Abstract
Background
Trials of immune checkpoint inhibitors (ICIs) have published patient-reported quality of life (QOL), but the size and heterogeneity of this literature can make patient education difficult. This meta-analysis aimed to describe change in QOL and symptomatology in patients receiving ICIs for cancer.
Methods
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, databases were searched through November 2019 for articles or abstracts of prospective, original studies reporting longitudinal QOL in adult cancer patients treated with ICIs. The prespecified primary outcomes were change in global QOL among patients treated with ICIs and difference in change since baseline in global QOL between patients treated with ICI vs non-ICI active treatment. Secondary outcomes included physical functioning and symptomatology. All statistical tests were 2-sided.
Results
Of 20 323 publications, 26 met inclusion criteria. Global QOL did not change over time in patients treated with ICIs (k = 26, n = 6974; P = .19). Larger improvements in global QOL was observed in patients receiving ICI vs non-ICI regimens (k = 16, ICI: n = 3588; non-ICI: n = 2948; P < .001). Physical functioning did not change in patients treated with ICIs (k = 14, n = 3169; P = .47); there were no differences in mean change between ICI vs non-ICI regimens (k = 11, n = 4630; P = .94). Regarding symptoms, appetite loss, insomnia, and pain severity decreased, but dyspnea severity increased in patients treated with ICIs (k = 14, n = 3243-3499; P < .001). Insomnia severity was higher in patients treated with ICIs than non-ICI regimens (k = 11, n = 4791; P < .001).
Conclusions
This study is among the first to quantitatively summarize QOL in patients treated with ICIs. Findings suggest ICI recipients report no change in global QOL and higher QOL than patients treated with non-ICI regimens.
Immunotherapies have generated widespread scientific and clinical excitement for their ability to prolong survival in cancer patients with poor prognoses (1,2). Immune checkpoint inhibitors (ICIs) (ie, atezolizumab, avelumab, durvalumab, ipilimumab, nivolumab, pembrolizumab) are now routinely used in standard care for treatment of metastatic melanoma, metastatic Merkel cell carcinoma, non-small cell lung cancer (NSCLC), small-cell lung cancer, hepatocellular carcinoma, metastatic triple-negative breast cancer, head and neck squamous cell cancer, renal cell carcinoma, Hodgkin lymphoma, bladder cancer, urothelial carcinoma, and some subtypes of metastatic colorectal cancer. These agents are also being tested in a variety of other cancer types (eg, ovarian, prostate) and in combination with other treatments (eg, radiation, chemotherapy) (3). Thus, there is a large and growing number of patients for whom ICIs are clinically appropriate.
One particular challenge of these agents is uncertainty over their impact on quality of life (QOL). Although QOL data have been collected as a secondary outcome on numerous clinical trials, the recency, size, and heterogeneity of this literature preclude easy summarization for patients wondering what to expect on treatment. To our knowledge, there is only 1 previous meta-analysis of patient-reported QOL in ICIs. Nishijima and colleagues (4) reported on 13 randomized trials of single-agent PD-1/PD-L1 inhibitors. Results indicated that follow-up QOL was better among ICI recipients than patients treated with other treatments. CTLA-4 inhibitors were not examined except as a comparator, and numerous new trials have published QOL data with novel agents and for different indications. Thus, the goal of this meta-analyses was to provide a comprehensive and generalizable summary of global QOL (primary outcome) and physical functioning and symptomatology (secondary outcomes) during treatment with ICIs and to examine additional, clinically important moderators of global QOL. With a focus on patient education, we selected moderators that would be known prior to initiation of ICIs, including regimen, disease site, age, sex, and duration of follow-up. We also examined risk of study bias as a potential moderator.
Methods
Framework
To ensure a rigorous methodology, the meta-analyses were conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (5). We also used the Covidence platform (Melbourne, Australia), an internet-based platform for screening and extracting data, to facilitate screening and data extraction.
Search Strategy
PubMed, EMBASE, and Web of Science were searched (see Supplementary Methods, available online). Because QOL results for some trials may have been reported in a conference abstract, a secondary hand search of conference abstracts and proceedings from 21 relevant professional societies was conducted (see Supplementary Table 1, available online). A smaller subset of keywords was used to identify conference abstracts because of limitations of search functions on some professional society websites. Reference lists from publications retrieved were also examined to identify abstracts. The search was inclusive through November 2019; no start date to the search window was used.
Selection Strategy
Selection of abstracts for full-text review was conducted by pairs of raters using Covidence. Each rater reviewed the abstracts independently and identified studies to retrieve for full-text review. Discrepancies were resolved by senior authors (BG and HJ). Five inclusion criteria were applied. First, each must have reported on adult cancer patients (ie, age 18 years or older). Second, abstracts must have reported data for participants treated with 1 or more PD-1, PD-L1, or CTLA-4 immune checkpoint inhibitors. The following agents were included: atezolizumab, avelumab, BMS 936559, durvalumab, ipilimumab, nivolumab, pembrolizumab, pidilizumab, tremelimumab, ticilimumab. Cemiplimab was not included in our original search because it had yet to gain US Food and Drug Administration (FDA) approval during study conceptualization and was not included in our results because we were unable to find any articles or abstracts published during the review period that reported on patient-reported QOL. Third, the abstract must have reported prospective, original data. Observational studies, interventional trials, and expanded access trials were included. Fourth, the abstract must have provided data regarding longitudinal change in patient-reported QOL; there were no restrictions on the QOL measure used. Fifth, abstracts must have been peer reviewed as a conference abstract or published paper.
Data were independently extracted and checked by rater pairs. Discrepancies were resolved by consensus by senior authors (BG, HJ). Information extracted included QOL data (ie, means, standard deviations, 95% confidence intervals [CIs], sample size), study design characteristics (ie, disease site, ICI regimen, comparison regimen, timing of assessments), and sample characteristics (ie, mean age, percent female). When no other statistics were reported, numerical data were independently extracted from figures using the free online tool WebPlotDigitizer (6). This allows the extractor to select relevant datapoints from figures and to export numerical values. When necessary, attempts were made to request the information from authors and/or study sponsors. A formal review protocol was developed (7), and a PRISMA checklist is available in the Supplementary Methods (available online).
Statistical Analyses
Use of specific QOL measures was not required for inclusion in analyses. Meta-analyses of global QOL used the 30-item European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) (8) global health status score and the EQ-5D (9) visual analog scale because of the similarity in the measures (ie, both are 0-100 scales with higher scores indicating better QOL) and because 1 or both were reported in all eligible publications. The EORTC QLQ-C30 was used when available, otherwise the EQ-5D visual analog scale was used. Analyses of physical functioning and symptomatology (ie, appetite loss, constipation, diarrhea, dyspnea, fatigue, insomnia, nausea and/or vomiting, and pain) used EORTC QLQ-C30 subscales. All meta-analysis outcomes (ie, global QOL, physical functioning, symptomology) were continuous variables. When not reported, effect sizes were calculated using means and standard deviations, standard error, or 95% confidence intervals. Consistent with published guidelines (10), we used report standardized mean differences using Cohen d effect sizes. These were calculated as the difference between baseline and follow-up scores divided by the pooled standard deviation and calculated for group comparisons as the difference in change since baseline divided by the pooled standard deviation. Data are presented as mean change from baseline within the ICI group and as a difference in mean change between the ICI and non-ICI groups. In publications with multiple follow-up assessments, the assessment within or closest to 12-24 weeks after initiation of therapy was selected because most publications reported a follow-up assessment during this time. In publications with multiple ICI study arms (11-17), the study arm that received the regimen most similar to an FDA-approved regimen was selected. Two reviewers (SE and KB) independently rated the methodological rigor of each study selected for inclusion using the Cochrane Risk of Bias Assessment criteria (18). The reviewers’ ratings were based on information found in the publication, other study publications, appendices, and supplemental materials (eg, study protocol). Discrepancies in risk of bias were resolved by consensus. Three reviewers (BG, LO, and HJ) independently rated the quality of patient-reported outcome (PRO) reporting of each study selected for inclusion using the Consolidated Standards of Reporting Trials PRO extension (19), consistent with previously published studies (20). Discrepancies were resolved by consensus.
Pairs of meta-analyses were conducted for each outcome, which 1) examined within-group change in outcomes in patients treated with ICIs from pretreatment baseline to follow-up approximately 12-24 weeks later and 2) compared between-group change in outcomes in ICIs vs non-ICI regimens. All meta-analyses were grouped by ICI regimen. Heterogeneity across studies was assessed using Cochrane’s Q and I2 for global QOL. Funnel plots and trim and fill were used to assess publication bias for meta-analyses for global QOL. Sensitivity analyses of change in QOL among ICI recipients and of follow-up QOL between groups were conducted that retained only published papers and excluded published abstracts. We report below on statistically significant change from baseline to follow-up in QOL as well as statistically significant differences between groups at follow-up. Random effect models were used because of the studies’ heterogeneity, and all analyses used a 2-sided alpha level of .05. Where statistically significant differences were observed for global QOL and subscales, we described changes in mean scores or differences between groups on mean scores as either trivial, small, medium, or large effects according to published guidelines (21).
Moderators of the association between ICI and global QOL included ICI regimen, disease site, duration of follow-up, comparator group, mean sample age, sex, risk of study bias, and quality of PRO reporting (see the Supplementary Methods, available online). Analyses examining whether the duration of follow-up moderated the association between ICI and global QOL used continuous weeks since baseline. Moderator analyses comparing different non-ICI comparator groups were conducted among randomized trials and examined whether outcomes differed between trials using placebo, chemotherapy-based regimens, or other non-ICI regimens. Moderator analyses examining age and sex used continuous measures of mean age and percent of participants identifying as female, respectively. A dichotomous risk of study bias summary assessment was determined for each study based on whether the study had low or unclear risk across all domains or contained 1 or more high-risk domains. A continuous score of quality of PRO reporting was used, with higher scores indicating better reporting quality. Meta-regression analyses were used to determine the impact of moderators on the association between ICI and global QOL. Models examined individual study-level moderators of effect size. To reduce risk of Type I error, only ICI regimen was included in moderator analyses for the secondary outcomes of physical functioning and symptomatology. Random-effects models were selected because of the heterogeneous nature of the studies. Sensitivity moderator analyses were conducted that retained only published papers and excluded published abstracts. All meta-analyses were conducted in Comprehensive Meta-Analysis Version 3 (Biostat, Englewood, NJ).
Results
Study Selection
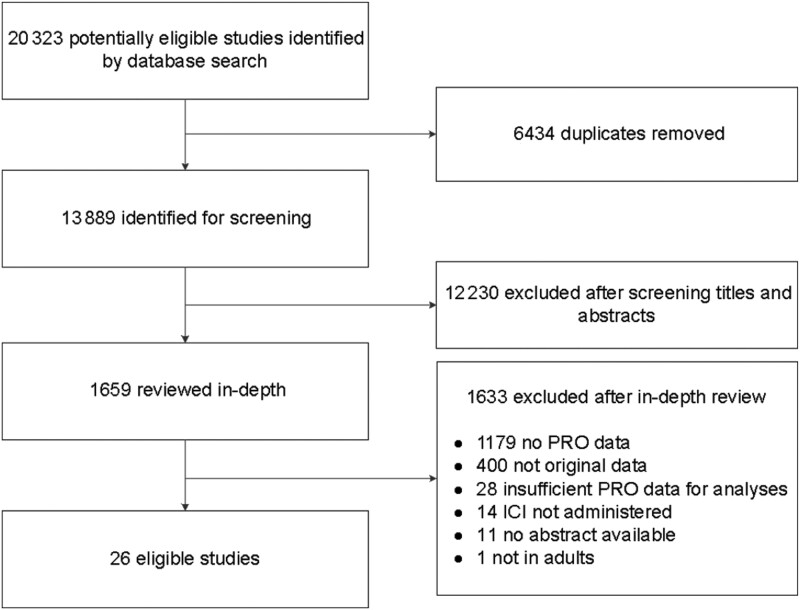
Figure 1 presents a flow diagram of the database search results and screening. Of the initial 20 323 publications retrieved, 6434 were duplicates, resulting in 13 889 unique publications. After removing 12 230 during initial screening, full-text reviews were conducted for the remaining 1659 publications. This process resulted in 52 eligible publications. Two additional publications were identified through hand search. Of the 54 publications that met inclusion criteria, sufficient data were not available to compute mean change for 28 abstracts (eg, after requesting information from study authors and/or sponsors). The remaining 26 publications with usable data were included in analyses.
Figure 1.
Study selection. ICI = immune checkpoint inhibitor; PRO = patient-reported outcome.
Descriptions of the included studies are presented in Table 1. Publications included phase I and II (k = 2), phase II (k = 4), phase II and III (k = 1), and phase III (k = 17) trials as well as 2 prospective observational studies. Of the 24 interventional studies, 19 reported a randomized design, and 5 reported a single-arm design. The most commonly evaluated ICI was nivolumab (k = 10), followed by pembrolizumab (k = 6), ipilimumab (k = 4), atezolizumab (k = 2), durvalumab (k = 2), and avelumab (k = 1). Among the 19 publications with a comparator group, ICI was compared with chemotherapy in 10 studies. The remaining publications compared ICIs with other ICI-based regimens (k = 4), a mix of non-ICI regimens (k = 2), placebo (k = 2), and gp100 plus placebo (k = 1). As shown in Supplementary Table 2 (available online), 8 publications were judged to have overall low risk of study bias, and 18 had high risk. Ratings of quality of PRO reporting are presented in Supplementary Table 3 (available online).
Table 1.
Characteristics of included studies
| Reference | Trial name | Phase | NCT number | Cancer type | Study type | QOL measure | Follow-up | Intervention arm |
Comparison arm |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | No. | Description | No. | ||||||||
| Ascierto et al. 2017 (22) | Not reported | III | NCT01515189 | Melanoma | Randomized trial | EORTC QLQ-C30 | Week 12 | Ipilimumab 10 mg/kg Q3W | 365 | Ipilimumab 3 mg/kg Q3W | 362 |
| Barlesi et al. 2019 (11) | KEYNOTE-010 | II/III | NCT01905657 | Non-small cell lung cancer | Randomized trial | EORTC QLQ-C30 | Week 12 | Pembrolizumab 2 mg/kg Q3W | 344 | Docetaxel 75 mg/m2 Q3W | 343 |
| Pembrolizumab 10 mg/kg Q3W | 346 | ||||||||||
| Bordoni et al. 2017 (23) | OAK | III | NCT02008227 | Non-small cell lung cancer | Randomized trial | EORTC QLQ-C30 | Week 15 | Atezolizumab 1200 mg Q3W | 425 | Docetaxel 75 mg/m2 Q3W | 425 |
| Brahmer et al. 2017 (24) | KEYNOTE-024 | III | NCT02142738 | Non-small cell lung cancer | Randomized trial | EORTC QLQ-C30 | Week 15 | Pembrolizumab 200 mg/kg Q3W | 151 | Investigator-choice platinum-doublet chemotherapy Q3W | 148 |
| Cella et al. 2016 (25) | CheckMate 025 | III | NCT01668784 | Renal cell carcinoma | Randomized trial | EQ-5D | Week 12 | Nivolumab 3 mg/kg Q2W | 362 | Everolimus 10 mg daily | 344 |
| Coens et al. 2017 (26) | EORTC 18071 | III | NCT00636168 | High-risk stage III melanoma | Randomized trial | EORTC QLQ-C30 | Week 24 | Ipilimumab 10 mg/kg 4x Q3W | 475 | Placebo 10 mg/kg 4x Q3W | 476 |
| El-Khoueiry et al. 2017 (27) | CheckMate 040 | I/II | NCT01658878 | Hepatocellular carcinoma | Single group trial | EQ-5D | Week 25 | Nivolumab (dose-expansion phase) | 214 | NA | |
| Harrington et al. 2017 (28) | CheckMate 141 | III | NCT02105636 | Squamous head and neck | Randomized trial | EORTC QLQ-C30 | Week 15 | Nivolumab 3 mg/kg Q2W | 240 | Chemotherapy (methotrexate, docetaxel, or cetuximab) | 121 |
| Hui et al. 2019 (29) | PACIFIC | III | NCT02125461 | Non-small cell lung cancer | Randomized trial | EORTC QLQ-C30 | Week 52 | Durvalumab 10 mg/kg Q2W | 476 | Placebo 10 mg/kg 2 W | 237 |
| Kaufman et al. 2017 (30) | JAVELIN Merkel 200 | II | NCT02155647 | Merkel cell carcinoma | Single group trial | EQ-5D | Week 13 | Avelumab 10 mg/kg Q2W | 72 | NA | |
| Larkin et al. 2018 (31) | CheckMate 037 | III | NCT01721746 | Melanoma | Randomized trial | EORTC QLQ-C30 | Week 12 | Nivolumab 3 mg/kg Q2W | 272 | Investigator’s choice (Dacarbazine 1000 mg/m2 or Carboplatin AUC 6 + Paclitaxel 175 mg/m2) Q3W | 133 |
| Long et al. 2016 (32) | CheckMate 066 | III | NCT01721772 | Melanoma | Randomized trial | EORTC QLQ-C30 | Weeks 61 | Nivolumab 3 mg/kg Q2W | 210 | Dacarbazine 1000 mg/m2 Q3W | 208 |
| Mathias et al. 2015 (33)a | IMAGE | N/A | NCT01511913 | Advanced cutaneous melanoma | Observational | EORTC QLQ-C30 | Week 26 | Ipilimumab | 196 | Non-ipilimumab–treated cohort | 100 |
| Mazieres et al. 2018 (34)a | KEYNOTE-407 | III | NCT02775435 | Non-small cell lung cancer | Randomized trial | EORTC QLQ-C30 | Week 18 | Pembrolizumab 200 mg + Investigator’s Choice (Pacitaxel 200 mg/m2 on Day 1 or NAB-Paclitaxel 100 mg/m2 on Days 1, 8, 15) + Carboplatin AUC6 Day 1, Q3W | 254 | Placebo 200 mg + investigator’s choice (Pacitaxel 200 mg/m2 on day 1 or NAB-Paclitaxel 100 mg/m2 on days 1, 8, 15) + Carboplatin AUC6 day 1, Q3W | 264 |
| O’Donnell et al. 2018 (35)a | MEDI4736 | I/II | NCT01693562 | Urothelial carcinoma | Single group trial | EORTC QLQ-C30 | Week 16 | Durvalumab, 10 mg/kg Q2W | 182 | NA | |
| Perol et al. 2019 (36)a | EVIDENS | N/A | NCT03382496 | Non-small cell lung cancer | Observational | EQ-5D | Week 36 | Nivolumab | 1394 | NA | |
| Petrella et al. 2017 (12) | KEYNOTE-006 | III | NCT01866319 | Melanoma | Randomized trial | EORTC QLQ-C30 | Week 12 | Pembrolizumab 10 mg/kg Q2W | 270 | Ipilimumab 3 mg/kg Q3W | 240 |
| Pembrolizumab 10 mg/kg Q3W | 266 | ||||||||||
| Powles et al. 2018 (37) | IMvigor211 | III | NCT02302807 | Urothelial carcinoma | Randomized trial | EORTC QLQ-C30 | Week 12 | Atezolizumab 1200 mg Q3W | 467 | Investigator’s choice: Docetaxel 75 mg/m2 or Paclitaxel 175 mg/m2 or Vinflunine 320 mg/m2 Q3W | 464 |
| Reck et al. 2018 (38) | CheckMate 017 | III | NCT01642004 | Non-small cell lung cancer | Randomized trial | EQ-5D | Week 66 | Nivolumab 3 mg/kg Q2W | 135 | Docetaxel 75 mg/m2 Q3W | 137 |
| Revicki et al. 2012 (13) | MDX010-20 | III | NCT00094653 | Melanoma | Randomized trial | EORTC QLQ-C30 | Week 12 | Ipilimumab (3 mg/kg) + gp 100 (1 mg) Q3W | 403 | gp100 (1 mg) + placebo Q3W | 136 |
| Ipilimumab (3 mg/kg) + placebo Q3W | 137 | ||||||||||
| Schadendorf et al. 2016 (14) | KEYNOTE-002 | II | NCT01704287 | Melanoma | Randomized trial | EORTC QLQ-C30 | Week 12 | Pembrolizumab 2 mg/kg Q3W | 180 | Investigator’s choice: carboplatin + paclitaxel, paclitaxel, dacarbazine, or temozolomide | 179 |
| Pembrolizumab 10 mg/kg Q3W | 181 | ||||||||||
| Schadendorf et al. 2017 (15) | CheckMate 067 | III | NCT01844505 | Melanoma | Randomized trial | EORTC QLQ-C30 | Week 55 | Nivolumab 3 mg/kg Q2W + placebo for ipilimumab W 1 and W 4+ placebo for nivolumab W 4 | 270 | Ipilimumab 3 mg/kg Q3W + placebo matching with nivolumab W 3, 5 | 259 |
| Nivolumab 1 mg/kg Q3W + ipilimumab 3 mg/kg Q3W | 274 | ||||||||||
| Sharma et al. 2017 (39) | CheckMate 275 | II | NCT02387996 | Bladder cancer | Single group trial | EORTC QLQ-C30 | Week 17 | Nivolumab 3 mg/kg Q2W | 270 | NA | |
| Vaughn et al. 2018 (40) | KEYNOTE-045 | III | NCT02256436 | Urothelial carcinoma | Randomized trial | EORTC QLQ-C30 | Week 15 | Pembrolizumab 200 mg Q3W | 266 | Paclitaxel 175 mg/m2 IV or Docetaxel 75 mg/m2 IV or Vinflunine 320 mg/m2 IV, Q3W | 253 |
| Weber et al. 2017 (16) | CheckMate 238 | III | NCT02388906 | Melanoma | Randomized trial | EORTC QLQ-C30 | Week 17 | Nivolumab and placebo matching ipilimumab 3 mg/kg Q2W | 453 | Ipilimumab and placebo matching nivolumab 10 mg/kg Q3W | 453 |
| Younes et al. 2016 (41) | CheckMate 205 | II | NCT02181738 | Hodgkin lymphoma | Clinical trial | EORTC QLQ-C30 | Week 17 | Nivolumab 3 mg/kg Q2W | 80 | NA | |
Published abstract. IV = intravenous; NA = not applicable; Q2W = every 2 weeks; Q3W = every 3 weeks; QOL = quality of life; NCT = ClinicalTrials.gov identifier.
Participant Characteristics
The number of participants in each publication ranged from 72 to 1394. Patient populations included those with melanoma (k = 10), NSCLC (k = 7), urothelial cancer (k = 3), renal cell carcinoma (k = 1), hepatocellular carcinoma (k = 1), head and neck cancer (k = 1), Merkel cell carcinoma (k = 1), bladder cancer (k = 1), and Hodgkin lymphoma (k = 1). For intervention arms, the mean sample age ranged from 37 to 70 years, and 18%-43% of participants were female. For comparison arms, mean ages ranged from 52 to 65 years and were 15%-46% female.
Global QOL
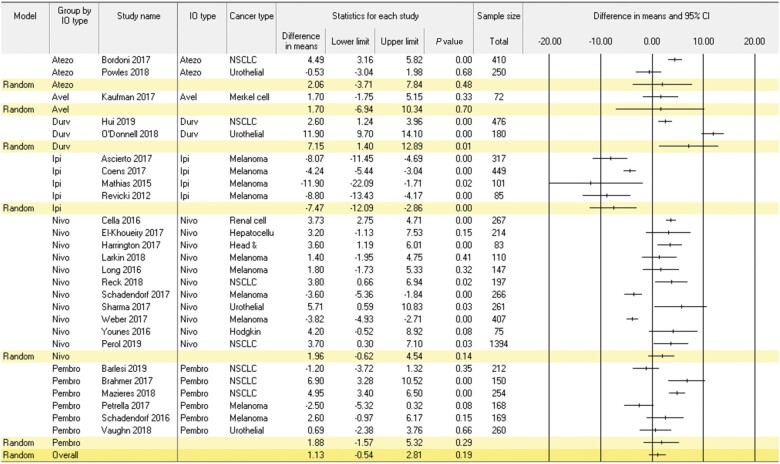
Results of the meta-analysis examining change from baseline to follow-up in global QOL among patients receiving ICIs are presented in Figure 2. This meta-analysis encompassed 26 studies and 6974 patients. Global QOL did not change statistically significantly from baseline to follow-up (mean change = 1.13, 95% CI = -0.54 to 2.81; P = .19). Statistically significant heterogeneity was observed across studies (Q = 442.0; P < .001; I2 = 94.3%). Moderators associated with change in global QOL were ICI regimen, cancer type, sex, mean age, and risk of bias (P < .03); however, age was no longer a statistically significant moderator in sensitivity analyses excluding published abstracts. Regarding ICI regimen, patients receiving ipilimumab reported small reductions in global QOL over time (mean change = -7.47, 95% CI = -12.09 to -2.86; P < .001), whereas patients treated with atezolizumab, avelumab, nivolumab, and pembrolizumab reported no change (mean change = 1.70-1.96; P > .14), and those treated with durvalumab reported small improvements in global QOL (mean change = 7.15, 95% CI = 1.40 to 12.89; P = .01). Regarding cancer type, melanoma patients reported trivial reduction in global QOL (mean change = -3.09, 95% CI = -5.16 to -1.03; P = .003). NSCLC and urothelial cancer patients reported small improvement in QOL (mean change range = 3.55-4.49; P < .007). Head and neck, hepatocellular, Hodgkin lymphoma, Merkel cell carcinoma, renal cell carcinoma patients did not report statistically significant change in QOL (mean change = 1.70-3.73; P > .19). Studies with a greater percentage of men (P = .003), older age (P = .03), and higher risk of bias (P = .003) reported improved global QOL. Duration of follow-up and quality of PRO reporting were not associated with change in global QOL (P ≥ .10).
Figure 2.
Meta-analysis of within-group change in global quality of life in patients receiving immune checkpoint inhibitor therapy. Positive values indicate improvement. Random effect models were used with a 2-sided alpha level of.05. Error bars indicate the 95% confidence intervals (CI). NSCLC = non-small cell lung cancer; IO = immune checkpoint inhibitor.
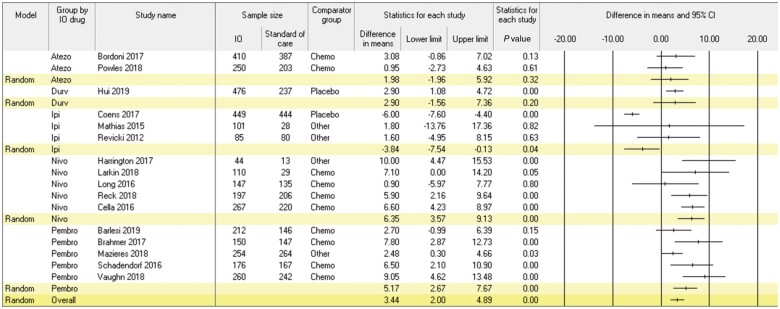
Figure 3 presents results of the meta-analysis comparing differences in mean change in global QOL from baseline to follow-up in patients treated with ICIs vs non-ICI regimens. This meta-analysis encompassed 16 studies and 6536 patients (ie, ICI: n = 3588; non-ICI: n = 2948). Patients receiving ICIs reported larger improvements in global QOL than patients receiving non-ICI regimens in the trivial range (mean change difference = 3.44, 95% CI = 2.00 to 4.89; P < .001). Statistically significant heterogeneity was observed across studies (Q = 145.4; P < .001; I2 = 89.7%). Moderators of group differences in change in global QOL included ICI regimen, mean participant age, and risk of bias (P < .05); the same pattern of results was observed in sensitivity analyses excluding published abstracts. Regarding ICI regimen, patients treated with ipilimumab reported less favorable change in global QOL than control patients (mean change difference = 3.84, 95% CI = -7.54 to -0.13; P = .04), whereas patients treated with atezolizumab and durvalumab reported no difference (mean change differences = 1.98-2.90; P > .20) and those treated with nivolumab (mean change difference = 6.35, 95% CI = 3.57 to 9.13; P < .001) and pembrolizumab (mean change difference = 5.17, 95% CI = 2.67 to 7.68; P < .001) reported more favorable change in global QOL in the trivial to small range. Differences in change in global QOL between patients treated with ICI and non-ICI regimens were larger in studies with higher mean participant age and higher risk of bias (P < .03). Cancer type, type of comparator group, sex, duration of follow-up, and quality of PRO reporting were not statistically significant moderators (P > .07).
Figure 3.
Meta-analysis of differences in mean change in global quality of life from baseline to follow-up in patients treated with immune checkpoint inhibitors vs other regimens. Positive values favor immune checkpoint inhibitors. Random effect models were used with a 2-sided alpha level of.05. Error bars indicate the 95% confidence intervals (CI). IO = immune checkpoint inhibitor.
Physical Functioning
Results of the meta-analysis examining change from baseline to follow-up in physical functioning among patients receiving ICIs are shown in Supplementary Figure 1 (available online). This meta-analysis encompassed 14 studies and 3169 patients. Across all ICI regimens, there was no statistically significant change in physical functioning from baseline to follow-up (mean change = 0.46, 95% CI = -0.79 to 1.71; P = .47). Patients treated with pembrolizumab reported worsening physical functioning in the trivial range (mean change = -3.13, 95% CI = -6.12 to -0.14; P = .04), those treated with durvalumab (single study) reported improved physical functioning in the trivial range (mean change = 2.30, 95% CI = 0.73 to 3.87; P = .004), and those treated with atezolizumab, ipilimumab, or nivolumab reported no change (P > .05).
Results of the meta-analysis comparing differences in mean change in physical functioning from baseline to follow-up in patients treated with ICIs vs those treated with non-ICI regimens are shown in Supplementary Figure 2 (available online). This meta-analysis encompassed 11 studies and 4630 patients (ie, ICI n = 2495; non-ICI n = 2135). Across all ICI regimens, there were no group differences in change in physical functioning between patients treated with ICIs vs those treated with non-ICI regimens (mean difference = -0.03, 95% CI = -0.75 to 0.70; P = .94). However, patients treated with pembrolizumab reported better physical functioning relative to comparator regimens in the trivial range (mean difference = 3.96, 95% CI = 1.07 to 6.86; P = .007). There were no other group differences in physical functioning by ICI regimen (P > .05).
Symptomatology
Results of meta-analyses examining change from baseline to follow-up in symptomatology among patients receiving ICIs are shown in Supplementary Figure 3 (available online). The meta-analysis of fatigue encompassed 15 studies and 3499 patients; meta-analyses of other symptoms encompassed 14 studies and 3243 to 3249 patients. Across ICI regimens, results indicated improved appetite loss, insomnia, and pain but worsening dyspnea (all in trivial range; P < .001). Patients treated with ipilimumab reported worsening appetite loss (small range), dyspnea (small range), fatigue (small range), and nausea and/or vomiting (trivial range; P < .007). Patients treated with nivolumab or pembrolizumab reported improved insomnia (both in trivial range; P < .007). All other symptoms demonstrated improvement in single studies (ie, atezolizumab, durvalumab) or no change.
Results of meta-analyses comparing differences in mean change in symptoms from baseline to follow-up in patients treated with ICIs vs those treated with non-ICI regimens are presented in Supplementary Figure 4 (available online). The meta-analysis of fatigue encompassed 12 studies and 5252 patients (ie, ICI n = 2825; non-ICI n = 2427). The meta-analyses of other symptoms encompassed 11 studies and 4789 to 4802 patients (ie, ICI n = 2618-2627; non-ICI n = 2170-2175). Across ICI regimens, patients treated with ICIs reported less insomnia than control patients (trivial range; P < .001). Patients treated with atezolizumab reported less dyspnea, fatigue, and pain and more insomnia (P < .008) than those treated with non-ICI regimens. Patients treated with durvalumab (k = 1) reported more appetite loss, diarrhea, dyspnea, fatigue, nausea and/or vomiting, and pain than control patients (P < .003). Patients treated with ipilimumab reported more insomnia than those treated with non-ICI regimens (P < .001). Patients treated with nivolumab reported less nausea and/or vomiting than those treated with non-ICI regimens (P = .04). Patients treated with pembrolizumab reported less appetite loss, constipation, diarrhea, dyspnea, fatigue, insomnia, nausea and/or vomiting, and pain than those treated with non-ICI regimens (P < .03).
Potential Publication Bias
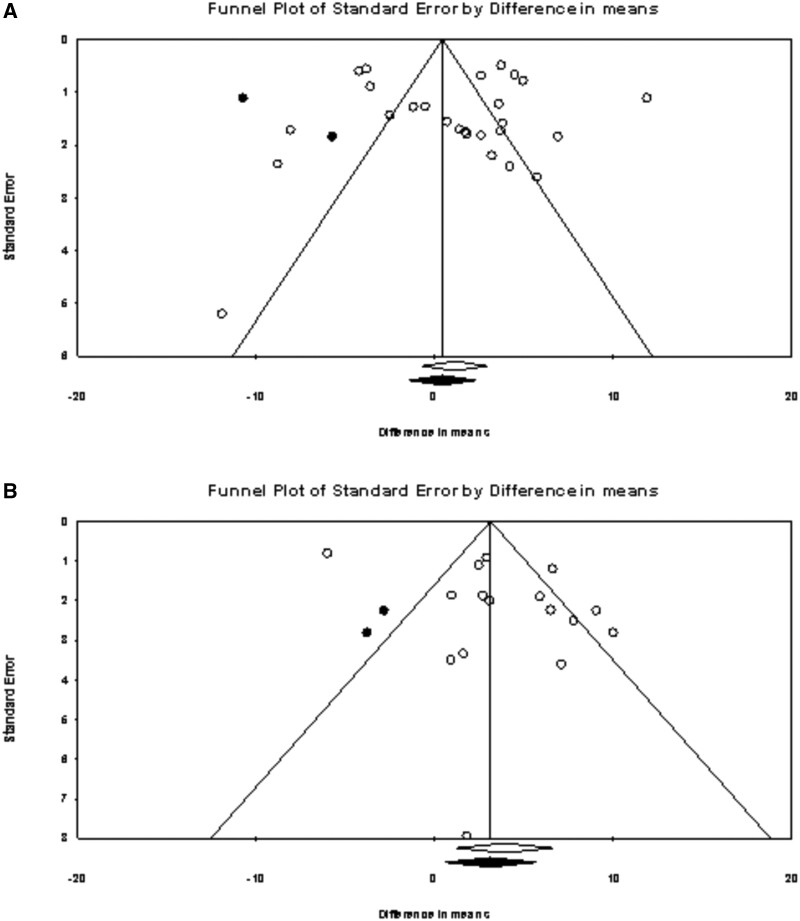
Results of the funnel plots examining publication bias are presented in Figure 4. For studies that examined change in QOL since baseline in patients treated with ICIs, the funnel plot indicated larger effects to the right of the mean. The trim and fill procedure imputed 2 studies, and the estimate is 0.38 (95% CI = -1.52 to 2.29), which suggests a slightly smaller estimate of the observed effect of 1.13 (95% CI = -0.54 to 2.81). Similarly, for studies that compared QOL at follow-up in ICIs vs non-ICI regimens the funnel plot indicated larger effects to the right of the mean. The trim and fill procedure imputed 2 studies, and the estimate is 3.11 (95% CI = 0.58 to 5.64), which suggests a slightly smaller estimate of the observed effect of 3.44 (95% CI = 2.00 to 4.89) but does not indicate statistically significant bias of the effect.
Figure 4.
Funnel plots of difference in means by standard error. The observed studies are indicated by circle outlines and imputed studies indicated by solid circles. Studies are plotted on standard error (vertical axis) and effect size (horizontal axis). Publication bias would be represented by a larger quantity of studies at the bottom of the plot and an asymmetrical distribution. Panel A shows the immune checkpoint inhibitor group change. Panel B shows the comparisons of between-group differences in quality of life at follow-up in patients treated with immune checkpoint inhibitors vs other regimens.
Discussion
This study quantitatively summarized a heterogeneous literature on patient-reported global QOL (primary outcome) and physical functioning and symptomatology (secondary outcomes) in patients treated with ICIs. The goal of the study is to facilitate patient education about what to expect when receiving these therapies. Pairs of meta-analyses were conducted for each outcome. One focused on within-group change in QOL among patients treated with ICIs, and the other focused on between-group differences in change in outcomes from baseline to follow-up in patients treated with ICIs compared with non-ICI regimens. Results indicated stable global QOL among patients treated with ICIs and statistically significantly better global QOL at follow-up compared with patients treated with non-ICI regimens. Physical functioning was also stable among patients treated with ICIs and similar to comparison groups. Regarding symptoms, patients treated with ICIs reported improvements in insomnia. Compared with patients treated with non-ICI regimens, they also reported less appetite loss, insomnia, and pain, but more dyspnea. These findings are broadly consistent with previous qualitative reviews (42-44) and a meta-analysis (4) on this topic. However, the only previous meta-analysis available did not examine CTLA-4 inhibitors, except as a comparator, and numerous recent trials have been conducted using novel agents and for different indications. The current study extends previous findings through rigorous statistical analysis of a larger, more inclusive search of interventional and observational studies encompassing both anti–PD-1/PD-L1 and anti–CTLA-4 agents.
Statistically significant heterogeneity was observed in QOL by regimen and disease site. Patients treated with ipilimumab and those diagnosed with melanoma reported statistically significantly worsening global QOL over time, whereas patients treated with other ICI regimens for other cancer types reported improved or stable global QOL. Worsening global QOL in melanoma patients was secondary to receipt of ipilimumab, as post hoc analyses indicated that melanoma patients treated with ipilimumab, but not pembrolizumab or nivolumab, reported worsening global QOL (data not shown). Similarly, patients treated with ipilimumab reported worse global QOL than patients treated with non-ICI regimens, whereas patients treated with atezolizumab, durvalumab, nivolumab, and pembrolizumab reported statistically significantly better global QOL. Patients treated with ipilimumab also reported more appetite loss, dyspnea, fatigue, and nausea and/or vomiting than patients treated with non-ICI regimens. More symptomatology may account for worse global QOL in ipilimumab-treated patients. Because only 1 study of combined ipilimumab and nivolumab for melanoma met criteria for inclusion (45), we were unable to assess the effects of combination ICI on global QOL. This study, a randomized, double-blind, phase III trial comparing single-agent ipilimumab, single-agent nivolumab, and combination nivolumab and ipilimumab for advanced melanoma (ie, CheckMate 067), reported comparable global QOL across all 3 groups despite better disease outcomes in the combination group (45). These findings are surprising in light of the higher incidence of adverse events in the combination group. They suggest that global QOL benefits of the combination because of better disease control may have been offset by worse side effects. More research on this topic is needed, however.
Additional moderators of global QOL included greater improvements in studies with a higher percentage of men, greater group differences in follow-up in studies with greater mean age, and better QOL outcomes in both meta-analyses among studies judged to be at high risk of bias. Although differences by sex and age were not observed in both global QOL meta-analyses, they are consistent with previously published reports suggesting that ICIs may be more efficacious in improving survival in men (46,47), and older cancer patients tend to report better QOL during active treatment than younger patients (48). The finding that better global QOL was reported in studies with higher risk of bias underscores the importance of assessing global QOL in blinded randomized trials. Nevertheless, it is also recognized that participants in clinical trials tend to be younger and healthier and have higher socioeconomic status than patients treated outside of clinical trials (49,50), which can introduce its own bias. Lastly, attrition in the included studies because of illness, toxicities, or other factors may also introduce bias in QOL findings.
Regarding the clinical significance of change in global QOL, patients treated with ipilimumab reported an average worsening of 7.48 points, which corresponds to slight worsening (51). In contrast, improvements in global QOL in patients treated with atezolizumab, durvalumab, nivolumab, and pembrolizumab ranged in size from 9.46 to 14.63 points, which corresponds to moderate improvement (51). Group differences in QOL at follow-up between patients treated with ICI vs non-ICI regimens ranged from -3.84 (ipilimumab) to 10.18 (nivolumab), below the cutoff of 12.8 (ie, 0.5 SD) for clinically significant differences in recurrent and metastatic cancer patients (52,53). Thus, although patients treated with ICIs reported statistically better global QOL than patients treated with non-ICI regimens, this difference was not clinically significant.
Notably, comparison regimen (ie, chemotherapy, placebo, other) did not statistically significantly impact group differences in global QOL at follow-up. This finding may be due in part to the statistically significant heterogeneity of comparison regimens across studies (eg, different types of chemotherapy) and even within the same study (eg, investigator’s choice). The finding may also be because chemotherapy may result in better disease control than placebo, offsetting the deleterious impact of side effects of chemotherapy on global QOL. Similarly, duration of follow-up was not a moderator of change in global QOL or group differences in global QOL. This finding may be because the various toxicities of ICIs tend to appear at different times after treatment initiation, with skin toxicities often appearing in 2-3 weeks, followed by gastrointestinal and hepatic toxicities at 4-7 weeks and liver and endocrine toxicities after 9 weeks (54,55).
The current study is characterized by numerous strengths, including a clinically important research question, thorough search strategy, and rigorous statistical analyses. Nevertheless, limitations should also be noted. Our search strategy may have missed QOL data presented at conferences that were not searched. In addition, there were 23 eligible studies for which we were unable to obtain data from the authors and/or sponsors. In some cases, this was because the trial investigators had yet to publish primary findings. Trim and fill plots found minimal bias of omitted data on results, however. We were unable to provide more fine-grained moderation analyses of non-ICI comparison groups, as many were heterogeneous in terms of regimens (eg, investigator’s choice). Regarding secondary outcomes, in several of the analyses, atezolizumab and durvalumab were represented by only 1 study. Therefore, caution is warranted when interpreting findings in physical functioning and symptoms in patients treated with these agents. Moreover, in light of the rapid pace of research on ICIs, there will likely be many additional studies published in the near future that are not included in these meta-analyses. These may include future studies of long-term and late effects, which are only starting to be described empirically (56). Nevertheless, a growing number of patients are receiving ICIs and need evidence-based information regarding what to expect on treatment. Lastly, some studies were published as peer-reviewed abstracts rather than papers; however, findings from sensitivity analyses excluding abstracts identified few and minor differences.
In summary, the current meta-analysis represents the intersection of 3 trends in oncology: 1) the paradigm shift of targeting immune cells in cancer treatment, 2) the use of PRO measures to better understand patients’ perspectives about their treatment, and 3) the application of advanced statistical and mathematical techniques to solve important problems in oncology. Of note, this study is among the first to aggregate data about patient-reported outcomes of immunotherapy, which allows greater statistical power to detect change over time and relationships among variables than single studies.
These data can be used to reassure patients and their families that they can expect stable or improved global QOL on average with PD-1/PD-L1 inhibitors and slight decrements in global QOL in CTLA-4 inhibitors.
Funding
Funding provided by NCI grant K01 CA211789 (PI: Gonzalez).
Notes
Role of the funder: The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: BG reports personal fees from SureMed Compliance, KemPharm, Elly Health, Inc. outside the submitted work. AD reports personal fees from Roche, EMD Serono, Celldex, Janssen, Cybrexa, Self Care Catalysts, Oncohost, ThirdBridge, Noxopharm, Varian, Accordant, and Moleculin outside the submitted work. HJ reports personal fees from RedHill BioPharma, Janssen Scientific Affairs, and Merck outside the submitted work. All other authors have no disclosures to report .
Author contributions: Conceptualization: BDG, HSLJ. Data curation: BDG, SLE, KEB, AIH, BWJ, BJS, SS, KAH, HWB, SMC, JM, AMN, RA, KM, BK, EL, NLW, SJ, MAP, APD, HSLJ. Formal analysis: SLE, BJS, HSLJ. Methodology: BDG, SLE, KEB, BJS, HSLJ. Project administration: BDG, SLE, KEB, HSLJ. Writing—original draft: BDG, HSLJ. Writing—review and editing: All authors.
Prior presentations: Results were presented at ESMO Congress 2020.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Contributor Information
Brian D Gonzalez, PhD, Department of Health Outcomes and Behavior, Moffitt Cancer Center, Tampa, FL, USA.
Sarah L Eisel, PhD, Department of Health Outcomes and Behavior, Moffitt Cancer Center , Tampa, FL, USA.
Kristina E Bowles, MPH, Department of Health Outcomes and Behavior , Moffitt Cancer Center, Tampa, FL, USA.
Aasha I Hoogland, PhD, Department of Health Outcomes and Behavior, Moffitt Cancer Center, Tampa, FL, USA.
Brian W James, BS, Morsani College of Medicine, University of South Florida, Tampa, FL, USA.
Brent J Small, PhD, School of Aging Studies, University of South Florida, Tampa, FL, USA.
Susan Sharpe, MA, Moffitt Biomedical Library, Moffitt Cancer Center, Tampa, FL, USA.
Kelly A Hyland, PhD, Department of Health Outcomes and Behavior, Moffitt Cancer Center, Tampa, FL, USA.
Hailey W Bulls, PhD, Department of Health Outcomes and Behavior, Moffitt Cancer Center, Tampa, FL, USA.
Shannon M Christy, PhD, Department of Health Outcomes and Behavior, Moffitt Cancer Center, Tampa, FL, USA.
Jori Mansfield, BS, Morsani College of Medicine, University of South Florida, Tampa, FL, USA.
Ashley M Nelson, PhD, Department of Psychiatry, Massachusetts General Hospital, Boston, MA, USA.
Raviteja Alla, BS, Morsani College of Medicine, University of South Florida, Tampa, FL, USA.
Kelly Maharaj, MPH, Department of Health Outcomes and Behavior, Moffitt Cancer Center, Tampa, FL, USA .
Brittany Kennedy, BA, Department of Health Outcomes and Behavior, Moffitt Cancer Center, Tampa, FL, USA.
Elizabeth Lafranchise, BS, Morsani College of Medicine, University of South Florida, Tampa, FL, USA.
Noelle L Williams, MD, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Sarah Jennewein, Department of Health Outcomes and Behavior, Moffitt Cancer Center, Tampa, FL , USA.
Laura B Oswald, PhD, Department of Health Outcomes and Behavior, Moffitt Cancer Center, Tampa , FL, USA.
Michael A Postow, MD, Southeast Radiation Oncology Group, Levine Cancer Institute at Atrium Health, Charlotte, NC, USA.
Adam P Dicker, MD, PhD, Department of Radiation Oncology, Thomas Jefferson University, Philadelphia, PA, USA.
Heather S L Jim, PhD, Department of Health Outcomes and Behavior, Moffitt Cancer Center, Tampa, FL, USA.
References
- 1. Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–2519. [DOI] [PubMed] [Google Scholar]
- 2. Sznol M, Longo DL. Release the hounds! Activating the T-cell response to cancer. N Engl J Med. 2015;372(4):374–375. [DOI] [PubMed] [Google Scholar]
- 3. Wargo JA, Reuben A, Cooper ZA, Oh KS, Sullivan RJ. Immune effects of chemotherapy, radiation, and targeted therapy and opportunities for combination with immunotherapy. Semin Oncol. 2015;42(4):601–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishijima TF, Shachar SS, Muss HB, Tamura K. Patient-reported outcomes with PD-1/PD-L1 inhibitors for advanced cancer: a meta-analysis. Oncologist. 2019;24(7):e565–e573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, Altman DG; for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rohatgi A. WebPlotDigitizer (4.2.) https://automeris.io/WebPlotDigitizer. Published 2019. Accessed February 8, 2021.
- 7.Gonzalez B, Eisel S, Bowles K, et al. Protocol: Meta-Analysis of Quality of Life in Cancer Patients Treated with Immune Checkpoint Inhibitors. INPLASY protocol; 2020. doi:10.37766/inplasy2020.4.0203. [DOI] [PMC free article] [PubMed]
- 8. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 9.EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- 10. Higgins J, Li T, Deeks J. . Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Chichester, UK: Cochrane; 2021:143–174. [Google Scholar]
- 11. Barlesi F, Garon EB, Kim DW, et al. Health-related quality of life in KEYNOTE-010: a phase II/III study of pembrolizumab versus docetaxel in patients with previously treated advanced, programmed death ligand 1-expressing NSCLC. J Thorac Oncol. 2019;14(5):793–801. [DOI] [PubMed] [Google Scholar]
- 12. Petrella TM, Robert C, Richtig E, et al. Patient-reported outcomes in KEYNOTE-006, a randomised study of pembrolizumab versus ipilimumab in patients with advanced melanoma. Eur J Cancer. 2017;86:115–124. [DOI] [PubMed] [Google Scholar]
- 13. Revicki DA, van den Eertwegh AJ, Lorigan P, et al. Health related quality of life outcomes for unresectable stage III or IV melanoma patients receiving ipilimumab treatment. Health Qual Life Outcomes. 2012;10(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schadendorf D, Dummer R, Hauschild A, et al. Health-related quality of life in the randomised KEYNOTE-002 study of pembrolizumab versus chemotherapy in patients with ipilimumab-refractory melanoma. Eur J Cancer. 2016;67:46–54. [DOI] [PubMed] [Google Scholar]
- 15. Schadendorf D, Larkin J, Wolchok J, et al. Health-related quality of life results from the phase III CheckMate 067 study. Eur J Cancer. 2017;82:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV Melanoma. N Engl J Med. 2017;377(19):1824–1835. [DOI] [PubMed] [Google Scholar]
- 17. Ascierto PA, Long GV, Robert C, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2019;5(2):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JP, Altman DG, Gotzsche PC, et al. ; for the Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD; for the CONSORT PRO Group. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309(8):814–822. [DOI] [PubMed] [Google Scholar]
- 20. Efficace F, Fayers P, Pusic A, et al. ; for the European Organization for Research and Treatment of Cancer Quality-of-Life Group (Patient-Reported Outcome Measurements Over Time in Oncology Registry). Quality of patient-reported outcome reporting across cancer randomized controlled trials according to the CONSORT patient-reported outcome extension: a pooled analysis of 557 trials. Cancer. 2015;121(18):3335–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011;29(1):89–96. [DOI] [PubMed] [Google Scholar]
- 22. Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611–622. [DOI] [PubMed] [Google Scholar]
- 23. Bordoni R, Ciardiello F, Von Pawel J, et al. Patient-reported outcomes (PROs) in OAK: a phase III study of atezolizumab vs docetaxel in non-small-cell lung cancer (NSCLC). J Thorac Oncolo. 2017;12(11):S1914- S1914. [DOI] [PubMed] [Google Scholar]
- 24. Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18(12):1600–1609. [DOI] [PubMed] [Google Scholar]
- 25. Cella D, Grunwald V, Nathan P, et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coens C, Suciu S, Chiarion-Sileni V, et al. Health-related quality of life with adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): secondary outcomes of a multinational, randomised, double-blind, phase 3 trial. Lancet Oncol. 2017;18(3):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harrington KJ, Ferris RL, Blumenschein G Jr, et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017;18(8):1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hui R, Özgüroğlu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(12):1670–1680. [DOI] [PubMed] [Google Scholar]
- 30. Kaufman HL, Hunger M, Hennessy M, Schlichting M, Bharmal M. Nonprogression with avelumab treatment associated with gains in quality of life in metastatic Merkel cell carcinoma. Future Oncol. 2018;14(3):255–266. [DOI] [PubMed] [Google Scholar]
- 31. Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in Checkmate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36(4):383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Long GV, Atkinson V, Ascierto PA, et al. Effect of nivolumab on health-related quality of life in patients with treatment-naive advanced melanoma: results from the phase III CheckMate 066 study. Ann Oncol. 2016;27(10):1940–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mathias SD, Kotapati S, Le TK, Sabater J, Abernethy AP. Health-related quality of life (HRQoL) and patient experience in advanced melanoma: 6-month results from the image study. Qual Life Res. 2015;24(S1):17–18. [Google Scholar]
- 34. Mazieres J, Kowalski D, Luft A, et al. Health-related quality of life (HRQoL) for pembrolizumab or placebo plus carboplatin and paclitaxel or nab-paclitaxel in patients with metastatic squamous NSCLC: Data from KEYNOTE-407. Ann Oncol. 2018;29:viii748–viii749. [Google Scholar]
- 35. O’Donnell PH, Arkenau HT, Sridhar SS, et al., , Patient-reported outcomes (PROs) in patients with urothelial carcinoma (UC) treated with durvalumab (second-line or above) in phase 1/2 dose-escalation study 1108. J Clin Oncol. 2018;36(15):4532. [Google Scholar]
- 36. Perol M, Dixmier A, Barlesi F, et al. Health-related quality of life (HRQoL) of non-small cell lung cancer (NSCLC) patients treated with nivolumab in real-life: the EVIDENS study. Ann Oncol. 2019;30(suppl 2):ii48. [Google Scholar]
- 37. Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748–757. [DOI] [PubMed] [Google Scholar]
- 38. Reck M, Taylor F, Penrod JR, et al. Impact of nivolumab versus docetaxel on health-related quality of life and symptoms in patients with advanced squamous non-small cell lung cancer: results from the checkmate 017 study. J Thorac Oncol. 2018;13(2):194–204. [DOI] [PubMed] [Google Scholar]
- 39. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. [DOI] [PubMed] [Google Scholar]
- 40. Vaughn DJ, Bellmunt J, Fradet Y, et al. Health-related quality-of-life analysis from KEYNOTE-045: a phase III study of pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer. J Clin Oncol. 2018;36(16):1579–1587. [DOI] [PubMed] [Google Scholar]
- 41. Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abdel-Rahman O, Oweira H, Giryes A. Health-related quality of life in cancer patients treated with PD-(L)1 inhibitors: a systematic review. Expert Rev Anticancer Ther. 2018;18(12):1231–1239. [DOI] [PubMed] [Google Scholar]
- 43. Narayan V, Kahlmeyer A, Dahm P, et al. Pembrolizumab monotherapy versus chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum-containing chemotherapy. A Cochrane Rapid Review. Cochrane Database Syst Rev. 2018;7(7):CD012838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hall ET, Singhal S, Dickerson J, et al. ; for the AAHPM Research Committee Writing Group. Patient-reported outcomes for cancer patients receiving checkpoint inhibitors: opportunities for palliative care-a systematic review. J Pain Symptom Manage. 2019;58(1):137–156. e131. [DOI] [PubMed] [Google Scholar]
- 45. Abbas-Aghababazadeh F, Li Q, Fridley BL. Comparison of normalization approaches for gene expression studies completed with high-throughput sequencing. PLoS One. 2018;13(10):e0206312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Conforti F, Pala L, Bagnardi V, et al. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. 2019;111(8):772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19(6):737–746. [DOI] [PubMed] [Google Scholar]
- 48. Cheng KK, Lim EY, Kanesvaran R. Quality of life of elderly patients with solid tumours undergoing adjuvant cancer therapy: a systematic review. BMJ Open. 2018;8(1):e018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106(3):dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483–491. [DOI] [PubMed] [Google Scholar]
- 51. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. [DOI] [PubMed] [Google Scholar]
- 52. Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 Reference Values. Brussels, Belgium: EORTC Quality of Life Group; 2008.
- 53. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 54. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. [DOI] [PubMed] [Google Scholar]
- 55. Fay AP, Moreira RB, Nunes Filho PRS, Albuquerque C, Barrios CH. The management of immune-related adverse events associated with immune checkpoint blockade. Expert Rev Qual Life Cancer Care. 2016;1(1):89–97. [Google Scholar]
- 56. Mamoor M, Postow MA, Lavery JA, et al. Quality of life in long-term survivors of advanced melanoma treated with checkpoint inhibitors. J Immunother Cancer. 2020;8:e000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.