Abstract
Growth of Colletotrichum gloeosporioides in pectolytic enzyme-inducing medium (PEIM) increased the pH of the medium from 3.8 to 6.5. Pectate lyase (PL) secretion was detected when the pH reached 5.8, and the level of secretion increased up to pH 6.5. PL gene (pel) transcript production began at pH 5.0 and increased up to pH 5.7. PL secretion was never detected when the pH of the inducing medium was lower than 5.8 or when C. gloeosporioides hyphae were transferred from PL-secreting conditions at pH 6.5 to pH 3.8. This behavior differed from that of polygalacturonase (PG), where pg transcripts and protein secretion were detected at pH 5.0 and continued up to 5.7. Under in vivo conditions, the pH of unripe pericarp of freshly harvested avocado (Persea americana cv. Fuerte) fruits, resistant to C. gloeosporioides attack, was 5.2, whereas in ripe fruits, when decay symptoms were expressed, the pericarp pH had increased to 6.3. Two avocado cultivars, Ardit and Ettinger, which are resistant to C. gloeosporioides attack, had pericarp pHs of less than 5.5, which did not increase during ripening. The present results suggest that host pH regulates the secretion of PL and may affect C. gloeosporioides pathogenicity. The mechanism found in avocado may have equivalents in other postharvest pathosystems and suggests new approaches for breeding against and controlling postharvest diseases.
Colletotrichum gloeosporioides (Penz.) Penz. & Sacc [teleomorph Glomerella cingulata (Stonem). Spauld et Schrenk] infects avocado fruits, via spore germination, appressorium formation, and penetration. The pathogen attacks fruits early in their development and remains as a germinated appressorium during fruit growth (quiescent infection) (17). After harvest and fruit ripening, fast-developing brown-black spots on the pericarp and soft rot in the mesocarp, the symptoms of anthracnose, appear (17). Prusky (17) has suggested several hypotheses to explain the resistance mechanism of unripe fruits: (i) nutrients available to the pathogen may be limited in unripe hosts, (ii) preformed antifungal compounds present in unripe fruits decline during ripening, (iii) inducible antifungal compounds in unripe fruits decline during ripening, and (iv) fungal pathogenicity factors may be activated mainly in ripening fruits. Antifungal compounds are implicated in the resistance of avocado fruits to C. gloeosporioides (19), but no reports have described the role of enzyme secretion as a factor in fungal attack.
C. gloeosporioides produces an array of pectolytic enzymes, including polygalacturonase (PG) (20), pectin lyase (2), pectin methyl esterase (15), and pectate lyase (PL) (28). No reports regarding the involvement of PG or pectin methyl esterase in C. gloeosporioides attack have been published. However, targeted disruption of pectin lyase from C. gloeosporioides did not reduce virulence (2). The importance of PL secretion during C. gloeosporioides attack in avocado fruits has been suggested by the inhibition of decay development during coinoculation of C. gloeosporioides spores with PL antibodies and also by the reduced pathogenicity of a Colletotrichum magna mutant with limited PL secretion (26, 27). These results suggest that PL is a limiting factor during pathogenesis (27).
Environmental conditions can affect protein production and secretion in various organisms (1, 4, 12). Dean and Timberlake (5) found that Aspergillus nidulans secretes PG at low pH values and PL when the medium pH increases and becomes conducive to PL activity. St. Leger et al. (24) hypothesized that ambient pH affects pathogenicity by altering the expression of cuticle-degrading enzymes and hydrophobin in the insect pathogen Metarhizium anisopliae.
Our working hypothesis is that host environmental conditions affect PL secretion and prevent the activation of C. gloeosporioides colonization in unripe fruits. In the present study, we demonstrated the importance of pH as a regulator of pectolytic enzyme secretion in C. gloeosporioides. Pathogenicity of C. gloeosporioides is dependent on its ability to secrete PL enzyme and not on pel gene expression. These results indicate that avocado susceptibility is regulated by more than one mechanism: a decrease in the level of antifungal compounds and an increase in pericarp pH which modulates PL secretion.
MATERIALS AND METHODS
Strains, media, and growth conditions.
C. gloeosporioides isolate Cg-14 was obtained from decayed avocado fruits and used in all experiments. Spores were stored in 10 mM Na phosphate buffer (pH 7.2) in 40% glycerol at −80°C. Cultures were initiated with 0.5 × 106 to 1.5 × 106 spores/ml.
Spores from 10- to 20-day-old cultures were harvested from M3S medium (25). Erlenmeyer flasks (500 ml) containing 250 ml of medium with Na polypectate and pectin as the sole carbon sources (pectolytic enzyme-inducing medium [PEIM]) (20) were inoculated with isolate Cg-14 (0.5 × 106 spores/flask) and grown at 24°C on an orbital shaker (150 rpm). The PEIM consisted of the following (per liter): polygalacturonic acid-Na, 5 g; pectin from citrus fruit (Sigma, St. Louis, Mo.), 5 g; KNO3, 5 g; KH2PO4, 4 g; MgSO4 · 7H2O, 2 g; CaCl2 · 7H2O, 0.3 g, and FeCl3, 10 mg. The initial pH of the PEIM was 3.8. In some experiments, the medium was adjusted to pH 6.0 with 5 N NaOH. In some experiments, mycelium was subsequently transferred to fresh PEIM after 4 days of growth in liquid M3S medium.
Fruits, inoculation conditions, and statistical analysis.
Avocado fruits, Persea americana Mill. var. drymifolia (Schidl. and Cham.) S. F. Blake ‘Fuerte’ cultivars Fuerte, Ardit, and Ettinger, were harvested from an orchard at Kibbutz Givat Brenner, Israel. Firmness, a parameter of fruit ripening, was checked at two places on the longer transverse axis of each fruit with a Penetrometer (Chatillon & Son Inc., Kew Gardens, N.Y.) with a conical probe. Results are given in newtons.
Inoculation was carried out on 10 freshly harvested fruits by placing 10 μl of spore suspension (106 spores/ml) at six points, three on each side of the longitudinal axis of the fruit. The fruits were then incubated at 22°C, in 90% humidity, for 10 to 12 days.
In vitro experiments were repeated five times. The results of one representative experiment are presented. In vivo experiments were repeated at least three times during two consecutive harvesting seasons. Standard deviations of the means were calculated, and differences between means were analyzed by analysis of variance.
pH measurements.
pH was measured with a flat electrode (Sensorex, Stanton, Calif.) in 3-ml aliquots sampled at different times after fungal inoculation. Pericarp pH was determined after an approximately 0.2-mm-deep cut was made with a scalpel blade. Mesocarp pH was determined after peeling the pericarp to a depth of 2 mm. pH measurements were taken by placing the flat electrode directly against the exposed tissue. The depth of the pericarp cut was determined based on the location of C. gloeosporioides infection (17). All measurements were repeated on three fruits at three different places (nine measurements) on the longer transverse axis of each fruit. The standard error of the mean of pH measurements was never higher than 2.5%. To test the hypothesis that direct pH measurement was a reliable indicator of the environment within the fruit, the direct measurement was compared to the pH determined by the common homogenization method (10), in which 5 g of pericarp tissue is ground in the presence of 10 ml of double-distilled water. Direct and homogenatization method pH measurements were compared at three different stages of fruit growth in 10 P. americana cv. Fuerte fruits (30 measurements). The regression coefficient (r) between the measurements was 0.999. Similar results were obtained with P. americana cultivars Ettinger and Ardit.
Detection of PL and PG in liquid media and fungal hyphae.
C. gloeosporioides hyphae were separated from the culture medium by centrifugation (12,000 × g, 10 min) at 4°C. The hyphae were washed twice with sterile water (250 ml each time), frozen under liquid nitrogen, lyophilized, and stored at −80°C until used. The culture medium supernatant was concentrated to 15 ml with a Rotavapor (Buchii, Flawil, Switzerland) at 42°C. The concentrated culture filtrate was dialyzed overnight (12,000-molecular-weight cutoff; Sigma) against 5 liters of Tris-HCl (50 mM, pH 8.5) concentrated to 5 ml as above, and 5 μg per lane was subjected to Western blot analysis.
Nonsecreted PL was extracted from fungal hyphae with TRI REAGENT (Sigma). Lyophilized hyphae were ground three times with a mortar and pestle under liquid nitrogen. Proteins were extracted by using 4 ml of TRI REAGENT for every 0.2 g of lyophilized hyphae in accordance with the manufacturer's instructions (TRI REAGENT; Sigma Technical Bulletin MB-205). To determine the presence of glycosylated PL, 10-μg protein samples were subjected to N-glycosidase F in accordance with the manufacturer's instructions (Boehringer Mannheim GmbH, Mannheim, Germany) and analyzed by Western blot analysis.
Each protein sample was quantified by the Bio-Rad Laboratories (Hercules, Calif.) protein assay, with bovine serum albumin as the standard. Samples were boiled for 4 min in loading buffer as described by Sambrook et al. (22), with 10% β-mercaptoethanol as a reducing agent. Samples were loaded onto a sodium dodecyl sulfate (SDS)–12.5% polyacrylamide gel electrophoresis (PAGE) gel (Mini-Protean II; Bio-Rad Laboratories) and run for 1 h at a constant 150 V. Western blot analysis was performed with PL (27) and PG (D. Prusky and N. T. Keen, unpublished data) antibodies diluted 1:500. For PL, preimmune ascitic fluid was used as a control, and anti-mouse immunoglobulin (IgG)-alkaline phosphatase (AP) conjugate (Promega, Madison, Wis.) was used as a secondary antibody. PG antibodies were prepared by injecting the glycosylated purified PG into a rabbit and collecting the resultant serum. Nonimmune serum was used as a control. Anti-rabbit IgG-AP conjugate (Promega) was used as the secondary antibody. Both secondary antibodies were used at a dilution of 1:6,000.
RNA extraction and Northern blot analysis.
Lyophilized hyphae were ground three times with a mortar and pestle under liquid nitrogen, and total RNA was extracted by using 4 ml of TRI REAGENT (Sigma) for every 0.2 g of lyophilized hyphae. Following homogenization, samples were prepared in accordance with the manufacturer's instructions (Sigma Technical Bulletin MB-205). RNA was quantified by GeneQuant (Pharmacia Biotech, Cambridge, United Kingdom).
Northern blot analysis was conducted by running 10 μg of total RNA on a 1.1% formaldehyde denaturing agarose gel (22). The RNA was blotted to a Hybond+ nylon membrane (Amersham, Buckinghamshire, United Kingdom) by the capillary method (22), using 20× SSC (1× SSC is 0.017 M NaCl plus 0.17 M sodium citrate). The RNA was fixed by baking for 2 h at 80°C and subjected to hybridization using the 1.1-kb pel full-length clone (GenBank accession no. U329242) as a probe. All hybridizations were carried out at 63°C, and the products were washed with 0.1× SSC. PG was probed with the 487-bp putative PG clone (cgpg1; GenBank accession no. AF116507) obtained by PCR cloning. The cgpg1 clone was obtained by PCR from 3 μg of genomic DNA of C. gloeosporioides, using two oligonucleotides (5′-CCTCAACGGCATCAAGGTACC-3′ as the forward primer and 5′-CAGGCATGCGTCCTGGTTGTA-3′ as the reverse primer). The primers were constructed on the basis of the alignment of clpg1 and clpg2 from Colletotrichum lindemuthianum (3). The following cycles were used: 95°C for 5 min, 1 cycle; 95°C for 1 min, 60°C for 1.5 min, 72°C for 1 min, 10 cycles; 94°C for 1 min, 60°C for 1.5 min, 72°C for 1 min, 20 cycles; and 72°C for 10 min, 1 cycle. The fragment was subcloned into pGEM-T Easy (Promega) and transferred to DH5-α by means of a Gene Pulser II (Bio-Rad Laboratories). Colonies were selected on Luria-Bertani (LB) medium supplemented with 100 μg of ampicillin per ml, with blue or white color selection. DNA was sequenced at the Weizmann Institute's Biological Services, Rehovot, Israel. The results were analyzed with the Wisconsin package, GCG version, by comparison with GenBank data. An ethidium bromide-stained gel was used for RNA quantification.
Antifungal diene extraction.
A 10-g sample of avocado pericarp (1- to 2-mm deep) was homogenized in 95% ethanol in an Omni-Mixer (Sorvall; DuPont Company, Newtown, Conn.) at full speed for 3 min. The ethanol extract was dried in a rotary evaporator at 40°C and redissolved in 10 ml of distilled water, and the organic phase was extracted by fractionation with dichloromethane. Following two extractions, the organic phases were pooled, dried with anhydrous MgSO4 (Riedel-deHaen, Seelze, Germany), and evaporated to dryness. Samples were redissolved in 1 ml of ethanol (analytical grade; Bio Lab, Jerusalem, Israel) and analyzed by high-performance liquid chromatography (21). The average values of three separate extractions are presented.
RESULTS
pH changes and PL secretion in PEIM by C. gloeosporioides.
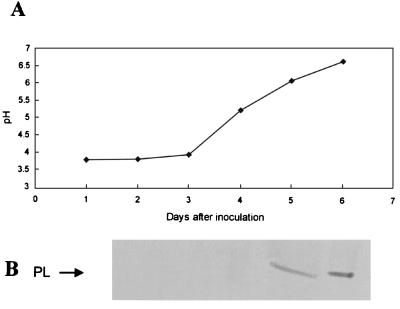
Three days after inoculation of PEIM cultures with C. gloeosporioides spores, the pH of the medium was 3.8, increasing to 6.5 by day 6 (Fig. 1A). We detected PL in the culture filtrate when the medium pH exceeded 6.0 (Fig. 1B), and the amount secreted increased with further increases in pH. PL activity at pH 6.5 was 3.2 × 10−3 U/min. To determine whether the absence of PL secretion was dependent on pH rather than on limited fungal development, the following experiments were initiated from fungal mass.
FIG. 1.
Changes in pH values (A) and PL protein secretion (B) in PEIM by C. gloeosporioides. Spores (0.5 × 106) were inoculated into 250 ml of PEIM, and the medium was shaken at 150 rpm at 22°C. The culture medium was concentrated, dialyzed, and further concentrated before being analyzed for the presence of PL, by Western blot analysis (5 μg of protein/lane).
Relationship between pH and PL secretion.
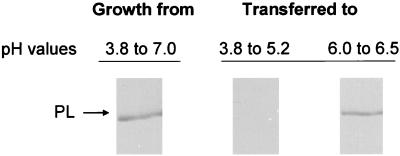
C. gloeosporioides mycelium from a culture grown in PEIM for 6 days, from pH 3.8 to PL secretion conditions at pH 7.0, was divided equally and transferred to PEIM at either pH 3.8 or 6.0. The hyphae did not secrete PL when transferred to pH 3.8 and grown to pH 5.2, 24 h later. However, when the mycelium was transferred to pH 6.0 and grown to pH 6.5, PL secretion was detected within 24 h (Fig. 2). The dry weights of the hyphae developed under both pH conditions during the 24-h period were the same. When mycelium grown under noninducing conditions (M3S medium) was transferred to PEIM at pH 3.8 and grown for 4 days to a pH not higher than 4.9 or 5.4, PL was not secreted. PL was detected in PEIM only when the pH reached 5.8 (Fig. 3). At the end of the experiment, the dry weight of the fungal hyphae at the low pH was 5 to 10% less than that of the hyphae grown at the high pH. When mycelium grown under noninducing conditions was transferred to PEIM adjusted to pH 6.0, PL was secreted within 24 h (Fig. 3).
FIG. 2.
Effect of PEIM pH on PL secretion by C. gloeosporioides following transfer from a 6-day-old culture grown in PEIM. Spores (1.5 × 106) were inoculated into PEIM and allowed to grow for 6 days until the pH reached 7.0. The mycelium mass was then washed, split equally, and transferred to PEIM at pH 3.8, and the pH of PEIM was adjusted to 6.0. The mycelium transferred to PEIM at pH 3.8 was grown for 24 h until the pH reached 5.2 and then the medium was sampled. The mycelium transferred to PEIM at pH 6.0 was grown for 24 h until the pH reached 6.5 and then the medium was sampled. The culture media were concentrated, dialyzed, and further concentrated for SDS-PAGE (5 μg of protein/lane), and then they were analyzed for the presence of PL by Western blot analysis.
FIG. 3.
Secretion of PL by C. gloeosporioides when grown on PEIM up to specific pH values. Spores (1.5 × 106) were inoculated into M3S medium and allowed to grow for 4 days. The mycelium was then washed thoroughly and transferred to PEIM that was adjusted with 32% HCl during growth to pH values not exceeding 4.9, 5.4, and 5.8. At the same time, the pH of the control changed from 6.0 to 7.0. The culture medium was concentrated, dialyzed, further concentrated before being loaded onto an SDS-PAGE gel (5 μg of protein/lane), and analyzed for the presence of PL by Western blot analysis.
pH levels in pericarp and mesocarp of avocado fruit during development and ripening.
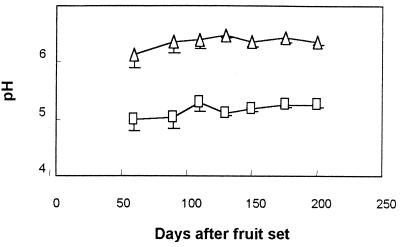
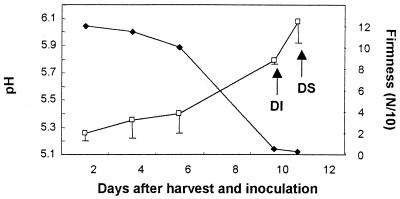
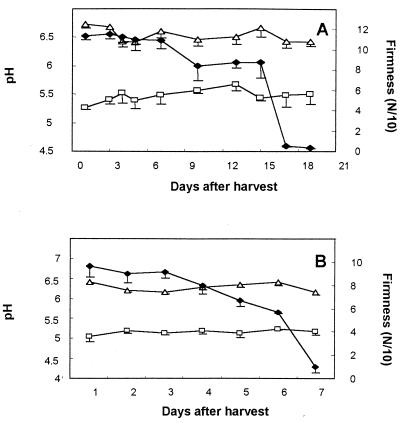
The pH of the pericarp, from 50 days after fruit set (immature fruits) to 200 days later (mature fruits), was approximately 5.2 (Fig. 4). No symptoms of decay were observed during this period. When P. americana cv. Fuerte fruits were harvested at the regular harvesting period and stored at 20°C to ripen, the pH in the pericarp increased from 5.2 to 6.1, with decay initiation (decay diameter < 0.5 cm) at pH 5.8 and decay symptoms (decay diameter > 0.5 cm) at pH 6.1 (Fig. 5). Mesocarp pH values averaged 6.4 during the ripening period (data not shown).
FIG. 4.
Changes in the pericarp (□) and mesocarp (▵) pH values of cultivar Fuerte avocado fruits on different days after fruit set. Bars represent the standard deviations of the mean of three independent tests from one representative experiment. The differences between the means of pericarp and mesocarp pH are significant (P < 0.05).
FIG. 5.
Changes in pH values of the pericarp (□) of cultivar Fuerte avocado fruits during postharvest ripening. Firmness (⧫) is presented as a parameter of ripening. Arrow indicates the time of decay initiation (DI) and of decay symptoms (DS) of C. gloeosporioides, following inoculation of freshly harvested fruits. Bars represent the standard deviations of the mean from one representative experiment. Cultivar Fuerte fruits were harvested at midseason.
The concentrations of the preformed antifungal diene in the pericarp of resistant cultivars Ardit and Ettinger was subfungitoxic (210 ± 10 and 330 ± 20 μg/g [fresh weight {FW}], respectively, compared to 960 ± 110 μg/g [FW] in cultivar Fuerte fruits). Interestingly, cultivar Ardit and Ettinger fruits had average pericarp pH levels of 5.5 and 5.1, respectively, and the average pH of the mesocarp of both cultivars was 6.5 (Fig. 6).
FIG. 6.
Changes in pH values of the pericarp (□) and mesocarp (▵) of cultivar Ardit (A) and Ettinger (B) avocado fruits during postharvest ripening. Firmness (⧫) is presented as a parameter of ripening. Bars represent standard deviations of the mean from one representative experiment. Cultivars Ettinger and Ardit were harvested at the same time, Ettinger late in its harvesting season and Ardit early in its harvesting season. The differences between the means of pericarp and mesocarp pH values in both cultivars are significant (P < 0.01).
Level of PL and PG regulation.
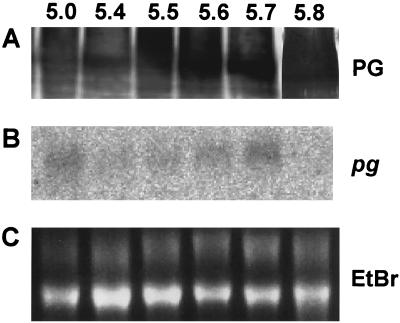
Northern blot analysis of C. gloeosporioides hyphae harvested from PEIM at final pH levels of 3.8 to 5.8 detected pel transcripts at pH 5.0. The transcript amount increased steadily up to pH 5.7 (Fig. 7B). pel expression was not accompanied by detectable PL in the culture medium. PL secretion was detected only when the medium pH rose above 5.8 (Fig. 7A). However, the expression (Fig. 8B) and secretion (Fig. 8A) of PG were detected from pH 5.0 to 5.7.
FIG. 7.
Effect of PEIM pH on mRNA expression of pel and secretion of PL from C. gloeosporioides. C. gloeosporioides was grown in PEIM whose pH was initially 3.8. At different pH levels, hyphae and culture media were harvested. Hyphae were subjected to RNA extraction, and culture medium was concentrated and dialyzed before PL analysis. Hybridizations were carried out at 63°C. The radioactive probe for pel was a full-length cDNA pel clone. (A) Western blot analysis; (B) Northern blot analysis; (C) ethidium bromide (EtBr) RNA loading.
FIG. 8.
Effect of PEIM pH on pg expression and secretion of PG from C. gloeosporioides. C. gloeosporioides was grown in PEIM whose pH was initially 3.8. At different pH levels, hyphae and culture media were harvested. Hyphae were subjected to RNA extraction, and culture medium was concentrated and dialyzed before PG analysis. Hybridization was carried out at 63°C. The radioactive probe for pg was a 487-bp fragment obtained by PCR. (A) Western blot analysis; (B) Northern blot analysis; (C) ethidium bromide (EtBr) RNA loading.
DISCUSSION
Resistance of unripe avocado fruits to C. gloeosporioides depends on antifungal compounds (17–19, 23). However, two cultivars, Ettinger and Ardit, have unripe fruits with concentrations of the antifungal diene that are significantly lower than the 50% effective dose of 450 μg/ml (ca. 330 μg of diene/g [FW] of pericarp) but are still resistant to C. gloeosporioides attack (17). This result suggests that the absence of fungal colonization may depend on the inhibition of secretion of pathogenicity factors, such as pectolytic enzymes (17). In the Colletotrichum-avocado system, high concentrations of the flavonoid epicatechin, an inhibitor of PL and PG, in the pericarp of unripe fruits was hypothesized to affect fungal colonization (26). However, the specific effect of host environmental conditions on the secretion of pectolytic enzymes has not been described.
C. gloeosporioides did not colonize avocado fruits when the pericarp pH was lower than 5.8. These low pH values are detected in cultivar Fuerte fruit pericarp during all periods of fruit growth before ripening and in the ripe Ettinger and Ardit cultivars. However, inoculation of C. gloeosporioides on either mesocarp or pericarp tissue of ripe fruits with pH levels higher than 5.8 caused decay symptoms within 2 days. C. gloeosporioides can cause disease in unripe mesocarp (8) that has a pH higher than 5.8, suggesting that the physiological stage of fruit ripening does not determine susceptibility to fungal colonization. When mycelium grown in PEIM at pH 6.5 was transferred to fresh PEIM at a pH of ≤5.7, no PL secretion was detected. PL also was not secreted if the medium was adjusted to pH levels of ≤5.7 at any time during the 4-day incubation. This behavior differed from that of PG, where pg transcripts and protein secretion occur between pH 5.0 and 5.8.
How pH affects the secretion of PL in C. gloeosporioides is still unknown. pH may affect a series of regulatory processes in fungi and yeast (5–7, 9, 11, 13, 14, 16). In the present study, the lack of PL secretion until transcript levels peaked suggests that PL is translated but that the protein remains in the mycelia until a secretion-permissive pH level is reached. The lack of PL secretion in PEIM at low pH following the transfer of mycelium from conditions where PL was being secreted further supports the hypothesis that PL secretion is regulated by pH. The presence of PL in fungal hyphae grown in PEIM at pHs lower than 5.7 suggests that PL is preformed and then released from the fungal mycelium by a pH-dependent mechanism. N-glycosidase F treatment of hyphal protein extracts significantly reduced the molecular weight of the putative intermediate PL-processed proteins, suggesting that the nonsecreted protein is glycosylated (data not shown).
Since C. gloeosporioides easily macerates avocado tissue (20), we hypothesize that the basal level of PG secretion may locally digest avocado pericarp cell walls, disrupt cell compartmentation, and expose the developing hyphae to the low-pH environment of the vacuole. Present and previous results (28) emphasize the importance of PL secretion for decay development in ripening fruits. Our results suggest that modulation of PL secretion by the host is an important mechanism that works together with preformed antifungal compounds to inhibit fungal colonization (17). The reduction in acidity and pH increase in ripening and senescing fruits and the consequent decay development occurring during this period also suggest that the effect of pH on pectolytic enzyme secretion may have a broader significance than for avocado fruits alone and could be used for breeding and control of decay occurring in other postharvest pathosystems.
ACKNOWLEDGMENTS
This research was supported by the Binational Agricultural Research and Development Fund (BARD), the German-Israel Agricultural Research Agreement (GIARA) for the Benefit of the Third World, and the U.S.-Israel Cooperative Development Research (CDR) Program.
We thank N. T. Keen for critically reviewing the manuscript and for suggesting significant changes.
Footnotes
Contribution 407-2000 from the Agricultural Research Organization, The Volcani Center, Bet Dagan, Israel.
REFERENCES
- 1.Annis L S, Goodwin H P. Recent advances in the molecular genetics of plant cell wall-degrading enzymes produced by plant pathogenic fungi. Eur J Plant Pathol. 1997;103:1–14. [Google Scholar]
- 2.Bowen K J, Templeton D M, Sharrock R K, Crowhurst N R, Rikkerink A H E. Gene inactivation in the plant pathogen Glomerella cingulata: three strategies for the disruption of the pectin lyase gene pnlA. Mol Gen Genet. 1995;246:196–205. doi: 10.1007/BF00294682. [DOI] [PubMed] [Google Scholar]
- 3.Centis S, Guillas I, Sejalon N, Esquerre-Tugaye M-T, Dumas B. Endopolygalacturonase genes from Colletotrichum lindemuthianum: cloning of CLPG2 and comparison of its expression to that of CLPG1 during saprophytic and parasitic growth of the fungus. Mol Plant-Microbe Interact. 1997;10:769–775. doi: 10.1094/MPMI.1997.10.6.769. [DOI] [PubMed] [Google Scholar]
- 4.Condemine G, Robert-Baudouy J. Synthesis and secretion of Erwinia chrysanthemi virulence factors are coregulated. Mol Plant-Microbe Interact. 1995;8:632–636. [Google Scholar]
- 5.Dean A R, Timberlake E W. Regulation of the Aspergillus nidulans pectate lyase gene (pelA) Plant Cell. 1989;1:275–284. doi: 10.1105/tpc.1.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espeso A E, Tilburn J, Arst N H, Penalva A M. pH regulation is a major determinant in expression of a fungal penicillin biosynthetic gene. EMBO J. 1993;12:3947–3956. doi: 10.1002/j.1460-2075.1993.tb06072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller P N, Nesbitt C, Sarr B, Phillips D T, Burow B G. pH regulation of sterigmatocystin and aflatoxin biosynthesis in Aspergillus spp. Phytopathology. 1997;87:643–648. doi: 10.1094/PHYTO.1997.87.6.643. [DOI] [PubMed] [Google Scholar]
- 8.Kobiler I, Prusky D, Midland S, Sims J J, Keen N T. Compartmentation of antifungal compounds in oil cells of avocado fruit mesocarp and its effect on susceptibility to Colletotrichum gloeosporioides. Physiol Mol Plant Pathol. 1993;43:319–328. [Google Scholar]
- 9.Lichter A, Mills D. Control of pigmentation of Ustilago hordei: the effect of pH, thiamin, and involvement of cAMP cascade. Fungal Genet Biol. 1998;25:63–74. doi: 10.1006/fgbi.1998.1087. [DOI] [PubMed] [Google Scholar]
- 10.Lurie S, Pesis E. Effect of acetaldehyde and anaerobiosis as postharvest treatments on the quality of peaches and nectarines. Postharvest Biol Technol. 1992;1:317–326. [Google Scholar]
- 11.Maccheroni W, Jr, May S G, Martinez-Rossi M N, Rossi A. The sequence of palF, an environmental pH response gene in Aspergillus nidulans. Gene. 1997;194:163–167. doi: 10.1016/s0378-1119(97)00095-4. [DOI] [PubMed] [Google Scholar]
- 12.MacKenzie A D, Jeenes D J, Belshaw N J, Archer B D. Regulation of secreted protein production by filamentous fungi: recent developments and perspectives. J Gen Microbiol. 1993;139:2295–2307. doi: 10.1099/00221287-139-10-2295. [DOI] [PubMed] [Google Scholar]
- 13.Muhlschlegel A F, Fonzi A W. PHR2 of Candida albicans encodes a functional homolog of the pH-regulated gene PHR1 with an inverted pattern of pH-dependent expression. Mol Cell Biol. 1997;17:5960–5967. doi: 10.1128/mcb.17.10.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orejas M, Espeso A E, Tilburn J, Sarkar S, Arst N H, Jr, Penalva A M. Activation of the Aspergillus PacC transcription factor in response to alkaline ambient pH requires proteolysis of the carboxy-terminal moiety. Gen Dev. 1995;9:1622–1632. doi: 10.1101/gad.9.13.1622. [DOI] [PubMed] [Google Scholar]
- 15.Ortega J. Pectolytic enzymes produced by the phytopathogenic fungus Colletotrichum gloeosporioides. Tex J Sci. 1996;48:123–128. [Google Scholar]
- 16.Otero C R, Gaillardin C. Dominant mutation affecting expression of pH-regulated genes in Yarrowia lipolytica. Gen Genet. 1996;252:311–319. doi: 10.1007/BF02173777. [DOI] [PubMed] [Google Scholar]
- 17.Prusky D. Pathogen quiescence in postharvest diseases. Annu Rev Phytopathol. 1996;34:413–434. doi: 10.1146/annurev.phyto.34.1.413. [DOI] [PubMed] [Google Scholar]
- 18.Prusky D, Keen N T. Involvement of preformed antifungal compounds in the resistance of subtropical fruit decay. Plant Dis. 1993;77:114–119. [Google Scholar]
- 19.Prusky D, Kobiler I, Jacoby B. Involvement of epicatechin in cultivar susceptibility of avocado fruits to Colletotrichum gloeosporioides after harvest. Phytopathol Z. 1988;123:140–146. [Google Scholar]
- 20.Prusky D, Gold S, Keen N T. Purification and characterization of an endopolygalacturonase produced by Colletotrichum gloeosporioides. Physiol Mol Plant Pathol. 1989;35:121–133. [Google Scholar]
- 21.Prusky D, Plumbley R A, Kobiler I. The relationship between antifungal diene levels and fungal inhibition during quiescent infection of unripe avocado fruits by Colletotrichum gloeosporioides. Plant Pathol. 1991;40:45–52. [Google Scholar]
- 22.Sambrook J, Fritsch F E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Simmonds J H. Latent infection in tropical fruits discussed in relation to part played by species of Gloeosporium and Colletotrichum. Proc R Soc Queensl. 1941;52:92–120. [Google Scholar]
- 24.St. Leger R J, Joshi L, Roberts D. Ambient pH is a major determinant in the expression of cuticle-degrading enzymes and hydrophobin by Metarhizium anisopliae. Appl Environ Microbiol. 1998;64:709–713. doi: 10.1128/aem.64.2.709-713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu J C. An improved Mathur's medium for growth, sporulation and germination of spores of Colletotrichum lindemuthianum. Microbiosis. 1985;44:87–93. [Google Scholar]
- 26.Wattad C, Dinoor A, Prusky D. Purification of pectate lyase produced by Colletotrichum gloeosporioides and its inhibition by epicatechin: a possible factor involved in the resistance of unripe avocado fruits to anthracnose. Mol Plant-Microbe Interact. 1994;7:293–297. doi: 10.1094/mpmi-7-0293. [DOI] [PubMed] [Google Scholar]
- 27.Wattad C, Freeman S, Dinoor A, Prusky D. A nonpathogenic mutant of Colletotrichum magna is deficient in extracellular secretion of pectate lyase. Mol Plant-Microbe Interact. 1995;8:621–626. [Google Scholar]
- 28.Wattad C, Kobiler D, Dinoor A, Prusky D. Pectate lyase of Colletotrichum gloeosporioides attacking avocado fruits: cDNA cloning and involvement in pathogenicity. Physiol Mol Plant Pathol. 1997;50:197–212. [Google Scholar]