Dear Sir:
Cerebral small vessel disease (SVD) is an important cause of stroke and dementia and is associated with a progressive, but highly unpredictable clinical course and increased mortality [1]. Serum neurofilament light chain (sNfL) has shown to be a candidate marker of various neurological diseases, including cerebral SVD [2]. sNfL is a component of the neuroaxonal cytoskeleton, which plays a critical role in neuroaxonal stability and electrical conductivity. We previously found that sNfL was associated with both severity and progression of SVD [3,4]; however, it is unknown whether sNfL may also serve as an easy measurable predictor of a more unfavorable outcome in SVD, e.g., mortality. In this study, we investigated whether baseline sNfL is associated with a 14-year risk of all-cause mortality, as well as mortality related to vascular events or dementia in SVD patients. We included 503 participants with symptomatic sporadic SVD from the prospective Radboud University Nijmegen Diffusion tensor and Magnetic resonance imaging Cohort (RUN DMC) cohort study [5].
All participants underwent magnetic resonance imaging (MRI), and neuropsychological testing at baseline and collection of blood in 2006. sNfL was measured at an experienced center using ultrasensitive single-molecule array assay and was age-normalized using samples from 485 healthy controls [6], to adjust for the exponential association between age and sNfL [7]. SVD-markers on MRI were rated according to the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) criteria [8].
Participants were followed up until their death or December 9, 2020. Information on vital status was retrieved from the Dutch Municipal Personal Records database. Information on the cause of mortality was obtained from medical records from general practitioners or treating physicians at time of death. We classified cause of death based on the classification scheme of the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10).
Predictors of mortality were identified via multivariable Cox regression models by performing a penalized lasso regression for variable selection, by using the R package ‘glmnet’ (R Foundation for Statistical Computing, Vienna, Austria). A quadratic term was added to age, since mortality-risk increases exponentially with age. Causes of death other than the studied group-cause of mortality (e.g., vascular death, dementia, etc.) were considered a competing risk, by using the proportional hazards model of Fine and Gray. Sensitivity analyses were done to identify predictors associated with vascular and dementia related deaths.
Out of 503 patients (mean±standard deviation [SD] age, 65.7±8.8 years), 182 (36.3%) died during a median follow-up of 14.0 years (interquartile range [IQR], 10.9 to 14.4). Two patients were lost to follow-up due to emigration after 13.2 and 8.8 years and were censored. Cause of death was retrieved in 170 (93.4%) of the 182 deceased patients. Among deceased patients, vascular cause of death was reported in 60 (33.0%) patients, whereas dementia-related deaths occurred in 30 (16.5%) individuals. Deceased patients were older, showed lower cognitive performance and had higher SVD-burden (median WMH volume: deceased 8.93 mL [IQR, 3.3 to 17.4]; survivors 2.44 mL [IQR, 0.8 to 7.1]), and lower brain volumes on MRI at baseline. Median sNfL of the total population was 53.4 pg/mL (IQR, 38.1 to 77.1), with survivors exhibiting a median sNfL of 44.3 pg/mL (IQR, 32.7 to 62.0) and deceased patients a median sNfL of 76.0 pg/mL (IQR, 54.7 to 119.2) (Figure 1).
Figure 1.
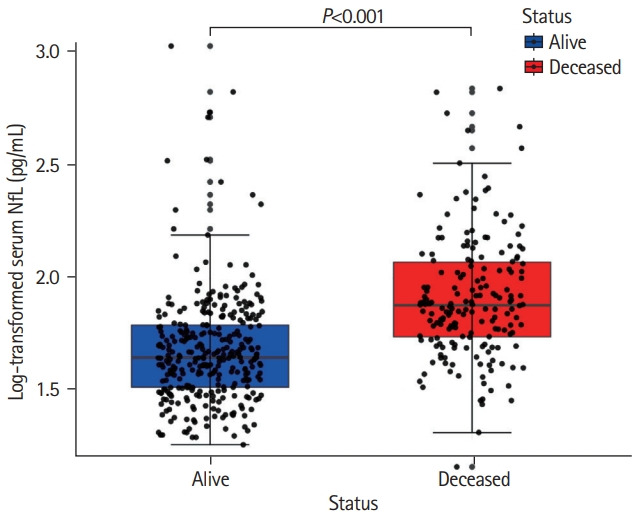
Distribution of serum neurofilament light chain (sNfL) between survivors and deceased patients. Deceased patients (in red) exhibiting higher age-normalized sNfL levels at baseline compared to survivors (in blue) after a 14-year follow-up. Data represent individual measures of both groups including 1.5 interquartile range error bars.
The 14-year survival probability was lower in the high sNfL group compared to the low sNfL group (dichotomization was based on the median of sNfL; log-rank test P<0.001) (Figure 2). Elevated sNfL level at baseline was independently associated with an increased risk of all-cause mortality, after adjusting for age, vascular comorbidity, cognition, and MRI-markers of SVD (hazard ratio [HR], 1.23 per 1-SD increase; 95% confidence interval [CI], 1.08 to 1.42; P=0.003) (Table 1). The following variables were also independently associated with allcause mortality: age-squared (HR, 2.03 per 1-SD increase; 95% CI, 1.63 to 2.53; P<0.001), cognitive index (HR, 0.70 per 1-SD increase; 95% CI, 0.58 to 0.85; P<0.001), ever-smoking (HR, 1.63; 95% CI, 1.13 to 2.37; P=0.01), and white matter volume (HR, 0.84 per 1-SD increase; 95% CI, 0.70 to 0.99; P=0.04) (Table 1). Sensitivity analyses showed that sNfL was associated with vascular death (HR, 1.28 per 1-SD increase; 95% CI, 1.02 to 1.63; P=0.04); however, it was not associated with mortality related to dementia (HR, 1.24 per 1-SD increase; 95% CI, 0.90 to 1.73; P=0.19).
Figure 2.
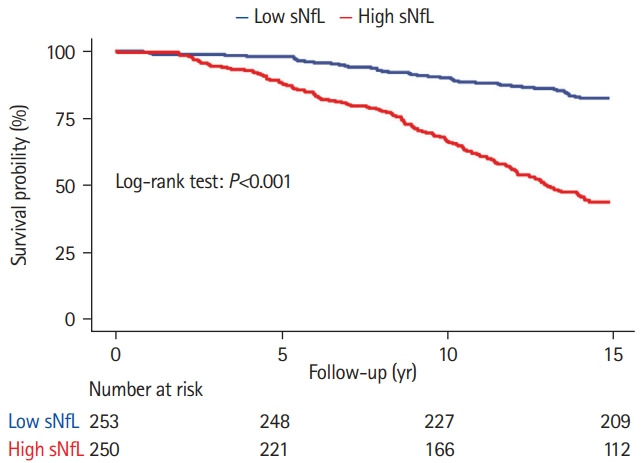
Survival probability between patients with low and high serum neurofilament light chain (sNfL). Differences in survival probability between patients with low and high sNfL at baseline. Patients were categorized based on the median sNfL of total group.
Table 1.
Variables selected in the multivariable cox regression model for prediction of all-cause mortality
| Variable | HR (95% CI) | P |
|---|---|---|
| sNfL (pg/mL) per 1-SD increase*,† | 1.233 (1.084–1.423) | 0.003 |
| Demographics | ||
| Age squared per 1-SD increase | 2.031 (1.632–2.531) | <0.001 |
| Sex | NS | NS |
| Cognition | ||
| Cognitive index | 0.704 (0.581–0.853) | <0.001 |
| Vascular risk factors | ||
| Hypertension | NS | NS |
| Diabetes | 1.263 (0.870–1.832) | 0.231 |
| Smoking | 1.630 (1.133–2.371) | 0.012 |
| Comorbidity | ||
| Cardiovascular events | 1.192 (0.854–1.671) | 0.314 |
| MRI characteristics | ||
| WMH (mL) per 1-SD increase* | NS | NS |
| GMV (mL) per 1-SD increase | 0.871 (0.720–1.064) | 0.180 |
| WMV (mL) per 1-SD increase | 0.840 (0.702–0.991) | 0.041 |
| MD white matter 10-3 mm2/sec per 1-SD increase‡ | 1.052 (0.943–1.170) | 0.382 |
| Presence of microbleeds | NS | NS |
| Presence of lacunes | 1.243 (0.881–1.752) | 0.224 |
Variables selected after applying lasso regression.
HR, hazard ratio; CI, confidence interval; sNfL, serum neurofilament light chain; SD, standard deviation; NS, not selected (by lasso regression); MRI, magnetic resonance imaging; WMH, white matter hyperintensities; GMV, grey matter volume; WMV, white matter volume; MD, mean diffusivity.
Log-transformed;
Age-normalized;
Three patients excluded because of insufficient scan quality.
In this study, we found that baseline sNfL is associated with a 14-year risk of mortality in SVD patients, independent of age, cognition, vascular comorbidity, and SVD-burden. Previously, sNfL was shown to be associated with progression of both, imaging and cognitive measures in SVD [3,9-11]. Our study extends these findings by showing association with mortality in SVD patients.
Our observed association between sNfL and vascular death shows that sNfL is also an important indicator for neuroaxonal damage from cerebrovascular diseases, probably resulting from (systemic) vascular diseases’ activity. It also underscores the importance of taking SVD into account when interpreting sNfL levels in the context of other neurodegenerative diseases, also because of the high prevalence of SVD in older individuals. However, our results also showed that sNfL is not only an independent predictor for vascular mortality, but also for allcause mortality (in contrast to MRI-markers of SVD). This finding indicates that sNfL levels not only capture SVD burden, but also presence of ongoing neurodegenerative processes that are associated with increased death. sNfL may thus be of value for clinical research and has the potential to serve as a marker for vulnerability and higher risk of poor outcomes and implement a more rigorous clinical management. The sensitivity analysis on vascular and dementia-related mortality should be interpreted with caution owing to limited statistical power given the low numbers in each group.
A strength of this study is the use of data from a large and well-phenotyped cohort of participants with sporadic SVD, with a comprehensive and standardized clinical and neuroimaging work-up. Furthermore, the longitudinal design provides insights in the long-term causes of mortality of SVD, with—to the best of our knowledge—the longest follow-up period in SVD patients and with only two subjects lost to follow-up. Serum NfL may thus serve as an easily obtainable and measurable predictive marker in SVD, potentially for both scientific investigations as well as clinical practice.
This study was conducted according to the principles of the Declaration of Helsinki (version 60, 19 October 2013) and the Dutch law for human research (WMO). Written informed consent was obtained from all participants. Ethical approval was obtained from the Medical Review Ethics Committee region Arnhem-Nijmegen.
Acknowledgments
This study was supported by Dutch Brain Foundation personal fellowship H04- 12;F2009(1)-16 (Prof. Dr. Frank-Erik de Leeuw) and by VIDI innovational grant 016.126.351 from The Netherlands Organization for Scientific Research (Dr. Frank-Erik de Leeuw). Anil M. Tuladhar is a junior staff member of the Dutch Heart Foundation (grant number 2016T044). Anil M. Tuladhar is supported by the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2018-28 & 2012-06 Heart Brain Connection), Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development, and the Royal Netherlands Academy of Sciences. Mengfei Cai is supported by China Scholarship Council (201706100189). Furthermore, this study was supported by the Neurology Research Pool, University Hospital Basel.
Footnotes
Disclosure
The authors have no financial conflicts of interest.
References
- 1.Ikram MA, Vernooij MW, Vrooman HA, Hofman A, Breteler MM. Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiol Aging. 2009;30:450–456. doi: 10.1016/j.neurobiolaging.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron. 2016;91:56–66. doi: 10.1016/j.neuron.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Peters N, van Leijsen E, Tuladhar AM, Barro C, Konieczny MJ, Ewers M, et al. Serum neurofilament light chain is associated with incident lacunes in progressive cerebral small vessel disease. J Stroke. 2020;22:369–376. doi: 10.5853/jos.2019.02845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duering M, Konieczny MJ, Tiedt S, Baykara E, Tuladhar AM, Leijsen EV, et al. Serum neurofilament light chain levels are related to small vessel disease burden. J Stroke. 2018;20:228–238. doi: 10.5853/jos.2017.02565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Norden AG, de Laat KF, Gons RA, van Uden IW, van Dijk EJ, van Oudheusden LJ, et al. Causes and consequences of cerebral small vessel disease. The RUN DMC study: a prospective cohort study. Study rationale and protocol. BMC Neurol. 2011;11:29. doi: 10.1186/1471-2377-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baranzini SE, Wang J, Gibson RA, Galwey N, Naegelin Y, Barkhof F, et al. Genome-wide association analysis of susceptibility and clinical phenotype in multiple sclerosis. Hum Mol Genet. 2009;18:767–778. doi: 10.1093/hmg/ddn388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalil M, Pirpamer L, Hofer E, Voortman MM, Barro C, Leppert D, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11:812. doi: 10.1038/s41467-020-14612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egle M, Loubiere L, Maceski A, Kuhle J, Peters N, Markus HS. Neurofilament light chain predicts future dementia risk in cerebral small vessel disease. J Neurol Neurosurg Psychiatry. 2021;92:582–589. doi: 10.1136/jnnp-2020-325681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattringer T, Pinter D, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology. 2017;89:2108–2114. doi: 10.1212/WNL.0000000000004645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu Y, Tan CC, Shen XN, Li HQ, Cui M, Tan L, et al. Association of plasma neurofilament light with small vessel disease burden in nondemented elderly: a longitudinal study. Stroke. 2021;52:896–904. doi: 10.1161/STROKEAHA.120.030302. [DOI] [PubMed] [Google Scholar]


