Abstract
Background
While general anesthesia (GA) is the most commonly used anesthetic method during lumbar microendoscopic discectomy (MED), local ± epidural anesthesia (LA) has been gaining popularity as an alternate method. Theoretical advantages of LA include reduced morbidity of anesthesia and improved surgeon-patient communication facilitating less nerve root manipulation and yielding improved surgical outcomes. The objective of this systematic review is to examine the impact of anesthesia type on patient reported outcomes (PROs) and complications with MED.
Methods
A systematic review and meta-analysis of the available literature examining MED performed under GA or LA was performed. The PubMed, EMBASE and SCOPUS databases were searched from inception to August 16, 2021, utilizing strict inclusion and exclusion criteria with all studies reporting greater than 6 months of follow-up and PRO data. PROs including Visual Analog Scale (VAS)-leg/back, Oswestry Disability Index (ODI), Japanese Orthopedic Association (JOA) and/or 36-Item Short Form (SF-36) physical component scores were collected. Complication, recurrent disc herniation, durotomy and reoperation rates as well as surgical factors were collected. All outcomes were compared between pooled studies examining GA or LA. Risk of bias was assessed with the Newcastle-Ottawa Scale.
Results
A total of 23 studies consisting of 2,868 patients (1,335 GA, 1,533 LA) were included in the meta-analysis. There were no significant differences between GA and LA groups in regard to overall complication rate, durotomy rate, recurrent disc herniation rate, reoperation rate, blood loss, or surgical time (p > 0.05). Both groups demonstrated significant improvements in ODI and JOA (p<0.0004), however leg and back VAS was only improved in GA (p<0.0025) and not in LA (p>0.058), and SF-36 only in LA (p=0.003).
Conclusions
Patients undergoing MED under both anesthetic techniques demonstrated significant improvements in ODI and JOA, with no significant differences in complication or reoperation rates. However, patients undergoing GA demonstrated significant improvement in VAS leg and back pain at last follow-up while LA did not. LA may be offered to carefully selected patients and prior studies have demonstrated reduced costs and risks with LA. Conclusions are limited by a high level of study bias and heterogeneity. Further investigation is needed to assess the true effects of GA and LA on outcomes after MED.
Key Words: Microendoscopic discectomy, General anesthesia, Local/epidural anesthesia, Patient-reported outcomes, Surgical outcomes, Meta-analysis, Systematic review, Spine
Background
Although the standard procedures for symptomatic lumbar disc herniation remain open discectomy or microdiscectomy, there is a growing trend towards increasingly minimally invasive techniques using microendoscopic technique to further minimize blood loss, maintain ligamentous structural integrity, decrease operative time, and shorten hospital length of stay (LOS) [1,2]. Microendoscopic discectomy (MED) was initially described in 1997 and allows surgeons to work through a small-diameter, tubular, operating table-mounted retractor with the use of an endoscope or microscope for visualization [3]. This technique has been associated with reduced postoperative pain, surgical trauma, length of hospital stay and time to return to work [4], [5], [6], [7], [8], [9]. Anesthesia type is also suggested to play a role in patient outcomes after lumbar discectomy surgery. Therefore, there has been a recent increase in literature comparing outcomes between general anesthesia (GA) and local/epidural anesthesia (LA) use in both open and MED. However, no systematic reviews have specifically addressed the effects of GA and LA on surgical and patient outcomes after MED.
Determination of anesthetic type involves a joint discussion between the surgeon, anesthesiologist, and patient. LA allows continuous clinical monitoring and patient conversation, providing another avenue of intraoperative feedback for the surgeon. However, studies have described patients expressing fear about undergoing percutaneous endoscopic lumbar discectomy (PELD) with LA alone [10,11]. In contrast, studies have demonstrated longer operative times and hospital LOS in a GA patient group when compared to LA, although these differences were of questionable clinical significance. Ultimately, secondary outcomes such as adverse reactions, Oswetry Disability Index scores, visual analog scores and postoperative patient satisfaction rates were similar between both anesthesia groups [11]. A retrospective study out of Turkey also demonstrated similar results with an added analysis of cost savings in favor of epidural anesthesia (EA) over GA [12].
Despite increasing literature on the topic, there remains a lack of higher level studies examining the use of GA compared to LA in MED and it is unclear if one specific technique results in superior outcomes. As such, a systematic review and meta-analysis was performed comparing the use of GA versus LA in regard to surgical and patient-reported outcomes (PROs) in patients undergoing lumbar MED.
Methods
This study was conducted with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [13] and was exempt from institutional review board approval.
Search strategy
The PubMed, EMBASE and SCOPUS databases were used to identify relevant studies. A search was conducted on August 16th, 2021, of these three databases. The search strategy consisted of combinations of key words and commonly used synonyms and abbreviations. Two authors (AS, JM) performed separate literature searches. The advanced search for articles in each database included the terms (((conscious sedation[Title/Abstract]) OR (awake[Title/Abstract]) OR (without general anesthesia[Title/Abstract]) OR (local[Title/Abstract])) AND (lumbar discectomy[Title/Abstract]))) to identify relevant articles for the LA group. Articles for the GA group were identified with the following terms: ((minimally invasive lumbar discectomy[Title/Abstract]) OR (percutaneous endoscopic lumbar discectomy[Title/Abstract])) OR (microendoscopic lumbar discectomy[Title/Abstract]). References cited in the eligible studies were also evaluated to identify any additional studies.
Records were screened by several reviewers working independently including JM and either AS, NE, AM, BM and NL. Reviewers JM, AS, NE, AM, BM and NL independently collected the data from the final list of included studies and this data was reviewed by JM. Any discrepancies were adjudicated by a third reviewer.
Inclusion and exclusion criteria
Study inclusion criteria were: 1) first-time single or two-level lumbar discectomy between L1-S1; 2) patient age 18; 3) surgical procedure performed using a tubular/microendoscopic approach; 4) > 10 patients; 5) study published in the English language; 6) patients with at least 6 months of reported follow-up with 7) documented pre and post-operative PROs in one or more of the categories including visual analog scale (VAS) leg, VAS back, Oswestry Disability Index (ODI), Japanese Orthopedic Association (JOA) score, McNab Score or 36-item short form physical component score (SF-36 PCS) 7) inclusion of standard deviation values for reported PROs and any other continuous variables for inclusion in the meta-analysis.
Microendoscopic discectomy (MED) was defined as any lumbar discectomy utilizing a minimally invasive tubular retractor in combination with a microscope. This definition excluded all cases involving a purely endoscopic approach performed percutaneously.
For the LA group, studies were required to have a patient cohort with > 90% of patients undergoing surgery with LA with or without lumbar epidural injection and conscious sedation. For the GA group, studies were required to have a patient cohort with > 90% of patients undergoing general anesthesia prior to surgery.
Meta-analyses, review articles, case reports, studies involving pathologies other than disc herniation, studies involving revision surgeries, non-English language, open surgical procedures and lumbar fusion procedures were excluded. Studies involving patients < 18 were excluded in addition to studies with a lack of adequate follow-up (6 months) or lack of PRO data categories.
Data collection and analysis
Studies were grouped as having patient cohorts undergoing either GA or LA. If a study was comparative between GA and LA, the cohorts were analyzed separately in their respective groups for the meta-analysis. Information on patient demographics, follow-up time in addition to PROs including VAS general, VAS back/leg, JOA score, McNab Score (Percent Excellent), ODI and SF-36 PCS were extracted from the studies. Additional information including mean surgery time, blood loss, complication rate, durotomy rate, length of stay, disc recurrence rate and reoperation rates were extracted from each study when available, pooled and compared between the LA and GA groups. Only studies that reported data with standard deviation values for a given category were included in the final analyses. Any missing data points were excluded from the analysis.
Outcomes
Primary outcomes were complication rate, durotomy rate, recurrent disc herniation rate, reoperation rate, and length of surgery, as these were the most consistently reported values across all studies and found to be the most objective. Complications included transient paresthesias, nerve root injuries, infection, wound dehiscence, sexual dysfunction, urinary retention, durotomy, discitis and post-operative hematoma.
Secondary outcome data including the PROs VAS general, VAS back/leg, JOA score, McNab Score, ODI score and SF-36 PCS score were recorded when available pre-operatively, at the first post-operative follow-up (within 1 month) and at the 3, 6, 12 and 24 month follow-up timepoints. Given the range in follow-up between studies, only the first post-operative follow-up and last follow-up time-points were pooled for comparison. Changes in PROs from pre-op to the first and last post-op follow-up appointment were also calculated and compared between LA and GA groups.
Statistical analysis
Descriptive statistics were calculated for baseline, surgical, and outcomes data using R (R Foundation for Statistical Computing, Vienna, Austria). For categorical variables, absolute counts were extracted to calculate proportions. For continuous variables, means and associated standard deviations were extracted. After Freeman-Tukey double arcsine transformation for count variables, weighted pooled means and associated 95% confidence intervals (CI) were computed using the DerSimonian-Laird method. A random effects model was used due to the assumptions of clinical diversity and differences in methodology among the included studies. The LA and GA cohorts were compared using a subgroup meta-analysis. Forest plots and the I2 statistic were used to investigate heterogeneity. Heterogenity between different studies were evaluated by χ2 and I2, and p < 0.05 was considered statistically significant. I2 values of < 25%, 25% to 75%, and > 75% represent mild, moderate and severe heterogeneity respectively.
The Newcastle-Ottowa Scale6 was used to assess the quality of the included studies and further risk of bias was assessed with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessment. Several reviewers reviewed each study working independently and discrepancies were adjudicated by a third author. All statistical tests were two-tailed, and p<0.05 was considered statistically significant.
Results
Search results
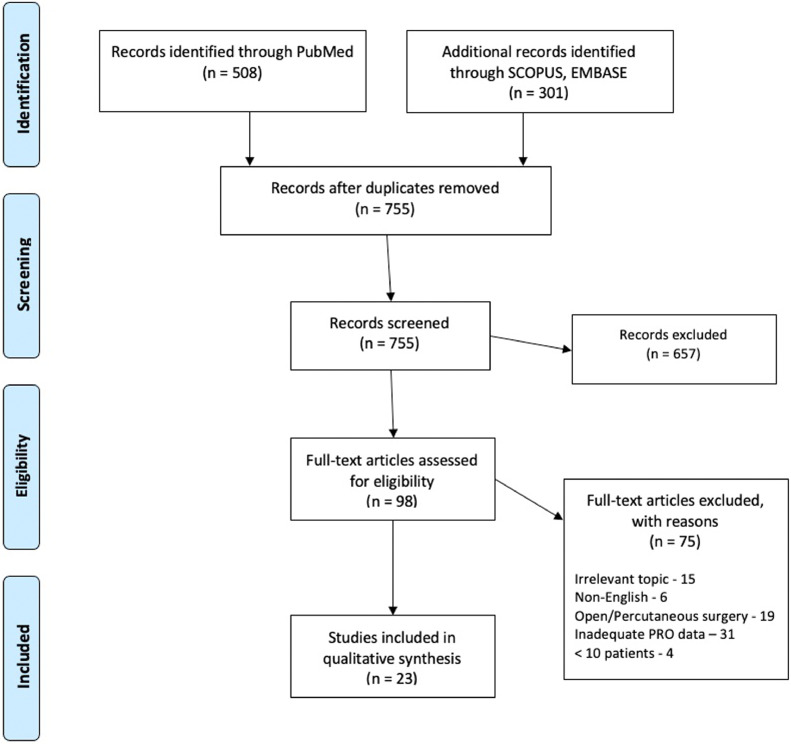
After the initial search of the electronic databases, a total of 755 articles were identified. Articles were further screened by titles, abstracts and full texts according to the specific inclusion and exclusion criteria. Ultimately, 657 articles were excluded and 98 were considered eligible for inclusion in this meta-analysis. The final articles included consisted of 23 articles (14 in the GA group, 9 in the LA group) (Fig. 1). These 23 articles included a total of 2,868 patients (1335 GA, 1533 LA). Descriptive data related to the included articles is presented in Table 1. The risk of bias as assessed with the Newcastle-Ottawa Scale for non-randomized studies is summarized in Table 2. The risk of bias as assessed with the Newcastle-Ottawa Scale for randomized studies is summarized in Table 3. The quality of evidence is summarized in Supplementary Table.
Fig. 1.
Flow diagram depicting the literature review, search strategy and selection process.
Table 1.
Study characteristics – primary surgical outcomes and operative data.
| STUDY | Study Design | No. Patients | Mean Age (Std. Dev.) | Mean Follow-Up (months) | Complication Rate (%) | Durotomy Rate (%) | Recurrent Disc Herniation Rate (%) | Reoperation Rate (%) | Length of Surgery (mins) | Blood Loss (cc) |
|---|---|---|---|---|---|---|---|---|---|---|
| GENERAL ANESTHESIA | ||||||||||
| Gibson et al., 2017 [29] | RCT | 70 | 39 (9.0) | 24 | 1.4 | 0.0 | 2.9 | 2.9 | 65 | NA |
| Patil, et al., 2018 [30] | Retrospective | 300 | NA | 6 | 6.0 | 1.0 | 2.0 | 3.3 | 82 | NA |
| Song et al. 2021 [31] | Retrospective | 116 | 49 (10.9) | 36 | 12.1 | 1.7 | 6.0 | NA | 81.1 | 71.3 |
| Yoon et al., 2012 [32] | Prospective | 26 | 56.5 (NA) | 20 | 7.7 | 3.8 | 3.8 | 0.0 | 178.8 | 153.8 |
| Yang et al., 2021 [33] | Retrospective | 19 | 48.5 (12.1) | 24 | 10.5 | 5.3 | NA | NA | 65 | NA |
| Porto et al. 2021 [34] | Retrospective | 38 | 53.6 (16.3) | 7.7 | 2.6 | NA | 28.9 | 13.2 | 57.5 | NA |
| Liu et al., 2021 [35] | Retrospective | 60 | 53.4 (14.3) | 20 | 20.0 | 0.0 | 0.0 | 3.3 | 51.1 | 39.83 |
| Yu et al., 2021 [36] | Retrospective | 421 | 40.1 (12.4) | 31.7 | 12.8 | 1.4 | 5.0 | 4.0 | 69.4 | 45.2 |
| Ren et al., 2020 [37] | Prospective | 51 | 42 (11.6) | 36 | 9.8 | 0.0 | 7.8 | NA | 99.2 | NA |
| Liu et al., 2018 [38] | Retrospective | 63 | 33.1 (6.7) | 29.6 | 9.5 | 4.8 | 3.2 | 3.2 | 57 | 23 |
| Li et al. 2015 [39] | Retrospective | 30 | 37.8 (6.6) | 15.4 | 0.0 | 0.0 | 0.0 | 0.0 | 58.5 | NA |
| Kunert et al., 2010 [40] | Retrospective | 13 | 34 (10.0) | 21 | 0.0 | 0.0 | 0.0 | 0.0 | 78 | NA |
| Righesso et al., 2007 [41] | RCT | 21 | 42 (10.7) | 36.2 | 14.3 | 4.8 | 4.8 | 4.8 | 82.6 | 50 |
| Ranjan et al., 2006 [42] | Prospective | 107 | NA | 12.9 | 6.5 | 1.9 | 1.9 | 0.9 | 120 | NA |
| LOCAL ± EPIDURAL ANESTHESIA | ||||||||||
| Abudurexiti et al., 2018 [43] | Prospective | 134 | 36.3 (8.6) | 6 | 8.2 | 0.7 | NA | NA | 85.0 | 137.0 |
| Song et al., 2017 [44] | Case/Control | 30 | 53.6 (6.4) | 18 | NA | NA | NA | NA | 76.6 | 100.7 |
| Jing et al., 2021 [45] | Retrospective | 31 | 50.2 (9.4) | 24 | 19.4 | 3.2 | 3.2 | NA | 61.7 | 30.6 |
| Pang et al., 2020 [46] | Retrospective | 48 | 45.3 (6.4) | 6 | 4.2 | 0.0 | 2.1 | NA | 72.6 | 35.4 |
| Chen et al., 2020 [47] | RCT | 111 | 41 (10.8) | 24 | 9.9 | 2.7 | 4.5 | 4.5 | 100.2 | NA |
| Liu et al., 2010 [48] | Retrospective | 82 | 42.1 (10.2) | 77.04 | 2.4 | 0.0 | 0.0 | 2.4 | NA | NA |
| Zhou et al., 2009 [49] | Retrospective | 151 | 39 (NA) | 60 | 5.3 | 3.3 | 3.3 | 3.3 | NA | NA |
| Wu et al., 2006 [50] | Retrospective | 873 | 41.5 (NA) | 28 | 4.0 | 1.6 | 0.9 | 2.3 | 56.0 | 44.0 |
| Chen et al., 2018 [51] | RCT | 73 | 40.7 (11.1) | 12 | 12.3 | 1.4 | 4.1 | 4.1 | 91.7 | NA |
RCT – randomized control trial
Table 2.
Assessment of the quality of included studies according to the Newcastle-Ottawa quality assessment scale for non-RCTs.
| STUDY | Representativeness of Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Outcome of Interest | Comparability of Cohorts | Assessment of Outcome | Adequate Duration of Follow-Up | Adequate Follow-up of Cohort |
|---|---|---|---|---|---|---|---|---|
| GENERAL | ||||||||
| Patil, et al., 2018 | * | - | * | * | - | * | * | * |
| Song et al. 2021 | * | - | * | * | - | * | * | * |
| Yoon et al., 2012 | * | - | * | * | - | * | * | * |
| Yang et al., 2021 | * | - | * | * | - | * | * | * |
| Porto et al. 2021 | * | * | * | * | - | * | * | * |
| Liu et al., 2021 | * | - | * | * | - | * | * | * |
| Yu et al., 2021 | * | - | * | * | * | * | * | * |
| Ren et al., 2020 | * | - | * | * | * | * | * | * |
| Liu et al., 2018 | * | - | * | * | - | * | * | * |
| Li et al. 2015 | * | * | * | * | * | * | * | * |
| Kunert et al., 2010 | * | - | * | * | - | * | * | * |
| Ranjan et al., 2006 | * | - | * | * | * | * | * | * |
| LOCAL ± EPIDURAL | ||||||||
| Abudurexiti et al., 2018 | * | - | * | * | - | * | * | * |
| Song et al., 2017 | * | * | * | * | * | * | * | * |
| Jing et al., 2021 | * | * | * | * | * | * | * | * |
| Pang et al., 2020 | * | - | * | * | - | * | * | * |
| Liu et al., 2010 | * | * | * | * | * | * | * | * |
| Zhou et al., 2009 | * | - | * | * | - | * | * | * |
| Wu et al., 2006 | * | * | * | * | * | * | * | * |
Table 3.
Assessment of the quality of included studies according to the newcastle-ottawa quality assessment scale for RCTs.
| Selection |
Comparability | Exposure |
||||||
|---|---|---|---|---|---|---|---|---|
| STUDY | Case Definition | Case Representativeness | Selection of Controls | Definition of Controls | Comparability | Ascertainment of Exposure | Same Method for Cases and Controls | Non-response Rate |
| GENERAL | ||||||||
| Gibson et al., 2017 | * | * | * | * | * | * | * | * |
| Righesso et al., 2007 | * | * | * | * | * | * | * | * |
| LOCAL ± EPIDURAL | ||||||||
| Chen et al., 2020 | * | * | * | * | * | * | * | * |
| Chen et al., 2018 | * | * | * | * | * | * | * | * |
Overall complications
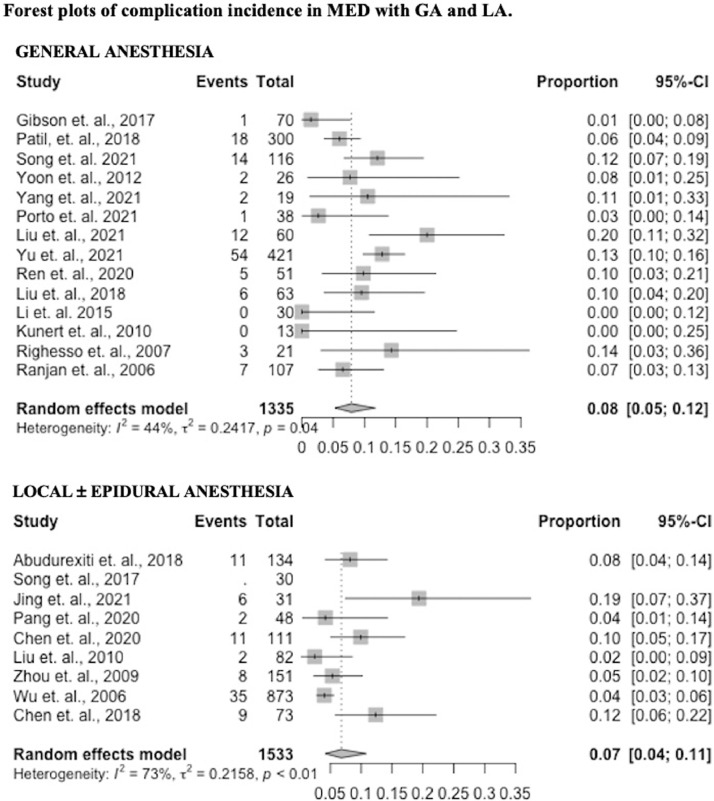
Twenty-two studies reported data on complications (14 GA – 100%, 8 LA – 89%). The complication incidence was 0.08 (95% confidence interval [CI] 0.05 – 0.12) in the GA group and 0.07 (95% CI 0.04 – 0.11) in the LA group (p = 0.57). These results are presented in Fig. 2 as forest plots.
Fig. 2.
Forest plots of complication incidence in MED with GA and LA.
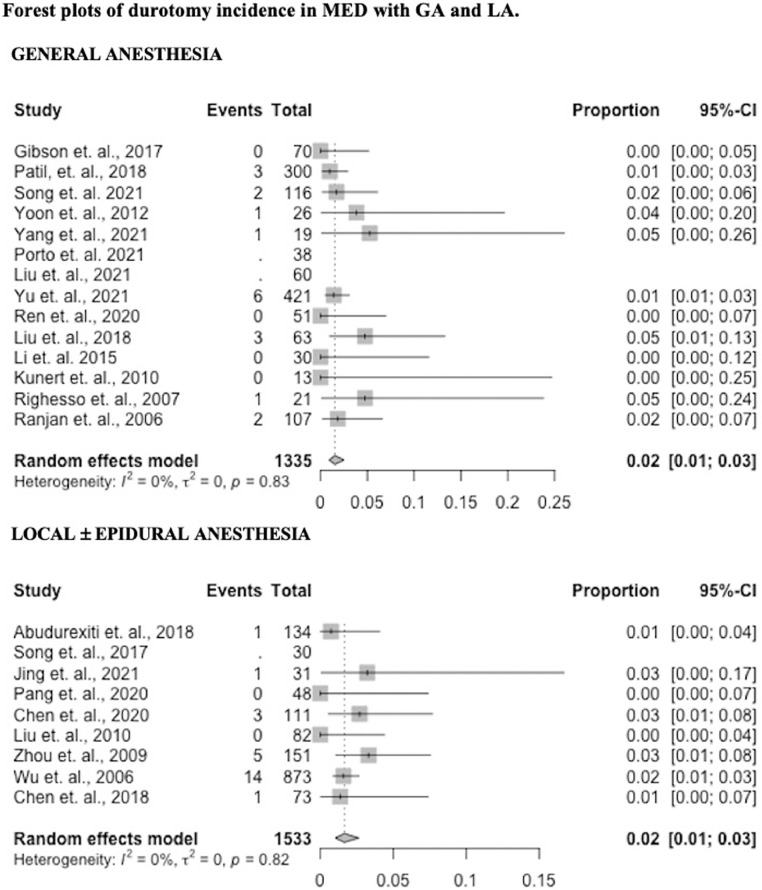
Durotomy
Twenty studies reported data on durotomy incidence (12 GA – 86%, 8 LA – 89%). Durotomy incidence in the GA group was 0.02 (95% CI 0.01 – 0.03) and 0.02 (95% CI 0.01 – 0.03) in the LA group (p = 0.79). Results by study are presented in Fig. 3 as forest plots.
Fig. 3.
Forest plots of durotomy incidence in MED with GA and LA.
Recurrent disc herniation
Twenty studies reported data on recurrent disc herniation incidence (13 GA – 93%, 7 LA – 78%). Recurrent disc herniation incidence in the GA group was 0.04 (95% CI 0.02 – 0.07) and 0.02 (95% CI 0.01 – 0.04) in the LA group (p = 0.23). Results by study are presented in Supplementary Figure 1 as forest plots.
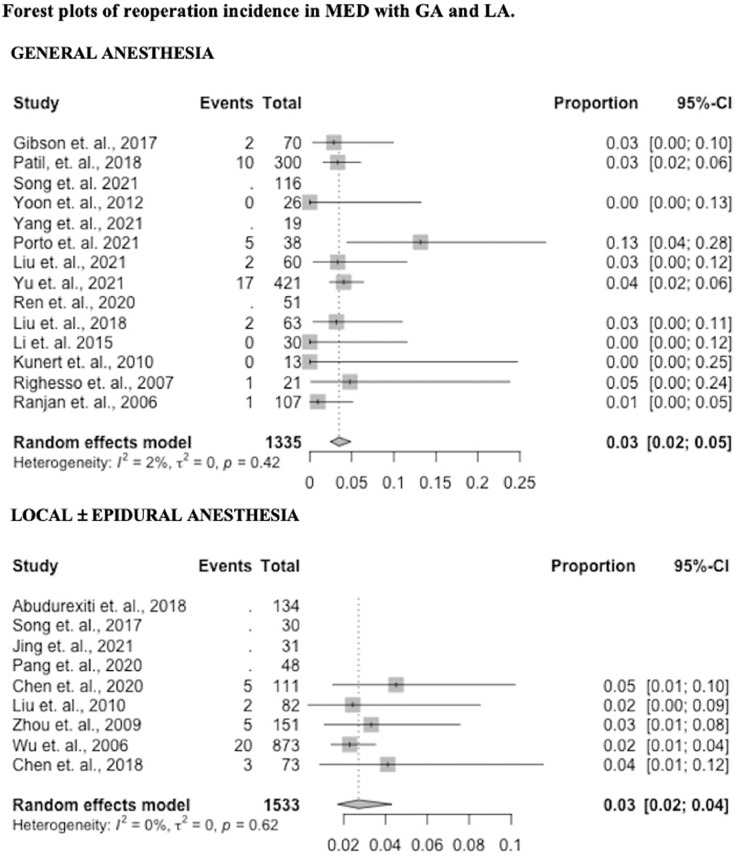
Reoperations
Sixteen studies reported data on reoperation incidence (11 GA – 79%, 5 LA – 56%). Reoperation incidence in the GA group was 0.04 (95% CI 0.03 – 0.05) and 0.03 (95% CI 0.02 – 0.04) in the LA group (p = 0.27). Results by study are presented in Fig. 4 as forest plots.
Fig. 4.
Forest plots of reoperation incidence in MED with GA and LA.
Operative data
Fifteen studies reported data on length of surgery (9 GA – 64%, 6 LA – 67%). Length of surgery in the GA group was 69.0 minutes (95% CI 56.9 – 81.1) and 80.8 minutes (95% CI 66.4 – 95.2) in the LA group (p = 0.12). Results by study are presented in Supplementary Figure 2 as forest plots. Eight studies (4 GA – 29%, 4 LA – 44%) reported data on blood loss. Blood loss in the GA group was 44.8cc (95% CI 13.0 – 76.6) and 75.8cc (95% CI 6.77 – 158.3) in the LA group (p = 0.27).
Patient reported outcomes
The greatest number of studies presented data on VAS back/leg (11 studies: 8 GA – 57%, 3 LA – 33%) and ODI scores (15 studies: 8 GA – 57%, 7 LA – 78%). Pre-operative VAS back, leg, and ODI scores were similar between the GA and LA groups. Only two studies (1 GA – 7%, 1 LA – 11%) reported JOA scores which overall were lower in the GA group pre-operatively (10.9 vs 12.26, p = 0.001). Five studies reported SF-36 scores (3 GA – 21%, 2 LA – 22%) and these were similar pre-operatively between groups (Table 4).
Table 4.
Baseline age and patient-reported outcomes.
| LOCAL ± EPIDURAL |
GENERAL |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | # Studies Pooled | Pooled Value | SD | # Studies Pooled | General | SD | P-value |
| Age | 7 | 44.1 | 7.5 | 11 | 42.8 | 8.1 | 0.69 |
| VAS back | 3 | 4.9 | 4.8 | 8 | 4.5 | 1.8 | 0.78 |
| VAS leg | 3 | 5.9 | 1.5 | 8 | 6.7 | 1.2 | 0.12 |
| JOA | 1 | 12.3 | 0.3 | 1 | 10.9 | 0.3 | 0.001 |
| SF-36 PCS | 2 | 52.6 | 3.5 | 3 | 50.4 | 30.9 | 0.78 |
| ODI | 7 | 45.7 | 20.3 | 8 | 51.5 | 18.9 | 0.49 |
SD – standard deviation; VAS – visual analog scale; JOA – Japanese Orthopedic Association score; ODI – Oswestry Disability Index; SF-36 PCS – 36-item short form physical component score
At the first post-operative follow-up (within 1 month), the LA group had significant improvements in VAS leg (5.9 to 1.7, p = 0.01), ODI (45.7 to 22.2, p = 0.04), and SF-36 scores (52.6 to 81.5, p = 0.009) compared to pre-op while the GA group only experienced significant improvements in VAS leg (6.7 to 2.4, p <0.001) and ODI scores (51.5 to 26.3, p = 0.02) (Table 5).
Table 5.
Changes in patient-reported outcomes – preoperative, postoperative and last follow-up.
| PRE-OPERATIVE |
FIRST POSTOPERATIVE |
LAST FOLLOW-UP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRO | # Studies Pooled | Pooled Value | SD | # Studies Pooled | Pooled Value | SD | P-value* | # Studies Pooled | Pooled Value | SD | P-value** |
| LOCAL ± EPIDURAL | |||||||||||
| VAS back | 3 | 4.9 | 4.8 | 3 | 1.7 | 1.3 | 0.33 | 3 | 1.1 | 2.8 | 0.3 |
| VAS leg | 3 | 5.9 | 1.5 | 3 | 1.7 | 1.0 | 0.01 | 3 | 1.0 | 2.8 | 0.06 |
| JOA | 1 | 12.3 | 0.3 | NA | NA | NA | NA | 1 | 27.3 | 0.3 | <0.001 |
| ODI | 7 | 45.7 | 20.3 | 5 | 22.2 | 10.3 | 0.04 | 7 | 9.5 | 12.7 | 0.002 |
| SF-36 PCS | 2 | 52.6 | 3.5 | 1 | 81.5 | 1.6 | 0.009 | 1 | 97 | 0.5 | 0.003 |
| GENERAL | |||||||||||
| VAS back | 8 | 4.5 | 1.8 | 5 | 3.0 | 1.4 | 0.14 | 8 | 1.7 | 1.3 | 0.003 |
| VAS leg | 8 | 6.7 | 1.2 | 5 | 2.4 | 0.8 | <0.001 | 8 | 1.3 | 1.1 | <0.001 |
| JOA | 1 | 10.9 | 0.3 | NA | NA | NA | NA | 1 | 24.0 | 0.1 | <0.001 |
| ODI | 8 | 51.5 | 18.9 | 5 | 26.3 | 11.1 | 0.02 | 8 | 15.7 | 9.0 | <0.001 |
| SF-36 PCS | 3 | 50.4 | 30.9 | 2 | 69.2 | 23.8 | 0.53 | 3 | 75.7 | 55.6 | 0.53 |
SD – standard deviation; VAS – visual analog scale; JOA – Japanese Orthopedic Association score; ODI – Oswestry Disability Index;
SF-36 PCS – 36-item short form physical component score
At the last follow-up appointment ( 6 months) the LA group did not have significant improvements in VAS back or leg pain but showed significant improvements in JOA score (12.3 to 27.3, p < 0.001), ODI score (45.7 to 9.5, p = 0.002) and SF-36 score (52.6 to 97, p = 0.003). In comparison, at the last follow-up appointment the GA group demonstrated significant improvements in VAS back pain (4.5 to 1.7, p = 0.003), VAS leg pain (6.7 to 1.3, p < 0.001), JOA score (10.9 to 24.0, p < 0.001) and ODI score (51.5 to 15.7, p < 0.001) but not SF-36 score (50.4 to 75.7, p = 0.53) (Table 5).
McNab scores were reported in 12 studies (6 GA, 6 LA). Incidence of those achieving an “Excellent” outcome in the GA group was 0.58 (95% CI 0.45 – 0.69) and 0.67 (95% CI 0.51 – 0.8) in the LA group (p = 0.21). Results by study are presented in Supplementary Figure 3 as forest plots.
Discussion
Minimally invasive approaches to treat lumbar disc herniations have evolved significantly since open discectomy was first reported in 1934 [14]. After the operating microscope was introduced in 1978, the microdisectomy technique was born and is now routinely performed to treat lumbar disc herniations [15], [16], [17], [18], [19], [20]. Since then, several other techniques have been developed incorporating even smaller incisions with the use of tubular retractors and advanced optical techniques in effort to minimize tissue trauma and increase visualization [21]. MED is one such technique that utilizes the endoscope and/or microscope along with a tubular retraction system with proponents claiming faster recovery and better cosmetic results [2,[22], [23], [24], [25], [26]].
Prior studies have suggested that anesthetic technique may impact surgical outcomes due to the known associated complications and side-effects of general anesthesia [11,12,22,27]. Additionally, it is possible that anesthetic technique may influence a surgeon's decision-making intraoperatively due to the added factor of patient discomfort that must be considered in awake spinal surgery. Awake surgery involves increased surgeon awareness of nerve root manipulation as well as a possible desire to shorten operative times based on patient discomfort. These factors may influence discectomy extent resulting in higher disc recurrence, durotomy and reoperation rates. Thus, investigation of the impact of anesthesia on outcomes after MED is warranted.
While MED and other minimally invasive techniques can be performed under LA, there remains a paucity of literature comparing outcomes in those undergoing MED with GA versus LA. This has become increasingly important from an economic standpoint as LA has been shown to reduce hospital costs [12,27]. Due to limited existing data on the topic, a systematic review and meta-analysis design was chosen for this study in attempt to pool and analyze the largest amounts of patient data examining the GA and LA techniques.
In the present study, anesthesia type did not affect the primary outcomes of overall complications, durotomies, recurrent disc herniations and reoperations with the GA and LA groups demonstrating similar rates for all. While prior studies have examined GA vs LA for open microdiscectomy [12,28] and PELD, [1] there have been no prior studies examining the effects of GA versus LA on surgical and patient outcomes after MED.
Ulutas et al. retrospectively examined a group of 850 open lumbar microdiscectomy patients undergoing GA (n = 277) and epidural anesthesia (EA, n = 573) finding significantly shorter total operating room (OR) times with EA. However, duration of surgery was similar between these groups, likely explained by the shorter duration of perioperative tasks such as patient positioning, induction of anesthesia and lack of need for extubation in those undergoing EA [12]. Dagistan et al. reviewed perioperative data in 180 patients undergoing spinal anesthesia (SA) versus GA for lumbar microdiscectomy. The authors found less surgical site bleeding, significantly lower blood pressure, a lower rate of tachycardia, decreased analgesia requirement and less postoperative nausea/vomiting in the SA group. GA patients were found to have a higher rate of pulmonary complications requiring treatment [28].
Chen et al. examined GA vs LA in patients undergoing PELD and is the only study to compare PROs between these two anesthetic techniques. The authors found statistically significant improvements in VAS back/leg and ODI scores in both groups without significant differences between groups; however, they did find a shorter hospital stay in the LA group [1]. In the present meta-analysis, anesthetic technique did not affect VAS leg scores or ODI at the first postoperative appointment, which were significantly improved in both groups.
Interestingly, compared to GA, the significant improvement in VAS leg scores in the LA group did not extend into their last follow up. This may suggest that MED under GA confers a more sustained benefit for the treatment of radiculopathy. The ability to adequately mobilize the nerve root for inspection of residual disc material to achieve optimal decompression is paramount. While the present study did not examine peri- or intraoperative patient comfort, patient discomfort and dysesthesias experienced during MED with LA may lead to inadequate nerve root decompression with eventual recurrence of radicular symptoms. However, the data in this analysis did not demonstrate a significant difference in recurrent disc herniation or reoperation rate to fully support this hypothesis.
Proponents of LA state continuous patient feedback during the operation may help reduce nerve root injury and post-operative dysesthesias. Chen et al. demonstrated significantly less post-operative dysesthesias in an LA group [1]. The present study results did not demonstrate any significant difference in these complications at first or final follow up. Based on prior studies, it is possible that MED under GA confers a higher risk of prolonged, transient dysesthesias that eventually improve over a longer time span. However, the potential benefit of greater long-term symptomatic improvement in leg pain with GA noted in the present meta-analysis must also be considered.
VAS back scores were only improved in the GA group at final follow up, however as the primary goal of MED is not relief of back pain, this difference between groups is not felt to represent clinical signi-ficance.
In contrast to prior studies, the present study did not demonstrate any difference in hospital LOS between LA and GA groups. This could be attributed to the inclusion of more international studies in the LA group, where in-hospital rehab counts in the LOS metric.
In general, the results of the present study demonstrate that LA may be utilized as a safe and effective alternative to GA in appropriately selected patients with similar rates of complications, durotomy rates and overall comparable PROs to those undergoing GA. Prior studies have demonstrated the potential of this anesthetic technique to reduce costs to the healthcare system with shorter hospitalizations and operative times. Ulutas et al. assessed cost effectiveness of LA over GA for microdiscectomy and found the use of LA to result in a 43.28% cost reduction [12]. Morris et al. similarly found spinal anesthesia to be 9.93% less expensive than general anesthesia with no significant of patient outcomes and diminished pain and recovery times [27]. Additionally, avoidance of the risk associated with GA as well as diminished post-operative nausea and vomiting make LA an attractive anesthetic technique. While the current study did not investigate or confirm all of these findings, these should be the targets of future large-scale prospectively designed trials.
While the PRO findings in this study demonstrated greater long-term leg-pain relief in the GA group, these results must be interpreted with caution given the small number and heterogeneous nature of the included studies. Future standardized prospective studies examining the effects of GA and LA on short and long-term PROs are warranted to further confirm this finding. Results from the PROs JOA and SF-36 PCS should particularly be interpreted with caution given the inclusion of only a few studies for each.
Limitations
The limitations of this study are primarily related to the limited body of literature directly examining the topic. Cohorts of patients undergoing LA and GA for MED were combined in attempt to overcome this, however, only 23 total studies met the inclusion criteria. Additionally, outcomes assessed were not present uniformly across all studies leading to underpowered metrics in several outcome categories. While several randomized control trials were included, the majority of included studies were retrospective or case-control designs of poor quality. This meta-analysis demonstrated significant heterogeneity within and between groups, limiting the strength of our conclusions. Included studies were conducted in a wide-range of geographic locations with many international studies, resulting in significant variation in patient assessment and outcome measurements. To help limit this, primary outcomes were chosen as those that were reported in the greatest number of studies and felt to be the most objective. Additionally, a majority of studies originated from China limiting the broad applicability of the findings from this review. Despite these limitations, this is the first systematic review and meta-analysis on the topic of anesthetic use in MED and generates several novel hypotheses that are worthy of further investigation.
Conclusions
The results of this meta-analysis demonstrate similar rates of overall complication, durotomy, recurrent disc herniation and reoperation rates in patients undergoing MED with GA and LA. Patients undergoing MED with GA were noted to have statistically significant improvements in VAS leg and back pain at the final follow-up appointment while the LA group was not. LA is a safe and effective alternative to GA in appropriately selected patients. Further prospective and randomized study is needed to assess the true effects of GA and LA on perioperative, surgical and patient-reported outcomes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Disclosures
None.
Acknowledgments
None.
Footnotes
Financial support: none.
PICO STATEMENT: In adult patients (>18yo) undergoing first time 1-2 level discectomy between L1-S1 for lumbar disc herniation (population), what is the impact of local anesthesia (intervention) versus general anesthesia (comparison) on complication rates, durotomy rates, recurrent disc herniation rates, reoperation rates, length of surgery (primary outcomes) and patient-reported outcomes (PROs – secondary outcomes) after at least 6 months of follow-up (time).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.xnsj.2022.100129.
Appendix. Supplementary materials
References
- 1.Chen H.T., Tsai C.H., Chao S.C., et al. Endoscopic discectomy of L5-S1 disc herniation via an interlaminar approach: Prospective controlled study under local and general anesthesia. Surgical neurology international. 2011;2:93. doi: 10.4103/2152-7806.82570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasouli M.R., Rahimi-Movaghar V., Shokraneh F., Moradi-Lakeh M., Chou R. Minimally invasive discectomy versus microdiscectomy/open discectomy for symptomatic lumbar disc herniation. Cochrane Database Syst Rev. 2014;(9) doi: 10.1002/14651858.CD010328.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Cruet M.J., Foley K.T., Isaacs R.E., et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery. 2002;51(5 Suppl):S129–S136. [PubMed] [Google Scholar]
- 4.Nakagawa H., Kamimura M., Uchiyama S., Takahara K., Itsubo T., Miyasaka T. Microendoscopic discectomy (MED) for lumbar disc prolapse. J Clin Neurosci. 2003;10(2):231–235. doi: 10.1016/s0967-5868(02)00337-5. [DOI] [PubMed] [Google Scholar]
- 5.Hussein M., Abdeldayem A., Mattar M.M. Surgical technique and effectiveness of microendoscopic discectomy for large uncontained lumbar disc herniations: a prospective, randomized, controlled study with 8 years of follow-up. Eur Spine J. 2014;23(9):1992–1999. doi: 10.1007/s00586-014-3296-9. [DOI] [PubMed] [Google Scholar]
- 6.Arts M.P., Brand R., van den Akker M.E., et al. Tubular diskectomy vs conventional microdiskectomy for the treatment of lumbar disk herniation: 2-year results of a double-blind randomized controlled trial. Neurosurgery. 2011;69(1):135–144. doi: 10.1227/NEU.0b013e318214a98c. discussion 44. [DOI] [PubMed] [Google Scholar]
- 7.Arts M., Brand R., van der Kallen B., Lycklama à Nijeholt G., Peul W. Does minimally invasive lumbar disc surgery result in less muscle injury than conventional surgery? A randomized controlled trial. Eur Spine J. 2011;20(1):51–57. doi: 10.1007/s00586-010-1482-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg B., Nagraja U.B., Jayaswal A. Microendoscopic versus open discectomy for lumbar disc herniation: a prospective randomised study. J Orthop Surg (Hong Kong) 2011;19(1):30–34. doi: 10.1177/230949901101900107. [DOI] [PubMed] [Google Scholar]
- 9.Huang T.J., Hsu R.W., Li Y.Y., Cheng C.C. Less systemic cytokine response in patients following microendoscopic versus open lumbar discectomy. J Orthop Res. 2005;23(2):406–411. doi: 10.1016/j.orthres.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.McLain R.F., Kalfas I., Bell G.R., Tetzlaff J.E., Yoon H.J., Rana M. Comparison of spinal and general anesthesia in lumbar laminectomy surgery: a case-controlled analysis of 400 patients. J Neurosurg Spine. 2005;2(1):17–22. doi: 10.3171/spi.2005.2.1.0017. [DOI] [PubMed] [Google Scholar]
- 11.Ye X.F., Wang S., Wu A.M., et al. Comparison of the effects of general and local anesthesia in lumbar interlaminar endoscopic surgery. Ann Palliat Med. 2020;9(3):1103–1108. doi: 10.21037/apm-20-623. [DOI] [PubMed] [Google Scholar]
- 12.Ulutas M., Secer M., Taskapilioglu O., et al. General versus epidural anesthesia for lumbar microdiscectomy. J Clin Neurosci. 2015;22(8):1309–1313. doi: 10.1016/j.jocn.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mixter W.J., Barr J.S. Rupture of the Intervertebral Disc with Involvement of the Spinal Canal. New England Journal of Medicine. 1934;211(5):210–215. [Google Scholar]
- 15.Arts M.P., Peul W.C., Koes B.W., Thomeer R.T. Management of sciatica due to lumbar disc herniation in the Netherlands: a survey among spine surgeons. J Neurosurg Spine. 2008;9(1):32–39. doi: 10.3171/SPI/2008/9/7/032. [DOI] [PubMed] [Google Scholar]
- 16.Deen H.G., Fenton D.S. Lamer TJ. Minimally invasive procedures for disorders of the lumbar spine. Mayo Clin Proc. 2003;78(10):1249–1256. doi: 10.4065/78.10.1249. [DOI] [PubMed] [Google Scholar]
- 17.Gibson J.N.A., Waddell G. Surgical interventions for lumbar disc prolapse. Cochrane Database of Systematic Reviews. 2007;(2) doi: 10.1002/14651858.CD001350.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haines S.J., Jordan N., Boen J.R., Nyman J.A., Oldridge N.B., Lindgren B.R. Discectomy strategies for lumbar disc herniation: results of the LAPDOG trial. J Clin Neurosci. 2002;9(4):411–417. doi: 10.1054/jocn.2002.1120. [DOI] [PubMed] [Google Scholar]
- 19.Thomé C., Barth M., Scharf J., Schmiedek P. Outcome after lumbar sequestrectomy compared with microdiscectomy: a prospective randomized study. J Neurosurg Spine. 2005;2(3):271–278. doi: 10.3171/spi.2005.2.3.0271. [DOI] [PubMed] [Google Scholar]
- 20.Tullberg T., Rydberg J., Isacsson J. Radiographic changes after lumbar discectomy. Sequential enhanced computed tomography in relation to clinical observations. Spine (Phila Pa 1976) 1993;18(7):843–850. [PubMed] [Google Scholar]
- 21.Mathews H.H., Long B.H. Minimally Invasive Techniques for the Treatment of Intervertebral Disk Herniation. JAAOS - Journal of the American Academy of Orthopaedic Surgeons. 2002;10(2):80–85. doi: 10.5435/00124635-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Arts M.P., Brand R., van den Akker M.E., Koes B.W., Bartels R.H., Peul W.C. Tubular diskectomy vs conventional microdiskectomy for sciatica: a randomized controlled trial. Jama. 2009;302(2):149–158. doi: 10.1001/jama.2009.972. [DOI] [PubMed] [Google Scholar]
- 23.Dasenbrock H.H., Juraschek S.P., Schultz L.R., et al. The efficacy of minimally invasive discectomy compared with open discectomy: a meta-analysis of prospective randomized controlled trials. J Neurosurg Spine. 2012;16(5):452–462. doi: 10.3171/2012.1.SPINE11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs W.C., Arts M.P., van Tulder M.W., et al. Surgical techniques for sciatica due to herniated disc, a systematic review. Eur Spine J. 2012;21(11):2232–2251. doi: 10.1007/s00586-012-2422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nellensteijn J., Ostelo R., Bartels R., Peul W., van Royen B., van Tulder M. Transforaminal endoscopic surgery for symptomatic lumbar disc herniations: a systematic review of the literature. Eur Spine J. 2010;19(2):181–204. doi: 10.1007/s00586-009-1155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivakumar Jaikumar, MD DHKM, Andrew C.Kam., MBBS FRACS History of Minimally Invasive Spine Surgery. Neurosurgery. 2002;51(2) S2-1-S2-14. [PubMed] [Google Scholar]
- 27.Morris M.T., Morris J., Wallace C., et al. An Analysis of the Cost-Effectiveness of Spinal Versus General Anesthesia for Lumbar Spine Surgery in Various Hospital Settings. Global Spine J. 2019;9(4):368–374. doi: 10.1177/2192568218795867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dagistan Y., Okmen K., Dagistan E., Guler A., Ozkan N. Lumbar Microdiscectomy Under Spinal and General Anesthesia: A Comparative Study. Turk Neurosurg. 2015;25(5):685–689. doi: 10.5137/1019-5149.JTN.10300-14.1. [DOI] [PubMed] [Google Scholar]
- 29.Gibson J.N.A., Subramanian A.S., Scott C.E.H. A randomised controlled trial of transforaminal endoscopic discectomy vs microdiscectomy. Eur Spine J. 2017;26(3):847–856. doi: 10.1007/s00586-016-4885-6. [DOI] [PubMed] [Google Scholar]
- 30.Patil A., Chugh A., Gotecha S., et al. Microendoscopic discectomy for lumbar disc herniations. J Craniovertebr Junction Spine. 2018;9(3):156–162. doi: 10.4103/jcvjs.JCVJS_61_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Z., Ran M., Luo J., et al. Follow-up results of microendoscopic discectomy compared to day surgery using percutaneous endoscopic lumbar discectomy for the treatment of lumbar disc herniation. BMC Musculoskelet Disord. 2021;22(1):160. doi: 10.1186/s12891-021-04038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon S.M., Ahn S.S., Kim K.H., Kim Y.D., Cho J.H., Kim D.H. Comparative Study of the Outcomes of Percutaneous Endoscopic Lumbar Discectomy and Microscopic Lumbar Discectomy Using the Tubular Retractor System Based on the VAS, ODI, and SF-36. Korean J Spine. 2012;9(3):215–222. doi: 10.14245/kjs.2012.9.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang F., Ren L., Ye Q., et al. Endoscopic and Microscopic Interlaminar Discectomy for the Treatment of Far-Migrated Lumbar Disc Herniation: A Retrospective Study with a 24-Month Follow-Up. J Pain Res. 2021;14:1593–1600. doi: 10.2147/JPR.S302717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porto G.B.F., Cisewski S.E., Wolgamott L., Frankel B.M. Clinical outcomes for patients with lateral lumbar radiculopathy treated by percutaneous endoscopic transforaminal discectomy versus tubular microdiscectomy: A retrospective review. Clin Neurol Neurosurg. 2021;208 doi: 10.1016/j.clineuro.2021.106848. [DOI] [PubMed] [Google Scholar]
- 35.Liu L., Xue H., Jiang L., et al. Comparison of Percutaneous Transforaminal Endoscopic Discectomy and Microscope-Assisted Tubular Discectomy for Lumbar Disc Herniation. Orthop Surg. 2021;13(5):1587–1595. doi: 10.1111/os.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu P., Zan P., Zhang X., et al. Comparison of Percutaneous Transforaminal Endoscopic Discectomy and Microendoscopic Discectomy for the Surgical Management of Symptomatic Lumbar Disc Herniation: A Multicenter Retrospective Cohort Study with a Minimum of 2 Years' Follow-Up. Pain Physician. 2021;24(1) E117-e25. [PubMed] [Google Scholar]
- 37.Ren C., Qin R., Li Y., Wang P. Microendoscopic Discectomy Combined with Annular Suture Versus Percutaneous Transforaminal Endoscopic Discectomy for Lumbar Disc Herniation: A Prospective Observational Study. Pain Physician. 2020;23(6) E713-e21. [PubMed] [Google Scholar]
- 38.Liu X., Yuan S., Tian Y., et al. Comparison of percutaneous endoscopic transforaminal discectomy, microendoscopic discectomy, and microdiscectomy for symptomatic lumbar disc herniation: minimum 2-year follow-up results. J Neurosurg Spine. 2018;28(3):317–325. doi: 10.3171/2017.6.SPINE172. [DOI] [PubMed] [Google Scholar]
- 39.Li M., Yang H., Yang Q. Full-Endoscopic Technique Discectomy Versus Microendoscopic Discectomy for the Surgical Treatment of Lumbar Disc Herniation. Pain Physician. 2015;18(4):359–363. [PubMed] [Google Scholar]
- 40.Kunert P., Kowalczyk P., Marchel A. Minimally invasive microscopically assisted lumbar discectomy using the METRx X-Tube system. Neurol Neurochir Pol. 2010;44(6):554–559. doi: 10.1016/s0028-3843(14)60152-3. [DOI] [PubMed] [Google Scholar]
- 41.Righesso O., Falavigna A., Avanzi O. Comparison of open discectomy with microendoscopic discectomy in lumbar disc herniations: results of a randomized controlled trial. Neurosurgery. 2007;61(3):545–549. doi: 10.1227/01.NEU.0000290901.00320.F5. discussion 9. [DOI] [PubMed] [Google Scholar]
- 42.Ranjan A., Lath R. Microendoscopic discectomy for prolapsed lumbar intervertebral disc. Neurol India. 2006;54(2):190–194. [PubMed] [Google Scholar]
- 43.Abudurexiti T., Qi L., Muheremu A. Amudong A. Micro-endoscopic discectomy versus percutaneous endoscopic surgery for lumbar disk herniation. J Int Med Res. 2018;46(9):3910–3917. doi: 10.1177/0300060518781694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song H.P., Sheng H.F., Xu W.X. A case-control study on the treatment of protrusion of lumbar intervertebral disc through PELD and MED. Exp Ther Med. 2017;14(4):3708–3712. doi: 10.3892/etm.2017.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jing Z., Li L., Song J. Percutaneous transforaminal endoscopic discectomy versus microendoscopic discectomy for upper lumbar disc herniation: a retrospective comparative study. Am J Transl Res. 2021;13(4):3111–3119. [PMC free article] [PubMed] [Google Scholar]
- 46.Pang J.Y., Tan F., Chen W.W., et al. Comparison of microendoscopic discectomy and open discectomy for single-segment lumbar disc herniation. World J Clin Cases. 2020;8(14):2942–2949. doi: 10.12998/wjcc.v8.i14.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Z., Zhang L., Dong J., et al. Percutaneous Transforaminal Endoscopic Discectomy Versus Microendoscopic Discectomy for Lumbar Disc Herniation: Two-Year Results of a Randomized Controlled Trial. Spine (Phila Pa 1976) 2020;45(8):493–503. doi: 10.1097/BRS.0000000000003314. [DOI] [PubMed] [Google Scholar]
- 48.Liu W.G., Wu X.T., Guo J.H., Zhuang S.Y., Teng G.J. Long-term outcomes of patients with lumbar disc herniation treated with percutaneous discectomy: comparative study with microendoscopic discectomy. Cardiovasc Intervent Radiol. 2010;33(4):780–786. doi: 10.1007/s00270-009-9720-6. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Y., Wang M., Wang J., Chu T.W., Zhang Z.F., Li C.Q. Clinical experience and results of lumbar microendoscopic discectomy: a study with a five-year follow-up. Orthop Surg. 2009;1(3):171–175. doi: 10.1111/j.1757-7861.2009.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu X., Zhuang S., Mao Z., Chen H. Microendoscopic discectomy for lumbar disc herniation: surgical technique and outcome in 873 consecutive cases. Spine (Phila Pa 1976) 2006;31(23):2689–2694. doi: 10.1097/01.brs.0000244615.43199.07. [DOI] [PubMed] [Google Scholar]
- 51.Chen Z., Zhang L., Dong J., et al. Percutaneous transforaminal endoscopic discectomy compared with microendoscopic discectomy for lumbar disc herniation: 1-year results of an ongoing randomized controlled trial. J Neurosurg Spine. 2018;28(3):300–310. doi: 10.3171/2017.7.SPINE161434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.