Abstract
This study investigated the biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons (PAHs) in liquid media and soil by bacteria (Stenotrophomonas maltophilia VUN 10,010 and bacterial consortium VUN 10,009) and a fungus (Penicillium janthinellum VUO 10,201) that were isolated from separate creosote- and manufactured-gas plant-contaminated soils. The bacteria could use pyrene as their sole carbon and energy source in a basal salts medium (BSM) and mineralized significant amounts of benzo[a]pyrene cometabolically when pyrene was also present in BSM. P. janthinellum VUO 10,201 could not utilize any high-molecular-weight PAH as sole carbon and energy source but could partially degrade these if cultured in a nutrient broth. Although small amounts of chrysene, benz[a]anthracene, benzo[a]pyrene, and dibenz[a,h]anthracene were degraded by axenic cultures of these isolates in BSM containing a single PAH, such conditions did not support significant microbial growth or PAH mineralization. However, significant degradation of, and microbial growth on, pyrene, chrysene, benz[a]anthracene, benzo[a]pyrene, and dibenz[a,h]anthracene, each as a single PAH in BSM, occurred when P. janthinellum VUO 10,201 and either bacterial consortium VUN 10,009 or S. maltophilia VUN 10,010 were combined in the one culture, i.e., fungal-bacterial cocultures: 25% of the benzo[a]pyrene was mineralized to CO2 by these cocultures over 49 days, accompanied by transient accumulation and disappearance of intermediates detected by high-pressure liquid chromatography. Inoculation of fungal-bacterial cocultures into PAH-contaminated soil resulted in significantly improved degradation of high-molecular-weight PAHs, benzo[a]pyrene mineralization (53% of added [14C]benzo[a]pyrene was recovered as 14CO2 in 100 days), and reduction in the mutagenicity of organic soil extracts, compared with the indigenous microbes and soil amended with only axenic inocula.
Polycyclic aromatic hydrocarbons (PAHs) occur in various ecosystems and are priority pollutants due to their potential toxicity, mutagenicity, and carcinogenicity (26). Low-molecular-weight PAHs (containing less than four benzene rings) are acutely toxic, with some having effects on the reproduction and mortality rates of aquatic animals, and most high-molecular-weight PAHs (containing four or more benzene rings) are mutagenic and carcinogenic. Due to their hydrophobic nature, most PAHs in aquatic and terrestrial ecosystems bind to particulates in soil and sediments, rendering them less available for biological uptake, and they also bioaccumulate in food chains (32).
Microbial degradation represents the major route responsible for the ecological recovery of PAH-contaminated sites (11); however, the success of bioremediation projects has been limited by the failure to remove high-molecular-weight PAHs (44). The recalcitrance of high-molecular-weight PAHs to microbial degradation has led to research focused on evaluating a wide phylogenetic spectrum of microorganisms for their degradative ability. This has resulted in the identification of a diverse group of bacteria and fungi that partially degrade, cometabolically oxidize, or mineralize some high-molecular-weight PAHs to detoxified products. Reports to date indicate that the highest-molecular-weight PAHs that are mineralized as sole carbon and energy sources by bacteria contain four benzene rings, such as pyrene and chrysene. The species involved include Rhodococcus sp. (42), Burkholderia cepacia (22, 23), Stenotrophomonas maltophilia (6), Mycobacterium sp. (7, 21, 25), Alcaligenes denitrificans (43), and Sphingomonas paucimobilis (33, 46). Many of these strains are also able to degrade five-benzene-ring PAHs partially, forming oxidized products. In contrast to bacteria, fungi generally do not utilize PAHs as their sole carbon and energy source but transform these compounds cometabolically to detoxified metabolites (39). The most extensive studies have focused on white rot fungi such as Phanerochaete chrysosporium (2, 8, 9), Pleurotus ostreatus (3, 41), and Trametes versicolor (1, 15, 41). These fungi are able to degrade some five-benzene-ring PAHs and detoxify PAH-polluted soils and sediments due to the production of extracellular lignin-degrading enzymes. Nonlignolytic fungi, such as Cunninghamella elegans, Penicillium janthinellum, and Syncephalastrum sp., can transform a variety of PAHs, including pyrene, chrysene, and benzo[a]pyrene, to polar metabolites (27, 29, 35, 45). However, reports on the mineralization of five-benzene-ring PAHs by pure microbial cultures are few. There is only one report that describes benzo[a]pyrene mineralization by bacteria (46). In this case, benzo[a]pyrene was mineralized by a resting-cell suspension of S. paucimobilis, but this strain could not grow on benzo[a]pyrene as a sole carbon and energy source. Phanerochaete spp. are the only fungus species reported to mineralize benzo[a]pyrene, and this occurred cometabolically (2, 4, 10, 37). The failure to isolate a single microorganism capable of growing on and mineralizing PAHs with five or more benzene rings suggests that mineralization of these compounds in nature depends largely upon the cooperative metabolic activities of mixed microbial populations.
For use in bioremediation, PAH degraders should ideally mineralize and grow on PAHs as sole carbon and energy source. This is important for minimizing the production of toxic, water-soluble degradation by-products and reducing the risk of isolates failing to survive at contaminated sites due to the lack of suitable growth substrates. Our previous work has focused on isolating bacterial strains from separate local PAH-contaminated sites, with an emphasis on selecting strains capable of growing on individual compounds with four or more benzene rings (6, 22, 23). From one of these sites, we noted that degradation of five-ring PAHs as sole carbon sources in basal salts medium (BSM) occurred only when a bacterial consortium grew alongside a fungal strain, and when they were separated, growth did not occur for either the fungus or the consortium. This present study evaluated the metabolic profile of the fungal isolate and the consortium when grown alone or as a coculture. Furthermore, it reports the mineralization of benzo[a]pyrene as sole carbon and energy source for this mixed population and for a defined coculture composed of the fungus plus a single strain of S. maltophilia (6), using liquid cultures and contaminated soils.
MATERIALS AND METHODS
Chemicals, media, and soils.
Fluorene was purchased from Aldrich Chemical Co. (Milwaukee, Wis.); phenanthrene, fluoranthene, pyrene, chrysene, benz[a]anthracene, benzo[a]pyrene, dibenz[a,h]anthracene [4,5,9,10-14C]pyrene (58.7 mCi/mmol; radiochemical purity, >98%), and [7-14C]benzo[a]pyrene (26.6 mCi/mmol; radiochemical purity, >98%) were purchased from Sigma Chemical Company (St. Louis, Mo.). All PAHs were high-purity grade. Dichloromethane (DCM), N′,N′-dimethylformamide, methanol, and other solvents and chemicals, except where specified, were obtained in analytical grade from BDH Laboratory Supplies (Poole, England). Bacteriological media, including nutrient agar, nutrient broth, yeast extract, and agar, were purchased from Oxoid (Unipath Ltd., Basingstoke, Hampshire, England). Potato dextrose agar (PDA) and malt extract were obtained from Difco Laboratories (Detroit, Mich.). The composition of the BSM has been described previously (22). Basal salt-glucose medium (BSM-glucose) contained 1% glucose. MYPD broth contained 0.3% malt extract, 0.3% yeast extract, 0.5% peptone, and 1% dextrose (pH 6.0). Soil from an uncontaminated local site with no history of hydrocarbon contamination consisted of 93% sand, 11.6% clay, and less than 0.5% silt. Soil from a PAH-contaminated site in Sydney contained high levels of C10 to C14 (350 ppm), C15 to C28 (6,700 ppm), and C29 to C36 (1,300 ppm) long-chain hydrocarbons; lead (570 ppm); zinc (260 ppm); and the following PAHs: naphthalene (186 ppm), acenaphthylene (43 ppm), fluorene (87 ppm), phenanthrene (156 ppm), anthracene (53 ppm), fluoranthene (137 ppm), pyrene (99 ppm), benz[a]anthracene (33 ppm), benzo[a]pyrene (15 ppm), and dibenz[a,h]anthracene (12 ppm).
Microorganisms.
P. janthinellum VUO 10,201 and bacterial consortium VUN 10,009 were isolated in this study from soil collected from a site in Warracknabeal, Victoria, Australia, that was believed to be contaminated with creosote. S. maltophilia VUN 10,010 was previously isolated from soil collected from a disused manufactured-gas plant in Port Melbourne (6).
Use of PAHs in BSM and soil.
All PAH stock solutions were prepared in N′,N′-dimethylformamide. When used as single PAHs in BSM, the final concentrations were 250 mg of pyrene or 50 mg of benz[a]anthracene, chrysene, benzo[a]pyrene, and dibenz[a,h]anthracene liter−1. For PAH mixtures, the final concentrations of PAHs were as follows (milligrams per liter): fluorene, 100; phenanthrene and pyrene, 250; and fluoranthrene, benz[a]anthracene, chrysene, benzo[a]pyrene, and dibenz[a,h]anthracene, 10. Uncontaminated soil was spiked to obtain the following final concentrations of PAHs (milligrams per kilogram of soil): fluorene, 100; phenanthrene and pyrene, 250; and fluoranthrene, benz[a]anthracene, chrysene, benzo[a]pyrene, and dibenz[a,h]anthracene, 50.
Enrichment, isolation, and identification of PAH-degrading microorganisms.
For the Warracknabeal site, 20 g (wet weight) of soil was firstly shaken overnight in 100 ml of Ringer's solution (BDH Laboratory Supplies) at 30°C and 175 rpm, and then 5 ml of the supernatant was used to inoculate BSM (45 ml) containing 50 mg of benzo[a]pyrene liter−1. When growth was visible, enrichment was continued by serially subculturing several times in the same medium, using a 10% inoculum from the previous culture.
Initial attempts to separate the resulting fungal-bacterial consortium involved subculture into fresh BSM containing cycloheximide (0.1 g liter−1) plus benzo[a]pyrene and inoculation with the enriched culture (0.1% [vol/vol]). However, bacterial growth did not occur. Subsequently, BSM-pyrene (100 mg liter−1)-cycloheximide was used as described above in successive transfers, the absence of fungi being confirmed by plating onto PDA after every transfer. The resulting bacterial consortium was named VUN 10,009, and this was maintained by subculture in BSM-pyrene broth. To isolate fungi, the benzo[a]pyrene-enriched culture was diluted 10-fold, and 0.1-ml samples were spread onto PDA plates supplemented with penicillin G (60 mg ml−1), streptomycin sulfate (100 μg ml−1), and benzo[a]pyrene (50 μg ml−1). After various incubation periods (up to several weeks) at room temperature, fungal colonies were selected and replated on the same medium without antibiotics until pure colonies were obtained. As all single fungal colonies had similar macroscopic characteristics, a representative colony was selected for storage. P. janthinellum VUO 10,201 was identified by Anne Lawrie, Royal Melbourne Institute of Technology University.
Preparation of bacterial and fungal inocula.
Bacterial inocula were grown at 30°C and 175 rpm in BSM (500 ml in 1-liter flasks) supplemented with pyrene (250 mg liter−1) until growth reached late exponential phase, or in 10-liter cultures in a 15-liter fermentor. Cells were harvested by centrifugation, washed twice with sterile BSM, and concentrated in an appropriate volume of BSM, and then this suspension was used as inoculum. Killed bacterial inocula were prepared by adding HgCl2 (0.7 g liter−1). Fungal inocula were prepared by growing P. janthinellum VUO 10,201 on PDA plates at 30°C for 7 days. Spores were harvested into 25 ml of MYPD broth, and 10 ml of the suspension was used to inoculate 250 ml of MYPD. After 48 h at 30°C and 175 rpm, the small mycelial pellets were collected by filtration through Whatman no. 1 paper and washed twice with sterile BSM, and these suspensions were used as inocula in subsequent experiments. Killed fungal inocula were prepared by adding HgCl2 (0.7 g liter−1) to 7-day MYPD cultures.
PAH degradation in liquid cultures.
Growth media were prepared by adding 0.1 ml of single or mixed PAH stocks to 10 ml of broth in 30-ml reaction vials. BSM-PAH was inoculated with either bacteria to provide an initial cell population of approximately 104 cells ml−1 (ca. 1 ml of inoculum) or mycelial pellets to give an initial fungal biomass of 0.025 g (wet weight) ml−1; cocultures were inoculated similarly with both types of organisms. Cometabolic PAH degradation by P. janthinellum was investigated using BSM-glucose or MYPD broths supplemented with single PAHs. Cometabolic studies with bacteria were performed with the respective PAH in BSM supplemented with pyrene (250 mg liter−1), inoculated as described above. All cultures were aseptically flushed for 5 s with filtered (0.22-μm pore size) air (0.2 liters min−1) at inoculation and then every 7 days. Abiotic controls were sterile medium containing only PAH(s). Killed-cell controls contained the appropriate PAHs and base media plus HgCl2-killed cells to achieve a bacterial population of approximately 106 cells ml−1 and a fungal biomass of 0.04 g (wet weight) ml−1. The experimental cultures were conducted in triplicate, and all controls were in duplicate, with incubation in the dark at 30°C and 175 rpm. Replicate samples were sacrificed periodically, and the entire 10 ml of each was used for analysis of biomass and PAH concentration, with means and standard deviations calculated for sets of replicates.
PAH degradation in soil.
Sterile uncontaminated soil was artificially contaminated by adding a defined PAH mixture, prepared in DCM, to a sterile jar, allowing the solvent to evaporate, and then adding soil to the jar. After thorough mixing, the homogeneity of distribution was confirmed by testing the PAH concentration in five random samples of the soil: the standard deviation between samples following extraction and recovery was <1.5%. After subdivision of the soil into 200-g (dry weight) lots in 1.5-liter jars, these were inoculated to give an initial bacterial population of 106 cells g of soil−1 or 25 g (wet weight) of fungal biomass kg of soil−1 for both axenic cultures and cocultures. HgCl2-killed cell controls were set up similarly, and abiotic soil controls contained PAHs but lacked inocula. All soil cultures were supplemented with sterile BSM solution to approximately 65% of the soil's water-holding capacity and were incubated at 25°C in the dark. Triplicate samples of 1 g of soil from each jar were collected periodically for analysis of PAH concentration and measurement of the microbial population.
Biomass determinations.
Most probable number (MPN) estimates for bacteria were made in 96-well microtiter plates. Liquid culture samples were 10-fold serially diluted in 0.1% peptone-water, and 1 g of soil was suspended in Ringer's solution (9 ml) and then mixed by vortexing. After soil particles had settled, 1 ml of supernatant was removed and 10-fold serially diluted. A volume (100 μl) from each dilution was inoculated into each of three or five replicate wells containing 100 μl of double-strength nutrient broth. All cultures were incubated at 30°C for 2 to 7 days, and turbidity arising from growth was scored relative to controls (uninoculated medium and peptone-water-inoculated medium). MPN of bacteria was estimated from the appropriate MPN table (14). To determine fungal biomass, broth cultures were filtered using Whatman no. 1 paper, and mycelia were washed with 200 ml of deionized water and then dried to constant weight at 105°C. Dry weights were corrected for organic and inorganic components in the medium by subtracting measurements made for filtered uninoculated medium controls.
PAH mineralization.
PAH mineralization was measured by quantifying 14CO2 evolution in duplicate biometer flasks (250 ml). [14C]benzo[a]pyrene (1 μl; specific radioactivity of 1 mCi ml−1) and unlabeled benzo[a]pyrene (50 mg liter−1) or PAH mixture were added to sterile BSM (100 ml) in flasks, and the 50-ml side arm contained 0.5 M NaOH (5 ml) to trap CO2. The side arm was sealed with a rubber stopper pierced by a 15-gauge needle (15 cm long) that was used to withdraw NaOH samples periodically for measuring 14CO2 production. Cultures were either prepared using single cultures or set up as cocultures inoculated with ca. 104 cells of pyrene-grown bacteria ml−1 or 0.025 g (wet weight) of 2-day-old fungal pellets ml−1, and then the biometers were sealed with a rubber stopper and incubated in the dark at 30°C and 175 rpm. Abiotic medium and HgCl2-killed cell cultures served as controls. For [14C]benzo[a]pyrene mineralization in soils, 100 g of PAH-spiked soil or PAH-contaminated soil from Sydney was mixed thoroughly with [14C]benzo[a]pyrene in the biometer flasks. This soil was inoculated with either axenic cultures or cocultures as described for uncontaminated soil, and the moisture was adjusted to approximately 65% of the water-holding capacity of the soil. All flasks were incubated at 25°C in the dark. Periodically, the NaOH solution was collected from the side arms for 14CO2 analysis, and this was replaced with fresh NaOH. Before the final samples were taken, a few drops of concentrated H2SO4 solution were added to the cultures to release dissolved 14CO2.
Mass balance analysis on liquid cultures.
Mass balance determinations for [14C]benzo[a]pyrene were quantified as the percentages of radioactivity recovered in alkali solution, biomass, or DCM-extractable fractions relative to the amount of radiolabel added initially. 14CO2 was measured as described by Fedorak et al. (17) using a liquid scintillation counter (Wallac 1410; Pharmacia). Percent conversion of radiolabeled PAH to bacterial biomass was determined by pelleting cells followed by resuspension in water. To remove PAHs adsorbed to the biomass, the resuspended cells were extracted with DCM using vigorous shaking, and radioactivity in the organic extract and resuspended cells was measured. The cell-free supernatant obtained after the initial pelleting of bacterial cells was quantified to determine the percentage of PAH converted into water-soluble products. This aqueous phase was also extracted with DCM to determine the amount of organic soluble 14C-products present. Fungal cultures were sonicated (microtip; Branson Sonifier 450; duty cycle of 50%) for 3 min using 30-s bursts. The sonicated material was then extracted three times each with 10 ml of DCM, and these extracts were pooled. The residual mycelial debris was separated from the aqueous fraction by filtration through glass wool, and the radioactivity was measured in 1-ml samples of the DCM extract, the aqueous fractions, and the entire mycelial fraction. For soil samples, only 14CO2 evolution was determined as described above relative to the initial radiolabel added.
Mutagenicity assays.
Mutagenicity was assayed using Salmonella enterica serovar Typhimurium strain TA100 as described by Maron and Ames (30), and mutagenicity assays were performed on the PAH compounds and crude DCM extracts of metabolites formed during PAH degradation. All test substances were exchanged into dimethyl sulfide (DMSO). To determine the direct mutagenicity activity, 0.1 ml of the test substance was mixed with 0.1 ml of an overnight culture of strain TA100 in 2.5 ml of molten top agar (50 μM l-histidine, 50 μM d-biotin, 0.5% NaCl, 0.6% agar) and poured onto minimal glucose plates (Vogel-Bonner medium E containing 2.0% glucose and 1.5% agar). Assays were also performed in the presence of hepatic postmitochondrial supernatant (S9) (Moltox; Boone, N.C.) by adding 0.5 ml of an S9 mixture (0.2 ml of S9, 1.0 ml of MgCl2-KCl salt, 0.25 ml of 1 M glucose-6-phosphate, 2.0 ml of 0.1 M NADP, 25 ml of 0.2 M sodium phosphate buffer [pH 7.4], adjusted to a total volume of 50 ml) to 2 ml of molten top agar in addition to 0.1 ml of test substance plus 0.1 ml of TA100 culture (30). Controls included plates prepared without a test substance, DMSO alone, and a positive control with a known mutagen (aflatoxin) added. All assays were performed in triplicate. Revertant colonies were counted after 48 h of incubation at 37°C.
PAH extraction and analytical procedures.
The efficiency of PAH extraction was evaluated by comparing the amount of PAH and 2,3-benzo[b]fluorene internal standard recovered following DCM extraction of known amounts of PAH added to liquid medium or spiked soil, with tests performed in the presence and absence of killed microbial inocula. Sequential DCM extractions using three successive repeats were conducted, and the PAH concentrations were measured after each extraction.
Samples of bacterial cultures were vigorously shaken for 20 s with an equal volume of DCM (typically 1 ml) after adding 2,3-benzo[b]fluorene as an internal standard (100 μl of a stock solution containing 1 mg ml−1 in DCM). To separate the emulsion, the mixture was held at room temperature for 1 to 2 h before freezing overnight at −20°C. The organic phase was collected for analysis by gas chromatography. Fungal cultures were placed on ice and sequentially extracted with DCM using ultrasonication, applying conditions described above for measuring radioactivity. The emulsions were separated from the mycelial debris by filtration through glass wool, and the mycelia were extracted twice more. The emulsions were pooled, the phases were allowed to separate, and then the DCM phase was collected and dried over anhydrous Na2SO4. The DCM extract was evaporated to approximately 1 ml. Soil samples (1 g) were mixed thoroughly with an equal amount of anhydrous Na2SO4 and then extracted by sequential ultrasonication as described for fungal cultures.
Gas chromatography and high-pressure liquid chromatography (HPLC) analysis.
PAH concentrations in the DCM extracts were measured on a Varian Star 3400 gas chromatograph, equipped with a flame ionization detector, using a BPX-5 capillary column (25 m by 0.22 mm) and operated as described previously (6). The peak areas of both internal standard and PAH were used to calculate peak area ratios. Ratios obtained for each sample as well as controls were compared to those of PAH standards. For the biodegradation experiments, the standard curves were linear in the concentration range of 0.5 to 250 mg liter−1 for pyrene and 0.5 to 50 mg liter−1 for chrysene, benz[a]anthracene, benzo[a]pyrene, and dibenz[a,h]anthracene.
Triplicate samples from biodegradation experiments were used for the extraction of PAH degradation products. The contents from each culture vial (10 ml) were transferred to separating funnels (100 ml) and extracted twice with an equal volume of DCM. Cultures containing a fungal inoculum were sonicated as described above before extraction. The pH was adjusted to 2.5 with concentrated HCl, and cultures were extracted twice again with an equal volume of DCM. The organic extracts were pooled and dried over anhydrous Na2SO4. The DCM phase was evaporated to partial dryness with a rotary evaporator and then dried completely using nitrogen gas. Dried samples were dissolved in methanol (200 μl) for HPLC analysis. Reverse-phase HPLC was performed on a Varian Star liquid chromatograph system comprising a 9012 solvent delivery system, a 9100 autosampler, and a 9050 variable-wavelength UV-visible light detector; this system was controlled by Varian Star chromatographic software (version 4.01). Methanol-dissolved extracts of tests and controls were appropriately diluted and injected (70 μl) onto a Spherex 5 C18 column (250 by 4.6 mm [inside diameter]; Phenomenex, Torrance, Calif.). The solvent consisted of water and methanol using the following gradient: 0 min, 50:50; 0 to 30 min, ramp to 0:100; 30 to 50 min, isocratic at 0:100. The flow rate was 0.7 ml min−1. Compounds in the eluate were detected at 254 nm.
RESULTS
Efficiency of the PAH-extraction methods.
Various methods of DCM extraction of PAHs and the internal standard from microbial cultures were tested to maximize the recovery of these compounds. For bacterial cultures in BSM, more than 99% of added PAHs were recovered following the first extraction by using a simple, but vigorous, shaking procedure. However, this technique was inappropriate for fungal cultures, since PAH recovery was adversely affected by adsorption of the PAHs to the fungal mycelia. Three sequential extractions, combining ultrasonication and vigorous shaking, were required for the fungal cultures, and cocultures, to recover more than 97% of the added PAHs. This sequential ultrasonication procedure was also suitable for extracting PAHs from PAH-spiked soil, where recovery was greater than 98% and comparable to the efficiency seen with standard Soxhlet extraction methods (data not shown). Results obtained from the sequential ultrasonication extraction method were reproducible with no significant differences between replicates according to variance analysis at 95% confidence level.
Enrichment, isolation, and identification of PAH-degrading microorganisms.
A mixed microbial population was obtained from soil from a contaminated site in Warracknabeal when the soil cultures were enriched by using benzo[a]pyrene as a sole source of carbon and energy in BSM. Each successive transfer of the enrichment culture was found to contain both bacteria and fungi. A pure fungal isolate, identified as P. janthinellum based on macroscopic and microscopic characteristics (A. Lawrie [Royal Melbourne Institute of Technology University], personal communication), was obtained from this enriched culture and assigned a Victoria University of Technology culture collection number of VUO 10,201. By inhibition of fungal growth with cycloheximide, a consortium of bacteria which grew on pyrene as a sole carbon and energy source was isolated; we noted that attempts to isolate the bacteria on benzo[a]pyrene as a sole carbon and energy source failed. This bacterial population was designated bacterial consortium VUN 10,009. The identity of S. maltophilia VUN 10,010, isolated previously from the Port Melbourne site (6), was confirmed by 16S ribosomal DNA sequence analysis.
Degradation of high-molecular-weight PAHs by bacterial and fungal isolates.
The bacterial consortium VUN 10,009 and S. maltophilia VUN 10,010 were able to degrade PAHs containing up to five benzene rings as the sole carbon source in BSM (Table 1). However, the degradation of chrysene, benz[a]anthracene, benzo[a]pyrene, and dibenz[a,h]anthracene was very slow, only 6 to 12% of these compounds was removed over 56 days, and there was no significant growth of either S. maltophilia VUN 10,010 or bacterial consortium VUN 10,009. PAH disappearance was interpreted as biodegradation, since the decrease in PAH concentration exceeded the amount that disappeared from the abiotic and HgCl2-killed cell controls, and this is accounted for in the data presented in Table 1.
TABLE 1.
Degradation of high-molecular-weight PAHs by fungal and bacterial isolates in axenic cultures
| PAHb | PAH removal (%)a
|
||||||
|---|---|---|---|---|---|---|---|
| VUN 10,009
|
VUN 10,010
|
P. janthinellum VUO 10,201
|
|||||
| BSMc | BSM + PYRd | BSMc | BSM + PYRd | BSMc | BSM-glucosee | MYPDe | |
| Pyrene | 100.0 ± 0.0 | NT | 100.0 ± 0.0 | NT | 3.3 ± 0.3 | 80.3 ± 2.8 | 93.6 ± 2.8 |
| Chrysene | 8.2 ± 0.5 | 12.1 ± 0.4 | 9.1 ± 1.2 | 28.3 ± 1.2 | 12.2 ± 0.4 | 25.6 ± 1.9 | 31.7 ± 3.7 |
| Benz[a]anthracene | 7.8 ± 0.3 | 9.6 ± 0.7 | 8.2 ± 1.6 | 19.8 ± 0.3 | 9.1 ± 1.4 | 18.1 ± 2.3 | 23.1 ± 1.2 |
| Benzo[a]pyrene | 6.7 ± 0.9 | 23.3 ± 0.5 | 12.3 ± 1.0 | 32.2 ± 1.2 | 16.9 ± 0.2 | 56.5 ± 2.4 | 72.2 ± 3.2 |
| Dibenz[a,h]anthracene | 5.9 ± 0.5 | 13.8 ± 0.9 | 7.8 ± 0.6 | 26.7 ± 0.7 | 12.9 ± 0.3 | 24.4 ± 2.7 | 36.7 ± 1.7 |
Percent degradation was calculated relative to the amount of PAH supplied, accounting for disappearance in abiotic and HgCl2-killed controls; averages are from triplicate experiments. NT, not tested. Culture conditions are described in the text.
Initial PAH concentrations were 250 mg liter−1 for pyrene and 50 mg liter−1 for each of the PAHs chrysene, benz[a]anthracene, benzo[a]pyrene, and dibenz[a,h]anthracene. Inoculation and growth conditions are described in the text.
Data for 56-day cultures.
The medium was BSM with pyrene (PYR; 250 mg liter−1) plus either chrysene, benz[a]anthracene, benzo[a]pyrene, or dibenz[a,h]anthracene. Data are for degradation of the latter over 28 days.
Data for 28-day cultures.
A substantial improvement in the rate of PAH degradation by axenic bacterial cultures occurred when pyrene (250 mg liter−1) was added to the BSM-PAH cultures. Under these cometabolic conditions and in comparison to the single PAH-containing cultures, PAH degradation rates by bacterial consortium VUN 10,009 increased by 1.5- to 6-fold, and the lowest increase was observed for benz[a]anthracene while the highest increase occurred for benzo[a]pyrene (Table 1). For S. maltophilia VUN 10,010 under cometabolic conditions, the PAH degradation rate increases seen were in the range of 3.8- to 5.8-fold, the former increase being observed for benz[a]anthracene and the latter being observed for dibenz[a,h]anthracene. Compared to bacterial consortium VUN 10,009, S. maltophilia VUN 10,010 degraded twice as much chrysene, benz[a]anthracene, and dibenz[a,h]anthracene and 1.4-fold more benzo[a]pyrene under these cometabolic conditions when inoculated similarly. Axenic cultures of P. janthinellum VUO 10,201 degraded substantial amounts of the four- and five-benzene-ring PAHs in MYPD or BSM-glucose, but these PAHs were slowly degraded and failed to support growth when supplied as a sole carbon source in BSM.
PAH degradation by fungal-bacterial cocultures.
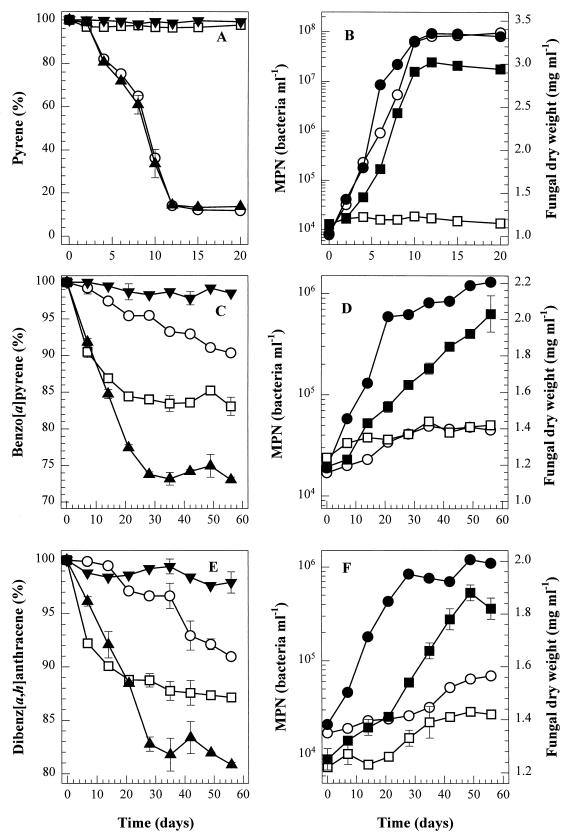
Although there was no substantial growth of bacterial consortium VUN 10,009 or P. janthinellum VUO 10,201 on benzo[a]pyrene when this was supplied as a sole carbon and energy source, the coenrichment of these cultures on benzo[a]pyrene in BSM suggested a mutual dependence under these conditions. Therefore, the growth of bacterial consortium VUN 10,009 and P. janthinellum VUO 10,201 was investigated in cocultures (designated coculture A) in BSM containing a single high-molecular-weight PAH as the sole source of carbon and energy. In coculture, P. janthinellum VUO 10,201 and bacterial consortium VUN 10,009 both grew on benzo[a]pyrene or dibenz[a,h]anthracene as a sole carbon and energy source (Fig. 1); only slight growth on these PAHs was observed in the parallel cultures of fungus or consortium alone. The bacterial population in coculture A increased by at least 2 logs (from 104 to 106 cells ml−1), and fungal dry weight increased by around 50 to 70% over a 56-day period. Coculture A degraded 27% of the benzo[a]pyrene and 19% of the dibenz[a,h]anthracene supplied in 56 days, which is significantly greater than the amounts degraded by individual cultures of bacterial consortium VUN 10,009 (9.7% of the benzo[a]pyrene and 9.2% of the dibenz[a,h]anthracene were degraded) or P. janthinellum VUO 10,201 (17% of the benzo[a]pyrene and 13% of the dibenz[a,h]anthracene were degraded). Rates of pyrene degradation by coculture A and the bacterial consortium VUN 10,009 culture were similar; however, P. janthinellum VUO 10,201 could grow on pyrene as a sole carbon source only in the presence of bacterial consortium VUN 10,009.
FIG. 1.
PAH degradation by P. janthinellum VUO 10,201 (□), bacterial consortium VUN 10,009 (○), or coculture A (▴). BSM contained 250 mg of pyrene (A and B), 50 mg of benzo[a]pyrene (C and D), or 50 mg of dibenz[a,h]anthracene (E and F) per liter. Test cultures were sampled in triplicate, and HgCl2-killed coculture A (▾) was sampled in duplicate, for measurement of bacterial numbers (axenic cultures [○] and coculture A [●]) and fungal dry weight (axenic cultures [□] and coculture A [■]).
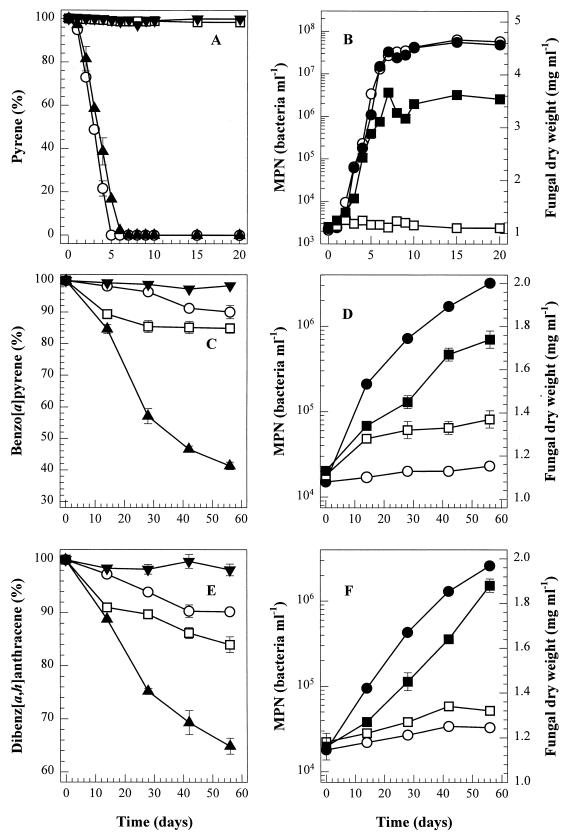
PAH degradation by fungal-bacterial coculture was further examined by combining P. janthinellum VUO 10,201 with S. maltophilia VUN 10,010 (designated coculture B). Preliminary experiments using dual cultures on PDA and nutrient agar plates indicated no apparent antagonism between P. janthinellum VUO 10,201 and S. maltophilia VUN 10,010 (data not shown). Coculture B and axenic cultures were inoculated into BSM containing a single high-molecular-weight PAH as the sole carbon and energy source. Under these conditions, P. janthinellum VUO 10,201 and S. maltophilia VUN 10,010 both grew on benzo[a]pyrene or dibenz[a,h]anthracene when these two organisms were coincubated (Fig. 2); there was no substantial microbial growth on these PAHs in the axenic cultures. The rate of five-benzene-ring PAH degradation by coculture B was higher than that observed with the axenic cultures and with coculture A. After 56 days, coculture B degraded 59% of the benzo[a]pyrene and 35% of the dibenz[a,h]anthracene. In BSM containing pyrene, the pyrene degradation rate (ca. 41.7 mg liter−1 day−1) and fungal biomass yield (3.6 mg [dry weight] ml−1) was greater with coculture B than with coculture A (ca. 18.3 mg liter−1 day−1; 3.1 mg [dry weight] ml−1). The improved pyrene degradation rate was probably due to the superior pyrene-degrading ability of S. maltophilia VUN 10,010 compared to that of bacterial consortium VUN 10,009, rather than the coculture per se.
FIG. 2.
PAH degradation by P. janthinellum VUO 10,201 (□), S. maltophilia VUN 10,010 (○), or coculture B (▴). BSM contained 250 mg of pyrene (A and B), 50 mg of benzo[a]pyrene (C and D), or 50 mg of dibenz[a,h]anthracene (E and F) per liter. Test cultures were sampled in triplicate, and HgCl2-killed coculture B (▾) was sampled in duplicate, for measurement of bacterial numbers (axenic cultures [○] and coculture B [●]) and fungal dry weight (axenic cultures [□] and coculture B [■]).
PAH degradation in soil by fungal-bacterial cocultures.
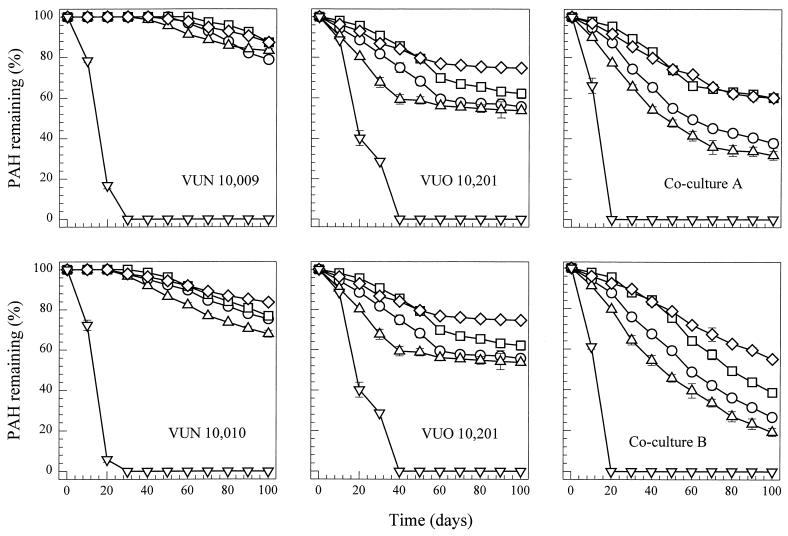
Degradation of a PAH mixture by cocultures and single cultures of P. janthinellum VUO 10,201, S. maltophilia VUN 10,010, and bacterial consortium VUN 10,009 was tested in unpolluted soil spiked with a PAH mixture. Pyrene was rapidly degraded in PAH-spiked soil inoculated with only S. maltophilia VUN 10,010 or bacterial consortium VUN 10,009, with all the pyrene (250 mg kg of soil−1) degraded in 20 to 30 days (Fig. 3). The rate of degradation of chrysene, benz[a]anthracene, benzo[a]pyrene, and dibenz[a,h]anthracene was low in soil inoculated with only bacterial consortium VUN 10,009 or S. maltophilia VUN 10,010. The amount of each of these PAHs degraded over 100 days was in the range of 12 to 22% (VUN 10,009) and 16 to 32% (VUN 10,010), and the lag periods before the onset of degradation were around 40 to 60 days (VUN 10,009) and 30 days (VUN 10,010). Conversely, there was no detectable lag period for soils inoculated with P. janthinellum VUO 10,201, and the PAH degradation rate was relatively high in the first 30 to 40 days compared to that for soils inoculated with only the bacteria. Chrysene, benz[a]anthracene, benzo[a]pyrene, and dibenz[a,h]anthracene degradation by P. janthinellum VUO 10,201 after 100 days was in the range of 24 to 48%; however, the degradation of these PAHs all but ceased after 60 days. Although fungal growth was not monitored in the soil cultures, viable fungal biomass was still present in the soil after 100 days (data not shown).
FIG. 3.
Degradation of pyrene (▿), benz[a]anthracene (□), chrysene (○), benzo[a]pyrene (▵), and dibenz[a,h]anthracene (◊) in PAH-spiked soil inoculated with either coculture A, coculture B, or axenic cultures of bacterial consortium VUN 10,009, S. maltophilia VUN 10,010, or P. janthinellum VUO 10,201. The soil was spiked with the following (milligrams per kilogram): fluorene, 100; phenanthrene and pyrene, 250; and fluoranthene, benz[a]anthracene, chrysene, benzo[a]pyrene, and dibenz[a,h]anthracene, 50. Data presented account for PAH disappearance in the HgCl2-killed controls, and all samples were assayed in triplicate.
PAH degradation substantially improved in the PAH-spiked soil when it was inoculated with either coculture A or coculture B. Chrysene, benz[a]anthracene, benzo[a]pyrene, and dibenz[a,h]anthracene degradation after 100 days was in the range of 40 to 68% (coculture A) and 44 to 80% (coculture B). Furthermore, there was no lag period before PAH degradation started, and this continued throughout the entire incubation period. Bacterial growth was similar (bacterial populations increased 100-fold) for soils inoculated either with only bacteria or when these were present as part of cocultures A and B (data not shown).
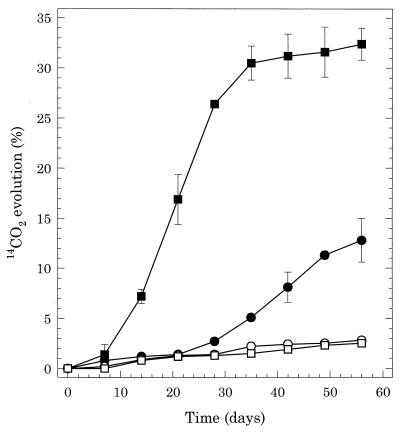
Benzo[a]pyrene mineralization.
S. maltophilia VUN 10,010 had been shown previously to mineralize pyrene as a sole carbon and energy source (6). Bacterial consortium VUN 10,009 was also found to mineralize pyrene (56% of added radiolabeled pyrene was recovered as 14CO2 in 20 days) as a sole carbon source in BSM (data not shown). Benzo[a]pyrene mineralization by S. maltophilia VUN 10,010 and bacterial consortium VUN 10,009 was investigated under cometabolic conditions by adding [14C]benzo[a]pyrene to BSM containing pyrene (250 mg liter−1) and benzo[a]pyrene (50 mg liter−1). Both S. maltophilia VUN 10,010 and bacterial consortium VUN 10,009 cometabolically mineralized benzo[a]pyrene in the presence of pyrene (Fig. 4). The rate of benzo[a]pyrene mineralization by S. maltophilia VUN 10,010 was greater than that of bacterial consortium VUN 10,009, and the amount of added radiolabel recovered as 14CO2 after 56 days was 32.4 and 12.8%, respectively. The remaining labeled carbon was found mainly in the DCM extract, most likely as undegraded benzo[a]pyrene and nonpolar degradation products (Table 2). [14C]benzo[a]pyrene was not mineralized when added to axenic P. janthinellum VUO 10,201 cultures comprising benzo[a]pyrene and pyrene in BSM (Table 2); benzo[a]pyrene in MYPD or BSM-glucose was not mineralized by P. janthinellum VUO 10,201 (data not shown). A high amount of 14C was recovered in the aqueous phase of P. janthinellum VUO 10,201 cultures. Cocultures A and B mineralized a greater amount of benzo[a]pyrene in BSM-benzo[a]pyrene containing added pyrene than did the axenic cultures, and unlike the axenic cultures, significant radioactivity was measured in the biomass fraction (Table 2).
FIG. 4.
Comineralization of [14C]benzo[a]pyrene in BSM containing pyrene (250 mg liter−1) and benzo[a]pyrene (50 mg liter−1) by either bacterial consortium VUN 10,009 (●) or S. maltophilia VUN 10,010 (■) and their HgCl2-killed controls (VUN 10,009 [○] and VUN 10,010 [□]). The degree of mineralization was calculated as the cumulative percentage of 14CO2 evolved relative to the amount of added label.
TABLE 2.
Distribution of 14C-residues after incubation of PAH-degrading isolates with [14C]benzo[a]pyrene in liquid cultures
| Medium and microorganism | % Recovery of added radiolabel from various culture fractionsa
|
||||
|---|---|---|---|---|---|
| 14CO2 | Biomass | Aqueous phase | DCM phase | Total recovery | |
| BSM containing benzo[a]pyrene (50 mg liter−1) and pyrene (250 mg liter−1) | |||||
| VUN 10,009b | 12.8 | 0.7 | 2.3 | 84.3 | 100.1 |
| VUN 10,010b | 32.4 | NA | 2.7 | 64.8 | 99.9 |
| VUO 10,201b | NA | NA | 14.5 | 84.1 | 98.6 |
| Coculture A | 37.2 | 7.3 | 1.1 | 53.7 | 99.3 |
| Coculture B | 58.1 | 8.1 | NA | 30.9 | 97.1 |
| BSM containing benzo[a]pyrene (50 mg liter−1) | |||||
| VUN 10,009b | NA | NA | 1.2 | 99.1 | 100.3 |
| VUN 10,010b | NA | NA | 1.6 | 98.5 | 100.1 |
| VUO 10,201b | NA | NA | 13.7 | 85.4 | 99.1 |
| Coculture A | 16.3 | 7.2 | 2.2 | 73.4 | 99.1 |
| Coculture B | 25.5 | 9.3 | 1.7 | 62.9 | 99.4 |
| BSM containing a PAH mixture | |||||
| VUN 10,009b | 8.2 | 1.2 | 2.1 | 87.7 | 99.2 |
| VUN 10,010b | 32.8 | 1.7 | 2.3 | 62.6 | 99.4 |
| VUO 10,201b | NA | NA | 13.2 | 85.7 | 98.9 |
| Coculture A | 35.7 | 6.9 | 1.4 | 55.3 | 99.3 |
| Coculture B | 53.0 | 8.5 | 0.6 | 35.8 | 97.9 |
Mean values of percentage of recovery for 56-day cultures. The standard deviation for recovery of added label was less than 5%. NA, not appreciable.
Axenic inocula were used.
Cocultures A and B also mineralized benzo[a]pyrene as a sole carbon and energy source in BSM. The amount of radioactivity recovered as 14CO2 was 16.3 and 25.5% after 56 days by coculture A and coculture B, respectively (Fig. 5A and B). Less than 3% of the 14C supplied was found in the aqueous phase, and the majority (approximately 63 to 74%) was recovered in the DCM extract (Table 2). A relatively high amount of 14C was measured in the coculture biomass, indicating its incorporation into cellular material. No significant 14CO2 evolution was detected from axenic cultures or the killed-cell controls containing benzo[a]pyrene as a sole carbon source. Coculture A and coculture B also mineralized substantial amounts of [14C]benzo[a]pyrene in BSM containing a PAH mixture (three to five benzene rings): the amount of added radiolabel recovered as 14CO2 was 35.7 and 53%, respectively, after 56 days (Fig. 5B and F); 8.2% (bacterial consortium VUN 10,009) and 32.8% (S. maltophilia VUN 10,010) of the radiolabel were recovered as 14CO2 over 56 days for the respective cultures containing the bacteria only. The remaining radioactivity in the cocultures was found mostly in the DCM extract and the biomass (Table 2).
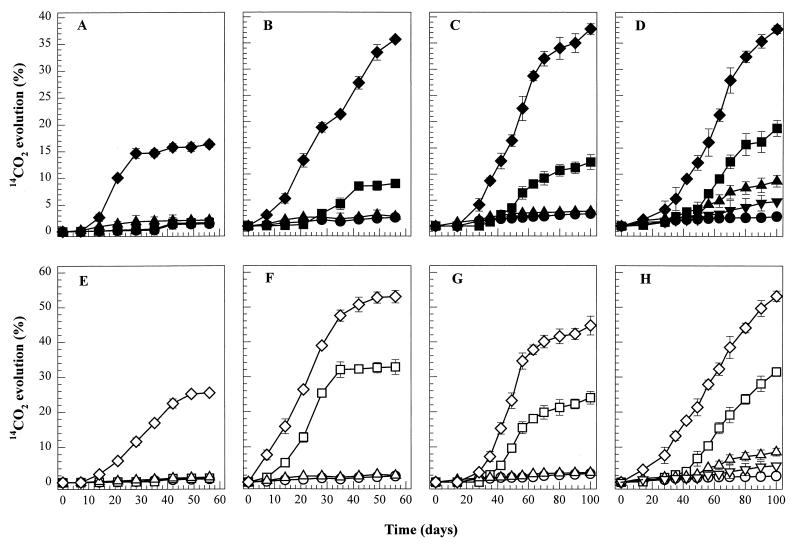
FIG. 5.
Benzo[a]pyrene mineralization in liquid and soil cultures. [14C]benzo[a]pyrene was added to BSM containing benzo[a]pyrene (50 mg liter−1) (A and E), BSM with a PAH mixture (B and F), PAH-spiked soil (C and G), and PAH-contaminated soil from Sydney (D and H). The upper panels show data for axenic cultures inoculated with either P. janthinellum VUO 10,201 (▴), bacterial consortium VUN 10,009 (■), or coculture A (⧫). The lower panels show data for axenic cultures of either VUO 10,201 (▵) or S. maltophilia VUN 10,010 (□) and coculture B (◊). Cumulative 14CO2 evolution (percentage relative to added label) is also shown for HgCl2-killed controls of cocultures A (●) and B (○) and for uninoculated, PAH-contaminated soil (▾ and ▿), which was presumably due to the indigenous microbial activity.
Benzo[a]pyrene mineralization by cocultures A and B was tested in PAH-spiked soil and PAH-contaminated soil from Sydney. For the PAH-spiked soil, no 14CO2 evolved during the first 14 days of incubation, but 37.7 and 44.7% of the added radioactivity were recovered over the next 86 days as 14CO2 from cocultures A and B, respectively (Fig. 5C and G). Benzo[a]pyrene mineralization by each coculture was greater than that seen for soil inoculated with only bacteria, where the amount of radiolabel recovered as 14CO2 was 12.3% (bacterial consortium VUN 10,009) and 24.1% (S. maltophilia VUN 10,010); no 14CO2 evolution was recorded for PAH-spiked soils inoculated with only P. janthinellum VUO 10,201 (Table 3) or for uninoculated soils (data not shown). Similar results were observed for PAH-contaminated soil: benzo[a]pyrene mineralization by the coculture was apparently unaffected by the more adverse soil environment (Fig. 5D and H). In fact, coculture B mineralized benzo[a]pyrene to a greater extent (53.2% of initial 14C recovered as 14CO2 after 100 days) and without an apparent lag period compared to its mineralization of benzo[a]pyrene in the PAH-spiked soil. This may be due to the indigenous microflora cooperatively mineralizing benzo[a]pyrene with the cocultures. Evidence of this is seen with the PAH-contaminated soil inoculated with axenic P. janthinellum VUO 10,201 inoculum in which 8.7% of the added radioactivity was recovered as 14CO2; this did not occur in PAH-spiked soil, which did not contain a measurable indigenous microbial population. The indigenous microbial population in uninoculated PAH-contaminated soil mineralized 4.8% of the added [14C]benzo[a]pyrene to 14CO2 over 100 days (Table 3).
TABLE 3.
Distribution of 14C-residues after incubation of PAH-degrading isolates with [14C]benzo[a]pyrene in soil cultures
| Soil and microorganism | % Recovery of added radiolabel from various culture fractionsa
|
||
|---|---|---|---|
| 14CO2 | DCM phase | Total recovery | |
| PAH-spiked soil | |||
| VUN 10,009b | 12.3 | 84.8 | 97.1 |
| VUN 10,010b | 24.1 | 73.9 | 98.0 |
| VUO 10,201b | NA | 97.7 | 97.7 |
| Coculture A | 37.7 | 61.4 | 99.1 |
| Coculture B | 44.7 | 53.6 | 98.3 |
| PAH-contaminated soil from Sydney | |||
| Indigenousc | 4.8 | 93.1 | 97.9 |
| VUN 10,009b | 18.8 | 80.6 | 99.4 |
| VUN 10,010b | 31.5 | 67.3 | 98.8 |
| VUO 10,201b | 8.7 | 89.1 | 97.8 |
| Coculture A | 37.7 | 60.8 | 98.5 |
| Coculture B | 53.2 | 44.2 | 97.4 |
Mean values of percentage of recovery for duplicate 100-day cultures. The standard deviation for recovery of added label was less than 5%. The amount of radiolabel in aqueous extracts and biomass fractions was not determined. NA, not appreciable.
Axenic inocula were used.
Uninoculated soil.
HPLC analysis.
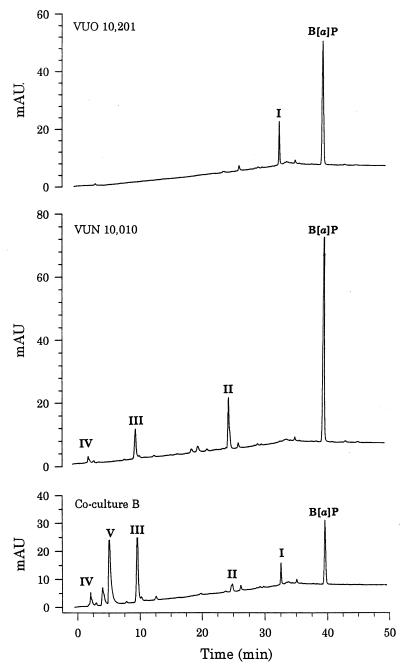
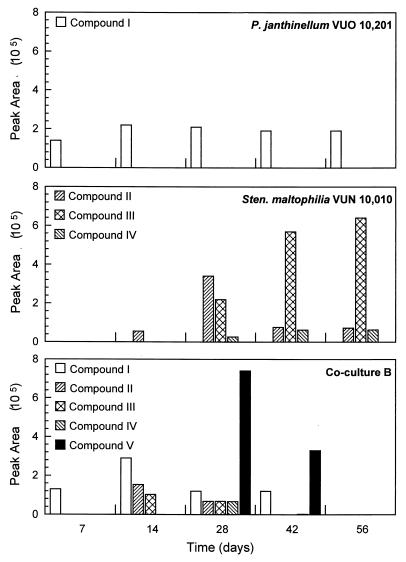
DCM extracts from the cocultures and axenic cultures, comprising BSM and benzo[a]pyrene as a sole carbon and energy source, were analyzed for PAH degradation products by HPLC. The axenic cultures and cocultures were inoculated to provide high initial biomass concentrations (ca. 2 × 106 cells ml−1 for S. maltophilia VUN 10,010 and 0.04 g [wet weight] ml−1 for P. janthinellum VUO 10,201) due to the lack of growth on benzo[a]pyrene in axenic cultures. One degradation compound (designated compound I) accumulated in axenic P. janthinellum VUO 10,201 cultures over the 56-day incubation (Fig. 6 and 7). In coculture B, compound I was the first peak to appear, but unlike axenic P. janthinellum VUO 10,201 cultures, this compound decreased and disappeared in the later stages of the incubation. Compound I was not present in axenic cultures of S. maltophilia VUN 10,010; however, three other degradation compounds (designated II, III, and IV) were detected (Fig. 6 and 7). Compound II was the first peak to appear in axenic S. maltophilia VUN 10,010 cultures, and its concentration initially increased but then decreased to a constant level during the remainder of the 56-day incubation. Compounds III and IV first appeared in the 28-day sample; their concentrations initially increased and were then relatively constant during the remainder of the 56-day incubation. Compounds II, III, and IV were also present in the 14- and 28-day samples of coculture B, but these compounds were degraded by the coculture to undetectable levels in the 56-day sample. A degradation compound (compound V) appeared in the coculture that was not detected in the axenic cultures. The concentration of compound V increased up to 28 days, then it decreased, and the compound was not detected in the 56-day sample. Only the benzo[a]pyrene peak was observed in HPLC profiles of abiotic and HgCl2-killed-cell control cultures (data not shown). Similar results were seen for bacterial consortium VUN 10,009 cultures and coculture A (data not shown).
FIG. 6.
Production of unidentified compounds by axenic cultures of P. janthinellum VUO 10,201 or S. maltophilia VUN 10,010 and by coculture B during incubation in BSM containing benzo[a]pyrene (50 mg liter−1). The HPLC profiles for DCM extracts of 28-day samples are shown, with unidentified compounds arbitrarily named I to V. mAU, milliabsorbance units.
FIG. 7.
Time course of compounds formed in axenic cultures of P. janthinellum VUO 10,201 or S. maltophilia VUN 10,010 and by coculture B during incubation in BSM containing benzo[a]pyrene (50 mg liter−1). The data represent peak areas of compounds detected by HPLC during degradation of benzo[a]pyrene as sole carbon and energy source, where compounds are numbered as described in the legend to Fig. 6.
Mutagenicity assessment.
The mutagenic potential of DCM extracts of cultures containing different PAHs was assessed following incubation with either a coculture or axenic cultures. Most of the axenic cultures failed to reduce the mutagenic potential significantly in BSM containing either benzo[a]pyrene or dibenz[a,h]anthracene as the sole carbon and energy source (Table 4). One exception was the axenic P. janthinellum VUO 10,201 culture, which reduced the mutagenicity of benzo[a]pyrene in BSM by around 50%. Both coculture A and coculture B significantly reduced the mutagenicity of BSM containing only benzo[a]pyrene (ca. 60 to 63%) or dibenz[a,h]anthracene (ca. 35 to 40%); these results were similar for the two cocultures, although coculture B always reduced the mutagenicity to a slightly greater extent than coculture A. The mutagenic potential of DCM extracts from PAH-spiked soil and PAH-contaminated soil was not significantly reduced over 100 days following amendment with axenic bacterial inocula (Table 4). On the other hand, inoculation of these soils with P. janthinellum VUO 10,201 led to a 47% (PAH-spiked soil) and 35% (PAH-contaminated soil) reduction in the mutagenicity of the DCM extracts. The mutagenic potential of DCM extracts from these soils was reduced further by both cocultures, with the reduction in mutagenicity being in the range of 58 to 62% (PAH-spiked soil) and 42 to 43% (PAH-contaminated soil); the difference in reduction of mutagenicity by coculture A and coculture B was not significant.
TABLE 4.
Mutagenicity of DCM extracts from PAH-contaminated liquid and soil cultures
| Culture from which DCM extracts were obtained | No. of revertants/platea
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benzo[a]pyreneb
|
Dibenz[a,h]anthraceneb
|
PAH-spiked soilc
|
PAH-contaminated soilc
|
|||||||||
| Day 0 | Day 28 | Day 56 | Day 0 | Day 28 | Day 56 | Day 0 | Day 56 | Day 100 | Day 0 | Day 56 | Day 100 | |
| Uninoculated control | 687 ± 6 | 704 ± 8 | 701 ± 6 | 444 ± 5 | 438 ± 6 | 447 ± 7 | 689 ± 11 | 671 ± 14 | 693 ± 6 | 718 ± 4 | 711 ± 8 | 689 ± 4 |
| VUN 10,009d | 696 ± 8 | 688 ± 9 | 657 ± 6 | 437 ± 7 | 411 ± 6 | 388 ± 1 | 702 ± 14 | 686 ± 9 | 594 ± 7 | 724 ± 9 | 703 ± 11 | 677 ± 12 |
| VUN 10,010d | 682 ± 3 | 651 ± 8 | 632 ± 13 | 441 ± 4 | 407 ± 5 | 384 ± 2 | 695 ± 7 | 623 ± 10 | 501 ± 16 | 722 ± 20 | 697 ± 6 | 648 ± 4 |
| VUO 10,201d | 673 ± 10 | 337 ± 13 | 339 ± 4 | 452 ± 7 | 392 ± 3 | 382 ± 8 | 697 ± 5 | 477 ± 12 | 367 ± 8 | 716 ± 7 | 634 ± 3 | 461 ± 8 |
| Coculture A | 682 ± 6 | 306 ± 11 | 272 ± 7 | 449 ± 10 | 296 ± 8 | 291 ± 4 | 705 ± 3 | 392 ± 10 | 295 ± 11 | 722 ± 8 | 617 ± 13 | 420 ± 6 |
| Coculture B | 687 ± 14 | 294 ± 5 | 253 ± 9 | 460 ± 16 | 277 ± 10 | 276 ± 3 | 698 ± 7 | 367 ± 15 | 263 ± 6 | 719 ± 4 | 576 ± 6 | 409 ± 4 |
Assay was performed using serovar Typhimurium TA100 with the postmitochondrial activation system (S9). DCM extracts were exchanged into DMSO: the number of revertants per plate for the DMSO control was 124 ± 3.
Mutagenicity assay on DCM extracts of BSM containing either benzo[a]pyrene or dibenz[a,h]anthracene as a sole carbon and energy source.
Mutagenicity assay on DCM extracts of PAH-spiked soil cultures and PAH-contaminated soil cultures.
Axenic inocula were used.
DISCUSSION
The focus of PAH research in recent years on the degradation of high-molecular-weight PAHs has resulted in the isolation of a number of microorganisms that can mineralize and grow on four-ring PAHs as a sole carbon and energy source (7, 6, 21, 23, 25, 33, 42, 43). Some of these isolates have been used to identify the biochemical pathways involved in the catabolism of these PAHs. Microorganisms capable of degrading PAHs containing five benzene rings have been more difficult to obtain. Our studies have demonstrated that cultures of bacterial consortium VUN 10,009, S. maltophilia VUN 10,010, or P. janthinellum VUO 10,201 can degrade a number of tetracyclic and pentacyclic PAHs including chrysene, dibenz[a,h]anthracene, and benzo[a]pyrene when present as a sole carbon and energy source. However, under these conditions there was no significant microbial growth on the five-benzene-ring PAHs, and benzo[a]pyrene was not mineralized.
Mineralization of [14C]benzo[a]pyrene when added to soils and sediments has been monitored in other studies; however, pure microbial cultures capable of degrading, and growing on, benzo[a]pyrene have not been isolated from these matrices (19, 20, 24). Cometabolic mineralization of benzo[a]pyrene by pure microbial cultures is not widely reported; however, P. chrysosporium will mineralize benzo[a]pyrene in a medium containing various carbon substrates (2, 4, 10, 37). Bacteria have not been shown to cometabolically mineralize benzo[a]pyrene in pure culture; however, a high population of resting S. paucimobilis EPA505 cells were reported to mineralize benzo[a]pyrene in a phosphate buffer (46). High cell populations were required, since EPA505 could not grow on benzo[a]pyrene as a sole carbon and energy source. EPA505 could grow in the presence of benzo[a]pyrene when another growth-supporting PAH was present, but benzo[a]pyrene mineralization by EPA505 was not tested under these cometabolic conditions. S. maltophilia VUN 10,010 and bacterial consortium VUN 10,009 could cometabolically mineralize benzo[a]pyrene in pure culture with pyrene supplied alone or with other PAHs. The degradation of at least a portion of the benzo[a]pyrene to CO2 and its incorporation into biomass by S. maltophilia VUN 10,010 and bacterial consortium VUN 10,009 indicated that a benzo[a]pyrene catabolic pathway is present in these bacteria. The reason why benzo[a]pyrene alone cannot support the growth of S. maltophilia VUN 10,010 and bacterial consortium VUN 10,009 is not clear, but it may be related to regulation of PAH-catabolic enzyme activity by benzo[a]pyrene or its degradation products at the level of the enzymes or their synthesis. Pyrene, or its degradation products, may compensate for this lack of enzyme activity via induction of benzo[a]pyrene catabolic enzymes (5) or because pyrene and benzo[a]pyrene share a common lower catabolic pathway which is regulated by pyrene. Investigation of the underlying mechanisms is warranted.
The use of defined fungal-bacterial cocultures to degrade PAHs has not been reported previously. In our work, the coculture containing P. janthinellum VUO 10,201 and bacterial consortium VUN 10,009 was able to mineralize and grow on benzo[a]pyrene as a sole carbon and energy source. Higher rates of benzo[a]pyrene mineralization and degradation were achieved when P. janthinellum VUO 10,201 was cocultured with S. maltophilia VUN 10,010. This result is significant since S. maltophilia VUN 10,010 was isolated from a different site from P. janthinellum VUO 10,201, indicating that fungal-bacterial cooperative mineralization of benzo[a]pyrene may not be restricted to species isolated from the same site, and hence having experienced similar selective pressures. Microbial growth on dibenz[a,h]anthracene as a sole carbon and energy source has not previously been reported, and yet the fungal-bacterial cocultures described here were able to grow on this compound as a sole carbon source. These cocultures may also be able to mineralize dibenz[a,h]anthracene, but this requires verification. However, the use of microbial cocultures in biodegradation studies is not without precedence: a coculture of four different bacterial species was shown to improve rates of degradation of PAHs including anthracene, pyrene, and benzo[a]pyrene in a nutrient-supplemented medium (40). Outside the field of PAH biodegradation, bacterial cocultures have been used to increase the rate of degradation of sulfonated aromatics (16) and chloronitrobenzenes (34). In the latter case, the combination of Pseudomonas putida HS12 and Rhodococcus sp. strain HS51 resulted in the mineralization of 3- and 4-chloronitrobenzenes in the presence of another carbon source; these compounds could not be mineralized by the coculture as a sole carbon and energy source. Features of the fungal-bacterial cocultures used in our study include increased PAH degradation rates plus considerable microbial growth on five-benzene-ring PAHs and benzo[a]pyrene mineralization when these compounds were provided as a sole carbon and energy source, while no significant microbial growth or benzo[a]pyrene mineralization was observed with axenic cultures.
The observation that neither P. janthinellum VUO 10,201, S. maltophilia VUN 10,010, nor bacterial consortium VUN 10,009 could independently mineralize or grow on benzo[a]pyrene as a sole carbon and energy source suggests that a mutually dependent relationship exists between the fungus and these bacteria during PAH degradation by the cocultures. HPLC data showed the accumulation of different benzo[a]pyrene degradation products in axenic P. janthinellum (compound I) and bacterial (compounds II, III, and IV) cultures which themselves were only further substantially degraded in the cocultures. The appearance and disappearance of compound V, which was not detected in the axenic cultures, were further evidence of the cooperative catabolism between the fungus and the bacteria. These degradation products were not identified; however, a P. janthinellum strain has been reported to oxidize benzo[a]pyrene to 9-hydroxy-benzo[a]pyrene (29). Mycobacterium sp. strain RJGII-135 and Beijerinckia sp. have been reported to oxidize benzo[a]pyrene initially to cis-dihydrodiols in the 4/5, 7/8, or 9/10 positions and then to 4,5-chrysene-dicarboxylic acid, 7,8-dihydro-pyrene-7-carboxylic acid, or 7,8-dihydro-pyrene-8-carboxylic acid (38). Characterizing the further degradation of these reported metabolites has been constrained by the lack of bacterial isolates that degrade benzo[a]pyrene beyond the initial oxidation steps. A more complete map of the benzo[a]pyrene, and possibly dibenz[a,h]anthracene, catabolism in microorganisms may be obtainable using P. janthinellum VUO 10,201 and S. maltophilia VUN 10,010 in coculture with studies of their cooperative activities.
There is some evidence to suggest that the cooperative benzo[a]pyrene catabolic route used by the fungal-bacterial cocultures involves the initial oxidation of benzo[a]pyrene by the fungal partner. For example, benzo[a]pyrene degradation and mineralization in soil and BSM (containing benzo[a]pyrene and pyrene) by axenic bacterial cultures commenced after considerable lag periods; such degradation lag periods were absent in axenic P. janthinellum VUO 10,201 cultures in soil. These lag periods were also absent in the cocultures, which would not be expected if the bacteria were responsible for initiating benzo[a]pyrene oxidation. Furthermore, compound I seen as a degradation product in P. janthinellum VUO 10,201 cultures was the first degradation product to appear in cocultures containing benzo[a]pyrene as the sole carbon and energy source. This is consistent with a number of previous reports that suggest that PAH degradation in nature is a consequence of sequential breakdown by fungi and bacteria, with the fungi performing the initial oxidation step (28, 31, 36). The strongest evidence to support this concept was reported recently: the rate of benzo[a]pyrene mineralization for a 15-day-old pure culture of white rot fungus was substantially increased when it was inoculated with a PAH-adapted sludge, containing an indigenous bacterial community (28). The benzo[a]pyrene mineralization rates were significantly lower when the 15-day-old fungal culture and PAH-adapted sludge were incubated separately. The authors proposed that the improved mineralization rate of the combined culture may be due to the greater bioavailability to the bacterial community of water-soluble compounds arising from fungal preoxidation of the benzo[a]pyrene. If this were true for the fungal-bacterial cocultures in our work, then it is conceivable that a fungal product, such as compound I, may serve as a substrate for bacterial metabolism. This is consistent with the observed accumulation of compound I in axenic fungal cultures compared to its production and then disappearance in the cocultures. However, such a role for compound I would need to be experimentally verified. The cross-feeding of metabolites in the fungal-bacterial coculture appears to occur in both directions, since P. janthinellum VUO 10,201 is able to grow in BSM containing a single PAH only when the bacteria are present.
The perceived drawback with using cocultures in practice is that the soil environment, being a heterogeneous mix of organics and microbes, may destabilize the coculture, possibly resulting in poor degradation rates, failure to degrade some PAHs, and/or the production of toxic, water-soluble intermediates. Our results suggest otherwise, since we have observed faster and more extensive degradation of low- and high-molecular-weight PAHs, especially benzo[a]pyrene mineralization, in PAH-contaminated soil using fungal-bacterial cocultures compared to axenic inocula or the indigenous microflora. A small amount of benzo[a]pyrene was mineralized in the PAH-contaminated soil inoculated with only P. janthinellum VUO 10,201. Since the fungus alone cannot mineralize this PAH, it is suspected that the indigenous bacteria and P. janthinellum VUO 10,201 were cooperatively mineralizing benzo[a]pyrene. This is consistent with the observation that benzo[a]pyrene mineralization was not observed in the sterile PAH-spiked soil inoculated only with P. janthinellum VUO 10,201. As seen for the liquid cultures, there was an exceptional decrease in the mutagenic potential of the contaminated soil inoculated with the coculture compared to that for soil with axenic inocula. This is probably due to the higher rates of degradation and the formation of less toxic degradation byproducts, e.g., CO2, by the coculture than by the bacterial cultures alone.
Our data have shown that defined fungal-bacterial cocultures can grown on five-benzene-ring PAHs and mineralize benzo[a]pyrene as sole carbon and energy sources. Inoculation of these cocultures into PAH-contaminated soil demonstrated their competitiveness in the degradation and mineralization of high-molecular-weight PAHs, and consequent reduction in mutagenicity, compared to axenic inocula and the indigenous microflora. The defined coculture containing a single bacterial and fungal species provides a useful model for investigating the cooperative catabolism of complex PAHs as well as developing practical applications for the complete bioremediation of PAH-contaminated sites.
ACKNOWLEDGMENTS
We are grateful to Brent Davey (Australian Defence Industry Environmental Services) for providing soil samples. We also thank Anne Lawrie, Department of Applied Biology and Biotechnology, Royal Melbourne Institute of Technology University, Melbourne, for identification of the P. janthinellum isolate, and Madol Serafica, University of Melbourne, for confirming the identity of VUN 10,010 by 16S ribosomal DNA sequencing.
The Australian Agency for International Development (AUSAID) is acknowledged for providing the Ph.D. scholarship for Sudarat Boonchan.
REFERENCES
- 1.Anderson B E, Henrysson T. Accumulation and degradation of dead-end metabolites during treatment of soil contaminated with polycyclic aromatic hydrocarbons with five strains of white-rot fungi. Appl Microbiol Biotechnol. 1996;46:647–652. [Google Scholar]
- 2.Barclay C D, Farquhar G F, Legge R L. Biodegradation and sorption of polyaromatic hydrocarbons by Phanerochaete chrysosporium. Appl Microbiol Biotechnol. 1995;42:958–963. doi: 10.1007/BF00191197. [DOI] [PubMed] [Google Scholar]
- 3.Bezalel L, Hadar Y, Fu P P, Freeman J P, Cerniglia C E. Initial oxidation products in the metabolism of pyrene, anthracene, fluorene, and dibenzothiophene by the white rot fungus Pleurotus ostreatus. Appl Environ Microbiol. 1996;62:2554–2559. doi: 10.1128/aem.62.7.2554-2559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogan B W, Lamar R T. Polycyclic aromatic hydrocarbon-degrading capabilities of Phanerochaete laevis HHB-1625 and its extracellular ligninolytic enzymes. Appl Environ Microbiol. 1996;62:1597–1603. doi: 10.1128/aem.62.5.1597-1603.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boldrin B, Tiehm A, Fritsche C. Degradation of phenanthrene, fluorene, fluoranthene, and pyrene by a Mycobacterium sp. Appl Environ Microbiol. 1993;59:1927–1930. doi: 10.1128/aem.59.6.1927-1930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boonchan S, Britz M L, Stanley G A. Surfactant-enhanced biodegradation of high molecular weight polycyclic aromatic hydrocarbons by Stenotrophomonas maltophilia. Biotechnol Bioeng. 1998;59:482–494. doi: 10.1002/(sici)1097-0290(19980820)59:4<482::aid-bit11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Bouchez M, Blancher D, Vandecasteele J-P. Degradation of polycyclic aromatic hydrocarbons by pure strains and by defined strain associations: inhibition phenomena and cometabolism. Appl Microbiol Biotechnol. 1995;43:156–164. doi: 10.1007/BF00170638. [DOI] [PubMed] [Google Scholar]
- 8.Brodkorb T S, Legge R L. Enhanced biodegradation of phenanthrene in oil tar-contaminated soils supplemented with Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:3117–3121. doi: 10.1128/aem.58.9.3117-3121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bumpus J A. Biodegradation of polycyclic aromatic hydrocarbons by Phanerochaete chrysosporium. Appl Environ Microbiol. 1989;55:154–158. doi: 10.1128/aem.55.1.154-158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bumpus J A, Tien M, Wright D, Aust S D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985;228:1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- 11.Cerniglia C E. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation. 1992;3:351–368. [Google Scholar]
- 12.Cerniglia C E, Gibson D T. Oxidation of benzo[a]pyrene by the filamentous fungus Cunninghamella elegans. J Biol Chem. 1979;254:12174–12180. [PubMed] [Google Scholar]
- 13.Cerniglia C E, Gibson D T. Fungal oxidation of benzo[a]pyrene and (±)-trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene: evidence for the formation of benzo[a]pyrene 7,8-diol-9,10-epoxide. J Biol Chem. 1980;255:5159–5163. [PubMed] [Google Scholar]
- 14.Collins C H, Lyne P M, Grange J M. Microbiological methods. 6th ed. London, United Kingdom: Butterworths; 1989. [Google Scholar]
- 15.Collins P J, Kotterman M J J, Field J A, Dobson A D W. Oxidation of anthracene and benzo[a]pyrene by laccases from Trametes versicolor. Appl Environ Microbiol. 1996;62:4563–4567. doi: 10.1128/aem.62.12.4563-4567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dangmann E, Stolz A, Kuhm A E, Hammer A, Feigel B, Noisommit-Rizzi N, Rizzi M, Reuss M, Knackmuss H-J. Degradation of 4-aminobenzenesulfonate by a two-species bacterial coculture. Biodegradation. 1996;7:223–229. doi: 10.1007/BF00058181. [DOI] [PubMed] [Google Scholar]
- 17.Fedorak P M, Foght J M, Westlake D W S. A method for monitoring mineralization of 14C-labeled compounds in aqueous samples. Water Res. 1982;16:1285–1290. [Google Scholar]
- 18.Gibson D T, Mahadevan V, Jerina D M, Yagi H, Yeh H J C. Oxidation of the carcinogens benzo[a]pyrene and benz[a]anthracene to dihydrodiols by a bacterium. Science. 1975;189:295–297. doi: 10.1126/science.1145203. [DOI] [PubMed] [Google Scholar]
- 19.Grosser R J, Warshawsky D, Vestal J R. Indigenous and enhanced mineralization of pyrene, benzo[a]pyrene, and carbazole in soils. Appl Environ Microbiol. 1991;57:3462–3469. doi: 10.1128/aem.57.12.3462-3469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosser R J, Warshawsky D, Vestal J R. Mineralization of polycyclic and N-heterocyclic aromatic compounds in hydrocarbon-contaminated soils. Environ Toxicol Chem. 1995;14:375–382. [Google Scholar]
- 21.Heitkamp M A, Freeman J P, Miller D W, Cerniglia C E. Pyrene-degradation by a Mycobacterium sp.: identification of oxidation and ring fission products. Appl Environ Microbiol. 1988;54:2556–2565. doi: 10.1128/aem.54.10.2556-2565.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juhasz A L, Britz M L, Stanley G A. Degradation of high molecular weight polycyclic aromatic hydrocarbons by Pseudomonas cepacia. Biotechnol Lett. 1996;18:577–582. [Google Scholar]
- 23.Juhasz A L, Britz M L, Stanley G A. Degradation of fluoranthene, pyrene, benz[a]anthracene and dibenz[a,h]anthracene by Burkholderia cepacia. J Appl Microbiol. 1997;83:189–198. [Google Scholar]
- 24.Kanaly R, Bartha R, Fogel S, Findlay M. Biodegradation of [14C]benzo[a]pyrene added in crude oil to uncontaminated soil. Appl Environ Microbiol. 1997;63:4511–4515. doi: 10.1128/aem.63.11.4511-4515.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kästner M, Breuer-Jammali M, Mahro B. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons (PAH) Appl Microbiol Biotechnol. 1994;41:267–273. [Google Scholar]
- 26.Keith L H, Telliard W A. Priority pollutants I—a perspective view. Environ Sci Technol. 1979;13:416–423. [Google Scholar]
- 27.Kiehlmann E, Pinto L, Moore M. The transformation of chrysene to trans-1,2-dihydroxy-1,2-dihydrochrysene by filamentous fungi. Can J Microbiol. 1996;42:604–608. [Google Scholar]
- 28.Kotterman M J J, Vis E H, Field J A. Successive mineralization and detoxification of benzo[a]pyrene by the white rot fungus Bjerkandera sp. strain BOS55 and indigenous microflora. Appl Environ Microbiol. 1998;64:2853–2858. doi: 10.1128/aem.64.8.2853-2858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Launen L, Pinto L, Wiebe C, Kiehlmann E, Moore M. The oxidation of pyrene and benzo[a]pyrene by nonbasidiomycete soil fungi. Can J Microbiol. 1995;41:477–488. doi: 10.1139/m95-064. [DOI] [PubMed] [Google Scholar]
- 30.Maron D M, Ames B N. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 31.Meulenberg R, Rijnaarts H H M, Doddema H J, Field J A. Partially oxidized polycyclic aromatic hydrocarbons show an increased bioavailability and biodegradability. FEMS Microbiol Lett. 1997;152:45–49. doi: 10.1111/j.1574-6968.1997.tb10407.x. [DOI] [PubMed] [Google Scholar]
- 32.Morehead N R, Eadie B J, Lake B, Landrum P D, Berner D. The sorption of PAH onto dissolved organic matter in Lake Michigan waters. Chemosphere. 1986;15:403–412. [Google Scholar]
- 33.Mueller J G, Chapman P J, Blattmann B O, Pritchard P H. Isolation and characterization of a fluoranthene-utilizing strain of Pseudomonas paucimobilis. Appl Environ Microbiol. 1990;56:1079–1086. doi: 10.1128/aem.56.4.1079-1086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park H-S, Lim S-J, Chang Y K, Livingston A G, Kim H-S. Degradation of chloronitrobenzenes by a coculture of Pseudomonas putida and a Rhodococcus sp. Appl Environ Microbiol. 1999;65:1083–1091. doi: 10.1128/aem.65.3.1083-1091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pothuluri J V, Selby A, Evans F E, Freeman J P, Cerniglia C E. Transformation of chrysene and other polycyclic aromatic hydrocarbon mixtures by the fungus Cunninghamella elegans. Can J Bot. 1994;73:1025–1033. [Google Scholar]
- 36.Sack U, Heinze T M, Deck J, Cerniglia C E, Martens R, Zadrazil F, Fritsche W. Comparison of phenanthrene and pyrene degradation by different wood-decaying fungi. Appl Environ Microbiol. 1997;63:3919–3925. doi: 10.1128/aem.63.10.3919-3925.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanglard D, Leisola M S A, Fiechter A. Role of extracellular ligninase in biodegradation of benzo[a]pyrene by Phanerochaete chrysosporium. Enzyme Microb Technol. 1986;8:209–212. [Google Scholar]
- 38.Schneider J, Grosser R, Jayasimhulu K, Xue W, Warshawsky D. Degradation of pyrene, benzo[a]anthracene, and benzo[a]pyrene by Mycobacterium sp. strain RJGII-135, isolated from a former coal gasification site. Appl Environ Microbiol. 1996;62:13–19. doi: 10.1128/aem.62.1.13-19.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutherland J B. Detoxification of polycyclic aromatic hydrocarbons by fungi. J Ind Microbiol. 1992;9:53–62. doi: 10.1007/BF01576368. [DOI] [PubMed] [Google Scholar]
- 40.Trzesicka-Mlynarz D, Ward O P. Degradation of polycyclic aromatic hydrocarbons (PAHs) by a mixed culture and its component pure cultures, obtained from PAH-contaminated soil. Can J Microbiol. 1995;41:470–476. doi: 10.1139/m95-063. [DOI] [PubMed] [Google Scholar]
- 41.Vyas B R M, Bakowski S, Sasek V, Matucha M. Degradation of anthracene by selected white rot fungi. FEMS Microbiol Ecol. 1994;14:65–70. [Google Scholar]
- 42.Walter U, Beyer M, Klein J, Rehm H J. Degradation of pyrene by Rhodococcus sp. UW1. Appl Microbiol Biotechnol. 1991;34:671–676. [Google Scholar]
- 43.Weissenfels W D, Beyer M, Klein J. Degradation of fluoranthene by pure bacterial cultures. Appl Microbiol Biotechnol. 1990;32:479–484. doi: 10.1007/BF00903787. [DOI] [PubMed] [Google Scholar]
- 44.Wilson S C, Jones K C. Bioremediation of soils contaminated with polynuclear aromatic hydrocarbons (PAHs): a review. Environ Pollut. 1993;88:229–249. doi: 10.1016/0269-7491(93)90206-4. [DOI] [PubMed] [Google Scholar]
- 45.Wunder T, Marr J, Kremer S, Sterner O, Anke H. 1-Methyoxypyrene and 1,6-dimethoxypyrene: two novel metabolites in fungal metabolism of polycyclic aromatic hydrocarbons. Arch Microbiol. 1997;167:310–316. doi: 10.1007/s002030050449. [DOI] [PubMed] [Google Scholar]
- 46.Ye D, Siddiqi M A, Maccubbin A E, Kumar S, Sikka H C. Degradation of polynuclear aromatic hydrocarbons by Sphingomonas paucimobilis. Environ Sci Technol. 1996;30:136–142. [Google Scholar]