Abstract
Objective: The expression, prognosis, and related mechanisms of ANXA1 are investigated in glioma, with the objective to find potential therapeutic molecular targets for glioma.
Methods: We analyzed the gene expression of ANXA1 using glioma-related databases, including the Chinese Glioma Genome Atlas (CGGA) database, The Cancer Genome Atlas (TCGA) database, and the Gene Expression Omnibus (GEO) database. Moreover, we collected the sample tissues and corresponding paracancerous tissues of 23 glioma patients and then conducted a Western blot experiment to verify the expression and correlate survival of ANXA1. Moreover, we generated survival ROC curves, performing univariate and multivariate Cox analyses and the construction of the nomogram. Differential expression analysis was conducted by high and low grouping based on the median of the ANXA1 gene expression values. We conducted Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis and Gene Set Enrichment Analysis (GSEA) to explore possible mechanisms, and gene co-expression analysis was also performed.
Results: The results showed that the ANXA1 expression level was higher in gliomas than in normal tissues, and a high expression level of ANXA1 in gliomas was associated with poorer prognosis. The independent prognosis analysis showed that the ANXA1 gene was an independent prognostic factor of glioma. In the analysis of KEGG and Gene Set Enrichment Analysis (GSEA), it is shown that ANXA1 may play an important role in glioma patients by affecting extracellular matrix (ECM)–receptor interaction and the focal adhesion signal pathway. The core genes, including COL1A1, COL1A2, FN1, ITGA1, and ITGB1, were screened for gene correlation and prognosis analysis. The expression level of the five genes was verified by qPCR in glioma. We concluded that these five core genes and ANXA1 could play a synergistic role in gliomas.
Conclusion: The results indicated that a high expression level of ANXA1 leads to worse prognosis and ANXA1 is an independent prognostic factor and a potentially important target for the treatment of gliomas.
Keywords: ANXA1, glioma, prognosis, ECM, focal adhesion, molecular target
Introduction
Glioma is an intracranial tumor originating from glial cells, and it is the most common intracranial primary tumor, accounting for approximately 80% of intracranial malignant tumors (Ostrom et al., 2014). Glioma is often accompanied by local invasion, especially glioblastoma (GBM), which invades and destroys the surrounding normal brain tissue, and the degree of malignancy is high with a poor prognosis (Weller et al., 2015). High-grade gliomas (HGGs) are the most common primary brain malignancies, predicting a 5-year survival rate of less than 5% (Ostrom et al., 2014). These tumors may arise de novo as isocitrate dehydrogenase (IDH)–wildtype GBMs or develop from progressive lower-grade gliomas (LGGs; defined as World Health Organization (WHO) grades 2–3) (Aldape et al., 2015). According to the WHO, gliomas were divided into grades I–IV by pathological characteristics; grade II–IV gliomas are the largest entity in the group of intracranial brain tumors, with a survival rate of more than 10 years (grade II) to less than 1 year (grade IV) (Cordier et al., 2016). However, the therapeutic effect is limited with conventional treatment of gliomas, including complete surgical resection and postoperative radiotherapy and chemotherapy (Stupp et al., 2005; Bush et al., 2017). In recent years, molecular-targeted therapy and immunotherapy have achieved good results in many tumors, such as colorectal cancer, breast cancer, and lung cancer (Naylor et al., 2016; Esteva et al., 2019; Ganesh et al., 2019; Piawah and Venook, 2019). However, due to the existence of the blood–brain barrier in the brain and the unique tumor microenvironment in glioma, the therapeutic effect is poor (Quail and Joyce, 2017). However, at present, molecular-targeted therapy and immunotherapy have achieved some effective results. For example, vemurafenib has demonstrated long-lasting antitumor activity in some patients with BRAFV600E mutant glioma (Kaley et al., 2018). Neoadjuvant administration of PD-1 blockers enhances local and systemic antitumor immune responses (Cloughesy et al., 2019). With the advent of the molecular era, the classification of gliomas has changed. Combined with pathological and molecular characteristics, the classification of gliomas by the WHO in 2016 included molecular markers, such as IDH mutation and 1p19q (Louis et al., 2016). In 2021, the latest central nervous system tumor guidelines of the fifth edition of the WHO confirmed the importance of molecular typing. In the grading of gliomas, the importance of grading according to molecular characteristics is more prominent. For example, loss of CDKN2A/B homozygosity is closely related to poor prognosis, especially in WHO grade III glioma with loss of CDKN2A/B homozygosity, where the prognosis is similar to WHO grade IV glioma. Therefore, CDKN2A/B has been included in the molecular diagnosis of gliomas (Louis et al., 2021). However, molecular typing still needs to be further explored to identify more molecular targets for better diagnosis and treatment of clinical patients. Therefore, we explore new targets of glioma to provide new therapeutic targets.
Annexin is a well-known calcium-regulated and phospholipid-dependent membrane-binding protein (Lim and Pervaiz, 2007). ANXA1 is a member of the annexin family, which is involved in many important biological processes, such as inflammation, phagocytosis, proliferation, differentiation, and apoptosis (Monastyrskaya et al., 2009; Xia et al., 2020). Many studies have shown that ANXA1 is associated with the occurrence, invasion, and metastasis of cancer (Mussunoor and Murray, 2008; Guo et al., 2013). ANXA1 has been extensively studied in gastric cancer and breast cancer (Maschler et al., 2010; Cheng et al., 2012). Some studies in gliomas have shown that ANXA1 is a risk prognostic factor (Xu et al., 2017; Luo et al., 2021; Wei et al., 2021) Therefore, we investigated the expression, prognosis, and related mechanisms of ANXA1 using a public database to provide new markers and potential therapeutic targets for glioma patients.
Materials and Methods
Data Acquisition and Download
The GSE4290, GSE7696, GSE29796, and GSE50161 expression sequences and clinical data were downloaded from the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) database for gene expression analysis. The GSE4290 dataset included 23 normal samples, which were obtained from epilepsy patients and used as nontumor samples, and 153 tumor samples, which included 26 astrocytomas, 50 oligodendrogliomas, and 77 glioblastomas (Sun et al., 2006). The GSE7696 dataset included 80 glioblastoma specimens and four nontumor brain samples (Murat et al., 2008). The GSE29796 dataset included 20 normal samples and 52 tumor samples, and these samples were based on matching patients with disease and/or pathology, including 20 cases of epileptic pathology and 52 cases of glioma (Auvergne et al., 2013). The GSE50161 dataset collected samples from surgical brain tumors and normal brains, including 13 normal samples and 117 tumor samples (Griesinger et al., 2013). In addition, the expression sequences and clinical data of 1,018 samples were downloaded from the Chinese Glioma Genome Atlas (CGGA, http://www.cgga.org.cn/) database, and the expression sequences and clinical data of 592 samples were downloaded from The Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.gov/) database (Jiang et al., 2016), which included 449 LGG samples and 143 glioblastoma (GBM) samples. Before further analysis, we performed a log2 transformation on RNA-sequencing data. All sample databases were screened to remove samples that had omitted clinical information.
Acquisition of 23 Patient Tissues and Collection of Clinical Information
We collected sample tissues and corresponding paracancerous tissues (distance from tumor edge >2 cm) from 23 glioma patients, including eight GBM samples and their corresponding paracancerous tissues and 15 LGG samples and their corresponding paracancerous tissues. Under the guidance of neurosurgery experts, gliomas and corresponding paracancerous tissues were obtained from surgery. All samples were obtained with the informed consent of the patients and their families, and the present study was approved by the Ethics Committee of the Second Hospital of Hebei Medical University. We followed up 23 glioma patients from January 2015 to December 2021, and we collected the following information: patient age, gender, grade, radiotherapy chemotherapy, and IDH mutation.
Expression Analysis of the ANXA1 Gene
The GSE4290, GSE7696, GSE29796, and GSE50161 datasets were downloaded from the GEO database. The expression values of ANXA1 in glioma and normal brain tissues were imported into GraphPad Prism 8 software for analysis followed by verification with Gene Expression Profiling Interactive Analysis (GEPIA, http://gepia.cancer-pku.cn/) and Human Protein Atlas online analysis (https://www.proteinatlas.org/). Moreover, Western blot analysis was also performed to verify the expression level of ANXA1.
Analysis of Clinical Prognosis and Clinicopathological Features of ANXA1
The clinical prognosis of ANXA1 was analyzed based on the transcriptome data and clinical information of 1,018 cases from the CGGA database. The expression level of ANXA1 was divided into high and low groups according to the median value. The survival curves of different expression levels of ANXA1 were drawn using the “survival” and “survivminer” software packages in R version 4.0.5. We used the “survival ROC” software package and the Kaplan–Meier method to calculate the receiver operator characteristic (ROC) curve of ANXA1 at 1, 3, and 5 years. The prognostic value of ANXA1 was further evaluated by univariate and multivariate Cox regression analyses, with a significance level of p < 0.001. To verify the accuracy of the ANXA1 prognosis, TCGA data were used to generate the survival and ROC curves and to perform univariate and multivariate analyses. Finally, based on the CGGA data, we used the “survival” and “RMS” software packages in R version 4.0.5 to construct a nomogram for 1-year, 2-year, and 3-year survival using the clinicopathological characteristics of ANXA1 expression. Subsequently, calibration curves were drawn to assess the accuracy of matching between predicted survival and actual survival. In addition, the correlation between the expression of ANXA1 and clinicopathological features was analyzed using the “beeswarm’’ package in R version 3.6.3.
Analysis of Differentially Expressed Genes and Signal Pathway Mechanism
The mRNA sequencing data of the glioma database were normalized using the CGGA. The median expression value of ANXA1 was divided into high and low groups for differential expression analysis, and the Differentially Expressed Genes (DEGs), including significantly upregulated and downregulated genes, were screened by adjusted p < 0.05 and absolute log2 fold change (FC) > 1. The “limma” and “ggplot2” software packages (Ritchie et al., 2015) were used to generate a volcano plot to visualize the DEGs, and the 30 genes with the most significant upregulation and downregulation were selected to generate a heat map of the DEGs using the “pheatmap” software package in R version 4.0.5. Finally, mechanism analysis of the DEGs was performed using Metascape (https://metascape.org/). In addition, GSEA (https://www.gsea-msigdb.org/) was also utilized to indirectly explain the potential mechanism of ANXA1 function. When NES > 1, p < 0.05, and FDR < 0.05, the gene set was considered the enrichment group.
Construction of the Protein–Protein Interaction Network of Differentially Expressed Genes
The median expression value of ANXA1 was divided into high and low groups using the cutoff of adjusted p < 0.05 and absolute log2 fold change (FC) > 1 to screen the DEGs, and a high threshold (0.7) binding degree was set for node screening through the STRING (https://string-db.org/) analysis to filter out the unconnected proteins. The remaining 1,034 proteins were imported into Cytoscape visualization software (version: 3.7.2). After installing the cytoHubba plug-in, the top 50 hub genes were screened by degree topology analysis.
Coexpression Analysis of ANXA1 and Core Genes
We obtained the core genes of ECM–receptor interaction and focal adhesion pathways by GSEA. Through a protein–protein interaction (PPI) network analysis, the DEGs were imported into Cytoscape visualization software to screen the top 50 hub genes by using degree topology analysis. Five core genes were obtained through the intersection of the Venn diagram for gene correlation analysis and coexpression analysis. The relative expression levels of the five genes in glioma and their corresponding paracancerous tissues were further analyzed by qPCR.
Chemical Reagents and Antibodies
The RIPA lysate (product number: p00138b) was purchased from Beyotime (Shanghai, China). Protein phosphatase inhibitors and BCA protein concentration determination kits were purchased from Solarbio (Beijing, China). ANXA1 antibody (catalog number: 21990-1-AP) and β-tubulin antibody (catalog number: 10094-1-ap) were obtained from Proteintech (Chicago, IL, United States). The anti-rabbit IgG HRP-conjugated antibody was purchased from Cell Signaling Technology. An ECL chromogenic solution (product number: BL520A) was obtained from Biosharp.
Tissue Protein Extraction and Western Blot
Tissue samples were removed from the −80°C freezer and thawed on ice. An RIPA lysis buffer, containing protein phosphatase inhibitor, was added to each sample, and the samples were homogenized using a homogenizer. The samples were centrifuged at 4°C and 12,000 rpm for 10 min, and the supernatants were collected. The protein concentrations were determined using a commercially available BCA protein concentration kit. After denaturation, 10–20 µg of the protein was separated with 10% SDS-PAGE and then transferred onto PVDF membranes (Merck Millipore Ltd.). The membranes were then blocked with 5% nonfat milk powder for 1 h and incubated with primary antibodies (ANXA1 antibody, 1:2000; and β-tubulin antibody, 1:1000) at 4°C overnight. The membranes were then washed thrice with TBST (10 min each wash) followed by incubation with the appropriate secondary antibody (anti-rabbit IgG, 1:2000). The membranes were then washed thrice with TBST (10 min each wash) followed by development and imaging using a chemiluminescence photodocumentation system.
RNA Isolation and qPCR
Total RNA was extracted from the remaining eight glioma samples and corresponding paracancerous tissues with TRIzol reagent (Invitrogen). The quality of the total RNA of these samples was evaluated by Nanodrop 2000c and agarose gel electrophoresis. Complementary DNA (cDNA) was synthesized using a revert aid first-strand cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, United States). Quantitative PCR was performed with SYBR Green (Hieff qPCR SYBR Green Master Mix, YEASEN, Shanghai, China) on a qPCR system (Model No. CFX96TM Option Module, Bio-Rad, United States). The specific primers of each gene were used to analyze the expression of these extracted tissue samples. All samples were standardized according to the expression of the gene-encoding human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a reference. Relative expression levels were calculated as 2−[(Ct of target gene)−(Ct of GAPDH)]. The sequences of the primers are listed in Supplementary Table S1.
Statistical Analysis
Based on public databases, the following software programs and online tools were used for analysis: GraphPad Prism 8 software was used for gene expression analysis; R software (version: 4.0.1, http://www.r-project.org/) was used for survival prognosis analysis and gene correlation analysis; R software (version: 3.6.3, http://www.r-project.org/) was used for clinicopathological characteristics analysis; Metascape online website and GSEA software were used for mechanism analysis; and the GEPIA online website was used for core gene prognosis analysis. When the results met the requirements of p < 0.05 and FDR < 0.05, they were considered statistically significant. In addition, we used Western blot analysis to verify the expression levels of 23 gliomas and their corresponding paracancerous tissues. The protein gray values were measured by ImageJ software, and the results were imported into GraphPad Prism 8 software. qPCR was used to verify the expression of the five core genes in the remaining eight pairs of samples. p < 0.05 was considered statistically significant.
Results
Article Workflow and Sample Information
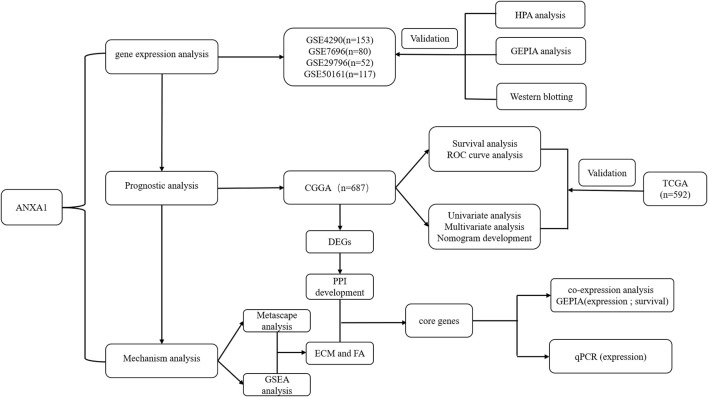
The workflow of the present study is shown in Figure 1. We downloaded the glioma sample information of the GSE4290, GSE7696, GSE29796, and GSE50161 datasets from the GEO database with 153, 80, 52, and 117 glioma samples and 23, 4, 20, and 52 normal samples, respectively. The clinicopathological information, including age, grade, category, 1p19q deletion status, and IDH mutation status, was obtained from the CGGA and TCGA databases. However, the TCGA database lacked clinical chemoradiotherapy and MGMT methylation data, which were only obtained from the CGGA database. The clinical information and categorical data of glioma patients from the CCGA and TGGA databases are shown in Table 1. The clinical information for the 23 glioma patients who provided samples for Western blot analysis is shown in Supplementary Table S2.
FIGURE 1.
Working diagram of the study. Abbreviations: GEO: Gene Expression Omnibus; CGGA: Chinese Glioma Genome Atlas; DEGs: differentially expressed genes; GSEA: Gene Set Enrichment Analysis; PPI: protein–protein interaction; ECM: extracellular matrix; FA: focal adhesion; HPA: Human Protein Atlas; GEPIA: Gene Expression Profiling Interactive Analysis; TCGA: The Cancer Genome Atlas.
TABLE 1.
Clinical information materials of gliomas. CGGA: 686 glioma samples; TCGA: 592 glioma samples; clinical features: grade, gender, age, IDH mutation, 1p19q codeletion, MGMT, radiotherapy, and chemistry.
| CGGA (n = 686) | TCGA (n = 592) | |||
|---|---|---|---|---|
| Case | Proportion (%) | Case | Proportion (%) | |
| WHO grade | ||||
| II | 177 | 25.8 | 211 | 35.6 |
| III | 226 | 32.9 | 238 | 40.2 |
| IV | 283 | 41.3 | 143 | 24.2 |
| Gender | ||||
| Male | 399 | 58.2 | 344 | 58.1 |
| Female | 287 | 41.8 | 248 | 41.9 |
| Age | ||||
| ≥42 | 379 | 55.2 | 349 | 59.0 |
| <42 | 307 | 44.8 | 243 | 41.0 |
| IDH mutation | ||||
| Yes | 371 | 54.1 | 372 | 62.8 |
| No | 315 | 45.9 | 220 | 37.2 |
| 1p19q codeletion | ||||
| Yes | 141 | 20.6 | 149 | 25.2 |
| No | 545 | 79.4 | 443 | 74.8 |
| MGMT methylation | ||||
| Yes | 386 | 56.3 | — | — |
| No | 300 | 43.7 | — | — |
| Radiotherapy | ||||
| Yes | 544 | 79.3 | — | — |
| No | 142 | 20.7 | — | — |
| Chemotherapy | ||||
| Yes | 501 | 73.0 | — | — |
| No | 185 | 27.0 | — | — |
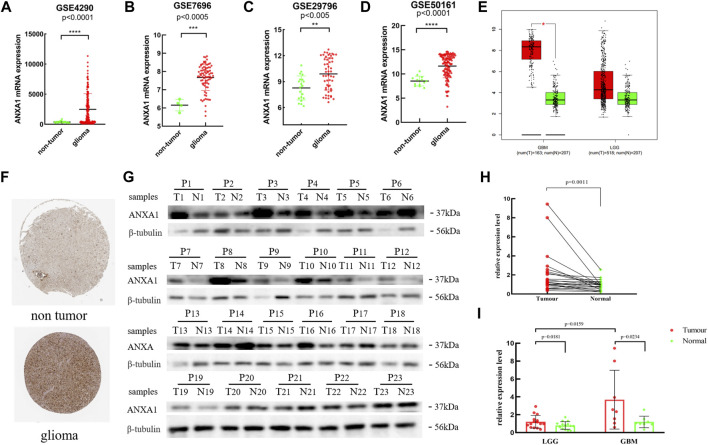
ANXA1 is Overexpressed in Glioma
Based on the expression data for gliomas and normal tissues in the GSE4290, GSE7696, GSE29796, and GSE50161 datasets, the ANXA1 gene was significantly highly expressed in gliomas (Figures 2A–D), and the results were verified by GEPIA and Human Protein Atlas online analyses (Figures 2E,F). In addition, we evaluated 23 glioma sample tissues and their corresponding paracancerous tissues by Western blot analysis (Figure 2G), and the results showed that the ANXA1 expression levels were significantly higher in the 23 glioma sample tissues than their corresponding paracancerous tissues (p < 0.05, Figure 2H). A paired-sample t-test was performed separately in 15 LGG samples and their corresponding paracancerous tissues and in eight GBM samples and their corresponding paracancerous tissues. In addition, an independent-sample t-test was also performed in eight GBM samples and 15 LGG samples. The results demonstrated that ANXA1 was overexpressed in gliomas with higher expression in GBM samples than in LGG samples (p < 0.05, Figure 2I), supporting that ANXA1 is correlated with a higher grade of gliomas.
FIGURE 2.
Expression level of the ANXA1 gene in glioma and normal tissues. (A) GSE4290 data set. (B) GSE7696 data set. (C) GSE29796 data set. (D) GSE50161 data set. (E) Based on the GEPIA online website, the expression level of ANXA1 in glioma and normal samples. (F) Based on The Human Protein Atlas online website, the expression level of ANXA1 in the brain tissues of glioma (Intensity: strong; Quantity: 75%–25%) and normal controls (Intensity: weak; Quantity: 75%–25%). (G) Western blotting images of 23 glioma tissues and their corresponding paracancerous samples (P1–P8: GBM; P9–P23: LGGs). (H) Paired-sample t-test of 23 glioma tissues and their corresponding paracancerous tissues. (I) Paired-sample t-test of GBM and LGG tumor tissues and their corresponding paracancerous tissues, and independent-sample t-test of eight GBM and 15 LGG samples.
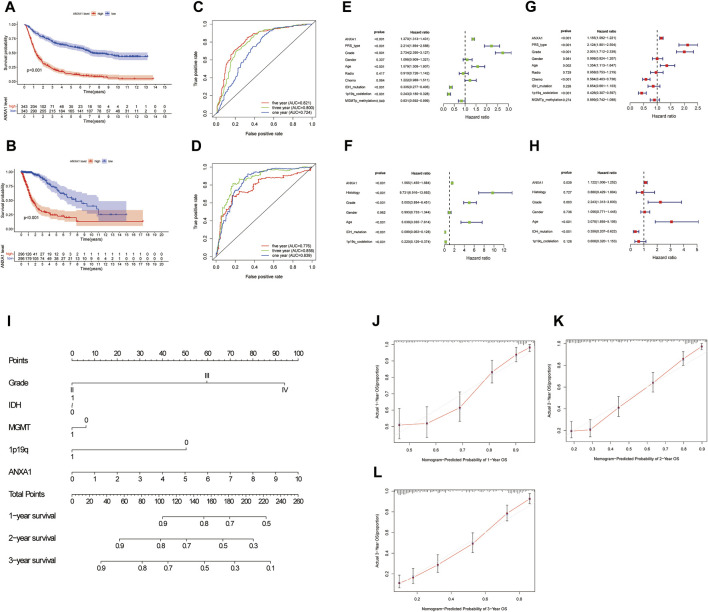
High Expression of ANXA1 has a Poor Prognosis
The Kaplan–Meier survival method was performed to analyze the survival prognosis using the sample information from the CGGA database, which showed that high expression of ANXA1 had worse prognosis (p < 0.001, Figure 3A), and these results were verified by analysis of the TCGA database (p < 0.001, Figure 3B). Using the CGGA and TCGA databases, the ROC curve was generated. The ROC curve of ANXA1 for 1-, 3-, and 5-year outcomes had AUC values of 0.724, 0.800, and 0.821, respectively, in the CGGA database (Figure 3C). The ROC curve of the ANXA1 gene was verified in the TCGA database, with AUC values of 0.839, 0.858, and 0.776 for 1-, 3-, and 5-year outcomes, respectively (Figure 3D). Univariate Cox regression analyses showed that the expression of ANXA1 [HR = 1.370; 95% CI (1.313–1.431); p < 0.001], type, grade, age, IDH mutation, and 1p19q expression status were significantly correlated with survival prognosis (Figure 3E). Moreover, multivariate Cox regression analyses showed that the expression of ANXA1 [HR = 1.155; 95% CI (1.092–1.221); p < 0.001], type, grade, age, chemotherapy, and 1p19q expression status were also correlated with survival prognosis (Figure 3F). The univariate and multivariate regression analyses were verified in the TCGA database (Figures 3G,H). Therefore, these findings indicated that ANXA1 is an independent prognostic indicator of glioma. In addition, based on the clinical characteristics of the CGGA, including grade, IDH mutation, methylation level, 1p19q expression, and ANXA1 expression level, we constructed a nomogram for quantitative prediction (Figure 3I). Finally, we found that the actual and predicted survival times were better matched by the calibration curve in patients with 3-year survival (Figures 3J–L).
FIGURE 3.
Survival analysis and independent prognostic analysis of the ANXA1 gene in glioma. (A,B) Survival analysis of ANXA1. (C,D) Survival ROC curve of ANXA1 at 1, 3, and 5 years. (E,F) Univariate analysis of ANXA1. (G,H) Multivariate analysis of ANXA1. (I) Based on the clinical information of the CGGA database, a prognostic nomogram model was constructed. (J–L) Calibration curve was constructed according to the CGGA database information. The scores of ANXA1, grade, IDH, MGMT, and 1p19q factors were used to predict the calibration curve of the 1-year, 2-year, and 3-year prognostic nomogram.
Correlation Between ANXA1 Gene Expression and Clinicopathological Features
The correlation analysis of ANXA1 expression and clinicopathological features demonstrated that ANXA1 was overexpressed in patients older than 42 years (p < 0.001, Figure 4A). In addition, the expression of ANXA1 also increased with the increase of tumor grade (p < 0.001, Figure 4B); among the type of pathological features, ANXA1 was more highly expressed in recurrent patients (p < 0.001, Figure 4C), and ANXA1 expression was low in patients with IDH mutation and combined 1p19q expression (p < 0.001, Figures 4D,E).
FIGURE 4.
Independent analysis of the ANXA1 gene and clinicopathological features. Based on 686 tumor samples in the CGGA database, the ANXA1 and clinical characteristics were analyzed independently: (A) Expression of ANXA1 in age (≥42 years, <42 years). (B) Expression of ANXA1 in glioma grade (WHO II, WHO III, and WHO IV). (C) Expression of ANXA1 in glioma types (primary, recurrent, and secondary). (D) Expression of ANXA1 in IDH mutation state (mutant and wild-type). (E) Expression of ANXA1 in 1p19q codeletion status (Codel and non-codel).
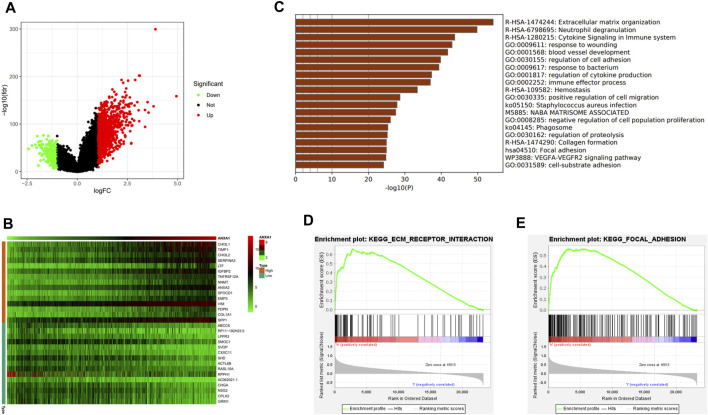
Differentially Expressed Genes and Enrichment Analysis of the ANXA1 Gene
In the differential expression analysis of ANXA1, 1,256 upregulated genes and 227 downregulated genes were obtained and visualized by a volcano map (Figure 5A), and 30 genes with significant upregulation and downregulation were utilized to generate a heat map (Figure 5B). GSEA indicated that the ANXA1 gene was enriched in the ECM receiver interaction (NSE = 1.96, NOM p-val = 0.002, and FDR q-val = 0.023) and focal adhesion (NES = 1.99, NOM p-val = 0.004, and FDR q-val = 0.039) pathways (Figures 5C, D), and Metascape online analysis showed that the DEGs were enriched in the process of ECM and focal adhesion (Figure 5E).
FIGURE 5.
DEG analysis and ANXA1 gene mechanism analysis. (A) Visual volcano plot of differentially expressed genes. (B) Heat map of DEGs was drawn, showing DEGs that were significantly upregulated and significantly downregulated. (C) Visual heat map for the mechanism analysis of DEGs on the Metascape online website. (D,E) Enriched ECM–receptor interaction and focal adhesion signal pathway by GSEA.
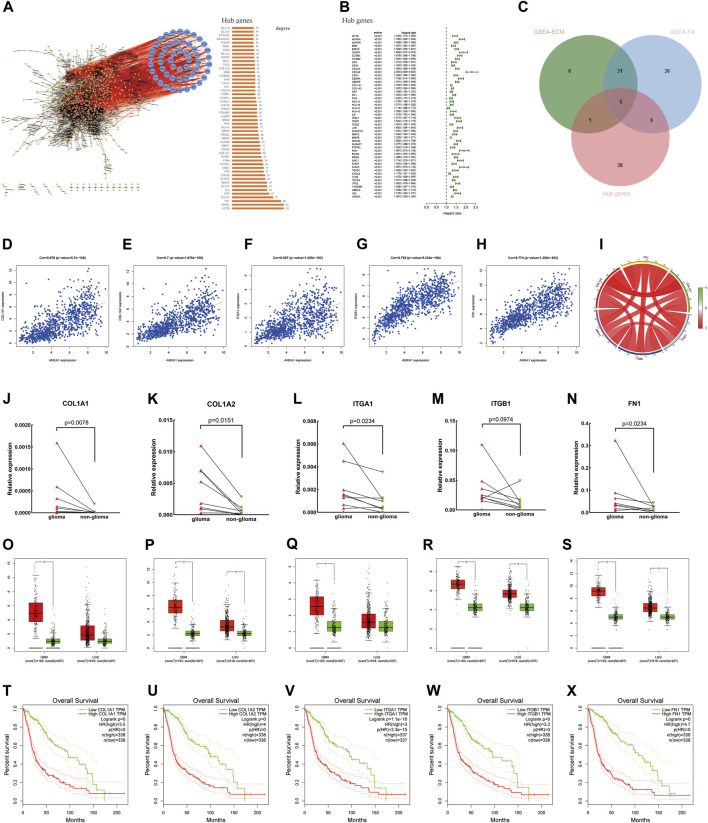
Construction of Protein–Protein Interaction of Differentially Expressed Genes, Filtration of Core Genes, Core Gene Correlation Analysis, and Prognostic Analysis
Based on the STRING online analysis, 1,034 proteins were screened and input into Cytoscape visualization software. The top 50 hub genes were obtained by a degree topology analysis using the cytoHubba plugin (Figure 6A). Univariate regression analysis was performed on the top 50 hub genes, which showed that these genes were risk factors for glioma (p < 0.001, Figure 6B). A Venn diagram was used to visualize the intersection of the top 50 hub genes with core genes enriched in the pathway, and five core genes were obtained, namely, COL1A1, COL1A2, FN1, ITGA1, and ITGB1 (Figure 6C). In addition, gene correlation analysis showed that ANXA1 was significantly correlated with COL1A1, COL1A2, FN1, ITGA1, and ITGB1 as indicated by the gene correlation circle diagram and scatter diagram (Figures 6D–I). In the remaining eight glioma samples (included four GBM and four LGG) and their corresponding paracancerous tissues, the relative expression levels of the five core genes were verified by qPCR (Figures 6J–N). The relative expression level is described in the Method section. The statistics were carried out by the paired-sample t-test, and the p values were 0.0078, 0.0151, 0.0234, 0.0974, and 0.0234. The expression of these genes was higher in gliomas by GEPIA (Figures 6O–S). The higher expression of these genes in gliomas was often accompanied by worse survival prognosis (Figures 6T–X).
FIGURE 6.
Screening of core genes, gene correlation analysis, and prognostic analysis. (A) PPI was constructed by the STRING online website and Cytoscape visualization software, and the top 50 Hub proteins with degree values were screened. (B) Univariate regression analysis of the top 50 Hub genes. (C) Intersection between ECM–receptor interaction and focal adhesion pathway genes and 50 Hub genes and displayed by the Venn diagram. (D–I) Gene correlation circle diagram and scatter diagram. (J–N) Relative expression levels of the five core genes were detected by qPCR. (O–S) Expression level of core genes in glioma and normal tissues according to the analysis of the GEPIA online website. (T–X) High expression level of the core genes has poor prognosis in gliomas.
Discussion
Glioma is the most common brain malignant tumor, and its incidence rate has increased in recent years (Wen and Kesari, 2008). Radiotherapy and chemotherapy have become the standard of care in the treatment of gliomas (Nabors et al., 2017). Due to different molecular types and grades of gliomas, GBM, in particular, is often accompanied by high invasiveness and high recurrence. Even after routine surgery and concurrent chemoradiotherapy, the prognosis of glioma patients is still poor (Wang and Jiang, 2013). In recent years, targeted therapy and immunotherapy have improved the prognosis in other common tumors. Although some glioma patients have responded to targeted therapies or immunotherapies, there is still a lack of obvious effect in most patients with glioma (Chen et al., 2017; Xu et al., 2020). Therefore, new predictive targets and potential therapeutic targets for glioma need to be further explored and studied.
In our study, we analyzed the expression of ANXA1 in glioma through the GEO database, which demonstrated that ANXA1 was highly expressed in glioma patients. The GEPIA and Human Protein Atlas online analyses verified these results. Moreover, the overexpression of ANXA1 in glioma was verified by Western blotting analysis. Survival prognosis, ROC curve, and univariate and multivariate Cox regression analyses were performed using the CGGA and TCGA databases, which further showed that ANXA1 played an important role in the development of glioma and supported that ANXA1 was an independent prognostic index of glioma. In addition, the expression of ANXA1 was investigated according to the clinical case characteristics of glioma, and a clinical correlation nomogram was constructed using the CGGA database, which supported that ANXA1 was an important predictor of glioma. Finally, calibration curves were used to verify the matching of actual and predicted survival, and the matching degree of the 3-year survival is higher. Therefore, these findings suggested that ANXA1 warrants further study in glioma.
Previous studies have linked ANXA1 with thrombosis and inflammation (Parente and Solito, 2004; Senchenkova et al., 2019). In addition, it has been reported that ANXA1 is closely related to tumor development and invasion (Boudhraa et al., 2016; Foo et al., 2019). For example, the prognosis of lung cancer patients with a high ANXA1 expression is poor, and the growth of lung cancer cells is reduced by downregulating ANXA1 using small interference RNA (siRNA) (Chuang et al., 2021). Overexpression of ANXA1 promotes metastasis in breast cancer patients, resulting in poor prognosis (de Graauw et al., 2010). Therefore, ANXA1 plays an important role in tumors.
The tumor microenvironment is the internal and external environment for tumor survival. The components of the tumor microenvironment include not only tumor cells but also peripheral blood vessels, ECM, and some molecular signal factors (Hui and Chen, 2015). In the tumor microenvironment, tumor cells change and maintain their own development needs through autocrine and paracrine factors. There is a two-way driving effect between the tumor microenvironment and tumor cell progression, invasion, and metastasis (Meurette and Mehlen, 2018), in which the molecular mechanism is complex. Moreover, due to the complex process of the occurrence, development, invasion, and infiltration of tumor cells (Parker et al., 2020) and the resourcefulness of tumor cells themselves (Dunn et al., 2002), it is difficult to effectively control tumors. GBM, a malignant brain tumor with rapid progression and recurrence, is more difficult to clinically control (Weller et al., 2015). After an analysis of the potential molecular targets of glioma, we found that ANXA1 may play an important role in glioma through ECM–receptor interaction and focal adhesion signal pathways. As an important part of the tumor microenvironment, the ECM has a critical role in tumor occurrence, development, and invasion (Mohan et al., 2020). Focal adhesion is a subcellular structure, which not only has strong adhesion to the ECM but also promotes intracellular reorganization, resulting in a series of dynamic changes in cell function and morphology. Focal adhesion also plays an important role in the process of tumorigenesis, development, and infiltration (Legate et al., 2009). Multifunctional and multiprotein focal adhesion complexes play a key role in the underlying mechanism, which not only promote contact with the ECM but also promote the close conjunction between the ECM and actin cytoskeleton. Therefore, these complexes control the external morphology and internal signals of cells in terms of structure and function to promote cell growth and development, proliferation, differentiation, and motility (Legate and Fässler, 2009; Eke and Cordes, 2015). The main component of the ECM is collagen (Nissen et al., 2019). Type I collagen exists in most connective and embryonic tissues. In general, type I collagen consists of two chains of the alpha 1 chain (COL1A1) and one chain of the alpha 2 chain (COL1A2) (Gelse et al., 2003; Exposito et al., 2010). The alpha 1 chain of type I collagen is encoded by the COL1A1 gene and has been reported to be expressed in a variety of cancers, such as gastric cancer (Wang and Yu, 2018) and glioma (Balbous et al., 2014). COL1A1 is considered to be a marker of mesenchymal osteoblasts (Mori-Akiyama et al., 2003) and is also defined as a glioma endothelial marker selectively expressed in microvessels (Liu et al., 2010). COL1A2 is one of the most abundant collagen types in the human body, and it is involved in the process of angiogenesis. It has been reported that COL1A2 is upregulated in cancer (Greco et al., 2010) and that it promotes the proliferation and invasion of various cancers, such as gastric cancer and pancreatic cancer (Wu et al., 2019; Pan et al., 2021). FN1 is a type of adhesion glycoprotein involved in the ECM function of tumor cells, including cell adhesion, proliferation, and migration (Ko et al., 2020). Integrins play an important role in tumorigenesis, progression, and metastasis because they mediate the adhesion, migration, proliferation, invasion, and tumorigenicity of cancer cells (Pinon and Wehrle-Haller, 2011; Boudjadi et al., 2017). Among them, integrin α1 (ITGA1) and integrin β1 (ITGB1) are important members of the integrin family (Humphries et al., 2006; Hu et al., 2017).
Finally, through the intersection of the enriched core genes of the two pathways and the hub genes, we found that COL1A1, COL1A2, FN1, ITGA1, and ITGB1 were highly correlated with the ANXA1 gene, and these genes were overexpressed in glioma compared to normal brain tissues, which was verified in small samples of gliomas by the qPCR experiment. Through GEPIA, we found that the overexpression of these five core genes had poor prognosis in gliomas. Overall, the present study indicated that these five core genes and the ANXA1 gene participated in the occurrence and development of gliomas and suggested that they may play a synergistic role in the prognosis of gliomas. Therefore, the impact of these genes on gliomas should be further studied to seek the outcomes for glioma patients.
Conclusion
Glioma is a common primary tumor in the brain. The high recurrence and invasion of gliomas endanger the survival of patients and pose a serious challenge to human health. Therefore, we investigated the expression of the ANXA1 gene in glioma and found that ANXA1 was overexpressed in gliomas. Moreover, we found that the prognosis of glioma patients with a high ANXA1 expression was worse and that ANXA1 was an independent prognostic index of gliomas. The present findings also indicated that ANXA1 may play an important role in the occurrence, development, invasion, and infiltration by affecting the ECM–receptor interaction and the focal adhesion signal pathways. Finally, by using gene correlation analysis, we found that the COL1A1, COL1A2, FN1, ITGA1, ITGB1, and ANXA1 genes may play a synergistic role in glioma patients. Therefore, the present study using public data recommends that ANXA1 may be an important molecular target for glioma. Blocking the overexpression of the ANXA1 gene is likely to improve the prognosis of glioma patients. Because the present study was based on online databases and glioma tissue samples, there were several limitations, indicating that additional clinical experimental studies are needed to improve the reliability of ANXA1 in glioma research.
Acknowledgments
We thank all participating members in the Central Laboratory of the Second Hospital of Hebei Medical University and the Radiotherapy Department of the Second Hospital of Hebei Medical University.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Second Hospital of Hebei Medical University. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by the Second Hospital of Hebei Medical University. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
DZ and WW conceived and/or designed experiments, conducted experiments, analyzed data, prepared charts, wrote and/or reviewed drafts of manuscripts, and approved final drafts. HZ conceived and/or designed the experiment, wrote and/or reviewed drafts of manuscripts, and approved the final draft. LS, XH, XZ, WH, and YW conducted data analysis and/or prepared charts, participated in the writing of the article, and reviewed the draft manuscript. XX conceived and designed the experiment, guided the writing and review of the draft manuscript, and approved the final draft.
Funding
This work was supported by The Key Research and Development Project of Hebei Province- Special Project of Biomedicine (Grant Number 19277737D).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.851505/full#supplementary-material
References
- Aldape K., Zadeh G., Mansouri S., Reifenberger G., von Deimling A. (2015). Glioblastoma: Pathology, Molecular Mechanisms and Markers. Acta Neuropathol. 129, 829–848. 10.1007/s00401-015-1432-1 [DOI] [PubMed] [Google Scholar]
- Auvergne R. M., Sim F. J., Wang S., Chandler-Militello D., Burch J., Al Fanek Y., et al. (2013). Transcriptional Differences between Normal and Glioma-Derived Glial Progenitor Cells Identify a Core Set of Dysregulated Genes. Cell Rep. 3, 2127–2141. 10.1016/j.celrep.2013.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbous A., Cortes U., Guilloteau K., Villalva C., Flamant S., Gaillard A., et al. (2014). A Mesenchymal Glioma Stem Cell Profile is Related to Clinical Outcome. Oncogenesis 3, e91. 10.1038/oncsis.2014.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudhraa Z., Bouchon B., Viallard C., D' Incan M., Degoul F. (2016). Annexin A1 Localization and its Relevance to Cancer. Clin. Sci. Lond. 130, 205–220. 10.1042/CS20150415 [DOI] [PubMed] [Google Scholar]
- Boudjadi S., Bernatchez G., Sénicourt B., Beauséjour M., Vachon P., Carrier J., et al. (2017). Involvement of the Integrin α1β1 in the Progression of Colorectal Cancer. Cancers 9, 96. 10.3390/cancers9080096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush N. A. O., Chang S. M., Berger M. S. (2017). Current and Future Strategies for Treatment of Glioma. Neurosurg. Rev. 40, 1–14. 10.1007/s10143-016-0709-8 [DOI] [PubMed] [Google Scholar]
- Chen R., Smith-Cohn M., Cohen A. L., Colman H. (2017). Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics 14, 284–297. 10.1007/s13311-017-0519-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T.-Y., Wu M.-S., Lin J.-T., Lin M.-T., Shun C.-T., Huang H.-Y., et al. (2012). Annexin A1 is Associated with Gastric Cancer Survival and Promotes Gastric Cancer Cell Invasiveness through the Formyl Peptide Receptor/extracellular Signal-Regulated Kinase/integrin Beta-1-Binding Protein 1 Pathway. Cancer 118, 5757–5767. 10.1002/cncr.27565 [DOI] [PubMed] [Google Scholar]
- Chuang M.-C., Lung J.-H., Chen Y.-C., Lin Y.-C., Li Y.-C., Hung M.-S. (2021). The Association of Annexin A1 and Chemosensitivity to Osimertinib in Lung Cancer Cells. Cancers 13, 4106. 10.3390/cancers13164106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloughesy T. F., Mochizuki A. Y., Orpilla J. R., Hugo W., Lee A. H., Davidson T. B., et al. (2019). Neoadjuvant Anti-PD-1 Immunotherapy Promotes a Survival Benefit with Intratumoral and Systemic Immune Responses in Recurrent Glioblastoma. Nat. Med. 25, 477–486. 10.1038/s41591-018-0337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier D., Krolicki L., Morgenstern A., Merlo A. (2016). Targeted Radiolabeled Compounds in Glioma Therapy. Seminars Nucl. Med. 46, 243–249. 10.1053/j.semnuclmed.2016.01.009 [DOI] [PubMed] [Google Scholar]
- de Graauw M., van Miltenburg M. H., Schmidt M. K., Pont C., Lalai R., Kartopawiro J., et al. (2010). Annexin A1 Regulates TGF-β Signaling and Promotes Metastasis Formation of Basal-like Breast Cancer Cells. Proc. Natl. Acad. Sci. U.S.A. 107, 6340–6345. 10.1073/pnas.0913360107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G. P., Bruce A. T., Ikeda H., Old L. J., Schreiber R. D. (2002). Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat. Immunol. 3, 991–998. 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- Eke I., Cordes N. (2015). Focal Adhesion Signaling and Therapy Resistance in Cancer. Seminars Cancer Biol. 31, 65–75. 10.1016/j.semcancer.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Esteva F. J., Hubbard-Lucey V. M., Tang J., Pusztai L. (2019). Immunotherapy and Targeted Therapy Combinations in Metastatic Breast Cancer. Lancet Oncol. 20, e175–e186. 10.1016/S1470-2045(19)30026-9 [DOI] [PubMed] [Google Scholar]
- Exposito J.-Y., Valcourt U., Cluzel C., Lethias C. (2010). The Fibrillar Collagen Family. Int. J. Mol. Sci. 11, 407–426. 10.3390/ijms11020407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo S. L., Yap G., Cui J., Lim L. H. K. (2019). Annexin-A1 - A Blessing or a Curse in Cancer? Trends Mol. Med. 25, 315–327. 10.1016/j.molmed.2019.02.004 [DOI] [PubMed] [Google Scholar]
- Ganesh K., Stadler Z. K., Cercek A., Mendelsohn R. B., Shia J., Segal N. H., et al. (2019). Immunotherapy in Colorectal Cancer: Rationale, Challenges and Potential. Nat. Rev. Gastroenterol. Hepatol. 16, 361–375. 10.1038/s41575-019-0126-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelse K., Pöschl E., Aigner T. (2003). Collagens-structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 55, 1531–1546. 10.1016/j.addr.2003.08.002 [DOI] [PubMed] [Google Scholar]
- Greco C., Bralet M.-P., Ailane N., Dubart-Kupperschmitt A., Rubinstein E., Le Naour F., et al. (2010). E-cadherin/p120-catenin and Tetraspanin Co-029 Cooperate for Cell Motility Control in Human Colon Carcinoma. Cancer Res. 70, 7674–7683. 10.1158/0008-5472.CAN-09-4482 [DOI] [PubMed] [Google Scholar]
- Griesinger A. M., Birks D. K., Donson A. M., Amani V., Hoffman L. M., Waziri A., et al. (2013). Characterization of Distinct Immunophenotypes across Pediatric Brain Tumor Types. J. Immunol. 191, 4880–4888. 10.4049/jimmunol.1301966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Liu S., Sun M.-Z. (2013). Potential Role of Anxa1 in Cancer. Future Oncol. 9, 1773–1793. 10.2217/fon.13.114 [DOI] [PubMed] [Google Scholar]
- Hu C., Ni Z., Li B.-s., Yong X., Yang X., Zhang J.-w., et al. (2017). hTERT Promotes the Invasion of Gastric Cancer Cells by Enhancing FOXO3a Ubiquitination and Subsequent ITGB1 Upregulation. Gut 66, 31–42. 10.1136/gutjnl-2015-309322 [DOI] [PubMed] [Google Scholar]
- Hui L., Chen Y. (2015). Tumor Microenvironment: Sanctuary of the Devil. Cancer Lett. 368, 7–13. 10.1016/j.canlet.2015.07.039 [DOI] [PubMed] [Google Scholar]
- Humphries J. D., Byron A., Humphries M. J. (2006). Integrin Ligands at a Glance. J. Cell. Sci. 119, 3901–3903. 10.1242/jcs.03098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Mao Y., Ma W., Mao Q., You Y., Yang X., et al. (2016). CGCG Clinical Practice Guidelines for the Management of Adult Diffuse Gliomas. Cancer Lett. 375, 263–273. 10.1016/j.canlet.2016.01.024 [DOI] [PubMed] [Google Scholar]
- Kaley T., Touat M., Subbiah V., Hollebecque A., Rodon J., Lockhart A. C., et al. (2018). BRAF Inhibition in BRAFV600-Mutant Gliomas: Results from the VE-BASKET Study. J. Clin. Oncol. 36, 3477–3484. 10.1200/JCO.2018.78.9990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y.-C., Lai T.-Y., Hsu S.-C., Wang F.-H., Su S.-Y., Chen Y.-L., et al. (2020). Index of Cancer-Associated Fibroblasts Is Superior to the Epithelial-Mesenchymal Transition Score in Prognosis Prediction. Cancers 12, 1718. 10.3390/cancers12071718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate K. R., Fässler R. (2009). Mechanisms that Regulate Adaptor Binding to β-integrin Cytoplasmic Tails. J. Cell. Sci. 122, 187–198. 10.1242/jcs.041624 [DOI] [PubMed] [Google Scholar]
- Legate K. R., Wickström S. A., Fässler R. (2009). Genetic and Cell Biological Analysis of Integrin Outside-In Signaling. Genes Dev. 23, 397–418. 10.1101/gad.1758709 [DOI] [PubMed] [Google Scholar]
- Lim L. H. K., Pervaiz S. (2007). Annexin 1: The New Face of an Old Molecule. FASEB J. 21, 968–975. 10.1096/fj.06-7464rev [DOI] [PubMed] [Google Scholar]
- Liu Y., Carson-Walter E. B., Cooper A., Winans B. N., Johnson M. D., Walter K. A. (2010). Vascular Gene Expression Patterns are Conserved in Primary and Metastatic Brain Tumors. J. Neurooncol. 99, 13–24. 10.1007/s11060-009-0105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis D. N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W. K., et al. (2016). The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a Summary. Acta Neuropathol. 131, 803–820. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- Louis D. N., Perry A., Wesseling P., Brat D. J., Cree I. A., Figarella-Branger D., et al. (2021). The 2021 WHO Classification of Tumors of the Central Nervous System: a Summary. Neuro Oncol. 23, 1231–1251. 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Liu L., Li X., Chen W., Lu Z. (2021). Tat-NTS Suppresses the Proliferation, Migration and Invasion of Glioblastoma Cells by Inhibiting Annexin-A1 Nuclear Translocation. Cell. Mol. Neurobiol. 10.1007/s10571-021-01134-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschler S., Gebeshuber C. A., Wiedemann E. M., Alacakaptan M., Schreiber M., Custic I., et al. (2010). Annexin A1 Attenuates EMT and Metastatic Potential in Breast Cancer. EMBO Mol. Med. 2, 401–414. 10.1002/emmm.201000095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurette O., Mehlen P. (2018). Notch Signaling in the Tumor Microenvironment. Cancer Cell 34, 536–548. 10.1016/j.ccell.2018.07.009 [DOI] [PubMed] [Google Scholar]
- Mohan V., Das A., Sagi I. (2020). Emerging Roles of ECM Remodeling Processes in Cancer. Seminars Cancer Biol. 62, 192–200. 10.1016/j.semcancer.2019.09.004 [DOI] [PubMed] [Google Scholar]
- Monastyrskaya K., Babiychuk E. B., Draeger A. (2009). The Annexins: Spatial and Temporal Coordination of Signaling Events during Cellular Stress. Cell. Mol. Life Sci. 66, 2623–2642. 10.1007/s00018-009-0027-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori-Akiyama Y., Akiyama H., Rowitch D. H., de Crombrugghe B. (2003). Sox9 is Required for Determination of the Chondrogenic Cell Lineage in the Cranial Neural Crest. Proc. Natl. Acad. Sci. U.S.A. 100, 9360–9365. 10.1073/pnas.1631288100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murat A., Migliavacca E., Gorlia T., Lambiv W. L., Shay T., Hamou M.-F., et al. (2008). Stem Cell-Related "Self-Renewal" Signature and High Epidermal Growth Factor Receptor Expression Associated with Resistance to Concomitant Chemoradiotherapy in Glioblastoma. J. Clin. Oncol. 26, 3015–3024. 10.1200/JCO.2007.15.7164 [DOI] [PubMed] [Google Scholar]
- Mussunoor S., Murray G. (2008). The Role of Annexins in Tumour Development and Progression. J. Pathol. 216, 131–140. 10.1002/path.2400 [DOI] [PubMed] [Google Scholar]
- Nabors L. B., Portnow J., Ammirati M., Baehring J., Brem H., Butowski N., et al. (2017). NCCN Guidelines Insights: Central Nervous System Cancers, Version 1.2017. J. Natl. Compr. Canc Netw. 15, 1331–1345. 10.6004/jnccn.2017.0166 [DOI] [PubMed] [Google Scholar]
- Naylor E. C., Desani J. K., Chung P. K. (2016). Targeted Therapy and Immunotherapy for Lung Cancer. Surg. Oncol. Clin. N. Am. 25, 601–609. 10.1016/j.soc.2016.02.011 [DOI] [PubMed] [Google Scholar]
- Nissen N. I., Karsdal M., Willumsen N. (2019). Collagens and Cancer Associated Fibroblasts in the Reactive Stroma and its Relation to Cancer Biology. J. Exp. Clin. Cancer Res. 38, 115. 10.1186/s13046-019-1110-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom Q. T., Bauchet L., Davis F. G., Deltour I., Fisher J. L., Langer C. E., et al. (2014). The Epidemiology of Glioma in Adults: A "state of the Science" Review. Neuro Oncol. 16, 896–913. 10.1093/neuonc/nou087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Ding Y., Jiang Y., Wang X., Rao J., Zhang X., et al. (2021). LncRNA LIFR-AS1 Promotes Proliferation and Invasion of Gastric Cancer Cell via miR-29a-3p/COL1A2 axis. Cancer Cell Int. 21, 7. 10.1186/s12935-020-01644-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente L., Solito E. (2004). Annexin 1: More Than an Anti-phospholipase Protein. Inflamm. Res. 53, 125–132. 10.1007/s00011-003-1235-z [DOI] [PubMed] [Google Scholar]
- Parker T. M., Henriques V., Beltran A., Nakshatri H., Gogna R. (2020). Cell Competition and Tumor Heterogeneity. Seminars Cancer Biol. 63, 1–10. 10.1016/j.semcancer.2019.09.003 [DOI] [PubMed] [Google Scholar]
- Piawah S., Venook A. P. (2019). Targeted Therapy for Colorectal Cancer Metastases: A Review of Current Methods of Molecularly Targeted Therapy and the Use of Tumor Biomarkers in the Treatment of Metastatic Colorectal Cancer. Cancer 125, 4139–4147. 10.1002/cncr.32163 [DOI] [PubMed] [Google Scholar]
- Pinon P., Wehrle-Haller B. (2011). Integrins: Versatile Receptors Controlling Melanocyte Adhesion, Migration and Proliferation. Pigment. Cell Melanoma Res. 24, 282–294. 10.1111/j.1755-148X.2010.00806.x [DOI] [PubMed] [Google Scholar]
- Quail D. F., Joyce J. A. (2017). The Microenvironmental Landscape of Brain Tumors. Cancer Cell 31, 326–341. 10.1016/j.ccell.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M. E., Phipson B., Wu D., Hu Y., Law C. W., Shi W., et al. (2015). Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 43, e47. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senchenkova E. Y., Ansari J., Becker F., Vital S. A., Al-Yafeai Z., Sparkenbaugh E. M., et al. (2019). Novel Role for the AnxA1-Fpr2/ALX Signaling Axis as a Key Regulator of Platelet Function to Promote Resolution of Inflammation. Circulation 140, 319–335. 10.1161/CIRCULATIONAHA.118.039345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R., Mason W. P., van den Bent M. J., Weller M., Fisher B., Taphoorn M. J. B., et al. (2005). Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 352, 987–996. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- Sun L., Hui A.-M., Su Q., Vortmeyer A., Kotliarov Y., Pastorino S., et al. (2006). Neuronal and Glioma-Derived Stem Cell Factor Induces Angiogenesis within the Brain. Cancer Cell 9, 287–300. 10.1016/j.ccr.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Wang Y., Jiang T. (2013). Understanding High Grade Glioma: Molecular Mechanism, Therapy and Comprehensive Management. Cancer Lett. 331, 139–146. 10.1016/j.canlet.2012.12.024 [DOI] [PubMed] [Google Scholar]
- Wang Q., Yu J. (2018). MiR-129-5p Suppresses Gastric Cancer Cell Invasion and Proliferation by Inhibiting COL1A1. Biochem. Cell Biol. 96, 19–25. 10.1139/bcb-2016-0254 [DOI] [PubMed] [Google Scholar]
- Wei L., Li L., Liu L., Yu R., Li X., Luo Z. (2021). Knockdown of Annexin-A1 Inhibits Growth, Migration and Invasion of Glioma Cells by Suppressing the PI3K/Akt Signaling Pathway. ASN Neuro 13, 175909142110012. 10.1177/17590914211001218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M., Wick W., Aldape K., Brada M., Berger M., Pfister S. M., et al. (2015). Glioma. Nat. Rev. Dis. Prim. 1, 15017. 10.1038/nrdp.2015.17 [DOI] [PubMed] [Google Scholar]
- Wen P. Y., Kesari S. (2008). Malignant Gliomas in Adults. N. Engl. J. Med. 359, 492–507. 10.1056/NEJMra0708126 [DOI] [PubMed] [Google Scholar]
- Wu J., Liu J., Wei X., Yu Q., Niu X., Tang S., et al. (2019). A Feature-Based Analysis Identifies COL1A2 as a Regulator in Pancreatic Cancer. J. Enzyme Inhibition Med. Chem. 34, 420–428. 10.1080/14756366.2018.1484734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W., Zhu J., Wang X., Tang Y., Zhou P., Hou M., et al. (2020). ANXA1 Directs Schwann Cells Proliferation and Migration to Accelerate Nerve Regeneration through the FPR2/AMPK Pathway. FASEB J. 34, 13993–14005. 10.1096/fj.202000726RRR [DOI] [PubMed] [Google Scholar]
- Xu Y., Wang J., Xu Y., Xiao H., Li J., Wang Z. (2017). Screening Critical Genes Associated with Malignant Glioma Using Bioinformatics Analysis. Mol. Med. Rep. 16, 6580–6589. 10.3892/mmr.2017.7471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Tang L., Li X., Fan F., Liu Z. (2020). Immunotherapy for Glioma: Current Management and Future Application. Cancer Lett. 476, 1–12. 10.1016/j.canlet.2020.02.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.