Abstract
Background
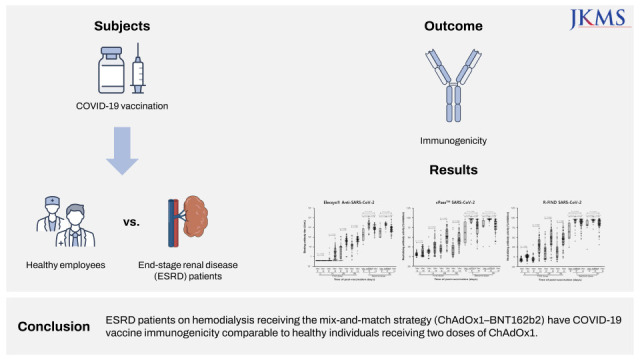
The objective of this study was to evaluate the immunogenicity of coronavirus disease 2019 (COVID-19) vaccination in patients with end-stage renal disease (ESRD) on hemodialysis.
Methods
ESRD patients at the hemodialysis center of a tertiary-care university-affiliated hospital and healthy employees at the clinical laboratory center were prospectively recruited between March and June 2021. For severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody analysis, blood samples were collected serially on days 0, 14, 28, and 56 after the first vaccine dose, and on days 7 and 50 after the second dose. Antibodies against the SARS-CoV-2 spike protein were quantified, and SARS-CoV-2 neutralizing antibodies were measured in the serum and plasma.
Results
Thirty-one ESRD patients and 55 healthy employees were regularly monitored. Twenty-five (80.6%) ESRD patients on hemodialysis received a mix-and-match strategy with ChAdOx1-BNT162b2 (AZ–Pf group) and six (19.4%) received two doses of ChAdOx1 (AZ–AZ group). ESRD patients on hemodialysis showed lower binding antibody titers and neutralizing antibody activities compared to healthy participants following the first vaccination with ChAdOx1. After the second dose, AZ-Pf group had higher immunogenicity than healthy people on days 7 and 50. The binding antibody titer and neutralizing antibody activities on days 7 and 50 were significantly higher in the AZ–Pf group than in the AZ–AZ group.
Conclusion
ESRD patients on hemodialysis receiving the mix-and-match strategy (ChAdOx1–BNT162b2) have COVID-19 vaccine immunogenicity comparable to healthy individuals receiving two doses of ChAdOx1.
Trial Registration
ClinicalTrials.gov Identifier: NCT04871945
Keywords: COVID-19, Vaccine, ESRD, Mix-and-Match
Graphical Abstract
INTRODUCTION
Since the emergence of coronavirus disease 2019 (COVID-19) in China in late December 2019, it has spread rapidly across the globe, resulting in a historical pandemic.1 To overcome such an unprecedented disastrous situation, some effective vaccines target the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were developed at the end of 2020 and many countries have been operating the national immunization program for COVID-19 using newly developed vaccines.2,3
Although its high efficacy of COVID-19 vaccination in reducing the severity of COVID-19 has been well established, its immunogenicity and effectiveness seem to be lower in immunocompromised patients than in healthy populations.4 Accordingly, in many countries, the additional dose of vaccination has been highly recommended for immunocompromised patients, including transplant recipients.5
End-stage renal disease (ESRD) is a well-known clinical condition that tends to show a reduced response to vaccination because of the impaired immune response associated with uremia; especially, patients on hemodialysis are at high risk of death from COVID-19.6,7 Despite this, there is insufficient data regarding the immune response against the COVID-19 vaccine among patients with ESRD. The objective of this study was to evaluate the immunogenicity of COVID-19 vaccination in patients with ESRD on hemodialysis.
METHODS
Study setting
A prospective observational cohort study was conducted at the hemodialysis center of a tertiary-care university-affiliated hospital (with 123 ESRD patients) and at a clinical laboratory center (with 872 employees) in Korea. Patients with ESRD at the hemodialysis center and employees at the clinical laboratory center were recruited for the study between March and June 2021. The inclusion criteria were: 1) those who had intended to get vaccinated with the COVID-19 vaccine according to the National Immunization Program and 2) those who provided informed consent to the study protocol. The exclusion criteria were as follows: 1) age < 18 years, 2) pregnancy, 3) medical history of COVID-19 before vaccination, and 4) history of vaccination with any type of COVID-19 vaccine before participation in the study. The recruitment advertisement that included the inclusion/exclusion criteria was posted on the bulletin boards of each institution, and those wishing to participate were enrolled in the study. The study protocol was also registered on ClinicalTrials.gov (NCT04871945) and is completed status.
National Immunization Program against COVID-19 in Korea during the study period
As part of the National Immunization Program against COVID-19, employees at medical institutions in Korea were mainly vaccinated with ChAdOx1 nCoV-19 adenovirus-based vector vaccine (ChAdOx1; Vaxzevria, AstraZeneca, Oxford, UK). Accordingly, all participants from the clinical laboratory center were vaccinated with two doses of ChAdOx1 (at 0 and 12 weeks). In comparison, patients with ESRD could choose either two doses of ChAdOx1 or a mix-and-match strategy, wherein they received ChAdOx1 as the first dose followed by the mRNA vaccine BNT162b2 (Comirnaty, BioNTech, Mainz, Germany) (second dose) after 11 weeks.
Data and sample collection
Demographic and clinical data, including age, sex, height, weight, underlying comorbidities, and the use of medications were collected after the administration of the first dose. Furthermore, local, and systemic adverse events within a week after vaccination were recorded for both the first and second doses.
For the analysis of antibodies for SARS-CoV-2, blood samples were collected serially from the participants at days 0, 14, 28, and 56 after administration of the first dose, and on days 7 and 50 after administration of the second dose (Supplementary Fig. 1). A time difference of ± 3 days was allowed for each point of sampling except day 0 where a time difference of only ± 1 day was allowed. For patients with ESRD, blood samples were obtained from an arterial needle prior to connecting the arterial blood tubing or flushing the needle before starting hemodialysis. No saline and/or heparin were present in the arterial needle or tubing prior to drawing the blood sample.
Laboratory tests
From serum and plasma samples, antibodies against the SARS-CoV-2 spike protein were quantified using Roche Elecsys® Anti-SARS-CoV-2 S (Roche Diagnostics GmbH, Mannheim, Germany) and SARS-CoV-2 neutralizing antibodies were measured using both cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript USA Inc., Piscataway, NJ, USA) and R-FIND SARS-CoV-2 Neutralizing Antibody ELISA (SG Medical Inc., Seoul, Korea). The cut-off value for each test was set following the value provided by the manufacturer: > 0.8 U/mL for Elecsys® Anti-SARS-CoV-2 S and > 30% inhibition for both tests for neutralization.
In patients with hemodialysis, the results of biochemical tests, performed as a regular examination before and after the second hemodialysis session of the week immediately prior to enroll, were obtained as the baseline laboratory values.8 Serum calcium concentration was measured using a Hitachi 7600 series automatic biochemical analyzer (Hitachi High-Technologies, Tokyo, Japan) and corrected serum calcium was calculated using the following formula9:
Corrected Serum Calcium (mg/dL) = Measured Total Calcium (mg/dL) + 0.8 × (4.0 − Serum Albumin [g/dL]).
Early responsiveness to ChAdOx1 vaccination among ESRD patients on hemodialysis
To find out the indicators that predict early responsiveness to ChAdOx1 vaccination among ESRD patients on hemodialysis, more detailed clinical and laboratory data were collected. We defined participant who obtained SARS-CoV-2 nAbs ≥ 30% inhibition at 28th days after priming vaccination as an early responder and the others as a delayed responder.
Statistical analyses
Categorical variables were analyzed using the χ2 test or Fisher’s exact test, as appropriate, and continuous variables were analyzed using the Mann–Whitney U test or the independent t-test, as appropriate.
Serial changes in binding and neutralizing antibody titers between study days were compared using the Wilcoxon signed-rank test, and the Cochrane–Armitage test for trend was used to compare the proportions of categorical variables between groups.
Multiple Cox-proportional hazard model was performed to determine independent variables affecting early responsiveness to ChAdOx1 vaccination among ESRD patients, and Gray’s test was used for checking if cumulative incidence curves were the same at all-time points.
A two-tailed P value of < 0·05 was considered statistically significant. Statistical Analysis Software version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB) of Hanyang University Seoul Hospital (IRB number: 2021-02-033), and written informed consent was obtained from all participants.
RESULTS
Baseline characteristics of participants
A total of 33 patients with ESRD on hemodialysis and 55 healthy employees were enrolled in the study and regular monitoring was performed on the participants according to the study protocol. Two patients with ESRD on hemodialysis dropped out during the study period; one dropped out due to death (not associated with the study) and another was transferred to another hemodialysis center. Finally, regular monitoring was performed on 31 patients with ESRD on hemodialysis and 55 healthy employees according to the study protocol. Of the patients with ESRD on hemodialysis, 25 (80.6%) participants received the mix-and-match strategy (AZ–Pf group), and six (19.4%) received two doses of ChAdOx1 (AZ–AZ group). All healthy employees received two doses of ChAdOx1.
The median age of patients with ESRD on hemodialysis was 60 years (interquartile range [IQR], 53–62), which was higher than that of healthy employees (median age, 36; IQR, 28–44) (P < 0.001). The Charlson’s comorbidity index (CCI) score was higher in patients with ESRD on hemodialysis when compared with healthy employees (3.29 ± 1.16 vs. 0.04 ± 0.19, P < 0.001). However, there was no significant difference in the proportion of female sex (51.6% vs. 45.5%, P = 0.583) and body mass index (BMI, 22.8 ± 4.3 vs. 23.1 ± 32.7; P = 0.660) between patients with ESRD and healthy employees. Furthermore, there was no significant difference in age, sex, BMI, and CCI between the AZ–Pf and AZ–AZ groups.
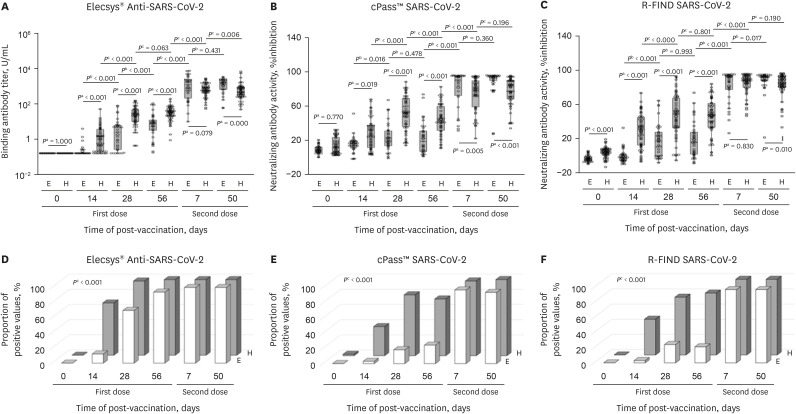
Serial change in humoral immunogenicity of COVID-19 vaccination
The overall immunogenicity was summarized in Table 1. We found that patients with ESRD on hemodialysis had not only consistently lower binding antibody titers and neutralizing antibody activities, but also poor vaccination efficacy compared to healthy people during the observation period following the first vaccination with ChAdOx1 (Fig. 1). However, it was noted that, on day 7 after vaccination with the second dose, the binding antibody titer and neutralizing antibody activities of patients with ESRD on hemodialysis increased to a similar degree as those of the healthy participants. On day 50 after the second vaccination, these increases were more prominent and patients with ESRD on hemodialysis had higher binding antibody titer and neutralizing antibody activities as compared with the control group. Finally, all participants in both groups obtained reactive (positive) binding and neutralizing antibody values.
Table 1. Summary of immunogenicity of COVID-19 vaccination between healthy people and patients with end-stage renal disease.
| Variables | Healthy people, two doses of ChAdOx1 | ESRD patients, ChAdOx1-BNT162b2 | P valuea | ESRD patients, two doses of ChAdOx1 | P valueb | |
|---|---|---|---|---|---|---|
| SARS-CoV-2 binding antibody titer measured by Elecsys® Anti-SARS-CoV-2 S, AU/mL | ||||||
| Day 0, from 1st dose | 0.4 ± 0.0 | 0.4 ± 0.0 | 1.000 | 0.4 ± 0.0 | 1.000 | |
| Day 14, from 1st dose | 6.4 ± 13.2 | 0.8 ± 1.5 | < 0.001 | 0.5 ± 0.2 | 0.003 | |
| Day 28, from 1st dose | 62.3 ± 60.3 | 15.0 ± 28.8 | < 0.001 | 23.3 ± 46.9 | 0.020 | |
| Day 56, from 1st dose | 69.0 ± 53.3 | 27.1 ± 39.0 | < 0.001 | 39.7 ± 69.3 | 0.030 | |
| Day 7, from 2nd dose | 1,192.0 ± 881.7 | 3,028.3 ± 2,687.2 | 0.004 | 523.9 ± 672.9 | 0.042 | |
| Day 50, from 2nd dose | 1,149.9 ± 1,493.4 | 2,373.9 ± 1,075.9 | < 0.001 | 891.9 ± 1,256.8 | 0.229 | |
| SARS-CoV-2 neutralizing antibody activity measured by cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit, % inhibition | ||||||
| Day 0, from 1st dose | 10.1 ± 10.4 | 4.5 ± 4.6 | 0.116 | 9.5 ± 5.1 | 0.697 | |
| Day 14, from 1st dose | 24.7 ± 18.5 | 14.0 ± 9.1 | 0.016 | 6.5 ± 10.2 | 0.024 | |
| Day 28, from 1st dose | 50.4 ± 21.2 | 20.4 ± 16.6 | < 0.001 | 20.7 ± 19.4 | 0.010 | |
| Day 56, from 1st dose | 42.5 ± 18.5 | 17.7 ± 18.4 | < 0.001 | 27.7 ± 25.4 | 0.096 | |
| Day 7, from 2nd dose | 74.8 ± 20.3 | 86.7 ± 18.2 | < 0.001 | 61.4 ± 29.4 | 0.291 | |
| Day 50, from 2nd dose | 77.0 ± 17.6 | 91.7 ± 14.4 | < 0.001 | 74.8 ± 26.1 | 0.832 | |
| SARS-CoV-2 neutralizing antibody activity measured by R-FIND SARS-CoV-2 Neutralizing Antibody ELISA, % inhibition | ||||||
| Day 0, from 1st dose | 0.3 ± 6.4 | −7.2 ± 4.3 | < 0.001 | −6.2 ± 4.3 | 0.015 | |
| Day 14, from 1st dose | 27.2 ± 20.8 | −3.9 ± 9.2 | < 0.001 | −5.6 ± 7.4 | 0.001 | |
| Day 28, from 1st dose | 45.4 ± 25.7 | 14.2 ± 20.7 | < 0.001 | 23.7 ± 22.6 | 0.063 | |
| Day 56, from 1st dose | 45.7 ± 22.0 | 14.4 ± 22.1 | < 0.001 | 26.5 ± 22.1 | 0.055 | |
| Day 7, from 2nd dose | 86.3 ± 13.5 | 87.8 ± 18.2 | 0.210 | 64.5 ± 27.4 | 0.030 | |
| Day 50, from 2nd dose | 84.6 ± 14.2 | 93.4 ± 5.5 | 0.002 | 76.1 ± 28.6 | 0.485 | |
| Proportion of positive result of SARS-CoV-2 binding antibody measured by Elecsys® Anti-SARS-CoV-2 S | ||||||
| Day 0, from 1st dose | 0 | 0 | 0 | |||
| Day 14, from 1st dose | 38 (69) | 3 (12) | < 0.001 | 1 (17) | 0.020 | |
| Day 28, from 1st dose | 54 (98) | 18 (72) | < 0.001 | 4 (67) | 0.024 | |
| Day 56, from 1st dose | 55 (100) | 23 (92) | 0.106 | 6 (100) | ||
| Day 7, from 2nd dose | 55 (100) | 25 (100) | 6 (100) | |||
| Day 50, from 2nd dose | 55 (100) | 25 (100) | 6 (100) | |||
| Proportion of positive result of SARS-CoV-2 neutralizing antibody activity measured by cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit | ||||||
| Day 0, from 1st dose | 1 (2) | 0 | 1.000 | 0 | 1.000 | |
| Day 14, from 1st dose | 21 (38) | 1 (4) | < 0.001 | 0 | 0.085 | |
| Day 28, from 1st dose | 44 (80) | 5 (20) | < 0.001 | 1 (17) | 0.004 | |
| Day 56, from 1st dose | 41 (75) | 5 (20) | < 0.001 | 3 (50) | 0.335 | |
| Day 7, from 2nd dose | 54 (98) | 25 (100) | 1.000 | 5 (83) | 0.189 | |
| Day 50, from 2nd dose | 55 (100) | 24 (96) | 0.313 | 5 (83) | 0.098 | |
| Proportion of positive result of SARS-CoV-2 neutralizing antibody activity measured by R-FIND SARS-CoV-2 Neutralizing Antibody ELISA | ||||||
| Day 0, from 1st dose | 0 | 0 | 0 | |||
| Day 14, from 1st dose | 26 (47) | 1 (4) | < 0.001 | 0 | 0.033 | |
| Day 28, from 1st dose | 42 (76) | 5 (20) | < 0.001 | 3 (50) | 0.179 | |
| Day 56, from 1st dose | 45 (82) | 5 (20) | < 0.001 | 2 (33) | 0.021 | |
| Day 7, from 2nd dose | 55 (100) | 25 (100) | 5 (83) | 0.098 | ||
| Day 50, from 2nd dose | 55 (100) | 25 (100) | 5 (83) | 0.098 | ||
Results are expressed as mean ± standard deviation or frequencies (and proportions).
COVID-19 = coronavirus disease 2019, ESRD = end-stage renal disease, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, ELISA = enzyme-linked immunosorbent assay.
aComparison between Healthy people, two doses of ChAdOx1 and ESRD patients, ChAdOx1-BNT162b2.
bComparison between Healthy people, two doses of ChAdOx1and ESRD patients, two doses of ChAdOx1.
Fig. 1. Comparison of immunogenicity of COVID-19 vaccination between healthy people and patients with ESRD on hemodialysis.
(A) Binding antibody titer measured by Elecsys® Anti-SARS-CoV-2 S. (B) Neutralizing antibody activity measured by cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit. (C) Neutralizing antibody activity measured by R-FIND SARS-CoV-2 Neutralizing Antibody ELISA. (D) Proportion of participants with a positive result of binding antibody measured by Elecsys® Anti-SARS-CoV-2 S. (E) Proportion of participants with a positive result of neutralizing antibody measured by cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit. (F) Proportion of participants with a positive result of neutralizing antibody measured by R-FIND SARS-CoV-2 Neutralizing Antibody ELISA.
COVID-19 = coronavirus disease 2019, ESRD = end-stage renal disease, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, ELISA = enzyme-linked immunosorbent assay, E = patients with ESRD, H = healthy people.
aP values are the result of Mann–Whitney U test; bP values are the result of Wilcoxon signed-rank test; and cP values are the result of the Cochrane–Armitage test for trend.
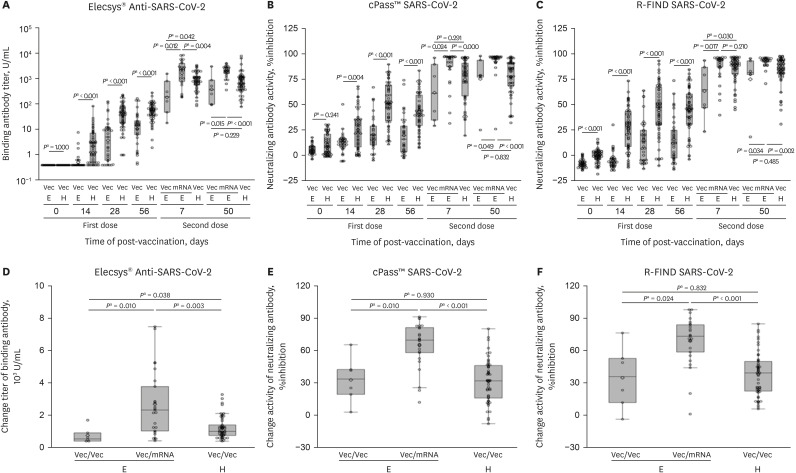
We found that there was some difference in the immunogenicity of patients with ESRD on hemodialysis according to the type of second vaccination used. In the AZ–AZ group, patients with ESRD on hemodialysis had lower binding antibody titers and neutralizing antibody activity at 7 days post-second vaccination. In the AZ–Pf group, patients with ESRD on hemodialysis had higher immunogenicity than healthy people (Fig. 2, Supplementary Fig. 2). After the second dose of vaccination, the binding antibody titer and neutralizing antibody activities on days 7 and 50 were significantly higher in the AZ–Pf group than in the AZ–AZ group (Fig. 2A-C). For further analysis, we compared the immunogenicity according to the type of second vaccination used, by measuring the change between day 56 after the first vaccination and day 7 after the second vaccination. We found that the range of change in immunogenicity was larger in AZ-Pf group than AZ-AZ group (Fig. 2D-F).
Fig. 2. Comparison of immunogenicity of COVID-19 vaccination between patients with ESRD on hemodialysis who received ChAdOx1–BNT162b2 and those who received two doses of ChAdOx1.
(A) Binding antibody titer measured by Elecsys® Anti-SARS-CoV-2 S. (B) Neutralizing antibody activity measured by cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit. (C) Neutralizing antibody activity measured by R-FIND SARS-CoV-2 Neutralizing Antibody ELISA. (D) Change in the level of binding antibody between day 56 from the first dose and day 7 from the second dose measured by Elecsys® Anti-SARS-CoV-2 S. (E) Change in the level of neutralizing antibody between day 56 from the first dose and day 7 from the second dose measured by cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit. (F) Change in the level of neutralizing antibody between day 56 from the first dose and day 7 from the second dose measured by R-FIND SARS-CoV-2 Neutralizing Antibody ELISA.
COVID-19 = coronavirus disease 2019, ESRD = end-stage renal disease, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, ELISA = enzyme-linked immunosorbent assay, Vec = ChAdOx1, mRNA = BNT162b2, E = patients with ESRD, H = healthy people.
aP values are the result of Mann–Whitney U test.
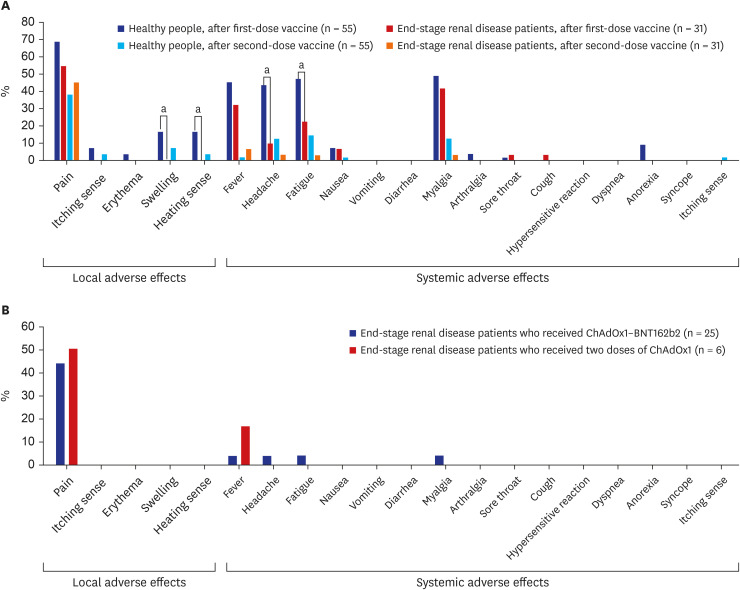
Adverse effects of COVID-19 vaccination
Regarding adverse effects, the proportion of participants with injection site swelling, heating sensation at the injection site, headache, and fatigue were higher within 7 days of the first dose among healthy people when compared to patients with ESRD on hemodialysis. In comparison, there was no significant difference in the proportion of participants with adverse effects after the second vaccination dose between healthy people and patients with ESRD on hemodialysis (Fig. 3).
Fig. 3. Adverse effects after COVID-19 vaccination.
(A) Comparison between healthy people and patients with ESRD on hemodialysis. (B) Comparison between patients with ESRD on hemodialysis who received ChAdOx1–BNT162b2 and those who received two doses of ChAdOx1.
COVID-19 = coronavirus disease 2019, ESRD = end-stage renal disease.
aP value <0.05.
Clinical indicator(s) predicting the early responsiveness to ChAdOx1 vaccination among ESRD patients on hemodialysis
Of ESRD patients on hemodialysis, there were no differences in age and sex between early and delayed responders. Five participants with glomerulonephritis as underlying cause of ESRD were prescribed with either steroid or mycophenolate mofetil to control extra-renal manifestation of underlying diseases. Participants with delayed immune system’s response were likely to be steroid users and have increased serum corrected calcium concentration, but they were statistically significant. Other baseline characteristics were shown in Table 2.
Table 2. Baseline characteristics of patients with end-stage renal disease on hemodialysis according to responsiveness to the priming ChAdOx1 vaccination.
| Variables | Delayed respondera (n = 25) | Early responderb (n = 8) | P value | |
|---|---|---|---|---|
| Age, yr | 58.3 ± 9.0 | 58.8 ± 4.0 | 0.900 | |
| Sex (male), % | 14 (56) | 4 (50) | 1.000 | |
| Body mass index, kg/m2 | 22.6 ± 3.8 | 23.6 ± 5.8 | 0.851 | |
| SBP before initiation of HD, mmHg | 139.8 ± 25.2 | 142.9 ± 8.3 | 0.533 | |
| DBP before initiation of HD, mmHg | 68.4 ± 19.3 | 60.5 ± 12.0 | 0.407 | |
| Cause of end-stage renal disease | ||||
| Diabetes mellitus | 10 (40) | 4 (50) | 0.695 | |
| Hypertension | 7 (28) | 2 (25) | 1.000 | |
| Chronic glomerulonephritis | 5 (20) | 0 | 0.302 | |
| Others | 3 (12) | 2 (25) | 0.574 | |
| Medical history | ||||
| Vintage, mon | 104.7 ± 85.6 | 175.0 ± 148.4 | 0.265 | |
| Charlson comorbidity index | 3.4 ± 1.7 | 3.2 ± 1.6 | 0.808 | |
| Cardiovascular disease | 9 (36) | 2 (25) | 0.681 | |
| Cerebrovascular disease | 2 (8) | 1 (13) | 1.000 | |
| Mycophenolate mofetil user | 1 (4) | 1 (13) | 1.000 | |
| Breast cancer | 0 | 1 (13) | 0.250 | |
| ARB user | 15 (60) | 4 (50) | 0.695 | |
| CCB user | 10 (40) | 6 (75) | 0.220 | |
| β-blocker user | 13 (52) | 3 (38) | 0.688 | |
| α-blocker user | 1 (4) | 1 (13) | 0.432 | |
| Sulfonylurea user | 3 (12) | 1 (13) | 1.000 | |
| DPP-4 inhibitor user | 8 (32) | 3 (38) | 1.000 | |
| Statin user | 12 (48) | 6 (75) | 0.242 | |
| Steroid user | 4 (16) | 0 (0) | 0.550 | |
| Vitamin D user | 17 (68) | 3 (38) | 0.338 | |
| Ca-based P binder user | 1 | 0 | 1.000 | |
| Non-Ca-based P binder user | 22 (88) | 8 (100) | 0.560 | |
| ESA user | 23 (92) | 7 (88) | 0.456 | |
| Laboratory test | ||||
| White blood cell, 109/L | 5.6 ± 2.1 | 6.1 ± 2.3 | 0.677 | |
| Hemoglobin, g/dL | 11.0 ± 1.0 | 10.4 ± 0.8 | 0.209 | |
| Platelet, 103/μL | 154.0 ± 57.4 | 168.8 ± 47.1 | 0.467 | |
| Neutrophil, 109/L | 3.7 ± 1.5 | 3.9 ± 1.7 | 0.753 | |
| Lymphocyte, 109/L | 1.1 ± 0.5 | 1.3 ± 0.4 | 0.240 | |
| Sodium, mmol/L | 136.3 ± 2.6 | 136.3 ± 1.8 | 0.967 | |
| Potassium, mmol/L | 4.7 ± 0.4 | 4.6 ± 0.6 | 0.371 | |
| Chloride, mmol/L | 99.0 ± 3.1 | 98.1 ± 2.6 | 0.544 | |
| Total CO2, mmol/L | 23.5 ± 2.7 | 24.1 ± 1.8 | 0.467 | |
| Total protein, g/dL | 7.3 ± 0.6 | 7.3 ± 0.4 | 0.876 | |
| Albumin, g/dL | 4.0 ± 0.3 | 4.1 ± 0.1 | 0.539 | |
| Corrected calcium, mg/dL | 9.2 ± 0.8 | 8.6 ± 0.5 | 0.062 | |
| Phosphorus, mg/dL | 4.8 ± 1.5 | 4.9 ± 1.5 | 0.967 | |
| Fasting blood glucose, mg/dL | 143.3 ± 53.0 | 125.4 ± 23.9 | 0.400 | |
| Hemoglobin A1c, % | 6.2 ± 1.0 | 6.1 ± 0.4 | 1.000 | |
| Cr before initiation of HD, mg/dL | 10.5 ± 2.5 | 9.6 ± 1.9 | 0.396 | |
| BUN before initiation of HD, mg/dL | 63.2 ± 19.4 | 65.4 ± 14.3 | 0.647 | |
| Standard Kt/Vurea | 1.6 ± 0.3 | 1.7 ± 0.3 | 0.384 | |
| Uric acid, mg/dL | 5.9 ± 1.3 | 6.0 ± 1.6 | 0.900 | |
| Total bilirubin, mg/dL | 0.7 ± 0.3 | 0.7 ± 0.4 | 0.677 | |
| Alkaline phosphatase, IU/L | 129.0 ± 78.6 | 138.3 ± 51.1 | 0.257 | |
| AST, IU/L | 21.4 ± 7.0 | 25.4 ± 23.3 | 0.418 | |
| ALT, IU/L | 15.6 ± 11.2 | 24.6 ± 35.9 | 0.867 | |
| γ-Glutamyl transferase, IU/L | 31.7 ± 55.7 | 31.1 ± 21.8 | 0.418 | |
| Lactate dehydrogenase, IU/L | 189.1 ± 35.4 | 197.3 ± 26.2 | 0.791 | |
| Triglyceride, mg/dL | 128.9 ± 91.0 | 125.3 ± 79.7 | 0.917 | |
| HDL-cholesterol, mg/dL | 44.2 ± 14.3 | 43.3 ± 19.2 | 0.647 | |
| LDL-cholesterol, mg/dL | 60.7 ± 18.6 | 52.0 ± 8.1 | 0.209 | |
| CRP, mg/dL | 0.6 ± 1.4 | 0.2 ± 0.2 | 0.468 | |
| ESR, mm/h | 31.3 ± 19.0 | 35.5 ± 19.9 | 0.934 | |
| Intact PTH, pg/mL | 452.6 ± 390.4 | 323.9 ± 314.7 | 0.352 | |
| 25-hydroxy vitamin D, ng/mL | 19.5 ± 13.6 | 14.6 ± 4.8 | 0.395 | |
| β2-microglobulin, mg/L | 35.1 ± 7.3 | 34.1 ± 8.2 | 0.851 | |
Results are expressed as mean ± standard deviation or frequencies (and proportions).
ChAdOx1 = ChAdOx1 nCoV-19 adenovirus-based vector vaccine, SBP = systolic blood pressure, DBP = diastolic blood pressure, HD = hemodialysis, ARB = angiotensin II receptor blocker, CCB = calcium channel blocker, DPP4 = dipeptidyl peptidase-4, ESA = erythropoiesis-stimulating agent, Cr = creatinine, BUN = blood urea nitrogen, AST = aspartate aminotransferase, ALT = alanine aminotransferase, HDL = high-density lipoprotein, LDL = low-density lipoprotein, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, PTH = parathyroid hormone.
aDefined as a SARS-CoV-2 neutralizing antibody < 30% inhibition at 28th days after priming vaccination.
bDefined as a SARS-CoV-2 neutralizing antibody ≥ 30% inhibition at 28th days after priming vaccination.
We performed Cox proportional-hazard model, using age and sex as covariates, to find possible clinical predictor of humoral immunogenicity after the first vaccination with ChAdOx1 (Table 3). We found that the vaccine-specific humoral responsiveness was deeply associated with steroid use (adjusted hazard ratio [HR], 0.719; 95% confidence interval [CI], 0.590–0.876), serum corrected calcium concentration (adjusted HR, 0.776; 95% CI, 0.627–0.961), and c-reactive protein level (adjusted HR, 0.933; 95% CI, 0.886–0.981).
Table 3. Cox proportional hazard model for early responsiveness of the priming ChAdOx1 vaccination.
| Variables | Adjustment for age and sex | |||
|---|---|---|---|---|
| HR | 95% CI | P value | ||
| Body mass index, kg/m2 | 1.022 | 0.970–1.076 | 0.413 | |
| SBP at initiation of HD, mmHg | 0.998 | 0.993–1.003 | 0.528 | |
| DBP at initiation of HD, mmHg | 0.999 | 0.990–1.009 | 0.901 | |
| Cause of end-stage renal disease | ||||
| Diabetes mellitus | 0.908 | 0.600–1.375 | 0.649 | |
| Hypertension | 1.052 | 0.682–1.621 | 0.819 | |
| Chronic glomerulonephritis | 0.723 | 0.574–0.911 | 0.006 | |
| Others | 1.579 | 0.824–3.028 | 0.169 | |
| Medical history | ||||
| Vintage, mon | 1.001 | 0.999–1.003 | 0.312 | |
| Charlson comorbidity index | 0.936 | 0.810–1.082 | 0.370 | |
| Cardiovascular disease | 0.943 | 0.620–1.435 | 0.784 | |
| Cerebrovascular disease | 1.082 | 0.442–2.651 | 0.863 | |
| Mycophenolate mofetil user | 0.696 | 0.528–0.917 | 0.010 | |
| Breast cancer | 3.693 | 1.992–6.846 | < 0.001 | |
| Diabetic (vs. non-diabetic) | 0.979 | 0.609–1.572 | 0.929 | |
| Hypertensive (vs. non-hypertensive) | 1.047 | 0.702–1.561 | 0.821 | |
| ARB use (vs. non-use) | 0.869 | 0.593–1.274 | 0.473 | |
| CCB use (vs. non-use) | 1.150 | 0.801–1.652 | 0.448 | |
| β-blocker use (vs. non-use) | 0.907 | 0.649–1.267 | 0.567 | |
| α-blocker use (vs. non-use) | 1.268 | 0.475–3.382 | 0.636 | |
| Sulfonylurea use (vs. non-use) | 0.793 | 0.363–1.732 | 0.561 | |
| DPP-4 inhibitor Use (vs. non-use) | 0.869 | 0.526–1.437 | 0.585 | |
| Statin use (vs. non-use) | 1.212 | 0.854–1.722 | 0.282 | |
| Steroid use (vs. non-use) | 0.719 | 0.590–0.876 | 0.001 | |
| Vitamin D use (vs. non-use) | 0.688 | 0.261–1.811 | 0.449 | |
| ESA use (vs. non-use) | 0.999 | 0.526–1.865 | 0.976 | |
| Laboratory test | ||||
| White blood cell, 109/L | 1.022 | 0.970–1.076 | 0.365 | |
| Hemoglobin, g/dL | 0.866 | 0.600–1.249 | 0.441 | |
| Platelet, 103/μL | 1.002 | 0.998–1.005 | 0.329 | |
| Neutrophil, 109/L | 1.105 | 0.954–1.280 | 0.605 | |
| Lymphocyte, 109/L | 1.113 | 0.723–1.714 | 0.261 | |
| Sodium, mmol/L | 1.044 | 0.968–1.127 | 0.262 | |
| Potassium, mmol/L | 0.763 | 0.453–1.287 | 0.311 | |
| Chloride, mmol/L | 1.012 | 0.949–1.080 | 0.717 | |
| Total CO2, mmol/L | 1.034 | 0.969–1.104 | 0.306 | |
| Total protein, g/dL | 0.984 | 0.774–1.251 | 0.894 | |
| Albumin, g/dL | 1.207 | 0.579–1.824 | 0.927 | |
| Corrected calcium, mg/dL | 0.776 | 0.627–0.961 | 0.020 | |
| Phosphorus, mg/dL | 0.972 | 0.859–1.100 | 0.652 | |
| Fasting blood glucose, mg/dL | 0.999 | 0.996–1.001 | 0.260 | |
| Hemoglobin A1c, % | 0.009 | 0.001–999.9 | 0.846 | |
| Cr before initiation of HD, mg/dL | 0.964 | 0.894–1.041 | 0.350 | |
| BUN before initiation of HD, mg/dL | 1.001 | 0.993–1.009 | 0.795 | |
| Standard Kt/Vurea | 0.924 | 0.409–2.088 | 0.850 | |
| Uric acid, mg/dL | 0.972 | 0.822–1.148 | 0.735 | |
| Total bilirubin, mg/dL | 0.935 | 0.373–2.343 | 0.886 | |
| Alkaline phosphatase, IU/L | 1.000 | 0.998–1.002 | 0.981 | |
| AST, IU/L | 1.009 | 0.986–1.033 | 0.458 | |
| ALT, IU/L | 1.006 | 0.992–1.021 | 0.414 | |
| γ-Glutamyl transferase, IU/L | 1.000 | 0.997–1.002 | 0.707 | |
| Lactate dehydrogenase, IU/L | 1.005 | 0.998–1.011 | 0.170 | |
| Triglyceride, mg/dL | 0.999 | 0.998–1.001 | 0.456 | |
| HDL-cholesterol, mg/dL | 1.003 | 0.986–1.020 | 0.708 | |
| LDL-cholesterol, mg/dL | 0.994 | 0.986–1.002 | 0.133 | |
| C-reactive protein, mg/dL | 0.933 | 0.886–0.981 | 0.007 | |
| ESR, mm/h | 1.001 | 0.977–1.026 | 0.925 | |
| Intact PTH, pg/mL | 1.000 | 0.999–1.001 | 0.347 | |
| 25-hydroxy vitamin D, ng/mL | 0.997 | 0.988–1.006 | 0.522 | |
| β2-microglobulin, mg/L | 0.997 | 0.971–1.021 | 0.718 | |
ChAdOx1 = ChAdOx1 nCoV-19 adenovirus-based vector vaccine, HR = hazard ratio, CI = confidence interval, SBP = systolic blood pressure, DBP = diastolic blood pressure, HD = hemodialysis, ARB = angiotensin II receptor blocker, CCB = calcium channel blocker, DPP4 = dipeptidyl peptidase-4, ESA = erythropoiesis-stimulating agent, Cr = creatinine, BUN = blood urea nitrogen, AST = aspartate aminotransferase, ALT = alanine aminotransferase, HDL = high-density lipoprotein, LDL = low-density lipoprotein, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, PTH = parathyroid hormone.
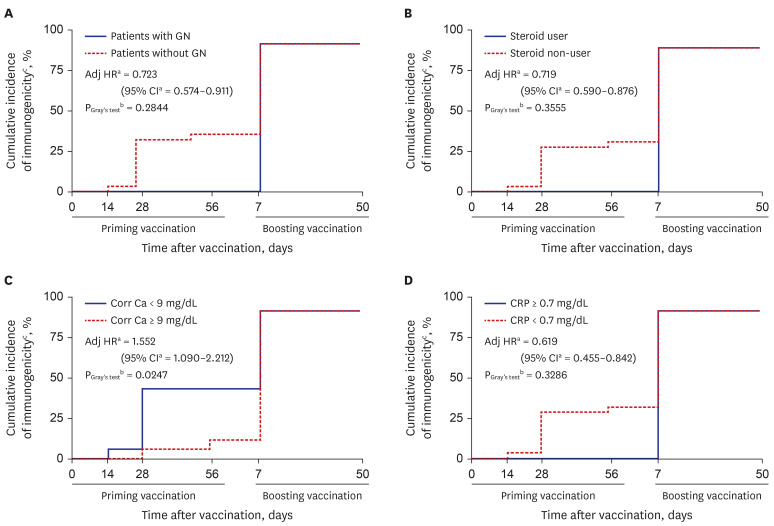
Subsequent cumulative-incidence analyses with multiple Cox-proportional hazard regression analysis and Gray’s test were performed to compare cause-specific cumulative incidence functions between two groups (Fig. 4). Participants with serum corrected calcium concentration < 9.0 mg/dL, C-reactive protein (CRP) < 0.7 mg/dL or steroid non-user had better immunogenicity. However, our result showed that its difference was significant only between participants with corrected calcium concentration < 9.0 and ≥ 9.0 mg/dL (PGray’s test = 0.0247).
Fig. 4. Cumulative incidence function curves of COVID-19 vaccination humoral immunogenicity stratified by (A) patient with GN as underlying cause of hemodialysis, (B) steroid treatment, (C) Corr Ca, and (D) CRP.
COVID-19 = coronavirus disease 2019, GN = glomerulonephritis, Adj HR = adjuster hazard ratio, CI = confidential interval, Corr Ca = corrected serum calcium, CRP = C-reactive protein.
aAdjusted for age and sex; bEvaluate hypotheses of equality of cause-specific cumulative incidence functions between two groups; cDefined as a SARS-CoV-2 nAbs ≥ 30% inhibition at each specific time points.
DISCUSSION
To the best of our knowledge, this is the first prospective study to evaluate the immunogenicity of a mix-and-match COVID-19 vaccination in patients with ESRD on hemodialysis. Given that the titers of S protein-targeting antibodies showed a good correlation with the neutralization capacity, the efficacy of vaccination among patients with ESRD on hemodialysis can be predicted from our study findings.10
There are some studies that have reported on the immunogenicity of BNT162b2 among patients with ESRD on hemodialysis and the seroconversion that occurs in the majority of patients, but the said immunogenicity seems to be lower than in healthy population.11,12 According to a recent cohort study, antibody titers following two doses of BNT162b2 were similar between patients with ESRD on hemodialysis and healthy participants, whereas patients with ESRD on hemodialysis who received ChAdOx1 had a lower neutralizing antibody response compared to that of the healthy controls.13
In fact, there is insufficient data on the mix-and-match strategy in ESRD patients on hemodialysis. A recent study in the UK showed that persons who received a second dose of the Moderna vaccine (mRNA-1273) after an initial ChAdOx1 or BNT162b2 had higher binding and neutralizing antibody responses and no difference in the serious adverse effects compared with persons who received two doses of either ChAdOx1 or BNT162b2.14 A similar result was found in a Swedish study; those who vaccinated mRNA-1273 after initial ChAdOx1 had a higher level of neutralizing antibody compared with those who were vaccinated with two doses of ChAdOx1.15 Based on the results of the previous studies and the result of this study, we suggest that the mRNA vaccine seems to stimulate the SARS-CoV-2-specific memory B-cell that has been generated by initial vaccination with ChAdOx1 more efficiently than ChAdOx1. Also, as shown in our study, the use of the mRNA vaccine as a second dose for ESRD patients on hemodialysis who were vaccinated with initial ChAdOx1 seems to be acceptable. Given that the world is still suffering from the COVID-19 pandemic and that there are still many places where the supply of vaccines is not stable, the introduction of the mix-and-match strategy is worth considering in patients with ESRD on hemodialysis as well as in healthy populations.
Our adjusted Cox proportional hazards models revealed that not only steroid usage, but also CRP level at baseline was deeply associated with early humoral immunogenicity of ChAdOx1 vaccination among ESRD patients on hemodialysis. There is evidence that shows the altered immune function and long-term low-grade systemic inflammation are common features in patients suffering from comorbidities related to metabolic syndrome, and those patients had reduced vaccine efficacy.16 Furthermore, an animal study showed that increased CRP level was deeply related with poor humoral immune system’s response of vaccine.17 Such findings suggested that baseline CRP may predict subclinical inflammation and reduced immunogenicity after vaccination in ESRD patients on hemodialysis.
Our cumulative incidence curve analysis revealed that participants with serum corrected calcium < 9 mg/dL had a better vaccination response at each time point. It is not yet known how serum calcium levels are related to post-vaccination immune responses. However, such finding is consistent with previous observational studies showing that serum corrected calcium concentration was lower in patients with non-severe COVID-19 infection as compared with matched healthy controls and hypocalcemia was a clinical discriminator of severe/critical clinical outcomes from COVID-19 patients.18,19,20,21 Because it is not exactly known how serum calcium levels are related with immune-inflammatory responses following COVID-19 infection or active immunization, there is need of more extensive study on this issue.
There are several potential limitations of the present study. First, an age difference exists between patients with ESRD on hemodialysis and healthy employees. Therefore, the difference in immunogenicity after administration of the first dose of vaccination might be attributable to the age difference between the two groups.22 Second, the subjects were not randomized and a small number of participants were enrolled. In particular, only a small number of patients with ESRD on hemodialysis who were vaccinated with two doses of ChAdOx1 were included. The findings need to be verified on a larger scale and with a randomized design. Third, a direct comparison of responses after the second dose was impossible between healthy people and ESRD patients on hemodialysis. To overcome it, subgroup analysis was analyzed between healthy people and AZ–Pf group or AZ–AZ group. Finally, the durability and persistence of immunity could not be evaluated using the results of the present study.
In conclusion, the immunogenicity of the COVID-19 vaccination in patients with ESRD on hemodialysis using the mix-and-match strategy (ChAdOx1–BNT162b2) is comparable to that in healthy participants using two-doses of ChAdOx1.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Lee CH, Kim B.
- Data curation: Hong S, Park JS, Minn D.
- Formal analysis: Hong S, Park JS.
- Investigation: Park JS, Minn D, Hong S, Kim S, Lee SH, Kim B.
- Methodology: Park JS, Minn D, Lee CH, Kim B.
- Project administration: Kim B.
- Resources: Hong S, Kim S.
- Software: Park JS.
- Supervision: Lee CH, Kim B.
- Validation: Park JS, Kim B.
- Visualization: Park JS.
- Writing - original draft: Park JS, Kim B.
- Writing - review & editing: Kim B.
SUPPLEMENTARY MATERIALS
The schedule of sample collection.
Comparison of immunogenicity of COVID-19 vaccination between healthy people and patients with ESRD on hemodialysis.
References
- 1.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, Elbaz M, Nesher L, Stein M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021;27(11):1652–1657. doi: 10.1016/j.cmi.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinits-Pensy M, Forrest GN, Cross AS, Hise MK. The use of vaccines in adult patients with renal disease. Am J Kidney Dis. 2005;46(6):997–1011. doi: 10.1053/j.ajkd.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 7.Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JS, Kim GH, Kang CM, Lee CH. Application of cystatin C reduction ratio to high-flux hemodialysis as an alternative indicator of the clearance of middle molecules. Korean J Intern Med. 2010;25(1):77–81. doi: 10.3904/kjim.2010.25.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Zhang X, Xie X, Yang X, Liu H, Tang R, et al. Risk factors for calciphylaxis in Chinese hemodialysis patients: a matched case-control study. Ren Fail. 2021;43(1):406–416. doi: 10.1080/0886022X.2021.1884094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 11.Espi M, Charmetant X, Barba T, Koppe L, Pelletier C, Kalbacher E, et al. The ROMANOV study found impaired humoral and cellular immune responses to SARS-CoV-2 mRNA vaccine in virus-unexposed patients receiving maintenance hemodialysis. Kidney Int. 2021;100(4):928–936. doi: 10.1016/j.kint.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanay NB, Freiman S, Shapira M, Wishahi S, Hamze M, Elhaj M, et al. Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int. 2021;99(6):1496–1498. doi: 10.1016/j.kint.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr EJ, Wu M, Harvey R, Wall EC, Kelly G, Hussain S, et al. Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet. 2021;398(10305):1038–1041. doi: 10.1016/S0140-6736(21)01854-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuart ASV, Shaw RH, Liu X, Greenland M, Aley PK, Andrews NJ, et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022;399(10319):36–49. doi: 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Normark J, Vikström L, Gwon YD, Persson IL, Edin A, Björsell T, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N Engl J Med. 2021;385(11):1049–1051. doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan Z, Yang M, Lai CL. COVID-19 vaccinations: a comprehensive review of their safety and efficacy in special populations. Vaccines (Basel) 2021;9(10):1097. doi: 10.3390/vaccines9101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romiszewski P, Kostro K, Lisiecka U. Effects of subclinical inflammation on C-reactive protein and haptoglobin levels as well as specific humoral immunity in dogs vaccinated against canine distemper and parvovirus. BMC Vet Res. 2018;14(1):70. doi: 10.1186/s12917-018-1383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal R, Ram S, Zohmangaihi D, Biswas I, Suri V, Yaddanapudi LN, et al. High prevalence of hypocalcemia in non-severe COVID-19 patients: a retrospective case-control study. Front Med (Lausanne) 2021;7:590805. doi: 10.3389/fmed.2020.590805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alemzadeh E, Alemzadeh E, Ziaee M, Abedi A, Salehiniya H. The effect of low serum calcium level on the severity and mortality of Covid patients: a systematic review and meta-analysis. Immun Inflamm Dis. 2021;9(4):1219–1228. doi: 10.1002/iid3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Chen D, Wang L, Zhao Y, Wei L, Chen Z, et al. Low serum calcium: a new, important indicator of COVID-19 patients from mild/moderate to severe/critical. Biosci Rep. 2020;40(12):BSR20202690. doi: 10.1042/BSR20202690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Filippo L, Formenti AM, Rovere-Querini P, Carlucci M, Conte C, Ciceri F, et al. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine. 2020;68(3):475–478. doi: 10.1007/s12020-020-02383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu Jabal K, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6):2100096. doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The schedule of sample collection.
Comparison of immunogenicity of COVID-19 vaccination between healthy people and patients with ESRD on hemodialysis.