Abstract
To take cancer survivorship research to the next level, it’s important to gain insight in trajectories of changing patient-reported outcomes and impaired recovery after cancer. This is needed as the number of survivors is increasing and a large proportion is confronted with changing health after treatment. Mechanistic research can facilitate the development of personalized risk-stratified follow-up care and tailored interventions to promote healthy cancer survivorship. We describe how these trajectories can be studied by taking the recently extended Dutch population-based Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship (PROFILES) registry as an example. PROFILES combines longitudinal assessment of patient-reported outcomes with novel, ambulatory and objective measures (eg, activity trackers, blood draws, hair samples, online food diaries, online cognitive tests, weighing scales, online symptoms assessment), and cancer registry and pharmacy databases. Furthermore, we discuss methods to optimize the use of a multidomain data collection–like return of individual results to participants, which may improve not only patient empowerment but also long-term cohort retention. Also, advanced statistical methods are needed to handle high-dimensional longitudinal data (with missing values) and provide insight into trajectories of changing patient-reported outcomes after cancer. Our coded data can be used by academic researchers around the world. Registries like PROFILES, which go beyond boundaries of disciplines and institutions, will contribute to better predictions of who will experience changes and why. This is needed to prevent and mitigate long-term and late effects of cancer treatment and to identify new interventions to promote health.
Worldwide, more than 50 million people have been diagnosed with cancer in the past 5 years and are still alive (1). With continuing improvement in early detection, treatment and care, and the aging of the population, the number of survivors is growing, and they are at risk for a variety of adverse physical and psychosocial effects (2).
In the past, cancer survivorship research predominantly focused on describing the long-term and late effects, applying a single exposure-to-outcome approach without including other possible exposure variables (2). Also, this approach almost solely focused on deterioration instead of changes. More recently, the focus shifted toward studies of longitudinal trajectories as our understanding of who reports changes in patient-reported outcomes (PROs) and why it is still in its infancy (3). Understanding various trajectories is imperative to identify patients at risk, to prevent and mitigate long-term and late effects, and to identify new interventions to promote health. The UK National Cancer Survivorship Initiative already stated in 2011 that we need to be able to identify the risk of an individual developing ongoing problems following treatment (4). The American Society of Clinical Oncology (5) and the European Academy of Cancer Sciences (6) argue that the dearth of research on long-term survivors of unselected groups of patients is an important gap to fill, and the initiation of prospective observational studies based on population-based registries is one of the recommendations (6). Both societies, and the National Cancer Institute (3), identify a research gap in our understanding of the potential trajectories of risk for long-term and late effects and emphasize the need to include biologic and behavior domains in survivorship research.
This paper describes the importance and challenges of population-based cancer survivorship registries that include data from multiple domains as they enable us to identify who will experience changes in PROs or lack of recovery and why. We will use the recently extended interdisciplinary Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship (PROFILES) registry (7) as an example.
The Dutch PROFILES Registry
PROFILES is a registry to collect data of cancer patients and noncancer controls to estimate the impact of cancer, beyond normal aging and the presence of comorbidities. PROFILES enables the investigation of the physical and psychosocial impact of cancer and its treatment in large groups of survivors, including adolescents and young adults or older patients; those with comorbidities, rare cancers, or palliative stage; and is acknowledged as a unique infrastructure for survivorship research (8).
PROFILES was established in 2009 and awarded an investment grant to leverage a successful registry with innovative data collections. It currently contains data from more than 30 000 survivors, linked to detailed clinical data from the population-based Netherlands Cancer Registry (NCR) (9). The NCR registers all newly diagnosed cancer patients in the Netherlands. PROFILES recently enriched the data collection with novel, ambulatory, and objective measures to create a multidomain data source for mechanistic cancer survivorship research (Box 1). Approval for data collection within the various PROFILES studies is always obtained from a certified medical ethics committee, and all patients sign informed consent.
Box 1. Specific innovative features of the extended PROFILES infrastructurea
Data collection of novel and established biological markers by blood sample collection and assaying
Objective ambulatory monitoring of physical activity, physiological functioning, and sleep of patients by means of activity trackers like Fitbits, ActiGraphs or biosensors
-
Assessment of dietary intake with an online food diary
Measurement of changes in body mass index and body composition by means of an 8-contact electrode bioelectrical impedance analysis system within a household scale
Online neuropsychological assessment of cognitive functioning by incorporating a computerized neuropsychological screening instrument that measures attention, concentration, memory, executive functioning, and cognitive and psychomotor processing speed in PROFILES
Extended online and paper-and-pencil questionnaire assessment with new instruments not currently in PROFILES
The SYMPRO app to monitor symptoms during chemotherapy and, if necessary, intervene by, for example, adjusting dosage or referring patients to specialized care
Integrate pharmacy data into the PROFILES registry by establishing linkage between existing pharmacy database and PROFILES
Methodological and technical innovation by 1) the adaption of online questionnaires for smartphone and tablet use and by providing PROFILES participants’ online feedback on their PROs and 2) optimizing safety and privacy vs patient access by integration of for example, Google Authenticator for 3-way identification
Extend inclusion of PROFILES participants with a special focus on cancer survivors of rare cancers
Extend normative control population of 2000 individuals with 200 men and women aged 75 years and older and 200 people between 18 and 35 years for optimal comparison of PROs with various cancer samples
aPROFILES = Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship; PROs = patient-reported outcomes.
Participants
Dynamic Patient Inclusion
A key feature of PROFILES is its dynamic patient inclusion: Every day, newly diagnosed cancer patients are invited to participate, striving to include them before treatment. This inclusion is set up in close collaboration with the hospital staff who actively approach patients. In studies among long-term cancer survivors who do not visit the hospital often, survivors are invited to participate by sending them an invitation by mail through their former treating physician. Once patients consent to participate, they receive invitations for measurements directly from PROFILES to decrease the workload for the participating health-care providers.
The response rates of PROFILES studies range between 50% and 80% and are dependent on the tumor, research questions, workload in the hospitals, and competing studies. In recent years, we have noticed that the response rates in general are slowly decreasing, probably due to the increasing number of studies for which patients are asked to participate. This is a serious problem that we try to solve by collaborating as much as possible with health-care providers so that we can collect PRO measure data together and do not have to burden patients twice. Nevertheless, through its unique linkage with the NCR, PROFILES has information about nonrespondents. Analyses of more than 14 000 patients showed that PROFILES participants have a statistically significantly better survival than nonparticipants, suggesting that even in studies with high participation rates, observed outcomes may represent the healthier patient with better outcomes (10). Participation has also been associated with male gender, being 60-70 years, high socioeconomic status, receiving any cancer treatment, the absence of comorbid conditions, and a cancer diagnosis between 2 and 3 years before study invitation. Furthermore, those who fully participated in PROFILES reported statistically significantly better health-related quality of life (HRQOL), functioning, and psychosocial symptoms than those who dropped out earlier (11).
An important feature of PROFILES is the population-based sampling frame, which enhances the generalizability compared with studies that include only a selection of patients from single or multiple (specialized) hospital(s). Because of its population-based inclusion, PROFILES also enables the international investigation of survivors of relatively rare cancers such as sarcoma (12,13), esophageal, pancreatic (14), and thyroid cancer (15), or specific groups like adolescents and young adults (16) or ultrarare cancers (17).
Normative Population
To determine the functional impairment and symptom burden after cancer beyond normal aging (3) and comorbidity, PROFILES annually collects information on PROs, biological and behavioral variables in a cohort of 2000 adult individuals without cancer, from the general Dutch population managed by the Dutch research institute CentERdata (www.centerdata.nl) (18-20). The panel is representative of the Dutch-speaking population in the Netherlands, including those without internet access. Those households that do not have access to the internet at the time of recruitment are given broadband access and a personal computer.
Study Measures
Multidomain Data Collection Following the Revised Wilson and Cleary Model
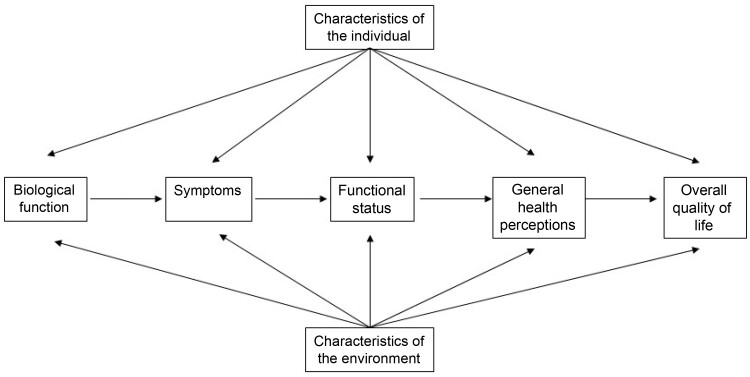
Wilson and Cleary have proposed a conceptual model linking biological and physiological variables to the measurement of health domains like HRQOL (21). Later, Ferrans and colleagues added individual and environmental characteristics (22). The revised Wilson and Cleary model (Figure 1) provides clear conceptual and operational definitions and emphasizes the importance of a multidomain data collection approach in survivorship research (23). The use of this framework in PROFILES allows the selection of appropriate measurement parameters.
Figure 1.
Shows a revised Wilson and Cleary model (22), which has been adapted from the model first published by Wilson and Cleary in their 1995 work (21).
PROFILES offers various levels of participation to patients, ranging from the basics, only completing surveys (linked to clinical data from the NCR) and blood draws, to optional comprehensive measurements of all domains as described below (eg, weighing scales with bioelectrical impedance analysis system, online food diaries, activity trackers). These optional measurements are offered at the same time as the questionnaires and blood draws (ie, baseline, 6 and 12 months, and then annually). By offering certain measurements as optional, we try to keep burden on the patient as low as possible while keeping the participation rates as high as possible. Therefore, PROFILES also offers all measurements (except blood draws) in the home situation. Researchers performing PROFILES studies can choose if, when, and how often they want to offer certain measures to the participants in their study. This depends mostly on the research questions relevant to the cancer patient group being followed.
Characteristics of the Individual
Sociodemographics
Sociodemographic factors collected in PROFILES like sex and age are derived from the NCR, whereas education, occupational status, and marital status are assessed with questionnaires. Health literacy is receiving increasing attention as it may explain variations in health status and health behaviors among cancer patients (24). It is therefore an imperative component of understanding healthy survivorship. In PROFILES, the Chew set of brief screening questions is used to evaluate health literacy (25).
Psychological Factors
A review including 330 studies indicated that stress-related psychosocial factors are associated with poorer cancer survival (26), which might be mediated through behavioral or pathophysiological pathways. The latter include the release of stress hormones that, for example, influence the tumor microenvironment, the cellular processes involved in the repair of damaged DNA, and the production of pro-inflammatory cytokines such as interleukin-6 (26). Personality, distress, and cognitive appraisal of disease are included as risk factors, whereas anxiety and depression have also been evaluated as outcomes.
Body Mass Index (BMI) and Body Composition
BMI and body composition (eg, fat and fat-free mass, body fat distribution) have been shown to be associated with survival, cancer recurrence, comorbidities, self-reliance, development and recovery of symptoms, and HRQOL (27-29). Percentage weight loss (>5% in ≤6 months or >10% in >6 months) (30) is the most important criterion of malnutrition with a negative impact on treatment outcome, survival, and HRQOL (31). Weight gain after diagnosis leading to obesity also negatively affects survival, HRQOL, and the risk of comorbidity and recurrence (28,32).
Besides self-reported BMI and self-measured waist and hip circumference (adding a measuring tape with instructions to the questionnaires), PROFILES collects the absolute amount and percentage fat and fat-free mass with an 8-contact electrode bioelectrical impedance analysis system within a household scale (Inbody Dial [Inbody, Seoul, Korea]). These scales are sent to patients’ homes.
Characteristics of the Environment
Social environmental characteristics are the interpersonal or social influences (eg, social support) on health outcomes, whereas physical environmental characteristics are settings such as home, neighborhood, and work place (including possibilities to exercise, access to healthy food, access to health care) (Figure 1) (22).
Smoking and Alcohol Intake
Smoking is not only an established risk factor for cancer but also it severely increases the risk of recurrence and mortality (33). Alcohol can also increase the risk of developing cancer and negatively affects prognosis (34). However, studies on its effect on survivorship or mortality are limited. In PROFILES, current, past, type, and dose of smoking and alcohol intake are measured by means of questionnaires as well as good diaries (see Dietary Intake).
Dietary Intake
Dietary intake has been shown to be associated with survival, recurrence, development of comorbidity, symptoms, and HRQOL (35-38). In PROFILES, dietary intake can be recorded using an online food diary (the Eetmeter) connected to the Dutch Nutrients Database (http://nevo-online.rivm.nl/) to quantify macro- and micronutrients. This method provides an acceptable estimation of nutritional intake (39) and is less burdensome and labor intensive compared with paper-based diaries.
Physical Activity, Physiological, and Functional Status
Consistent and compelling evidence exists on the role of physical activity in the prevention of cancer and the improvement of longevity (34,40). Self-reported physical activity is susceptible to recall bias or misclassification (41). Previous studies demonstrated a discrepancy between physical activity measured retrospectively with questionnaires and physical activity measured using activity trackers (42-46). Ambulatory activity trackers enable researchers to monitor the type, quantity, and quality of patients’ everyday activities (47,48), and some also assess sleep quality and quantity. Activity trackers may capture many of the preclinical signs of treatment toxicities with limited patient burden (49). Machine learning–derived algorithms applied to this data could contribute to earlier detection and management of toxicity.
In PROFILES, we use the Fitbit Inspire HR (Fitbit, San Fransisco, USA), the ActiGraph (Actigraph, Pensacola, USA), and a wearable biosensor to assess physical activity, physiological parameters (eg, heart rate, temperature, electrocardiogram, and sleep) in various studies. The activity trackers are sent to the participant’s home.
Cancer Treatments and Other Medication Use
Traditionally, cancer survivorship research investigates the impact of treatment exposure on long-term and late effects. PROFILES also includes detailed information about primary and secondary cancer treatments from the linkage with the NCR. Furthermore, linkage with PHARMO has been established from which detailed information on medication use beyond cancer treatment can be obtained from both inpatient and outpatient pharmacies. This high-quality linkage has an overall specificity of 99.5% and a sensitivity of 98.3% (50).
Biological Function
Evidence is mounting for a biological basis of subjective experiences including fatigue, pain, and HRQOL (51,52). In recent years, attention is being paid to the genetic susceptibility for toxicity of cancer treatments. The polygenic risk score is expected to become part of clinical care (53); for example, studies aim to identify any genetics associated with long-term neurotoxicity and ototoxicity (54) or genetic variants that increase an individual’s risk for radiation toxicity (55).
Furthermore, commonly experienced symptoms such as fatigue, pain, and depression suggest a shared neuroendocrine-immune pathophysiologic mechanism (56), involving disbalance of inflammatory cytokines, the monoamine system, and the hypothalamic-pituitary-adrenal (HPA) axis (57). Further, lab, animal, and noncancer population studies suggest that imbalance of the kynurenine pathway is an underlying mechanism of symptoms such as depression (58). Another emerging concept is that cancer treatments induce accelerated aging processes, which are linked to fatigue and depression (2).
It is vital to compare biological and physiological function of cancer survivors who have limited late effects and high HRQOL to those with many physical and psychosocial problems, enabling identification of pathways involved. Additionally, the inclusion of a noncancer norm population enables the comparison of the found associations considering normal aging and the presence of comorbidities. In PROFILES, patients and a norm population are asked to donate blood samples. Biomarkers of the neuroendocrine system, HPA axis, kynurenine pathway, cellular aging, and chronic inflammation including application of global-omics technologies (59) are assessed. Blood is stored at a central biobank, Biobank Maastricht, the Netherlands.
Chronic stress is characterized by dysfunction of the HPA axis and excessive production of the stress hormone cortisol (60-64). Chronic stress can be assessed using scalp hair that has a predictable growth rate of approximately 1 cm per month. The most proximal 1-cm segment to the scalp approximates the last month’s cortisol production; the second most proximal 1-cm segment approximates the production during the month before that and so on (65). In some PROFILES studies, hair samples are collected once after diagnosis to assess stress levels before diagnosis.
Symptoms, Functional Status, General Health Perceptions, and Overall HRQOL
Traditionally, PROs like symptoms, functioning, and HRQOL have been captured after cancer treatment by questionnaires, like the European Organisation for Research and Treatment of Cancer QLQ-C30 (66). More recently, increasing attention has been drawn to assess PRO symptoms during cancer treatment (67). Patients complete an online symptom diary that generates alerts when exceeding a threshold for a particular symptom. This alert is forwarded to the patient and/or health-care provider to introduce more timely intervention. This is known to result in lower symptom grades, improved HRQOL and survival, and fewer hospital admissions (68). In PROFILES, we can monitor side effects using the integrated SYMPRO web application that is linked to a website with the latest systemic therapies and their known side effects (69). From diagnosis until follow-up, patients complete a list of cancer-specific symptoms. SYMPRO provides patients with information on possible side effects, a digital symptom diary, alerts, and data feedback to patients and health-care providers.
Research shows that impairments in memory, attention, clarity of thought, executive functioning, and information processing speed among cancer patients have a considerable impact on everyday life (70-72). In PROFILES, cognitive functioning is assessed with the Amsterdam Cognition Scan, an online neuropsychological test battery that measures a broad variety of cognitive functions like attention, information processing, learning and memory, executive functioning, and psychomotor speed (73,74). It is preferably applied before and after treatment to measure the unique impact of treatment on cognitive functioning.
Methodological and Statistical Developments and Challenges
Online Questionnaires
Although PROFILES has been designed to facilitate completion of online questionnaires (7), patients are still given the opportunity to participate on paper. This allows us to reduce the digital divide (62) and obtain optimal generalizability of findings. Nevertheless, in the past years, we have observed a steady increase of 35% to 50% of patients who prefer to complete questionnaires online. The COVID-19 crisis stimulated people to use online solutions for everyday life and health-care issues (63) and further stimulated online completion rates.
Patient Engagement, Cohort Retention, and Return of Individual Results
Recently, there has been an increasing focus on patient involvement in prioritizing research questions and conducting research. PROFILES recently established a formal partnership with the Dutch Federation of Cancer Patient Organizations to maintain a dialogue on prioritization of research questions. Also, PROFILES has a patient in its advisory board and involves patients in the development of interventions, questionnaires, and feedback of results.
There is a growing demand by patients to gain access to their individual results (64,75-77). Returning individual PRO results enables patients to monitor their functioning and to recognize and be aware of symptoms. Furthermore, it offers patients the opportunity to compare their scores with peers (75) to evaluate if their scores are normal, discuss them with their health-care practitioner, and incorporate this information into personal decision making (76). In addition, return of individual results may also be an incentive for cohort retention. In PROFILES, a system for return of individual results was developed. If preferred, patients can compare their scores to age- and sex-matched survivors of the same cancer type and to an age- and sex-matched normative population without cancer (75,78 ). Furthermore, the Eetmeter provides personalized feedback about what participants ate or drank and whether that matches the current guidelines to patients who complete the food diary (79). SYMPRO provides an overview of the course of side effects over time, including complaints that have triggered alerts.
Statistical Challenges and Opportunities
Combining PRO data with data from the cancer registry, pharmacy, activity trackers, blood draws, hair samples, food diaries, cognitive tests, and weighing scales requires statistical modeling approaches that can handle high-dimensional longitudinal data, in presence of blocks of missing values. Furthermore, to understand the effect of the different exposures on multiple outcomes, a multivariate approach is needed. For high-dimensional multivariate data, regularized graphical models and dimension reduction techniques have been shown to be particularly useful and are often named as the way to go (80). Dimension reduction techniques that jointly account for the correlation between exposure variables and the correlation of these variables to the outcomes like sparse partial least squares and sparse principal covariates regression have been successfully applied in many contexts where data were of a high-dimensional and multivariate nature. Major advantages are that they give insight in the pathways at play and are less prone to overfitting. The multiple variables are summarized by a limited number of dimensions that represent the underlying factors or pathways giving rise to the correlations between exposure variables.
As sparse partial least squares approaches have been mainly developed in the context of genomics where data are usually complete and not of a longitudinal nature, further developments are needed to adapt these methods to integrate various biological processes with environmental and behavioral factors in relation to disease onset, progression, and outcomes.
Dissemination
Open Data
Sharing data within and outside a specific discipline increases collaboration, can push boundaries, and leads to new crosscutting insights. Also, it improves confidence in findings (81). Finally, by sharing, data are used more extensively, which decreases patient burden and increases the efficiency of research resources. PROFILES data are freely available according to the FAIR (Findable, Accessible, Interoperable, Reusable) principles (82) for noncommercial scientific research, subject only to privacy and confidentiality restrictions.
PROFILES data is made available through Questacy and can be accessed through the website (www.profilesregistry.nl) (83). To arrange optimal long-term data warehousing and dissemination, PROFILES follows the quality guidelines that are formulated in the Data Seal of Approval (www.datasealofapproval.org), developed by Data Archiving and Networked Services (The Hague, the Netherlands). Since 2011, 53 researchers from 7 countries (the Netherlands, United States, Sweden, United Kingdom, Australia, New Zealand, and Singapore) used PROFILES data.
Open Use of Infrastructure
The PROFILES registry is unique by also sharing its infrastructure for data collection. Various Dutch universities and hospitals integrated the PROFILES management system in their daily data collection routine. Data managers are trained in using the application, and research groups receive support like knowledge and experience with instruments, optimal invitational approaches, and patient engagement. This, for example, supported the development of the Dutch nationwide comprehensive gastrointestinal (esophageal, pancreas, and colon cancer) cohorts (14). Currently, the PROFILES approach is being copied in other countries where the PROFILES team advises colleagues about setting up and implementing similar infrastructures. The Royal Marsden Cancer Hospital in London has been using PROFILES since 2019, which stimulates collaboration in the study of rare cancers and enables comparison between the English and Dutch cancer care (84,85).
Discussion
Cancer survivorship registries that include a multidomain data collection facilitate the investigation of trajectories of changing PROs (decline and recovery) among cancer survivors. This knowledge is critical to develop follow-up guidelines, new treatment strategies, and other interventions that improve care and outcomes for survivors (86). Unfortunately, only a few exist. This is probably because of the high investment in both time and money involved in the development and long-term continuation, which is far greater than can be financed with the usual grants for research projects. It is probably also for this reason that most studies do not include a longitudinal data collection in a normative population. Fortunately, there are an increasing number of national, but also European, developments that enable start-up and maintenance of these kinds of large infrastructures for studies. Recently, the European Union and European Federation of Pharmaceutical Industries and Associations (a public private partnership) have launched the Big Data For Better Outcomes Program that aims to improve health outcomes and health-care systems by maximizing the potential of Big Data. In the field of oncology, the disease-specific HARMONY (www.harmony-alliance.eu) and PIONEER (prostate-pioneer.eu) projects are funded to establish big data platforms for hematological and prostate cancer survivors, respectively.
There are several challenges and lessons learned from the PROFILES registry that could be of benefit for other cohorts and registries as well. When setting up or linking cohorts or registries to obtain multidomain datasets, it is important that there is a conceptual framework or model, to avoid collecting data that is ultimately not used to answer research questions. Further, a representative inclusion of patients is highly desirable for the generalizability of findings and relevance to daily clinical practice. However, even population-based studies like PROFILES have been influenced by selective participation (11). Decreasing this selective participation is a major challenge. Offering questionnaires on paper, besides online questionnaires, is an important means to reduce the digital divide and thus selective participation. Also, ambulatory or at home assessments of variables associated with changes in patient outcomes potentially decrease selective participation. They also decrease patient and clinical burden. Further, making sure that health-care providers must do the absolute minimum to enroll patients decreases clinical burden and increases the number of patients included in studies. In this light, linkages of existing registries or studies are considered increasingly important because they decrease registration burden. Cohort retention (keeping patients engaged in studies over several years) is challenging as well. An important lesson we have learned is that return of individual results not only may empower patients but can also be an incentive for cohort retention. Another major challenge of cancer survivorship research is to determine the functional impairment and symptom burden after cancer beyond normal aging and comorbidity. Our lesson learned is that comparison to an age- and sex-matched normative population is very important in the interpretation of findings. Longitudinal tracking of a normative population is therefore imperative.
Data sharing is a responsibility we have as a research community to reduce patient burden and use research resources more efficiently. Sharing PROFILES data and the infrastructure for data collection increased the knowledge on cancer survivorship in a very efficient way. Not only directly, by having more output, but also indirectly, because it led to new collaborations, new ideas, and new insights. Finally, a multidisciplinary team of clinicians, epidemiologists, health scientists, psychologists, and statisticians or data scientists is needed to address the interdisciplinary research questions.
Ultimately, knowledge derived from multidomain registry studies can 1) support informed and shared decision making in which HRQOL can be balanced against the expected survival; 2) give direction to surveillance of survivors who are at high risk for late consequences of cancer treatment; 3) help develop interventions aimed to improve care across the cancer survivorship continuum; 4) help patient organizations dedicate their efforts to get facilities for cancer survivors to optimize social rehabilitation; and 5) offer patients the opportunity for self-tracking their personalized health and improve patient empowerment (87). This is much needed as we face a steep increase of the number of cancer survivors of whom a large proportion experiences long-term and late effects of cancer and its treatment, negatively impacting healthy aging and burdening public health.
Funding
The present research was supported by the Netherlands Comprehensive Cancer Organisation, Utrecht, the Netherlands; the Center of Research on Psychological disorders and Somatic diseases (CoRPS), Tilburg University, the Netherlands; and an Investment Grant Large (2016/04981/ZONMW-91101002) of the Dutch Research Council (the Hague, the Netherlands).
Notes
The role of the funders: The funders had no role in the design and conduct of the study; preparation of the manuscript; and decision to submit the manuscript for publication.
Disclosures: The authors have no disclosures.
Author contributions: Conceptualization: LVP-F, NH, DS, SB, NPME, OH, SO, SBS, GH, KVD, CH, ME, FM. Funding acquisition: LVP-F, GJH, FM, NH, DS, SB, NPME, OH, SO. Project administration: NH. Supervision: LVP-F, NH, ME, FM. Writing—original draft: LP, FM. Writing—review & editing: LVP-F, NH, DS, SB, NPME, OH, SO, SBS, GH, KVD, CH, ME, FM.
Acknowledgements: We would like to thank all patients and their doctors for their (ongoing) participation in PROFILES. Also, we would like to thank CentERdata for their continuing efforts to further develop the PROFILES registry and maintain its websites and panel management system.
Data Availability
PROFILES data is available upon request through www.profilesregistry.nl.
Contributor Information
Lonneke V van de Poll-Franse, Department of Research & Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, the Netherlands; CoRPS—Center of Research on Psychological Disorders and Somatic Diseases, Department of Medical and Clinical Psychology, Tilburg University, Tilburg, the Netherlands; Department of Psychosocial Research, Division of Psychosocial Research & Epidemiology, Netherlands Cancer Institute, Amsterdam, the Netherlands.
Nicole Horevoorts, Department of Research & Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, the Netherlands; CoRPS—Center of Research on Psychological Disorders and Somatic Diseases, Department of Medical and Clinical Psychology, Tilburg University, Tilburg, the Netherlands.
Dounya Schoormans, CoRPS—Center of Research on Psychological Disorders and Somatic Diseases, Department of Medical and Clinical Psychology, Tilburg University, Tilburg, the Netherlands.
Sandra Beijer, Department of Research & Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, the Netherlands.
Nicole P M Ezendam, Department of Research & Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, the Netherlands; CoRPS—Center of Research on Psychological Disorders and Somatic Diseases, Department of Medical and Clinical Psychology, Tilburg University, Tilburg, the Netherlands.
Olga Husson, Department of Medical Oncology, Netherlands Cancer Institute, Amsterdam, the Netherlands; Department of Surgical Oncology, Erasmus MC Cancer Institute, Erasmus University Medical Center, Rotterdam, the Netherlands.
Simone Oerlemans, Department of Research & Development , Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, the Netherlands.
Sanne B Schagen, Department of Psychosocial Research, Division of Psychosocial Research & Epidemiology, Netherlands Cancer Institute, Amsterdam, the Netherlands.
Geja J Hageman, Department of Pharmacology & Toxicology, Research Institute NUTRIM, School of Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, the Netherlands.
Katrijn Van Deun, Department of Methodology and Statistics, Tilburg University, Tilburg, the Netherlands.
Corina van den Hurk, Department of Research & Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, the Netherlands.
Mies van Eenbergen, Department of Research & Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, the Netherlands.
Floortje Mols, Department of Research & Development, Netherlands Comprehensive Cancer Organisation (IKNL) , Utrecht, the Netherlands; CoRPS—Center of Research on Psychological Disorders and Somatic Diseases, Department of Medical and Clinical Psychology, Tilburg University, Tilburg, the Netherlands.
the PROFILES Registry Group:
Belle de Rooij, Natasja Raijmakers, Carla Vlooswijk, Cynthia Bonhof, Afke Ekels, Meeke Hoedjes, S J M van Cappellen – van Maldegem, Laurien Ham, Danielle van de Graaf, and Janneke van Roij
References
- 1.International Agency for Research on Cancer. World Fact Sheet. Globocan; 2020 . https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf. Accessed January 1, 2020.
- 2. Shapiro CL. Cancer survivorship. N Engl J Med. 2018;379(25):2438–2450. [DOI] [PubMed] [Google Scholar]
- 3. Gallicchio L, Tonorezos E, de Moor JS, et al. Evidence gaps in cancer survivorship care: a report from the 2019 National Cancer Institute Cancer survivorship workshop. J Natl Cancer Inst. 2021;113(9):1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richards M, Corner J, Maher J. The National Cancer Survivorship Initiative: new and emerging evidence on the ongoing needs of cancer survivors. Br J Cancer. 2011;105(suppl 1):S1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacobsen PB, Rowland JH, Paskett ED, et al. Identification of key gaps in cancer survivorship research: findings from the American Society of Clinical Oncology survey. J Oncol Pract. 2016;12(3):190–193. [DOI] [PubMed] [Google Scholar]
- 6. Lagergren P, Schandl A, Aaronson NK, et al. ; for the European Academy of Cancer Sciences. Cancer survivorship: an integral part of Europe’s research agenda. Mol Oncol. 2019;13(3):624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van de Poll-Franse LV, Horevoorts N, van Eenbergen M, et al. ; for the Profiles Registry Group. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47(14):2188–2194. [DOI] [PubMed] [Google Scholar]
- 8. Cancer survivors: still room for improvement. Lancet Oncol. 2011;12(7):609. [DOI] [PubMed] [Google Scholar]
- 9. Janssen-Heijnen MLG, Louwman WJ, Van de Poll-Franse LV, Coebergh JWW. Results of 50 Years Cancer Registry in the South of the Netherlands: 1955-2004 (In Dutch). Eindhoven: Eindhoven Cancer Registry; 2005. [Google Scholar]
- 10. de Rooij BH, Ezendam NPM, Mols F, et al. Cancer survivors not participating in observational patient-reported outcome studies have a lower survival compared to participants: the population-based PROFILES registry. Qual Life Res. 2018;27(12):3313–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramsey I, de Rooij BH, Mols F, et al. Cancer survivors who fully participate in the PROFILES registry have better health-related quality of life than those who drop out. J Cancer Surviv. 2019;13(6):829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soomers V, Desar IME, van de Poll-Franse LV, et al. The perceived impact of length of the diagnostic pathway is associated with health-related quality of life of sarcoma survivors: results from the Dutch nationwide SURVSARC study. Cancers. 2020;12(8):2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drabbe C, Grunhagen DJ, Van Houdt WJ, et al. Diagnosed with a rare cancer: experiences of adult sarcoma survivors with the healthcare system: results from the SURVSARC Study. Cancers (Basel). 2021;13(4):679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coebergh van den Braak RRJ, van Rijssen LB, van Kleef JJ, et al. ; for the Dutch Pancreatic Cancer Group, Dutch Upper GI Cancer Group and PLCRC working group. Nationwide comprehensive gastro-intestinal cancer cohorts: the 3P initiative. Acta Oncol. 2018;57(2):195–202. [DOI] [PubMed] [Google Scholar]
- 15. Schoormans D, Wijnberg L, Haak H, Husson O, Mols F. Negative illness perceptions are related to poorer health-related quality of life among thyroid cancer survivors: results from the PROFILES registry. Head Neck. 2020;42(9):2533–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Husson O, Prins JB, Kaal SE, et al. Adolescent and young adult (AYA) lymphoma survivors report lower health-related quality of life compared to a normative population: results from the PROFILES registry. Acta Oncol. 2017;56(2):288–294. [DOI] [PubMed] [Google Scholar]
- 17. Weidema ME, Husson O, van der Graaf WTA, et al. Health-related quality of life and symptom burden of epithelioid hemangioendothelioma patients: a global patient-driven Facebook study in a very rare malignancy. Acta Oncol. 2020;59(8):975–982. [DOI] [PubMed] [Google Scholar]
- 18. Mols F, van de Poll-Franse LV, Vreugdenhil G, et al. Reference data of the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-CIPN20 Questionnaire in the general Dutch population. Eur J Cancer. 2016;69:28–38. [DOI] [PubMed] [Google Scholar]
- 19. van de Poll-Franse LV, Mols F, Gundy CM, et al. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 2011;47(5):667–675. [DOI] [PubMed] [Google Scholar]
- 20. Mols F, Husson O, Oudejans M, Vlooswijk C, Horevoorts N, van de Poll-Franse LV. Reference data of the EORTC QLQ-C30 questionnaire: five consecutive annual assessments of approximately 2000 representative Dutch men and women. Acta Oncol. 2018;57(10):1381–1391. [DOI] [PubMed] [Google Scholar]
- 21. Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. [PubMed] [Google Scholar]
- 22. Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health-related quality of life. J Nurs Scholarsh. 2005;37(4):336–342. [DOI] [PubMed] [Google Scholar]
- 23. Bakas T, McLennon SM, Carpenter JS, et al. Systematic review of health-related quality of life models. Health Qual Life Outcomes. 2012;10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith SK, Nutbeam D, McCaffery KJ. Insights into the concept and measurement of health literacy from a study of shared decision-making in a low literacy population. J Health Psychol. 2013;18(8):1011–1022. [DOI] [PubMed] [Google Scholar]
- 25. Fransen MP, Van Schaik TM, Twickler TB, Essink-Bot ML. Applicability of internationally available health literacy measures in the Netherlands. J Health Commun. 2011;16(suppl 3):134–149. [DOI] [PubMed] [Google Scholar]
- 26. Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Rev Clin Oncol. 2008;5(8):466–475. [DOI] [PubMed] [Google Scholar]
- 27. Meyerhardt JA, Niedzwiecki D, Hollis D, et al. ; for the Cancer and Leukemia Group B 89803. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26(25):4109–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12(4):282–294. [DOI] [PubMed] [Google Scholar]
- 29. van Baar H, Bours MJL, Beijer S, et al. Body composition and its association with fatigue in the first 2 years after colorectal cancer diagnosis. J Cancer Surviv. 2021;15(4):597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cederholm T, Jensen GL, Correia M, et al. ; for GLIM Working Group. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38(1):1–9. [DOI] [PubMed] [Google Scholar]
- 31. Martin L, Senesse P, Gioulbasanis I, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol. 2015;33(1):90–99. [DOI] [PubMed] [Google Scholar]
- 32. Nichols HB, Trentham-Dietz A, Egan KM, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ligibel J. Lifestyle factors in cancer survivorship. J Clin Oncol. 2012;30(30):3697–3704. [DOI] [PubMed] [Google Scholar]
- 34.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective continuous update project expert report; 2018. dietandcancerreport.org. Accessed June 1, 2021.
- 35. Jochems SHJ, Van Osch FHM, Bryan RT, et al. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: a systematic review of current epidemiological literature. BMJ Open. 2018;8(2):e014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Westhoff E, Wu X, Kiemeney LA, et al. Dietary patterns and risk of recurrence and progression in non-muscle-invasive bladder cancer. Int J Cancer. 2018;142(9):1797–1804. [DOI] [PubMed] [Google Scholar]
- 37. Barchitta M, Maugeri A, Magnano San Lio R, et al. The effects of diet and dietary interventions on the quality of life among breast cancer survivors: a cross-sectional analysis and a systematic review of experimental studies. Cancers (Basel). 2020;12(2):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Veen MR, Mols F, Bours MJL, Weijenberg MP, Kampman E, Beijer S. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations for cancer prevention is associated with better health-related quality of life among long-term colorectal cancer survivors: results of the PROFILES registry. Support Care Cancer. 2019;27(12):4565–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cameron ME, Van Staveren WA. Manual on Methodology for Food Consumption Studies. New York: Oxford University Press; 1988. [Google Scholar]
- 40. Patel AV, Friedenreich CM, Moore SC, et al. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51(11):2391–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Courneya KS, Vallance JK, Culos-Reed SN, et al. The Alberta moving beyond breast cancer (AMBER) cohort study: a prospective study of physical activity and health-related fitness in breast cancer survivors. BMC Cancer. 2012;12:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goedendorp MM, Peters ME, Gielissen MF, et al. Is increasing physical activity necessary to diminish fatigue during cancer treatment? Comparing cognitive behavior therapy and a brief nursing intervention with usual care in a multicenter randomized controlled trial. Oncologist. 2010;15(10):1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grossman P, Deuring G, Garland SN, Campbell TS, Carlson LE. Patterns of objective physical functioning and perception of mood and fatigue in posttreatment breast cancer patients and healthy controls: an ambulatory psychophysiological investigation. Psychosom Med. 2008;70(7):819–828. [DOI] [PubMed] [Google Scholar]
- 44. Rogers LQ, Hopkins-Price P, Vicari S, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41(4):935–946. [DOI] [PubMed] [Google Scholar]
- 45. Servaes P, Verhagen CA, Bleijenberg G. Relations between fatigue, neuropsychological functioning, and physical activity after treatment for breast carcinoma: daily self-report and objective behavior. Cancer. 2002;95(9):2017–2026. [DOI] [PubMed] [Google Scholar]
- 46. Timmerman JG, Dekker-van Weering MG, Tonis TM, Hermens HJ, Vollenbroek-Hutten MM. Relationship between patterns of daily physical activity and fatigue in cancer survivors. Eur J Oncol Nurs. 2015;19(2):162–168. [DOI] [PubMed] [Google Scholar]
- 47. Broderick JM, Ryan J, O’Donnell DM, Hussey J. A guide to assessing physical activity using accelerometry in cancer patients. Support Care Cancer. 2014;22(4):1121–1130. [DOI] [PubMed] [Google Scholar]
- 48. Derksen JWG, Beijer S, Koopman M, Verkooijen HM, van de Poll-Franse LV, May AM. Monitoring potentially modifiable lifestyle factors in cancer survivors: a narrative review on currently available methodologies and innovations for large-scale surveillance. Eur J Cancer. 2018;103:327–340. [DOI] [PubMed] [Google Scholar]
- 49. Ward WH, Meeker CR, Handorf E, et al. Feasibility of fitness tracker usage to assess activity level and toxicities in patients with colorectal cancer. J Clin Oncol Clin Cancer Inform. 2021;5(5):125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Herk-Sukel MP, van de Poll-Franse LV, Lemmens VE, et al. New opportunities for drug outcomes research in cancer patients: the linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur J Cancer. 2010;46(2):395–404. [DOI] [PubMed] [Google Scholar]
- 51. Sprangers MA, Sloan JA, Barsevick A, Chauhan C, et al. ; for the Geneqol Consortium. Scientific imperatives, clinical implications, and theoretical underpinnings for the investigation of the relationship between genetic variables and patient-reported quality-of-life outcomes. Qual Life Res. 2010;19(10):1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sprangers MA, Thong MS, Bartels M, et al. ; for the GeneQol Consortium. Biological pathways, candidate genes, and molecular markers associated with quality-of-life domains: an update. Qual Life Res. 2014;23(7):1997–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sugrue LP, Desikan RS. What are polygenic scores and why are they important? JAMA. 2019;321(18):1820–1821. [DOI] [PubMed] [Google Scholar]
- 54. Dolan ME, El Charif O, Wheeler HE, et al. Clinical and genome-wide analysis of cisplatin-induced peripheral neuropathy in survivors of adult-onset cancer. Clin Cancer Res. 2017;23(19):5757–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fachal L, Gomez-Caamano A, Barnett GC, et al. A three-stage genome-wide association study identifies a susceptibility locus for late radiotherapy toxicity at 2q24.1. Nat Genet. 2014;46(8):891–894. [DOI] [PubMed] [Google Scholar]
- 56. Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim HJ, Barsevick AM, Fang CY, Miaskowski C. Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nurs. 2012;35(6):E1–E20. [DOI] [PubMed] [Google Scholar]
- 58. Savitz J. Role of kynurenine metabolism pathway activation in major depressive disorders. Curr Top Behav Neurosci. 2017;31:249–267. [DOI] [PubMed] [Google Scholar]
- 59. Coughlin SS. Toward a road map for global-omics: a primer on -omic technologies. Am J Epidemiol. 2014;180(12):1188–1195. [DOI] [PubMed] [Google Scholar]
- 60. Selye H. Stress in Health and Disease. Butterworths, Boston: Butterworth-Heinemann; 2013. [Google Scholar]
- 61.. Cohen S, Gianaros PJ, Manuck SB. A stage model of stress and disease. Perspect Psychol Sci. 2016;11(4):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Horevoorts NJ, Vissers PA, Mols F, Thong MS, van de Poll-Franse LV. Response rates for patient-reported outcomes using web-based versus paper questionnaires: comparison of two invitational methods in older colorectal cancer patients. J Med Internet Res. 2015;17(5):e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van de Poll-Franse LV, de Rooij BH, Horevoorts NJE, et al. Perceived care and well-being of patients with cancer and matched norm participants in the COVID-19 crisis: results of a survey of participants in the Dutch PROFILES registry. JAMA Oncol. 2021;7(2):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Downey AS, Busta ER, Mancher M, Botkin JR, eds.; for the National Academies of Sciences, Engineering, and Medicine. Returning Individual Research Results to Participants: Guidance for a New Research Paradigm. Washington, DC: National Academies Press; 2018. [PubMed] [Google Scholar]
- 65. Noppe G, de Rijke YB, Dorst K, van den Akker EL, van Rossum EF. LC-MS/MS-based method for long-term steroid profiling in human scalp hair. Clin Endocrinol. 2015;83(2):162–166. [DOI] [PubMed] [Google Scholar]
- 66. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 67. Basch E, Leahy AB, Dueck AC. Benefits of digital symptom monitoring with patient-reported outcomes during adjuvant cancer treatment. J Clin Oncol. 2021;39(7):701–703. [DOI] [PubMed] [Google Scholar]
- 68. Oldenmenger WH, van den Hurk CJG, Howell D, Utilizing technology to manage symptoms. In: Charalambous A., ed. Developing and Utilizing Digital Technology in Healthcare for Assessment and Monitoring. Springer Nature Switzerland: Springer; 2020:55–72. [Google Scholar]
- 69. Billingy NE, Tromp V, Veldhuijzen E, et al. Symptom monitoring with patient-reported outcomes using a web application among patients with lung cancer in the Netherlands (SYMPRO-Lung): study protocol for a stepped-wedge randomised controlled trial. BMJ Open. 2021;11(9):e052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv. 2009;3(4):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Reid-Arndt SA. Breast cancer and “chemobrain”: the consequences of cognitive difficulties following chemotherapy and the potential for recovery. Mo Med. 2009;106(2):127–131. [PubMed] [Google Scholar]
- 72. Oerlemans S, Schagen SB, van den Hurk CJ, Husson O, Schoormans D, van de Poll-Franse LV. Self-perceived cognitive functioning and quality of life among cancer survivors: results from the PROFILES registry. J Cancer Surviv. 2021. [DOI] [PubMed] [Google Scholar]
- 73. Feenstra HE, Vermeulen IE, Murre JM, Schagen SB. Online self-administered cognitive testing using the Amsterdam cognition scan: establishing psychometric properties and normative data. J Med Internet Res. 2018;20(5):e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Feenstra HEM, Murre JMJ, Vermeulen IE, Kieffer JM, Schagen SB. Reliability and validity of a self-administered tool for online neuropsychological testing: the Amsterdam Cognition Scan. J Clin Exp Neuropsychol. 2018;40(3):253–273. [DOI] [PubMed] [Google Scholar]
- 75. Oerlemans S, Arts LP, Horevoorts NJ, van de Poll-Franse LV. “Am I normal?” The wishes of patients with lymphoma to compare their patient-reported outcomes with those of their peers. J Med Internet Res. 2017;19(8):e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shalowitz DI, Miller FG. Disclosing individual results of clinical research: implications of respect for participants. JAMA. 2005;294(6):737–740. [DOI] [PubMed] [Google Scholar]
- 77. Wong CA, Hernandez AF, Califf RM. Return of research results to study participants: uncharted and untested. JAMA. 2018;320(5):435–436. [DOI] [PubMed] [Google Scholar]
- 78. Arts LPJ, van de Poll-Franse LV, van den Berg SW, et al. Lymphoma InterVEntion (LIVE) – patient-reported outcome feedback and a web-based self-management intervention for patients with lymphoma: study protocol for a randomised controlled trial. Trials. 2017;18(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gezondheidsraad. Richtlijnen Goede Voeding 2015. The Hague, Netherlands; 2015.
- 80. Buck Louis GM, Sundaram R. Exposome: time for transformative research. Stat Med. 2012;31(22):2569–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Data sharing and the future of science. Nat Commun. 2018;9(1):2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wilkinson MD, Dumontier M, Aalbersberg IJ, et al. The FAIR guiding principles for scientific data management and stewardship. Sci Data. 2016;3:160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Amin A, Edwards M, Hopt O, et al. Questacy: Documenting and Disseminating Longitudinal Data Online Using DDI 3; 2009. http://www.ddialliance.org/sites/default/files/QuestasyDocumentingAndDisseminatingLongitudinalDataUsingDDI3.pdf. Accessed June 1, 2021.
- 84. Younger E, Jones RL, Desar IME, Peckitt C, van der Graaf WTA, Husson O. Health-related quality of life in patients with advanced soft tissue sarcomas treated with chemotherapy (the HOLISTIC study): protocol for an international observational cohort study. BMJ Open. 2020;10(6):e035171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lidington E, McGrath SE, Noble J, et al. Evaluating a digital tool for supporting breast cancer patients: a randomized controlled trial protocol (ADAPT). Trials. 2020;21(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5):825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Absolom K, Warrington L, Hudson E, et al. Phase III randomized controlled trial of eRAPID: eHealth intervention during chemotherapy. J Clin Oncol. 2021;39(7):734–747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
PROFILES data is available upon request through www.profilesregistry.nl.