Abstract
It has recently been noted that a diversity of hyperthermophilic microorganisms have the ability to reduce Fe(III) with hydrogen as the electron donor, but the reduction of Fe(III) or other metals by these organisms has not been previously examined in detail. When Pyrobaculum islandicum was grown at 100°C in a medium with hydrogen as the electron donor and Fe(III)-citrate as the electron acceptor, the increase in cell numbers of P. islandicum per mole of Fe(III) reduced was found to be ca. 10-fold higher than previously reported. Poorly crystalline Fe(III) oxide could also serve as the electron acceptor for growth on hydrogen. The stoichiometry of hydrogen uptake and Fe(III) oxide reduction was consistent with the oxidation of 1 mol of hydrogen resulting in the reduction of 2 mol of Fe(III). The poorly crystalline Fe(III) oxide was reduced to extracellular magnetite. P. islandicum could not effectively reduce the crystalline Fe(III) oxide minerals goethite and hematite. In addition to using hydrogen as an electron donor for Fe(III) reduction, P. islandicum grew via Fe(III) reduction in media in which peptone and yeast extract served as potential electron donors. The closely related species P. aerophilum grew via Fe(III) reduction in a similar complex medium. Cell suspensions of P. islandicum reduced the following metals with hydrogen as the electron donor: U(VI), Tc(VII), Cr(VI), Co(III), and Mn(IV). The reduction of these metals was dependent upon the presence of cells and hydrogen. The metalloids arsenate and selenate were not reduced. U(VI) was reduced to the insoluble U(IV) mineral uraninite, which was extracellular. Tc(VII) was reduced to insoluble Tc(IV) or Tc(V). Cr(VI) was reduced to the less toxic, less soluble Cr(III). Co(III) was reduced to Co(II). Mn(IV) was reduced to Mn(II) with the formation of manganese carbonate. These results demonstrate that biological reduction may contribute to the speciation of metals in hydrothermal environments and could account for such phenomena as magnetite accumulation and the formation of uranium deposits at ca. 100°C. Reduction of toxic metals with hyperthermophilic microorganisms or their enzymes might be applied to the remediation of metal-contaminated waters or waste streams.
Recent studies have suggested that the capacity for dissimilatory Fe(III) reduction is a common characteristic of hyperthermophilic Archaea and Bacteria (58). However, these initial studies only represented a very preliminary screening of the potential for dissimilatory metal reduction at high temperature. For example, growth studies were only conducted on two organisms, Thermotoga maritima and Pyrobaculum islandicum. In those studies, soluble Fe(III) citrate was provided as the electron acceptor whereas the environmentally relevant Fe(III) forms are insoluble Fe(III) oxides (28). Furthermore, no stoichiometric data was available to demonstrate that Fe(III) reduction was the sole sink for hydrogen consumption.
Further investigation of Fe(III) reduction at temperatures of ca. 100°C is warranted because of the potential environmental and evolutionary significance of this form of respiration. For example, as recently reviewed (62), common physiological characteristics of hyperthermophilic Bacteria and Archaea are often considered to provide insights into the physiological properties of the earliest microorganisms because hyperthermophiles are the extant organisms most closely related to the last common ancestors of modern life. Thus, the finding that all hyperthermophiles that have been investigated have a constitutive ability to reduce Fe(III) has led to the suggestion that early microorganisms had the capacity for Fe(III) reduction (58). This concept is consistent with geochemical evidence which suggests that conditions on pre-biotic Earth were conducive to hydrogen oxidation coupled to Fe(III) reduction and that Fe(III) reduction was one of the earliest forms of microbial respiration (7, 11, 28, 60).
Geochemical evidence suggests that Fe(III) reduction also is, or has been, an important process in hot biospheres other than early Earth. For example, the recovery of larger quantities of ultrafine-grained magnetite at depths of 5.5 to 6.7 km in the terrestrial subsurface, was proposed as evidence for a modern deep, hot subsurface biosphere (15) because the magnetite was morphologically similar to magnetite known to be produced by dissimilatory Fe(III)-reducing bacteria (27, 44). It was further speculated that there could be similar hot biospheres below the surfaces of other planets (15), and ultrafine-grained magnetite was subsequently found in meteorite ALH84001, which some believe contains evidence of previous life on Mars (46). Modern hydrothermal fluids generally contain high concentrations of Fe(II), which can be oxidized to Fe(III) upon exposure to oxygen with the subsequent deposition of Fe(III) oxides within hot anaerobic environments (3, 20, 21), and it has been suggested that microbial Fe(III) reduction warrants “special attention” to help better understand carbon and electron flow in marine hydrothermal vents (21).
Many mesophilic microorganisms that have the ability use Fe(III) as a terminal electron acceptor can also reduce a variety of metals and metalloids other than Fe(III) (29, 33). After Fe(III), Mn(IV) is the most abundant metal likely to be found as a potential electron acceptor in sedimentary environments, and most Fe(III) reducers have the capacity to reduce Mn(IV) (31, 33). Trace metals reduced by Fe(III)-reducing microorganisms include the oxidized forms of the radioactive metals uranium (41, 43) and technetium (26), as well as the other trace metals and metalloids, such as cobalt (6, 16), chromium (29), arsenic (24), and selenium (50). Many of these metals and metalloids are environmental contaminants, and reduction of contaminant metals with Fe(III)-reducing microorganisms has been shown to have potential for the removal of contaminant metals from waters and waste streams and to immobilize metals in subsurface environments (30, 32). Microbial reduction of some of these metals may also play an important role in the formation of metal deposits, which might be especially important in hot environments containing metal-rich waters. A thermophilic Thermus species was found to reduce the contaminant metals uranium, chromium, and cobalt at 60°C (23). However, the potential for hyperthermophiles to reduce metals other than Fe(III) does not appear to have been previously evaluated.
P. islandicum is an anaerobic hyperthermophile that was recovered from hydrothermal groundwater (19). It was initially found to grow at an optimum temperature of 100°C with hydrogen as the electron donor and S° as the electron acceptor or with complex organics and a variety of sulfur compound electron acceptors (19). Previous studies suggested that Pyrobaculum islandicum us a suitable model organism for studying Fe(III) reduction in hyperthermophilic Archaea (58). The purpose of the studies reported here was to further investigate enzymatic reduction of Fe(III) oxides at 100°C with P. islandicum and to determine its potential for the reduction of other metals. The results demonstrate that not only can P. islandicum reduce Fe(III) oxide to Fe(II), it can reduce a variety of other metals of environmental interest that might account for important geological phenomena and have applications for remediation of metal-contaminated waters.
MATERIALS AND METHODS
Source of organisms.
P. islandicum (DSM 4184) was purchased from the German Collection of Microorganisms (DSM), Braunschweig, Germany. P. aerophilum (DSM 7523) was a gift from Imke Schroeder (Department of Microbiology and Molecular Genetics, University of California, Los Angeles).
Culturing techniques.
Strict anaerobic techniques (2, 47) were used throughout, as previously described (38). All incubations were 100°C in the dark, unless otherwise specified. For routine maintenance of cultures, 10 ml of medium was dispensed into anaerobic pressure tubes (Bellco Glass, Inc., Vineland, N.J.) and sparged with the appropriate gas mixture (as described below) for 10 min in order to remove dissolved oxygen from the medium. For the cell growth studies, 50 to 100 ml of medium was dispensed into 120-ml serum bottles and sparged for ca. 30 min with the appropriate gas mixture.
For growth of P. islandicum with hydrogen as the electron donor and poorly crystalline Fe(III) oxide as the electron acceptor, DSM medium 390, which is suggested for growth P. islandicum, was modified by replacing the thiosulfate with poorly crystalline Fe(III) oxide (100 mmol/liter) prepared as previously described (37). Yeast extract (0.01%) and sodium bicarbonate (1.93 g/liter) were added, and l-cysteine (0.25 mM) was substituted for the sodium sulfide. Vitamins and trace minerals were added from stock solutions (35). The headspace was H2-CO2 (80:20, vol/vol). The pH of the autoclaved medium was 6.2.
For studies on the stoichiometry of hydrogen uptake and reduction of poorly crystalline Fe(III) oxide, the headspace of the medium was N2-CO2 (80:20) and ca. 1 ml of hydrogen was added to initiate the study. Fe(II) and hydrogen were measured, as described below, at the start of the incubation and after most of the hydrogen had been consumed.
For growth with organic compounds as the electron donor, the bicarbonate was omitted from the medium described above, the concentration of yeast extract was increased to 0.02% and 0.05% peptone was added. The gas phase was N2. The pH was 6.2. Fe(III)-citrate was present at 20 mM. For comparison of the reduction of various Fe(III) oxides, goethite, hematite, or poorly crystalline Fe(III) oxide was provided at 100 mmol/liter as previously described (37).
Medium for evaluating the growth of P. aerophilum on Fe(III) was a modification of the peptone medium previously used to culture this organism (22). Fe(III) was provided as Fe(III) citrate (20 mM). The pH was adjusted to 6.8 to 7.0 with HCl. The gas phase was N2-CO2 (80:20, vol/vol).
Cell suspension studies.
For cell suspension studies, P. islandicum was grown in Fe(III)-citrate as the electron acceptor and organic compounds as the electron donor in the medium described above. Cells from 800-ml cultures grown in 1-liter bottles were harvested under N2 with centrifugation. The cells were suspended in 80 ml of anaerobic bicarbonate buffer (23 mM) under N2-CO2 and repelleted with centrifugation, and then this procedure repeated. The cells were resuspended in 8 ml of the bicarbonate buffer under N2-CO2. In order to determine the potential for the cells to reduce various metals, an aliquot of the cell suspension (ca. 0.1 ml) was added to metal-amended bicarbonate buffer (10 ml; pH 6) under H2-CO2 to provide ca. 0.025 mg of cell protein per ml.
For studies on U(VI) reduction, U(VI) was provided as uranyl acetate. Mn(IV) was added as MnO2 (15 mmol/liter) synthesized as previously described (38). Cr(VI) was added as potassium chromate. Co(III)-EDTA was generated by oxidizing Co(II) in the presence of MnO2 and EDTA as previously described (16) and added at a final concentration of 250 μM. Tc(VII) was added as ammonium pertechnetate to provide a final concentration of 250 μM. As(V) was supplied as sodium arsenate (100 μM), and Se(VI) was provided as sodium selenate (100 μM). Incubations were done at 100°C in the dark, except for the studies conducted with technetium, in which incubation was done at 90°C.
Analytical techniques.
Production of HCl-extractable Fe(II) was measured with ferrozine as previously described (36). For the studies on the stoichiometry of hydrogen uptake and Fe(III) oxide reduction, Fe(III) and Fe(II) were determined with the anaerobic oxalate extraction technique as previously described (51). Hydrogen concentrations were monitored with gas chromatography as previously described (34). Concentrations of U(VI) in cell suspension studies were measured on filtrates (0.2-μm pore diameter) injected into a Dionex DX-500 Ion Chromatograph with an HPIC-AS5 column and 0.1 M MgSO4–0.05 M H2SO4 as the eluent. U(VI) was monitored as A650 following an Arsenazo-III postcolumn derivitization (10). For Mn(II) determinations, Mn(II) was solubilized in 0.5 N HCl and the acidic extract was injected into a Dionex IonPac CS5A column with a solution of 1.4 mM pyridine-2,6-dicarboxylic acid, 13.2 mM potassium hydroxide, 1.12 mM potassium sulfate, and 14.8 mM formic acid as the eluent. Mn(II) was detected at A530 after postcolumn derivitization with a solution containing 1.0 M 2-dimethylaminoethanol, 0.5 M ammonium hydroxide, 0.3 M sodium bicarbonate, and 0.06 g of 4-(2-pyridylazo)resorcinol per liter. Co(II) was detected in a manner similar to that used for Mn(II). Cr(VI) concentrations were monitored with the diphenylcarbazide method as previously described (40). Technetium reduction was analyzed with a PhosphorImager technique as previously described (26).
Cells were counted with acridine orange staining and epifluorescence microscopy as previously described (38). Particulate iron forms were dissolved by the oxalate method as previously described (38), except that Fe(II) chloride (final concentrations, ca. 5 mM) was added to the samples to accelerate dissolution.
Protein concentrations were determined as previously described (55). Bovine serum albumin was the reference protein.
RESULTS
Reduction of Fe(III).
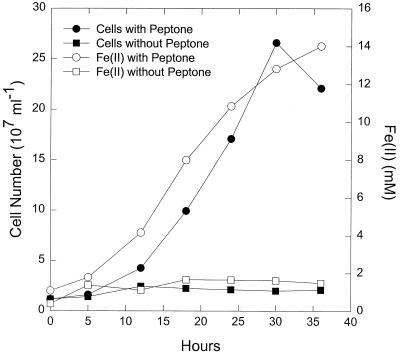
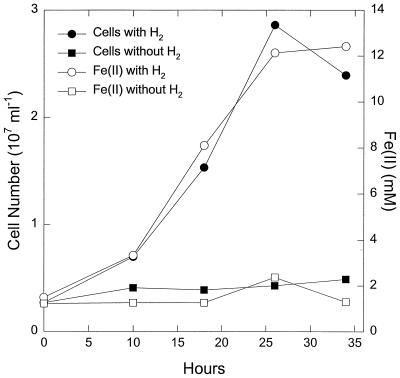
P. islandicum grew in medium with hydrogen as the electron donor and Fe(III)-citrate as the electron acceptor (Fig.1). The increase in cell numbers per mole of Fe(III) reduced was ca. 10-fold higher than the cell yield reporter in a comparable previous study with this organism (58). As discussed below, this difference was the result of a calculation error in the earlier study. P. islandicum also grew in medium in which peptone was provided as a potential electron donor (Fig.2). The closely related species P. aerophilum grew in a similar manner (K. Kashefi; unpublished data). The number of cells of P. islandicum produced per mole of Fe(III) reduced was higher in the complex medium (Fig. 2) than in medium in which hydrogen served as the electron donor for Fe(III) reduction (Fig. 1).
FIG. 2.
Growth of P. islandicum with peptone as the electron donor and Fe(III)-citrate as the electron acceptor. The medium also contained 0.02% yeast extract. The results are the means of triplicate cultures.
FIG. 1.
Growth of P. islandicum in medium with hydrogen as the electron donor and Fe(III)-citrate as the electron acceptor. The medium also contained 0.01% yeast extract. The results are the means of triplicate cultures.
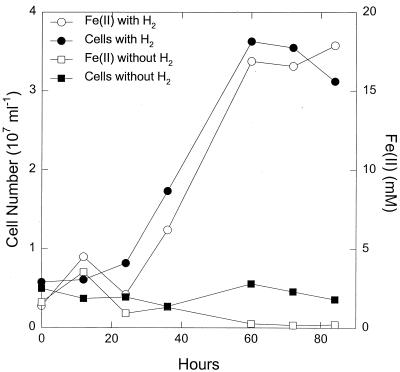
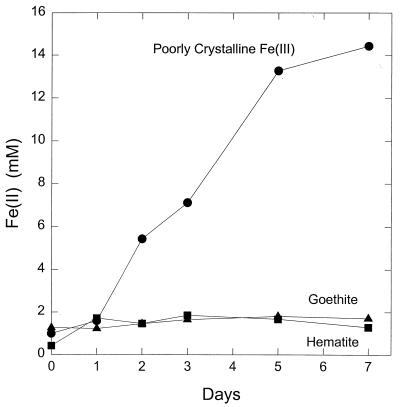
P. islandicum could also use poorly crystalline Fe(III) oxide as the electron acceptor for hydrogen oxidation (Fig. 3). The number of cells produced per mole of Fe(III) reduced when Fe(III) oxide was the electron acceptor was comparable to that with Fe(III)-citrate. Hydrogen did not abiotically reduce the Fe(III) oxide, and in the presence of cells, hydrogen was required for Fe(III) reduction and growth. Measurements of hydrogen consumption and Fe(III) oxide reduction in five replicate cultures demonstrated that 2.06 ± 0.16 (mean ± standard error) mol of Fe(III) was reduced per mol of hydrogen consumed, which is consistent with the stoichiometry expected from the following reaction: H2 + 2 Fe(III) → 2 Fe(II) + 2 H+. P. islandicum also reduced poorly crystalline Fe(III) oxide when grown in the peptone-yeast extract medium (Fig. 4). In contrast, the more crystalline Fe(III) oxide forms goethite and hematite were only poorly reduced, if at all (Fig. 4).
FIG. 3.
Growth of P. islandicum in medium with hydrogen as the electron donor and poorly crystalline Fe(III) oxide as the electron acceptor. The medium also contained 0.01% yeast extract. The results are the means of triplicate cultures.
FIG. 4.
Fe(III) reduction by P. islandicum grown on various forms of Fe(III) in peptone-yeast extract medium. The results are the means of duplicate cultures.
Reduction of poorly crystalline Fe(III) oxide was associated with a visible change in the iron. The nonmagnetic, reddish brown, poorly crystalline Fe(III) oxide was converted to a black magnetic precipitate (Kashefi, unpublished). X-ray diffraction analysis indicated that this precipitate is comprised of magnetite, and electron microscopy revealed that the magnetite is deposited extracellularly (Kashefi, unpublished).
Reduction of other metals.
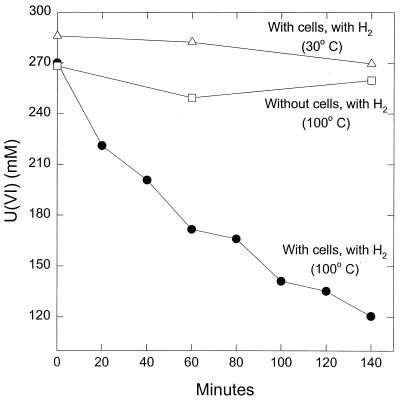
Washed cell suspensions of Fe(III)-grown P. islandicum were capable of reducing a variety of other metals. For example, U(VI) was readily reduced with hydrogen as the electron donor (Fig. 5). When U(VI) was added at a more environmentally relevant initial concentration of 10 μM, the U(VI) was completely reduced within 1 h (Kashefi, unpublished). Hydrogen did not abiotically reduce U(VI) at 100°C, and there was no reduction of U(VI) when the cells were incubated at a temperature too low for enzymatic activity of P. islandicum (Fig. 5). During U(VI) reduction, a dark precipitate formed. X-ray diffraction analysis of this product indicated that it contained the U(IV) mineral uraninite, which is deposited outside the cell (Kashefi, unpublished).
FIG. 5.
Reduction of U(VI) in cell suspensions of P. islandicum. The results are the means of duplicate incubations. The cell protein concentration was 0.025 mg/ml.
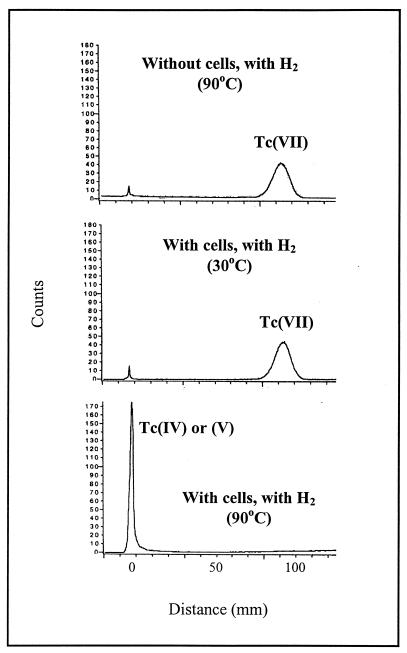
When Tc(VII) was added to cell suspensions of P. islandicum with hydrogen as the electron donor, the radioactivity added as Tc(VII) was recovered as a reduced form after overnight incubation (Fig. 6). In contrast, the radioactivity remained in the Tc(VII) pool if the cell suspensions were incubated at 30°C or if the cells were omitted from the incubation.
FIG. 6.
PhosphorImager analysis of paper chromatograms of supernatant from cell suspensions of P. islandicum incubated overnight in the presence of Tc(VII). The cell protein concentration was 0.012 mg/ml.
Cell suspensions of P. islandicum also rapidly reduced Mn(IV) and Co(III)-EDTA, as evidenced by accumulation of Mn(II) and Co(II) over time (Kashefi, unpublished). Mn(IV) reduction was associated with rapid disappearance of the dark brown Mn(IV) oxide and formation of a white precipitate that was presumably the manganese carbonate rhodochrosite. Attempts to grow P. islandicum with Mn(IV) as the sole electron acceptor were not successful.
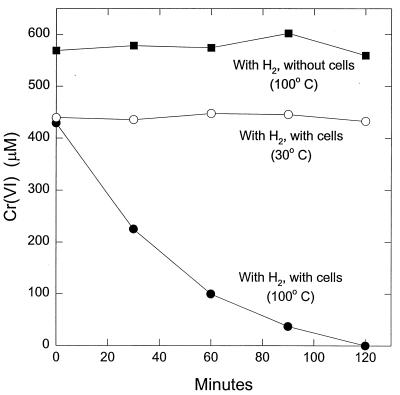
Cr(VI) was also reduced in cell suspensions (Fig. 7). The yellow color, characteristic of Cr(VI) in solution, disappeared as the concentration of Cr(VI) diminished, and there was the formation of a white colloidal suspension, characteristic of Cr(III) hydroxide. This indicates that the loss of Cr(VI) was the result of Cr(VI) reduction to Cr(III). There was no abiotic reduction of Cr(VI) at 100°C and no reduction at 30°C in the presence of cells.
FIG. 7.
Cr(VI) reduction in cell suspensions of P. islandicum. The results are the means of duplicate incubations. The cell protein concentration was 0.025 mg/ml.
DISCUSSION
This study represents the first detailed study of metal reduction by a hyperthermophilic microorganism. The results demonstrate that P. islandicum can readily reduce poorly crystalline Fe(III) oxide with either hydrogen or complex organics as the electron donor and is also capable of reducing a wide variety of trace metals of geological and environmental significance. As detailed below, these results provide further insight into the potential role of hyperthermophiles in the reduction of Fe(III) in hot environments, suggest potential mechanisms for the deposition of minerals at high temperatures, and may provide new potential approaches for the bioremediation of metal contamination.
Energy conservation via Fe(III) reduction.
Although a diversity of hyperthermophilic microorganisms have been shown to have the capacity to reduce Fe(III) (58), to date, P. islandicum and P. aerophilum are the only Archaea that have definitely been shown to conserve energy to support growth from dissimilatory Fe(III) reduction. Hydrogen-dependent growth of P. islandicum associated with the reduction of soluble Fe(III) was previously demonstrated (58). The increase in cell numbers reported here with hydrogen as the electron donor and Fe(III) citrate as the electron acceptor is ca. 10-fold higher than that previously reported under similar conditions (58). This apparent difference is due to a miscalculation in computing the previously reported cell numbers (Kashefi, unpublished). A similar error was made in the calculation of the growth of Thermatoga maritima in that previous study. The increase in cell numbers of P. islandicum per Fe(III) millimolar reduced reported here is comparable to the growth previously reported for several other hydrogen-oxidizing, Fe(III)-reducing mesophilic microorganisms (5, 9, 42).
The studies on the stoichiometry of hydrogen consumption and Fe(III) reduction indicate that hydrogen can serve as the primary electron donor for Fe(III) reduction in P. islandicum. P. islandicum has not been found to be capable of autotrophic growth with hydrogen and Fe(III), as small amounts of yeast extract are required for growth. This contrasts with the ability of this organism to grow autotrophically with hydrogen and S° as the electron acceptor (19). All of the mesophilic Fe(III)-reducing microorganisms reported to data were also found to require the addition of carbon sources for growth on Fe(III) with hydrogen as the electron donor (1, 5, 42). However, an autotrophic hydrogen-oxidizing, Fe(III)-reducing hyperthermophile has recently been isolated (K. Kashefi, J. M. Tor, and D. R. Lovley, unpublished data).
P. islandicum produced more cells per mole of Fe(III) reduced in a complex medium that contained peptone as a potential electron donor than in medium with hydrogen as the electron donor. This is similar to the greater yield in cell numbers when mesophilic Shewanella (5, 42) and Geobacter (6) species are grown with organic electron donors rather than hydrogen.
This report also provides the first data on the growth of a hyperthermophilic microorganism with Fe(III) oxide as an electron acceptor. Such data are important because Fe(III) oxides are likely to be the dominant forms of Fe(III) available for microbial reduction in most environments (28). The results presented here demonstrate that P. islandicum readily reduces poorly crystalline Fe(III) oxides but that more crystalline Fe(III) oxides are poorly reduced, if at all. This result is similar to that observed with many mesophilic Fe(III) reducers (28) and the thermophile Deferribacter thermophilus (17). The ability of P. islandicum to reduce Fe(III) oxide contrasts with a thermophilic Thermus species which only poorly reduced Fe(III) oxide and did not grow with Fe(III) oxide as the electron acceptor (23). However, several other pure cultures of other thermophilic Bacteria (17, 53), as well as thermophilic enrichment cultures (25, 54), have been found to reduce poorly crystalline Fe(III) oxide.
Geochemical implications.
The oxidation of hydrogen and organic matter coupled to the reduction of Fe(III) oxide carried out by P. islandicum provides a biological model for important geochemical reactions on early Earth. As previously reviewed (7, 14, 60), ocean chemistry in the Archaean is likely to have been dominated by hydrothermal systems and the oldest rocks on Earth contain evidence for microbial reduction of Fe(III) to magnetite. It has been proposed (7 and references therein) that prior to development of life on Earth, there was production of Fe(III) and hydrogen as high levels of UV radiation impinging on Archaean seas hydrolyzed the abundant dissolved Fe(II) according to the following reaction:hv2 Fe(II) + 2 H+ → 2 Fe(III) + H2
Other potential sources of Fe(III) were Fe(III) emitted in hydrothermal fluids (7) and oxidation of Fe(II) by abiotically formed nitrogen oxides (57). Hydrogen was also available from other geological sources (59). Other potential electron acceptors, such as oxygen, nitrate, or sulfate, were probably not abundant (8, 13, 61). If this geological scenario is correct, then conditions were highly favorable for the development of a biological entity that could take advantage of the energy available from hydrogen oxidation coupled to Fe(III) reduction. Thus, P. islandicum provides a pure-culture model of how microbial life might have benefited from environmental conditions on early Earth, leading to the massive accumulations of magnetite in the earliest Banded Iron Formations.
The studies with P. islandicum reported here also suggest that hyperthermophilic Fe(III)-reducing microorganisms could be growing in modern hydrothermal environments. For example, submarine hydrothermal vents emit high concentrations of dissolved Fe(II), which is oxidized in the oxygen-containing seawater, and the resulting Fe(III) oxides precipitate in the nearby sediments (20, 21, 52). Hot water percolating through these sediments may provide appropriate conditions for the growth of Fe(III)-reducing hyperthermophilic microorganisms. Similar opportunities for the growth of hyperthermophilic Fe(III)-reducing microorganisms may exist near terrestrial hot-spring environments (3).
The ultrafine-grained magnetite produced by P. islandicum is similar to the magnetite that has been recovered form the hot, deep terrestrial subsurface (15). It was previously speculated that the magnetite from the deep terrestrial subsurface was of microbial origin (15), but this study with P. islandicum is the first demonstration that such magnetite can be produced during the growth of a hyperthermophilic microorganism. The apparent production of rhodochrosite during Mn(IV) reduction by P. islandicum provides evidence for the potential of other minerals to be formed as the result of microbial metal reduction at 100°C.
The discovery that uranium can be enzymatically reduced at 100°C increases the known temperature range over which microorganisms may have an important influence on uranium geochemistry. Until the discovery of microbial U(VI) reduction at mesophilic temperatures, uranium geochemistry in anaerobic environments was considered to be controlled by abiotic processes ( 39, 41). However, studies on the relative significance of proposed abiotic processes for U(VI) reduction versus biological U(VI) reduction have led to the suggestion that microbial reductive precipitation of uranium is an important factor in geochemically important processes such as the precipitation of uranium in marine sediments, the formation of uranium reduction spots, and uranium ore accumulations (39, 41, 43).
Reductive precipitation of uranium at ca. 100°C is known to have led to the accumulation of important subsurface uranium deposits. Typical sandstone-type uranium deposits are considered to have been formed at 100°C (18). Another well-known example is the naturally generated Oklo nuclear reactor, in which U(VI) dissolved in groundwaters of 100 to 150°C was reduced to U(IV), which precipitated and formed the large uranium accumulations in this environment (4). It is probably impossible to determine whether microorganisms were responsible for the initial precipitation of uranium at this site which took place in the PreCambrian period. However, the results presented here suggest that in the modeling of the formation of this and other, more recent, uranium deposits formed in a similar manner (12), the possibility that microbial U(VI) reduction could be important should be considered. This is further emphasized by the finding that the lignite organic matter often associated with U(IV) deposits does not abiotically reduce U(VI) at temperatures below 120°C (48).
The ability of P. islandicum to reduce a variety of other metals is also of potential significance to the geochemistry of hydrothermal environments because hydrothermal fluids are typically enriched in a variety of metals and metalloids. However, the results indicate that hyperthermophilic microorganisms may not be able to reduce all metals and metalloids. P. islandicum could not reduce As(V) or Se(VII). As(V) reduction at 100°C is of interest because arsenic can be an important metalloid constituent of hydrothermal fluids and arsenic adsorbs onto Fe(III) oxides as they precipitate near hydrothermal vents (52). The inability of P. islandicum to reduce As(V) is not unexpected, as many mesophilic Fe(III)-reducing microorganisms are also incapable of As(V) reduction. Only one Fe(III)-reducing microorganism, Sulfurospirillum barnesii (formerly strain SES-3), is known to be capable of growing with As(V) as the electron acceptor (24). The other known As(V)-reducing microorganisms do not reduce Fe(III) (45, 49, 56).
Bioremediation implications.
Many mesophilic Fe(III)-reducing microorganisms have the potential to reduce toxic metals, converting them to less-soluble forms which are less mobile in groundwater or can be precipitated from waste streams or soil washings (30, 32). Thus, microbial metal reduction may be a strategy for in situ and ex situ remediation of metal contamination. A Thermus sp. was found to reduce U(VI), Cr(VI), and Co(III) at 60°C (23), and a fermentative microorganism enhanced reduction of Cr(VI) and Co(III) at temperatures of up to 65°C (63). The finding that P. islandicum can reduce toxic and radioactive metals at 100°C increases the known temperature range at which bioremediation of such metals may be possible.
Subsurface disposal of high-level radioactive waste represents one instance in which microbial reduction of U(VI), Tc(VII), and Co(III) at elevated temperatures could play an important role in preventing contaminant mobility. The environment around such wastes can be expected to have elevated temperatures (4), and thus, hyperthermophilic metal-reducing microorganisms could help prevent migration of these contaminants by reducing them to less mobile forms. Hot radioactive or metal-containing industrial wastes could potentially be treated in bioreactors containing microorganisms with a metabolism like that of P. islandicum or their enzymes.
In summary, the results demonstrate that P. islandicum has the ability to conserve energy to support growth by using Fe(III) as a terminal electron acceptor and has the capacity to transfer electrons to a wide variety of other metals. These results expand the known temperature range over which dissimilatory metal-reducing microorganisms may have an important impact on metal geochemistry. Studies on the distribution and activity of hyperthermophilic microorganisms in hot environments are warranted to determine the geochemical significance of this metabolism.
ACKNOWLEDGMENTS
We thank John Lloyd and Betsy Blunt-Harris for technical assistance.
This research was supported by grant DEB-9714285 from the LExEN program of the National Science Foundation.
REFERENCES
- 1.Balashova V V, Zavarzin G A. Anaerobic reduction of ferric iron by hydrogen bacteria. Microbiology. 1980;48:635–639. [PubMed] [Google Scholar]
- 2.Balch W E, Gox G E, Magrum L J, Woese C R, Wolfe R S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979;43:260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brock T D, Petersen C S, S, Mosser J L. Biogeochemistry and bacteriology of ferrous iron oxidation in geothermal habitats. Geochim Cosmochim Acta. 1976;40:493–500. [Google Scholar]
- 4.Brookins D G. Radionuclide behavior at the Oklo nuclear reactor, Gabon. Waste Management. 1990;10:285–296. [Google Scholar]
- 5.Caccavo F, Jr, Blakemore R P, Lovley D R. A hydrogen-oxidizing, Fe(III)-reducing microorganism from the Great Bay Estuary, New Hampshire. Appl Environ Microbiol. 1992;58:3211–3216. doi: 10.1128/aem.58.10.3211-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caccavo F, Jr, Lonergan D J, Lovley D R, Davis M, Stolz J F, McInerney M J. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol. 1994;60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairns-Smith A G, Hall A J, Russell M J. Mineral theories of the origin of life and an iron sulfide example. Orig Life Evol Biosphere. 1992;22:161–180. [Google Scholar]
- 8.Cameron E M. Sulphate and sulphate reduction in early Precambrian oceans. Nature. 1982;296:145–148. [Google Scholar]
- 9.Coates J D, Lonergan D J, Jenter H, Lovley D R. Isolation of Geobacter species from diverse sedimentary environments. Appl Environ Microbiol. 1996;62:1531–1536. doi: 10.1128/aem.62.5.1531-1536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Beer H, Coezce P. Ion chromatographic separation and spectrophotometric determination of U(VI) and U(IV) Radiochim Actya. 1992;57:113–117. [Google Scholar]
- 11.de Duve C. Vital dust. New York, N.Y: Basic Books; 1995. [Google Scholar]
- 12.Doi K, Hirono S. Behavior or uranium migration in epigenetic uranium ore deposits with reference to radioactive waste isolation in geologic media. Waste Management. 1990;10:275–284. [Google Scholar]
- 13.Eastoe C J, Gustin M S, Hurlbut D F, Orr R L. Sulfur isotopes in early Proterozoic volcanogenic massive sulfide deposits: new data from Arizona and implications for ocean chemistry. Precambrian Res. 1990;46:353–364. [Google Scholar]
- 14.Glasby G P. Earliest life in the Archean: rapid dispersal of CO2-utilizing bacteria from submarine hydrothermal vents. Episodes. 1998;21:252–256. [Google Scholar]
- 15.Gold T. The deep, hot biosphere. Proc Natl Acad Sci USA. 1992;89:6045–6049. doi: 10.1073/pnas.89.13.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorby Y A, Caccavo F, Jr, Bolton H., Jr Microbial reduction of cobaltIIIEDTA− in the presence and absence of manganese(IV) oxide. Environ Sci Technol. 1998;32:244–250. [Google Scholar]
- 17.Greene A C, Patel B K C, Sheehy A J. Deferribacter thermophilus gen. nov., sp. nov., a novel thermophilic manganese- and iron-reducing bacterium isolated from a petroleum reservoir. Int J Syst Bacteriol. 1997;47:505–509. doi: 10.1099/00207713-47-2-505. [DOI] [PubMed] [Google Scholar]
- 18.Hostetler P B, Garrels R M. Transportation and precipitation of uranium and vanadium at low temperatures with special reference to sandstone-type uranium. Econ Geol. 1962;57:137–167. [Google Scholar]
- 19.Huber R, Kristjansson J K, Stetter K O. Pyrobaculum gen. nov., a new genus of neutrophilic, rod-shaped archaebacteria form continental solfataras growing optimally at 100°C. Arch Microbiol. 1987;149:95–101. [Google Scholar]
- 20.Jannasch H W. Microbial interactions with hydrothermal fluids. Seafloor hyrothermal systems: physical, chemical, biological, and geological interactions. Geophys Monogr. 1995;91:273–296. [Google Scholar]
- 21.Karl D M. Ecology of free-living hydrothermal vent microbial communities. In: Karl D M, editor. The Microbiology of deep-sea hydrothermal vents. New York, N.Y: CRC Press; 1995. pp. 35–124. [Google Scholar]
- 22.Kashefi K. Cloning of the nitrate reductase gene from Desulfovibrio desulfuricans (ATCC 27774), an anaerobic sulfate-reducing bacterium. Ph.D. dissertation. London, England: University of London; 1996. [Google Scholar]
- 23.Kieft T L, Fredrickson J K, Onstott T C, Gorby Y A, Kostandarithes H M, Bailey T J, Kennedy D W, Li W, Plymale A E, Spadoni C M, Gray M S. Dissimilatory reduction of Fe(III) and other electron acceptors by a Thermus isolate. Appl Environ Microbiol. 1999;65:1214–1221. doi: 10.1128/aem.65.3.1214-1221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laverman A M, Switzer Blum J, Schaefer J K, Phillips E J P, Lovley D R, Oremland R S. Growth of strain SES-3 with arsenate and other diverse electron acceptors. Appl Environ Microbiol. 1995;61:3556–3561. doi: 10.1128/aem.61.10.3556-3561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S V, Zhou J, Zhang C, Cole D R, Gajdarziska-Josifovska M, Phelps T J. Thermophilic Fe(III)-reducing bacteria from the deep subsurface: the evolutionary implications. Science. 1997;277:1106–1109. [Google Scholar]
- 26.Lloyd J R, Macaskie L E. A novel PhosphorImager-based technique for monitoring the microbial reduction of technetium. Appl Environ Microbiol. 1996;62:578–582. doi: 10.1128/aem.62.2.578-582.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovley D R. Magnetite formation during microbial dissimilatory iron reduction. In: Frankel R B, Blakemore R P, editors. Iron biominerals. New York, N.Y: Plenum Press; 1990. pp. 151–166. [Google Scholar]
- 28.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovley D R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 30.Lovley D R. Bioremediation of organic and metal contaminants with dissimilatory metal reduction. J Ind Microbiol. 1995;14:85–93. doi: 10.1007/BF01569889. [DOI] [PubMed] [Google Scholar]
- 31.Lovley, D. R. Fe(III)- and Mn(IV)-reducing prokaryotes. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer and E. Stackebrandt (ed.), The prokaryotes, in press. Springer-Verlag, Inc., New York, N.Y.
- 32.Lovley D R, Coates J D. Bioremediation of metal contamination. Curr Opin Biotechnol. 1997;8:285–289. doi: 10.1016/s0958-1669(97)80005-5. [DOI] [PubMed] [Google Scholar]
- 33.Lovley D R, Coates J D, Saffarini D A, Lonergan D J. Dissimilatory iron reduction. In: Winkelman G, Carrano C J, editors. Iron and related transition metals in microbial metabolism. Zurich, Switzerland: Harwood Academic Publishers; 1997. pp. 187–215. [Google Scholar]
- 34.Lovley D R, Goodwin S. Hydrogen concentrations as an indicator of the predominant terminal electron accepting reactions in aquatic sediments. Geochim Cosmochim Acta. 1998;52:2993–3003. [Google Scholar]
- 35.Lovley D R, Greening R C, Ferry J G. Rapidly growing rumen methanogenic organism that synthesizes coenzyme M and has a high affinity for formate. Appl Environ Microbiol. 1984;48:81–87. doi: 10.1128/aem.48.1.81-87.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovley D R, Phillips E J P. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol. 1986;52:751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovley D R, Phillips E J P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lovley D R, Phillips E J P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron on manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lovley D R, Phillips E J P. Reduction of uranium by Desulfovibrio desulfuricans. Appl Environ Microbiol. 1992;58:850–856. doi: 10.1128/aem.58.3.850-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lovley D R, Phillips E J P. Reduction of chromate by Desulfovibrio vulgaris (Hildenborough) and its c3 cytochrome. Appl Environ Microbiol. 1994;60:726–728. doi: 10.1128/aem.60.2.726-728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lovley D R, Phillips E J P, Gorby Y A, Landa E R. Microbial reduction of uranium. Nature. 1991;350:413–416. [Google Scholar]
- 42.Lovley D R, Phillips E J P, Lonergan D J. Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl Environ Microbiol. 1989;55:700–706. doi: 10.1128/aem.55.3.700-706.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovley D R, Roden E E, Phillips E J P, Woodward J C. Enzymatic iron and uranium reduction by sulfate-reducing bacteria. Marine Geol. 1993;113:41–53. [Google Scholar]
- 44.Lovley D R, Stolz J F, Nord G L, Phillips E J P. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature. 1987;330:252–254. [Google Scholar]
- 45.Macy J M, Nunan K, Hagen K D, Dixon D R, Harbour P J, Cahill M, Sly L I. Chrysiogenes arsenatis gen. nov. sp. nov., a new arsenate-respiring bacterium isolated from gold mine wastewater. J Syst Bacteriol. 1996;46:1153–1157. doi: 10.1099/00207713-46-4-1153. [DOI] [PubMed] [Google Scholar]
- 46.McKay D S, Gibson E K, Jr, Thomas-Deprta K L, Vali H, Romanek C S, Clement S J, Chillier X D F, Maechling C R, Zare R N. Search for past life on Mars: possible relic biogenic activity in Martial meteorite ALH84001. Science. 1996;273:924–930. doi: 10.1126/science.273.5277.924. [DOI] [PubMed] [Google Scholar]
- 47.Miller T L, Wolin M J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974;27:985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakashima S, Disnar J R, Perruchot A, Trichet J. Experimental study of mechanisms of fixation and reduction of uranium by sedimentary organic matter under diagenetic or hydrothermal conditions. Geochim Cosmochim Acta. 1984;48:2331–2329. [Google Scholar]
- 49.Newman D K, Kennedy E K, Coates J D, Ahmann D, Ellis D J, Lovley D R, Morel F M M. Dissimilatory As(V) and S(VI) reduction in Desulfotomaculum aurpigmentum, sp. nov. Arch Microbiol. 1998;36:380–388. doi: 10.1007/s002030050512. [DOI] [PubMed] [Google Scholar]
- 50.Oremland R S. Biogeochemical transformations of selenium in anoxic environments. In: Frankenberger W T J, Benson S N, editors. Selenium in the environment. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 389–419. [Google Scholar]
- 51.Phillips E J P, Lovley D R. Determination of Fe(III) and Fe(II) in oxalate extracts of sediment. Soil Sci Soc Am J. 1987;51:938–941. [Google Scholar]
- 52.Pichler T, Veizer J, Hall G E M. Natural input of arsenic into a coral-reef ecosystem by hydrothermal fluids and its removal by Fe(III) oxyhydroxides. Environ Sci Technol. 1999;33:1373–1378. [Google Scholar]
- 53.Slobodkin A, Reysenbach A-L, Strutz N, Dreier M, Wiegel J. Thermoterrabacterium ferrireducens gen. nov., sp. nov., a thermophilic anaerobic dissimilatory Fe(III)-reducing bacterium from a continental hot spring. Int J Syst Bacteriol. 1997;47:541–547. doi: 10.1099/00207713-47-2-541. [DOI] [PubMed] [Google Scholar]
- 54.Slobodkin A, Wiegel J. Fe(III) as an electron acceptor for H2 oxidation in thermophilic anaerobic enrichment cultures from geothermal areas. Extremophiles. 1997;2:106–109. doi: 10.1007/s007920050022. [DOI] [PubMed] [Google Scholar]
- 55.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzaon M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 56.Stolz J F, Ellils D J, Switzer Blum J, Ahmann D, Lovley D R, Oremland R S. Sulfurospirillum barnesii sp. nov., Sulfurospirillum arsenophilum sp. nov., new members of the Sulfurospirillum clade of the Epsilon Proteobacteria. Int J Syst Bacteriol. 1999;49:1177–1180. doi: 10.1099/00207713-49-3-1177. [DOI] [PubMed] [Google Scholar]
- 57.Summers D P, Chang S. Prebiotic ammonia from reduction of nitrite by iron(II) on the early Earth. Nature. 1993;365:630–633. doi: 10.1038/365630a0. [DOI] [PubMed] [Google Scholar]
- 58.Vargas M, Kashefi K, Blunt-Harris E L, Lovley D R. Microbiological evidence for Fe(III) reduction on early Earth. Nature. 1998;395:65–67. doi: 10.1038/25720. [DOI] [PubMed] [Google Scholar]
- 59.Walker J C G. Atmospheric constraints on the evolution of metabolism. Origins of Life. 1980;10:93–104. doi: 10.1007/BF00928660. [DOI] [PubMed] [Google Scholar]
- 60.Walker J C G. Was the Archaean biosphere upside down? Nature. 1987;329:710–712. doi: 10.1038/329710a0. [DOI] [PubMed] [Google Scholar]
- 61.Walker J C G, Brimblecombe P. Iron and sulfur in the pre-biologic ocean. Precambrian Res. 1985;28:205–222. doi: 10.1016/0301-9268(85)90031-2. [DOI] [PubMed] [Google Scholar]
- 62.Wiegel J, Adams M W W. Thermophiles: the keys to molecular evolution and the origin of life? Philadelphia, Pa: Taylor and Francis; 1998. p. 346. [Google Scholar]
- 63.Zhang C, Liu S, Logan J, Mazumder R, Phelps T J. Enhancement of Fe(III), Co(III), and Cr(VI) reduction at elevated temperatures by a thermophilic bacterium. Appl Biochem Biotechnol. 1996;57/58:923–932. [Google Scholar]