Abstract
Strongyloides stercoralis is a soil-transmitted helminth endemic to tropical and subtropical regions and can be acquired due to parasite penetration through the skin. It can remain dormant in the gastrointestinal system for decades after the primary infection. In immunocompromised patients, this parasite can cause autoinfection with progression to hyperinfection syndrome. Here we report a unique case of pulmonary strongyloidiasis in a 32-year-old female, originally from Guatemala, with a significant clinical history of Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia diagnosed in 2019. The patient is status post chemotherapy with tyrosine kinase inhibitor plus hyper-CVAD regimen (Cyclophosphamide, Vincristine sulfate, Doxorubicin hydrochloride (Adriamycin), and Dexamethasone). History of drug-induced hyperglycemia and obesity was also noted. Her current chief complaint included dyspnea, tachycardia, and chest pain. Chest computerized tomography (CT) scan showed diffuse interstitial pulmonary edema with septal thickening, scattered ground-glass opacities, and small pericardial effusion. Due to normal ejection fraction, the differential diagnosis included non-cardiogenic pulmonary edema, pneumonitis secondary to chemotoxicity, and infection. She rapidly progressed to acute hypoxic respiratory failure, and a bronchoalveolar lavage study revealed numerous larvae consistent with Strongyloides hyperinfection. Further workup revealed eosinophilia with negative Strongyloides IgG antibody. Given the rarity of this infection in the United States and the patient’s place of birth, acquired latent Strongyloides infection is favored as the initial source of infection. The reactivation of the infection process was most likely secondary to her chemotherapy treatment. Strongyloides hyperinfection diagnosis can be challenging to establish and entails a high level of suspicion. Cytology evaluation is an essential factor for diagnosis.
Keywords: Strongyloidiasis, Strongyloides stercoralis, Autoinfection, Hyperinfection, Acute lymphoblastic leukemia
Introduction
Strongyloidiasis is a chronic parasite infection caused by Strongyloides stercoralis. It is endemic in tropical and subtropical areas [1]. Poor sanitary conditions and skin contact with contaminated soil are major risk factors for disease. The parasite can penetrate the human skin and migrate through the circulation to the lungs once the rhabditiform larvae developed into filariform larvae in moist soil [2], [3]. Eventually, these larvae escape the alveolar spaces and migrate to the pharynx. Larvae can settle in the small intestine and mature into adults after being swallowed in pharyngeal secretion. Nematode parasitic adults are only females (3 mm) and for reproduction, use either parthenogenesis (reproduction without fertilization) inside the host’s small intestine or sexual reproduction (with fertilization) outside the host. Larvae can be shed in the stool or become filiform larvae capable of penetrating the intestinal tract, causing internal or external (skin) autoinfection. In immunocompromised patients, the parasite can cause autoinfection without further exogenous infestation [2]. Hyperinfection syndrome can occur from autoinfection leading to massive and disseminated life-threatening parasitic infection. While multiple organs can be affected in hyperinfection syndrome, most presentations involve the lungs and gastrointestinal tract. In non-endemic areas, strongyloidiasis often presents as a diagnostic challenge [4], [5].
Case report
The patient, a 32-year-old female with Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia diagnosed in 2019, presented with a ten-day history of increasing dyspnea, tachycardia, and chest pain. She is originally from Guatemala; however, she had not returned to Guatemala for approximately fifteen years. Her medical history is also notable for drug-induced hyperglycemia and obesity.
In April 2019, she was admitted for a one-month history of fatigue, lethargy, and diffuse pain in her spine and upper and lower extremities. At the time, she had noticed new bruising in her skin without any significant trauma. Blood tests showed a white blood cell count of 55,000 × 109/L with increased blasts. Her peripheral blood smear showed normochromic, normocytic anemia, thrombocytopenia, and 88% blasts. CT imaging studies demonstrated a right breast mass and spinal lesions. Bone marrow aspiration and biopsy showed acute B-lymphoblastic leukemia (B-ALL) with t(9;22)(q34.1;q11.2) BCR-ABL1. Analysis by FISH was abnormal and indicated approximately 90% of nuclei with BCR/ABL1 fusion and a partial ETV6 deletion as well as a partial CDKN2A deletion in 61.5% of nuclei. She underwent induction chemotherapy with AYA protocol (AALL 0232), which consisted of a tyrosine kinase inhibitor plus a hyper-CVAD regimen and bone marrow transplant. Additionally, she received central nervous system (CNS) prophylaxis with intrathecal methotrexate and ARA-C per protocol. On day 28, after bone marrow transplant, she was negative for minimal residual disease and was discharged on dasatinib.
In August 2019, she was started on maintenance with a POMP regimen (6-mercaptopurine, vincristine, methotrexate, prednisone), and in January 2020, she relapsed with blasts in the cerebrospinal fluid (CSF). The POMP regimen was switched to high-dose methotrexate. Due to persistent CNS disease, she received intrathecal methotrexate, cytarabine, and hydrocortisone every two weeks for five cycles. The treatment was briefly interrupted due to COVID-19 infection and resumed in July of 2020. In November 2020, the patient was admitted with complete vision loss in the right eye. Magnetic resonance imaging (MRI) showed concern for leukemic infiltration of the right optic nerve and a new pineal mass. The patient underwent whole-brain radiotherapy in 10 fractions. In January of 2021, she was re-admitted with leukocytosis and circulating blasts. At this time, a CT scan showed hepatosplenomegaly and numerous lytic lesion in the axial skeleton. She received one cycle of etoposide, ifosfamide, and mitoxantrone. In February 2021, the patient started systemic chemotherapy with hyper-CVAD, and in May 2021, cycle 3 part A was interrupted because of interval development of acute appendicitis requiring emergent surgery. The surgical specimen only showed pathologic alterations associated with appendicitis. In June of 2021, the patient completed cycle 3, part B.
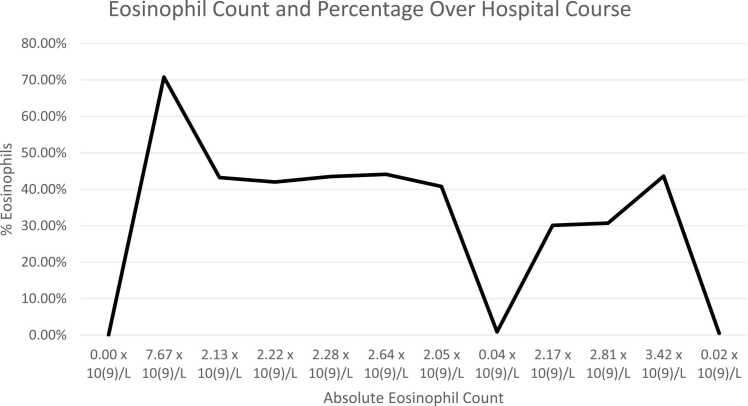
A month later, at the time of admission for her scheduled hyper-CVAD cycle 4 part B treatment, she developed shortness of breath, central and left chest pain that worsened with inspiration, non-productive cough, back pain, and epigastric pain. Additionally, she developed acute hypoxic respiratory failure, so the chemotherapy was held. On admission, she was afebrile and had an elevated ESR of 46.0 mm/hr. Initial absolute eosinophil count was within normal range, however, twelve days later, it was increased at 7.67 × 10(9)/L and twenty-three days later decreased to 2.13 × 10(9)/L. (Fig. 1). Concurrently, she developed a diffuse pruritic maculopapular rash bilaterally on the scapular region, right thigh, and knee. The primary differential diagnosis was herpes zoster infection related to dermatitis. However, dermatology was consulted and attributed the cause of her rash to her prescribed Percocet.
Fig. 1.
: Documented eosinophil count over two-month period during patient’s hospital course.
Blood cultures were obtained; one out of two were positive for coagulase-negative staphylococci, likely a contaminant and fungal cultures were negative. Additionally, despite of being tested positive for Mycobacterium tuberculosis by QuantiFERON-TB Gold three months before this admission and treated with Isoniazid for latent tuberculosis infection, current respiratory cultures for Mycobacteria were negative.
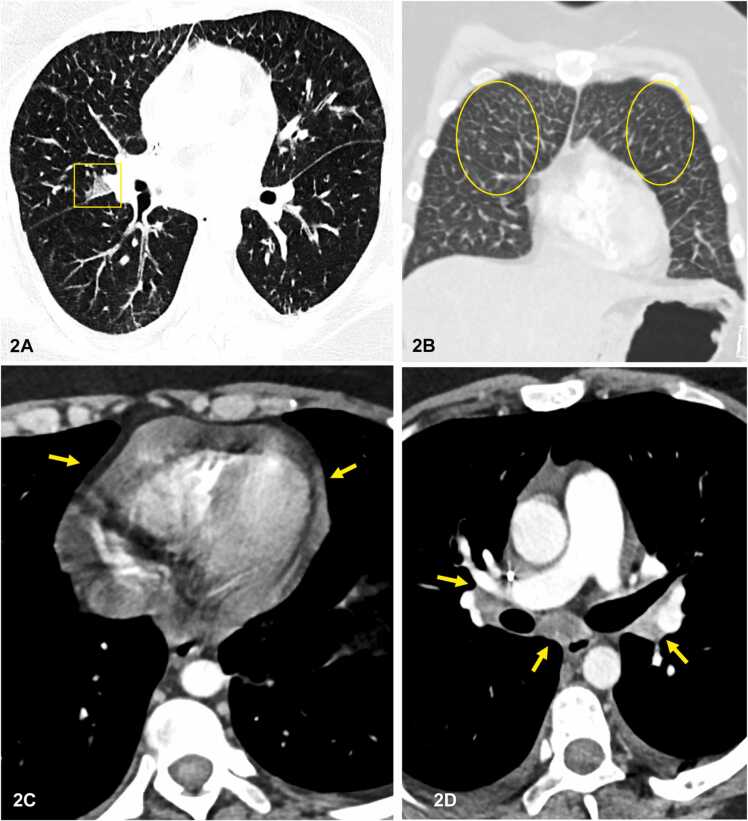
A new chest CT scan demonstrated bilateral pulmonary focal ground-glass opacities and interlobular septal thickening, consistent with pulmonary edema. Small pericardial effusion and mild bilateral hilar and mediastinal lymphadenopathy were also identified. Overall findings, in conjunction with an ejection fraction of 65% on echocardiogram, non-cardiogenic pulmonary edema, and pericardial effusion were favored (Fig. 2) [6], [7]. Due to the treatment with multiple cardiotoxic chemotherapeutic agents since 2019, possible pneumonitis secondary to chemotoxicity was included as a probable differential diagnosis. The patient’s chest CT scan findings lacked diffuse ground-glass opacities (GGOs), common to many infectious processes and incredibly opportunistic infections. Cyclophosphamide, cytarabine, doxorubicin, and Methotrexate are capable of causing interstitial non-cardiogenic pulmonary edema and pericardial effusion. The patient received intravenous methylprednisolone, and a bronchoscopy with brochoalveolar lavage (BAL) was performed. Forty milliliters of pink fluid with mucoid material was placed in ThinPrep® CytoLyt solution (methanol-based buffered preservative solution) and sent to cytology for further processing and analysis. A ThinPrep slide and two cytospins, one stained with Giemsa stain and the second one stained with Grocott’s Methenamine Silver stains (GMS), were performed. Numerous filariform larvae with short buccal cavity and prominent genital primordium were identified, consistent with Strongyloides. These morphologic findings were consistent with Strongyloides, thus leading to the diagnosis of Strongyloides hyperinfection (Fig. 3, Fig. 4). Stool analysis was negative for ova and parasites (O&P). She was started on Ivermectin (200 mcg/kg/day) for two weeks. Thirteen days later, pathology confirmed the newly submitted specimen after treatment was negative for parasites, despite persistent eosinophilia (absolute eosinophil count of 2.64 ×10(9)/L). The patient's follow-up course was further complicated with right-sided blindness, latent tuberculosis infection, and chronic pain at direct admission for chemotherapy. Her stool O&P was negative for Strongyloides larvae. Given the patient's multiple systemic comorbidities secondary to her relapsed ALL, the palliative care team was consulted to collaborate in future care and management of this patient.
Fig. 2.
Contrast-enhanced CT chest. A) axial image lung window showing patchy area of ground glass. opacification (yellow box). B) coronal image lung window demonstrating interlobular septal thickening, consistent with pulmonary edema (yellow circles). C, D) axial mediastinal window images showing pericardial. effusion (yellow arrows) in (C) and non-specific midly enlarged mediastinal and bilateral hilar lymph nodes. (yellow arrows) in (D).
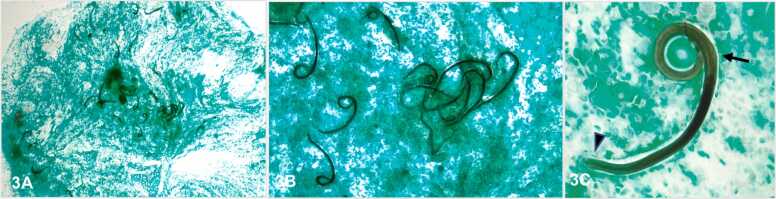
Fig. 3.
Bronchoalveolar lavage, Grocott-Gomori methenamine-silver (GMS) stain. A) 20x, numerous Strongyloides stercoralis larva. B) 100x, numerous Strongyloides stercoralis larva. C) 400x, single Strongyloides stercoralis larva with short buccal grove (arrow head) and prominent genital primordium (arrow).
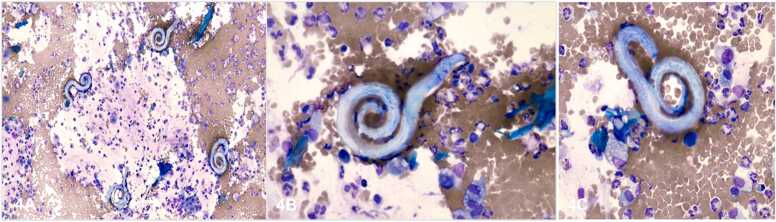
Fig. 4.
Bronchoalveolar lavage, Wright-Giemsa stain. A). 400x, four Strongyloides stercoralis larva. B) 400x, a single Strongyloides stercoralis larva. C) 400x, a single Strongyloides stercoralis larva.
Discussion
The patient, originally from Guatemala, presented with dyspnea and eosinophilia. When taken together with BAL findings, her chronic eosinophilia and recurrent rashes are most consistent with chronic strongyloidiasis. However, the patient’s complex comorbidities and the CT scan findings made this an atypical presentation of strongyloidiasis.
The clinical history of leukemia and immunosuppression secondary to multiple chemotherapeutic agents increased the likelihood of developing acute respiratory distress syndrome secondary to cardiotoxicity. A wide spectrum of imaging findings, such as diffuse or focal ground-glass opacities, pulmonary consolidation, interlobular thickening, interstitial infiltrates/fibrosis, cavitary lesions, and miliary nodular pattern, can be identified in patients with strongyloidiasis [6]. Our patient presented with focal ground-glass opacification and interlobular septal thickening on chest CT scan imaging. In early-onset chemotherapy-induced toxicity, CT scan imaging shows infiltrates, pulmonary edema, pleural effusion, ground-glass infiltrates, while during late-onset demonstrates infiltrates or fibrosis [7]. The described findings in our case favor the possibility of chemotherapy-induced toxicity and are consistent with the conclusions reported in the literature. The complex and atypical chest CT scan findings and progression of the patient’s acute respiratory distress and clinical symptoms prompted the performance of bronchoscopy.
Broncheoalveolar lavage obtained for cytology confirmed the diagnosis of Strongyloides stercoralis infection. The submitted specimen was processed by high-speed centrifugation using standard smear preparation, and only few small larvae passed through the filter. The additional cytospins, made with the remaining material, yielded better results than the ThinPrep since the larvae in the fluid were concentrated and placed directly on the smear. In this particular scenario, cytospin method is superior than standar liquid base smear preparation (either ThinPrep or SurePath) since the microorganisms present in the specimen show a better preserved morphology. Moreover, cytospins can be stained with GMS and/or Giemsa special stains which highlight even more the pathogens’ morphology, essencial for diagnosis and correct classification. Contrary to cytospins, due to the alcohol (methanol) present in CytoLyt, only Papanicolaou stain can be done in ThinPrep. This stain makes the larvae to look transparent and shiny when the condenser attached to the microscope is removed.
Ova and parasite testing have very low sensitivity in Strongyloidiasis due to the low number of larva shed in the stool [8], [9]. Many studies focus on detecting the parasite by stool examination despite a yield that does not exceed 46% even after three stool examinations [10]. Increased attention should be paid to searching the parasite in multiple tissues, particularly the gastrointestinal tract or bronchi. A sizeable parasitic burden is present during parasitic dissemination, leading to a high yield in the lung, bronchial, or small bowel biopsies [7].
In immunocompromised patients, eosinophilia may be absent. Thus, increased attention to travel history and clinical presentation is critical.
Notable, in this case, was the Strongyloides IgG antibody negative result. It has been previously described that Strongyloides IgG antibody testing is more sensitive but can be negative in acute infection, immunosuppression, or if infected with similar non-Strongyloides stercoralis species Halicephalobus and Pelodera [11], [12]. For those patients receiving immunosuppressant or steroid therapy, like in this particular case, the critical role of cytology in diagnosing Strongyloides stercoralis is emphasized. Cytologic examination of BAL is essential for pathogens identification, like Strongyloides, that do not grow in routine culture. The Strongyloides larva contains a short buccal cavity and prominent genital primordium(Fig. 3C). These particular findings are important to differentiate Strongyloides (tapeworm) from Hookworms. Hookworms have a longer buccal cavity and less prominent genital primordium. These anatomical details can be easily assessed in cytospins stained with Grocott's Methenamine Silver Stain (GMS) or Giemsa stain (Fig. 3, Fig. 4). In this particular case, cytological evaluation was essential to confirm the presence of the Strongyloides larvae and proceed with the appropriate treatment.
The first-line treatment for uncomplicated strongyloidiasis is Ivermectin, with a reported eradication rate of approximately 80% [13], [14]. A single-dose or two-dose of 200 mcg/kg/day of Ivermectin is often sufficient to eradicate the parasite in immunocompetent patients [15]. A four-dose regimen (200 mcg/kg daily for two days, repeated at two weeks) is the recommended treatment course in immunocompromised patients. If hyperinfection is present the dose remains 200 mcg/kg daily until stool exam is negative for 2 weeks [17]. Diagnostic studies such as BAL and stool samples must be repeated to determine the persistence of Strongyloides infection if symptoms do not resolve after initial therapy. The resolution of the patient's eosinophilia does not always indicate the elimination of this tapeworm. Since it is challenging to confirm eradication of the infection clinically, many experts prefer to repeat a two-day course of therapy one week after the initial course, with careful follow-up in patients with persistent symptoms and infection [15], [16]. In the United States, screening for Strongyloides is not a routine practice. However, extra attention and follow-up should be made in cases like the one discussed in this report.
Conclusion
Diagnosis of Strongyloides hyperinfection syndrome and disseminated disease can be challenging to establish and entails a high level of suspicion. Clinical suspicion should be raised in an immunosuppressed patient as the patient presented in this case.
The patient’s complex comorbidities and the chest CT scan findings made this an atypical presentation of strongyloidiasis. Her leukemia and immunosuppression secondary to multiple chemotherapeutic agents combined with the lack of diffuse ground-glass opacities (GGOs) common to many infectious processes, especially opportunistic infections on CT imaging, made chemotoxicity the most likely cause of her acute respiratory distress. Bronchoscopy was able to confirm the diagnosis. Cytological examination of bronchoscopy samples is vital for pathogens assessment, like Strongyloides, that do not grow in routine culture.
CRediT authorship contribution statement
Caitlyn J. Smith: Conceptualization, Writing − original draft. Carla R. Caruso: Supervision, Writing – review & editing. Kelly Bowers: Providing histopathology pictures, Writing – review & editing. Chase Baxter: Writing – review & editing. Ayman H. Gaballah: Supervision, Providing radiographic images, Writing – review & editing.
Conflicts of Interest
All authors have no financial disclosures or conflicts of interest.
References
- 1.Schär F., Trostdorf U., Giardina F., et al. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl Trop Dis. 2013;7 doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bethony J., Brooker S., Albonico M., Geiger S.M., Loukas A., Diemert D., et al. Soil transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 3.Lam C.S., Tong M.K., Chan K.M., et al. Disseminated strongyloidiasis: a retrospective study of clinical course and outcome. Eur J Clin Microbiol Infect Dis. 2006;25:14–18. doi: 10.1007/s10096-005-0070-2. [DOI] [PubMed] [Google Scholar]
- 4.Sato Y., Kobayashi J., Toma H., et al. Efficacy of stool examination for detection of Strongyloides infection. Am J Trop Med Hyg. 1995;53:248–250. doi: 10.4269/ajtmh.1995.53.248. [DOI] [PubMed] [Google Scholar]
- 5.Arifin N., Hanafiah K.M., Ahmad H., Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol, Immunol Infect. 2019;52(3):371–378. doi: 10.1016/j.jmii.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Nabeya D., Haranaga S., Parrott G.L., et al. Pulmonary strongyloidiasis: assessment between manifestation and radiological findings in 16 severe strongyloidiasis cases. BMC Infect Dis. 2017;17:320. doi: 10.1186/s12879-017-2430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torrisi L., Schwartz L.H., Gollub M.J., et al. CT findings of chemotherapy-induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology. 2011;258(1):41–56. doi: 10.1148/radiol.10092129. [DOI] [PubMed] [Google Scholar]
- 8.Salvador F., Sulleiro E., Sánchez-Montalvá A., et al. Usefulness of Strongyloides stercoralis serology in the management of patients with eosinophilia. Am J Trop Med Hyg. 2014;90(5):830–834. doi: 10.4269/ajtmh.13-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genta R.M. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev Infect Dis. 1989;11:755–767. doi: 10.1093/clinids/11.5.755. [DOI] [PubMed] [Google Scholar]
- 10.Thompson B.F., Fry L.C., Wells C.D., et al. The spectrum of GI strongyloidiasis: an endoscopic-pathologic study. Gastrointest Endosc. 2004;59:906–910. doi: 10.1016/s0016-5107(04)00337-2. [DOI] [PubMed] [Google Scholar]
- 11.Boggild A.K., Libman M., Greenaway C., McCarthy A.E. Committee to Advise on Tropical Medicine; Travel (CATMAT). CATMAT statement on disseminated strongyloidiasis: Prevention, assessment and management guidelines. Can Commun Dis Rep. 2016;42(1):12–19. doi: 10.14745/ccdr.v42i01a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirisi M., Salvador E., Bisoffi Z., et al. Unsuspected strongyloidiasis in hospitalised elderly patients with and without eosinophilia. Clin Microbiol Infect. 2006;12:787–792. doi: 10.1111/j.1469-0691.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- 13.Suputtamongkol Y., Premasathian N., Bhumimuang K., Waywa D., Nilganuwong S., et al. Efficacy and safety of single and double doses of ivermectin versus 7-day high dose albendazole for chronic strongyloidiasis. PLOS Negl Trop Dis. 2011;5(5) doi: 10.1371/journal.pntd.0001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fardet L., Généreau T., Poirot J.L., Guidet B., Kettaneh A., Cabane J. Severe strongyloidiasis in corticosteroid-treated patients: case series and literature review. J Infect. 2007;54:18–27. doi: 10.1016/j.jinf.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Henriquez-Camacho C., Gotuzzo E., Echevarria J., et al. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016;2016(1) doi: 10.1002/14651858.CD007745.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez E.A., Abraham T., Williams F.K. Severe strongyloidiasis with negative serology after corticosteroid treatment. Am J Case Rep. 2015;16:95–98. doi: 10.12659/AJCR.892759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mejia R., Nutman T.B. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis. 2012;25(4):458–463. doi: 10.1097/QCO.0b013e3283551dbd. [DOI] [PMC free article] [PubMed] [Google Scholar]