Abstract
Background
Independent of CD4 cell count, a low CD4/CD8 ratio in people with HIV (PWH) is associated with deleterious immune senescence, activation, and inflammation, which may contribute to carcinogenesis and excess cancer risk. We examined whether low CD4/CD8 ratios predicted cancer among PWH in the United States and Canada.
Methods
We examined all cancer-free PWH with 1 or more CD4/CD8 values from North American AIDS Cohort Collaboration on Research and Design observational cohorts with validated cancer diagnoses between 1998 and 2016. We evaluated the association between time-lagged CD4/CD8 ratio and risk of specific cancers in multivariable, time-updated Cox proportional hazard models using restricted cubic spines. Models were adjusted for age, sex, race and ethnicity, hepatitis C virus, and time-updated CD4 cell count, HIV RNA, and history of AIDS-defining illness.
Results
Among 83 893 PWH, there were 5628 incident cancers, including lung cancer (n = 755), Kaposi sarcoma (n = 501), non-Hodgkin lymphoma (n = 497), and anal cancer (n = 439). The median age at cohort entry was 43 years. The overall median 6-month lagged CD4/CD8 ratio was 0.52 (interquartile range = 0.30-0.82). Compared with a 6-month lagged CD4/CD8 of 0.80, a CD4/CD8 of 0.30 was associated with increased risk of any incident cancer (adjusted hazard ratio = 1.24 [95% confidence interval = 1.14 to 1.35]). The CD4/CD8 ratio was also inversely associated with non-Hodgkin lymphoma, Kaposi sarcoma, lung cancer, anal cancer, and colorectal cancer in adjusted analyses (all 2-sided P < .05). Results were similar using 12-, 18-, and 24-month lagged CD4/CD8 values.
Conclusions
A low CD4/CD8 ratio up to 24 months before cancer diagnosis was independently associated with increased cancer risk in PWH and may serve as a clinical biomarker.
Although people with HIV (PWH) are living longer with use of effective antiretroviral therapy (ART), they remain at an increased risk for cancer compared with the general US population (1-5). In addition to AIDS-defining cancers (ADCs), including Kaposi sarcoma, non-Hodgkin lymphomas (NHLs) and cervical cancer, risk of several non-ADC remains increased in PWH (6-9). Cancer mortality is also high among PWH (10,11). Approximately 10% of all deaths among PWH prescribed ART in the United States between 1995 and 2009 were attributed to cancer, with NHL, lung, and liver cancer contributing the highest number of deaths (10).
Cancer risk in PWH is attributed to immunodeficiency, immune dysfunction, and high prevalence of oncogenic virus coinfection (including human papillomavirus [HPV] and hepatitis C virus [HCV]) and behavioral risk factors, including smoking (12-15). In addition to immunodeficiency, decreased immune surveillance and increased cellular replication associated with immune senescence, activation, and inflammation increase carcinogenesis (16,17). In PWH, CD4 and CD8 cell counts are clinically readily available. A low CD4/CD8 ratio inversely correlates with T lymphocyte replicative senescence, activation, and exhaustion, each a measure of immune senescence and systemic inflammation (18-20). A low CD4/CD8 ratio is also associated with older age, cytomegalovirus coinfection, and low CD4 cell count nadir, and has been associated with risk of non-AIDS outcomes (18,21-27). We sought to determine whether the CD4/CD8 ratio, as a marker of immune dysfunction, was associated with cancer risk, with the hypothesis that the CD4/CD8 ratio would be inversely associated with incident cancer diagnoses among PWH.
Methods
Study Population
We examined incident cancer diagnoses and longitudinal CD4/CD8 data from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) (28). Participating cohorts contributed patient-level demographic, behavioral, and clinical data, including diagnoses, medications, and laboratory values, to the central Data Management Center for quality assessment and harmonization. All cancer diagnoses were validated by standardized medical record documentation that included abstraction of histopathologic information or linkage with cancer registries (4). The institutional review boards at participating cohorts, Johns Hopkins University, and Vanderbilt University have approved human patient activities conducted within the NA-ACCORD related to this study.
NA-ACCORD includes PWH aged 18 years or older with at least 2 clinic visits within the first year of cohort enrollment. A total of 22 observational cohorts in NA-ACCORD collected validated cancer diagnoses from January 1998 to December 2016. Seven cohorts were excluded where 25% or more of PWH had no CD8 cell counts data. All PWH with any cancer diagnosis before or within the first 6 months of cohort entry or a cancer with an unknown diagnosis date were excluded. Only PWH with a minimum of 12 months of clinic follow-up were included in the primary analysis to allow for our lagged exposure (see below). PWH contributed person-time of observation from 6 months after cohort entry until cancer diagnosis, death, last laboratory value if longer than 18 months before cohort data closure, or date of validated cancer data closure. PWH with extended periods out of care (defined as >18 months without laboratory values) were allowed to reenter the observation period.
Our primary outcomes were incident invasive cancers, excluding basal and squamous cell skin cancers (due to inadequate capture or data in some cancer registries). We examined any incident cancer diagnosis as a composite outcome as well as individual ADCs (Kaposi sarcoma, NHL, and cervical cancer), other viral-associated cancers (Hodgkin lymphoma, liver, anal, head and neck cancers), and nonviral associated cancers (lung, breast, colorectal cancer, and prostate cancers). For those PWH with multiple cancers, only the first such diagnosis was included as an outcome.
We used time-updated, lagged values of CD4/CD8 ratio in our time-to-event analyses. We hypothesized a delayed effect of CD4/CD8 ratio and cancer diagnosis; therefore, we evaluated lagged CD4/CD8 ratio values, referencing the values recorded during defined intervals in the past in estimation of cancer risk during observation time (Supplementary Figure 1, available online). Individual observation time was constructed based on laboratory values and their corresponding dates of measurement. CD4 and CD8 cell counts were time-varying and carried forward for a maximum of 1 year. In the setting of a gap in laboratory values of more than 1 year, the remaining person-time was coded as missing laboratory values, and the gap period was excluded from the analysis. Person-time of observation, including cancer diagnoses occurring during periods of missing laboratory values, were censored. Our primary analysis examined the 6-month lagged CD4/CD8 ratio value to minimize risk of reverse causality of CD4/CD8 ratio changes and cancer diagnosis. Given that the time between a low CD4/CD8 ratio and cancer development is not known, we repeated analyses using 12-month, 18-month, and 24-month lagged CD4/CD8 ratios.
Statistical Analysis
Multivariable Cox proportional hazards models estimated cause-specific hazard ratios for CD4/CD8 ratio and cancer risk (29). Assumption of proportionality of the primary exposure variable was tested by a global test based on Schoenfeld residuals and visual residual plots checking. Individuals were followed to their first cancer outcome or were censored if they died or had no event during their observed follow-up time. Multivariable models included covariates selected a priori based on clinical knowledge and number of events (degrees of freedom) as potential confounders of the association between CD4/CD8 ratio and each cancer outcome, other cancers, or death. All models included age at study entry, race and ethnicity (Black, Latinx, other [which included Asian, Indigenous, Multiracial, Other, and unknown race], or White), sex, cohort entry year, any history of HCV infection, and time-varying history of any AIDS-defining illness (excluding ADC). Statistical interaction terms were added to examine whether associations between race and ethnicity and CD4/CD8 ratio altered risk for any cancer. Models examining risk factors for liver cancer additionally adjusted for prior hepatitis B virus infection, injection drug use, heavy alcohol use (defined by documented diagnoses, medical record review, and survey results), and time-varying, lagged diagnosis of cirrhosis. Models including time-varying lagged CD4 cell count and HIV RNA examined the effect of CD4/CD8 ratio (independent of absolute CD4 cell count) and accounted for the strong correlation between HIV viremia and the absolute CD4 and CD8 cell count. HIV RNA (log10 transformed), CD4 count (square root transformed), and CD4/CD8 ratio (natural logarithm transformed) were modeled as time-varying covariates. HIV RNA was used as a surrogate marker for time-varying ART use, and values below the limit of detection were calculated as (lower limit of detection − 1 copy/mL). To avoid assuming a linear relationship, most continuous covariates were included using restricted cubic splines, including CD4/CD8 ratio (30). Consistent referent values were important for comparison of effects for each cancer analysis. We present adjusted hazard ratios for the CD4/CD8 ratio comparing the 0.3 and 0.5 with 0.8 values for comparison and approximation to the 25th, 50th, and 75th percentile values of the 6-month lagged CD4/CD8 ratio values for the study cohort. P values were computed using Wald statistics. Breast and cervical cancer analyses included only females; prostate cancer analyses included only males. Covariates were selected a priori based on biologic plausibility and not by stepwise selection. The number of covariates for each model was based on the number of outcomes and available degrees of freedom (approximately 10 events per covariate). Due to the small number of events, covariates for models for breast and cervical cancers included those hypothesized to be the most important potential confounders (CD4 cell count, HIV RNA, age, race and ethnicity, and prior AIDS-defining event). We stratified all Cox analyses by cohort to allow for separate baseline hazards.
In sensitivity analyses, primary multivariable models were repeated, replacing the CD4/CD8 ratio with time-lagged, square-root transformed absolute CD8 cell count and using restricted cubic splines to explore individual effects of the CD4/CD8 ratio. Given the effects of ART on the CD4/CD8 ratio, we additionally repeated 6- and 12-month lagged CD4/CD8 ratio multivariable analyses for all cancers censoring person-time corresponding to laboratory results obtained before ART initiation. Another analysis fitted models that included HIV acquisition risk factor; smoking status (ever vs never); and 6-month lagged, time-varying body mass index. When smoking status or alcohol was missing for individual patients, we used covariates and the outcome data to multiply impute (20 times) using predictive mean matching. Estimates from the imputation-specific analyses were then combined using Rubin’s rule (31).
All analyses were conducted in R version 3.6.2, and the analysis code is available online at https://biostat.app.vumc.org/wiki/Main/ArchivedAnalyses. All P values were 2-sided, and the cut point for statistical significance was .05.
Results
The study population from the 15 eligible cohorts with contributing data is shown in Supplementary Figure 2 (available online). Characteristics of PWH included in the primary and sensitivity analyses are shown in Table 1. In the primary analysis using 6-month lagged laboratory data, 83 893 PWH contributed 744 415 person-years of observation and 5628 incident cancer diagnoses. Of all observation time, 167 816 person-years (22.5%) were censored for the missing CD4/CD8 ratio or HIV RNA levels. The median 6-month lagged CD4/CD8 ratio was 0.52 (interquartile range = 0.30-0.82). PWH in both the primary and sensitivity analyses were predominantly male, and less than one-half were White. The median CD4 cell count, HIV RNA, and history of AIDS-defining illness at the start of observation time was similar between PWH included in the primary and sensitivity analyses. Table 1 shows the frequency of the most common cancers diagnosed. For the primary analyses, there was a total of 5628 incident cancers, of which Kaposi sarcoma (n = 501), NHL (n = 497), prostate cancer (n = 817), lung cancer (n = 755), and anal cancer (n = 439) were the most frequent.
Table 1.
Baseline patient characteristics and outcomes of people living with HIV included in primary and sensitivity analyses of 6-month lagged laboratory data
| Baseline characteristics and outcomes | Primary analysis (N = 83 893) |
Sensitivity analysis (N = 40 975) |
|---|---|---|
| Sex, No. (%) | ||
| Male | 72 709 (86.7) | 32 420 (79.1) |
| Female | 11 184 (13.3) | 8555 (20.9) |
| Median age at study entry (IQR), y | 42.5 (35.1-50.4) | 39.5 (32.5-46.8) |
| Race and ethnicity, No. (%) | ||
| Black | 31 252 (37.3) | 12 283 (30.0) |
| Latinx | 9449 (11.3) | 5728 (14.0) |
| Other or unknowna | 6898 (8.2) | 3316 (8.1) |
| White | 36 294 (43.3) | 19 648 (48.0) |
| HIV transmission risk factor, No. (%) | ||
| Men who have sex with men | 28 725 (34.2) | 24 368 (59.5) |
| Injection drug use | 16 264 (19.4) | 4200 (10.3) |
| Heterosexual | 10 927 (13.0) | 9222 (22.5) |
| Other | 2015 (2.4) | 1007 (2.5) |
| Unknown or missing | 25 962 (30.9) | 2178 (5.3) |
| Chronic hepatitis C virus infection, No. (%) | 17 855 (21.3) | 6816 (16.6) |
| Chronic hepatitis B virus infection, No. (%) | 5991 (7.1) | 3023 (7.4) |
| Any history of heavy alcohol useb, No. (%) | 24 623 (29.4) | 11 500 (28.1) |
| Any history of tobacco usec, No. (%) | 28 373 (33.8) | 24 201 (59.1) |
| Median CD4 cell count (IQR), cells/µLd | 413 (243-610) | 433 (262-631) |
| Median CD4/CD8 ratioe (IQR) | 0.47 (0.27-0.76) | 0.50 (0.29-0.78) |
| Median Log10 HIV RNA (IQR), copies/mLf | 2.3 (1.7-3.9) | 2.1 (1.7-3.8) |
| HIV RNAf, No. (%) | ||
| <500 copies/mL | 41 470 (58.3) | 23 670 (59.7) |
| 500-4999 copies/mL | 9807 (13.6) | 5187 (13.1) |
| ≥5000 copies/mL | 19 844 (27.9) | 10 784 (27.2) |
| Median body mass index (IQR), kg/m2 g | 25.2 (22.7-28.5) | 25.2 (22.6-28.5) |
| History of AIDS-defining illness, No. (%) | 11 438 (13.6) | 7035 (17.2) |
| Geographic region of clinical careh, No. (%) | ||
| US Northeast | 10 788 (12.9) | 3632 (8.9) |
| US Midwest | 7403 (8.8) | 2993 (7.3) |
| US Southeast | 20 483 (24.4) | 7283 (17.8) |
| US West | 28 363 (33.8) | 19 209 (46.9) |
| US Southwest | 4444 (5.3) | 111 (0.3) |
| US Puerto Rico and Virgin Islands | 585 (0.7) | 2 (0.0) |
| Canada | 6733 (8.0) | 3293 (8.0) |
| Other | 12 (0.0) | 2 (0.0) |
| Unknown | 5082 (6.1) | 4450 (10.9) |
| Median duration of follow-up (IQR), y | 8.5 (4.0-13.8) | 7.7 (3.6-12.7) |
| Initiated antiretroviral therapy before study exiti, No. (%) | 75 201 (89.6) | 37 029 (90.4) |
| Proportion of follow-up time after antiretroviral therapy initiation, No. (%) | ||
| <10% | 834 (1.0) | 388 (0.9) |
| 10%-24% | 895 (1.1) | 382 (0.9) |
| 25%-49% | 2171 (2.6) | 857 (2.1) |
| 50%-74% | 4108 (4.9) | 1868 (4.6) |
| 75%-89% | 4042 (4.8) | 1935 (4.7) |
| ≥90% | 63 528 (75.7) | 31 806 (77.6) |
| Missing antiretroviral therapy data | 8315 (9.9) | 3739 (9.1) |
| Died, No. (%) | 14 345 (17.1) | 4062 (9.9) |
| Any incident cancerj, No. (%) | 5628 (6.7) | 2172 (5.3) |
| AIDS-defining cancers, No. (%) | ||
| Non-Hodgkin lymphoma | 497 | 251 |
| Kaposi sarcoma | 501 | 253 |
| Cervix | 47 | 41 |
| HIV-associated cancers, No. | ||
| Lung | 755 | 186 |
| Anus | 439 | 209 |
| Liver | 347 | 74 |
| Hodgkin lymphoma | 176 | 84 |
| Head and neck | 134 | 60 |
| Other, No. | ||
| Prostate | 817 | 163 |
| Colorectum | 221 | 69 |
| Breast | 79 | 61 |
| Other | 1615 | 721 |
Other race and ethnicity included Asian, Indigenous, Multiracial, Other, and unknown race. IQR = interquartile range.
Ever documentation of alcohol abuse or at-risk alcohol use. Total of 2165 (3%) missing in primary analysis, 80 (0%) missing in sensitivity analysis.
Ever documentation of tobacco usage. Total of 43 253 (52%) missing in primary analysis, 5660 (14%) missing in sensitivity analysis.
CD4 cell count at baseline (closest values within 6 months before or after). Total of 13 170 (16%) missing in primary analysis, 1197 (3%) missing in sensitivity analysis.
CD8 and CD4/CD8 ratio at baseline (closest values within 6 months before or after). Total of 15 915 (19%) missing in primary analysis, 2198 (5%) missing in sensitivity analysis.
HIV RNA at baseline (closest values with 6 months before or 30 days after). Total of 12 772 (15%) missing in primary analysis, 1334 (3%) missing in sensitivity analysis.
Body mass index at baseline (closest values within 6 months before or after). Total of 21 989 (26%) missing in primary analysis, 3994 (10%) missing in sensitivity analysis.
Geographic regions refers to location recorded closest to date of cohort entry. Other locations include Armed Forces Africa, Europe, Middle East, and Pacific and Guam.
Study exit includes earliest occurrence of first cancer, death, or administrative censoring. ART data missing for 8315 (9.9%) in primary analysis and 3739 (9%) in sensitivity analysis.
Any incident cancer excludes nonmelanoma skin cancers.
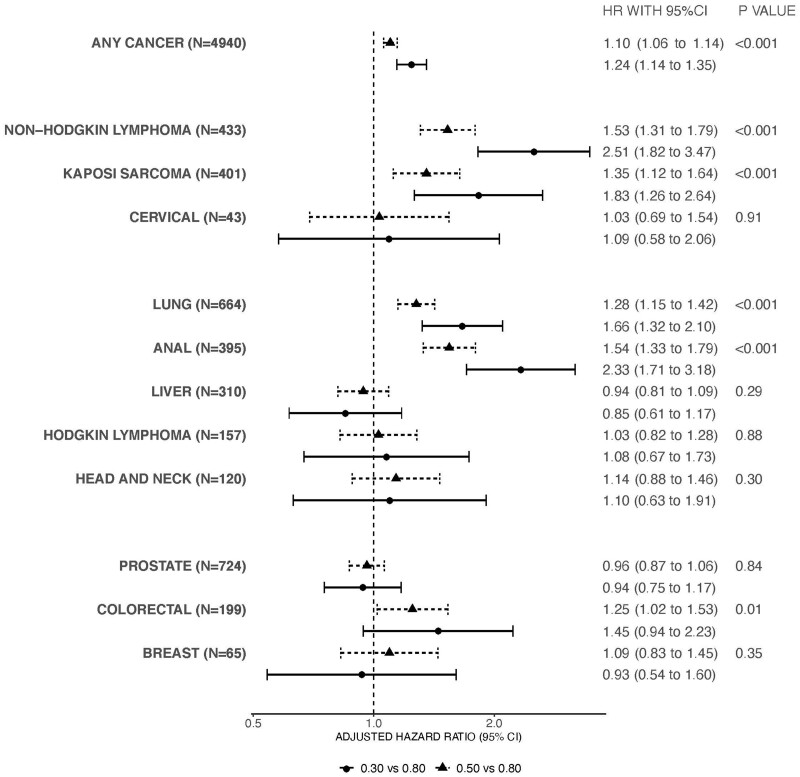
Figure 1 shows the adjusted hazard ratios and 95% confidence intervals (CIs) comparing the 0.3 and 0.5 with the 0.8 CD4/CD8 ratio values of our primary analysis. There was a 24% increased risk of any incident cancer for individuals with a 6-month lagged CD4/CD8 ratio of 0.3 compared with 0.8, independent of CD4 cell count, HIV RNA value, and other covariates (adjusted hazard ratio [aHR] = 1.24, 95% CI = 1.14 to 1.35). We observed the same pattern of increasing cancer risk with decreasing CD4/CD8 ratio for NHL, Kaposi sarcoma, lung cancer, anal cancer, and colorectal cancer. The 6-month-lagged CD4/CD8 ratio was not associated with risk of cervical cancer, Hodgkin lymphoma, head and neck cancer, prostate cancer, breast cancer, or liver cancer. We similarly observed an inverse association of CD4/CD8 ratio and risk of any cancer for all race and ethnicity groups, though the magnitude of the CD4/CD8 ratio effect on outcome varied by race (interaction term overall P value <.001) (Supplementary Figure 3, available online).
Figure 1.
Adjusted hazard ratios (HR) and 95% confidence interval (CI) for cancer risk comparing the 6-month lagged, time-varying CD4/CD8 ratio values. Models for any cancer, lung cancer, non-Hodgkin lymphoma, Hodgkin lymphoma, Kaposi sarcoma cancer, anal cancer, head and neck cancer, colorectal cancer included the covariates of age, sex, race and ethnicity, year of cohort entry, any history of chronic hepatitis C virus infection, and time-varying and time-updated CD4/CD8 ratio, CD4 cell count, HIV RNA, and history of AIDS-defining illness. The multivariable model for liver cancer included the covariates of age, sex, race, year of cohort entry, any history of chronic hepatitis C virus infection, history of chronic hepatitis B virus infection, history of heavy alcohol use, history of injection drug use, and time-varying and time-updated CD4/CD8 ratio, CD4 cell count, HIV RNA, history of AIDS-defining illness, and history of cirrhosis. The multivariable model for prostate cancer included only males and covariates age, race, year of cohort entry, any history of chronic hepatitis C virus infection, and time-varying and time-updated CD4/CD8 ratio, CD4 cell count, HIV RNA, and history of AIDS-defining illness. Models for breast cancer and cervical cancer included only females and included age, race (White vs non-White), and time-varying and lagged CD4/CD8 ratio, CD4 cell count, HIV RNA, and history of AIDS-defining illness. The error bars represent the 95% confidence intervals. Wald statistic was used to calculate P values (2-sided). Circles represent the adjusted hazard ratio comparing a CD4/CD8 value of 0.30 vs a CD4/CD8 value of 0.80. Triangles represent the adjusted hazard ratio comparing a CD4/CD8 value of 0.50 vs a CD4/CD8 value of 0.80.
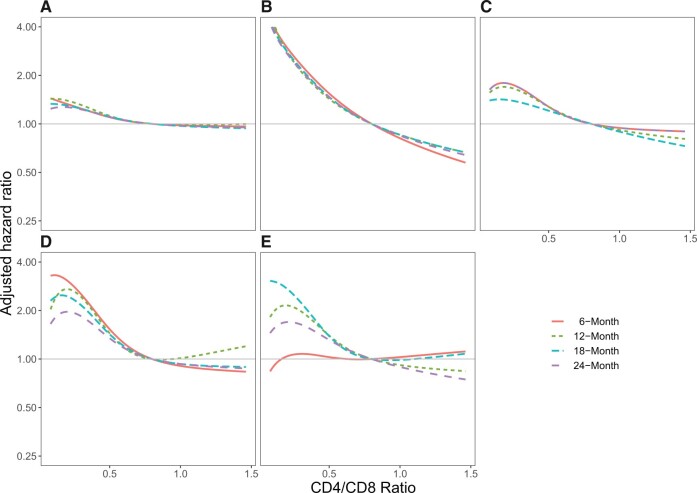
Supplementary Table 1 (available online) shows results examining the 6-month, 12-month, 18-month, and 24-month lagged CD4/CD8 ratio values. Adjusted hazard ratios for cancers common among PWH along a continuum of CD4/CD8 values compared with 0.8 and using different lags are shown in Figure 2. Overall, we observed differing patterns of timing of low CD4/CD8 ratio and cancer risk across cancer types. Low CD4/CD8 ratio values from 6 to 24 months prior were all associated with increased risk of any cancer (P < .001 for all time points). Similarly, a low CD4/CD8 ratio was strongly associated with increased risk of anal and lung cancers across all lagged time points (P < .001 for all time points for both cancers) (Figure 2). In contrast, although statistically significant at all lagged time points, a low CD4/CD8 ratio was associated with highest risk of NHL in the 6 months prior compared with 24 months (Figure 2D, P < .01 for all lagged time points). In contrast, a low CD4/CD8 ratio 6 months prior was not associated with risk of Hodgkin lymphoma (P = .88) but was associated with increased risk 12 and 18 months prior (P = .05 and .02, respectively) (Figure 2). Additional figures for specific cancer types and lagged CD4/CD8 ratio values are shown in Supplementary Figure 4 (available online). Results of models restricted to include only person-time after ART initiation were very similar to results including all person-time (Supplementary Table 2, available online). Results of 6-month lagged models that included absolute CD8 cell count rather than CD4/CD8 ratio were consistent with our primary analyses: high CD8 and low CD4 were generally associated with increased hazards of cancers (Supplementary Figure 5, available online).
Figure 2.
Adjusted hazard ratio for cancer risk and CD4/CD8 ratios lagged 6, 12, 18, and 24 months. Adjusted hazard ratio for CD4/CD8 ratio values lagged 6 months (solid orange lines), 12 months (dashed green lines), 18 months (dashed teal lines), and 24 months (dashed purple lines) for the following cancers (number of events for each model): A) any cancer (6 months n = 4940; 12 months n = 4597; 18 months n = 4308; 24 months n = 4043); B) anal cancer (6 months n= 395; 12 months n = 375; 18 months n = 361; 24 months n = 340); C) lung cancer (6 months n = 664; 12 months n = 645; 18 months n = 645; 24 months n = 564); D) non-Hodgkin lymphoma (6 months n = 433; 12 months n = 382; 18 months n = 333; 24 months n = 310); E) Hodgkin lymphoma (6 months n = 157; 12 months n = 145; 18 months n = 143; 24 months n = 124). Models included the covariates of age, year of cohort entry, sex, race and ethnicity, any history of chronic hepatitis C virus infection, and time-varying and lagged CD4/CD8 ratio, CD4 cell count, HIV RNA, and history of AIDS-defining illness.
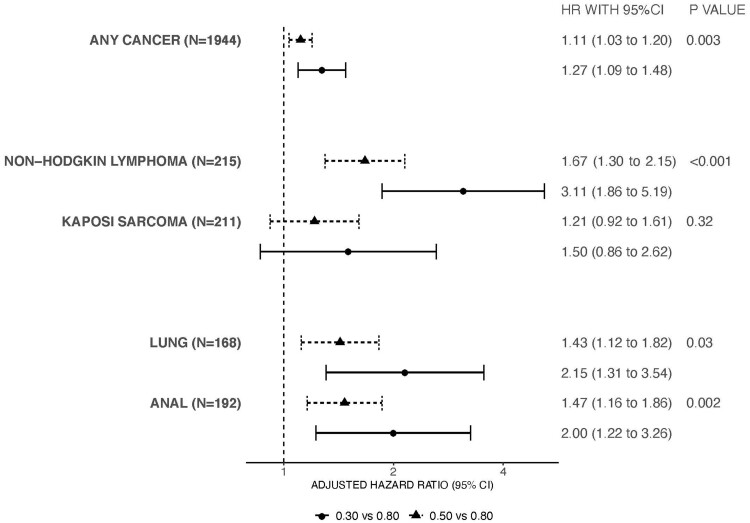
The multivariable analyses including the subset of patients with available smoking, alcohol, HIV acquisition risk factor, and body mass index data that assessed 6-month lagged CD4/CD8 ratio and cancer risk are shown in Figure 3. Given the smaller number of cancers included in the sensitivity analyses, only any cancer, NHL, Kaposi sarcoma, lung cancer, and anal cancer were assessed in multivariable models to avoid overfitting. The inverse association between low CD4/CD8 ratio and increased cancer risk persisted for any cancer, NHL, lung cancer, and anal cancer but was attenuated and no longer statistically significant for Kaposi sarcoma.
Figure 3.
Sensitivity analyses including additional behavioral and body mass index covariates for the adjusted hazard ratios and 95% confidence interval (CI) for cancer outcomes comparing the 6-month lagged, time-varying CD4/CD8 ratio values. Only models for any cancer, lung cancer, non-Hodgkin lymphoma, Kaposi sarcoma cancer, and anal cancer performed due to number of events. All models included the covariates of age, sex, race, year of cohort entry, any history of chronic hepatitis C virus infection, history of smoking, history of heavy alcohol use, HIV acquisition risk factor, and time-varying and time-updated CD4/CD8 ratio, CD4 cell count, HIV RNA, history of AIDS-defining illness, and body mass index. The error bars represent the 95% confidence intervals. Wald statistic was used to calculate P values (2-sided). Circles represent the adjusted hazard ratio comparing a CD4/CD8 value of 0.30 vs a CD4/CD8 value of 0.80. Triangles represent the adjusted hazard ratio comparing a CD4/CD8 value of 0.50 vs a CD4/CD8 value of 0.80.
Discussion
In this large study of PWH in care and with high uptake of ART between 1998 and 2016, we found that a low, time-lagged CD4/CD8 ratio was associated with increased risk of incident cancer diagnoses after accounting for CD4 cell count, HIV viral load, HIV acquisition risk, smoking, and other factors. The inverse association between CD4/CD8 ratio and cancer risk was observed for several specific cancers, including 3 of the most common cancers among PWH in the United States: NHL, lung cancer, and anal cancer. The association between low CD4/CD8 ratio and risk of cancer was not uniform, nor was the association exclusive for cancers associated with oncogenic viruses. Given the robust association up to 24 months before cancer diagnosis, the CD4/CD8 ratio may indicate underlying immune dysfunction affecting carcinogenesis and inform cancer screening and prevention interventions among PWH.
A low CD4/CD8 ratio predicted risk of any cancer, independent of age, sex, CD4 cell count, HIV viral load, chronic HCV infection, and smoking history measures. Among PWH, a low CD4/CD8 ratio is associated with older age, low CD4 nadir, male sex, HIV acquisition risk factors (particularly for men who whave sex with men), chronic viral coinfection (including HCV and cytomegalovirus), and large HIV reservoir (27,32-35). Despite immune reconstitution from effective ART, PWH with a low CD4/CD8 ratio have increased measures of adaptive immunosenescence, including skewed T-lymphocyte populations from naïve to terminally differentiated phenotypes, higher expression of cellular markers of CD8 T-cell activation and senescence, and a higher kynurenine to tryptophan ratio (18,20). A low CD4/CD8 ratio has also been associated with increased measures of monocyte activation and systemic inflammatory measures, including interleukin 6 (IL-6), C-reactive protein (CRP), and soluble CD14 (18,19). Adaptive immune senescence, T-cell activation, and inflammation have been identified as drivers of the excess risk of noncommunicable diseases observed in PWH, including cardiovascular disease and cancers (36-39). In PWH, a low CD4/CD8 ratio has been associated with increased risk of mortality and noncommunicable diseases (22,23,40). A low CD4/CD8 ratio has been associated with risk of non-ADCs in smaller studies of PWH (24,41). To our knowledge, this is the largest study to demonstrate an independent association between a low CD4/CD8 ratio and cancer risk in PWH, which we found was consistent up to 2 years preceding the cancer diagnosis.
NHL, lung cancer, and anal cancer, 3 of the leading causes of cancer-related morbidity and mortality in PWH in the United States, were strongly and consistently associated with a low CD4/CD8 ratio (42). Both lung cancer and anal cancer have higher incidence and are diagnosed at younger ages in PWH compared with the general population, suggesting accelerated aging biology, such as immunosenescence, may contribute to cancer risk in PWH (6,8,43,44). The large Veterans Aging Cohort Study found an independent association between a low, cumulatively averaged CD4/CD8 ratio and lung cancer risk in US veterans with HIV (25). Our results are consistent with their findings, supporting the hypothesis that immune dysfunction may contribute to cancer risk in PWH.
This is the largest study to show that a low peripheral CD4/CD8 ratio predicts anal cancer risk, independent of HIV acquisition risk factor, smoking, or immunodeficiency. A single-center study of PWH observed that a low CD4/CD8 ratio nadir and CD4/CD8 proximal to anal cancer screening were associated with high-grade anal dysplasia and cancer; however, the analyses did not adjust for concurrent CD4 cell count (45). The ratio of CD4/CD8 tumor-infiltrating and stromal lymphocytes in tissue biopsies of HPV-associated precancers of the cervix, anus, and head and neck have been associated with disease progression or regression; however, the circulating CD4/CD8 ratio has not been evaluated in anal precancers (46-49). ART has not been shown to be associated with the regression or clearance of anal lesions, suggesting immune restoration does not entirely eliminate the increased risk (50,51). That we did not find an association between CD4/CD8 ratio and cervical cancer may be due to the small number of cancer cases in this cohort. Although we did not find a statistically significant association between CD4/CD8 ratio and head and neck cancer, we could not distinguish cases that were associated with HPV. Further research is needed to assess CD4/CD8 ratio and HPV-associated and HPV-negative head and neck cancers.
It is worth noting that because our analyses adjusted for CD4 count, the observed inverse association between the CD4/CD8 ratio and risk of cancer reflects an association between higher CD8 cell count and higher risks of cancer. Our sensitivity analyses that replaced the CD4/CD8 ratio with absolute CD8 cell count demonstrated this relationship. We chose to present results using the CD4/CD8 ratio rather than CD8 cell count for consistency with the literature and the convenience of including CD4 and CD8 cell counts in a single measure; however, CD4 and CD8 could alternatively be considered in tandem as biomarkers for cancer.
There are important limitations of this study. Additional modeling and prospective studies are needed to translate how and when CD4/CD8 ratio can best be used in cancer screening in clinical care for PWH. These studies will need to account for differences in the association of the CD4/CD8 ratio for specific cancers as well as investigate differences by demographic and time-varying clinical (such as time since ART initiation, CD4 cell count nadir, and current CD4 cell count) characteristics among PWH. Our investigation revealed an interaction between race and ethnicity and the association CD4/CD8 ratio and any cancer outcome. Additional investigation into whether and how individual characteristics among PWH (including race, sex, age, and clinical history) affect the association of the CD4/CD8 ratio and specific cancer outcomes are needed. Our study population was disproportionately male, not only resulting in low numbers of cervical cancer and breast cancer but also limiting our ability to examine CD4/CD8 ratio and cancer risk in women with HIV more generally. HIV acquisition risk factor and behavioral data including smoking history were not available from all cohorts, though results from our sensitivity analyses were generally consistent with those from the primary analyses. However, for lung cancer in particular, our results may still be affected by residual confounding from smoking given the limited smoking information in our cohort. The CD4/CD8 ratio is lower in settings of chronic viral infections, and our data on prevalent oncogenic viruses were limited to HCV and hepatitis B virus. The cancer most associated with these viruses, liver cancer, was not statistically significantly associated with the CD4/CD8 ratio in multivariable models. Seroprevalence of cytomegalovirus, human herpes virus 8 (Kaposi sarcoma), Epstein-Barr virus (Hodgkin lymphoma and NHL), or infection with high-risk HPV (cervical, anal, and head and neck cancers) is not universally collected in clinical care of PWH. We used lagged CD4/CD8 ratio values to reduce the risk that cancer itself is causing a low CD4/CD8 ratio; however, it is possible that 6 months may not be sufficient to eliminate this possibility. Reassuringly, results examining 12-month and up to 24-month lagged values were consistent with the 6-month lagged results. Our data may also be subject to misclassification bias as a result of carrying forward CD4/CD8 data and periods of missing CD4/CD8 data.
In the largest study of PWH in the United States and Canada, we found that a lagged, low CD4/CD8 ratio was associated with increased risk of certain incident cancers. A low CD4/CD8 ratio was associated with increased risk of NHL, lung cancer, and anal cancer. The consistency of association observed up to 24 months before cancer diagnosis suggests the CD4/CD8 ratio may be a useful biomarker for screening for lung and anal cancer in PWH where the average age at diagnosis is younger than in the general population. Further investigation into the roles of immunosenescence, immune activation, and inflammation and cancer risk observed in PWH is needed.
Funding
This work was supported by National Institutes of Health grants P30CA068485-23S3, K23AI120875, K07CA225404, U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01DA011602, R01DA012568, R01 AG053100, R24AI067039, R34DA045592, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA03629, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24AA020794, U54GM133807, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR002378, Z01CP010214 and Z01CP010176; contracts CDC-200–2006-18797 and CDC-200–2015-63931 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; the Grady Health System; grants CBR-86906, CBR-94036, HCP-97105 and TGF-96118 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care, and the Government of Alberta, Canada. Additional support was provided by the National Institute Of Allergy And Infectious Diseases (NIAID), National Cancer Institute (NCI), National Heart, Lung, and Blood Institute (NHLBI), Eunice Kennedy Shriver National Institute Of Child Health and Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute for Mental Health (NIMH) and National Institute on Drug Abuse (NIDA), National Institute On Aging (NIA), National Institute Of Dental and Craniofacial Research (NIDCR), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Nursing Research (NINR), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). These data were collected by cancer registries participating in the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC).
Notes
Role of the funders: The funding sources of the study had no role in study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit manuscript for publication.
Disclosures: MJS has received a grant to his institution from Gilead Sciences for HIV research not directly related to this manuscript. VCM received research grants from CDC, Gilead Sciences, NIH, VA, and ViiV, received honoraria from Eli Lilly and Company, served as an advisory board member for Eli Lilly and Company and Novartis and participated as a study section chair for the NIH. JG is an ad Hoc member of Canadian national HIV advisory Boards to Merck, Gilead and ViiV. All other co-authors have no potential conflicts of interest.
Author contributions: Data curation and Resources by RDM, KNA, TRS, MMK, MJS, KS, MAH, MJG, AMM, DW, CJA, RJB, CSR, SN, RMN, WCM, JET, SBC, and JS. Conceptualization and Methodology by JLC, SLS, BES, CAJ, and TRS with significant contributions from KS, MJG, MMK, MJS, CJA, RJB, MAH, AMM, SBC, CSR, SN, RMN, WCM, JS, KNA, JS, TRS, and RDM. Investigation by JLC, SLS, AB, CAJ, and BES and Formal Analysis by AB. Visualization by JLC, SLS, and AB. Writing—Original Draft by JLC, SLS, AB, and BES. Writing—Review and Editing by JLC, AB, CAJ, BES, KS, JG, MMK, MJS, AMM, SBC, DW, CJA, VCM, RJB, MAH, CSR, SN, RMN, CM, JET, JS, KNA, RDM, TRS, and SLS. Funding Acquisition by JLC and SLS. Supervision by JLC, SLS, BES, TRS, KNA, and RDM.
Prior presentations: Abstract 71. 27th Conference on Retroviruses and Opportunistic Infections, Boston, March 2020.
Disclaimers: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability
NA-ACCORD welcomes interested investigators to collaborate with us for use of our data. Please visit https://naaccord.org/ for additional information.
Supplementary Material
Contributor Information
Jessica L Castilho, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Aihua Bian, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, TN, USA.
Cathy A Jenkins, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, TN, USA.
Bryan E Shepherd, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, TN, USA.
Keith Sigel, Division of Infectious Diseases, Department of Medicine, Mount Sinai School of Medicine, New York, NY, USA.
M John Gill, Department of Medicine, University of Calgary, Calgary, AB, Canada.
Mari M Kitahata, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, WA, USA.
Michael J Silverberg, Division of Research, Kaiser Permanente Northern California, Oakland, CA, USA.
Angel M Mayor, Retrovirus Research Center, Internal Medicine Department, Universidad Central del Caribe School of Medicine, Bayamón, PR, USA.
Sally B Coburn, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Dorothy Wiley, School of Nursing, University of California Los Angeles, Los Angeles, CA, USA.
Chad J Achenbach, Division of Infectious Diseases, Department of Medicine, Northwestern Feinberg School of Medicine, Chicago, IL, USA.
Vincent C Marconi, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine and Rollins School of Public Health, Atlanta, GA, USA.
Ronald J Bosch, Department of Biostatistics, T.H. Chan Harvard School of Public Health, Boston, MA, USA.
Michael A Horberg, Kaiser Permanente Mid-Atlantic Medical Group and Research Institute, Washington, DC, USA.
Charles S Rabkin, Division of Cancer Epidemiology and Genetics, Infections and Immunoepidemiology Branch, National Cancer Institute, Rockville, MD, USA.
Sonia Napravnik, Division of Infectious Diseases, Department of Medicine, University of North Carolina School of Medicine, Chapel Hill, NC, USA.
Richard M Novak, Division of Infectious Diseases, Department of Medicine, University of Illinois Chicago School of Medicine, Chicago, IL, USA.
W Christopher Mathews, Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, CA, USA.
Jennifer E Thorne, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Ophthalmology, Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Jing Sun, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Keri N Althoff, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Richard D Moore, Division of Infectious Diseases, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Timothy R Sterling, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Staci L Sudenga, Division of Epidemiology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of the International Epidemiology Databases to Evaluate AIDS (IeDEA):
Jessica L Castilho, Aihua Bian, Cathy A Jenkins, Bryan E Shepherd, Keith Sigel, M John Gill, Mari M Kitahata, Michael J Silverberg, Angel M Mayor, Sally B Coburn, Dorothy Wiley, Chad J Achenbach, Vincent C Marconi, Ronald J Bosch, Michael A Horberg, Charles S Rabkin, Sonia Napravnik, Richard M Novak, W Christopher Mathews, Jennifer E Thorne, Jing Sun, Keri N Althoff, Richard D Moore, Timothy R Sterling, and Staci L Sudenga
References
- 1. Samji H, Cescon A, Hogg RS, et al. ; for the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187–194. [DOI] [PubMed] [Google Scholar]
- 3. Hessol NA, Seaberg EC, Preston-Martin S, et al. Cancer risk among participants in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2004;36(4):978–985. [DOI] [PubMed] [Google Scholar]
- 4. Silverberg MJ, Lau B, Achenbach CJ, et al. ; North American AIDS Cohort Collaboration on Research and Design of the International Epidemiologic Databases to Evaluate AIDS. Cumulative incidence of cancer among persons with HIV in North America: a cohort study. Ann Intern Med. 2015;163(7):507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Althoff KN, Chandran A, Zhang J, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Life-expectancy disparities among adults with HIV in the United States and Canada: the impact of a reduction in drug- and alcohol-related deaths using the lives saved simulation model. Am J Epidemiol. 2019;188(12):2097–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hernandez-Ramirez RU, Shiels MS, Dubrow R, et al. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. 2017;4(11):e495–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel P, Hanson DL, Sullivan PS, et al. ; Adult and Adolescent Spectrum of Disease Project and HIV Outpatient Study Investigators. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008;148(10):728–736. [DOI] [PubMed] [Google Scholar]
- 8. Robbins HA, Pfeiffer RM, Shiels MS, et al. Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst. 2015;107(4):dju503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Engels EA, Yanik EL, Wheeler W, et al. ; for the North American AIDS Cohort Collaboration on Research and Design of the International Epidemiologic Databases to Evaluate AIDS. Cancer-attributable mortality among people with treated human immunodeficiency virus infection in North America. Clin Infect Dis. 2017;65(4):636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coghill AE, Pfeiffer RM, Shiels MS, et al. Excess mortality among HIV-infected individuals with cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(7):1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park LS, Hernandez-Ramirez RU, Silverberg MJ, et al. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS. 2016;30(2):273–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162(5):335–344. [DOI] [PubMed] [Google Scholar]
- 14. Altekruse SF, Shiels MS, Modur SP, et al. Cancer burden attributable to cigarette smoking among HIV-infected people in North America. AIDS. 2018;32(4):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Martel C, Shiels MS, Franceschi S, et al. Cancers attributable to infections among adults with HIV in the United States. AIDS. 2015;29(16):2173–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fulop T, Larbi A, Kotb R, et al. Immunology of aging and cancer development. Interdiscip Top Gerontol. 2013;38:38–48. [DOI] [PubMed] [Google Scholar]
- 17. Bottazzi B, Riboli E, Mantovani A. Aging, inflammation and cancer. Semin Immunol. 2018;40:74–82. [DOI] [PubMed] [Google Scholar]
- 18. Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10(5):e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sainz T, Serrano-Villar S, Diaz L, et al. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS. 2013;27(9):1513–1516. [DOI] [PubMed] [Google Scholar]
- 20. Serrano-Villar S, Gutierrez C, Vallejo A, et al. The CD4/CD8 ratio in HIV-infected subjects is independently associated with T-cell activation despite long-term viral suppression. J Infect. 2013;66(1):57–66. [DOI] [PubMed] [Google Scholar]
- 21. Serrano-Villar S, Perez-Elias MJ, Dronda F, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One. 2014;9(1):e85798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castilho JL, Shepherd BE, Koethe J, et al. CD4+/CD8+ ratio, age, and risk of serious noncommunicable diseases in HIV-infected adults on antiretroviral therapy. AIDS. 2016;30(6):899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mussini C, Lorenzini P, Cozzi-Lepri A, et al. ; Icona Foundation Study Group. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV. 2015;2(3):e98–e106. [DOI] [PubMed] [Google Scholar]
- 24. Hema MN, Ferry T, Dupon M, et al. ; ANRS CO 8 (APROCO/COPILOTE) study group. Low CD4/CD8 ratio is associated with non AIDS-defining cancers in patients on antiretroviral therapy: ANRS CO8 (Aproco/Copilote) prospective cohort study. PLoS One. 2016;11(8):e0161594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sigel K, Wisnivesky J, Crothers K, et al. Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. Lancet HIV. 2017;4(2):e67–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Triplette M, Attia EF, Akgun KM, et al. A low peripheral blood CD4/CD8 ratio is associated with pulmonary emphysema in HIV. PLoS One. 2017;12(1):e0170857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caby F, Guihot A, Lambert-Niclot S, et al. Determinants of a low CD4/CD8 ratio in HIV-1-infected individuals despite long-term viral suppression. Clin Infect Dis. 2016;62(10):1297–1303. [DOI] [PubMed] [Google Scholar]
- 28. Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol. 2007;36(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haller B, Schmidt G, Ulm K. Applying competing risks regression models: an overview. Lifetime Data Anal. 2013;19(1):33–58. [DOI] [PubMed] [Google Scholar]
- 30. Shepherd BE, Rebeiro PF, Caribbean C, et al. ; Caribbean, Central and South America Network for HIV Epidemiology. Brief report: assessing and interpreting the association between continuous covariates and outcomes in observational studies of HIV using splines. J Acquir Immune Defic Syndr. 2017;74(3):e60–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons; 2004. [Google Scholar]
- 32. Kuniholm MH, O'brien TR, Prokunina-Olsson L, et al. Association of hepatitis C virus infection with CD4/CD8 ratio in HIV-positive women. J Acquir Immune Defic Syndr. 2016;72(2):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Combes JD, Clifford GM, Egger M, et al. Human papillomavirus antibody response following HAART initiation among MSM. AIDS. 2017;31(4):561–569. [DOI] [PubMed] [Google Scholar]
- 34. Chun TW, Justement JS, Pandya P, et al. Relationship between the size of the human immunodeficiency virus type 1 (HIV-1) reservoir in peripheral blood CD4+ T cells and CD4+:CD8+ T cell ratios in aviremic HIV-1-infected individuals receiving long-term highly active antiretroviral therapy. J Infect Dis. 2002;185(11):1672–1676. [DOI] [PubMed] [Google Scholar]
- 35. Verboeket SO, Wit FW, Verheij E, et al. HIV-negative men who have sex with men have higher CD8+ T-cell counts and lower CD4+/CD8+ T-cell ratios compared to HIV-negative heterosexual men. J Infect Dis. 2021;224(7):1187–1197. doi: 10.1093/infdis/jiaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hearps AC, Martin GE, Rajasuriar R, et al. Inflammatory co-morbidities in HIV+ individuals: learning lessons from healthy ageing. Curr HIV/AIDS Rep. 2014;11(1):20–34. [DOI] [PubMed] [Google Scholar]
- 37. Kaplan RC, Sinclair E, Landay AL, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217(1):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–1259. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol. 2012;24(4):501–506. [DOI] [PubMed] [Google Scholar]
- 40. Serrano-Villar S, Moreno S, Fuentes-Ferrer M, et al. The CD4:CD8 ratio is associated with markers of age-associated disease in virally suppressed HIV-infected patients with immunological recovery. HIV Med. 2014;15(1):40–49. [DOI] [PubMed] [Google Scholar]
- 41. Lasry A, Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 2015;36(4):217–228. [DOI] [PubMed] [Google Scholar]
- 42. Engels EA, Yanik EL, Wheeler W, et al. ; for the North American AIDS Cohort Collaboration on Research and Design of the International Epidemiologic Databases to Evaluate AIDS. Cancer-attributable mortality among people with treated human immunodeficiency virus infection in North America. Clin Infect Dis. 2017;65(4):636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shiels MS, Althoff KN, Pfeiffer RM, et al. HIV infection, immunosuppression, and age at diagnosis of non-AIDS-defining cancers. Clin Infect Dis. 2017;64(4):468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shiels MS, Cole SR, Mehta SH, et al. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J Acquir Immune Defic Syndr. 2010;55(4):510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Geltzeiler CB, Xu Y, Carchman E, et al. CD4/CD8 ratio as a novel marker for increased risk of high-grade anal dysplasia and anal cancer in HIV+ patients: a retrospective cohort study. Dis Colon Rectum. 2020;63(12):1585–1592. [DOI] [PubMed] [Google Scholar]
- 46. Monnier-Benoit S, Mauny F, Riethmuller D, et al. Immunohistochemical analysis of CD4+ and CD8+ T-cell subsets in high risk human papillomavirus-associated pre-malignant and malignant lesions of the uterine cervix. Gynecol Oncol. 2006;102(1):22–31. [DOI] [PubMed] [Google Scholar]
- 47. Wolf GT, Chepeha DB, Bellile E, et al. ; University of Michigan Head and Neck SPORE Program. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: a preliminary study. Oral Oncol. 2015;51(1):90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saber CN, Gronhoj Larsen C, Dalianis T, et al. Immune cells and prognosis in HPV-associated oropharyngeal squamous cell carcinomas: review of the literature. Oral Oncol. 2016;58:8–13. [DOI] [PubMed] [Google Scholar]
- 49. Liu Y, Gaisa MM, Wang X, et al. Differences in the immune microenvironment of anal cancer precursors by HIV status and association with ablation outcomes. J Infect Dis. 2018;217(5):703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seaberg EC, Wiley D, Martinez-Maza O, et al. ; for the Multicenter AIDS Cohort Study (MACS). Cancer incidence in the multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer. 2010;116(23):5507–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palefsky JM, Holly EA, Efirdc JT, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19(13):1407–1414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
NA-ACCORD welcomes interested investigators to collaborate with us for use of our data. Please visit https://naaccord.org/ for additional information.