Abstract
Background
Most persistent poverty counties are rural and contain high concentrations of racial minorities. Cancer mortality across persistent poverty, rurality, and race is understudied.
Methods
We gathered data on race and cancer deaths (all sites, lung and bronchus, colorectal, liver and intrahepatic bile duct, oropharyngeal, breast and cervical [females], and prostate [males]) from the National Death Index (1990-1992; 2014-2018). We linked these data to county characteristics: 1) persistent poverty or not; and 2) rural or urban. We calculated absolute (range difference [RD]) and relative (range ratio [RR]) disparities for each cancer mortality outcome across persistent poverty, rurality, race, and time.
Results
The 1990-1992 RD for all sites combined indicated persistent poverty counties had 12.73 (95% confidence interval [CI] = 11.37 to 14.09) excess deaths per 100 000 people per year compared with nonpersistent poverty counties; the 2014-2018 RD was 10.99 (95% CI = 10.22 to 11.77). Similarly, the 1990-1992 RR for all sites indicated mortality rates in persistent poverty counties were 1.06 (95% CI = 1.05 to 1.07) times as high as nonpersistent poverty counties; the 2014-2018 RR was 1.07 (95% CI = 1.07 to 1.08). Between 1990-1992 and 2014-2018, absolute and relative disparities by persistent poverty widened for colorectal and breast cancers; however, for remaining outcomes, trends in disparities were stable or mixed. The highest mortality rates were observed among African American or Black residents of rural, persistent poverty counties for all sites, colorectal, oropharyngeal, breast, cervical, and prostate cancers.
Conclusions
Mortality disparities by persistent poverty endured over time for most cancer outcomes, particularly for racial minorities in rural, persistent poverty counties. Multisector interventions are needed to improve cancer outcomes.
Cancer is the second leading cause of death nationally (1), and disparities in mortality have been observed for decades (2). For example, cancer mortality among African American or Black (hereafter “Black”) individuals compared with White individuals is approximately 20% higher for males and approximately 13% higher for females (3). Disparities in cancer mortality can also be observed by contextual factors, such as rurality [mortality is 10% higher in rural compared with urban counties (4,5)] and area-level poverty [mortality is 19% higher in the most-deprived compared with least-deprived areas (6)].
Increasing attention has focused on health disparities in counties characterized by poverty, culminating with an executive order from President Joe Biden calling for efforts to advance equity for residents of persistent poverty counties (7). Persistent poverty counties are those that have had at least 20% of their residents living below the federal poverty line since 1980 (8). Persistent poverty counties have higher concentrations of racial minorities (9) and are disproportionately rural (10), and residents of persistent poverty counties face higher levels of cancer risks (11,12). Recently, we demonstrated that county-level cancer mortality is 12% higher in counties experiencing persistent poverty compared with all other counties and 7% higher compared with counties experiencing current (but not persistent) poverty (13). The reasons for these disparities are not well understood, but they could be related to multilevel characteristics of persistent poverty counties compared with other counties or compared with counties experiencing current poverty. For example, it is possible that county-level poverty, county-level rurality, and individual-level race jointly contribute to cancer disparities observed by persistent poverty.
This study will examine disparities in mortality rates for selected cancer outcomes by persistent poverty in 1990-1992 and 2014-2018, a period of approximately 25 years. In particular, we sought to examine how disparities have changed over time as well as how these disparities varied by rurality and race (Black and White race). These findings can contextualize cancer mortality disparities by providing a more nuanced examination of the interacting roles of persistent poverty and other markers of risk, which has implications for public health and clinical care.
Methods
Data Sources and Measures
United States Department of Agriculture (USDA) Economic Research Service (ERS): County-Level Characteristics
The USDA ERS classifies counties as persistent poverty if they had at least 20% of residents living below the federal poverty line according to decennial censuses in 1980, 1990, and 2000 and the American Community Survey’s 5-year (2007-2011) estimates (8). We categorized counties as persistent poverty or nonpersistent poverty.
USDA ERS also generates the 9-point county-level rural-urban continuum codes (14). Each county is assigned a code based on population size, level of urbanization, and adjacency to a metropolitan area (14). As is common practice in studies of rural and urban differences in health (15), we classified counties as urban (codes 1-3, metropolitan) or rural (codes 4-9, nonmetropolitan) for each time period.
National Death Index (NDI): Individual-Level Characteristic
From the NDI (16), we gathered race and anatomic cancer site for people who died of cancer in the United States in 1990-1992 and 2014-2018. (For data stability, we combined multiple years of deaths according to data availability.) Data on race came from death certificates, usually provided by a family member to a funeral director (16). We limited our analysis to individuals classified as Black or White to ensure adequate cell sizes and due to changes in reporting of racial categories over the study period (16).
We assessed overall cancer mortality, that is, “all sites.” In addition, we analyzed cancer sites with high mortality burden (17) and clinical opportunity for prevention or screening: lung and bronchus, colorectal, liver and intrahepatic bile duct, oropharyngeal, female breast, cervical, and prostate. For each outcome, age-adjusted mortality rates are expressed as the number of deaths per 100 000 people per year; breast and cervical cancers were measured among females only, and prostate cancer was measured among males only. Age-adjusted rates were standardized in reference to the 2000 US standard population.
Statistical Analysis
We used the Surveillance, Epidemiology, and End Results (SEER) *Stat software (18) to link NDI data to county-level characteristics using the Federal Information Processing Standards codes, which uniquely identify each county in the United States (19). We generated descriptive statistics to summarize the mean and standard error of annual age-adjusted cancer mortality rates for each time period, overall and by persistent poverty. Mean cancer mortality rates accounted for the population of each county within a given persistent poverty category. Cancer mortality rates by persistent poverty, rurality, and race for each time period appear in Supplementary Table 1 (available online).
Next, we used the Health Disparities Calculator (https://seer.cancer.gov/hdcalc/) (20) to quantify absolute and relative health disparities in cancer site mortality by persistent poverty for each time period. Specifically, we quantified disparities in cancer mortality using the range difference (RD), an absolute measure, and range ratio (RR), a relative measure (21,22). In the current study, RD is the difference in cancer death rates (per 100 000 people, females, or males per year) between persistent poverty and nonpersistent poverty counties, and RR is the ratio of cancer death rates in persistent poverty compared with nonpersistent poverty counties (21). Relative health disparity measures are commonly used in medical and public health research because they are easy to compare across outcomes, but absolute health disparity measures provide information on burden of disparity for the population (21). We evaluated the percent change in RD and RR between 1990-1992 and 2014-2018 for each outcome (23). Narrowing of absolute and relative health disparities in cancer mortality over time indicates reductions in differences and ratios, respectively; however, narrowing of absolute health disparities does not automatically indicate a narrowing of relative health disparities (or vice versa).
Finally, we examined patterns in cancer site mortality by cross-classifying county-level persistent poverty, county-level rurality, and individual-level race. We then generated the cancer site mortality rates for these subgroups at each time period to facilitate descriptive comparisons of the changes in rates and disparities over time across subgroups.
Analyses used a 2-sided P value of .05. Per federal regulations, this project was exempt from institutional review because it involved secondary analysis of publicly available datasets.
Results
County-Level Information
As of 2010, there were 3143 counties in the United States. Of these, 395 (12.6%) were persistent poverty counties. In 1990, 73.4% of all counties were rural, but in 2010, only 62.9% were rural. In 1990, the average county-level racial and ethnic composition was 8.6% Black and 89.0% White race; in 2010, these figures were 8.9% and 83.6%, respectively.
Patterns of Cancer Mortality by Persistent Poverty
Cancer Mortality Rates by Persistent Poverty
The 1990-1992 age-adjusted mortality rates were higher in persistent poverty than nonpersistent poverty counties for all sites, lung and bronchus, liver and intrahepatic bile duct, oropharyngeal, cervical, and prostate cancers (all P < .001) (Table 1). For example, mortality for all cancer sites was 226.33 (95% confidence interval [CI] = 225.02 to 227.64) in persistent poverty counties and 213.60 (95% CI = 213.25 to 213.95) in nonpersistent poverty counties (P < .001). Mortality for the 2 remaining cancer outcomes (colorectal and breast) did not differ by persistent poverty.
Table 1.
Cancer deaths and age-adjusted mortality rates by persistent poverty, National Death Index, 1990-1992 and 2014-2018
| Cancer outcome | Overall mortality |
By PP |
||||||
|---|---|---|---|---|---|---|---|---|
| Non-PP (k = 2748) |
PP (k = 395) |
% Difference in rates | P c | |||||
| No. | Mean ratea (95% CI) | No. | Mean rateb (95% CI) | No. | Mean rateb (95% CI) | |||
| 1990-1992 | ||||||||
| All sites | 1 540 609 | 214.48 (214.14 to 214.82) | 1 424 151 | 213.60 (213.25 to 213.95) | 116 458 | 226.33 (225.02 to 227.64) | 6.0 | <.001 |
| Lung and bronchus | 430 567 | 58.92 (58.74 to 59.10) | 398 435 | 58.72 (58.53 to 58.90) | 32 132 | 61.61 (60.93 to 62.29) | 4.9 | <.001 |
| Colorectal | 170 919 | 24.09 (23.97 to 24.20) | 158 709 | 24.11 (23.99 to 24.23) | 12 210 | 23.86 (23.43 to 24.29) | −1.0 | .27 |
| Liver and intrahepatic bile duct | 26 912 | 3.74 (3.69 to 3.78) | 24 490 | 3.67 (3.62 to 3.71) | 2422 | 4.69 (4.50 to 4.88) | 27.8 | <.001 |
| Oropharyngeal | 24 789 | 3.46 (3.42 to 3.51) | 22 706 | 3.41 (3.37 to 3.46) | 2083 | 4.11 (3.93 to 4.29) | 20.5 | <.001 |
| Breastd | 130 034 | 32.48 (32.30 to 32.66) | 120 702 | 32.50 (32.31 to 32.69) | 9332 | 32.32 (31.66 to 33.00) | −0.6 | .61 |
| Cervicald | 13 782 | 3.56 (3.50 to 3.62) | 12 241 | 3.41 (3.35 to 3.47) | 1541 | 5.54 (5.26 to 5.83) | 62.5 | <.001 |
| Prostatee | 100 177 | 39.04 (38.79 to 39.30) | 92 145 | 38.72 (38.46 to 38.98) | 8032 | 43.34 (42.37 to 44.32) | 11.9 | <.001 |
| 2014-2018 | ||||||||
| All sites | 2 984 000 | 155.46 (155.28 to 155.64) | 2 792 787 | 154.80 (154.61 to 154.98) | 191 213 | 165.79 (165.04 to 166.55) | 7.1 | <.001 |
| Lung and bronchus | 746 042 | 38.48 (38.39 to 38.57) | 697 911 | 38.30 (38.21 to 38.39) | 48 131 | 41.21 (40.84 to 41.59) | 7.6 | <.001 |
| Colorectal | 261 043 | 13.67 (13.62 to 13.73) | 242 818 | 13.53 (13.48 to 13.59) | 18 225 | 15.89 (15.65 to 16.12) | 17.4 | <.001 |
| Liver and intrahepatic bile duct | 131 816 | 6.62 (6.58 to 6.65) | 122 005 | 6.52 (6.48 to 6.55) | 9811 | 8.20 (8.03 to 8.36) | 25.8 | <.001 |
| Oropharyngeal | 49 612 | 2.53 (2.50 to 2.55) | 46 330 | 2.51 (2.49 to 2.53) | 3282 | 2.80 (2.70 to 2.90) | 11.6 | <.001 |
| Breastd | 208 686 | 20.11 (20.02 to 20.20) | 194 830 | 19.98 (19.89 to 20.08) | 13 856 | 22.00 (21.63 to 22.38) | 10.1 | <.001 |
| Cervicald | 20 823 | 2.23 (2.20 to 2.27) | 18 958 | 2.17 (2.14 to 2.20) | 1865 | 3.23 (3.08 to 3.39) | 48.8 | <.001 |
| Prostatee | 149 535 | 19.04 (18.94 to 19.14) | 139 552 | 18.86 (18.76 to 18.96) | 9983 | 21.86 (21.43 to 22.30) | 15.9 | <.001 |
The mean rates presented in the “Overall mortality” columns are average annual age-adjusted mortality rates. CI = confidence interval; PP = persistent poverty.
Mean rates presented in the “non-PP” and “PP” columns are means of the average annual age-adjusted mortality rates for each group of counties. Mean mortality rates are weighted by county population.
Comparisons used a 2-sided z test to calculate P values.
Among females.
Among males.
The 2014-2018 age-adjusted mortality rates were higher in persistent poverty than nonpersistent poverty counties for all outcomes (all P < .001) (Table 1). For example, mortality for all cancer sites was 165.79 (95% CI = 165.04 to 166.55) in persistent poverty counties and 154.80 (95% CI = 154.61 to 154.98) in nonpersistent poverty counties (P < .001). For most outcomes, mortality rates were lower in 2014-2018 than in 1990-1992.
Absolute Health Disparities in Cancer Mortality
In 1990-1992, mortality rates for 6 cancer outcomes demonstrated statistically significant absolute health disparities (ie, RD > 1) (Table 2), indicating higher cancer mortality rates in persistent poverty vs nonpersistent poverty counties (all sites, RD = 12.73 [95% CI = 11.37 to 14.09]; lung and bronchus, RD = 2.88 [95% CI = 2.18 to 3.59]; liver and intrahepatic bile duct, RD = 1.02 [95% CI = 0.83 to 1.22]; oropharyngeal, RD = 0.69 [95% CI = 0.51 to 0.88]; cervical, RD = 2.13 [95% CI = 1.84 to 2.42]; and prostate, RD = 4.62 [95% CI = 3.61 to 5.63]). For example, the RD for all sites in 1990-1992 indicates that persistent poverty counties had an additional 12.73 cancer deaths per 100 000 people per year compared with nonpersistent poverty counties (P < .05). There was no difference in mortality rates by persistent poverty for colorectal or breast cancers (ie, RDs were indistinguishable from 1).
Table 2.
Absolute (RD) and relative (RR) measures of overall health disparities by persistent poverty for age-adjusted cancer mortality rates, National Death Index, 1990-1992 vs 2014-2018a
| Cancer outcome | Absolute health disparity |
Relative health disparity |
||||
|---|---|---|---|---|---|---|
| 1990-1992 RD (95% CI) | 2014-2018 RD (95% CI) | % Changeb over time periods | 1990-1992 RR (95% CI) | 2014-2018 RR (95% CI) | % Changeb over time periods | |
| All sites | 12.73 (11.37 to 14.09) | 10.99 (10.22 to 11.77) | − | 1.06 (1.05 to 1.07) | 1.07 (1.07 to 1.08) | − |
| Lung and bronchus | 2.88 (2.18 to 3.59) | 2.91 (2.52 to 3.30) | − | 1.05 (1.04 to 1.06) | 1.08 (1.07 to 1.09) | 2.6 |
| Colorectal | 0.25 (−0.20 to 0.69) | 2.36 (2.11 to 2.60) | 849.6 | 1.01 (0.99 to 1.03) | 1.17 (1.16 to 1.19) | 16.2 |
| Liver and intrahepatic bile duct | 1.02 (0.83 to 1.22) | 1.68 (1.51 to 1.85) | 64.2 | 1.28 (1.23 to 1.33) | 1.26 (1.23 to 1.28) | − |
| Oropharyngeal | 0.69 (0.51 to 0.88) | 0.29 (0.19 to 0.39) | −58.8 | 1.20 (1.15 to 1.26) | 1.11 (1.07 to 1.16) | − |
| Breastc | 0.18 (−0.52 to 0.87) | 2.02 (1.63 to 2.40) | 1040.1 | 1.01 (0.98 to 1.03) | 1.10 (1.08 to 1.12) | 9.5 |
| Cervicalc | 2.13 (1.84 to 2.42) | 1.06 (0.91 to 1.22) | −50.1 | 1.63 (1.54 to 1.72) | 1.49 (1.42 to 1.57) | − |
| Prostated | 4.62 (3.61 to 5.63) | 3.00 (2.55 to 3.45) | −35.2 | 1.12 (1.09 to 1.15) | 1.16 (1.14 to 1.18) | − |
In the “% change” columns, positive entries indicated widening disparities, and negative entries indicate narrowing disparities. CI = confidence interval; RD = range difference; RR = range ratio.
bPercent change is the percent change for each health disparity measure from 1990-1992 to 2014-2018.
Among females.
Among males.
In 2014-2018, mortality rates for all cancer outcomes demonstrated statistically significant absolute health disparities (Table 2) (RD ranged from 0.29 for oropharyngeal to 10.99 for all sites). For example, the RD for all sites in 2014-2018 indicates that persistent poverty counties had an additional 10.99 (95% CI = 10.22 to 11.77) cancer deaths per 100 000 people per year compared with nonpersistent poverty counties (P < .05).
Over the 25-year period, absolute health disparities in cancer mortality widened for colorectal (+849.6%, or an 8.50-fold increase in the RD over time periods), liver and intrahepatic bile duct (+64.2%), and breast (+1040.1%) cancers, but narrowed for oropharyngeal (−58.8%), cervical (−50.1%), and prostate (−35.2%) cancers (Table 2). Absolute health disparities for all sites and for lung and bronchus cancer did not change.
Relative Health Disparities in Cancer Mortality
In 1990-1992, mortality rates for 6 cancer outcomes demonstrated statistically significant relative health disparities (ie, RR > 1) (Table 2), indicating relatively elevated death rates for persistent poverty vs nonpersistent poverty counties (all sites, RR = 1.06 [95% CI = 1.05 to 1.07]; lung and bronchus, RR = 1.05 [95% CI = 1.04 to 1.06]; liver and intrahepatic bile duct, RR = 1.28 [95% CI = 1.23 to 1.33]; oropharyngeal, RR = 1.20 [95% CI = 1.15 to 1.26]; cervical, RR = 1.63 [95% CI = 1.54 to 1.72]; and prostate, RR = 1.12 [95% CI = 1.09 to 1.15]). For example, the RR for all sites in 1990-1992 indicates that persistent poverty counties had mortality rates that were 1.05 times as high as the rates for nonpersistent poverty counties (P < .05). There was no difference in relative mortality rates for persistent poverty vs nonpersistent poverty counties for colorectal or breast cancers (ie, RRs were indistinguishable from 1).
In 2014-2018, mortality rates for all cancer outcomes demonstrated statistically significant relative health disparities (Table 2) (RR ranged from 1.07 for all sites to 1.49 for cervical [95% CI = 1.07 to 1.08]). For example, the RR for all sites in 2014-2018 indicates that persistent poverty counties had mortality rates that were 1.07 times as high as the rates for nonpersistent poverty counties (P < .05).
Over the 25-year period, relative health disparities in cancer mortality widened for lung and bronchus (+2.6%), colorectal (+16.2%), and breast (+9.5%) cancers, but did not change for the remaining outcomes (Table 2).
Cancer Mortality Rates by Persistent Poverty, Rurality, and Race
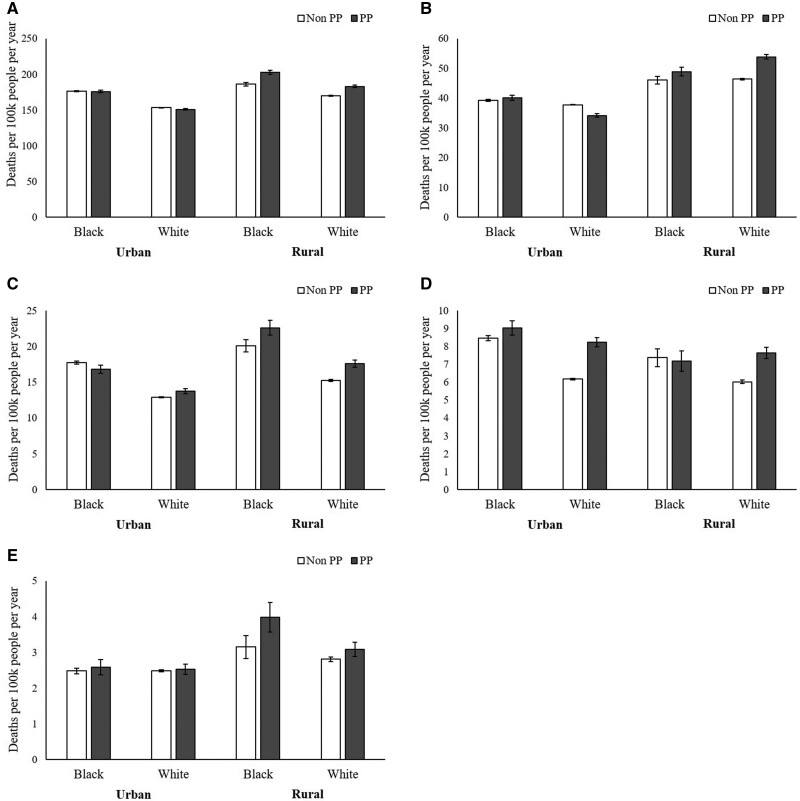
Analysis of 2014-2018 cancer mortality rates across subgroups defined by persistent poverty, rurality, and race provides more details about differences by persistent poverty (Figure 1: cancers that affect all sexes; Figure 2: cancers that affect females; Figure 3: cancers that affect males). Across subgroups, rates were generally higher in persistent poverty counties than nonpersistent poverty counties. Mortality rates were highest among Black residents of counties that were both rural and persistent poverty for 6 out of the 8 outcomes assessed (all sites, colorectal, oropharyngeal, breast, cervical, and prostate).
Figure 1.
Age-adjusted mortality rates per 100 000 people per year for (A) all sites, (B) lung and bronchus, (C) colorectal, (D) liver and intrahepatic bile duct, and (E) oropharyngeal cancers by county-level persistent poverty (PP), county-level rurality, and individual-level race, National Death Index, 2014-2018. White columns = PP counties; shaded columns = non-PP counties. Error bars indicate 95% confidence intervals.
Figure 2.
Age-adjusted mortality rates per 100 000 females per year for (A) breast and (B) cervical cancers by county-level persistent poverty (PP), county-level rurality, and individual-level race, National Death Index, 2014–2018. White columns = PP counties; shaded columns = non-PP counties. Error bars indicate 95% confidence intervals.
Figure 3.
Age-adjusted mortality rates per 100 000 males per year for prostate cancer by county-level persistent poverty (PP), county-level rurality, and individual-level race, National Death Index, 2014-2018. White columns = PP counties; shaded columns = non-PP counties. Error bars indicate 95% confidence intervals.
Supplementary Table 2 (available online) presents cancer mortality disparities between 1990-1992 and 2014-2018 for all the subgroups defined by combinations of persistent poverty, rurality, and race. Across all 8 subgroups, absolute and relative disparities increased over the study period for lung and bronchus cancer mortality (+24.5% and +23.4%, respectively). In contrast, absolute and relative disparities decreased for oropharyngeal (−57.2% and −27.7%, respectively) and cervical (−58.5% and −28.2%, respectively). The remaining cancers had stable or mixed trends in disparities over the study period. Supplementary Figures 1-8 (available online) illustrate changes in these mortality rates across subgroups from 1990-1992 to 2014-2018.
Discussion
In this analysis of age-adjusted cancer mortality comparing 2 time periods across 25 years in the United States, for almost all outcomes, persistent poverty counties had higher cancer mortality rates than nonpersistent poverty counties, resulting in absolute health disparities (ie, differences in death rates) and relative health disparities (ie, ratios of death rates) in 2014-2018. For colorectal and breast cancers, both absolute and relative health disparities as measured by cancer mortality rates widened over the study period; for lung and bronchus, relative (but not absolute) health disparities widened; for liver and intrahepatic bile duct, absolute (but not relative) health disparities widened; for all sites, absolute and health disparities remained stable; and for oropharyngeal, cervical, and prostate cancers, absolute disparities narrowed but relative health disparities remained stable. Additional markers or risk, that is, rurality and race, intersect with persistent poverty disparities such that, as of 2014-2018, Black residents of rural persistent poverty counties had the highest mortality rates for 6 out of 8 cancer outcomes. Taken together, these findings indicate enduring or widening disparities by persistent poverty in several cancer mortality outcomes, underscoring the recent Presidential executive order (EO 13985) calling for additional research and intervention in persistent poverty counties to promote health equity (7).
Absolute and/or relative disparities in mortality by persistent poverty widened over the study period for 4 cancer outcomes (lung and bronchus; colorectal; liver and intrahepatic bile duct; and breast). For colorectal and breast cancers, we found large increases in absolute health disparities (RD = +849.6% and +1040.1%, respectively) but moderate increases in relative health disparities (RR = +16.2% and +9.5%, respectively). This pattern occurred because the differences in death rates (ie, RD, a measure of absolute health disparities) by persistent poverty from these cancers were close to 0 in 1990-1992, but they were larger in 2014-2018; the change in the RD over time appears large because it is divided by a number close to 0 in the early time period. However, the ratio of death rates by persistent poverty for these 2 cancers (ie, RR, a measure of relative health disparities) considers the mortality rates themselves (not just the absolute difference between them) (24). These findings underscore the methodological importance of calculating and reporting both absolute and relative measures of disparities.
In contrast, absolute disparities in cancer mortality by persistent poverty narrowed over the study period (but relative disparities were stable) for 3 cancer sites: oropharyngeal, cervical, and prostate. These trends emerged because, even though persistent poverty and nonpersistent poverty counties both saw reductions in mortality over the study period, in absolute terms, these reductions were larger for persistent poverty counties. This pattern is most easily observed for cervical cancer mortality rates, which, between 1990-1992 and 2014-2018, decreased by 1.24 deaths per 100 000 women per year (or 36.4% of the baseline rate) in nonpersistent poverty counties but 2.31 deaths per 100 000 women per year (or 41.7% of the baseline rate) in persistent poverty counties. Even though the absolute reduction was almost twice as high for persistent poverty compared with nonpersistent poverty counties (ie, 2.31 vs 1.24), in the context of their baseline rates, the relative reductions were similar (ie, 41.7% vs 36.4%, respectively), resulting in narrowed absolute disparities but stable relative disparities over time. This pattern distinguishing oropharyngeal, cervical, and prostate cancers from other outcomes included in this analysis could be related to temporal changes in county- and individual-level risk behaviors or clinical prevention, diagnosis, or treatment modalities (25). Future studies should investigate these causal mechanisms. However, it should be noted that even though the absolute health disparities for these 3 cancers decreased over the study period, the 2014-2018 mortality rates from oropharyngeal, cervical, and prostate cancers are statistically significantly higher in persistent poverty counties than nonpersistent poverty counties, indicating that additional work is needed to promote health equity.
Persistent poverty counties face several clinical and public health challenges, including a shortage of health-care providers (26,27), resulting in decreased access to care and lower uptake of cancer prevention and screening services (28-30). In recent years, rural health-care facilities have eliminated service offerings or even closed (31,32); these closures have likely introduced challenges to health-care access in persistent poverty counties. Without concerted effort to improve health-care systems in persistent poverty counties, cancer mortality disparities may continue to worsen. Additional county- and individual-level risk factors are likely to contribute to cancer mortality disparities by persistent poverty. For example, cigarette smoking and chewing tobacco or snuff is more common for lower-income individuals (33) and communities (34) and in rural communities (35); disparities in tobacco-related cancers may be related to variation in cigarette smoking by persistent poverty (and by rurality and race). Multilevel interventions across the cancer control continuum (36) are needed to begin to ameliorate these disparities. These interventions will be difficult to implement in areas with the highest rates of cancer mortality, so stakeholders will need to be resourceful, creative, collaborative, and innovative.
Further, public health interventions must be sensitive to the complex social and structural environments in persistent poverty counties. These counties are predominantly rural (10), and rural populations have higher rates of cancer-causing behaviors [such as tobacco use (35)], lower cancer screening rates (30), higher cancer mortality (4,5), and greater delays in seeking medical treatment (37) compared with urban populations. Rural providers face challenges in delivering care, such as overlapping roles (eg, family practice includes psychiatry, orthopedics, and obstetrics), confidentiality issues, and resource limitations (38). Further, persistent poverty counties have higher concentrations of minority populations (10), and the undue burden of cancer mortality among racial minorities has been well described in the research literature for more than 65 years (2,39). Concerns about systemic and medical racism resulting in medical mistrust may be even more pertinent in persistent poverty counties due to the strained social, economic, and medical systems in those communities (40,41). These constructs are likely relevant in this study, because we found the highest mortality rates among Black residents of rural persistent poverty counties for 6 out of the 8 cancer outcomes. Disparities across levels of persistent poverty, rurality, and race widened for lung and bronchus cancer from 1990-1992 to 2014-2018, which is concerning given that lung cancer is the leading cause of cancer death (17). Conversely, these disparities narrowed for oropharyngeal and cervical cancers, which is an encouraging development. Reversing the negative trends among rural, persistent poverty, minority groups should be a priority for intervention work. Although all residents of persistent poverty counties would benefit from cancer prevention and control interventions, programs focused on specific risk behaviors [eg, smoking cessation (42)] and services [eg, human papillomavirus vaccination (43)] associated with the disparities previously discussed and tailored to the most vulnerable subpopulations, such as rural Black residents, may have the greatest impact on reducing cancer-related disparities.
In terms of study strengths, we used data from 2 time periods spanning 25 years from the NDI, a near-complete registry of deaths in the United States. With these data, we were able to analyze group-, time-, and site-specific cancer mortality rates. We examined several cancer sites to identify patterns of mortality by persistent poverty. In addition, we analyzed absolute and relative health disparities, which provide formal tests of the degree and changes in disparities in cancer mortality. These study strengths facilitated an in-depth analysis of disparities across multiple county- and individual-level markers of risk. In terms of study limitations, we did not conduct a full multilevel analysis to completely account for individual- and area-level correlates of cancer outcomes; future studies should use analytic techniques such as hierarchical modeling to assess changes in mortality rates for subgroups nested within counties over time. Mortality outcomes for other cancer sites were excluded from the current analysis, but informative patterns may be observed for those cancers. In addition, analysis of cancer incidence or incidence-based mortality could provide useful insights into the development of cancer mortality, especially given evidence that incidence rates do not necessarily demonstrate the same disparities that mortality rates do (44). Misclassification of (1) cause of death, (2) race, and (3) place of residence may have biased the results. Detailed information on race, ethnicity, and origin (which was not available for this analysis) would also provide greater insight into multilevel influences on disparities by persistent poverty. Future studies should attempt to overcome these limitations and fill additional research gaps, including investigating the roles of individual-level factors (eg, educational attainment, occupational exposure, behavioral risks) and contextual factors (eg, access to care, environmental risks) in influencing cancer burden for persistent poverty vs nonpersistent poverty counties.
In conclusion, we found enduring or widening health disparities for several cancer mortality outcomes by county-level persistent poverty, with particularly high rates for Black residents in persistent poverty counties that were also rural. As of 2014-2018, absolute and relative disparities in mortality were evident for all cancer outcomes under study, indicating elevated cancer mortality in persistent poverty compared with nonpersistent poverty counties. To promote public health and improve clinical care, multilevel interventions are needed to address challenges across the cancer control continuum and reduce the unjust burden of cancer mortality in persistent poverty counties in the United States.
Funding
This work was supported by the National Institutes of Health (active duty for JLM, SS, KAC, and RTC; and grant number K22 CA225705 to JLM).
Notes
Role of the funder: The funder played no role in the study design, study conduct, data analysis, interpretation of results, or preparation or approval of the manuscript. The decision to submit the manuscript for publication was made without input from the funder.
Disclosures: The authors have no potential conflicts of interest to disclose.
Author contributions: JLM: Conceptualization, data curation, formal analysis, validation, investigation, visualization, methodology, writing–original draft, writing–review and editing. CNP: Conceptualization, investigation, writing–review and editing. SS: Conceptualization, resources, supervision, writing–original draft, writing–review and editing. KAC: Conceptualization, resources, software, supervision, validation, investigation, methodology, writing–original draft, project administration, writing–review and editing. RTC: Conceptualization, resources, supervision, validation, writing–original draft, project administration, writing–review and editing.
Disclaimers: The opinions expressed in this article are the authors' own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Data Availability
All data are publicly available for access with SEER*Stat via a signed research data agreement. For full details, see https://seer.cancer.gov/mortality/.
Supplementary Material
Contributor Information
Jennifer L Moss, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD, USA; Department of Family and Community Medicine, Penn State College of Medicine, The Pennsylvania State University, Hershey, PA, USA; Department of Public Health Sciences, Penn State College of Medicine, The Pennsylvania State University, Hershey, PA, USA.
Casey N Pinto, Department of Family and Community Medicine, Penn State College of Medicine, The Pennsylvania State University, Hershey, PA, USA; Department of Public Health Sciences, Penn State College of Medicine, The Pennsylvania State University, Hershey, PA, USA.
Shobha Srinivasan, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD, USA.
Kathleen A Cronin, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD, USA.
Robert T Croyle, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD, USA.
References
- 1. Heron MP. Deaths: leading causes for 2016. Natl Vital Stat Rep. 2018;67(6):1–77. [PubMed] [Google Scholar]
- 2. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78–93. [DOI] [PubMed] [Google Scholar]
- 3. Henley SJ, Ward EM, Scott S, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2020;126(10):2225–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henley SJ, Anderson RN, Thomas CC, Massetti GM, Peaker B, Richardson LC. Invasive cancer incidence, 2004-2013, and deaths, 2006-2015, in nonmetropolitan and metropolitan counties - United States. MMWR Surveill Summ. 2017;66(14):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part I-all cancers and lung cancer and part II-colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biden JR. Executive order on advancing racial equity and support for underserved communities through the federal government. The White House. Published 2021. https://www.whitehouse.gov/briefing-room/presidential-actions/2021/01/20/executive-order-advancing-racial-equity-and-support-for-underserved-communities-through-the-federal-government/. Accessed November 30, 2021 .
- 8.US Department of Agriculture. Descriptions and maps: county economic types, 2015 edition. Published 2017. https://www.ers.usda.gov/data-products/county-typology-codes/descriptions-and-maps/. Accessed November 30, 2021.
- 9. Beale CL. The ethnic dimension of persistent poverty in rural and small-town areas. In: Swanson LL, ed. Racial/Ethnic Minorities in Rural Areas: Progress and Stagnation, 1980-90. Rural Economy Division, Economic Research Service, U.S. Department of Agriculture; 1980:26-32. [Google Scholar]
- 10. Miller KK, Weber BA. Persistent poverty across the rural-urban continuum. Rural Poverty Research Center Working Paper. Published 2003. https://ageconsearch.umn.edu/record/18910?ln=en. Accessed November 30, 2021.
- 11. Miller KK, Crandall MS, Weber BA, Persistent poverty and place: how do persistent poverty and poverty demographics vary across the rural-urban continuum. Paper presented at: Measuring Rural Diversity; July 2003; Montreal, Quebec, Canada.
- 12. Bennett KJ, Probst JC, Pumkam C. Obesity among working age adults: the role of county-level persistent poverty in rural disparities. Health Place. 2011;17(5):1174–1181. [DOI] [PubMed] [Google Scholar]
- 13. Moss JL, Pinto CN, Srinivasan S, Cronin KA, Croyle RT. Persistent poverty and cancer mortality rates: an analysis of county-level poverty designations. Cancer Epidemiol Biomarkers Prev. 2020;29(10):1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Department of Agriculture. Rural-urban continuum codes: overview. Published 2013. http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx. Accessed November 30, 2021.
- 15. Meilleur A, Subramanian SV, Plascak JJ, Fisher JL, Paskett ED, Lamont EB. Rural residence and cancer outcomes in the United States: issues and challenges. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1657–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. National Death Index. Published 2017. https://www.cdc.gov/nchs/ndi/index.htm. Accessed November 30, 2021.
- 17. Ward E, Sherman RL, Henley SJ, et al. Annual report to the nation on the status of cancer, 1999-2015. Featuring cancer in men and women ages 20-49. J Natl Cancer Inst. 2019;111(12):1279-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Cancer Institute Surveillance Research Program. SEER*Stat software (seer.cancer.gov/seerstat) version 8.3.2. Published 2021. https://seer.cancer.gov/seerstat/. Accessed November 30, 2021.
- 19.National Institute of Standards and Technology. Current FIPS. Published 2018. https://www.nist.gov/itl/current-fips. Accessed November 30, 2021.
- 20.National Cancer Institute. Health disparities calculator (HDCalc). Published 2013. https://seer.cancer.gov/hdcalc/. Accessed November 30, 2021.
- 21. Harper S, Lynch J. Methods for Measuring Cancer Disparities: Using Data Relevant to Healthy People 2010 Cancer-Related Objectives. Bethesda, MD: National Cancer Institute; 2005. [Google Scholar]
- 22. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman ME. An overview of methods for monitoring social disparities in cancer with an example using trends in lung cancer incidence by area-socioeconomic position and race-ethnicity, 1992-2004. Am J Epidemiol. 2008;167(8):889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schenker N, Gentleman JF. On judging the significance of differences by examining the overlap between confidence intervals. Am Stat. 2001;55(3):182–186. [Google Scholar]
- 24. Harper S. Selected comparisons of measures of health disparities: a review using databases relevant to healthy people 2010 cancer-related objectives. NCI Cancer Surveillance Monograph Series. Published 2007. https://seer.cancer.gov/publications/disparities2/. Accessed November 30, 2021.
- 25. Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930-1998. Cancer. 2003;97(S12):3133–3275. [DOI] [PubMed] [Google Scholar]
- 26. Streeter RA, Snyder JE, Kepley H, Stahl AL, Li T, Washko MM. The geographic alignment of primary care health professional shortage areas with markers for social determinants of health. PloS One. 2020;15(4):e0231443- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doescher MP, Fordyce MA, Skillman SM, Jackson JE, Rosenblatt RA. Persistent Primary Care Health Professional Shortage Areas (HPSAs) and Health Care Access in Rural America. WWAMI Rural Health Research Center; 2009. [Google Scholar]
- 28. Belasco EJ, Gong G, Pence B, Wilkes E. The impact of rural health care accessibility on cancer-related behaviors and outcomes. Appl Health Econ Health Policy. 2014;12(4):461–470. [DOI] [PubMed] [Google Scholar]
- 29. Continelli T, McGinnis S, Holmes T. The effect of local primary care physician supply on the utilization of preventive health services in the United States. Health Place. 2010;16(5):942–951. [DOI] [PubMed] [Google Scholar]
- 30. Moss JL, Liu B, Feuer EJ. Urban/rural differences in breast and cervical cancer incidence: the mediating roles of socioeconomic status and provider density. Womens Health Issues. 2017;27(6):683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaufman BG, Thomas SR, Randolph RK, et al. The rising rate of rural hospital closures. J Rural Health. 2016;32(1):35–43. [DOI] [PubMed] [Google Scholar]
- 32. Iglehart JK. The challenging quest to improve rural health care. N Engl J Med. 2018;378(5):473–479. [DOI] [PubMed] [Google Scholar]
- 33. Drope J, Liber AC, Cahn Z, et al. Who’s still smoking? Disparities in adult cigarette smoking prevalence in the United States. CA Cancer J Clin. 2018;68(2):106–115. [DOI] [PubMed] [Google Scholar]
- 34. Dwyer-Lindgren L, Mokdad AH, Srebotnjak T, Flaxman AD, Hansen GM, Murray CJL. Cigarette smoking prevalence in US counties: 1996-2012. Popul Health Metr. 2014;12(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roberts ME, Doogan NJ, Kurti AN, et al. Rural tobacco use across the United States: how rural and urban areas differ, broken down by census regions and divisions. Health Place. 2016;39:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012(44):2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vanderpool RC, Mills LA, Cancer prevention and control in rural communities. In: Crosby RA, Wendel ML, Vanderpool RC, Casey BR, eds. Rural Populations and Health: Determinants, Disparities, and Solutions. San Francisco, CA: Jossey-Bass; 2012:341–356. [Google Scholar]
- 38. Brems C, Johnson ME, Warner TD, Roberts LW. Barriers to healthcare as reported by rural and urban interprofessional providers. J Interprof Care. 2006;20(2):105–118. [DOI] [PubMed] [Google Scholar]
- 39. Dorn HF, Cutler SJ. Morbidity from cancer in the United States. I. Variation in incidence by age, sex, race, marital status, and geographic region. Public Health Monogr. 1955;29:1–121. [PubMed] [Google Scholar]
- 40. Feagin J, Bennefield Z. Systemic racism and U.S. health care. Soc Sci (Med). 2014;103:7–14. [DOI] [PubMed] [Google Scholar]
- 41. Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet (London, England). 2017;389(10077):1453–1463. [DOI] [PubMed] [Google Scholar]
- 42. Islami F, Bandi P, Sahar L, Ma J, Drope J, Jemal A. Cancer deaths attributable to cigarette smoking in 152 U.S. metropolitan or micropolitan statistical areas, 2013-2017. Cancer Causes Control. 2021;32(3):311–316. [DOI] [PubMed] [Google Scholar]
- 43. Spencer JC, Brewer NT, Coyne-Beasley T, Trogdon JG, Weinberger M, Wheeler SB. Reducing poverty-related disparities in cervical cancer: the role of HPV vaccination. Cancer Epidemiol Biomarkers Prev. 2021;30(10):1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O’Keefe EB, Meltzer JP, Bethea TN. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000-2010. Front Public Health. 2015;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are publicly available for access with SEER*Stat via a signed research data agreement. For full details, see https://seer.cancer.gov/mortality/.