Abstract
Background
The association between preterm birth and neurodevelopmental delays have been well examined, however, reliable estimates for the full range of gestational age (GA) are limited, and few studies explored the impact of post-term birth on child development.
Objective
This study aimed to examine the long-term neuropsychological outcomes of children born in a full range of GA with a national representative sample in China.
Methods
In this retrospective population-based cohort study, a total of 137,530 preschoolers aged 3–5 years old (65,295/47.5% females and 72,235/52.5% males) were included in the final analysis. The Ages and Stages Questionnaires-Third Edition (ASQ-3) was completed by parents to evaluate children's neurodevelopment. The associations between GA and neurodevelopment were analyzed by a generalized additive mixed model with thin plate regression splines. Logistic regression was also conducted to examine the differences in children's development with different GAs.
Results
There was a non-linear relationship between GA and children's neurodevelopmental outcomes with the highest scores at 40 weeks gestational age. The adjusted risks of GAs (very and moderately preterm, late-preterm, early-term, and post-term groups) on suspected developmental delays were observed in communication (OR were 1.83, 1.28, 1.13, and 1.21 respectively, each p < 0.05), gross motor skill (OR were 1.67, 1.38, 1.10, and 1.05 respectively, each p < 0.05), and personal social behavior (OR were 1.01, 1.36, 1.12, and 1.18 respectively, each p < 0.05). The adjusted OR of very and moderately preterm, late-preterm, and early-term were observed in fine motor skills (OR were 1.53, 1.22, and 1.09 respectively, each p < 0.05) and problem-solving (OR were 1.33, 1.12, and 1.06 respectively, each p < 0.05).
Conclusion
GAs is a risk factor for neurodevelopmental delays in preschoolers after controlling for a wide range of covariates, and 40–41 weeks may be the ideal delivery GA for optimal neurodevelopmental outcomes. Close observation and monitoring should be considered for early- and post-term born children as well as pre-term children.
Keywords: gestational age, early and post-term delivery, neurodevelopment, early childhood, national representative sample
Background
Neurodevelopmental or neurobehavioral disorders are defined as behavioral and cognitive disorders that become apparent in early childhood and involve significant difficulties in the acquisition and execution of specific intellectual, motor, cognition, or social functions (1). Various communication disorders affecting speech and language, Autism Spectrum Disorder (ASD), Attention Deficit Hyperactivity Disorder (ADHD), Learning Disorders (LD), and motor disorders such as Developmental Coordination Disorder (DCD) are major neurodevelopmental disorders.
The prevalence, as a group, for these neurodevelopmental disorders range from 5 to 20% in the general population (2, 3), however, the etiology of neurodevelopmental disorders is complex and cannot be elucidated in a large percentage of cases (4). Neurodevelopmental disorders usually have their onset from infancy and can persist over the lifespan (5). Unfortunately, clinical symptoms of these developmental delays can be difficult to identify in early childhood, and the early diagnosis of neurodevelopmental disorders can be difficult to make, which leads to a postponement of intervention and treatment (6).
The long-term neurodevelopmental problems associated with preterm birth have been well examined. It has been indicated that very and moderately preterm birth (<34 weeks gestation) is associated with neurodevelopmental delays (7–9). Late-preterm infants (34–36 weeks) have also been reported to be more likely to have lower intellectual and psychomotor developmental assessment scores than those born full-term (10–12).
However, few studies have focused on the effects of gestational on neurodevelopmental delays age within the full-term range (37–41 weeks). For example, studies have shown that early-term infants (37–38 weeks) are at a higher risk of neurodevelopmental delays (6, 13–15). and have more complications and higher mortality than full-term children (6, 16). Previous data has also indicated that longer gestation is associated with better cognitive and psychomotor development after controlling for the effect of potential confounds such as social-economic status (SES) and home environment (17–19). Evidence also suggests that cognitive abilities can improve with a longer gestational age toward term, reaching a peak at 40 weeks before decreasing toward late-term (40–41 weeks) (20–22). However, many studies are limited by missing data (23, 24). Inconsistent results were also reported and some studies did not find significant effects in several neurodevelopmental domains between late-term and full-term children (25, 26). Most studies remain underpowered to assess whether there is continued decline in these neurodevelopmental domains with late GA.
More importantly, very little is known about the neurodevelopmental outcomes of post-term born children (>41 weeks). Although it is often assumed that full-term and post-term births are homogeneous in terms of health outcomes, post-term delivery has been reported to be associated with an increased risk of intrapartum and postpartum obstetric complications and has a high rate of perinatal morbidity and mortality (27, 28). Post-term birth is associated with significant cognitive delays compared with full-term counterparts (29). Children born post-term were more likely to have emotional and behavioral problems assessed at both 18 and 36 months old compared to their full-term counterparts (8, 30). Post-term children also presented increased risk and symptomatology of ASD (8, 31) and ADHD (32) during childhood. Evidence also suggested that post-term children were more likely to have school difficulties (33) and worse school performance (22).
Therefore, in this population-based retrospective study, using a large nationally representative sample, we systematically examined the association between a full range of GAs and the neurodevelopment of children aged 3–5 years in five neurodevelopmental domains (communication, gross motor skills, fine motor skills, problem-solving, and personal-social behavior). We hypothesized that a higher risk of neurodevelopmental delays may occur in preterm (<37 weeks), early-term (37–38 weeks), and post-term (>41weeks) children. We aimed to 1) explore a linear or non-linear relationship between GA at birth and neurodevelopment; 2) analyze the strength of effects in preterm, early-term, and post-term born children when compared with full-term born children.
Methods
Participants
In this national retrospective cohort study, a stratified cluster sampling plan was used to ensure that the participants included in the current study were representative of the Chinese population. Data from the 2018–2019 National Census in China provided the basis for the stratification by geographic region, age, sex, and social-economic status (SES). Government-supported maternity and children's health centers in each city were selected to invite their local kindergartens to participate in the study. Class teachers were responsible for distributing the notification to parents to complete the online questionnaire; researcher contact information was provided in case parents had queries. An electronic questionnaire system was used to enhance the quality of the data by allowing the inclusion of pop-up instructions, error messages, links to further information, and to set conditions to ensure participants could not skip questions. A data coordination center was established to take charge of establishing, managing, and maintaining the database and website, and coordinating among health centers.
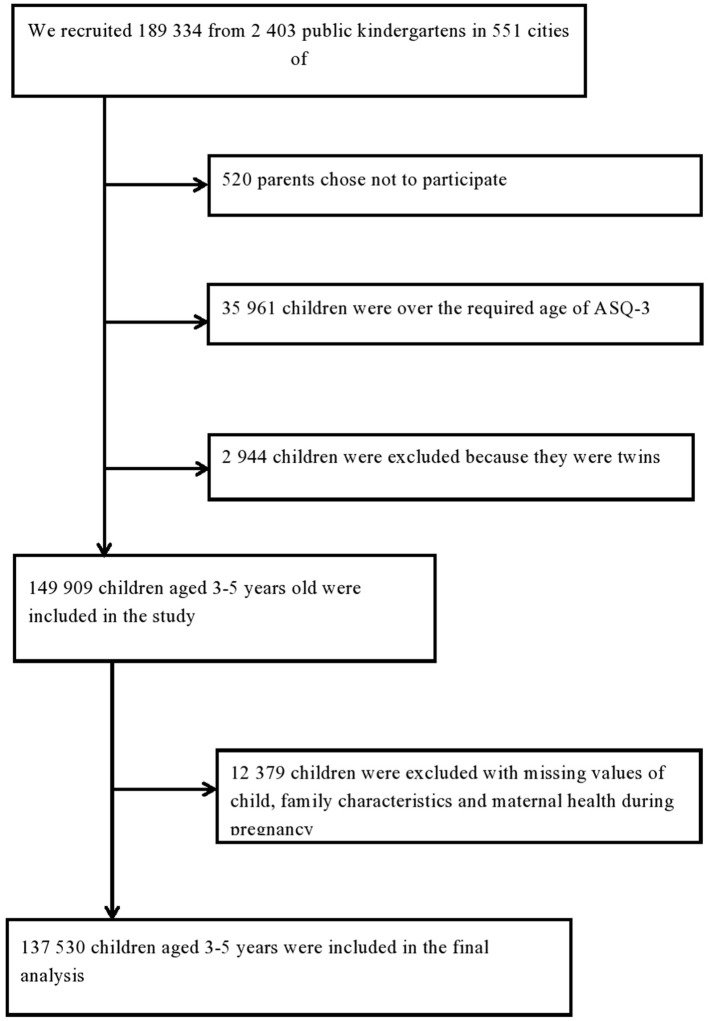
Only mainstream schools and nurseries were included in the study. Children with severe visual, hearing, intellectual impairments (according to examinations that took place before starting kindergarten), or other severe developmental disorders who were required to attend special education schools/nurseries – according to local regulations – were not included in the current study. A total of 2,403 kindergartens in 551 cities of China were invited to the study from April 2018 to December 2019. Of these participants, 149 909 children with singleton delivery aged 3–5 years old were included in the study. However, children with missing covariates (child, family, and maternal characteristics) were excluded. Finally, 137,530 children with completed questionnaires were included in the final analysis (Figure 1).
Figure 1.
Flowchart of the study population.
The study was approved by the Ethics Committee of Shanghai First Maternity and Infant Hospital (KS18156). The parents had given online consent to participate in the study before completing the online questionnaire. All information acquired was kept confidential and was only accessible by the researchers.
Outcomes
The preschooler's neurodevelopmental outcomes were assessed using the Ages & Stages Questionnaires-Third Edition (ASQ-3), completed by a parent of the child. This has been widely recognized and praised as a screening tool and has been validated in more than 70 languages and dialects including Chinese (34, 35). The Chinese version of ASQ-3 has fair reliability and validity in the Chinese population (36) with the Cronbach's alpha coefficient for the whole measure of 0.8.
As a screening tool designed to assess developmental status between 1 and 66 months of age, the ASQ-3 consists of 21 age-versions, each consisting of six questions per developmental domain (problem-solving, fine motor skills, gross motor skills, communication, and personal-social behavior) concerning key age-specific developmental milestones (37, 38). The scores of each domain were summed by the scores of their corresponding items. The higher score of each domain indicates the better performance of the child. According to the cutoff provided by the ASQ-3 user's guide, (39) a score that is more than two standard deviations below the mean indicates Suspected Developmental Delay (SDD) in each domain.
Predictors
Gestational age at birth was obtained from the mother's medical records, which was based on ultrasound examination and date of last menstrual period (LMP). Gestational weeks were divided into six categories according to previous literature (32). Full-term birth was defined as 39–40 weeks gestation. The other five categories were: very preterm and moderately preterm (<34 weeks), late-preterm (34–36 weeks), early-term (37–38 weeks), late-term (41 weeks) and post-term (>41 weeks).
Other Variates
We included the child, family, and maternal health characteristics as variables to control for when exploring the association between GA and children's ASQ-3 scores, based on the literature review (Supplementary Figure 1). All variables as shown in the columns in Table 1 were controlled for in the current study. Most of these variables were dichotomized into “yes” or “no”; BMI is an indicator of obesity which is based on height and weight [BMI = weight(kg)/height(m)] (40). Other developmental disorders included autism spectrum disorder, attention-deficit/hyperactivity disorder, learning disorders, etc. Family structures were classified into three types: three-generation (or more) family, nuclear family, and single-parent family. The “three-generation (or more) family” refers to the child living with his/her parents and grandparents, which is a traditional family structure in China; “nuclear family” refers to the child living only with his/her parents, and “single mother or father” means the child lives with one of his/her parents. We divided maternal age into three age bands: “ <30”, “30–34” and “>34” years old according to the literature (41). Higher education of mother or father refers to tertiary education leading to the award of an academic degree. Maternal complications of pregnancy and delivery were defined according to the International Classification of Diseases-Revision 10 (ICD-10). The classification is defined as having one of the following maternal complications during pregnancy, these include: vaginal bleeding during pregnancy, risk of miscarriage, use of antibiotics, use of fertility drugs, intrauterine distress, fetal asphyxia.
Table 1.
The child and family characteristics of the study population (n = 137,530).
| Characteristics | Total | Gender | |
|---|---|---|---|
|
Male
(n = 72,235) |
Female
(n = 65,295) |
||
| Child characteristics | |||
| Children's age (M, SD) | 4.348 (0.68) | 4.356 (0.67) | 4.338 (0.68) |
| BMI (M, SD) | 15.625 (1.63) | 15.783 (1.65) | 15.451 (1.60) |
| Right handedness (n %) | |||
| No | 9,884 (7.19) | 5,763 (7.98) | 4,121 (6.31) |
| Yes | 127,646 (92.81) | 66,472 (92.02) | 61,174 (93.69) |
| Eyesight (n %) | |||
| Normal | 124,053 (90.20) | 65,114 (90.14) | 58,939 (90.27) |
| Abnormal | 13,477 (9.80) | 7,121 (9.86) | 6,356 (9.73) |
| Birth weight (n %) | |||
| <2500g | 587 (4.70) | 297 (4.11) | 290 (4.44) |
| ≥2500g | 136,643 (95.30) | 71,938 (95.89) | 65,005 (95.45) |
| Gestational weeks at birth (n %) | |||
| <34 (very and moderately preterm) | 5,366 (3.90) | 2,905 (4.02) | 2,461 (3.77) |
| 34–36 (late-preterm) | 12,387 (9.01) | 6,809 (9.43) | 5,578 (8.54) |
| 37–38 (early-term) | 35,160 (25.57) | 19,364 (26.81) | 15,796 (24.19) |
| 39–40 (full-term) | 69,529 (50.56) | 35,733 (49.47) | 33,796 (51.76) |
| 41 (late-term) | 8,362 (6.0.8) | 4,045 (5.60) | 4,317 (6.61) |
| >41 (post-term) | 6,726 (4.89) | 3,379 (4.68) | 3,347 (5.13) |
| Delivery mode | |||
| Vaginal delivery | 72,169 (52.48) | 37,018 (51.25) | 35,151 (53.83) |
| Delivery with cesarean section | 65,361 (47.52) | 35,217 (48.75) | 30,144 (46.17) |
| NICU admission | |||
| No | 123,852 (90.05) | 64,548 (89.36) | 59,304 (90.82) |
| Yes | 13,678 (9.95) | 7,687 (10.64) | 5,991 (9.18) |
| Other developmental disordersa | |||
| No | 136,482 (99.24) | 71,606 (99.13) | 65,876 (99.36) |
| Yes | 1,048 (0.76) | 629 (0.87) | 419 (0.64) |
| Family characteristics | |||
| Higher education of motherb (n %) | |||
| No | 63,093 (45.88) | 33,568 (46.47) | 29,525 (45.22) |
| Yes | 74,437 (54.12) | 38,667 (53.53) | 35,770 (54.78) |
| Higher education of father b (n %) | |||
| No | 64,404 (46.83) | 34,177 (47.13) | 30,227 (46.29) |
| Yes | 73,126 (53.17) | 38,058 (52.69) | 35,068 (53.71) |
| Mother's occupation (n %) | |||
| Employed | 85,254 (61.99) | 45,082 (62.41) | 40,172 (55.62) |
| Unemployed | 52,276 (38.01) | 27,153 (37.59) | 25,123 (34.78) |
| Father's occupation (n %) | |||
| Employed | 108,383 (78.81) | 56,998 (78.91) | 51,385 (78.70) |
| Unemployed | 29,147 (21.19) | 15,237 (21.09) | 13,910 (21.30) |
| Family annual per-capita income (RMB)c (n %) | |||
| Below | 26,808 (19.49) | 13,910 (19.26) | 12,898 (19.75) |
| Above or equal to | 110,722 (80.51) | 58,325 (80.74) | 52,397 (80.25) |
| Family structure (n %) | |||
| Single families | 3,404 (2.38) | 1,718 (2.38) | 1,686 (2.58) |
| Nuclear families | 85,510 (62.17) | 45,090 (62.42) | 40,420 (61.91) |
| Extended families | 48,616 (35.35) | 25,427 (35.20) | 23,189 (35.51) |
| The number of children in the family (n %) | |||
| One | 61,431 (44.67) | 31,351 (43.40) | 30,080 (46.07) |
| Two or more | 76,099 (55.33) | 40,884 (56.60) | 35,215 (53.93) |
| Maternal health during pregnancy | |||
| Maternal age at delivery (n %) | |||
| <30 | 41,877 (30.45) | 21,829 (30.22) | 20,048 (30.70) |
| 30–34 | 66,432 (48.30) | 35,049 (48.52) | 31,383 (48.07) |
| ≥35 | 29,221 (21.25) | 15,357 (21.26) | 13,864 (21.23) |
| Smoking or passive smoking during pregnancy (n %) | |||
| No | 99,274 (72.18) | 52,101 (72.13) | 47,173 (72.25) |
| Yes | 38,356 (27.82) | 20,134 (27.87) | 18,122 (27.75) |
| Maternal complications during pregnancyd (n %) | |||
| No | 131,064 (95.30) | 68,910 (95.40) | 62,154 (95.19) |
| Yes | 6,466 (04.70) | 3,325 (4.60) | 3,141 (4.81) |
Other developmental disorders included autism spectrum disorder, attention-deficit/hyperactivity disorder, learning disorders.
Higher education of mother or father refers to tertiary education leading to the award of an academic degree.
The national average family per-capita income of the year before the survey time.
Having one of the following maternal complications during pregnancy including gestational diabetes, hypertensive disorders, vaginal bleeding during pregnancy, at risk of miscarriage, use of antibiotics, use of fertility drugs, intrauterine distress, fetal asphyxia.
Statistical Analysis
We used a generalized additive mixed model with thin plate regression splines to explore the non-linear relationship between gestational weeks and ASQ-3 scores. Based on the a stratified cluster sampling plan, a multi-level model was used which allows for grouping of child outcomes within nurseries and included residuals at the child and nursery school level, so as to represent the unobserved school characteristics that affect child outcomes. We hypothesized that there was no interaction between kindergartens and ASQ-3 scores, so we used the random intercept in the multilevel model. A mixed model utilizing a random intercept was used to investigate the associations of the different GA categories with all five sub-scores from the ASQ-3 when compared with full-term birth. A multilevel logistic regression model was also used to determine the strength of association for different GA categories and suspected neurodevelopmental delay in each domain of the ASQ-3 (0 = typically developing; 1 = SDD). A p-value less than 0.05 was denoted as statistically significant. LMER and GLMER procedure with R 4.0.1 was used for data statistical analysis.
Results
Of the 137,530 children aged 3–5 years old, 65,295 (47.48%) were females, and 72,235 (52.52%) were males. A total of 69,529 (50.56%) were full-term births (39–40 weeks); 5,366 (3.90%) were very and moderately preterm (<34 weeks), 12,387 (9.01%) were late preterm (34–36 weeks), 35,160 (25.57%) were early-term (37–38 weeks), 8 362 (6.08%) were late-term (41 weeks), and 6,726 (4.89%) were post-term (>41 weeks).
The scores of all five domains of the ASQ-3 were sorted by GA category (Table 2). Preterm and early-term children showed significantly lower scores in all five domains of the ASQ-3 compared with full-term children. For late-term children, their scores in gross motor skills and communication were significantly higher than those of full-term children, however, their scores in problem-solving, fine motor skills and personal-social behavior were better than early-term but worse than full-term children. All five ASQ-3 sub-scores for post-term children were significantly lower than full-term children but higher than preterm children.
Table 2.
ASQ-3 scores for each domain sorted by gestational age at birth (n = 137,530).
| ASQ-3 | |||||
|---|---|---|---|---|---|
| Gestational age | Communication | Gross motor | Fine motor | Problem-solving | Personal-social behavior |
| ASQ-3 scores Mean (SD) | |||||
| <34 (very and moderately preterm) | 51.69 (11.96) | 47.27 (13.76) | 46.58 (14.11) | 51.85 (11.89) | 52.98 (10.93) |
| 34–36 (late-preterm) | 52.74 (10.59) | 48.08 (13.07) | 47.27 (13.59) | 52.95 (10.68) | 54.01 (9.59) |
| 37–38 (early-term) | 53.82 (9.96) | 49.82 (12.11) | 48.45 (12.94) | 53.95 (9.84) | 54.49 (8.92) |
| 39–40 (full-term) | 54.34 (9.56) | 50.47 (11.74) | 49.14 (12.66) | 54.34 (9.53) | 54.82 (8.67) |
| 41 (late-term) | 54.45 (9.27) | 50.68 (11.54) | 49.12 (12.57) | 54.34 (9.35) | 54.78 (8.64) |
| >41 (post-term) | 53.11 (10.92) | 49.33 (12.67) | 48.49 (13.04) | 53.40 (10.56) | 54.43 (9.44) |
| Neurodevelopmental delay n (%) |
|||||
| <34 (very and moderately preterm) | 433 (8.09) | 987 (18.39) | 322 (6.00) | 261 (4.86) | 418 (7.79) |
| 34–36 (late-preterm) | 821 (6.63) | 2,160 (17.44) | 641 (5.17) | 481 (3.88) | 773 (6.24) |
| 37–38 (early-term) | 1,886 (5.36) | 4,546 (12.93) | 1,447 (4.12) | 1,094 (3.11) | 1,680 (4.78) |
| 39–40 (full-term) | 3,189 (4.58) | 8,066 (11.60) | 2,532 (3.64) | 2,081 (2.99) | 2,938 (4.23) |
| 41 (late-term) | 346 (4.14) | 925 (11.06) | 302 (3.61) | 230 (2.75) | 356 (4.26) |
| >41 (post-term) | 464 (6.90) | 982 (14.60) | 291 (4.30) | 255 (3.80) | 368 (5.47) |
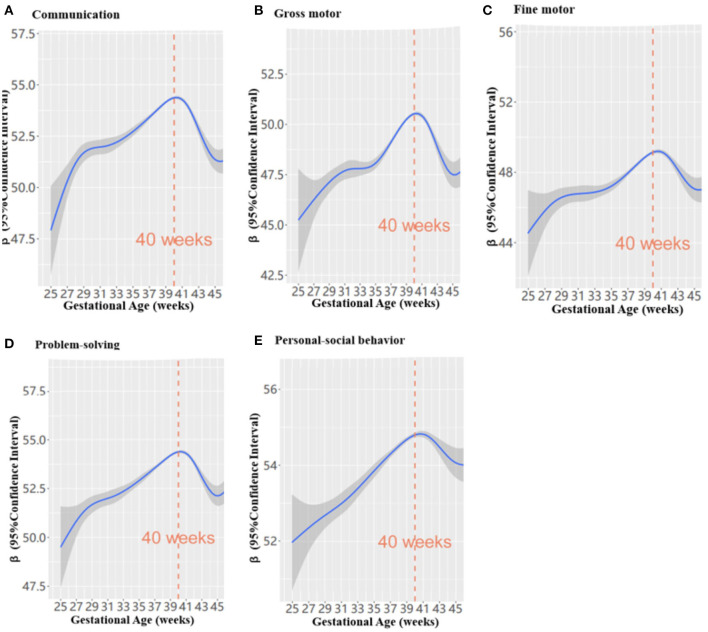
We observed a non-linear relationship of GA with scores of problem-solving, gross motor skill, fine motor skill, communication and personal-social behavior in the ASQ-3 (Figure 2). We found that the ASQ-3 scores in all five domains increased with rising gestational weeks before 40 weeks, and decreased with the fall of gestational weeks after 40 weeks (each p < 0.05). Children who were born at 40 weeks gestation performed the best in problem-solving, gross motor skills, fine motor skills, communication and personal-social behavior.
Figure 2.
The non-liner relationship between gestational weeks and ASQ-3 scores in preschoolers using a generalized additive mixed model (n = 137 530); (A–E) refers to different domains.
The crude and adjusted association between gestational age and scores on the ASQ-3 in preschoolers are shown in Table 3. Compared with the full-term group, associations between very and moderately preterm, late-preterm, early-term, late-preterm, and post-term groups in problem-solving (β were −0.19, 0.87, 0.25, 0.138, −0.56, each p < 0.05), gross motor skill (β were −2.02, −1.18, −0.39, −0.31, each p < 0.05), fine motor skill (β were −0.16, −0.93, −0.46, −0.10, each p < 0.05), communication (β were −1.84, −0.81, −0.31, −0.64 respectively, each p < 0.05), and personal-social behavior (β were −1.57, −0.59, −0.28, 0.07, −0.23, each p < 0.05) were found when adjusting for the child, family and maternal health characteristics. However, there were no statistically significant differences between late-term birth and ASQ-3 sub-scores when compared with full-term birth (each p > 0.05).
Table 3.
The association between gestational age and ASQ-3 scores in preschoolers (n = 137,530).
| Gestational week | Communication | Gross motor | Fine motor | Problem–solving | Personal social behavior | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude β | Adjusted βa | Crude β | Adjusted βa | Crude β | Adjusted βa | Crude β | Adjusted βa | Crude β | Adjusted βa | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| <34 (very and moderately preterm) |
−2.64 *** (−2.92, −2.37) |
−1.84*** (−2.12, −1.56) |
−3.20 *** (−3.53, −2.86) |
−2.02 *** (−2.36, −1.69) |
−2.56*** (−2.92, −2.20) |
−0.16*** (−1.95, −1.24) |
−2.50 *** (−2.77, −2.22) |
−0.19*** (−1.06, −0.68) |
−1.84*** (−2.09, −1.59) |
−1.57*** (−1.82, −1.32) |
| 34–36 (late–preterm) |
−1.59 *** (−1.78, −1.41) |
−0.80*** (−1.00, −0.61) |
−2.38*** (−2.62, −2.15) |
−1.18*** (−1.41, −0.95) |
−1.86*** (−2.11, −1.62) |
−0.93*** (−1.18, −0.69) |
−0.14*** (−1.58, −1.20) |
−0.87*** (−1.06, −0.68) |
−0.81*** (−0.98, −0.63) |
−0.59*** (−0.76, −0.41) |
| 37–38 (early–term) |
−0.51*** (−0.64, −0.39) |
−0.31*** (−0.44, −0.18) |
−0.65 *** (−0.81, −0.50) |
−0.39*** (−0.54, −0.24) |
−0.68*** (−0.85, 0.52) |
−0.46*** (0.62, −0.29) |
−0.39*** (−0.52, −0.27) |
−0.25*** (−0.38, −0.12) |
−0.33*** (−0.45, −0.22) |
−0.28*** (−0.40, −0.17) |
| 39–40 (full–term) | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 41 (late–term) |
0.11 (−0.11, 0.34) |
0.12 (−0.11, 0.34) |
0.21 (−0.06, 0.48) |
0.11 (−0.16, 0.38) |
−0.01 (−0.31, 0.28) |
0.11 (−0.18, 0.40) |
0.001 (−0.22, 0.22) |
0.13 (−0.09, 0.35) |
−0.04 (−0.24, 0.17) |
0.07 (−0.14, 0.027) |
| >41 (post–term) |
−1.22*** (−1.47, −0.98) |
−0.64 *** (−0.89, −0.39) |
−1.14*** (−1.44, −0.83) |
−0.31* (−0.61, −0.01) |
−0.65 *** (−0.97, −0.32) |
−0.10 (−0.42, 0.22) |
−0.94*** (−1.19, −0.69) |
−0.56*** (−0.81, −0.32) |
−0.0.39*** (0.61, −0.17) |
−0.23* (−0.46, −0.01) |
*p < 0.05, **p < 0.01, ***p < 0.001.
bAdjusting for the child, family characteristics and maternal health during pregnancy.
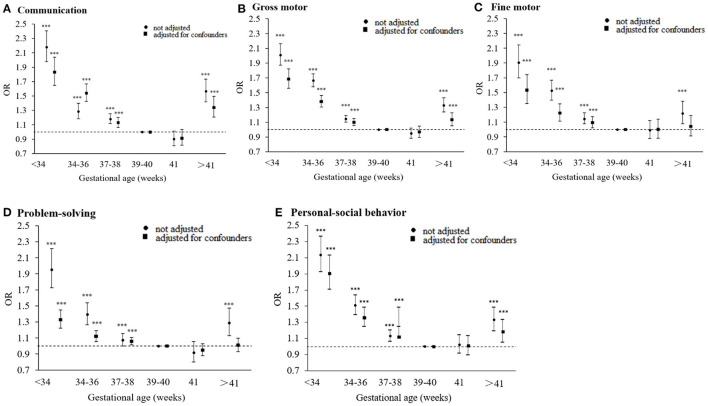
Associations were also found between GA categories and the risk of SDD when adjusting or not adjusting for the child, family, and maternal health characteristics (Figure 3). The adjusted risks of GA categories (very and moderately preterm, late-preterm, early-term, and post-term groups) on preschoolers' suspected neurodevelopmental delays were observed in communication (OR were 1.83, 1.28, 1.13, and 1.21 respectively, each p < 0.05), gross motor skill (OR were 1.67, 1.38, 1.10, and 1.05 respectively, each p < 0.05), and personal-social behavior (OR were 1.01, 1.36, 1.12, and 1.18 respectively, each p < 0.05). When adjusting for the child, family, and maternal health characteristics, we found the associations of GA categories (very and moderately preterm, late-preterm, and early-term) with fine motor skills (OR were 1.53, 1.22, and 1.09 respectively, each p < 0.05) and problem-solving (OR were 1.33, 1.12, and 1.06 respectively, each p < 0.05). However, no statistically significant difference was found for late-term birth on SDD when compared with full-term birth (each p > 0.05).
Figure 3.
Association between gestational age (weeks) and neurodevelopmental delays in preschoolers when adjusting or not adjusting for child, family characteristics and maternal health during pregnancy (n = 137 530); (A–E) refers to the different domains (***p < 0.001).
Comment
Principal Findings
To our knowledge, this is the first study to systematically examine the associations between GA across the full range of gestation and neurodevelopment based on a national population-based sample in China. A non-linear regression model was found to best explain the association between GA and children's neurodevelopment at 3–5 years old. Children's neurodevelopmental outcomes benefit from longer gestation before 40 gestational weeks but decrease after 40 gestational weeks. The novel findings of our study were that a higher risk of neurodevelopmental delays was found in early-term and post-term birth when compared with full-term birth.
Strengths and Limitations
The strengths of our study are that we involved a large national representative sample to examine the effects of GA across a full range and neurodevelopmental outcomes with a focus on early-term and post-term birth. We also included a comprehensive number of possible confounders including the child, family, and maternal health characteristics. Some limitations should also be considered in the current study. First, it should be noted that children with severe visual, hearing, intellectual impairments, or other severe developmental disorders who were required to attend special education schools/nurseries were not included in the current study. The previous studies (42, 43) suggested a strong association between preterm birth and special education needs in children with severe impairments, further studies should also include children with special education needs and examine the associations for children with severe impairments across the full range of gestation. Second, possible sample selection bias and recall bias might also exist: the current study only included children attending nurseries but did not include children who did not attend nurseries, and the accuracy of information provided using the ASQ-3 is dependent on the subjective judgment of parents. In addition, based on a retrospective cohort, our research may provide limited information when it comes to uncovering causal associations. In future studies, in-depth evaluation of developmental delays could be made to further verify the results of this study. Further research is needed to explain the mechanisms linking early- and post-term birth to neurodevelopmental delays. Randomized controlled trials could also be considered to provide evidence of the causal association between gestational age and neuropsychological development (44).
Interpretation
We observed an inverted U-shaped relationship between GA and neurodevelopmental outcome as measured by the ASQ-3 (in all five domains: problem-solving, fine motor skills, gross motor skills, communication, and personal-social behavior). We found that neurodevelopmental outcomes improved with the rising of gestational weeks before 40 gestational weeks, and decreased after 40 gestational weeks. The findings were consistent with previous studies that 40 gestational weeks at birth is the optimal GA for children's health (13, 23, 45–47). Similar evidence reported that gestation from 37 to 41 gestational weeks benefits both the cognitive and motor development of infants (19). Evidence from neuroimaging studies has indicated that a longer gestational duration was associated with region-specific increases in gray matter density (48) and a more efficient neural network (49). In particular, late gestational cerebellar growth is rapid as a linear increase in total gray matter volume of 1.4% per week can be seen from 29 to 41 gestational weeks (18, 50), and approximately 50% of the increase in cortical volume occur between 34 and 40 gestational weeks (51). Imaging studies have also suggested that a longer gestational duration was associated with region-specific increases (temporal lobes) in gray matter density (48), and children born at term with a shortened GA were observed to have a reduced neural network efficiency in the posterior medial cortex, including the precuneus, cuneus, and superior parietal regions (49). Another study has also suggested the benefits of longer gestation in temporal cortex development, one of the latest brain regions to mature during gestation, which is related to cognitive development (52).
For premature birth, we observed the strongest associations of very and moderately preterm with SDD, which is consistent with the reports of previous studies (8, 32, 53). Moreover, our results provide reliable evidence that late-preterm children also have an increased risk of neurodevelopmental delays based on a large national sample. The association between preterm birth and brain development has been well examined, and previous studies have shown that preterm birth is associated with altered hemispheric connections (54), loss of gray/white matter (55, 56), disrupted myelination, abnormal cortical folding (57, 58) and thalamocortical abnormalities (59). In particular, the abnormal connection and development of white matter and specific functional areas (especially the frontal cortex, corpus callosum, thalamus, and cingulate gyrus) are important causes of neurodevelopmental delay in preterm children (60). In addition, shortened gestation indicates that the fetus may be exposed to unfavorable intrauterine conditions. This may lead to modifications in the nervous system and an adjusted developmental trajectory to ensure survival for the fetus (61).
With our analysis, we found that even within the full-term range of GA (37–41 weeks), there were still significant differences among children born with different GAs. The early-term children (37–38 weeks) had a decreased neurodevelopmental outcomes and a higher risk of SDD when compared with their full-term counterparts. The results are consistent with previous studies in which early-term children have affected performance in cognition (14), motor (62), and language development (63, 64) during the early years of life. Our study has confirmed that such negative effects can last until 3–5 years of age, and also exists in social behaviors which are different from what has previously been reported in the literature (65). Our results support the findings from previous studies which emphasized the importance of the last few weeks of gestation (37 th and 38 th week) (66) The last few weeks are an important period for the development of the neurological system and the total volume of gray and white matter increases significantly between 35 and 41 weeks of gestation (67). Even at 38 weeks of gestation, the brain is still only 90% of the full-term weight (68).
More importantly, we found a stronger association between post-term birth and SDD when compared with late-preterm and early-term birth. In recent years, several studies have highlighted the significant negative effect on cognitive delays (29), emotional and behavioral problems (8, 30), and developmental disorders of post-term birth (8, 31). Our study confirms the negative effect of post-term birth on neurodevelopment in all five domains including problem-solving, communication, fine motor skills, gross motor skills, and personal-social behavior. The mechanisms underlying the negative effect of post-term birth on children's neurodevelopment can be explained by obstetric or neonatal complications marked by late-preterm birth, or a combination of both. First, a larger weight baby at birth, associated with a longer GA, has a higher risk for perinatal problems such as prolonged labor, which can cause a perinatal lack of oxygen, which has been shown to be associated with behavioral problems (69). Second, post-term birth is related to uteroplacental insufficiency (70), and compared with full-term birth, the placenta in post-term birth offers fewer nutrients and less oxygen which is associated with abnormal emotional and behavioral development (30). Finally, previous studies have shown that the placental secretion of corticotrophin-releasing hormone (71), the principal regulator of the maternal and fetal hypothalamic–pituitary–adrenal axis (72) is lower in women who deliver post-term than full-term. These neuroendocrine abnormalities might increase children's vulnerability to emotional and behavioral problems later in life (30).
Conclusions
Our study found a non-linear relationship between GA and children's neurodevelopmental outcomes, with 40 gestational weeks being the most optimal GA for children's neurodevelopment. Before 40 gestational weeks, neurodevelopmental outcomes increased with gestational weeks; and after 40 gestational weeks, neurodevelopmental outcomes decreased with gestational weeks. In addition to preterm birth, the risks of suspected neurodevelopmental delays increased in children born early-term and post-term. Our results can inform clinical professionals, parents, and teachers who should not neglect the long-term neurodevelopmental risks of being born in the early-term and post-term gestational period. The results of this study can inform clinical professionals when considering the optimal timing of birth during the full-term period. Elective birth interventions should only be recommended if the risk of continuing the pregnancy is higher than the delivery (post-term birth). The same as preterm birth, early-term and post-term born children also need close follow-up and additional assessment for detecting potential neurodevelopmental delays.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai First Maternity and Infant Hospital (KS18156). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
WD and JH: access to all of the data in the study, took responsibility for the integrity of the data, the accuracy of the data analysis, and concept and design. JH, YS, and GW: acquisition, analysis, or interpretation of data. JH: drafting of the manuscript. JH, WD, AB, and GW: critical revision of the manuscript for important intellectual content. YF, YZ, and YS: statistical analysis. JH and YS: obtained funding. WD and YS: administrative, technical, or material support. WD: supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Yao lin, Bolin Cui, Minhui Cao, Wenjing Wang, Xujie Mao, Haifeng Li, Guixia Chen, Lei Wang, Junyan Feng, Yingchun Liu, Lan Zhan, Ling Zhu, Tingting Weng, and Hongyan Guan for help with the material support and acquisition of data. We are grateful to the class teachers in all participated kindergarten for distributing the notification to parents to complete the online questionnaire. None of the contributors received compensation for their time. This study was supported by the National Natural Science Foundation of China (81673179), the Science and Technology Commission of Shanghai Municipality (19140903100), Shanghai Municipal Health Commission (2020YJZX0213), Pudong Municipal Health Commission (PW2020D-11), Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR1047B-003).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.860192/full#supplementary-material
References
- 1.WHO . ICD-11 for Mortality and Morbidity Statistics. Available online at: https://icd.who.int/browse11/lm/en/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1207960454. (2020).
- 2.American Psychiatric Association APADasmomdD-US . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington DC: American Psychiatric Association; (2013). 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- 3.Melillo R, Leisman G. Neurobehavioral Disorders of Childhood: An Evolutionary Perspective. Springer Science & Business Media. (2004). [Google Scholar]
- 4.Accardo PJ, Capute AJ. Capute & Accardo's Neurodevelopmental Disabilities in Infancy and Childhood. 3rd ed Baltimore: Paul H Brookes Pub. (2008). [Google Scholar]
- 5.Patel DR, Merrick J. Neurodevelopmental and neurobehavioral disorders. Transl Pediatr. (2020) 9:S1–2. 10.21037/tp.2020.02.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabie NZ, Bird TM, Magann EF, Hall RW, McKelvey SS. ADHD and developmental speech/language disorders in late preterm, early term and term infants. J Perinatol. (2015) 35:660–4. 10.1038/jp.2015.28 [DOI] [PubMed] [Google Scholar]
- 7.Girard LC, Doyle O, Tremblay RE. Breastfeeding, cognitive and noncognitive development in early childhood: a population study. Pediatrics. (2017) 139. 10.1542/peds.2016-1848 [DOI] [PubMed] [Google Scholar]
- 8.Persson M, Opdahl S, Risnes K, Gross R, Kajantie E, Reichenberg A, et al. Gestational age and the risk of autism spectrum disorder in Sweden, Finland, and Norway: a cohort study. PLoS Med. (2020) 17:e1003207. 10.1371/journal.pmed.1003207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Vliet EO, de Kieviet JF, van der Voorn JP, Been JV, Oosterlaan J, van Elburg RM. Placental pathology and long-term neurodevelopment of very preterm infants. Am J Obstet Gynecol. (2012) 206:489e481–487. 10.1016/j.ajog.2012.03.024 [DOI] [PubMed] [Google Scholar]
- 10.McGowan JE, Alderdice FA, Holmes VA, Johnston L. Early childhood development of late-preterm infants: a systematic review. Pediatrics. (2011) 127:1111–24. 10.1542/peds.2010-2257 [DOI] [PubMed] [Google Scholar]
- 11.Talge NM, Holzman C, Wang J, Lucia V, Gardiner J, Breslau N. Late-preterm birth and its association with cognitive and socioemotional outcomes at 6 years of age. Pediatrics. (2010) 126:1124–31. 10.1542/peds.2010-1536 [DOI] [PubMed] [Google Scholar]
- 12.Woythaler MA, McCormick MC, Smith VC. Late preterm infants have worse 24-month neurodevelopmental outcomes than term infants. Pediatrics. (2011) 127:e622–629. 10.1542/peds.2009-3598 [DOI] [PubMed] [Google Scholar]
- 13.Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ. (2012) 344:e896. 10.1136/bmj.e896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua J, Sun J, Cao Z, Dai X, Lin S, Guo J, et al. Differentiating the cognitive development of early-term births in infants and toddlers: a cross-sectional study in China. BMJ Open. (2019) 9:e025275. 10.1136/bmjopen-2018-025275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists . ACOG Committee Opinion No 579: definition of term pregnancy. Obstetr Gynecol. (2013) 122:1139–1140. 10.1097/01.AOG.0000437385.88715.4a [DOI] [PubMed] [Google Scholar]
- 16.Mally PV, Agathis NT, Bailey SM. Early term infants are at increased risk of requiring neonatal intensive care. World J Pediatr. (2016) 12:76–81. 10.1007/s12519-015-0049-8 [DOI] [PubMed] [Google Scholar]
- 17.Rose O, Blanco E, Martinez SM, Sim EK, Castillo M, Lozoff B, et al. Developmental scores at 1 year with increasing gestational age, 37-41 weeks. Pediatrics. (2013) 131:e1475–1481. 10.1542/peds.2012-3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. (2005) 115:688–95. 10.1542/peds.2004-1169 [DOI] [PubMed] [Google Scholar]
- 19.Espel EV, Glynn LM, Sandman CA, Davis EP. Longer gestation among children born full term influences cognitive and motor development. PLoS ONE. (2014) 9:e113758. 10.1371/journal.pone.0113758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang SPR, Kramer MS. Variation in child cognitive ability by week of gestation among healthy term births. Am J Epidemiol. (2010) 171:399–406. 10.1093/aje/kwp413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noble KG, FW, Rauh VA, et al. Academic achievement varies with gestational age among children born at term. Pediatrics. (2012) 130:e1–10. 10.1542/peds.2011-2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abel KHH, Wicks S, Rai D, Emsley R, Gardner R, Dalman C. Gestational age at birth and academic performance: population-based cohort study. Int J Epidemiol. (2017) 46:324–35. 10.1093/ije/dyw284 [DOI] [PubMed] [Google Scholar]
- 23.Yang S, Bergvall N, Cnattingius S, Kramer MS. Gestational age differences in health and development among young Swedish men born at term. Int J Epidemiol. (2010) 39:1240–9. 10.1093/ije/dyq070 [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Fontaine P. Common questions about late-term and postterm pregnancy. Am Fam Physician. (2014) 90:160–5. [PubMed] [Google Scholar]
- 25.Noble KG, Fifer WP, Rauh VA, Nomura Y, Andrews HF. Academic achievement varies with gestational age among children born at term. Pediatrics. (2012) 130:e257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Platt RW, Kramer MS. Variation in child cognitive ability by week of gestation among healthy term births. Am J Epidemiol. (2010) 171:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Los Santos-Garate AM V-GM, Villanueva-Garciá D et al. Perinatal morbidity and mortality in late-term and post-term pregnancy: NEOSANE perinatal network's experience in Mexico. J Perinatol. (2011) 31:789–93. 10.1038/jp.2011.43 [DOI] [PubMed] [Google Scholar]
- 28.Bruckner TACY, Caughey AB. Increased neonatal mortality among normal-weight births beyond 41 weeks of gestation in California. Am J Obstet Gynecol. (2008) 199:e1–7. 10.1016/j.ajog.2008.05.015 [DOI] [PubMed] [Google Scholar]
- 29.Glover Williams A, Odd D. Investigating the association between post-term birth and long term cognitive, developmental and educational impacts: a systematic review and Meta-analysis. J Matern Fetal Neonatal Med. (2020) 33:1253–65. 10.1080/14767058.2018.1514379 [DOI] [PubMed] [Google Scholar]
- 30.El Marroun H, Zeegers M, Steegers EA, van der Ende J, Schenk JJ, Hofman A, et al. Post-term birth and the risk of behavioural and emotional problems in early childhood. Int J Epidemiol. (2012) 41:773–81. 10.1093/ije/dys043 [DOI] [PubMed] [Google Scholar]
- 31.Movsas TZ, Paneth N. The effect of gestational age on symptom severity in children with autism spectrum disorder. J Autism Dev Disord. (2012) 42:2431–9. 10.1007/s10803-012-1501-4 [DOI] [PubMed] [Google Scholar]
- 32.Ask H, Gustavson K, Ystrom E, Havdahl KA, Tesli M, Askeland RB, et al. Association of gestational age at birth with symptoms of attention-deficit/hyperactivity disorder in children. JAMA Pediatr. (2018) 172:749–56. 10.1001/jamapediatrics.2018.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiingreen R, Greisen G, Svensson J, Hansen BM. Low gestational age at birth and difficulties in school-A matter of 'dose'. PLoS ONE. (2018) 13. 10.1371/journal.pone.0198482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yue A, Jiang Q, Wang B, Abbey C, Medina A, Shi Y, et al. Concurrent validity of the ages and stages questionnaire and the bayley scales of infant development III in China. PLoS ONE. (2019) 14:e0221675. 10.1371/journal.pone.0221675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steenis LJ, Verhoeven M, Hessen DJ, van Baar AL. Parental and professional assessment of early child development: the ASQ-3 and the Bayley-III-NL. Early Hum Dev. (2015) 91:217–25. 10.1016/j.earlhumdev.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 36.Wei M, Bian X, Squires J, Yao G, Wang X, Xie H, et al. Zhonghua er ke za zhi = Chin J Pediatr. (2015) 53:913–918. [PubMed] [Google Scholar]
- 37.Rubio-Codina M, Araujo MC, Attanasio O, Munoz P, Grantham-McGregor S. Concurrent validity and feasibility of short tests currently used to measure early childhood development in large scale studies. PLoS ONE. (2016) 11:e0160962. 10.1371/journal.pone.0160962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothstein A, Miskovic A, Nitsch K. Brief review of psychometric properties and clinical utility of the ages and stages questionnaires, third edition for evaluating pediatric development. Arch Phys Med Rehabil. (2017) 98:809–10. 10.1016/j.apmr.2016.11.001 [DOI] [Google Scholar]
- 39.Squires J, Twombly E, Bricker D, Potter L. Psychometric Studies of ASQ. ASQ-3 user's guide. (2009). [Google Scholar]
- 40.Consultation WHOE . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. (2004) 363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 41.Teng X, Shane MI, Pan S. The changing situation about maternal age, risk factors and pregnancy outcomes after the two-child policy: a retrospective cohort study. Ann Palliat Med. (2020) 9:824–34. 10.21037/apm.2020.04.27 [DOI] [PubMed] [Google Scholar]
- 42.MacKay DF, Smith GCS, Dobbie R, Pell JP. Gestational age at delivery and special educational need: retrospective cohort study of 407,503 Schoolchildren. PLoS Med. (2010) 7. 10.1371/journal.pmed.1000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore GS, Kneitel AW, Walker CK, Gilbert WM, Xing GB. Autism risk in small- and large-for-gestational-age infants. Am J Obstetr Gynecol. (2012) 206. 10.1016/j.ajog.2011.10.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hannah ME, Hannah WJ, Hellmann J, Hewson S, Milner R, Willan A. Induction of labor as compared with serial antenatal monitoring in postterm pregnancy - a randomized controlled trial. New Engl J Med. (1992) 326:1587–92. 10.1056/NEJM199206113262402 [DOI] [PubMed] [Google Scholar]
- 45.Altman M, Edstedt Bonamy AK, Wikstrom AK, Cnattingius S. Cause-specific infant mortality in a population-based Swedish study of term and post-term births: the contribution of gestational age and birth weight. BMJ Open. (2012) 2. 10.1136/bmjopen-2012-001152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and mortality in young adulthood. JAMA. (2011) 306:1233–40. 10.1001/jama.2011.1331 [DOI] [PubMed] [Google Scholar]
- 47.Jacob J, Lehne M, Mischker A, Klinger N, Zickermann C, Walker J. Cost effects of preterm birth: a comparison of health care costs associated with early preterm, late preterm, and full-term birth in the first 3 years after birth. Eur J Health Econ. (2017) 18:1041–6. 10.1007/s10198-016-0850-x [DOI] [PubMed] [Google Scholar]
- 48.Davis EP, Buss C, Muftuler LT, Head K, Hasso A, Wing DA, et al. Children's brain development benefits from longer gestation. Front Psychol. (2011) 2:1. 10.3389/fpsyg.2011.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim DJ, Davis EP, Sandman CA, Sporns O, O'Donnell BF, Buss C, et al. Longer gestation is associated with more efficient brain networks in preadolescent children. Neuroimage. (2014) 100:619–27. 10.1016/j.neuroimage.2014.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hüppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. (1998) 44:584–90. 10.1203/00006450-199810000-00019 [DOI] [PubMed] [Google Scholar]
- 51.Adams-Chapman I. Neurodevelopmental outcome of the late preterm infant. Clin Perinatol. (2006) 33:947–964. 10.1016/j.clp.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 52.Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A. (2010) 107:13135–40. 10.1073/pnas.1001229107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 288:728–737. 10.1001/jama.288.6.728 [DOI] [PubMed] [Google Scholar]
- 54.Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. (2000) 284:1939–47. 10.1001/jama.284.15.1939 [DOI] [PubMed] [Google Scholar]
- 55.Groeschel S, Tournier JD, Northam GB, Baldeweg T, Wyatt J, et al. Identification and interpretation of microstructural abnormalities in motor pathways in adolescents born preterm. Neuroimage. (2014) 87:209–19. 10.1016/j.neuroimage.2013.10.034 [DOI] [PubMed] [Google Scholar]
- 56.Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. (2008) 131:205–17. 10.1093/brain/awm282 [DOI] [PubMed] [Google Scholar]
- 57.Melbourne A, Kendall GS, Cardoso MJ, Gunny R, Robertson NJ, Marlow N, et al. Preterm birth affects the developmental synergy between cortical folding and cortical connectivity observed on multimodal MRI. Neuroimage. (2014) 89:23–34. 10.1016/j.neuroimage.2013.11.048 [DOI] [PubMed] [Google Scholar]
- 58.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. (2009) 8:110–124. 10.1016/S1474-4422(08)70294-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N, et al. The effect of preterm birth on thalamic and cortical development. Cereb Cortex. (2012) 22:1016–24. 10.1093/cercor/bhr176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rogers CE, Lean RE, Wheelock MD, Smyser CD. Aberrant structural and functional connectivity and neurodevelopmental impairment in preterm children. J Neurodev Disord. (2018) 10:38. 10.1186/s11689-018-9253-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandman CA, Davis EP. Neurobehavioral risk is associated with gestational exposure to stress hormones. Expert Rev Endocrinol Metab. (2012) 7:445–59. 10.1586/eem.12.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatziioannidis IKM, Exadaktylou S, Antoniou E, Zafeiriou D, Nikolaidis N. Neurological outcome at 6 and 12 months corrected age in hospitalised late preterm infants -a prospective study. Eur J Paediatric Neurol. (2018) 22:602–9. 10.1016/j.ejpn.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 63.Zambrana IM, Vollrath ME, Sengpiel V, Jacobsson B, Ystrom E. Preterm delivery and risk for early language delays: a sibling-control cohort study. Int J Epidemiol. (2016) 45:151–9. 10.1093/ije/dyv329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stene-Larsen K, Brandlistuen RE, Lang AM, Landolt MA, Latal B, Vollrath ME. Communication impairments in early term and late preterm children: a prospective cohort study following children to age 36 months. J Pediatr. (2014) 165:1123–8. 10.1016/j.jpeds.2014.08.027 [DOI] [PubMed] [Google Scholar]
- 65.Nielsen TM, Pedersen MV, Milidou I, Glavind J, Henriksen TB. Long-term cognition and behavior in children born at early term gestation: a systematic review. Acta Obstet Gynecol Scand. (2019) 98:1227–34. 10.1111/aogs.13644 [DOI] [PubMed] [Google Scholar]
- 66.Bolk J, Farooqi A, Hafstrom M, Aden U, Serenius F. Developmental Coordination Disorder and Its Association With Developmental Comorbidities at 6.5 Years in Apparently Healthy Children Born Extremely Preterm. JAMA Pediatr. (2018) 172:765–74. 10.1001/jamapediatrics.2018.1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol. (2006) 30:81–8. 10.1053/j.semperi.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 68.Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. (2006) 3:e265. 10.1371/journal.pmed.0030265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piesova M, Mach M. Impact of perinatal hypoxia on the developing brain. Physiol Res. (2020) 69:199–213. 10.33549/physiolres.934198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naeye RL. Causes of perinatal mortality excess in prolonged gestations. Am J Epidemiol. (1978) 108:429–33. 10.1093/oxfordjournals.aje.a112641 [DOI] [PubMed] [Google Scholar]
- 71.Smith R, Nicholson RC. Corticotrophin releasing hormone and the timing of birth. Front Biosci. (2007) 12:912–8. 10.2741/2113 [DOI] [PubMed] [Google Scholar]
- 72.Challis JR, Sloboda D, Matthews SG, Holloway A, Alfaidy N, Patel FA, et al. The fetal placental hypothalamic–pituitary–adrenal (HPA) axis, parturition and post natal health. Molec Cell Endocrinol. (2001) 185:135–144. 10.1016/S0303-7207(01)00624-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.