Abstract
The oxidation of either ferrous iron or sulfur by Thiobacillus ferrooxidans was selectively inhibited or controlled by various anions, inhibitors, and osmotic pressure. Iron oxidation was more sensitive than sulfur oxidation to inhibition by chloride, phosphate, and nitrate at low concentrations (below 0.1 M) and also to inhibition by azide and cyanide. Sulfur oxidation was more sensitive than iron oxidation to the inhibitory effect of high osmotic pressure. These differences were evident not only between iron oxidation by iron-grown cells and sulfur oxidation by sulfur-grown cells but also between the iron and sulfur oxidation activities of the same iron-grown cells. Growth experiments with ferrous iron or sulfur as an oxidizable substrate confirmed the higher sensitivity of iron oxidation to inhibition by phosphate, chloride, azide, and cyanide. Sulfur oxidation was actually stimulated by 50 mM phosphate or chloride. Leaching of Fe and Zn from pyrite (FeS2) and sphalerite (ZnS) by T. ferrooxidans was differentially affected by phosphate and chloride, which inhibited the solubilization of Fe without significantly affecting the solubilization of Zn.
Thiobacillus ferrooxidans is a gram-negative acidophilic chemolithoautotroph, using CO2 as a carbon source and obtaining its energy for growth from the oxidation of ferrous iron, sulfur, and reduced sulfur compounds (26). T. ferrooxidans was initially isolated from acidic copper-leaching waters and believed to be the dominant bacterium responsible for metal sulfide solubilization (18). Iron oxidation in response to pH (1, 28), organic acids (40), anions (2, 17), and cations (21, 38) has been extensively studied. Sulfur oxidation has received substantially less attention, with limited references to certain anions (26).
Thiobacilli have considerable economic importance in the treatment of acid mine drainage (10, 22) and desulfurization of waste gases (SO2 and H2S) (10, 12, 29). The use of bacteria in the mining industry is a growing field of interest (4, 25). Levels of tolerance of key metals by T. ferrooxidans growth on Fe2+ are as follows: Cd2+, 0.75 M; Ni2+, 1 M; Zn2+, 1 M; Cu2+, 0.6 M; Co2+, 0.15 M; Cr3+, 0.075 M; Pb2+, 1 mM; Hg+, 0.1 mM; Hg2+, 10 μM; and Ag+, 1 μM (21). The naturally occurring counterion sulfate is not inhibitory at 0.14 M (26, 39), and the concentration may reach as high as 1.25 M during bacterial leaching of sulfide minerals (7).
Metal extraction from mineral ore by T. ferrooxidans is achieved through two reactions: the oxidation of ferrous to ferric iron (2Fe2+ + 1/2 O2 + 2H+ → 2Fe3+ + H2O) and that of sulfide/sulfur to sulfuric acid (H2S + 2O2 → H2SO4 or S0 + 1 1/2O2 + H2O → H2SO4). Uranium solubilization from uraninite, for example, requires only iron oxidation (UO2 + 2Fe3+ → 2Fe2+ + UO22+, 2Fe2+ + 1/2 O2 + 2H+ → 2Fe3+ + H2O), while zinc solubilization from sphalerite necessitates sulfide oxidation (ZnS + 2O2 → ZnSO4). Metal extraction becomes complicated when ores contain mineral combinations (19, 27). In a pyrite-sphalerite mixture T. ferrooxidans will oxidize both sulfide (ZnS + 2O2 → ZnSO4) and iron plus sulfide [4FeS2 + 15O2 + 2H2O → 2Fe2(SO4)3 + 2H2SO4], creating difficulty in further zinc recovery from the leachate. Low concentrations of ferric sulfate are beneficial in the indirect leaching of mineral ores. Higher concentrations, however, result in the production of jarosite, a ferric iron precipitate which can cover the ore surface, preventing further leaching from occurring. Higher jarosite levels also produce an additional disposal problem.
We propose to show that the iron and sulfur oxidation activities of T. ferrooxidans can be differentially controlled through the use of specific anions and inhibitors. Under certain conditions iron oxidation can be blocked with little to no effect on sulfur oxidation and vice versa. Through this type of manipulation we hoped to achieve specific metal extraction from an ore sample, with the absence or at least reduction of contaminating metals.
MATERIALS AND METHODS
Media.
T. ferrooxidans strain SM-4 (20) was grown in modified 9K medium (M9K): 0.4 g of (NH4)2SO4, 0.1 g of K2HPO4, 0.4 g of MgSO4 · 7H2O, and 33.3 g of FeSO4 · 7H2O per liter, adjusted to pH 2.3 with H2SO4. Cells used for sulfur oxidation were grown in Starkey no. 1 medium (33) after adaptation on sulfur (37): 0.3 g of (NH4)2SO4, 3.5 g of KH2PO4, 0.5 g of MgSO4 · 7H2O, 0.25 g of CaCl2, and 18 mg of FeSO4 · 7H2O per liter, adjusted to pH 2.3 with H2SO4. Powdered sulfur (10 g of BDH sulfur per liter) was spread evenly over the surface after inoculation. Thiobacillus thiooxidans strain SM-6 grown on sulfur was used for most of the growth experiments on sulfur, since sulfur-adapted T. ferrooxidans was not available. The results of key experiments, however, were later confirmed with sulfur-adapted T. ferrooxidans strain SM-4.
Culture procedures.
Iron-grown cells were cultured in M9K using a 10% inoculum. The flasks were incubated at 25°C and placed on a rotary shaker at 150 rpm for 48 h. The culture was passed through Whatman no. 1 filter paper to remove the majority of the precipitated ferric iron. The supernatant was centrifuged at 8,000 × g for 10 min. The cell pellet was resuspended in 0.1 M β-alanine sulfate buffer (pH 2.3) and centrifuged at 1,000 × g for 5 min to allow further ferric iron sedimentation. The supernatant was transferred to a secondary tube and centrifuged at 10,000 × g for 10 min. The cells were centrifuged a fourth time, generating a final suspension of 50 mg of cells (wet weight) per ml in the same buffer. The protein concentration was determined using bovine serum albumin as the standard (37).
Sulfur-grown cells were cultured in Starkey no. 1 medium using a 2.5% inoculum. The stationary flasks were incubated at 28°C for 4 days. The cell collection procedure was identical to that for iron-grown cells.
Determination of iron and sulfur oxidation using cell suspensions.
The rates of iron and sulfur oxidation were measured using a Gilson oxygraph equipped with a Clark oxygen electrode at 25°C. The reaction vessel contained 10 μl of cell suspension (sulfur- or iron-grown cells), 0.1 ml of sulfur suspension (32 g of BDH S0 in 100 ml, plus 500 ppm of Tween 80) (for sulfur oxidation) or 0.5 μmol of FeSO4 · 7H2O (for iron oxidation), and various concentrations of potassium salts of anions, sucrose, or β-alanine buffer (all at pH 3.0 unless otherwise stated) to make a total volume of 1.2 ml. The effect of azide and cyanide was studied in 0.1 M β-alanine sulfate at pH 3. For sulfur oxidation by iron-grown cells, however, 100 μl of the cell suspension was required for accurate rate determinations. All tests whose results are shown in Fig. 1 were performed at pH 3 rather than pH 2.3 (growth pH) because of the lower sulfur oxidation activity at pH 2.3. Duplicate experiments using the same batch of cells were impossible to carry out for all of the conditions tested due to the instability of activity after more than 2 days of storage. Results, however, were reproducible with other batches of cells, although absolute activities varied from 10 to 20%. Standard deviations in the activity determinations fell within 10% of the stated values except for sulfur oxidation by iron-grown cells, where the values could deviate by as much as 20%. It should be noted that all of the experiments testing iron and sulfur oxidation were repeated with T. ferrooxidans ATCC 19859, with similar results.
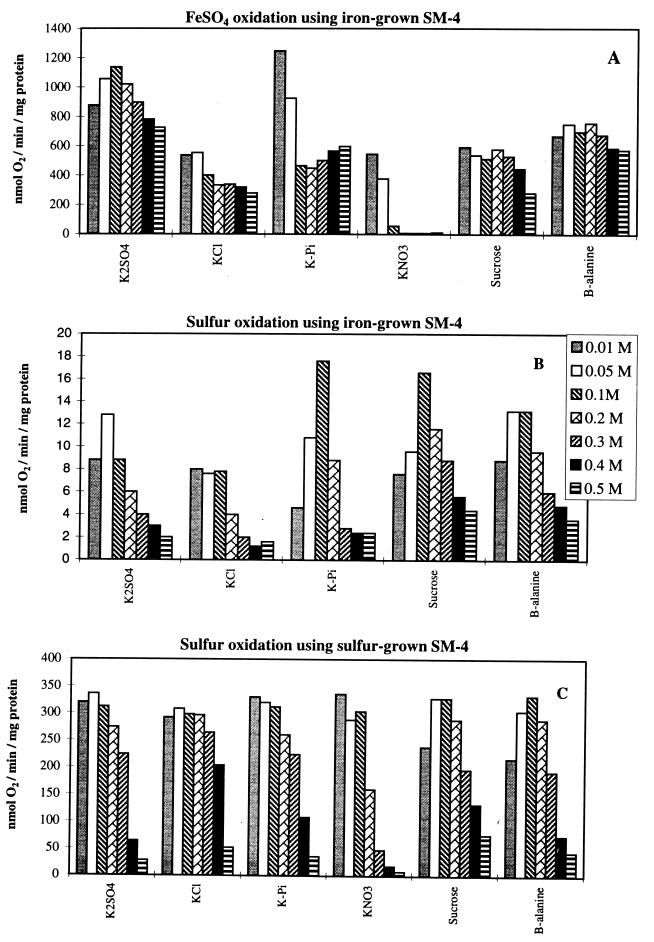
FIG. 1.
Effects of concentrations of various anions, sucrose, and β-alanine on the oxidation of ferrous iron and sulfur in iron-grown T. ferrooxidans strain SM-4 and of sulfur in sulfur-grown T. ferrooxidans strain SM-4. Please note the different scales on the y axes.
Determination of iron and sulfur oxidation and carbon dioxide fixation using growing cell cultures.
The rates of iron and sulfur oxidation in growing cell cultures were measured using a Micro-oxymax respirometer (Columbus Instruments) at Cominco Research Limited, Trail, British Columbia, Canada. The reaction vessel contained a 5% inoculum, 12 mmol of FeSO4 · 7H2O (for iron oxidation) or 1 g of BDH sulfur sprinkled on the surface plus 18 mg of FeSO4 · 7H2O per liter (for sulfur oxidation), various concentrations of anionic salts or inhibitors, and M9K at pH 2.3, making a total volume of 100 ml. The reaction was stirred with a magnetic stirrer, and both O2 consumption (oxidation) and CO2 consumption (autotrophic growth) were measured at 26°C.
Shake flask leaching of metals.
Flotation tailings, provided by Cominco Research Limited, contained 3.3% Zn as sphalerite and 5.5% Fe as pyrite (FeS2) and were in the form of a finely ground powder. Five grams of tailings was placed in a 250-ml Erlenmeyer flask with 100 ml of M9K at pH 2.3 with or without additional potassium phosphate. The flask was inoculated with 5 ml of T. ferrooxidans SM-4 (grown on FeSO4) and left stationary at 25°C for 24 h, followed by shaking on rotary shaker at 180 rpm for the remainder of the experiment. Five-milliliter samples were taken at time zero, 2 and 14 days, filtered through Whatman no. 1 filter paper, and analyzed for dissolved Fe and Zn content by atomic absorption spectrophotometry. The extent of metal leaching was calculated as percent extraction from the total metal content of the tailings.
RESULTS AND DISCUSSION
Effect of anions on iron and sulfur oxidation.
Cell suspensions were initially used to determine the effects of anions and selective inhibitors on the iron and sulfur oxidation activities of T. ferrooxidans. The reaction in each case was carried out at pH 3, an acidic pH comparable to natural growing conditions. The specific activities of iron (Fig. 1A) and sulfur (Fig. 1B and C) oxidation in buffer concentrations ranging from 0.01 to 0.5 M were determined. Enzymes required for iron and sulfur oxidation are differentially expressed depending on the bacterial growth substrate (14, 23, 37). Cells grown on ferrous iron showed only low levels of sulfur-oxidizing activities (Fig. 1B) compared to sulfur-grown cells (Fig. 1C). Although not shown in Fig. 1, iron oxidation experiments were also carried out at pHs 2.3 and 1.8, and they showed a stronger inhibition by the potassium salts. Sucrose was not inhibitory at all pH values, while β-alanine was less inhibitory than the potassium salts at lower pH values.
Sulfate is an anion that is normally associated with the environment in which the organism is found, e.g., acid mine drainage water. It was initially believed to act as a bridging ligand between ferrous iron and the cell (16, 17). Further experiments showed its role in the transfer of electrons from an iron sulfur cluster to the copper(II) ion of rusticyanin in the oxygen-dependent iron oxidation electron transport chain (8). A sulfate requirement for rusticyanin reduction by ferrous iron was also reported with a partially purified iron:rusticyanin oxidoreductase (3). Figure 1 shows that sulfate up to a concentration of 0.2 M had very little effect on either the iron or sulfur oxidation pathway. Beyond this point, iron oxidation was only marginally affected, while sulfur oxidation showed a dramatic drop in activity. This apparent preferential inhibition of sulfur oxidation at high sulfate concentrations is believed to be caused by changes in osmotic pressure, as it was observed in all other buffers at high concentrations (Fig. 1) except nitrate, a specific inhibitor of iron oxidation (17), as discussed below. High osmotic pressure inhibited sulfur oxidation in all the buffers tested both with iron-grown (Fig. 1B) and sulfur-grown (Fig. 1C) cells. Iron oxidation by the same iron-grown cells, however, was insensitive to high osmotic pressure (Fig. 1A).
Chloride is a known inhibitor of cell growth and ferrous iron oxidation (17, 26). It is an inhibitor of cell-free iron-cytochrome c reductase (6). A concentration of 0.14 M was reported as being toxic to the bacteria in its initial description in 1963 (26). Figure 1A shows that chloride is indeed inhibitory compared to sulfate for iron oxidation even at the lowest concentration used, 10 mM. Increased chloride concentrations resulted in a marginal added decrease in specific activity. Sulfur oxidation, on the other hand, was inhibited only at very high chloride concentrations. Iron-grown cells were inhibited at 0.2 M chloride (Fig. 1B), while sulfur-grown cells were relatively unaffected up to a concentration of 0.4 M (Fig. 1C).
Phosphate is required for normal bacterial function (2). Cells grown with phosphate limitation present a filamentous morphology due to a lack of cell division (31, 32). Phosphate starvation studies show changes in the degree of synthesis of at least 25 proteins, some of which are exclusively synthesized under starvation conditions (30–32). A number of these proteins have been linked to the bacterial surface, suggesting the existence of a phosphate scavenging system in T. ferrooxidans (13, 30). Phosphate concentrations used in this study were not limiting but rather in excess. The lowest phosphate concentration used, 10 mM, allowed for maximal iron and sulfur oxidation in iron- and sulfur-grown cells, respectively (Fig. 1A and C). Additional phosphate resulted in a sharp decrease in iron oxidation up to 0.1 M, followed by a moderate return of activity. Sulfur oxidation in iron-grown cells (Fig. 1B) showed both activation at low phosphate concentrations and inhibition at high phosphate concentrations. Sulfur-grown cells, on the other hand, showed inhibition only at high phosphate concentrations, similar to sulfate or chloride. Thus, iron-grown cells with low sulfur-oxidizing activities and sulfur-grown cells with high oxidizing activities responded differently at low phosphate concentrations yet similarly at high phosphate concentrations, distinct from their response in iron oxidation.
Nitrate is an inorganic anion known to inhibit oxidation by and growth of T. ferrooxidans on ferrous iron (17). Iron oxidation using cell suspensions was completely inhibited by sodium nitrate concentrations of 1 to 94 mM (17, 26). The large degree in variation is due to experimental design and strain specificity. T. ferrooxidans strain SM-4 was sensitive to nitrate at the lowest concentration used. Increased levels of nitrate completely inhibited iron oxidation. Sulfur oxidation was more resistant, showing little to no inhibition up to 0.1 M. Higher nitrate concentrations, however, resulted in a substantial drop in sulfur oxidation. It should be noted that nitrate was more strongly inhibitory in growth experiments on sulfur at pH 2.3, as shown below.
The sucrose and β-alanine buffers used in this study were adjusted with sulfuric acid. The purpose of these buffers was to show the effect of osmotic pressure on the two key reactions examined (iron and sulfur oxidation). Ferrous iron oxidation is believed to take place on the outer surface of the cell membrane (11). The ferrous ion is soluble and is expected to interact with the polynuclear iron coat surrounding the cell (11) or the iron-cytochrome c reductase on the exterior membrane (9). Only the electrons enter the cell, moving through the electron transport system, with the final reduction of oxygen to water. In this general scheme, osmotic pressure is not expected to have any significant effect on iron oxidation. Sucrose and β-alanine at high concentrations, i.e., high osmotic pressure, had little effect on iron oxidation (Fig. 1A). Sulfur oxidation, on the other hand, in both iron- and sulfur-grown cells (Fig. 1B and C) was inhibited by high osmotic pressure.
As shown in Fig. 1, phosphate, chloride, and nitrate all preferentially inhibited iron oxidation. Phosphate caused 50% inhibition of iron oxidation at 100 mM, the concentration at which sulfur oxidation was maximal. Sulfur oxidation activities of iron-grown cells and sulfur-grown cells were equally inhibited by increasing osmotic pressures, created by high concentrations of either inorganic salts (K2SO4, KCl, KPi, or KNO3), sucrose, or β-alanine sulfate (Fig. 1). Essentially identical results were obtained with T. ferrooxidans ATCC 19859. Since growth on sulfur induces new proteins in iron-grown T. ferrooxidans (23), it is surprising that the two types of cells responded similarly. This uniform effect must be directly related to the mechanism of sulfur oxidation, which is different from that of iron oxidation. Sulfur, unlike ferrous iron, is an insoluble substrate. At high osmotic pressure the cells lose water and the membranes shrink, making contact with the sulfur particles and their subsequent oxidation difficult. Similar inhibition of sulfur oxidation by high osmotic pressure has been obtained with T. thiooxidans (36). The general trend observed with cell suspensions (Fig. 1) was found to be applicable to growing cell cultures, as shown below.
Effects of azide and cyanide on iron and sulfur oxidation.
The sulfur and iron oxidation pathways and their interactions are surrounded by a great deal of controversy. Sugio et al. suggest that ferric iron reduction is coupled to sulfur oxidation under both aerobic and anaerobic conditions (34, 35). An alternate theory proposed by Corbett and Ingledew suggests that the iron and sulfur oxidation pathways are two separate entities but that ferric iron can replace oxygen as a terminal electron acceptor under anaerobic conditions (5). Iron- and sulfur-dependent oxygen uptake occurs via two separate oxidases (24). Azide at low concentrations is a specific inhibitor of the terminal oxidase of ferrous iron oxidation, but not that of sulfur oxidation (24). The results in Table 1 agree with the concept of two terminal oxidases being differentially inhibited by azide. Inhibition of iron oxidation by 50% required only 0.7 μM azide, while that of sulfur oxidation required 19 to 32 μM azide. Cyanide is also a specific inhibitor of the terminal oxidase. It behaved in a manner similar to that of azide, preferentially inhibiting iron oxidation. As shown in Table 1, only 3.2 μM cyanide was required to cut the iron oxidation in half, while 77 to 323 μM was necessary to produce a similar effect on sulfur oxidation.
TABLE 1.
Inhibition by azide and cyanide of sulfur and iron oxidation by T. ferrooxidans strain SM-4
| Inhibitor | Conc (μm) required for 50% inhibitiona
|
||
|---|---|---|---|
| Fe2+-grown cells
|
S0 oxidation by S0-grown cells | ||
| Fe2+ oxidation | S0 oxidation | ||
| NaN3 | 0.7 | 32 | 19 |
| KCN | 3.2 | 323 | 77 |
Percent inhibition was calculated using the activity in 0.05 M K2SO4-H2SO4 (pH 3) as 100%.
Effects of anions and inhibitors on cell growth.
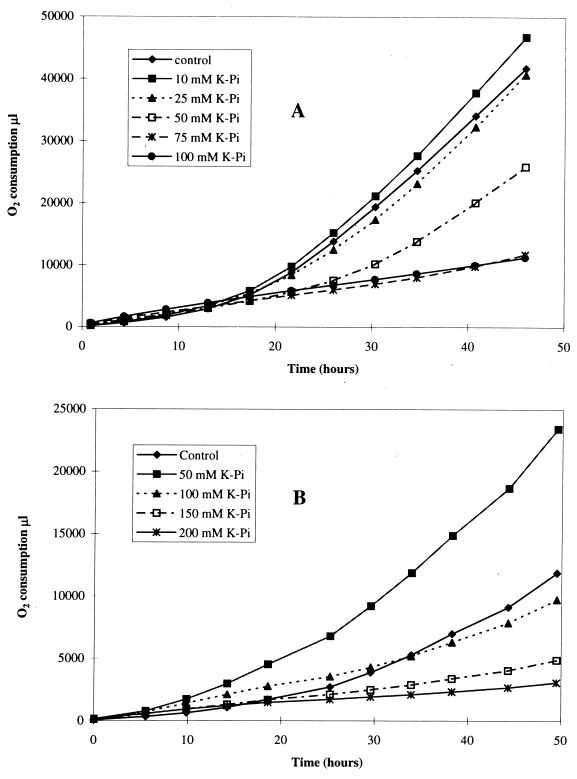
The second part of this paper deals with the use of the above-mentioned anions and inhibitors on growing cell cultures. Cells growing on single substrates were monitored in a Micro-oxymax respirometer. Iron oxidation and sulfur oxidation were measured in terms of cumulative oxygen consumption. Since we did not have sulfur-grown T. ferrooxidans SM-4 available for these growth experiments, we used sulfur-grown T. thiooxidans SM-6 instead for experiments on sulfur. Important findings were later confirmed with sulfur-adapted T. ferrooxidans. Figure 2 shows the effects of increasing concentrations of phosphate on iron and sulfur oxidation by growing cells. The control in each case contained a 5% inoculum along with ferrous sulfate or precipitated sulfur as the substrate. Cumulative oxygen consumption was plotted as a function of time for each of the reaction vessels. Iron oxidation (Fig. 2A) was decreased by half in the presence of 50 mM phosphate. Sulfur oxidation (Fig. 2B), on the other hand, increased to twice the control level in the presence of 50 mM phosphate. Similar results were obtained with 50 mM chloride, which also inhibited iron oxidation and stimulated sulfur oxidation, although slightly less extensively (data not shown).
FIG. 2.
Effect of phosphate concentration on oxygen consumption. (A) Growth of T. ferrooxidans on Fe2+; (B) growth of T. thiooxidans on S0.
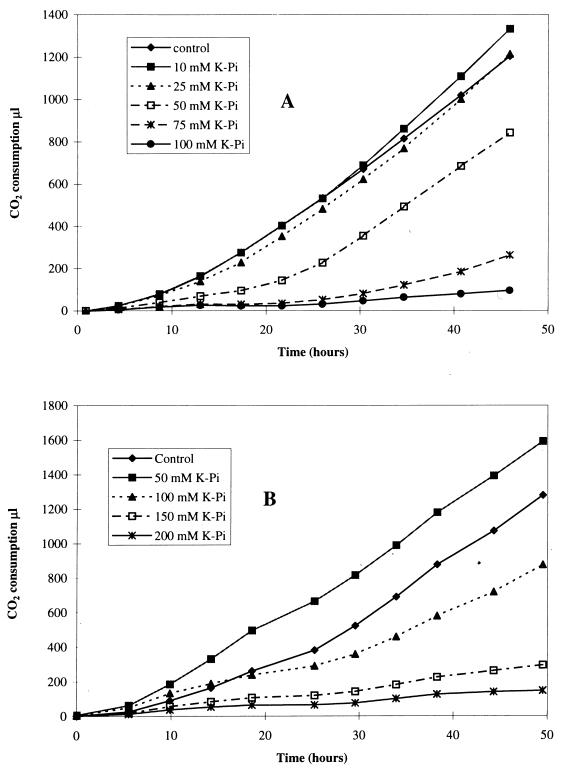
Cumulative carbon dioxide consumption was used to measure the cellular growth rate. Figure 3 shows the effects of phosphate ions on growth using either ferrous iron or elemental sulfur as a substrate. A phosphate concentration of 50 mM caused a 50% drop in iron oxidation and was equally effective in inhibiting growth on ferrous iron (Fig. 3A). Growth on elemental sulfur (Fig. 3B) required more than twice as much phosphate for 50% inhibition. At 50 mM, potassium phosphate stimulated growth on sulfur (Fig. 3B) as well as sulfur oxidation (Fig. 2B). The stimulatory effect of phosphate was confirmed with T. ferrooxidans adapted on sulfur; 75 mM potassium phosphate increased growth on sulfur by 20% and sulfur oxidation by 30%.
FIG. 3.
Effect of phosphate concentration on carbon dioxide consumption. (A) Growth of T. ferrooxidans on Fe2+; (B) growth of T. thiooxidans on S0.
Table 2 provides a summary of the effects of anions and inhibitors on cell growth (CO2 consumption) and iron and sulfur oxidation (O2 consumption). As mentioned above, half as much phosphate was required to inhibit 50% of the oxidation and growth on iron compared to sulfur. Chloride had a similar effect, inhibiting oxidation and growth on iron while stimulating those on sulfur at lower anion concentrations (data not shown). Nitrate was unique among the anions in its strong inhibitory effect on both cell cultures. Azide and cyanide both showed significant differences with respect to the two cell types. Iron growth was 2.5 times more sensitive to azide and 3 to 4 times more sensitive to cyanide inhibition than sulfur growth.
TABLE 2.
Inhibition of oxidation and growth on Fe2+ or S0
| Addition | Conc required for 50% inhibition ona:
|
|||
|---|---|---|---|---|
| Fe2+
|
S0
|
|||
| O2 | CO2 | O2 | CO2 | |
| KH2PO4-H3PO4 | 58 mM | 59 mM | 140 mM | 120 mM |
| KCl-HCl | 79 mM | 90 mM | 160 mM | 120 mM |
| KNO3-HNO3 | 43 mM | 56 mM | 63 mM | 42 mM |
| NaN3 | 0.4 μM | 0.4 μM | 0.9 μM | 1.0 μM |
| NaCN | 2.8 μM | 3.0 μM | 11 μM | 7.7 μM |
Micro-oxymax data on O2 and CO2 consumption after 42 h. The control (100%) flask contained the M9K without additions.
Effect of anions on metal leaching.
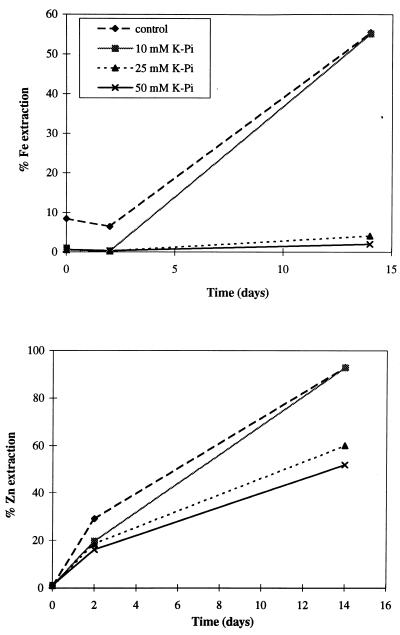
Leaching of Fe and Zn from pyrite and sphalerite by T. ferrooxidans was differentially affected by phosphate concentrations, as shown in Fig. 4. Phosphate at or above 25 mM inhibited the solubilization of Fe completely while allowing the zinc solubilization to proceed with only some rate reduction. The effect at 75 and 100 mM was essentially the same as that at 50 mM. Although not shown in Fig. 4, KCl at 50 mM inhibited Fe solubilization by 84 and 35% after 2 and 14 days, respectively, and did not inhibit Zn solubilization at all. KNO3 also inhibited the leaching of Fe more strongly than that of Zn, inhibiting Fe solubilization by 86% and Zn solubilization by only 16% at 50 mM after 14 days. The effect of NaN3 and NaCN at 0.1 to 5 μM was not apparent after 14 days because of their volatility at pH 2.3 as HN3 and HCN escaping from the flasks through the cotton plugs. After 2 days, however, 1 μM NaN3 and 5 μM NaCN inhibited Fe solubilization by 65% without significantly affecting Zn solubilization (14 and 25% inhibition for NaCN and NaN3, respectively). These preliminary leaching experiments support the concept of differential leaching of Fe and Zn by inhibiting iron oxidation but not sulfur oxidation, thus favoring the solubilization of Zn from ZnS over the solubilization of Fe from FeS2. Detailed studies (L. Harahuc, H. M. Lizama, and I. Suzuki, submitted for publication; I. Suzuki and L. Harahuc, June 1998, Canadian Patent Office) of selective solubilization of metals from sulfide ores by this method support its potential application in bacterial leaching.
FIG. 4.
Effect of phosphate concentration on the extraction of Fe and Zn from a mixture of pyrite and sphalerite.
Bioleaching has become an increasingly important process due to the growing need to use lower-grade ores, the relative ease of implementation, and the low start-up costs required compared to those of a conventional mining operation (4). Under natural conditions an ore body may show a tendency for selective solubilization of certain metals. The mineral that is extensively oxidized is (i) the most hydrophobic, (ii) the lowest of an electrochemical series, or (iii) behaving as the anode of a galvanic cell (14). We have demonstrated in this study that the bacterial activities responsible for metal leaching, the oxidation of ferrous iron and that of sulfur, can be selectively controlled by manipulation of the media, leading to differential leaching of Fe and Zn. This control of bacterial activities raises the potential of successful bacterial leaching beyond the three physical criteria listed above.
ACKNOWLEDGMENTS
We thank the Natural Sciences and Engineering Research Council of Canada for a grant to I.S. in support of the research and for the Industrial Postgraduate Scholarship to L.H., and we thank Cominco Research Limited for sponsoring the scholarship and for making their research facilities available to her.
REFERENCES
- 1.Amaro A M, Chamorro D, Seeger M, Arredondo R, Peirano I, Jerez C A. Effect of external pH perturbations on in vivo protein synthesis by the acidophilic bacterium Thiobacillus ferrooxidans. J Bacteriol. 1991;173:910–915. doi: 10.1128/jb.173.2.910-915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck J V, Shafia F M. Effect of phosphate ion and 2,4-dinitrophenol on the activity of intact cells of Thiobacillus ferrooxidans. J Bacteriol. 1964;88:850–857. doi: 10.1128/jb.88.4.850-857.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blake R C, II, Shute E A. Respiratory enzymes of Thiobacillus ferrooxidans. Kinetic properties of an acid-stable iron:rusticyanin oxidoreductase. Biochemistry. 1994;33:9220–9228. doi: 10.1021/bi00197a025. [DOI] [PubMed] [Google Scholar]
- 4.Brierley C L. Bacterial leaching. Crit Rev Microbiol. 1978;6:207–219. doi: 10.3109/10408417809090623. [DOI] [PubMed] [Google Scholar]
- 5.Corbett C M, Ingledew W J. Is Fe3+/2+ cycling an intermediate in sulfur oxidation by Fe2+-grown Thiobacillus ferrooxidans? FEMS Microbiol Lett. 1986;41:1–6. [Google Scholar]
- 6.Din G A, Suzuki I. Mechanism of Fe2+-cytochrome c reductase of Ferrobacillus ferrooxidans. Can J Biochem. 1967;45:1547–1556. doi: 10.1139/o67-184. [DOI] [PubMed] [Google Scholar]
- 7.Espejo R T, Romero J. Bacterial community in copper sulfide ores inoculated and leached with solution from a commercial-scale copper leaching plant. Appl Environ Microbiol. 1997;63:1344–1348. doi: 10.1128/aem.63.4.1344-1348.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fry I V, Lazaroff N, Packer L. Sulfate-dependent iron oxidation by Thiobacillus ferrooxidans: characterization of a new EPR detectable electron transport component on the reducing side of rusticyanin. Arch Biochem Biophys. 1986;246:650–654. doi: 10.1016/0003-9861(86)90321-8. [DOI] [PubMed] [Google Scholar]
- 9.Fukumori Y F, Yano T, Sato A, Yamanaka T. Fe(II)-oxidizing enzyme purified from Thiobacillus ferrooxidans. FEMS Microbiol Lett. 1988;50:169–172. [Google Scholar]
- 10.Imaizumi T. Some industrial applications of inorganic microbial oxidation in Japan. Biotechnol Bioeng Symp. 1986;16:363–371. [Google Scholar]
- 11.Ingledew W J, Cox J C, Halling P J. A proposed mechanism for energy conservation during F2+ oxidation by Thiobacillus ferrooxidans: chemiosmotic coupling to net H+ influx. FEMS Microbiol Lett. 1977;2:193–197. [Google Scholar]
- 12.Jensen A B, Webb C. Treatment of H2S-containing gases: a review of microbiological alternatives. Enzyme Microb Technol. 1995;17:2–10. [Google Scholar]
- 13.Jerez C A, Seeger M, Amaro A M. Phosphate starvation affects the synthesis of outer membrane proteins in Thiobacillus ferrooxidans. FEMS Microbiol Lett. 1992;98:29–34. doi: 10.1016/0378-1097(92)90127-a. [DOI] [PubMed] [Google Scholar]
- 14.Kupla C F, Mjoli N, Roskey M T. Comparison of iron and sulfur oxidation in Thiobacillus ferrooxidans: inhibition of iron oxidation by growth on sulfur. In: Ehrlich H L, Holmes D S, editors. Workshop on biotechnology for the mining, metal-refining and fossil fuel processing industries. New York, N.Y: John Wiley and Sons; 1986. pp. 289–295. [Google Scholar]
- 15.Lawrence J R, Kwong Y T J, Swerhone G D W. Colonization and weathering of natural sulfide mineral assemblages by Thiobacillus ferrooxidans. Can J Microbiol. 1997;43:178–188. [Google Scholar]
- 16.Lazaroff N. Sulfate requirement for iron oxidation by Thiobacillus ferrooxidans. J Bacteriol. 1963;85:78–83. doi: 10.1128/jb.85.1.78-83.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazaroff N. The specificity of the anionic requirements for iron oxidation by Thiobacillus ferrooxidans. J Gen Microbiol. 1977;101:85–91. [Google Scholar]
- 18.Leduc L G, Ferroni G D. The chemolithotrophic bacterium Thiobacillus ferrooxidans. FEMS Microbiol Rev. 1994;14:103–120. [Google Scholar]
- 19.Le Roux N W, Mehta K B. Examination of a copper ore after leaching with bacteria. In: Murr L E, Torma A E, Brierley J A, editors. Metallurgical applications of bacterial leaching and related microbiological phenomena. New York, N.Y: Academic Press; 1978. pp. 463–476. [Google Scholar]
- 20.Lizama H M, Suzuki I. Bacterial leaching of a sulfide ore by Thiobacillus ferrooxidans and Thiobacillus thiooxidans. I. Shake flask studies. Biotechnol Bioeng. 1988;32:110–116. doi: 10.1002/bit.260320116. [DOI] [PubMed] [Google Scholar]
- 21.Magnin J, Baillet F, Boyer A, Zlatev R, Luca M, Cheruy A, Ozil P. Augmentation, par régénération électrochimique du substrat, de la production d'une biomasse (Thiobacillus ferrooxidans DSM 583) pour un procédé biologique de récupération de métaux. Can J Chem Eng. 1998;76:978–984. [Google Scholar]
- 22.Murayama T, Konno Y, Sakata T, Imaizumi T. Application of immobilized Thiobacillus ferrooxidans for large-scale treatment of acid mine drainage. Methods Enzymol. 1987;136:530–540. [Google Scholar]
- 23.Ohmura N, Tsugita K, Koizumi J, Saiki H. Sulfur-binding protein of flagella of Thiobacillus ferrooxidans. J Bacteriol. 1996;178:5776–5780. doi: 10.1128/jb.178.19.5776-5780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pronk J T, Liem K, Bos P, Kuenen J G. Energy transduction by anaerobic ferric iron respiration in Thiobacillus ferrooxidans. Appl Environ Microbiol. 1991;57:2063–2068. doi: 10.1128/aem.57.7.2063-2068.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rawlings D E, Silver S. Mining with microbes. Bio/Technology. 1995;13:773–778. [Google Scholar]
- 26.Razzell W E, Trussell P C. Isolation and properties of an iron-oxidizing Thiobacillus. J Bacteriol. 1963;85:595–603. doi: 10.1128/jb.85.3.595-603.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakaguchi H, Torma A E, Silver M. Microbiological oxidation of synthetic chalcocite and covellite by Thiobacillus ferrooxidans. Appl Environ Microbiol. 1976;31:7–10. doi: 10.1128/aem.31.1.7-10.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sand W. Ferric iron reduction by Thiobacillus ferrooxidans at extremely low pH values. Biogeochemistry. 1989;7:195–201. [Google Scholar]
- 29.Satoh H, Yoshizawa J, Kametani S. Bacteria help desulfurize gas. Hydrocarbon Processing. 1988;67:76–78. [Google Scholar]
- 30.Seeger M, Jerez C A. Phosphate limitation affects global gene expression in Thiobacillus ferrooxidans. Geomicrobiol J. 1992;10:227–237. [Google Scholar]
- 31.Seeger M, Jerez C A. Phosphate-starvation induced changes in Thiobacillus ferrooxidans. FEMS Microbiol Lett. 1993;108:35–42. doi: 10.1111/j.1574-6968.1993.tb06070.x. [DOI] [PubMed] [Google Scholar]
- 32.Seeger M, Jerez C A. Response of Thiobacillus ferrooxidans to phosphate limitation. FEMS Microbiol Rev. 1993;11:37–42. [Google Scholar]
- 33.Starkey R L. Concerning the physiology of Thiobacillus thiooxidans, an autotrophic bacterium oxidizing sulfur under acid conditions. J Bacteriol. 1925;10:135–163. doi: 10.1128/jb.10.2.135-163.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugio T, Katagiri T, Inagaki K, Tano T. Actual substrate for elemental sulfur oxidation by sulfur:ferric ion oxidoreductase purified from Thiobacillus ferrooxidans. Biochim Biophys Acta. 1989;973:250–256. [Google Scholar]
- 35.Sugio T, Katagiri T, Moriyama M, Zhen Y L, Inagaki K, Tano T. Existence of a new type of sulfite oxidase which utilizes ferric ions as an electron acceptor in Thiobacillus ferrooxidans. Appl Environ Microbiol. 1988;54:153–157. doi: 10.1128/aem.54.1.153-157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki I, Lee D, Mackay B, Harahuc L, Oh J K. The effect of various ions, pH, and osmotic pressure on the oxidation of elemental sulfur by Thiobacillus thiooxidans. Appl Environ Microbiol. 1999;65:5163–5168. doi: 10.1128/aem.65.11.5163-5168.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki I, Takeuchi T L, Yuthasatrakosol T D, Oh J K. Ferrous iron and sulfur oxidation and ferric iron reduction activities of Thiobacillus ferrooxidans are affected by growth on ferrous iron, sulfur, or a sulfide ore. Appl Environ Microbiol. 1990;56:1620–1626. doi: 10.1128/aem.56.6.1620-1626.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torma A E. The role of Thiobacillus ferrooxidans in hydrometallurgical processes. Adv Biochem Eng. 1977;6:1–37. [Google Scholar]
- 39.Tuovinen O H, Kelly D P. Biology of Thiobacillus ferrooxidans in relation to the microbiological leaching of sulfide ores. Z Allg Mikrobiol. 1972;12:311–346. doi: 10.1002/jobm.3630120406. [DOI] [PubMed] [Google Scholar]
- 40.Tuttle J H, Dugan P R. Inhibition of growth, and sulfur oxidation in Thiobacillus ferrooxidans by simple organic compounds. Can J Microbiol. 1976;22:719–730. doi: 10.1139/m76-105. [DOI] [PubMed] [Google Scholar]