Dear Sir:
Cancer can cause arterial and venous thrombosis, and hypercoagulability has been suggested as the main cause of cancer-associated venous thrombosis. As arterial thrombosis may also be associated with hypercoagulability, guidelines recommend anticoagulation to prevent arterial thrombosis in patients with cancer [1]. However, histological studies of fresh thrombi retrieved from the cerebral arteries of acute stroke patients with cancer during mechanical thrombectomy indicate that thrombi are platelet-rich [2,3]. Moreover, it remains uncertain whether hypercoagulability and platelet activation contribute differently to venous and arterial thrombosis or whether it is a patient-specific feature.
We report the histological findings of fresh thrombi obtained from a patient with cancer who developed four stroke events and deep vein thrombosis. This study was approved by the Institutional Review Board of Yonsei University Health System (No. 2017-0904-001). The patient provided informed consent for the use of thrombi for research. A 73-year-old woman with metastatic gastric cancer presented with aphasia and rightside weakness to the emergency department. Computed tomographic angiography revealed occlusion of the left middle cerebral artery (MCA). The artery was successfully recanalized by mechanical thrombectomy. On day 7, the patient developed recurrent stroke with right MCA occlusion and bilateral renal infarctions. The occluded MCA was recanalized by mechanical thrombectomy. On day 9, she experienced pain in her left leg with edema. Computed tomographic angiography showed left common iliac vein occlusion. The vein was recanalized by mechanical thrombectomy and stenting. On days 17 and 18, she developed occlusions of the right MCA and left MCA, respectively, which were recanalized by mechanical thrombectomy (Figure 1). At every stroke or venous thrombosis event, the patient was administered anticoagulants (enoxaparin, apixaban, or dabigatran) or antiplatelet agents (aspirin and clopidogrel) or both.
Figure 1.
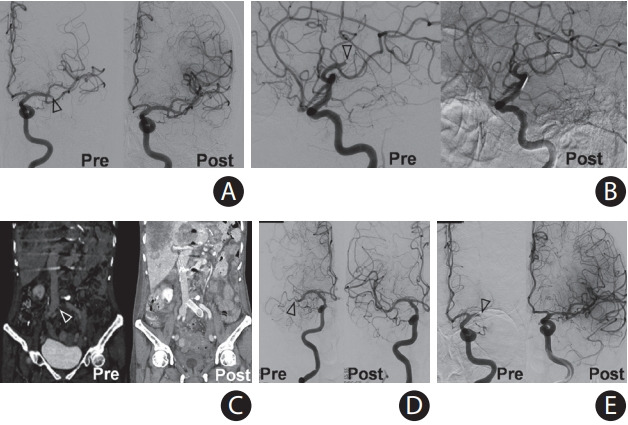
Angiography images acquired before and after mechanical thrombectomy at (A) first stroke, (B) second stroke, (C) deep vein thrombosis, (D) third stroke, and (E) fourth stroke. Occlusion in the middle cerebral arteries was successfully recanalized in all cases. Occlusion of the iliac vein was successfully recanalized with thrombectomy and stenting. Empty arrow heads indicate the site of occlusion.
The patient had no vascular risk factors and no evidence of atherosclerosis. On day 3, transesophageal echocardiography revealed a small patent foramen ovale (PFO), while transthoracic echocardiography was unremarkable. Follow-up transthoracic echocardiography on day 23 showed moderate mitral regurgitation due to thickened mitral valve and prolaptic motion of the anteromedial valve.
Thrombi were obtained during each thrombectomy. All thrombi from the cerebral arteries were grossly white, while those from the common iliac vein were red (Figure 2 and Supplementary Figure 1). Immunohistochemistry was performed using anti-CD42b (ab134087, 1:100, Abcam, Cambridge, UK) for platelets, anti-fibrinogen (ab34269, 1:200, Abcam) for fibrin/fibrinogen, and anti-glycophorin A (ab129024, 1:400, Abcam) for erythrocytes. Briefly, 4-µm-thick tissue sections were subjected to antigen retrieval using the IHC-Tek epitope retrieval solution and a steamer (IHC World, Woodstock, MD, USA), except for anti-CD42b. Sections were incubated overnight with primary antibodies at 4°C. After the secondary antibody reaction with an avidin-biotin-horseradish peroxidase complex (Vector Laboratories Ltd., Peterborough, UK), the color of the positive signals was developed by incubating the slides with a 3,3'-diaminobenzidine solution. After counterstaining with hematoxylin, the slides were mounted with Permount Mounting Medium (Fisher Scientific, Fair Lawn, NJ, USA). Details of the imaging analysis is discussed in Supplementary Methods. All four arterial thrombi had high platelet (55.2% to 72.6%) and fibrin (32.8% to 49.7%) fractions and very low erythrocyte fractions (0% to 0.2%). However, the venous thrombus had high erythrocyte (50.2%) and fibrin (49.2%) fractions and a very low platelet fraction (3.0%) (Figure 2 and Supplementary Figure 1). All arterial thrombi obtained from four separate stroke events were platelet-rich and erythrocyte-poor, while the venous thrombi were erythrocyte-rich and platelet-poor.
Figure 2.
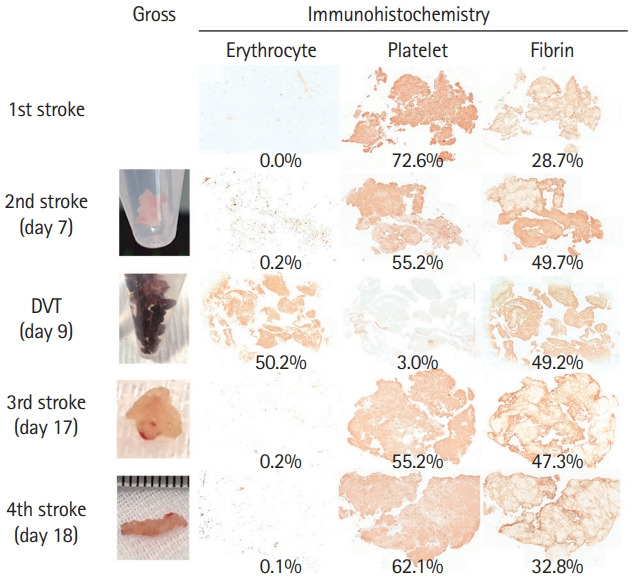
Gross and immunohistochemical findings of the arterial and venous thrombi. Thrombi from the cerebral arteries are white, while those from the vein are red. Arterial thrombi show the highest fraction of platelet, followed by fibrin. Few red blood cells are seen in the arterial thrombi. Venous thrombus shows high fractions of red blood cells and fibrin, and very low platelet fraction. Representative larger immunohistochemistry images are available in Supplementary Figure 1. The method of virtual slice acquisition and imaging analysis is discussed in Supplementary Methods.
No obvious etiology of the stroke was found in this patient. Cryptogenic stroke is common in patients with systemic cancer in that the mechanism was cryptogenic in approximately 50% of stroke patients with active cancer [4]. Common possible underlying mechanisms include cerebral intravascular coagulation, nonbacterial thrombotic endocarditis (NBTE), and paradoxical embolism [5]. PFO is a common cardiac abnormality found in approximately 25% of the general population and 45% of the patients with cryptogenic stroke [6]. In this case, the PFO was detected on echocardiography, but paradoxical embolism from pre-existing venous thrombi is unlikely because histological findings of the arterial thrombi were different from those of venous thrombi in this patient. Our findings indicate that the presence of PFO and coexisting venous thrombosis in cancer patients with cryptogenic stroke does not necessarily indicate that paradoxical embolism from venous thrombosis is the cause of stroke.
Although definite vegetation was not detected by echocardiography, we surmise that recurrent arterial thromboembolism in our patient may be associated with NBTE because of (1) mitral valve abnormalities on echocardiography; (2) similar histological features of the arterial thrombi to those previously reported in NBTE [2]; (3) recurrent stroke common in patients with NBTE [7]; and (4) frequent detection of NBTE on autopsy which was undetected on echocardiography.
Our findings indicate that arterial and venous thrombi pathology differs, even within the same patient. Tumor cells activate platelets and induce tumor platelet aggregates [8]. Platelets are essential for the survival of tumor cells in circulation and metastasis, and those around tumor cells form a physical shield, protecting tumor cells from recognition and lysis by natural killer cells and shear-induced damage.
Platelets also facilitate tumor arrest at the endothelium [9]. It is uncertain why platelet-rich thrombi (vegetation) are formed at the cardiac valve in cancer. However, a high shear stress activates the von Willebrand factor, which once activated interacts with platelets and mediates their adhesion to the endothelium. As blood flow is very fast across the cardiac valve leaflets, tumor platelet aggregates or tumor cell-activated platelets might more easily arrest the cardiac valve and form platelet-rich cardiac vegetation, explaining the exclusive development of NBTE in patients with metastatic cancer [7]. Tumor cells express high levels of tissue factor and promote thrombin generation [10], which may further enhance platelet and fibrin-rich cardiac vegetation. In the venous system where blood flow is slow, excessive thrombin generation and fibrin clot formation can lead to erythrocyte-and fibrin-rich thrombi (Supplementary Figure 2).
Arterial thrombosis frequently recurred in this patient despite treatment with various anticoagulants or antiplatelet agents, or both. Recurrent stroke is prevalent in patients with NBTE, 50% of patients within a few months after the initial event [7]. Thus, different therapeutic strategies may be necessary for effective prevention. Large randomized trials are required to determine the optimal preventive strategy in patients with cancer-associated stroke. Inhibition of both platelet aggregation and coagulation cascade may be considered for selected cancer patients with coexisting arterial and venous thrombosis.
Acknowledgments
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education (NRF-2021R1A2C2003658).
Footnotes
Disclosure
The authors have no financial conflicts of interest.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2021.04140.
A representative immunohistochemistry of arterial and venous thrombi. DVT, deep vein thrombosis.
A diagram showing the mechanism of arterial and venous thrombosis in cancer. TF, tissue factor; vWF, von Willebrand factor.
References
- 1.Salem DN, Stein PD, Al-Ahmad A, Bussey HI, Horstkotte D, Miller N, et al. Antithrombotic therapy in valvular heart disease: native and prosthetic. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):457S–482S. doi: 10.1378/chest.126.3_suppl.457S. [DOI] [PubMed] [Google Scholar]
- 2.Park H, Kim J, Ha J, Hwang IG, Song TJ, Yoo J, et al. Histological features of intracranial thrombi in stroke patients with cancer. Ann Neurol. 2019;86:143–149. doi: 10.1002/ana.25495. [DOI] [PubMed] [Google Scholar]
- 3.Heo JH, Nam HS, Kim YD, Choi JK, Kim BM, Kim DJ, et al. Pathophysiologic and therapeutic perspectives based on thrombus histology in stroke. J Stroke. 2020;22:64–75. doi: 10.5853/jos.2019.03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navi BB, Singer S, Merkler AE, Cheng NT, Stone JB, Kamel H, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology. 2014;83:26–33. doi: 10.1212/WNL.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bang OY, Chung JW, Lee MJ, Seo WK, Kim GM, Ahn MJ, et al. Cancer-related stroke: an emerging subtype of ischemic stroke with unique pathomechanisms. J Stroke. 2020;22:1–10. doi: 10.5853/jos.2019.02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koutroulou I, Tsivgoulis G, Tsalikakis D, Karacostas D, Grigoriadis N, Karapanayiotides T. Epidemiology of patent foramen ovale in general population and in stroke patients: a narrative review. Front Neurol. 2020;11:281. doi: 10.3389/fneur.2020.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo J, Choi JK, Kim YD, Nam HS, Park H, Lee HS, et al. Outcome of stroke patients with cancer and nonbacterial thrombotic endocarditis. J Stroke. 2020;22:245–253. doi: 10.5853/jos.2020.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet–cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 2004;143:819–826. doi: 10.1038/sj.bjp.0706013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu XR, Yousef GM, Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood. 2018;131:1777–1789. doi: 10.1182/blood-2017-05-743187. [DOI] [PubMed] [Google Scholar]
- 10.Hisada Y, Mackman N. Tissue factor and cancer: regulation, tumor growth, and metastasis. Semin Thromb Hemost. 2019;45:385–395. doi: 10.1055/s-0039-1687894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A representative immunohistochemistry of arterial and venous thrombi. DVT, deep vein thrombosis.
A diagram showing the mechanism of arterial and venous thrombosis in cancer. TF, tissue factor; vWF, von Willebrand factor.


