Abstract
Background and Purpose
We investigated the impact of stroke etiology on the endovascular treatment (EVT) procedure and clinical outcome of posterior circulation stroke (PCS) patients with EVT compared to anterior circulation stroke (ACS) patients.
Methods
We retrospectively analyzed ischemic stroke patients who underwent EVT between January 2012 and December 2020. Enrolled ACS and PCS patients were compared according to etiologies (intracranial arterial steno-occlusion [ICAS-O], artery-to-artery embolic occlusion [AT-O], and cardioembolic occlusion [CA-O]). EVT procedure and favorable clinical outcomes at 3 months (modified Rankin Scale 0–2) were compared between the ACS and PCS groups for each etiology.
Results
We included 419 patients (ACS, 346; PCS, 73) including 88 ICAS-O (ACS, 67; PCS, 21), 66 AT-O (ACS, 50; PCS, 16), and 265 CA-O (ACS, 229; PCS, 36) patients in the study. The onset-to-recanalization time was longer in the PCS group than in the ACS group (median 628.0 minutes vs. 421.0 minutes, P=0.01). In CA-O patients, the door-to-puncture time was longer, whereas the puncture-to-recanalization time was shorter in the PCS group than in the ACS group. The proportions of successful recanalization and favorable clinical outcomes were similar between the ACS and PCS groups for all three etiologies. Low baseline National Institutes of Health Stroke Scale (NIHSS) scores and absence of intracerebral hemorrhage at follow-up imaging were associated with favorable clinical outcomes in both groups, whereas successful recanalization (odds ratio, 11.74; 95% confidence interval, 2.60 to 52.94; P=0.001) was only associated in the ACS group.
Conclusions
The proportions of successful recanalization and favorable clinical outcomes were similar among all three etiologies between PCS and ACS patients who underwent EVT. Initial baseline NIHSS score and absence of hemorrhagic transformation were related to favorable outcomes in the PCS and ACS groups, whereas successful recanalization was related to favorable outcomes only in the ACS group.
Keywords: Ischemic stroke, Endovascular treatment, Etiology, Brain infarction
Introduction
Endovascular treatment (EVT) is now a well-established management strategy for patients with anterior circulation stroke (ACS) associated with large vessel occlusion (LVO) [1,2]. In contrast, the benefit of EVT for posterior circulation stroke (PCS) patients remains unclear. A large multicenter prospective registry [3] and recent randomized-controlled studies [4,5] did not clearly show the superiority of EVT over medical treatment. In clinical practice, however, EVT is occasionally performed in PCS patients based on observational studies that reported the benefits of EVT [6,7].
For PCS patients, the investigation of clinical characteristics and prognostic factors related to favorable clinical outcomes is important because careful patient selection is required. Predictors of prognosis [8], including the effects of etiology [9-11] have been investigated in ACS patients who underwent EVT. However, although previous studies explored prognostic factors after EVT in PCS patients [12-14], the effects of etiology on clinical outcomes remain uncertain [15-17] and whether the effect is similar or different from ACS counterparts is unknown.
In this study, we investigated differences in clinical outcomes according to etiology in PCS patients who received EVT in comparison with ACS patients. We also compared factors related to favorable outcomes in PCS and ACS patients.
Methods
Patient selection and grouping
Consecutive patients with acute ischemic stroke (AIS) who underwent EVT between January 2012 and December 2020 at the Asan Medical Center, Seoul, Korea, were prospectively registered. Patients were excluded if (1) they had uncommon etiologies (e.g., arterial dissection, Moyamoya disease, or vasculitis); (2) they had two or more possible etiologies; and (3) their modified Rankin Score (mRS) at 3 months was unobtainable. Enrolled patients were classified as PCS when relevant LVO was found in the vertebral artery (VA), basilar artery (BA), or posterior cerebral artery (PCA).
Patients’ baseline demographic and clinical characteristics, procedural times, National Institutes of Health Stroke Scale (NIHSS) scores, and imaging parameters were obtained from the registry. Stroke etiologies were categorized into intracranial arterial steno-occlusion (ICAS-O), artery-to-artery embolic occlusion (AT-O), and cardioembolic occlusion (CA-O) [18].
ICAS-O was defined when: (1) residual stenosis >70% in the target artery after thrombectomy; (2) moderate stenosis with flow and perfusion impairment on angiography or an evident tendency toward reocclusion; and (3) no source of embolism (e.g., significant proximal arterial stenosis or cardiac disease). Warfarin–Aspirin Symptomatic Intracranial Disease criteria were used to measure the degree of stenosis. AT-O was defined as follows: (1) >50% stenosis in the proximal artery (VA [V1–3 segment], BA, internal carotid artery, common carotid artery [up to the levels of C1], or severe atherosclerosis of the aorta, defined as the presence of atheroma at the aortic arch more than 4 mm in size, detected by transesophageal echocardiography or computed tomography [CT] angiography) associated with relevant, distal artery occlusion associated with relevant, distal artery occlusion, and (2) absence of a cardioembolic source. CA-O was defined when the cardiac disease of medium to high risk of stroke was present, defined by the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification [19].
The occlusion site was further classified as: extracranial VA (from the origin of the VA to the end of the V3 segment), intracranial VA (from the V4 segment to just before the vertebrobasilar junction), proximal BA (from the level of the vertebrobasilar junction to the anterior inferior cerebellar artery [AICA]), middle BA (from the AICA to the superior cerebellar artery [SCA]), and distal BA (distal to the SCA) [20].
This study was approved by the Institutional Review Board of the Asan Medical Center (IRB number: 2021-1302) and written informed consent was exempted due to the retrospective nature of the study.
Endovascular treatment
In our institute, both CT and magnetic resonance imaging (MRI) facilities are located adjacent to the emergency department for rapid imaging evaluation of stroke patients. All patients with suspected AIS first underwent CT. When hemorrhage was not observed, intravenous tissue plasminogen activator was administered if it could be given ≤4.5 hours after symptom onset. In the meantime, multi-modal MRI was performed, which included diffusion-weighted, perfusion-weighted, fluid-attenuated inversion recovery, and gradient-echo imaging along with magnetic resonance angiography (MRA). When magnetic resonance examination was not possible for any reason (e.g., implantable cardiac defibrillator, head and neck metal implants), CT perfusion and CT angiography were performed instead.
When LVO with a diffusion-perfusion mismatch, according to visual inspection, or diffusion-clinical mismatch was found, the eligibility of EVT was discussed among attending neurologists and neurointerventionists. Although the time widow for EVT is considered to be <24 hours after symptom onset [2], we occasionally performed EVT at ≥24 hours from symptom onset in selected patients if consensus was met. Two experienced neurointerventionists (D.C.S. and D.H.L.) performed EVTs as described elsewhere [21].
The choice of EVT techniques including direct stenting and/or balloon angioplasty, mechanical disruption, direct aspiration, and a stent retriever was at the discretion of the neurointerventionist. In cases of tandem lesions in AT-O patients, the attending neurointerventionist decided which lesion was to be addressed first (e.g., proximal-to-distal or distal-to-proximal).
Evaluation of clinical and angiographic outcomes
We measured times from symptom onset-to-door, door-togroin, puncture-to-recanalization, and onset-to-recanalization. Successful recanalization was defined as modified thrombolysis in cerebral infarction (mTICI) 2b–3. Reperfusion time was defined as the point of time at which successful recanalization was achieved. We calculated the onset-to-recanalization time and the puncture-to-recanalization time in patients in whom successful recanalization was achieved. At 24 to 48 hours post-procedure, follow-up imagings were obtained by MRI/MRA or CT/CT angiography to assess the hemorrhagic transformation and patency of recanalized vessels.
We used a method described by the European Cooperative Acute Stroke Study I trial for the classification of intracerebral hemorrhage (ICH) [22]. Symptomatic hemorrhage was defined as an increase of 4 points or more in the NIHSS score or an increase of 2 points or more in at least one of the 11 subcategories of NIHSS [23]. mRS at 3 months was obtained by structured telephone interview or at the outpatient clinic by a trained nurse clinical specialist. An mRS score ≤2 was regarded as a favorable clinical outcome.
Statistical analysis
Categorical variables were evaluated using Pearson’s chi-square or Fisher’s exact test as appropriate. Mann-Whitney U test was applied when comparing continuous variables, such as demographic variables, the NIHSS score, laboratory parameters, and procedural time. The Kruskal-Wallis test was used for three-group comparisons, and the Mann-Whitney U test was used for two-group comparisons. We performed multivariable binary logistic regression analysis to find independent factors affecting clinical outcomes. Included variables had a P<0.05 on the preceding univariable analysis. A two-sided P value of 0.05 was used to define statistical significance. Statistical analyses were performed using SPSS version 26.0 (IBM Co., Armonk, NY, USA).
Results
Baseline characteristics and clinical outcomes of the ACS and PCS patients
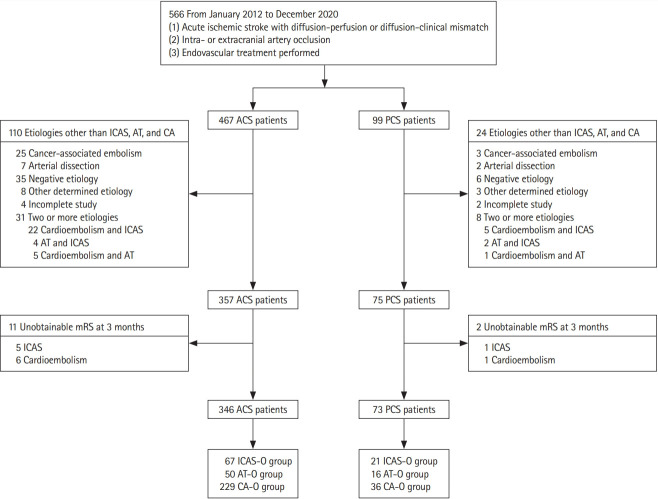
We evaluated 566 consecutive patients with AIS who underwent EVT between January 2012 and December 2020. After excluding 147 patients according to the exclusion criteria, there were 346 (82.6%) patients in the ACS group and 73 (17.4%) in the PCS group (Figure 1).
Figure 1.
Flowchart of the patient selection process. ACS, anterior circulation stroke; PCS, posterior circulation stroke; ICAS, intracranial arterial stenosis; AT, artery-to-artery embolism; CA, cardioembolism; mRS, modified Rankin Scale; ICAS-O, intracranial arterial steno-occlusion; AT-O, artery-to-artery embolic occlusion; CA-O, cardioembolic occlusion.
As shown in Table 1, patients in the PCS group were younger (median age, 67.0 years vs. 71.0 years, P=0.01) and had less frequent CA-O etiology (49.3% vs. 66.2%, P=0.03). Regarding EVT procedure, door-to-groin time (median 144.0 minutes vs. 178.0 minutes, P<0.01), and onset-to-recanalization time (median 421.0 minutes vs. 628.0 minutes, P=0.01) were longer in the PCS group than in the ACS group. At follow-up imaging, there were numerically more ICHs in the ACS group than in the PCS group (42.1% vs. 30.3%, P=0.08). The proportions of successful recanalization and favorable outcomes at 3 months were similar between the two groups, whereas 3 months mortality was higher in the PCS group than the ACS group (15.1% vs. 6.6%, P=0.02).
Table 1.
Baseline characteristics and clinical outcomes in enrolled acute stroke patients with endovascular treatment
| Characteristic | ACS (n=346) | PCS (n=73) | P |
|---|---|---|---|
| Age (yr) | 71.0 (63.8–78.0) | 67.0 (57.0–74.0) | 0.01 |
| Female sex | 136 (39.3) | 25 (34.2) | 0.42 |
| Initial NIHSS | 12.0 (8.0–16.0) | 12.0 (8.0–20.0) | 0.72 |
| Hypertension | 220 (63.6) | 49 (67.1) | 0.57 |
| Diabetes | 94 (27.2) | 18 (24.7) | 0.66 |
| Hyperlipidemia | 78 (22.5) | 24 (32.9) | 0.06 |
| Current smoker | 71 (20.5) | 18 (24.7) | 0.43 |
| Previous stroke | 71 (20.5) | 20 (27.4) | 0.20 |
| Atrial fibrillation | 195 (56.4) | 29 (39.7) | 0.01 |
| Clear onset | 184 (53.2) | 39 (53.4) | 0.97 |
| Intravenous tPA | 97 (28.0) | 19 (26.0) | 0.73 |
| Stroke etiology | 0.03 | ||
| ICAS-O | 67 (19.4) | 21 (28.8) | |
| AT-O | 50 (14.5) | 16 (21.9) | |
| CA-O | 229 (66.2) | 36 (49.3) | |
| Time variables (min) | |||
| Onset-to-door | 188.5 (50.0–501.3) | 265.0 (83.0–621.0) | 0.06 |
| Door-to-groin puncture | 144.0 (113.0–209.5) | 178.0 (133.5–281.5) | <0.01 |
| Puncture-to-recanalization | 58.0 (38.0–82.3) | 55.0 (29.0–77.0) | 0.13* |
| Onset-to-recanalization | 421.0 (263.8–825.8) | 628.0 (356.0–998.0) | 0.01† |
| Modalities of EVT | |||
| Suction thrombectomy | 180 (52.0) | 33 (45.2) | 0.30 |
| Angioplasty | 91 (26.3) | 30 (41.1) | 0.01 |
| Stent retriever | 234 (67.6) | 37 (50.7) | 0.01 |
| Stent insertion | 73 (21.1) | 26 (35.6) | 0.01‡ |
| Instant re-thrombosis | 13 (3.8) | 4 (5.5) | 0.50 |
| Tirofiban use | 13 (3.8) | 3 (4.1) | 0.75 |
| mTICI | 0.49 | ||
| mTICI 0–1 | 19 (5.5) | 1 (1.4) | |
| mTICI 2a | 14 (4.0) | 2 (2.7) | |
| mTICI 2b | 138 (39.9) | 29 (39.7) | |
| mTICI 3 | 175 (50.6) | 41 (56.2) | |
| Successful recanalization (mTICI 2b–3) | 313 (90.5) | 70 (95.9) | 0.13 |
| Recanalization in follow-up images | 296/321 (91.9) | 59/66 (89.4) | 0.50§ |
| Symptomatic ICH | 24/321 (7.5) | 1/66 (1.5) | 0.10 |
| ICH at follow-up imaging | 135/321 (42.1) | 20/66 (30.3) | 0.08 |
| Type of ICH | 0.03 | ||
| Hemorrhagic infarction 1 | 52 (38.5) | 13 (65.0) | |
| Hemorrhagic infarction 2 | 46 (34.1) | 3 (15.0) | |
| Parenchymal hemorrhage 1 | 21 (15.6) | 0 (0) | |
| Parenchymal hemorrhage 2 | 16 (11.9) | 4 (20.0) | |
| mRS 0–2 at 3 months | 148 (42.8) | 26 (35.6) | 0.26 |
| Mortality at 3 months | 23 (6.6) | 11 (15.1) | 0.02 |
Values are presented as median (interquartile range) or number (%).
ACS, anterior circulation stroke; PCS, posterior circulation stroke; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator; ICAS-O, intracranial arterial steno-occlusion; AT-O, artery-to-artery embolic occlusion; CA-O, cardioembolic occlusion; EVT, endovascular treatment; mTICI, modified thrombolysis in cerebral infarction; ICH, intracerebral hemorrhage; mRS, modified Rankin Scale.
Puncture-to-recanalization time and onset-to-recanalization time were calculated in patients for whom successful recanalization was achieved;
Stent insertion refers to intracranial stenting in patients with ICAS-O or CA-O and extracranial (proximal) stenting in patients with AT-O;
Follow-up images were available for 321 and 66 patients in the ACS and PCS groups, respectively.
Baseline characteristics of ACS and PCS patients according to etiology
Table 2 compared patients’ characteristics and outcomes according to each of three etiologies (ICAS-O, AT-O, CA-O) between the ACS and PCS groups; there were 67 (19.4%), 50 (14.5%), and 229 (66.2%) patients in the ACS group and 21 (28.8%), 16 (21.9%), and 36 (49.3%) patients in the PCS group, respectively. Baseline characteristics were similar between the ACS and PCS groups in each etiology except for a higher proportion of previous stroke history (18.0% vs. 50.0%, P=0.02) of AT-O patients in the PCS group.
Table 2.
Baseline characteristics and clinical outcomes of anterior and posterior ischemic stroke patients according to three etiologies
| Characteristic | ICAS-O (n=88) |
AT-O (n=66) |
CA-O (n=265) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ACS (n=67) | PCS (n=21) | P | ACS (n=50) | PCS (n=16) | P | ACS (n=229) | PCS (n=36) | P | ||
| Age (yr) | 65.5 (61.0–74.0) | 68.0 (56.0–73.5) | 0.86 | 68.5 (63.3–73.0) | 62.5 (57.0–71.0) | 0.20 | 74.0 (65.0–79.0) | 69.0 (60.5–77.8) | 0.06 | |
| Female sex | 13 (19.4) | 3 (14.3) | 0.75 | 6 (12.0) | 2 (12.5) | >0.99 | 117 (51.1) | 20 (55.6) | 0.62 | |
| Initial NIHSS | 9.0 (6.0–13.0) | 10.0 (9.0–17.0) | 0.06 | 10.0 (8.0–12.3) | 10.5 (5.5–20.5) | 0.70 | 14.0 (10.0–17.0) | 13.0 (7.0–22.0) | 0.94 | |
| Hypertension | 47 (70.1) | 18 (85.7) | 0.16 | 27 (54.0) | 10 (62.5) | 0.55 | 146 (63.8) | 21 (58.3) | 0.53 | |
| Diabetes | 20 (29.9) | 4 (19.0) | 0.33 | 15 (30.0) | 8 (50.0) | 0.14 | 59 (25.8) | 6 (16.7) | 0.24 | |
| Hyperlipidemia | 16 (23.9) | 9 (42.9) | 0.10 | 11 (22.0) | 6 (37.5) | 0.32 | 51 (22.3) | 9 (25.0) | 0.72 | |
| Current smoker | 26 (38.8) | 7 (33.3) | 0.65 | 24 (48.0) | 6 (37.5) | 0.46 | 21 (9.2) | 5 (13.9) | 0.38 | |
| Previous stroke | 13 (19.4) | 5 (23.8) | 0.76 | 9 (18.0) | 8 (50.0) | 0.02 | 49 (21.4) | 7 (19.4) | 0.79 | |
| Atrial fibrillation | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA | 195 (85.2) | 29 (80.6) | 0.48 | |
| Clear onset | 32 (47.8) | 14 (66.7) | 0.13 | 28 (56.0) | 9 (56.3) | >0.99 | 124 (54.1) | 16 (44.4) | 0.28 | |
| Intravenous tPA | 12 (17.9) | 6 (28.6) | 0.35 | 13 (26.0) | 3 (18.8) | 0.74 | 72 (31.4) | 10 (27.8) | 0.66 | |
| Time variables (min) | ||||||||||
| Onset-to-door | 505.0 (155.0–1,177.0) | 328.0 (190.0–573.0) | 0.23 | 274.0 (132.5–752.3) | 407.0 (156.5–861.8) | 0.45 | 120.0 (36.5–354.0) | 207.0 (40.0–510.0) | 0.14 | |
| Door-to-groin puncture | 212.0 (149.0–596.0) | 172.0 (129.0–238.0) | 0.09 | 156.5 (123.5–313.8) | 174.0 (135.0–376.3) | 0.43 | 132.0 (110.0–178.0) | 179.0 (133.5–276.3) | <0.01 | |
| Puncture-to-recanalization | 73.0 (57.0–94.0) | 72.0 (52.0–93.5) | 0.75* | 61.5 (45.5–96.0) | 62.0 (49.0–82.5) | 0.80* | 51.0 (36.0–70.5) | 36.5 (23.3–61.5) | <0.01* | |
| Onset-to-recanalization | 950.0 (528.0–1,960.0) | 659.0 (427.5–930.5) | 0.07† | 559.5 (320.5–1,351.5) | 973.0 (481.0–2,679.0) | 0.11† | 346.0 (237.5–571.0) | 511.5 (234.8–958.8) | 0.06† | |
| Modalities of EVT | ||||||||||
| Suction thrombectomy | 18 (26.9) | 3 (14.3) | 0.38 | 27 (54.0) | 6 (37.5) | 0.25 | 135 (59.0) | 24 (66.7) | 0.38 | |
| Angioplasty | 43 (64.2) | 17 (81.0) | 0.15 | 38 (76.0) | 12 (75.0) | >0.99 | 10 (4.4) | 1 (2.8) | >0.99 | |
| Stent retriever | 35 (52.2) | 8 (38.1) | 0.26 | 32 (64.0) | 11 (68.8) | 0.73 | 167 (72.9) | 18 (50.0) | 0.01 | |
| Stent insertion | 32 (47.8) | 13 (61.9) | 0.32‡ | 29 (58.0) | 11 (68.8) | 0.44‡ | 12 (5.2) | 2 (5.6) | >0.99‡ | |
| Instant re-thrombosis | 7 (10.4) | 4 (19.0) | 0.29 | 5 (10.0) | 0 (0.0) | 0.33 | 1 (0.4) | 0 (0) | >0.99 | |
| Tirofiban use | 7 (10.4) | 3 (14.3) | 0.70 | 4 (8.0) | 0 (0.0) | 0.57 | 2 (0.9) | 0 (0) | >0.99 | |
| mTICI | 0.27 | 0.18 | 0.38 | |||||||
| mTICI 0–1 | 1 (1.5) | 0 (0) | 2 (4.0) | 1 (6.3) | 16 (7.0) | 0 (0) | ||||
| mTICI 2a | 4 (6.0) | 0 (0) | 0 (0) | 1 (6.3) | 10 (4.4) | 1 (2.8) | ||||
| mTICI 2b | 15 (22.4) | 9 (42.9) | 26 (52.0) | 5 (31.3) | 97 (42.4) | 15 (41.7) | ||||
| mTICI 3 | 47 (70.1) | 12 (57.1) | 22 (44.0) | 9 (56.3) | 106 (46.3) | 20 (55.6) | ||||
| Successful recanalization (mTICI ≥2b–3) | 62 (92.5) | 21 (100.0) | 0.33 | 48 (96.0) | 14 (87.5) | 0.25 | 203 (88.6) | 35 (97.2) | 0.14 | |
| Recanalization in follow-up imaging | 55/62 (87.3) | 16/20 (80.0) | 0.45§ | 43/46 (93.5) | 11/14 (78.6) | 0.13§ | 198/213 (93.0) | 32/32 (100.0) | 0.23§ | |
| Symptomatic ICH | 2/62 (3.2) | 0/20 (0.0) | >0.99 | 2/46 (4.3) | 0/14 (0.0) | >0.99 | 20/213 (9.4) | 1/32 (3.1) | 0.33 | |
| ICH at follow-up imaging | 14/62 (22.6) | 3/20 (15.0) | 0.55 | 18/46 (39.1) | 4/14 (28.6) | 0.47 | 103/213 (48.4) | 13/32 (40.6) | 0.99 | |
| Types of ICH | 0.48 | 0.69 | 0.10 | |||||||
| HI-1 | 6 (42.9) | 3 (100.0) | 7 (38.9) | 3 (75.0) | 39 (37.9) | 7 (53.8) | ||||
| HI-2 | 5 (35.7) | 0 (0) | 7 (38.9) | 1 (25.0) | 34 (33.0) | 2 (15.4) | ||||
| PH-1 | 2 (14.3) | 0 (0) | 3 (16.7) | 0 (0) | 16 (15.5) | 0 (0) | ||||
| PH-2 | 1 (7.1) | 0 (0) | 1 (5.6) | 0 (0) | 14 (13.6) | 4 (30.8) | ||||
| mRS 0–2 at 3 months | 35 (52.2) | 7 (33.3) | 0.13 | 24 (48.0) | 7 (43.8) | 0.77 | 89 (38.9) | 12 (33.3) | 0.53 | |
| Mortality at 3 months | 2 (3.0) | 3 (14.3) | 0.09 | 3 (6.0) | 1 (6.3) | >0.99 | 18 (7.9) | 7 (19.4) | 0.06 | |
Values are presented as median (interquartile range) or number (%).
ICAS-O, intracranial arterial steno-occlusion; AT-O, artery-to-artery embolic occlusion; CA-O, cardioembolic occlusion; NIHSS, National Institutes of Health Stroke Scale; ACS, anterior circulation stroke; PCS, posterior circulation stroke; NA, not applicable; tPA, tissue plasminogen activator; EVT, endovascular treatment; mTICI, modified thrombolysis in cerebral infarction; ICH, intracerebral hemorrhage; HI, hemorrhagic infarction; PH, parenchymal hemorrhage; mRS, modified Rankin Scale.
Puncture-to-recanalization time and onset-to-recanalization time were calculated in patients for whom successful recanalization was achieved;
Stent insertion refers to intracranial stenting in patients with ICAS-O or CA-O and extracranial (proximal) stenting in patients with AT-O;
Follow-up images were available for 82, 60, and 245 patients in the ICAS-O, AT-O, and CA-O groups, respectively.
Procedural and clinical outcomes of ACS and PCS patients according to etiology
EVT procedure-related time variables, including onset-to-door and onset-to-recanalization times were not significantly different between the ACS and PCS groups in ICAS-O and AT-O patients (Table 2). However, among CA-O patients, the PCS group had longer door-to-groin puncture time (median 179.0 minutes vs. 132.0 minutes, P<0.01) than the ACS group, whereas puncture-to-recanalization time (median 36.5 minutes vs. 51.0 minutes, P<0.01) was shorter in the PCS group than in the ACS group. The use of EVT modalities and proportion of successful recanalization (mTICI 2b–3), as well as favorable 3-month clinical outcomes, were similar in all three etiologies between the ACS and PCS groups (Table 2 and Figure 2).
Figure 2.
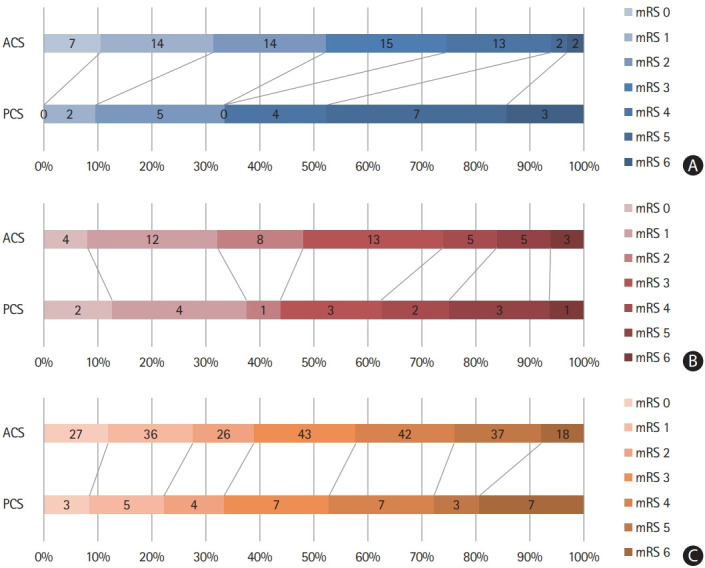
Score on the modified Rankin Scale (mRS) at 3 months in (A) intracranial arterial steno-occlusion, (B) artery-to-artery embolism, and (C) cardioembolic occlusion patients between anterior circulation stroke (ACS) and posterior circulation stroke (PCS) groups. Numbers in a bar represent the number of patients.
Baseline characteristics, and procedural and clinical outcomes of ACS patients according to etiology
We also analyzed the baseline characteristics and outcomes of patients according to etiology, confined to each ACS and PCS group. The results of ACS patients are summarized in Supplementary Table 1. The median age (ICAS-O, 65.5 years; AT-O, 68.5 years; CA-O, 74.0 years; P<0.01) and proportion of female sex (ICAS-O, 19.4%; AT-O, 12.0%; CA-O, 51.1%; P<0.01) were higher in the CA-O group. The initial NIHSS score was higher in the CA-O group (ICAS-O, 9.0; AT-O, 10.0; CA-O, 14.0; P<0.01). Procedural variables showed that CA-O patients had a shorter time interval in all aspects, including onset-to-door (ICAS-O, 505.0 minutes; AT-O, 274.0 minutes; CA-O, 120.0 minutes; P<0.01), door-to-puncture (ICAS-O, 212.0 minutes; AT-O, 156.5 minutes; CA-O, 132.0 minutes; P<0.01), and onset-to-recanalization time (ICAS-O, 950.0 minutes; AT-O, 559.5 minutes; CA-O, 346.0 minutes; P<0.01). The rate of successful recanalization, favorable clinical outcome, and mortality at 3 months were similar among the three etiologies.
Baseline characteristics, and procedural and clinical outcomes of PCS patients according to etiology
Among PCS group patients, the median age and initial NIHSS score were not significantly different, but female sex was more frequent in the CA-O group (ICAS-O, 14.3%; AT-O, 12.5%; CA-O, 55.6%; P<0.01) (Supplementary Table 2). Analysis of procedural time variables showed that onset-to-door and door-to-puncture times were similar among the three etiologies, whereas puncture-to-recanalization time (ICAS-O, 72.0 minutes; AT-O, 62.0 minutes; CA-O, 36.5 minutes; P<0.01) and onset-to-recanalization time (ICAS-O, 659.0 minutes; AT-O, 973.0 minutes; CA-O, 511.5 minutes; P=0.04) was the shortest in CA-O patients. The rate of TICI 2b–3, 3-month mRS 0–2, and 3-month mortality were similar among the three etiologies.
Factors related to favorable clinical outcomes
Univariable analysis showed that age, baseline NIHSS score, and ICH at follow-up imaging for the PCS group, and age, baseline NIHSS score, successful recanalization, and ICH at follow-up imaging for the ACS group were associated with clinical outcomes (Table 3). Multivariable analysis showed that baseline NIHSS score (odds ratio [OR], 0.84; 95% confidence interval [CI], 0.75 to 0.93; P=0.001) and ICH at follow-up imaging (OR, 0.20; 95% CI, 0.04 to 0.97; P=0.046) in the PCS group, and baseline NIHSS score (OR, 0.86; 95% CI, 0.81 to 0.91; P<0.001), successful recanalization (OR, 11.74; 95% CI, 2.60 to 52.94; P=0.001), and ICH at follow-up imaging (OR, 0.47; 95% CI, 0.29 to 0.77; P=0.003) in the ACS group were independently associated with favorable 3-month mRS scores.
Table 3.
Logistic regression analysis for factors affecting 3-month favorable outcomes (mRS 0–2) in anterior circulation and posterior circulation stroke patients
| Variable | Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | ||
| ACS group | |||||||
| Age (yr) | 0.97 | 0.95–0.99 | 0.001 | 0.98 | 0.96–1.00 | 0.057 | |
| Male sex | 0.80 | 0.52–1.23 | 0.304 | ||||
| Baseline NIHSS | 0.84 | 0.80–0.89 | <0.001 | 0.86 | 0.81–0.91 | <0.001 | |
| ICA occlusion | 0.91 | 0.59–1.40 | 0.671 | ||||
| Onset-to-recanalization time (min) | 1.00 | 1.00–1.001 | 0.740 | ||||
| Successful recanalization (mTICI 2b–3) | 11.72 | 2.75–50.04 | 0.001 | 11.74 | 2.60–52.94 | 0.001 | |
| ICH at follow-up imaging | 0.37 | 0.24–0.58 | <0.001 | 0.47 | 0.29–0.77 | 0.003 | |
| Occlusion etiology | |||||||
| CA-O | 1 (reference) | ||||||
| AT-O | 1.65 | 0.95–2.86 | 0.073 | ||||
| ICAS-O | 1.48 | 0.80–2.74 | 0.213 | ||||
| PCS group | |||||||
| Age (yr) | 0.95 | 0.91–0.99 | 0.021 | 0.95 | 0.89–1.01 | 0.075 | |
| Male sex | 0.98 | 0.36–2.67 | 0.961 | ||||
| Baseline NIHSS | 0.83 | 0.74–0.92 | <0.001 | 0.84 | 0.75–0.93 | 0.001 | |
| Proximal to middle BA occlusion | 1.28 | 0.46–3.55 | 0.642 | ||||
| Onset-to-recanalization time (min) | 1.00 | 1.00–1.001 | 0.074 | ||||
| Successful recanalization (mTICI 2b–3) | NA | NA | NA | ||||
| ICH at follow-up imaging | 0.21 | 0.06–0.80 | 0.022 | 0.20 | 0.04–0.97 | 0.046 | |
| Occlusion etiology | |||||||
| CA-O | 1 (reference) | ||||||
| AT-O | 1.00 | 0.31–3.13 | >0.999 | ||||
| ICAS-O | 0.50 | 0.47–5.20 | 0.473 | ||||
mRS, modified Rankin Scale; CI, confidence interval; ACS, anterior circulation stroke; NIHSS, National Institutes of Health Stroke Scale; ICA, internal carotid artery; mTICI, modified thrombolysis in cerebral infarction; ICH, intracerebral hemorrhage; CA-O, cardioembolic occlusion; AT-O, artery-to-artery embolic occlusion; ICAS-O, intracranial arterial steno-occlusion; PCS, posterior circulation stroke; NA, not applicable.
Discussion
In this study, we compared EVT procedure and clinical outcomes between the PCS and ACS groups, focusing on three etiologies, ICAS-O, AT-O, and CA-O. We found that PCS group patients were younger, had less frequent CA-O etiology, and longer onset-to-recanalization time and door-to-puncture time than ACS group patients. The proportions of successful recanalization and favorable clinical outcomes at 3 months were similar among the three etiologies between the ACS and PCS groups.
The onset-to-recanalization time, which is the sum of onset-to-door, door-to-puncture, and puncture-to-recanalization time, was longer in PCS patients than in ACS patients. As shown in Table 1, the difference mainly resulted from the longer onset-to-door and door-to-puncture times in PCS patients. The relatively longer onset-to-door time in the PCS group was probably attributed to symptoms such as dizziness, diplopia, visual dimness, or dysphagia, which were not regarded as serious neurologic symptoms by the patients. Although the reason for delayed door-to-puncture time in PCS patients is less clear, further analysis showed that the difference was obvious only in the CA-O patients (Table 2). Thus, the difference was in part related with different characteristics of CA-O between the PCS and ACS groups. Although the NIHSS score was highest in CA-O patients in the ACS group (Supplementary Table 1), it was similar among the three etiologies in the PCS group (Supplementary Table 2). This is probably due to the different symptom characteristics of CA-O patients between the two groups. Although CA-O in the ACS group produced abrupt and severe motor dysfunction associated with sudden middle cerebral artery or internal carotid occlusion (Supplementary Table 1), CA-O in the PCS group typically occluded distal basilar or PCAs [24-26] (Supplementary Table 2), which is less likely to produce severe motor dysfunction, thereby delaying the initiation of the EVT decision.
Interestingly, in the CA-O patients, puncture-to-recanalization was shorter in the PCS group than in the ACS group (Table 2). The reason remains unclear. Perhaps, in CA-O patients without proximal artery atherosclerosis, access to the occluded thrombi may be relatively difficult in ACS patients due to the presence of curved carotid syphons. Morphological anomalies such as tortuosity or coiling has been reported to common in the internal carotid artery [27]. Alternatively, the presence of the collateral flow of the posterior circulation, such as the posterior communicating arteries and superficial anastomosis [28], might have a role in spontaneous thrombus fragmentation. These anatomic characteristics may also partly explain why the proportion of favorable clinical outcomes was similar despite the relatively prolonged onset-to-recanalization time in the PCS group when compared with the ACS group (Table 2).
Among patients with embolic occlusion, we separated AT-O from CA-O. The efficacy and safety of EVT in tandem lesions in ACS patients have been described [29,30]. However, the appropriate treatment of tandem occlusion in PCS patients still needs to be investigated, although a considerable proportion of PCS patients receiving EVT are classified into this group [31-33]. In our study, the proportion of successful recanalization as well as 3-month favorable clinical outcomes in AT-O patients in the PCS group were all comparable with those of AT-O patients in the ACS group (Table 2), suggesting the efficacy and safety of EVT in this group of patients.
After the multivariable analysis, low baseline NIHSS score and the absence of cerebral hemorrhage were significantly related to favorable outcomes in PCS and ACS patients, whereas successful recanalization was an independent factor for ACS patients only. Previous studies found that PCS patients with EVT had a lower rate of functional independence despite a rate of successful recanalization similar to that of ACS patients [34,35]. This might be explained by a higher proportion of futile reperfusions in PCS compared to ACS, as previously suggested [34]. In addition, mRS score, which is a widely used scoring system to evaluate clinical outcomes, might not be an appropriate tool for PCS patients, because symptoms such as visual field defect, dizziness, ataxia, and paresthesia are not properly reflected in this scoring system [36]. Thus, the possible benefit of recanalization therapy may have been underestimated in studies using mRS as an outcome parameter.
There were several limitations in this study. First, because this was a single center, retrospective study, there may have been selection bias. Second, as the number of PCS patients was relatively small, although it was comparable to previously published studies [14,25]. Third, an assessment of collaterals was not systemically performed in this retrospective study. Lastly, patients with missing 3-month mRS were excluded from this study, which may introduced a selection bias.
Conclusions
In this study, we found that PCS group patients, especially in the CA-O group, had longer door-to-puncture and onset-to-recanalization times although favorable clinical outcomes were similar in the ACS and PCS groups, regardless of etiology. The lower baseline NIHSS score and the absence of hemorrhagic transformation were related to favorable outcomes in PCS patients, but not successful reperfusion, which was related to favorable outcomes in ACS patients. More studies are needed to examine the role of EVT in PCS patients.
Footnotes
Disclosure
The authors have no financial conflicts of interest.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2022.01095.
Baseline characteristics and clinical outcomes of anterior circulation stroke patients according to three etiologies
Baseline characteristics and clinical outcomes of posterior circulation stroke patients according to three etiologies
References
- 1.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 3.Schonewille WJ, Wijman CA, Michel P, Rueckert CM, Weimar C, Mattle HP, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol. 2009;8:724–730. doi: 10.1016/S1474-4422(09)70173-5. [DOI] [PubMed] [Google Scholar]
- 4.Langezaal LC, van der Hoeven EJ, Mont’Alverne FJ, de Carvalho JJ, Lima FO, Dippel DW, et al. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med. 2021;384:1910–1920. doi: 10.1056/NEJMoa2030297. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 2020;19:115–122. doi: 10.1016/S1474-4422(19)30395-3. [DOI] [PubMed] [Google Scholar]
- 6.Rentzos A, Karlsson JE, Lundqvist C, Rosengren L, Hellström M, Wikholm G. Endovascular treatment of acute ischemic stroke in the posterior circulation. Interv Neuroradiol. 2018;24:405–411. doi: 10.1177/1591019918762320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Houwelingen RC, Luijckx GJ, Mazuri A, Bokkers RP, Eshghi OS, Uyttenboogaart M. Safety and outcome of intra-arterial treatment for basilar artery occlusion. JAMA Neurol. 2016;73:1225–1230. doi: 10.1001/jamaneurol.2016.1408. [DOI] [PubMed] [Google Scholar]
- 8.Yoon W, Kim SK, Park MS, Baek BH, Lee YY. Predictive factors for good outcome and mortality after stent-retriever thrombectomy in patients with acute anterior circulation stroke. J Stroke. 2017;19:97–103. doi: 10.5853/jos.2016.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SJ, Seok JM, Bang OY, Kim GM, Kim KH, Jeon P, et al. MR mismatch profiles in patients with intracranial atherosclerotic stroke: a comprehensive approach comparing stroke subtypes. J Cereb Blood Flow Metab. 2009;29:1138–1145. doi: 10.1038/jcbfm.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiedt S, Herzberg M, Küpper C, Feil K, Kellert L, Dorn F, et al. Stroke etiology modifies the effect of endovascular treatment in acute stroke. Stroke. 2020;51:1014–1016. doi: 10.1161/STROKEAHA.119.028383. [DOI] [PubMed] [Google Scholar]
- 11.Kang DH, Kim YW, Hwang YH, Park SP, Kim YS, Baik SK. Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis. 2014;37:350–355. doi: 10.1159/000362435. [DOI] [PubMed] [Google Scholar]
- 12.Xun K, Mo J, Ruan S, Dai J, Zhang W, Lv Y, et al. A meta-analysis of prognostic factors in patients with posterior circulation stroke after mechanical thrombectomy. Cerebrovasc Dis. 2021;50:185–199. doi: 10.1159/000512609. [DOI] [PubMed] [Google Scholar]
- 13.Yoon W, Kim SK, Heo TW, Baek BH, Lee YY, Kang HK. Predictors of good outcome after stent-retriever thrombectomy in acute basilar artery occlusion. Stroke. 2015;46:2972–2975. doi: 10.1161/STROKEAHA.115.010840. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Hong JM, Choi JW, Park JH, Park B, Kang DH, et al. Predicting endovascular treatment outcomes in acute vertebrobasilar artery occlusion: a model to aid patient selection from the ASIAN KR Registry. Radiology. 2020;294:628–637. doi: 10.1148/radiol.2020191227. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, Rajah GB, Cosky EE, Wu X, Li C, Chen J, et al. Outcomes in endovascular therapy for basilar artery occlusion: intracranial atherosclerotic disease vs. embolism. Aging Dis. 2021;12:404–414. doi: 10.14336/AD.2020.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo G, Mo D, Tong X, Liebeskind DS, Song L, Ma N, et al. Factors associated with 90-day outcomes of patients with acute posterior circulation stroke treated by mechanical thrombectomy. World Neurosurg. 2018;109:e318–e328. doi: 10.1016/j.wneu.2017.09.171. [DOI] [PubMed] [Google Scholar]
- 17.Sefcik RK, Tonetti DA, Desai SM, Casillo SM, Lang MJ, Jadhav AP, et al. Does stroke etiology influence outcome in the posterior circulation?: an analysis of 107 consecutive acute basilar occlusion thrombectomies. Neurosurg Focus. 2021;51:E7. doi: 10.3171/2021.4.FOCUS2189. [DOI] [PubMed] [Google Scholar]
- 18.Lee D, Lee DH, Suh DC, Kim BJ, Kwon SU, Kwon HS, et al. Endovascular treatment in patients with cerebral artery occlusion of three different etiologies. J Stroke. 2020;22:234–244. doi: 10.5853/jos.2019.02404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 20.Archer CR, Horenstein S. Basilar artery occlusion: clinical and radiological correlation. Stroke. 1977;8:383–390. doi: 10.1161/01.str.8.3.383. [DOI] [PubMed] [Google Scholar]
- 21.Lee D, Lee DH, Suh DC, Kwon HS, Jeong DE, Kim JG, et al. Intra-arterial thrombectomy for acute ischaemic stroke patients with active cancer. J Neurol. 2019;266:2286–2293. doi: 10.1007/s00415-019-09416-8. [DOI] [PubMed] [Google Scholar]
- 22.Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999;30:2280–2284. doi: 10.1161/01.str.30.11.2280. [DOI] [PubMed] [Google Scholar]
- 23.von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049. [DOI] [PubMed] [Google Scholar]
- 24.Meyer L, Stracke CP, Jungi N, Wallocha M, Broocks G, Sporns PB, et al. Thrombectomy for primary distal posterior cerebral artery occlusion stroke: the TOPMOST Study. JAMA Neurol. 2021;78:434–444. doi: 10.1001/jamaneurol.2021.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baik SH, Park HJ, Kim JH, Jang CK, Kim BM, Kim DJ. Mechanical thrombectomy in subtypes of basilar artery occlusion: relationship to recanalization rate and clinical outcome. Radiology. 2019;291:730–737. doi: 10.1148/radiol.2019181924. [DOI] [PubMed] [Google Scholar]
- 26.Lindsberg PJ, Soinne L, Tatlisumak T, Roine RO, Kallela M, Häppölä O, et al. Long-term outcome after intravenous thrombolysis of basilar artery occlusion. JAMA. 2004;292:1862–1866. doi: 10.1001/jama.292.15.1862. [DOI] [PubMed] [Google Scholar]
- 27.Alverne FJ, Lima FO, Rocha FA, Bandeira DA, Lucena AF, Silva HC, et al. Unfavorable vascular anatomy during endovascular treatment of stroke: challenges and bailout strategies. J Stroke. 2020;22:185–202. doi: 10.5853/jos.2020.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindsberg PJ, Pekkola J, Strbian D, Sairanen T, Mattle HP, Schroth G. Time window for recanalization in basilar artery occlusion: speculative synthesis. Neurology. 2015;85:1806–1815. doi: 10.1212/WNL.0000000000002129. [DOI] [PubMed] [Google Scholar]
- 29.Malik AM, Vora NA, Lin R, Zaidi SF, Aleu A, Jankowitz BT, et al. Endovascular treatment of tandem extracranial/intracranial anterior circulation occlusions: preliminary single-center experience. Stroke. 2011;42:1653–1657. doi: 10.1161/STROKEAHA.110.595520. [DOI] [PubMed] [Google Scholar]
- 30.Wilson MP, Murad MH, Krings T, Pereira VM, O’Kelly C, Rempel J, et al. Management of tandem occlusions in acute ischemic stroke: intracranial versus extracranial first and extracranial stenting versus angioplasty alone: a systematic review and meta-analysis. J Neurointerv Surg. 2018;10:721–728. doi: 10.1136/neurintsurg-2017-013707. [DOI] [PubMed] [Google Scholar]
- 31.Compter A, van der Hoeven EJ, van der Worp HB, Vos JA, Weimar C, Rueckert CM, et al. Vertebral artery stenosis in the Basilar Artery International Cooperation Study (BASICS): prevalence and outcome. J Neurol. 2015;262:410–417. doi: 10.1007/s00415-014-7583-5. [DOI] [PubMed] [Google Scholar]
- 32.Piechowiak EI, Kaesmacher J, Zibold F, Dobrocky T, Mosimann PJ, Jung S, et al. Endovascular treatment of tandem occlusions in vertebrobasilar stroke: technical aspects and outcome compared with isolated basilar artery occlusion. J Neurointerv Surg. 2020;12:25–29. doi: 10.1136/neurintsurg-2019-014825. [DOI] [PubMed] [Google Scholar]
- 33.Cohen JE, Leker RR, Gomori JM, Eichel R, Rajz G, Moscovici S, et al. Emergent revascularization of acute tandem vertebrobasilar occlusions: endovascular approaches and technical considerations: confirming the role of vertebral artery ostium stenosis as a cause of vertebrobasilar stroke. J Clin Neurosci. 2016;34:70–76. doi: 10.1016/j.jocn.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Wang F, Wang J, He Q, Wang L, Cao Y, Zhang H, et al. Mechanical thrombectomy for posterior circulation occlusion: a comparison of outcomes with the anterior circulation occlusion: a meta-analysis. J Atheroscler Thromb. 2020;27:1325–1339. doi: 10.5551/jat.54221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huo X, Gao F, Ma N, Mo D, Sun X, et al. Characteristic and prognosis of acute large vessel occlusion in anterior and posterior circulation after endovascular treatment: the ANGEL registry real world experience. J Thromb Thrombolysis. 2020;49:527–532. doi: 10.1007/s11239-020-02054-2. [DOI] [PubMed] [Google Scholar]
- 36.Romano JG, Gardener H, Campo-Bustillo I, Khan Y, Tai S, Riley N, et al. Predictors of outcomes in patients with mild ischemic stroke symptoms: MaRISS. Stroke. 2021;52:1995–2004. doi: 10.1161/STROKEAHA.120.032809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics and clinical outcomes of anterior circulation stroke patients according to three etiologies
Baseline characteristics and clinical outcomes of posterior circulation stroke patients according to three etiologies