained with ethidium bromide. The molecular size standards are indicated on the left of the gel. Lane 1, Fi01f; lane 2, Fi10f; lane 4, Fi17f; lane 5, Fi19f; lane 6, Fi20f; lane 7, Fi22f; lane 8, Fi23f; lane 10, Fi26f; lane 11, Fi30f; lane 12, Fi71p; lane 13, Fi72p; lane 14, Fi03f; lane 15, Fi16f; all have pattern B. Lane 3, Fi14f; lane 9, Fi24f; both have pattern R.
TABLE 3.
DraI and MseI restriction fragments of 5S-23S intergenic spacer amplicons of isolates from ticks that fed on migratory birds
| Strain | Host | Tick sp. and stagea |
DraI
|
MseI
|
Species | ||
|---|---|---|---|---|---|---|---|
| RFLP pattern | Restriction fragment sizes (bp) | RFLP pattern | Restriction fragment sizes (bp) | ||||
| Study isolates | |||||||
| Fi01f | T. pallidus | H. flava, N | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Fi10f | T. pallidus | H. flava, L | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Fi14f | T. pallidus | H. flava, N | Rv1′ | 144, 52, 41 | Rv1 | 107, 51, 41, 38 | B. garinii |
| Fi17f | T. pallidus | H. flava, N | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Fi19f | T. pallidus | H. flava, N | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Fi20f | T. pallidus | H. flava, L | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Fi22f | T. pallidus | H. flava, N | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Fi23f | T. pallidus | H. flava, N | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Fi24f | T. pallidus | H. flava, N | Rv2′ | 188, 52 | Rv2 | 107, 82, 51 | B. garinii |
| Fi26f | T. pallidus | H. flava, N | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Fi30f | T. pallidus | H. flava, Fb | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Fi71p | T. pallidus | I. persulcatus, N | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Fi72p | T. pallidus | I. persulcatus, L | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Fi03f | T. cardis | H. flava, L | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Fi16f | E. spodocephala | H. flava, L | B′ | 201, 52 | B | 108, 95, 50 | B. garinii |
| Controls | |||||||
| 20047c | I. ricinus, A | B′ | 201, 52 | B | 108, 95, 50 | B. garinii | |
| 935Td | I. persulcatus, A | R′ | 185, 52 | R | 107, 79, 51 | B. garinii | |
| ChY13pe | I. persulcatus, A | R′ | 185, 52 | R | 107, 79, 51 | B. garinii | |
N, nymph; L, larva.
Female molted from a nymph that fed on T. pallidus.
French isolate.
Korean isolate.
Isolate from northeastern China.
TABLE 4.
Sequence similarity matrix of 5S-23S rRNA gene intergenic spacer of isolates from ticks that fed on migratory birdsa
| Species | Strain | Similarity (%) to strain:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 20047 | ASF | 935T | ChY13p | Fi14f | Fi24f | VS461 | NT28 | ||
| B. burgdorferi | B31 | 99.3 | 92.1 | 88.2 | 87.0 | 88.6 | 86.3 | 88.2 | 90.2 |
| B. garinii | 20047 | 98.0 | 92.5 | 90.1 | 92.1 | 89.8 | 91.3 | 93.3 | |
| ASF | 92.9 | 88.9 | 93.3 | 89.0 | 91.3 | 93.3 | |||
| 935T | 95.4 | 99.6 | 95.4 | 92.7 | 94.3 | ||||
| ChY13p | 95.0 | 97.9 | 94.3 | 91.9 | |||||
| Fi14f | 95.0 | 92.3 | 94.7 | ||||||
| Fi24f | 94.8 | 92.3 | |||||||
| B. afzelii | VS461 | 97.6 | |||||||
Strains Fi14f and Fi24f were isolated from nymphal H. flava feeding on T. pallidus. The other strains were used as comparative reference strains. Underlines indicate the highest-similarity values.
DISCUSSION
The prevalence of ticks on examined birds was relatively high among ground-feeding birds, especially on T. pallidus and T. cardis, while it was generally low among arboreal birds. Some juvenile ticks of I. persulcatus, a northern species associated with Lyme disease borreliae, were detected in some birds examined. This species has not been found previously at this survey site by flagging vegetation (unpublished data) but is known to be widely distributed in mountainous areas over 1,000 m above sea level in the eastern part of Fukui Prefecture (7, 8).
The transovarial transmission of Borrelia does not occur in I. persulcatus or Ixodes ovatus in Japan (22), although it has been demonstrated partly in I. ricinus, Ixodes pacificus, and I. scapularis in Europe and North America (12, 21, 31). The transovarial transmission of Borrelia in H. flava has not yet been examined. However, it is well known that all stages of field-collected H. flava have usually been negative for Borrelia, although there were rare cases in which Borrelia spp. were isolated from field-collected nymphs or adults of genus Haemaphysalis ticks in China (35) and Japan (7, 8). Thus, the present isolations of B. garinii from juveniles of H. flava and I. persulcatus that fed on birds confirm that some species of migratory birds possess B. garinii, and especially that T. pallidus, from which B. garinii-positive ticks were predominantly found, may be one of the important reservoirs. Of course, it is difficult to determine the absolute positivity rate for Borrelia in ticks (or that in host birds) by BSK culture alone, since BSK culture may have a bias for genospecies of Borrelia and since the frequency of Borrelia transmission may vary by feeding time or the engorgement condition of the tick on the host bird. Nevertheless, BSK culture of ticks easily estimated the prevalence of live borreliae in birds examined.
Most of the present isolates showed pattern B and pattern B′ by MseI and DraI digestion, respectively. These are common patterns in Borrelia from I. persulcatus in Eurasia and I. ricinus in Europe (16, 29). Nakao et al. (24) reported that isolates from bird-derived larvae of I. persulcatus were identified as ribotype II of B. garinii, the common subtype in Europe and far-eastern Asia, and most strains such as those of ribotype II generated pattern B on the 5S-23S rRNA intergenic spacer PCR-RFLP system (T. Masuzawa, unpublished data). Thus, our findings reconfirmed a strong affinity between birds and B. garinii.
Although not many Korean or Chinese borreliae have been clearly characterized, most Korean and northeastern Chinese strains isolated in previous surveys (11, 14, 34) were identified as B. garinii with pattern B or pattern C or B. afzelii with pattern D (14, T. Masuzawa, unpublished data); only two strains, 935T and ChY13p, were identified as having pattern R. Such a unique pattern had not been observed previously among isolates in Japan and far-eastern Russia. Our findings revealed that strains Fi14f and Fi24f, characterized in the pattern R group, were closely related to strains 935T in Korea and ChY13p in China, respectively. It has been reported that Turdus and E. spodocephala birds mainly migrate on the route shown in Fig. 1 in autumn. Therefore, our results strongly suggest that there is a gradual route of introducing Lyme disease-causing B. garinii from northeastern China via Korea to Japan by long-distance dispersal of ticks feeding on migratory birds. Our additional surveys reconfirmed that there were not many ticks on birds in spring, as the occurrence of juveniles of common ticks including I. persulcatus is known to drop from winter to spring. This suggests that migratory birds have not so many chances to be newly infected with Borrelia before leaving Japan and may not play a significant role in carrying Borrelia from Japan to the Asiatic continent.
Although the transstadial transmission of Borrelia under laboratory conditions has been experimentally demonstrated for I. persulcatus and I. scapularis (5, 23), that in Amblyomma americanum, Amblyomma andersonii, Dermacentor variabilis, and I. ovatus is unclear (5, 23, 32). In the present experiment, a female H. flava tick, which molted from a nymph that fed on T. pallidus, was positive for B. garinii, and the isolate showed the same PCR-RFLP pattern as most B. garinii isolates from unmolted partly engorged larvae or nymphs examined. This is the first study to show that H. flava transmits B. garinii transstadially. This suggests there is an eventual route of Borrelia transmission in nature, namely, the infected adult of H. flava may transmit Borrelia to large animals, although we hardly detected Borrelia in routine samples of field-collected H. flava. The probability of human cases of Lyme disease caused by H. flava is not yet established, although this species is well known to bite humans (33), and a few suspected cases associated with its bite have been seen in Japan (unpublished data).
ACKNOWLEDGMENTS
We thank Shigemoto Kometa, Yamashina Institute for Ornithology, Yasuo Ueki, Fukui Branch of the Wild Bird Society of Japan, and Yoshito Oosako, Fukui Nature Conservation Center, for helpful guidance for material collection.
This work was supported by research grant no. 0804431, 0804181, and 10041204 from the International Scientific Research Program of the Ministry of Education, Science and Culture, Japan.
REFERENCES
- 1.Anderson J F. Epizootiology of Borrelia in Ixodes tick vectors and reservoir hosts. Rev Infect Dis. 1989;11:1451–1459. doi: 10.1093/clinids/11.supplement_6.s1451. [DOI] [PubMed] [Google Scholar]
- 2.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J C, Assous M, Grimont P A D. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 3.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 4.Canica M M, Nato F, duMerle L, Mazie J C, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 5.Dolan M C, Maupin G O, Panella N A, Golde W T, Piesman J. Vector competence of Ixodes scapularis, I. spinipalpis, and Dermacentor andersonii (Acari:Ixodidae) in transmitting Borrelia burgdorferi, the etiologic agent of Lyme disease. J Med Entomol. 1997;34:128–135. doi: 10.1093/jmedent/34.2.128. [DOI] [PubMed] [Google Scholar]
- 6.Hubalek Z, Anderson J F, Halouzka J, Hajek V. Borreliae in immature Ixodes ricinus (Acari:Ixodidae) ticks parasitizing birds in the Czech Republic. J Med Entomol. 1996;33:766–771. doi: 10.1093/jmedent/33.5.766. [DOI] [PubMed] [Google Scholar]
- 7.Ishiguro F, Iida H, Hatano M, Yasuhiro Y, Takada N. Tick fauna and the prevalence of Lyme Borrelia in Fukui Prefecture. J Acarol Soc Jpn. 1992;1:27–35. . (In Japanese with English abstract.) [Google Scholar]
- 8.Ishiguro F, Iida H, Ma X, Yano Y, Fujita H, Takada N. Diversity of Borrelia isolates found in rodent-tick relationship in Fukui Prefecture and some additional areas. Jpn J Sanit Zool. 1994;45:141–145. [Google Scholar]
- 9.Ishiguro F, Takada N. Lyme Borrelia from Ixodes persulcatus and small rodents from northern and central parts of mainland Japan. Med Entomol Zool. 1996;47:183–185. [Google Scholar]
- 10.Jaenson T G T. The epidemiology of Lyme borreliosis. Parasitol Today. 1991;7:39–60. doi: 10.1016/0169-4758(91)90187-s. [DOI] [PubMed] [Google Scholar]
- 11.Kee S H, Yoon J H, Oh H B, Park Y P, Kim Y W, Cho M K, Park K S, Chang W H. Genetic analysis of Borrelia burgdorferi sensu lato in Korea using genomic hybridization and 16S rRNA gene sequence determination. Microbiol Immunol. 1996;40:599–605. doi: 10.1111/j.1348-0421.1996.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 12.Lane R S, Burgdorfer W. Transovarial and transstadial passage of Borrelia burgdorferi in the western black-legged tick, Ixodes pacificus (Acari: Ixodidae) Am J Trop Med Hyg. 1987;37:188–192. doi: 10.4269/ajtmh.1987.37.188. [DOI] [PubMed] [Google Scholar]
- 13.Levine J F, Sonenshine D E, Nicholson W L, Turner R T. Borrelia burgdorferi in ticks (Acari:Ixodidae) from coastal Virginia. J Med Entomol. 1991;28:668–674. doi: 10.1093/jmedent/28.5.668. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Masuzawa T, Takada N, Ishiguro F, Fujita H, Iwaki A, Wang H, Wang J, Kawabata M, Yanagihara Y. Lyme disease Borrelia species in northeastern China resemble those isolated from far eastern Russia and Japan. Appl Environ Microbiol. 1998;64:2705–2709. doi: 10.1128/aem.64.7.2705-2709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marconi R T, Liveris D, Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analysis of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J Clin Microbiol. 1995;33:2427–2434. doi: 10.1128/jcm.33.9.2427-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuzawa T, Iwaki A, Sato Y, Miyamoto K, Korenberg E I, Yanagihara Y. Genetic diversity of Borrelia burgdorferi sensu lato isolated in far eastern part of Russia. Microbiol Immunol. 1997;41:595–600. doi: 10.1111/j.1348-0421.1997.tb01897.x. [DOI] [PubMed] [Google Scholar]
- 17.Masuzawa T, Fukui T, Miyake M, Oh H B, Cho M K, Chang W H, Imai Y, Yanagihara Y. Determination of members of a Borrelia afzelii-related group isolated from Ixodes nipponensis in Korea as Borrelia valaisiana. Int J Syst Bacteriol. 1999;49:1409–1415. doi: 10.1099/00207713-49-4-1409. [DOI] [PubMed] [Google Scholar]
- 18.Masuzawa T, Komikado T, Iwaki A, Suzuki H, Kaneda K, Yanagihara Y. Characterization of Borrelia sp. isolated from Ixodes tanuki, I. turdus, and I. columnae in Japan by restriction fragment length polymorphism of rrf(5S)-rrl(23S) intergenic spacer amplicon. FEMS Microbiol Lett. 1996;142:77–83. doi: 10.1111/j.1574-6968.1996.tb08411.x. [DOI] [PubMed] [Google Scholar]
- 19.Matuschka F R, Fischer P, Heiler M, Richter D R, Spielman A. Capacity of European animals as reservoir hosts for the Lyme disease spirochete. J Infect Dis. 1992;165:479–483. doi: 10.1093/infdis/165.3.479. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto K, Sato Y, Okada K, Fukunaga M, Sato F. Competence of migratory bird, red-bellied thrush (Turdus chrysolaus), as an avian reservoir for the Lyme disease spirochetes in Japan. Acta Trop. 1997;65:43–51. doi: 10.1016/s0001-706x(97)00651-7. [DOI] [PubMed] [Google Scholar]
- 21.Mount G A, Haile D G, Daniels E. Simulation of blacklegged tick (Acari:Ixodidae) population dynamics and transmission of Borrelia burgdorferi. J Med Entomol. 1997;34:461–484. doi: 10.1093/jmedent/34.4.461. [DOI] [PubMed] [Google Scholar]
- 22.Nakao M, Miyamoto K. Negative finding in detection of transovarial transmission of Borrelia burgdorferi in Japanese ixodid ticks, Ixodes persulcatus and Ixodes ovatus. Jpn J Sanit Zool. 1992;43:343–345. [Google Scholar]
- 23.Nakao M, Miyamoto K. Susceptibility of Ixodes persulcatus and I. ovatus (Acari:Ixodidae) to Lyme disease spirochetes isolated from humans in Japan. J Med Entomol. 1994;31:467–473. doi: 10.1093/jmedent/31.3.467. [DOI] [PubMed] [Google Scholar]
- 24.Nakao M, Miyamoto K, Fukunaga M. Lyme disease spirochetes in Japan: enzootic transmission cycles in birds, rodents, and Ixodes persulcatus ticks. J Infect Dis. 1994;170:878–882. doi: 10.1093/infdis/170.4.878. [DOI] [PubMed] [Google Scholar]
- 25.Nakao M, Miyamoto K, Fukunaga M, Hashimoto Y, Takahashi H. Comparative studies on Borrelia afzelii isolated from a patient of Lyme disease, Ixodes persulcatus ticks, and Apodemus speciosus rodents in Japan. Microbiol Immunol. 1994;38:413–420. doi: 10.1111/j.1348-0421.1994.tb01801.x. [DOI] [PubMed] [Google Scholar]
- 26.Nicholls T H, Callister S M. Lyme disease spirochetes in ticks collected from birds in midwestern United States. J Med Entomol. 1996;33:379–384. doi: 10.1093/jmedent/33.3.379. [DOI] [PubMed] [Google Scholar]
- 27.Olsen B, Duffy D C, Jaenson T G T, Gylfe A, Bonnedahl J, Bergstrom S. Transhemispheric exchange of Lyme disease spirochetes by seabirds. J Clin Microbiol. 1995;33:3270–3274. doi: 10.1128/jcm.33.12.3270-3274.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen B, Jaenson T G T, Bergstrom S. Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl Environ Microbiol. 1995;61:3082–3087. doi: 10.1128/aem.61.8.3082-3087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Postic D, Assous M V, Grimont P A D, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf(5S)-rrl(23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 30.Rand P W, Lacombe E H, Smith R P, Jr, Ficker J. Participation of birds (aves) in the emergence of Lyme disease in southern Maine. J Med Entomol. 1998;35:270–276. doi: 10.1093/jmedent/35.3.270. [DOI] [PubMed] [Google Scholar]
- 31.Randolph S E, Craine N G. General framework for comparative quantitative studies on transmission of tick-borne diseases using Lyme borreliosis in Europe as an example. J Med Entomol. 1995;34:765–777. doi: 10.1093/jmedent/32.6.765. [DOI] [PubMed] [Google Scholar]
- 32.Sanders F H, Jr, Oliver J H., Jr Evaluation of Ixodes scapularis, Amblyomma americanum, and Dermacentor variabilis (Acari:Ixodidae) from Georgia as vectors of a Florida strain of the Lyme disease spirochete, Borrelia burgdorferi. J Med Entomol. 1995;32:402–406. doi: 10.1093/jmedent/32.4.402. [DOI] [PubMed] [Google Scholar]
- 33.Takada N. A pictorial review of medical acarology in Japan. Kyoto, Japan: Kinpodo; 1990. pp. 108–111. . (In Japanese.) [Google Scholar]
- 34.Takada N, Ishiguro F, Fujita H, Wang H, Wang J, Masuzawa T. Lyme disease spirochetes in ticks from northeastern China. J Parasitol. 1998;84:499–504. [PubMed] [Google Scholar]
- 35.Wanchun T, Zhikun Z, Moldenhauer S, Yixiu G, Qiuli Y, Lina W, Mei C. Detection of Borrelia burgdorferi from ticks (Acari) in Hebei Province, China. J Med Entomol. 1998;35:95–98. doi: 10.1093/jmedent/35.2.95. [DOI] [PubMed] [Google Scholar]