Abstract
Flow cytometric analyses of cellular staining with fluorescent viability dyes and direct microscopic observations of methylene blue exclusion were compared for evaluation of the effects of a chlorhexidine gluconate-based contact lens disinfectant solution and a polyhexamethylene biguanide solution against cysts and trophozoites of Acanthamoeba castellanii and Acanthamoeba polyphaga. The flow cytometric procedure with propidium iodide (used to stain dead cells) indicated that more than 90% of trophozoites of both species (inocula of 105 to 106/ml) at 22°C lost their viability after 4 h of exposure to chlorhexidine. When propidium iodide was used in combination with fluorescein diacetate (for live cells), the apparent number of propidium iodide-stained cells was reduced, but the relative efficacies of the two biguanide solutions appeared unchanged from those evident with the single dyes; the chlorhexidine solution was more effective than the polyhexamethylene biguanide solution. Similar data were obtained with the more cumbersome methylene blue exclusion procedure. Flow cytometric analyses provided a statistically reproducible and rapid procedure for determining the relative antiamoebal efficacies of the disinfecting solutions.
An average of 50% of contact lens wearers have contaminating microorganisms in their contact lens cases at some time during their use of lenses (16, 17). Gram-negative bacteria in lens cases, particularly Pseudomonas aeruginosa, present a risk for serious keratitis. Contamination of lens care systems, in particular with gram-negative bacteria, seems to be a prerequisite for the establishment of potentially infectious populations of Acanthamoeba spp. (1, 15). Compared to the incidence of contact lens-related bacterial keratitis, amoebic keratitis is a rare infection, but its recalcitrance to treatment and potential to cause blindness are a serious concern (8, 10, 12). The incidence of amoebic keratitis has been particularly centered among users of home-prepared saline solutions or tap water (particularly if the water supply was from a roof storage facility deficient in disinfectant) as a means of rinsing lenses (9, 13). In the United States, the discontinuation of salt tablets for home-prepared saline from the market in the mid-1980s resulted in a decrease in the incidence of Acanthamoeba keratitis.
Acanthamoeba spp. are widespread in fresh and marine aqueous environments, and they are common in moisture reservoirs in buildings. The genus is characterized by typically uninucleate trophozoites with fine protoplasmic acanthopodia. This active motile stage feeds by phagocytosis and pinocytosis and divides by binary fission. Trophozoites can undergo a transition to a double-walled cyst stage within a few hours (1). Early cyst stages often are less resistant than mature cysts to adverse environmental conditions, and mature cysts of a given strain may have varied resistance (1, 7). The relative susceptibilities of Acanthamoeba spp. to biguanide-type and other contact lens disinfectant solutions have been determined from postexposure enumeration of surviving amoebae from tracks or plaques in a bacterial lawn (3, 4) and by dye exclusion procedures (3, 5, 14). No single procedure for in vitro evaluation of antiamoebal compounds has been accepted, and disadvantages in methods used for Acanthamoeba testing have been presented (2).
Khunkitti et al. (6) determined the lethal effects of chlorhexidine diacetate and polyhexamethylene biguanide (PHMB) on Acanthamoeba castellanii by flow cytometry and plaque assay procedures. Minimal lethal concentrations (12.5 μg/ml) detected for trophozoites were generally the same by both procedures, whereas minimal lethal concentrations (25 to 50 μg/ml) detected for cysts were occasionally higher by the flow cytometry procedure. Herein we compare the dye exclusion and flow cytometry procedures.
MATERIALS AND METHODS
Cultivation of amoebae.
A. castellanii (ATCC 30234) and Acanthamoeba polyphaga (ATCC 30461) were obtained from the American Type Culture Collection, Manassas, Va. For preparation of inocula, trophozoites and cysts were transferred to a peptone (0.1%)-yeast extract (0.1%)-glucose (1.8%) (PYG) medium containing penicillin G sulfate (400 U) and streptomycin sulfate (400 μg) with incubation for 48 h. Axenic strains were maintained in 10 ml of PYG medium at 25°C in 75-cm2 tissue culture flasks or in 250 ml of PYG in 500-ml Erlenmeyer flasks. The flasks were incubated on a rotary shaker (150 rpm). Incubation times varied from 72 to 96 h for production of trophozoites (>98%) and from 3 to 6 weeks for development of cysts (70 to 97%). Trophozoites were harvested in the exponential growth phase and washed with phosphate-buffered saline (PBS [pH 7.4]; 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4, and 0.24 g of KH2PO4 per liter of distilled H2O). Washed amoebae were suspended in PBS and adjusted to a concentration of 107 cells/ml as determined from cell counts performed in a hemocytometer counting chamber (Hausser Scientific, Inc., Horsham, Pa.). Cyst inocula were prepared from cultures incubated in flasks for up to 6 weeks and were harvested as described above. Inocula with at least 85% cysts were used in the comparative experiments. Washed trophozoites or cysts were suspended to appropriate concentrations (∼106/ml) in the disinfectant solutions. All experiments were conducted at least in triplicate.
Disinfection.
A disinfection solution for use with soft and rigid gas-permeable contact lenses composed mainly of a borate-buffered saline with 50 μg of chlorhexidine per ml (Flexcare; Alcon, Fort Worth, Tex.) was compared with a borate-buffered saline solution containing 0.5 μg of PHMB per ml. Flexcare was employed as a standard positive solution. In our experience, this solution is one of the more active antiamoebal contact lens disinfection solutions, typically killing more than 90% of trophozoite inocula within 4 h. Our borate-buffered PHMB solution (pH 7.2) did not represent the activities of all current commercially available PHMB solutions for hydrogel lenses, but it was selected because it provided a medium range of activity (of such solutions available commercially) after 4 h at 25°C, i.e., treated-cell suspensions yielded recoverable cysts.
An inoculum of 0.01 ml of buffered saline containing 107 Acanthamoeba organisms/ml was added to the wells of microplates (24-well, flat-bottom, sterile polystyrene microplates with lid; Corning, Corning, N.Y.) containing 0.99 ml of disinfectant solution. The suspension was mixed with a single aspiration step from a 1,000-μl pipettor. The microplates were held in static culture at 25°C for 4 h. (No glass containers or pipettes were used in this protocol because of potential adsorption of biocide noted in preliminary experiments.) In separate experiments, the tests were conducted in polypropylene vials (sterile, 2-ml capacity, cryogenic vials; Nalgene, Rochester, N.Y.). The amoeba suspensions in disinfectant solutions for each test run were compared with the inoculum suspended in PBS. Between 2 and 10% of the cells suspended in PBS were found to lose viability over the 4-h period. PBS controls for each test series were employed to normalize the data obtained with the disinfectant solutions.
Flow cytometry.
The viability of trophozoites and cysts exposed to disinfectants was assessed by flow cytometry by procedures adapted from those of Khunkitti et al. (6). Suspensions of 1 ml containing exposed trophozoites or cysts were transferred from the polypropylene vials to 1.5-ml microcentrifuge tubes. Cells were stained either with fluorescein diacetate (FDA) (Molecular Probes, Eugene, Oreg.) in combination with propidium iodide (PI) (Molecular Probes) or with PI alone. FDA and PI were added to the amoeba suspensions to give final concentrations of 10 and 25 mg/liter, respectively. Flow cytometric analyses were performed with a FACSCalibur fluorescence-activated cell sorter system (Becton Dickinson, Heidelberg, Germany). Illumination was from a 15-mW, 488-nm argon-ion laser. Viable cells stained with FDA gave a green fluorescence with a 530/30 filter (FL1-H [height of fluorescence intensity]). Nonviable cells stained with PI fluoresced red with a 585/42 filter (FL2-H). Heat-killed cells (90°C for 20 min) that all fluoresced red with PI stain served as a control for the staining procedure. The dyes were used separately and simultaneously. Because of some overlap in the fluorescence emissions of FDA and PI, the stains routinely were used independently in the flow cytometric analyses. Suspensions containing viable untreated amoebae served as controls. Control amoeba populations that excluded PI (viable cells) were used to select analysis gates in FL2-H versus FSC-H (forward scattered light measure of cell size) dot plots. The dot plots were also gated by forward scatter to eliminate analysis of noise and smaller particles (<3 μm in diameter). Treated amoebae were superimposed into the previously selected analysis gates, and the percent viability based on the number of cells analyzed (at least 10,000 amoebae) was determined after treatment in chlorhexidine and PHMB. Setting of analysis gates (see figure legends for details), data handling, manipulation, and presentation were performed with CELLQuest software (Becton Dickinson).
Methylene blue exclusion.
After a 4-h incubation (the most common recommended disinfection time for cold disinfection systems), 0.1 ml of the amoeba-disinfectant suspension was removed and stained with 0.1 ml of 0.3% basic methylene blue. Unstained (viable) and stained (nonviable) cells were enumerated in the counting chamber within 10 min after stain addition. More than 100 amoebae were examined in each of triplicate samples. The relative percent killing was determined by comparison with triplicate amoeba suspensions in buffered saline. The percentage of viable amoebae of the initial inoculum was adjusted for mortality in PBS.
We used a low-speed centrifugation step (<3,000 × g) during the methylene blue exclusion procedure prior to staining. The trophozoites tended to gather on the surfaces of culture flasks and vials; with centrifugation, the trophozoites became spherical and more dispersed. When an outlying number was obtained in the triplicate counting of a given cell suspension in a cell counting chamber, we examined an additional sample and also counted at least 100 cells within 25 grids when numbers were less than 100 in the five standard grids.
RESULTS
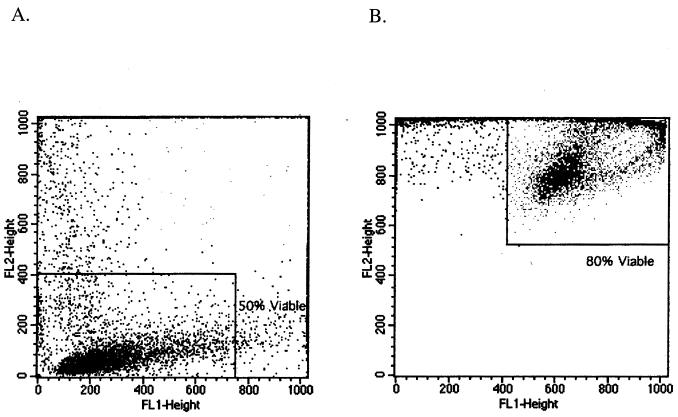
The numbers of trophozoites stained with PI and methylene blue after exposure in PBS to chlorhexidine and PHMB, normalized for loss of viability in PBS, are presented in Table 1. The percent stained varied in repeat experiments (±10%). In experiments with chlorhexidine, some trophozoites lysed within the first few minutes after exposure to the disinfectant (confirmed microscopically) and therefore were not detected on the flow cytometric dot plots. The percent loss of viability was calculated, in all cases, as the number of detected viable cells divided by the total number of cells originally in the sample analyzed (Fig. 1 and 2). Experiments showed that when amoeba cells were stained with FDA and PI simultaneously, the number of cells stained with PI (dead cells) was much lower than when only PI was used (Fig. 3).
TABLE 1.
Loss of viability of A. castellanii and A. polyphaga in disinfectant solutions containing chlorhexidine and PHMB as determined by PI staining and methylene blue exclusion
| Amoebaea | % Reduction byb:
|
|||||
|---|---|---|---|---|---|---|
| Flow cytometry
|
Methylene blue exclusion
|
|||||
| CH | PHMB | PBS | CH | PHMB | PBS | |
| A. castellanii | ||||||
| Trophozoitesc | 93–99 | 13–24 | 8–17 | 94–99 | 25–68 | 8–12 |
| Cystsc | 87–94 | 17–29 | 8–10 | 91–99 | 18–20 | 16–21 |
| A. polyphaga | ||||||
| Trophpozoites | 89–100 | 6–17 | 3–13 | 96–99 | 22–81 | 10–16 |
| Cysts | 73–94 | 14–19 | 4–10 | 92–97 | 17–21 | 5–9 |
Inocula of 105 to 106 amoebae/ml (n = 6).
CH, chlorhexidine (50 μg/ml). PHMB was used at 0.5 μg/ml.
Data normalized for loss of viability in PBS.
FIG. 1.
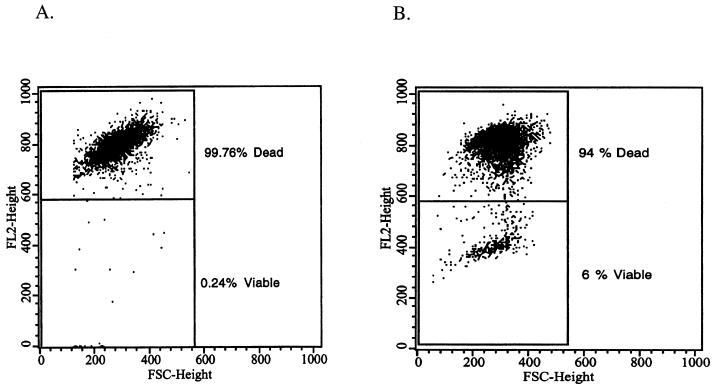
Effect of chlorhexidine solution (50 μg/ml) on trophozoites (A) and cysts (B) on A. polyphaga (2 × 106 cells/ml) stained with PI. FL2-H versus FSC-H dot plots were used to discriminate viable (PI excluded; lower quadrant) from dead (PI stained; upper quadrant) cells. The dot plots were gated by forward scatter to eliminate analysis of noise and smaller particles (<3 μm in diameter). Analysis gates were set with fluorescence from control live (FL2-H, ≤540) and heat-killed (FL2-H, >540) amoebae.
FIG. 2.
Effect of PHMB solution (0.5 μg/ml) on viability of trophozoites (A) and cysts (B) of A. castellanii; cells were stained with PI. Analysis gates were set as described previously.
FIG. 3.
Effect of staining with PI alone (A) and PI and FDA simultaneously (B) after exposure of A. polyphaga cysts to PHMB for 4 h. Of 10,000 treated cells, 50% appeared viable after staining with PI alone (A [lower quadrant]) and 80% appeared viable after staining with PI and FDA (B [upper right quadrant]). The analysis gates (from amoebae stained with PI and FDA) were set with fluorescence from control live (FL1-H, ≥400; FL2-H, ≥520) and heat-killed (FL1-H, <400; FL2-H, ≥520) cells. Analysis gates for cells stained with PI were set as previously described.
The relative disinfection capacities of the model disinfectant solutions, as indicated by flow cytometry with PI and the microscopy procedure with methylene blue exclusion, were similar (Table 1). Replicate samples of trophozoites of both species (106 cells) essentially were killed within 4 h by the chlorhexidine solution. In fact, most trophozoites in chlorhexidine were stained intensely within 30 s, whereas in the PHMB solution exposure, only about 75% of the trophozoite preparation was intensely stained after 4 h. The staining procedures for the methylene blue determinations were all performed within 10 min, because various numbers of viable cells were stained by 15 min, and nearly all cells were stained by 20 min. Trophozoites were recovered from the disinfectant solutions upon culture in PYG for 20 min, but not after 96 h following a 4-h exposure to the chlorhexidine. Amoebae were recovered in PYG after a 4-h exposure to the PHMB solution. The recovery steps included transfer to lawns of Stenotrophomonas maltophilia and inoculation of PYG broth (data not shown). Occasional cyst preparations stained mainly as dead cells, and the nonstained cysts lost viability with incubation in PBS and were not recoverable after 4 h. These sensitive cyst preparations were excluded from the data in Table 1. A gradual lysis of trophozoites or clumping occasionally was observed microscopically, more often in the presence of PHMB than with chlorhexidine. This gradual lysis phenomenon and the observation that the presence of PHMB (during the 4-h exposures) tended to delay the uptake of methylene blue resulted in occasional outlying data for numbers of live and dead cells.
DISCUSSION
The flow cytometry procedure and the methylene blue exclusion procedure in repeat tests with the same preparations of inocula gave comparable data on the relative efficacies of two disinfectant solutions, but the actual numbers killed, particularly for cysts, varied. When extensive lysis of trophozoites and precysts occurred after exposure to the disinfectants, insufficient numbers of cells were available for transfer to the cell counting chamber by the methylene blue procedure, and accuracy was reduced when the counting step was extended to the full 10-min period. The lysis events varied with the inocula. In contrast, flow cytometric analysis provided a rapid and quantitative evaluation of efficacy based on 104 individual cell counts within 3 min. Moreover, clumps of trophozoites and cysts that occasionally formed in the disinfectant solutions (an episodic obstacle for the methylene blue method) were dispersed. This dispersal most probably resulted from passage of the cells through the hydrodynamically focused sample core of the flow cytometer. This type of phenomenon was observed with several preparations of inocula analyzed with a Coulter counter (data not shown). The numbers of viable trophozoites and cysts detected after exposure to disinfectants typically were greater by the flow cytometric procedure than by the methylene blue procedure. Khunkitti et al. (6) had similar results with comparisons of flow cytometry (employing FDA and PI simultaneously) and a plaque assay. They proposed that dying cells retain their ability to hydrolyze fluorescein diacetate.
Khunkitti et al. (6) found minimal lethal concentrations of chlorhexidine diacetate and PHMB of 12.5 μg/ml for initial inocula of 106 trophozoites/ml and 25 to 50 μg of each inhibitor per ml for 106 cysts of A. castellanii per ml. The actual numbers of recoverable trophozoites and cysts in the inocula preparations were closer to 104/ml. The higher values (50 μg/ml) for cysts were obtained for both inhibitors by the flow cytometric procedure. Khunkitti et al. (7) reported that different encystment stages (pre-excystment cyst, pre-encystment trophozoite, and mature cysts) showed different sensitivities to these inhibitors. The biocidal values cited above for trophozoites and cysts with chlorhexidine diacetate and PHMB are similar to those found by a variety of procedures (including different contact times) by Hay et al. (5), Burger et al. (3), and Tirado-Angel et al. (15). Values in the latter paper are taken from their Table 1 (15). (Values in the text of their report are listed incorrectly as milligrams per milliliter.) All of the above values for PHMB exceed the 0.5-μg/ml concentration (a concentration at the lower range used in current commercial systems) used in this study. The chlorhexidine and PHMB formulations were both borate buffered and included emulsifiers. Borate formulations and various emulsifiers may potentiate the antimicrobial activity of biguanides in commercial preparations (3, 6, 11).
Our current interpretation of the data from the literature and our investigation suggest that relative efficacies of disinfectant solutions against Acanthamoeba are obtainable by a variety of methods, including flow cytometric analyses. The obvious advantages of the use of flow cytometric procedures over the exclusion of methylene blue and culture procedures were the speed with which results were obtained and avoidance of the microscopic counting procedure. Potential problems or miscalculations because of lysis were more quickly evaluated by flow cytometry. The stains used in both procedures were time and concentration based, but the 10-min staining period with methylene blue was less discerning than the shorter staining period in flow cytometric analyses. With both methods (and with others given in the literature), there is the possibility that unstained amoebae were nonrecoverable by the culture techniques used. Our data indicate the relative efficacies of disinfectants against a given inoculum. A major question in all studies of susceptibilities of cysts to biocides is variation within the cyst population and potentially reduced resistance of cysts induced from an axenic culture with MgCl2. More definitive data on physiology and regulation of trophozoite-cyst transition and excystment will be needed to validate a standardized quantitative method for the evaluation of disinfection efficacies. For the present, we recommend that contact lens solutions be compared for efficacy against cysts by using a chlorhexidine-borate control formulation similar to that studied herein. At least a 1-log reduction in viability of cysts of A. castellanii (104 to 105/ml) should be obtained with this solution. When lesser values are obtained, the cyst preparation should be considered unacceptable.
REFERENCES
- 1.Ahearn D G, Gabriel M M. Contact lenses, disinfectants, and Acanthamoeba keratitis. Adv Appl Microbiol. 1997;43:35–56. doi: 10.1016/s0065-2164(08)70222-3. [DOI] [PubMed] [Google Scholar]
- 2.Buck S L, Rosenthall R A. A quantitative method to evaluate neutralizer toxicity against Acanthamoeba castellanii. Appl Environ Microbiol. 1996;62:3521–3526. doi: 10.1128/aem.62.9.3521-3526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger R M, Franco R J, Drlica K. Killing acanthamoebae with polyaminopropyl biguanide: quantitation and kinetics. Antimicrob Agents Chemother. 1994;38:886–888. doi: 10.1128/aac.38.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies D J G, Anthony Y, Meakin B J, Kilvington S, Anger C B. Evaluation of the anti-acanthamoebal activity of five contact lens disinfectants. Int Contact Lens Clin. 1990;17:14–20. [Google Scholar]
- 5.Hay J, Kirkness C M, Seal D V, Wright P. Drug resistance and Acanthamoeba keratitis: the quest for alternative antiprotozoal chemotherapy. Eye. 1994;8:555–563. doi: 10.1038/eye.1994.137. [DOI] [PubMed] [Google Scholar]
- 6.Khunkitti W, Avery S V, Lloyd D, Furr J R, Russell A D. Effects of biocides on Acanthamoeba castellanii as measured by flow cytometry and plaque assay. J Antimicrob Chemother. 1997;40:227–233. doi: 10.1093/jac/40.2.227. [DOI] [PubMed] [Google Scholar]
- 7.Khunkitti W, Lloyd D, Furr J R, Russell A D. Acanthamoeba castellanii: growth, encystment, excystment and biocide susceptibility. J Infect. 1998;36:43–48. doi: 10.1016/s0163-4453(98)93054-7. [DOI] [PubMed] [Google Scholar]
- 8.Kilvington S, White D G. Acanthamoeba: biology, ecology, and human disease. Rev Med Microbiol. 1994;5:12–20. [Google Scholar]
- 9.Kirn T F. As the number of contact lens users increases, research seeks to determine risk factors and how best to prevent potential eye infections. JAMA. 1987;258:17–18. [PubMed] [Google Scholar]
- 10.Ledee D R, Hay J, Byers T J, Seal D V, Kirkness C M. Acanthamoeba griffini. Molecular characterization of a new corneal pathogen. Investig Ophthalmol Vis Sci. 1996;37:544–550. [PubMed] [Google Scholar]
- 11.Meisler D M, Ludwig I H, Rutherford I. Acanthamoeba and disinfection of soft contact lenses. Rev Infect Dis. 1991;13:390–391. doi: 10.1093/clind/13.supplement_5.s410. [DOI] [PubMed] [Google Scholar]
- 12.Modi P A, Gresh W, Shih K. Antimicrobial efficacy of two commercial rgp contact lens care systems. CLAO J. 1995;21:242–246. [PubMed] [Google Scholar]
- 13.Moore M B, Ubelaker J, Silvany R. Scanning electron microscopy of Acanthamoeba castellanii: adherence to surfaces of new and used contact lenses and to human corneal button epithelium. Rev Infect Dis. 1985;13:423–424. doi: 10.1093/clind/13.supplement_5.s423. [DOI] [PubMed] [Google Scholar]
- 14.Talamo J D, Larkin D S. Bilateral Acanthamoeba keratitis and gas-permeable contact lenses. Am J Ophthalmol. 1993;116:651. doi: 10.1016/s0002-9394(14)73216-7. [DOI] [PubMed] [Google Scholar]
- 15.Tirado-Angel J, Gabriel M M, Wilson L A, Ahearn D G. Effects of polyhexamethylene biguanide and chlorhexidine on four species of Acanthamoeba in vitro. Curr Eye Res. 1996;15:225–228. doi: 10.3109/02713689608997418. [DOI] [PubMed] [Google Scholar]
- 16.Ubelaker J E. Acanthamoeba spp.: opportunistic pathogens. Trans Am Microsc Soc. 1991;110:289–299. [Google Scholar]
- 17.Wilson L A, Sawant A D, Simmons R B, Ahearn D G. Microbial contamination of contact lens storage cases and solutions. Am J Ophthalmol. 1990;110:193–198. doi: 10.1016/s0002-9394(14)76991-0. [DOI] [PubMed] [Google Scholar]