Abstract
Alzheimer’s disease (AD) progressively inflicts impairment of synaptic functions with notable deposition of amyloid-β (Aβ) as senile plaques within the extracellular space of the brain. Accordingly, therapeutic directions for AD have focused on clearing Aβ plaques or preventing amyloidogenesis based on the amyloid cascade hypothesis. However, the emerging evidence suggests that Aβ serves biological roles, which include suppressing microbial infections, regulating synaptic plasticity, promoting recovery after brain injury, sealing leaks in the blood-brain barrier, and possibly inhibiting the proliferation of cancer cells. More importantly, these functions were found in in vitro and in vivo investigations in a hormetic manner, that is to be neuroprotective at low concentrations and pathological at high concentrations. We herein summarize the physiological roles of monomeric Aβ and current Aβ-directed therapies in clinical trials. Based on the evidence, we propose that novel therapeutics targeting Aβ should selectively target Aβ in neurotoxic forms such as oligomers while retaining monomeric Aβ in order to preserve the physiological functions of Aβ monomers.
Keywords: Alzheimer’s disease, Amyloid beta-peptides, Amyloidosis, Neurodegenerative diseases, Therapeutics
INTRODUCTION
Marked by progressive memory loss and cognitive decline, Alzheimer's disease (AD) is the most common neurodegenerative disease that continues to afflict patients and their families without a concrete understanding of its pathogenesis nor effective therapeutics to modify the disease [1-3]. So far, mounting evidence points to the dysregulated accumulation of amyloid-β (Aβ) and tau in the brain as both the cause and biomarker of developing AD. Aβ peptide is known to be derived from amyloid-β precursor protein (AβPP), which can undergo an amyloidogenic or a non-amyloidogenic pathway. When APP is normally cleaved by α-secretase, it goes through the non-amyloidogenic pathway and produces sAPPα and C83 fragment, which are harmless. On the other hand, the dysfunctional amyloidogenic pathway begins by the cleavage of APP by β-secretase and γ-secretase. Here, the β-secretase cleavage yields C99 fragments, and these fragments are further processed into Aβ peptide, which consists of 37 to 49 amino acid residues, depending on the site of γ-secretase cleavage. Aβ peptide mainly exists with 40 amino acid residues, Aβ40, or 42 amino acid residues, Aβ42 [4], which is less abundant, but more pathologically detrimental [5]. In turn, the amyloid cascade follows as Aβ peptides are released into the extracellular space and aggregated into soluble oligomers, fibrils, and plaques, known to damage synapses and neurons. Accordingly, the ‘amyloid cascade hypothesis’ has been postulated arguing that this abnormal deposition of Aβ oligomers and plaques alters the homeostasis of ions and causes synaptic dysfunction, marking Aβ as the primary cause of AD [6-10].
Based on this hypothesis, numerous attempts have been made to alter the course of AD pathogenesis with various means of therapeutics targeting Aβ. However, constant failures in the drug candidates aiming a reduction or complete elimination of Aβ plaques have been reported [11, 12]. For example, reduced levels of Aβ in the brain were observed in phase II trials of verubecestat, a β-secretase inhibitor drug, and solanezumab, a monoclonal antibody drug of Aβ peptide [12, 13], but they showed no efficacy in phase III trials with worsened cognition in some cases [14, 15]. Accordingly, there is an emerging suspicion that questions the conventional approaches of targeting Aβ, or even challenging the amyloid cascade hypothesis to some extent.
Thus, we would like to turn the attention to monomeric form of Aβ. In the past, the monomeric form of Aβ peptide had been widely recognized as a functionless protein generated by APP catabolism, although an evolutionary evidence suggests otherwise: the sequence of Aβ peptide in human has been conserved for at least 400 million years, pointing to the possibility that Aβ perhaps has unidentified, significant roles that confer an evolutionary advantage [16]. Accordingly, we have gathered a significant number of evidence suggesting non-pathological aspects of Aβ monomers and their potential physiological functions. A thorough review of manuscripts has revealed that Aβ may possess antimicrobial properties and play a role in regulating synaptic functions and memory. Also, Aβ may aid the recovery from brain injury with its interference with angiogenesis and even may exert an anticancer effect. Hence, the appreciation of the pathogenesis of AD can be further enhanced by identifying the physiological roles of Aβ monomers, and whether or not to keep Aβ monomers in brains can be determined accordingly to adjust the target of current AD therapeutics.
PROTECTION AGAINST MICROBIAL INFECTIONS
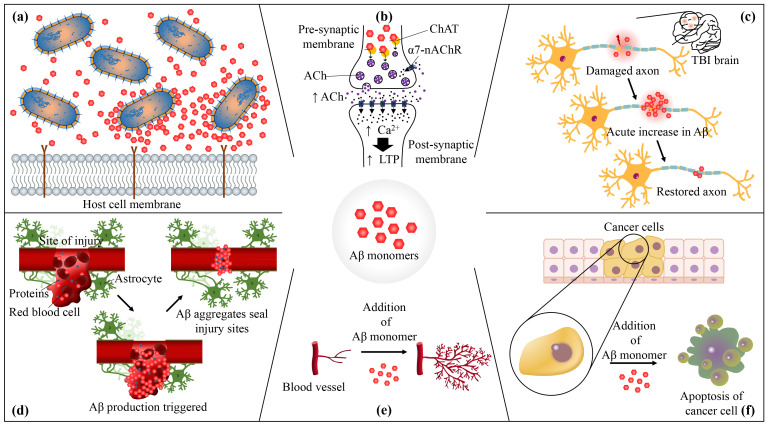
Aβ and microbes are seemingly unrelated, as the former causes neurodegeneration while infections by the latter result in headache, nausea, and so on; however, antimicrobial activities of Aβ have been postulated by Robinson and Bishop [17] with the ‘Bioflocculant Hypothesis’. The authors have proposed that Aβ binds to neurotoxins and pathogens, and it aggregates into plaques in order to lead the trapped microbes to phagocytes. Later, Moir et al. [18] extended the previous hypothesis by introducing the ‘Antimicrobial Protection Hypothesis’, which notes that Aβ functions as a part of innate immune system, but a certain dysregulation and dysfunction of this pathway lead to an increased Aβ deposition and eventually into a sustained neuroinflammation and neurodegeneration typical of AD patients. This renewed hypothesis not only captures the essence of the antimicrobial function of Aβ, but also provides a plausible explanation for Aβ aggregation.
In supporting these hypotheses, researchers often highlight structural and functional similarities between Aβ and antimicrobial peptides (AMPs), which target specific bacteria, viruses, fungi, and so on to modulate the innate immune system [19]. For instance, Pastore et al. [20] reviewed similarities in sequences and structures between Aβ40 and linear AMPs with highly helical conformations, and Lee et al. [21] summarized their similar propensity to self-assemble into supramolecular structures when faced with bacterial infections. Especially, LL-37, an archetypical human AMP [22], has been frequently compared with Aβ in different studies and review articles to establish the idea that Aβ acts as an AMP [18, 21, 23-27].
In addition to structural comparisons, in vitro interactions between Aβ and microbes have been widely studied using either synthetic or cell-derived Aβ peptides. A couple of studies showed the binding of synthetic Aβ peptides to the bacterial membrane and their antibacterial activity against eight types of bacteria including Gram-negative and Gram-positive bacteria [23, 28]. Similarly, cell-derived Aβ40 and 42 isoforms from cultured cells of human brain neuroglioma exhibited the antimicrobial activity against Candida albicans (C. albicans). Microscopic images and immunochemical analyses from this study revealed that both Aβ40 and Aβ42 showed inhibited adhesion levels to C. albicans and significantly higher agglutinating activities compared to non-transformed monolayers [24]. Here, another connection has been made between Aβ and AMP because a typical AMP activity against pathogens begins with preventing pathogens from attaching to host cells, followed by agglutination of the unattached to accumulate AMPs within the aggregates.
Not only the antibacterial and antifungal activities, but also antiviral activities of Aβ have also been reported in a series of in vitro studies. A direct interaction between Aβ peptides and H3N2 and H1N1 influenza A virus was detected, leading to neutralization and aggregation [25]. Interestingly, this viral aggregation caused by Aβ is absent in the mechanism of LL-37 [29], a point against the argument that Aβ is an AMP similar to LL-37. In other studies, different cell types including MRC-5 cells from human fetal lung fibroblasts, A549 cells from adenocarcinoma epithelial cells in lungs, H4 cells from human neuroglioma, and human neuronal-glial cells were incubated with Aβ peptides, and inhibition of HSV-1 activity was observed. The results illustrated that HSV-1 replication was significantly reduced and up-regulation of miRNA-146a, a typical process during HSV-1 infection, was prevented [27, 30].
However, compared to the results produced by the synthetic Aβ peptides, those of the cell-derived Aβ exhibited a greater level of adhesion inhibition and agglutination [24]. The answer to this difference may lie on the conformation of Aβ; while the Aβ oligomers are typically removed from the synthetic Aβ peptides, leaving the monomeric form only, the oligomeric form is retained in the cell-derived Aβ. Nonetheless, given that different isoforms of Aβ in physiologically normal human brain have been discovered [31], in vitro studies in which synthetic Aβ peptides were pretreated to overwhelmingly express specific isoforms might not be a realistic representation of the heterogeneous composition of Aβ in the brain. In fact, one study has demonstrated the enhanced antiviral ability of the oligomeric form of Aβ against herpes simplex virus-1 (HSV-1) compared to Aβ monomers; whereas low nanomolar concentrations of cell-derived Aβ with oligomers could inhibit HSV-1, micromolar concentrations were required for synthetic Aβ with monomers to take an effect [26]. Although further investigations are needed to explore the effect of different compositions of Aβ on its antimicrobial ability, it is a possibility that oligomers of soluble Aβ possess an enhanced, protective ability against certain pathogens.
Notably, regardless of methods used to prepare Aβ peptides, synthetic or cell-derived, Aβ42 isoform exhibited a greater antibiotic activity than Aβ40 [23, 24]. Accordingly, specific domains of Aβ responsible for the antimicrobial activities were identified as Iso41 and Ala42, which are C-terminal amino acids of Aβ42. The capability of these domains were evidenced by truncated peptides, such as Aβ22-42 and Aβ35-42, causing neutralization and aggregation of influenza A virus and Escherichia coli (E. coli) and inducing an elevated uptake of the pathogens, whereas fragments lacking the two C-terminal amino acids did not [32]. Additionally, another isoform of Aβ, Aβ25-35, was tested against bacterial lipopolysaccharide by incubating them in neuronal and microglial cells, and it displayed enhanced proliferation of the cells more than Aβ40 or Aβ42 [33]. Surprisingly, Aβ25-35 too exhibited a protective against LPS by preventing DNA damage in the cells, further demonstrating the antimicrobial capacity of different Aβ variants [34].
However, conflicting results were published in regard to the effect of different Aβ isoforms. In contrast to the results provided by Soscia et al. [23] that both Aβ40 and Aβ42 showed an antimicrobial activity against C. albicans, a study by Spitzer et al. [28] showed only Aβ40 as effective against the same species. This apparent discrepancy in the two sets of data can be attributed to different pretreatment methods of Aβ peptides used or different strains of bacteria selected. Regardless of the causes, standardized criteria for determining the inhibitive activity of Aβ against pathogens in vitro are in need for future investigations to reach a consensus on this matter.
In line with the in vitro studies that showed the antimicrobial activity of Aβ peptides against a series of microbial pathogens, its protective property was also observed in in vivo studies. 5XFAD transgenic mice that express human Aβ showed a significantly higher survival rate (p=0.009) than the wild-type mice against Salmonella enterica serotype Typhimurium (S. Typhimurium) injection, and the transgenic mice exhibited lower loads of S. Typhimurium in the cerebral region (p=0.03). A similar protective property of Aβ against S. Typhimurium was observed in Aβ-expressing nematodes as well [24]. Additionally, the antiviral activity of Aβ against HSV-1 was illuminated as the survival rate of 5XFAD mice with human Aβ was significantly higher than that of the wild-type. Here, it is important to note that the 5XFAD mice used in this study were five- to six-week-old that had not developed Aβ plaques, opening up the possibility that unaggregated form of Aβ might have had a protective role against HSV-1 [26]. Interestingly, this AMP-like behavior of Aβ was also observed in one study with human brain. The whole brain homogenates of AD patients demonstrated a higher inhibition rate of growth of C. albicans in temporal lobe region compared to those from non-AD subjects, with a significant correlation between the antimicrobial activity and the concentration of Aβ in the temporal lobe [23]. Taken together, these in vivo studies solidify the notion that Aβ acts as an AMP.
Besides the studies that investigated the direct interaction between pathogens and Aβ, adverse effects reported in clinical trials of β-secretase and γ-secretase inhibitors such as tarenflurbil, semagacestat, and elenbecestat (reviewed by Iqbal et al. [35]) also indirectly signify the potential ability of Aβ against microbial infections. Instead of exhibiting intended clinical benefits such as improved cognitive abilities, the rates of infection elevated in the subjects. Another study demonstrated the regulative effect of different β-secretase and γ-secretase inhibitors, GL-189 and N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine-t-butyl-ester (DAPT), on secretion levels of tumor necrosis factor α, interleukin (IL) 6 and 10, which are involved in immune response by macrophages [36]. Intriguingly, these results are in agreement with another in vivo studies demonstrating that Aβ may act as an innate defense system against pathogens in case of skin damage and thrombosis as the elevated level of Aβ40 peptides around brain and dermal blood vessels in mice were observed [37, 38]. Together, these results may explain the failure of some anti-Aβ drugs as a cure for AD because AD patients with thrombosis, or related conditions, could suffer from unexpected inflammatory effects when a defense mechanism by Aβ is removed.
Using the aforementioned in vitro and in vivo studies, groups of researchers hypothesized possible mechanisms of the antimicrobial activities of Aβ. Kumar et al. [24] proposed a new model based on their findings that Aβ induces agglutination and inhibits adhesion of pathogens to host cells: with its VHHQKL heparin-binding domain, non-dysfunctional oligomeric Aβ binds to the cell wall of microbes and agglutinates them by interfering the adhesion to host cells. This model is consistent with the accumulated evidence that Aβ resembles LL-37 because LL-37 too possesses a heparin-binding motif used to inhibit pathogens from attaching to host cells [39]. Alternatively, some studies suggested that Aβ with β-sheet structure perforates the plasma membrane by forming toxic ion channels, such as K+-permeable tetrameric channels and Ca++-permeable hexametric pores, which eventually lead to destruction of pathogens due to uncontrolled movement of solutes (see review by Kagan et al. [40]). This mechanism is comparable to that of defensins, human β-sheet AMPs that also possess the pore-forming ability for their antimicrobial activities [41]. Accordingly, these new models further imply that the pathogenesis of AD may originate from dysfunctional antimicrobial activities, while physiologically normal Aβ actively defends our body against bacterial and viral infections.
REGULATION OF SYNAPTIC FUNCTIONS
Contrary to the common notion that Aβ peptides, especially the soluble oligomeric forms, are responsible for impairing synaptic functions followed by disrupted long-term potentiation (LTP) and synaptic plasticity typically seen in AD patients [8-10], increasing evidence suggests that Aβ may play an essential role in synaptic plasticity and memory when maintained at a normal level [42-46].
To begin with, a positive correlation between the level of Aβ in interstitial fluid (ISF) and synaptic activity was reported in in vivo studies. When synaptic activity of the hippocampus in 3~5 months Tg2576 mice with mutated human APP was artificially stimulated using electricity, the ISF level of Aβ increased significantly. Conversely, when their neuronal activity was depressed using tetanus toxin, known to inhibit endocytosis of synaptic vesicles, the Aβ levels in ISF decreased accordingly, further highlighting the positive correlation between them [47]. However, these results still need to be verified to ensure that the predisposition to developing Aβ plaques and synaptic dysfunction prevalent in the transgenic mice with familial AD had not taken an effect in these results. Additionally, the potential relation between Aβ and synaptic vesicles was shown with the level of ISF Aβ significantly declining when a dynamin dominant-negative inhibitory peptide was added to inhibit the endocytosis of clathrin-coated vesicles [48]. Given that the elevated synaptic transmission no longer had an effect on increasing the level of ISF when endocytosis was inhibited, the positive correlation observed between Aβ levels in ISF and synaptic activity may be dependent on endocytosis. More detailed explanation on the interactions of Aβ with presynaptic proteins and kinases and thus their involvement in the synaptic vesicle cycle is reviewed by Fagiani et al. [49].
Furthermore, various methods, such as antibodies that specifically block Aβ, knockout (KO) mice model of Aβ and its related substrates, and Aβ injection, have been implemented to actively identify the potential role of Aβ peptides involved in synaptic functions, instead of simply observing the correlation between Aβ and synaptic activity. Starting with anti-Aβ antibodies, 4G8, DAKO, and HJ5.1 were separately injected to the hippocampi of rats and mice, and a significant performance decline in behavioral tests was observed compared to controls [50, 51]. LTP, a type of neuronal activity associated with memory [52], was shown to be impaired when the endogenous Aβ was suppressed with antirodent JRF/rAb2 antibody [53]. In addition to anti-Aβ antibodies that block endogenous Aβ, a peptide called DFFVG, which binds to the receptor site of Aβ and thus blocks the activity of Aβ [54], was shown to impair learning ability as well when intracerebroventricularly injected [51]. Together, the possible involvement of Aβ in short- and long-term memory formation and learning has been suggested.
Furthermore, KO or knockdown of Aβ-related substrates in mice models was used to observe the effect of suppressing APP expression and thereby inhibiting the production of Aβ. Both KO and knockdown of APP resulted in an increased level of post-synaptic proteins, especially AMPA receptor subunit GluA1, signifying the involvement of APP in normal synaptic composition [55]. Additionally, a significant reduction in LTP was observed with knockdown of APP expression using Pen1-APP-siRNA, which can induce ~60% decline in APP expression in hippocampal cultures [53, 56], consistent with the aforementioned results shown with anti-Aβ antibodies. Similarly, conditional KO of presenilin (PS) 1 and 2, responsible for cleaving APP and thus Aβ production, also resulted in impaired synaptic plasticity and deficits in hippocampal memory in vivo [57].
Despite the mounting evidence that suppression of Aβ production using anti-Aβ antibodies and KO/knockdown mice models resulted in impairment of LTP and memory [57-60], some researchers have expressed their concern regarding these methods to determine the physiological role of Aβ because there is a diversity of substrates and pathways that APP and the secretases can initiate and regulate [61-63]. Consequently, several research groups attempted to artificially inject synthetic Aβ into the hippocampi of mice as an alternative. Morley et al. [51] demonstrated that nanomolar concentrations of Aβ42 successfully enhanced the performance of mice in behavioral tests, signifying the enhancement of memory by Aβ. However, given that the normal physiological level of Aβ peptides has been estimated to be in picomolar concentrations [64-67], the nanomolar concentrations of Aβ administered in the study largely deviates from the endogenous level of Aβ. To address this issue, other research groups attempted to inject picomolar concentrations of Aβ, and the injected mice performed better in behavioral tests as well, confirming the enhancing effect of Aβ on synaptic plasticity and memory formation [50, 68].
Interestingly, this positive effect in low-dose Aβ directly contrasts with the inhibitive effect of high-dose Aβ against synaptic functions observed in AD patients [69], and thus it has been proposed that Aβ may operate under the mechanism of hormesis, having contrasting effects at low- and high-dose Aβ [46, 70]. Accordingly, Puzzo et al. [46, 53] identified the maximum concentrations of Aβ for the stimulatory and inhibitory hormetic responses against LTP to be 200 pM and 20 μM, respectively. Similarly, another research group demonstrated that Aβ12-28 fragment in micromolar concentrations impaired memory [71], whereas nanomolar concentrations of the same Aβ isoform resulted in a significant improvement in memory formation and learning [51]. These results suggest that the role of Aβ in regard to synaptic functions may be hormetic: inhibitory at high concentrations and stimulatory at low concentrations.
Yet, an exact isoform(s) or aggregation status of Aβ responsible for this stimulatory effect on synaptic plasticity still needs to be evaluated. In fact, some data demonstrated that it may be oligomeric Aβ42 that mediates synaptic plasticity in the hippocampus. When the production of endogenous Aβ was suppressed using anti-Aβ antibodies, 200 pM oligomeric Aβ42 rescued the impaired LTP and improved behavioral deficits, whereas a medium enriched with Aβ monomers did not have an effect [53, 72]. Also, the inhibitory effect of Aβ was only present in oligomers, while cerebral microinjection of a cell medium enriched with Aβ monomers maintained a normal LTP [10].
However, this Aβ perfusion/injection method is not without concerns. The precise concentrations of monomers and different oligomers in Aβ42 preparations perfused with each hippocampal slice cannot be accurately identified due to the propensity of the monomers to undergo conformational changes into other forms of Aβ such as dimers and oligomers [73]. In fact, when 200 pM Aβ42 oligomeric solutions were analyzed using transmission electron microscopy, the percentage of identifiable monomeric form was actually higher than that of oligomers (78.21% vs 21.78%) [72]. Also, Aβ concentration of 200 pM identified as inducing maximum enhancement in LTP [68] failed to take into an account of endogenous Aβ that the mice already had. To address these problems, perhaps novel methods or a combination of existing and new experiments could further support or reject the established hypothesis. For instance, one study devised the use of thiorphan, a competitive inhibitor to neprilysin, a presynaptic metalloprotease known to degrade endogenous Aβ, in order to increase the production of endogenous Aβ [74]. The result showed that the level of endogenous Aβ elevated in the synaptic cleft and led to overall enhancement of presynaptic strength [75]. In this fashion, more data with new experimental methods are needed to identify specific isoform(s) and aggregation status of Aβ responsible for synaptic functions.
Aside from the positive correlation reported between low concentrations of Aβ and LTP, Aβ has also been shown to target nicotinic acetylcholine receptors (nAChRs) [76, 77], which are known to promote synaptic plasticity, neuroprotection, learning and memory and regulate transmitter release in several brain regions including hippocampus [45, 78, 79]. Interestingly, mounting evidence points to the possibility that this interaction too operates under hormesis. Several studies exhibited that picomolar concentrations of Aβ42 can act as an agonist of α7-nAChRs [80, 81], resulting in increased acetylcholine production in hippocampus [51], activated PI3K [33] and elevated expression of MAP kinase, which eventually led to an increased level of LTP [82]. Additionally, another in vitro study demonstrated that soluble Aβ, especially Aβ42, can directly regulate the concentration of acetylcholine by acting as allosteric enhancers of choline acetyltransferase [83]. On the other hand, nanomolar concentration of Aβ has been shown to desensitize α7-nAChR [80, 81] by inhibiting the receptors in a poor reversible manner [84], demonstrating the hormetic involvement of Aβ in nAChRs. Building on these observations, the mechanism behind the activation of α7-nAChRs by Aβ peptides has been suggested to be by causing the influx of presynaptic Ca2+, possibly through Aβ binding with lipids on the surface of cell membrane and disrupting intracellular signal transduction mechanisms that are mediated by nicotinic receptors [85, 86].
Coupled with its hormetic interaction with nAChRs and its potential influence on presynaptic Ca2+, Aβ may conceivably interact with N-methyl-D-aspartate receptors (NMDAR) [87, 88]. Since α7-nAChR-dependent NMDAR endocytosis induces endocytosis of AMPA receptors and degradation of PSD-95, a postsynaptic scaffolding protein for synaptic development and plasticity [89], the binding of pathological Aβ at high concentrations to α7-nAChR resulted in Aβ-induced long-term depression (LTD) and synaptic dysfunction [90, 91]. Given that NMDARs can promote LTP or LTD depending on the intracellular Ca2+ concentration [92], it is possible that Aβ at picomolar concentrations may also have a physiologically normal interaction with NMDARs, though related investigations are currently lacking to reach a conclusion.
In addition to the hormetic effect of Aβ, an intriguing hypothesis on the function of Aβ on synaptic transmission has been proposed by Kamenetz et al. [93] that Aβ peptides may operate under a negative feedback loop. According to this model, the acute neuronal activity leads to an elevated production of Aβ from endogenous APP. After this increase, Aβ depresses synaptic transmission and keeps neuronal activity within a normal physiological range. Then, the level of Aβ restores back to normal. In other words, Aβ is normally maintained at an appropriate level by a negative feedback loop of Aβ, oscillating between synaptic potentiation and depression [93]. However, when this negative feedback loop of Aβ is disrupted with unknown reasons, the over-suppression of excitatory synaptic activity occurs by the accumulated Aβ and ultimately leads to the development of AD [93, 94].
Besides the physiological function of Aβ involved in synaptic plasticity and neurotransmitter receptors, a neuroprotective role of Aβ has been observed. In one study, monomeric Aβ42 activated type-1 insulin-like growth factor receptors as a positive allosteric modulator [95], which in turn induced the activation of phosphatidylinositol-3-kinase/AKT pathway [96], known to be a survival pathway for neurons [97]. Consistent with this result, Aβ monomers, especially 16~20 amino acid sequence KLVFF in the peptide, exhibited a protective activity against excitotoxic death of neurons [95, 96]. Furthermore, both synthetic and cell-derived Aβ42 monomers in nanomolar concentrations enhanced the phosphorylation of cyclic adenosine monophosphate response element binding protein (CREB), which possesses a regulative role on the expression of genes for neuronal functions such as memory and learning [98], as well as brain-derived neurotrophic factor (BDNF), which plays an important physiological role in neuronal functions and survival in normal brain [99, 100]. Taken together, these results suggest an unidentified, protective role of Aβ for cognitive and neuronal abilities by binding to insulin-like growth factor receptors and enhancing the gene expression of BDNF and CREB phosphorylation.
PROMOTION OF RECOVERY FROM BRAIN INJURY
Another occasion on which the level of Aβ peptides has been reported to elevate beside AD is in the case of traumatic brain injury (TBI). Accordingly, an epidemiological link between TBI and AD originating from Aβ pathology has been suggested in the past studies [101-105], though some studies have provided data against such a correlation (reviewed by Johnson et al. [106] and Tsitsopoulos et al. [107]). In fact, recent studies have reported distinct distributions of fibrillar Aβ in brains of AD and TBI patients [108] and the absence of progressive development of Aβ into plaques in long-term survivors of TBI [109]. Notably, the level of Aβ remained elevated in axons of the long-term TBI survivors without a discernable plaque formation [109]. Although their limited sample size calls for further verification to follow, the mechanisms of Aβ pathology might be different in the two diseases, possibly severing the link between AD and TBI.
Despite the unmet consensus on their association, elevated levels of Aβ in case of brain injury have consistently been published in both animal and human studies. In studies in which implemented non-transgenic mice/rodents with experimental TBI, an acute increase of rodent Aβ in damaged axons one day after injury was observed [110, 111], and even a long, persistent accumulation of Aβ was seen in some cases [112]. Interestingly, not just the levels of Aβ, but those of APP and PS1 were also restored to the normal within a week after the injury [110, 111], hinting the existence of Aβ clearance system after the initial increase. Furthermore, these results were replicated using transgenic AD model mice, engineered to express human Aβ. They exhibited elevated levels of Aβ in their axons [113-115], and the levels returned to sham levels by seven days post injury [116]. Similarly, both acute and long-term accumulations of Aβ in axons [109, 117, 118] and in cerebrospinal fluid [119-121] were confirmed in clinical trials of TBI patients, and one of the studies reported a restoration of the Aβ level to the control levels after three weeks [120], exhibiting a consistent trend with those from the animal studies. Not just a restoration of Aβ back to normal, but also fluctuation in ISF Aβ concentrations depending on neurological status of patients with acute brain injury has been observed, suggesting a potential link between neuronal activity and Aβ concentration [122]. Clearly, a positive correlation between Aβ level and brain injury exists with an undefined Aβ-clearance mechanism, and these evidence calls for a need to investigate whether Aβ is an agent for promoting recovery or a part of pathological cascade that follows after TBI [42].
In fact, some researchers have already extended their parameter to suggest a protective role of Aβ against brain injury using the BACE1 KO mouse models. However, the use of BACE1 KO mouse models rather yielded equivocal results in regard to the possible involvement of Aβ in brain injury. Loane et al. [110] reported a reduced level of cell loss and improved behavioral deficits when BACE1 was genetically inhibited in impacted mice, suggesting a deleterious role of Aβ on brain injury. Later, a contradictory result was published by Mannix and colleagues [123] that the KO of BACE1 in mice after controlled cortical impact (CCI) attenuated motor performance compared to controls. Accordingly, it has been pointed out by Mannix et al. [123] that methodological differences could be the reasons for the contrasting results. For example, Loane and associates [110] used aged (11~12 months) mice, whereas Mannix et al. [123] tested young (2~3 months) mice. Given that previous studies have suggested that the activity of BACE1 generally elevates and TBI worsens with aging [124, 125], an age-dependent effect of TBI on Aβ is also a possibility and thus call for further studies. Additionally, a detrimental effect of BACE1 KO was observed within a week in the study by Mannix et al. [123], whereas a beneficial effect of inhibition of BACE1 was only apparent 2~3 weeks after CCI, rendering a direct comparison rather difficult. Nonetheless, Mannix and colleagues published a follow-up study showing a rescuing effect of intracerebroventricularly injected Aβ40 against impaired motor memory in TBI-impacted BACE1-/- mice, highlighting a protective effect of Aβ [126]. Moreover, another evidence in support of the beneficial effect of Aβ in brain injury has been provided by Pajoohesh-Ganji et al., as reduced level of endogenous Aβ achieved using γ-secretase inhibitor DAPT or BACE1 KO mice exhibited attenuated functional recovery after spinal cord injury (SCI) [111].
However, the results from BACE1-/- rat models need to be interpreted with caution because other substrates of BACE1, instead of Aβ, could be responsible for the observed effect. In fact, several studies have shown that the inhibition of BACE1 either by KO mouse model or its inhibitors led to an elevated level of axonal regeneration after injury, and vice versa for the elevated BACE expression [127-129]. Also, inhibiting BACE1 in both axons and Schwann cells of injured rats resulted in a reduced level of remyelination [130, 131], a process significant for restoring the function of demyelinated axons from brain damage [132]. Nonetheless, these results have been concluded to be independent of Aβ; neuregulin-1, one of the substrates of BACE1, instead is likely to be responsible for the regulation of remyelination [133, 134]. Likewise, other substrates of BACE1, such as Jagged-1 and Jagged-2 [135], might affect the results from the BACE-/- model. Hence, further investigations are in need to identify the specific substrate(s) that is responsible for the observed effect of BACE1 KO on brain injury.
Aside from TBI, focal cerebral ischemia, which results in occlusion of blood vessels in brain, was also found to be correlated with Aβ accumulations [136, 137], and these findings open up a possibility that Aβ may have a functional role against other types of brain injury as well. One study demonstrated that a transgenic mouse model that overexpresses human APP (hAPP695) exhibited a significantly lower infarct volume in the cortex after experimental cerebral ischemia induction, compared to wild type [138]. However, this decrease in the infarct volume did not lead to recovery in their behavioral deficits; rather, the transgenic mice exhibited impaired behavioral performance. The authors from this study suggested that these contrasting results could be due to the protective role of APP and the accumulation of neurotoxic Aβ that impairs cognitive abilities [138]. However, as the aforementioned studies suggested the hormetic effect of Aβ on synaptic functions, that is to exert normal, physiological effects in picomolar concentrations and detrimental effects in nanomolar concentrations, it is also possible that the overexpression of APP in the mouse model resulted in exceeding the normal, endogenous level of Aβ in brain and thus cognitive impairment. Hence, further investigation is needed to confirm whether a normal endogenous level of Aβ could lead to functional recovery after cerebral ischemia and other types of brain injury.
Also, the effect of Aβ against experimental autoimmune encephalomyelitis (EAE), an inflammatory disease in brain, was tested by Grant et al. [139]. Their results from the intraperitoneal administration of Aβ40 and Aβ42 enriched in monomers and oligomers demonstrated that the treatment before the onset of the disease successfully decreased the number of incidence and severity of the disease, and the administration of Aβ after the onset diminished motor paralysis compared to the control group. In addition, Aβ was also shown effective against EAE accelerated by proinflammatory T helper cells (TH1 and TH17) as it significantly decreased the severity of the disease and prevented the production of TH1 and TH17 related proinflammatory cytokines such as IL-6, IFN-γ, and IL-17 [139]. Notably, this protective effect of Aβ was achieved without Aβ localizing in the CNS.
Although increasing evidence on the positive effect of Aβ in the cases of brain injury is available, conflicting results concerning the relationship between Aβ accumulation and TBI still call for a thorough evaluation of their limitations and further investigation. To begin with, many studies in the past have used transgenic mouse models with a genetic predisposition to human familial AD via overexpression of human APP and/or PS1/2 to prove a causal link between TBI and AD. However, the use of these models failed to consider the possibility that the overexpression of the transgenes followed by the accumulation of Aβ and pathological deficits from AD might have influenced the acquired outcomes. Specifically, it is hard to determine which came first: did a genetic predisposition to AD exacerbate TBI or did TBI lead to AD? Accordingly, a recent study led by Maigler and colleagues employed a transgenic mouse model of non-mutated, human APP with KO of the Aβ-degrading enzyme neprilysin (APPtg.NEP-/-) as an alternative to the conventional AD models and examined the effect of reduced Aβ clearance in TBI [140]. Consistent with the aforementioned studies conducted with the transgenic AD mice, the APPtg.NEP-/- mice demonstrated an elevated level of Aβ 1 day after TBI. Notably, the neprilysin-deficient mice performed better in behavioral tests compared to wild-type mice, and no significant difference was observed between impacted and non-impacted neprilysin-deficient mice [140]. Hence, the authors argued a protective role of Aβ against brain injury. Although it remains to be investigated whether it was Aβ or other substrates of neprilysin that were responsible for this positive effect, this normalized human APP mouse model could be used as an alternative for future studies on the effect of Aβ against brain injury.
In addition to the limitation of the use of AD mouse models, methodological inconsistencies among studies have been a primary drawback of animal studies examining the level of cerebral Aβ after TBI (reviewed by Bird et al. [141]). For instance, the animal models used have ranged from transgenic ones expressing human familial AD mutations such as APPswe and PS-1swe to non-transgenic ones such as CD1. Undoubtedly, more clinically relevant data would be obtained with transgenic mice with a normal level of human Aβ due to its difference with rodent Aβ [142-144]. Also, the surgical procedures used to incur TBI differed among studies, leading to various severities of TBI and diverse regions of brain being affected. For example, a contradictory result was observed when rats were subjected to blast overpressure exposure instead of controlled cortical impact, whereby the level of endogenous rodent Aβ decreased [145]. Additionally, variability in the ages of rats, durations of experiments, and types of Aβ assay used such as ELISA and IHC have made it difficult to directly compare the results of different studies.
However, it is not just animal studies that encompasses limitations; clinical results from TBI patients are also not so free of similar limitations. Limited sample sizes and heterogeneity of TBI in each patient have always hindered researchers from reaching a concrete conclusion. For example, while one study has reported no correlations between the ISF Aβ level and the level of consciousness in TBI patients [117], the data from another study has shown that the ISF Aβ correlated with the neurological status of their patients [122]. Therefore, more concrete evidence is needed to confirm the physiological role of Aβ in brain injury.
A SEALANT FOR THE BLOOD-BRAIN BARRIER AND MODULATION OF ANGIOGENESIS
Among different hypotheses for AD pathogenesis, ‘the vascular hypothesis’ states that acute changes in cerebral vasculature system such as disrupted blood-brain barrier (BBB) and neoangiogenesis could lead to AD, and amyloidosis has been suspected to trigger such an event [146-148]. However, there is emerging evidence that non-pathological Aβ can in fact regulate angiogenesis, an essential physiological process for developing new blood vessels in cases of developing organs, healing wound, and so on [149], and potentially protect leakages in blood brain barrier (BBB) in a hormetic manner [42, 150, 151]. Several studies on physiochemical properties of Aβ and its localization to injured cerebrovascular regions suggest that Aβ peptides may serve as a protective ‘scab’, in other words a sealant, that maintains the integrity of the BBB and seals its leakages (reviewed by Atwood et al. [150, 152]. These leakages are typical of cerebrovascular alterations such as decreased levels of endothelial cells and microvascular density found in both aged people and AD patients [153-156]. Notably, it has also been pointed out that cerebral microhemorrhage observed when deposited Aβ was removed by immunotherapy in a cerebral amyloid angiopathy mouse model is evidence that Aβ has a role in maintaining vascular integrity [157]. Another in vivo experiment demonstrated unexpected deposition of Aβ when a bacterium Chlamydia pneumoniae, which penetrates and alters the BBB, was sprayed into the nose of a BALB/c mouse model with no AD transgenes [158, 159]. In this fashion, Aβ seems to be progressively generated when the BBB is disturbed, working as a sealant.
Still, more research on the physiological role of Aβ in regard to angiogenesis and sealing BBB is in need because the general aim of previous studies has been finding the potential relation of Aβ-induced neoangiogenesis to AD pathology, rather than elucidating the sole effect of Aβ peptide. For example, Biron et al. [147] reported hypervascularity in transgenic AD mice (Tg2565) in 18- to 24-month-old, and another study has shown a positive correlation between vascular density in the hippocampus and Aβ loads in postmortem AD brain tissues using immunoactivity of αvβ3, a marker for angiogenesis [160].
Interestingly, in line with the previously mentioned studies indicating the hormetic effect of Aβ on several physiological functions, a series of in vitro and in vivo studies has demonstrated a dose-dependent behavior of Aβ on angiogenesis as well. Nanomolar concentrations of Aβ40, Aβ42, and its fragmented form Aβ25-35 induced proliferation of endothelial cells and led to angiogenesis, whereas their micromolar concentrations rather inhibited the proliferation and resulted in altered microvessel morphology and death of endothelial cells [161-164]. Furthermore, both ex vivo and in vivo studies demonstrated the ability of Aβ peptides to form new blood vessels, acting similarly to fibroblast growth factor-2, which regulates the functions of endothelium and acts an essential factor for the survival of endothelial cells [165, 166], and synergetic activity of Aβ with fibroblast growth factor-2 in mediating angiogenesis [161]. The effect of Aβ on vascular branching was further illustrated: human monomeric Aβ42 dose-dependently increased blood vessel branching in zebrafish embryos and retinas of adult zebrafish [167, 168]. Additionally, zebrafish embryos with APP-deficiency exhibited vascular abnormalities, while an artificial injection of human Aβ42 restored the level of central artery branching similar to that of control [169]. Also, the same research group tested the effect of β-secretase inhibitor on zebrafish embryos, and vascular defects were reported, solidifying the evidence for the physiological function of Aβ in capillary bed density [169]. However, as mentioned earlier, since Aβ peptides has been suggested to exist in the picomolar range [64-67], further evidence on the effect of picomolar Aβ on angiogenesis could enhance our understanding of its function.
In addition to the dose-dependent effect of Aβ, a conformation-dependent effect on angiogenesis has also been observed. The oligomeric form of Aβ peptides exhibited an anti-angiogenic activity, while fibrillar forms did not shown to be active in the same assay [170]. This result was further supported by the follow-up study that the amino acid sequence identified to be responsible for the anti-angiogenic effect is HHQKLVFF, of which includes the LVFF sequence known to be exposed in the oligomeric form of Aβ [171]. Conversely, a pro-angiogenic effect was seen in Aβ35-42, a motif that may protrude outwards in monomeric or dimeric states with its property of solvent-inaccessibility [171, 172]. Another study showed that incubation of Aβ42 monomers in 225 nM with human umbilical vein endothelial cells resulted in an elevated number of endothelial tip cell formation without any apparent apoptotic death [167]. Together, these results signify a potentially non-pathological effect of monomeric Aβ on angiogenesis.
INHIBITION OF PROLIFERATING CANCER CELLS
Despite the notorious notion that AD encompasses, meta-analyses have demonstrated that AD patients have lower risks of developing various types of cancers such as bladder, breast, lung, colorectal, head and neck cancers and hematologic malignancies compared to cognitively normal elderly individuals [173-176]. This notable negative correlation drew researchers’ attention to Aβ, and it has been hypothesized that Aβ may inhibit the growth of tumor cells [164, 177, 178]. In support of this hypothesis, a direct injection of freshly dissolved Aβ into human lung adenocarcinoma xenografts and human glioblastoma suppressed the tumor growth in nude mice [164]. In addition to nude mice, transgenic mice that overexpress Aβ exhibited 50% of reduction in tumor volume compared to wild-type when glioma tumors were intracranially injected [177]. Consistent result was observed with cell-derived Aβ from mammalian cells as well, preventing the proliferation of human glioblastoma cells, breast cancer cells and mouse skin cancer cells in a concentration-dependent manner [178].
Here, a potential mechanism proposed for the antitumor effect of Aβ is by promoting apoptosis. A couple of studies indicated that APP partially mediated the expression of p53, a gene responsible for controlling apoptosis [179] and that Aβ42 bound to the promotor of p53, eventually increasing the rate of transcription of p53 [180]. Also, high nanomolar concentrations of Aβ42 increased susceptibility to oxidative stress and resulted in a lower expression of X-linked inhibitor of apoptosis (XIAP), leading to downregulation of XIAP, which directly inhibits key proteases of the apoptosis cascade, such as caspase 3, 7 and 9 [181, 182]. Not limited to p53 and XIAP, but Bcl-2, a vital anti-apoptotic protein, was also shown to be negatively influenced by Aβ42, while the level of Bax, which promotes cell apoptosis, increased when 100 nM of Aβ42 was added to primary neuron cultures [183]. This result is noteworthy because Bcl-2 overexpression is commonly observed in many types of cancer [184], further highlighting the hypothesis that Aβ may contribute to reducing cancer risks by promoting apoptosis.
Despite the growing evidence, the antitumor role of Aβ is not conclusive due to limited data and methodology. In the aforementioned studies on the inhibitive effect of Aβ on the growth of tumor cells, either freshly solubilized Aβ or naturally secreted soluble Aβ were incubated with cancer cells, without further identifying the exact species, monomeric or further aggregated form, responsible for the observed effect [164, 177, 178]. Therefore, more research on a major contributor of Aβ species to the potential antitumor role of Aβ is required.
Aβ-DIRECTED THERAPEUTICS IN CLINICAL TRIALS AND IMPLICATIONS
Given the potential clinical significance and market effects of AD therapeutics, there have been many attempts to target Aβ using various approaches focusing on different stages of the amyloid cascade (Table 1) [185-228]. For example, inhibitors of Aβ-related secretases aim to decrease the production of Aβ itself as a preventative measure, while anti-Aβ immunotherapies induce partial or complete clearance of Aβ at the end of the amyloid cascade. However, a lack of efficiency and unexpected adverse side effects have hampered AD patients from receiving effective treatment and led to a reliance on drugs such as donepezil (AChE inhibitor) and memantine (NMDA receptor antagonist) that are designed to temporarily alleviate symptoms [229]. Recently, the US Food and Drug Administration (FDA) granted approval for aducanumab as the first disease-modifying therapy for AD; however, many professionals expressed concerns and criticism of this decision due to insufficient data showing the effectiveness of the drug on rescuing the loss of cognitive abilities. Therefore, an important question must be answered: why are Aβ-targeted drugs failing?
Table 1.
A list of therapeutic approaches to target Aβ and their adverse events in clinical trials
| Mechanism of action | Drug | Trial phase | Adverse events in clinical trials | Main reason for failure | References |
|---|---|---|---|---|---|
| BACE1 inhibitor | Verubecestat | III | Worsened cognition, increased mortality, and reduced brain volume in the whole-brain and hippocampal region | Lack of efficacy | Egan et al. (2018) [185], Sur et al. (2020) [186] |
| Umibecestat (CNP520) | II / III | Worsened cognition and brain atrophy | Toxicity | NCT02565511, NCT03131453 | |
| Atabecestat | II / III | Worsened cognition and liver toxicity | Toxicity | Novak et al. (2020) [187], Sperling et al. (2021) [188] | |
| Lanabecestat (AZD3293, LY3314814) | III | Worsened cognition | Lack of efficacy | Wessels et al. (2020) [189] | |
| BI1181181 | I | No SAEs reported | Further study required | NCT02044406, Nicolas et al. (2015) [190] | |
| LY2886721 | II | Liver toxicity | Toxicity | Lahiri et al. (2014) [191] | |
| AZD3839 | I | Heart rhythm disturbance | Toxicity | Yan (2016) [192], NCT01348737 | |
| RG7129 | I | Liver toxicity | Toxicity | NCT01664143, NCT01592331 | |
| Elenbecestat (E2609) | III | No SAEs reported | Lack of efficacy | NCT02956486, NCT03036280 | |
| LY3202626 | II | No SAEs reported | Lack of efficacy | Lo et al. (2021) [193] | |
| γ-secretase inhibitor | Semagacestat | III | Worsened cognition and increased skin cancers and infections | Toxicity and lack of efficacy | Doody et al. (2013) [194] |
| Begacestat (GSI-953) | I | No SAEs reported | Further study required | NCT00547560 | |
| Avagacestat | II | Worsened cognition and increased nonmelanoma skin cancer | Toxicity and lack of efficacy | Coric et al. (2012) [195], Coric et al. (2015) [196] | |
| MPC-7869 (Flurizan, Tarenflurbil) | III | No SAEs reported | Lack of efficacy |
NCT00322036 Green et al. (2009) [197] |
|
| Aβ antigen | AN-1792 (AIP001) | II | Brain inflammation (meningoencephalitis) | Toxicity and lack of efficacy | Gilman et al. (2005) [198], Boche et al. (2010) [199] |
| Affitope AD02 | II | No SAEs reported | Lack of efficacy | Schneeberger et al. (2015) [200] | |
| Vanutide cridificar (ACC-001) | II | No SAEs reported | Lack of efficacy | Pasquier et al. (2016) [201] | |
| CAD 106 (Amilomotide) | II | Worsened cognition, decreased cortical gray-matter volume, and ARIAs (ARIA-E & ARIA-H) | Lack of efficacy | Vandenberghe et al. (2017) [202] | |
| Monoclonal antibody | Aducanumab (BIIB037, Aduhelm) | III | ARIA-E | Lack of efficacy | Ferrero et al. (2016) [203], Sevigny et al. (2016) [204], NCT02477800, NCT02484547 |
| Bapineuzumab (AAB-001) | III | Increased risk of serious treatment-emergent adverse events and ARIA-E | Toxicity and Lack of efficacy | Salloway et al. (2014) [205], Vandenberghe et al. (2016) [206], Abushouk et al. (2017) [207] | |
| AAB-003 | I | ARIA-E | Further study required | Delnomdedieu et al. (2016) [208], NCT01193608 | |
| Gantenerumab | III | ARIAs | Lack of efficacy | Ostrowitzki et al. (2017) [209] | |
| Solanezumab (LY2062430) | III | ARIAs | Lack of efficacy | Doody et al. (2014) [210], Honig et al. (2018) [15], Siemers et al. (2016) [211] | |
| Crenezumab | III | No SAEs reported | Lack of efficacy | Yang et al. (2019) [212], NCT02670083 | |
| Ponezumab | II | No SAEs reported | Lack of efficacy | Landen et al. (2017) [213], NCT00722046 | |
| Immunoglobin | III | No SAEs reported | Lack of efficacy | Relkin et al. (2017) [214] | |
| Donanemab (LY3002813) | III | Reduced brain volume and ARIA-E | Lack of efficacy | Lowe et al. (2021) [215], Ayton (2021) [216] NCT04437511 |
|
| Lecanemab (BAN2401) | II | ARIA-E | Lack of efficacy | Swanson et al. (2021) [217] NCT03887455 |
|
| SAR228810 | I | No SAEs reported | Further study required | Pradier et al. (2018) [218], NCT01485302 | |
| MEDI1814 | I | No SAEs reported | Further study required | NCT02036645 | |
| GSK933776 | II | No SAEs reported | Lack of efficacy | Leyhe et al. (2014) [219], NCT01342926 | |
| Aβ vaccine | ACI-24 | II | No SAEs reported | Further study required | Ritchie et al. (2003) [220] |
| UB-311 | II | No SAEs reported | Further study required | Wang et al. (2017) [221], NCT02551809 | |
| ABVac40 | II | No SAEs reported | Further study required | Lacosta et al. (2018) [222] NCT03461276 | |
| Aβ aggregation inhibitor | Alzhemed (Tramiprosate, 3-APS) | III | No SAEs reported | Lack of efficacy | NCT00314912, Aisen et al. (2006) [223], Gauthier et al. (2009) [224] |
| Scyllo-inositol (AZD-103, ELND005) | II | Higher incidence of SAEs and respiratory tract infections in high dose groups | Lack of efficacy, Further study required | Salloway et al. (2011) [225] | |
| PBT1 (Clioquinol) | II | SAEs such as visual impairment and intracranial hemorrhage | Toxicity and lack of efficacy | Sampson et al. (2014) [226] | |
| PBT2 (Hydroxyquinoline) | II | No SAEs reported | Lack of efficacy | Lannfelt et al. (2008) [227] NCT00471211 | |
| GV-971 (Sodium oligo-mannurarate) | III | Higher incidence of hyperlipidemia and nasopharyngitis | Further study required | Xiao et al. (2021) [228] NCT02293915 |
SAE, serious adverse events; ARIA-E, amyloid-related imaging abnormalities with vasogenic edema; ARIA-H, amyloid-related imaging abnormalities with microhemorrhages.
To begin with, the earliest components of the amyloid cascade targeted by AD therapeutics are BACE1- and γ- secretases by their respective inhibitors. Co-cleavage of APP by BACE1- and γ- secretases is known to release Aβ into the extracellular space; inhibition of these secretases has therefore been proposed as a measure to block the Aβ production, regardless of the conformation of Aβ. However, the clinical outcomes of both BACE1- and γ- secretases have been disappointing because many were discontinued due to a lack of efficiency and worsen cognition among the tested patients compared to placebo group (Table 1). For example, a series of oral BACE1 inhibitors, including Verubecestat [185], Lanabecestat [189], and Atabecestat [230], which underwent clinical trials on mild AD patients were shown to exacerbate cognitive functions of the participants, despite the apparent reduction in CSF Aβ levels. Also, the clinical trials of γ- secretase inhibitors, Semagacestat [194] and Avagacestat [4, 196] were halted as their administration resulted in aggravated functional and cognitive abilities, and higher rates of skin cancer and infections [231]. These adverse events observed in the clinical trials of BACE1- and γ- secretase inhibitors were consistent with in vitro and in vivo evidence that Aβ may possess roles in regulating synaptic functions, defending the human body against various microbes, and even suppressing the growth of tumor, as discussed earlier.
In response to the apparent failure in the upstream regulation of the amyloid cascade via the secretase inhibitors, more recent attempts have been made with anti-Aβ immunotherapies. These immunization methods are intended to reduce the level of Aβ deposits in the brain through either an active approach using Aβ antigen that generate an antibody response against Aβ or a passive approach using humanized Aβ antibodies [232]. An initial attempt was made by Elan Pharmaceuticals with the AN-1792 Aβ antigen, which is a synthetic, aggregated Aβ42 combined with adjuvant, and the result showed a significant reduction in Aβ loads in the participants. Nonetheless, no improvement in their cognitive decline was observed [198, 233]. Notably, ~6% of the immunized patients exhibited meningoencephalitis, which is an inflammatory disease of the brain caused by infection [198, 234]. This unexpected adverse effect suggests that there may be a need to keep normal forms of Aβ rather than complete clearance, because it has been suggested that monomeric and oligomeric Aβ may exert an antimicrobial effect and have a protective role against brain inflammatory diseases by suppression of the production of proinflammatory cytokines [139]. Consequently, lack of clinical efficiency and adverse side effects such as cognitive worsening in active immunotherapies such as AD02 [200] and CAD106 [202] that followed after AN-1792 turned many researchers to move onto the passive Aβ immunotherapy.
Similarly, the passive immunotherapy aims to clear out Aβ using monoclonal or polyclonal anti-Aβ antibodies targeting neurotoxic Aβ. Among the antibodies tested, aducanumab by Biogen has received the greatest attention because of successful phase I results indicating the dose- and time-dependent clearance of Aβ plaques on positron emission tomography scans and slowed progressive cognitive decline of AD patients [204]. Aducanumab was the first such drug to be approved by the FDA as an AD therapeutic. However, many professionals, even some advisory members of the FDA, have criticized the approval of aducanumab due to inadequate evidence for clinical effects in the phase III trials (EMERGE and ENGAGE) and due to the subjectivity of the FDA as they worked with the sponsor to reevaluate the submitted data [235-237].
Adding on to the ambiguous findings for aducanumab and the apparent lack of efficiency of other Aβ immunotherapies, a new adverse effect, amyloid-related imaging abnormalities (ARIA), has been reported in clinical trials of a significant number of the immunized Aβ antibodies such aducanumab [203], bapineuzumab [206], gantenerumab [238], and solanezumab [15] (reviewed by DiFrancesco et al. [239]). Based on radiographic features of the subjects, ARIA is divided into two subgroups: ARIA-E if vasogenic edema is observed and ARIA-H by microhemorrhages with hemosiderin deposits in brain [240]. Interestingly, the subjects were either symptomatic with several neurological side effects or asymptomatic in some cases [203, 205]. One hypothesis suggested for pathophysiology of ARIA is the solubilization of parenchymal Aβ plaques by the immunotherapies leading to the accumulation of cerebrovascular Aβ in vessel walls. Then, this accumulation is hypothesized to be stimulating the clearance of vascular Aβ by relative lymphocytes and proteases and weakening of BBB, and thus edema and hemorrhages are spotted [240-242]. Although very limited evidence for risk factors and mechanisms of ARIA is available so far, any signs of ARIA in the brain of the patients must be carefully screened in future trials.
Continuous failures of the Aβ-targeting therapeutics and adverse effects highlight the need for further elucidation of the pathogenesis of AD and for the adjustment on AD therapeutics. One hypothesis to explain the lack of efficiency of the Aβ immunotherapies is that the AD patients in clinical trials were too advanced in their stage of AD and thus no longer exhibited the clinical effect observed in the preclinical stages [243]. It is possible that the antibodies may be effective only as a preventative measure, and do not engage with Aβ plaques once profound abnormalities in neuropathology have developed. Additionally, an increased level of soluble oligomeric Aβ after the clearance of Aβ plaques by aducanumab has been proposed as another possibility for the failure, as mounting evidence points to deleterious effects of diffusible Aβ oligomers [244]. The possibility that Aβ might not be a desirable target for clinical interventions in AD should also be considered; indeed, a recent meta-analysis of randomized trials of Aβ-directed drugs exhibited that Aβ clearance therapies did not cause a significant improvement in cognition [245]. Based on these considerations, many new approaches for AD therapeutics have been suggested, including modifications of targeted epitopes of Aβ, adjustments on administered doses, and direct-delivery methods across the BBB [246].
Based on the physiological roles of Aβ discussed earlier (Fig. 1), novel therapeutics for AD could be designed to selectively remove or disaggregate neurotoxic forms of Aβ while retaining its monomeric form in the brain. We hypothesize that the side effects reported in the clinical trials of Aβ-targeting drug candidates might have been resulted from the complete clearance of Aβ from the brain, which led to the unexpected loss of monomeric Aβ and their functions. For instance, the weakened BBB evident through ARIA might be related to normal Aβ peptides no longer being able to work as a protective scab for BBB. Also, the patients with reduced Aβ could have been more susceptible to microbial infections with reduced number of Aβ monomers with antimicrobial activity, which could explain the increased infection rates observed in the clinical trials. More importantly, the absence of monomeric Aβ that has been shown to regulate synaptic activity could be the reason why many subjects with reduced Aβ levels experienced worsened cognition in the trials. Although more direct evidence is required to verify this hypothesis, the restoration of monomeric Aβ in the brain has a potential to elicit cognitive improvement and satisfy the unmet clinical need for AD therapeutics.
Fig. 1.
The physiological roles of amyloid-β (Aβ). (a) Aβ has an antimicrobial property: Aβ induces agglutination and inhibits the adhesion of pathogens to host cells. (b) Aβ regulates synaptic functions: picomolar concentrations of Aβ42 increase the concentration of acetylcholine (ACh), by acting as an allosteric enhancer of choline acetyltransferase (ChAT), causing an influx of Ca2+ through α7-nicotinic acetylcholine receptors (α7-nAChRs), which eventually lead to an increase in long-term potentiation (LTP). (c) Aβ promotes recovery from brain injury: during the recovery of traumatic brain injury (TBI), the levels of Aβ elevate, hinting a protective role of Aβ against brain injury. (d) Aβ serves as a sealant of blood-brain barrier (BBB) leakages. (e) Monomeric forms of Aβ dose-dependently promote angiogenesis. (f) Aβ suppresses tumor growth by promoting apoptosis.
ACKNOWLEDGEMENTS
This research was supported by the Korea Health Technology R&D Project (Grant Number: HU21C0161) through the Korea Health Industry Development Institute (KHIDI) and Korea Dementia Research Center (KDRC), and Mid-Career Researcher Program (Grant Number: NRF-2021R1A2C2093916), and Basic Science Research Program (Grant Number: NRF-2018R1A6A1A03023718) through the National Research Foundation of Korea (NRF), funded by the Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea. This research was also supported by Yonsei Frontier Lab, Amyloid Solution, and POSCO Science Fellowship of POSCO TJ Park Foundation.
References
- 1.Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron. 2014;82:756–771. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56:127–129. doi: 10.1212/WNL.56.1.127. [DOI] [PubMed] [Google Scholar]
- 3.Shankar GM, Walsh DM. Alzheimer's disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop GM, Robinson SR. The amyloid hypothesis: let sleeping dogmas lie? Neurobiol Aging. 2002;23:1101–1105. doi: 10.1016/S0197-4580(02)00050-7. [DOI] [PubMed] [Google Scholar]
- 5.Storey E, Cappai R. The amyloid precursor protein of Alzheimer's disease and the Abeta peptide. Neuropathol Appl Neurobiol. 1999;25:81–97. doi: 10.1046/j.1365-2990.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 6.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 7.Ricciarelli R, Fedele E. The amyloid cascade hypothesis in Alzheimer's disease: it's time to change our mind. Curr Neuropharmacol. 2017;15:926–935. doi: 10.2174/1570159X15666170116143743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 9.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 11.Uddin MS, Kabir MT, Rahman MS, Behl T, Jeandet P, Ashraf GM, Najda A, Bin-Jumah MN, El-Seedi HR, Abdel-Daim MM. Revisiting the amyloid cascade hypothesis: from anti-Aβ therapeutics to auspicious new ways for Alzheimer's Disease. Int J Mol Sci. 2020;21:5858. doi: 10.3390/ijms21165858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeremic D, Jiménez-Díaz L, Navarro-López JD. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer's disease: a systematic review. Ageing Res Rev. 2021;72:101496. doi: 10.1016/j.arr.2021.101496. [DOI] [PubMed] [Google Scholar]
- 13.Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, Friedrich S, Dean RA, Gonzales C, Sethuraman G, DeMattos RB, Mohs R, Paul SM, Siemers ER. Safety and biomarker effects of solanezumab in patients with Alzheimer's disease. Alzheimers Dement. 2012;8:261–271. doi: 10.1016/j.jalz.2011.09.224. [DOI] [PubMed] [Google Scholar]
- 14.Egan MF, Kost J, Voss T, Mukai Y, Aisen PS, Cummings JL, Tariot PN, Vellas B, van Dyck CH, Boada M, Zhang Y, Li W, Furtek C, Mahoney E, Harper Mozley L, Mo Y, Sur C, Michelson D. Randomized trial of verubecestat for prodromal Alzheimer's disease. N Engl J Med. 2019;380:1408–1420. doi: 10.1056/NEJMoa1812840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, Hager K, Andreasen N, Scarpini E, Liu-Seifert H, Case M, Dean RA, Hake A, Sundell K, Poole Hoffmann V, Carlson C, Khanna R, Mintun M, DeMattos R, Selzler KJ, Siemers E. Trial of solanezumab for mild dementia due to Alzheimer's disease. N Engl J Med. 2018;378:321–330. doi: 10.1056/NEJMoa1705971. [DOI] [PubMed] [Google Scholar]
- 16.Moore DB, Gillentine MA, Botezatu NM, Wilson KA, Benson AE, Langeland JA. Asynchronous evolutionary origins of Aβ and BACE1. Mol Biol Evol. 2014;31:696–702. doi: 10.1093/molbev/mst262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson SR, Bishop GM. Abeta as a bioflocculant: implications for the amyloid hypothesis of Alzheimer's disease. Neurobiol Aging. 2002;23:1051–1072. doi: 10.1016/S0197-4580(01)00342-6. [DOI] [PubMed] [Google Scholar]
- 18.Moir RD, Lathe R, Tanzi RE. The antimicrobial protection hypothesis of Alzheimer's disease. Alzheimers Dement. 2018;14:1602–1614. doi: 10.1016/j.jalz.2018.06.3040. [DOI] [PubMed] [Google Scholar]
- 19.Zaiou M. Multifunctional antimicrobial peptides: therapeutic targets in several human diseases. J Mol Med (Berl) 2007;85:317–329. doi: 10.1007/s00109-006-0143-4. [DOI] [PubMed] [Google Scholar]
- 20.Pastore A, Raimondi F, Rajendran L, Temussi PA. Why does the Aβ peptide of Alzheimer share structural similarity with antimicrobial peptides? Commun Biol. 2020;3:135. doi: 10.1038/s42003-020-0865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee EY, Srinivasan Y, de Anda J, Nicastro LK, Tükel Ç, Wong GCL. Functional reciprocity of amyloids and antimicrobial peptides: rethinking the role of supramolecular assembly in host defense, immune activation, and inflammation. Front Immunol. 2020;11:1629. doi: 10.3389/fimmu.2020.01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 23.Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD. The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, Moir RD. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White MR, Kandel R, Tripathi S, Condon D, Qi L, Taubenberger J, Hartshorn KL. Alzheimer's associated β-amyloid protein inhibits influenza A virus and modulates viral interactions with phagocytes. PLoS One. 2014;9:e101364. doi: 10.1371/journal.pone.0101364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, Rodriguez AS, Mitchell T, Washicosky KJ, György B, Breakefield XO, Tanzi RE, Moir RD. Alzheimer's disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron. 2018;99:56–63.e3. doi: 10.1016/j.neuron.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourgade K, Garneau H, Giroux G, Le Page AY, Bocti C, Dupuis G, Frost EH, Fülöp T., Jr β-Amyloid peptides display protective activity against the human Alzheimer's disease-associated herpes simplex virus-1. Biogerontology. 2015;16:85–98. doi: 10.1007/s10522-014-9538-8. [DOI] [PubMed] [Google Scholar]
- 28.Spitzer P, Condic M, Herrmann M, Oberstein TJ, Scharin-Mehlmann M, Gilbert DF, Friedrich O, Grömer T, Kornhuber J, Lang R, Maler JM. Amyloidogenic amyloid-β-peptide variants induce microbial agglutination and exert antimicrobial activity. Sci Rep. 2016;6:32228. doi: 10.1038/srep32228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripathi S, Tecle T, Verma A, Crouch E, White M, Hartshorn KL. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J Gen Virol. 2013;94(Pt 1):40–49. doi: 10.1099/vir.0.045013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukiw WJ, Cui JG, Yuan LY, Bhattacharjee PS, Corkern M, Clement C, Kammerman EM, Ball MJ, Zhao Y, Sullivan PM, Hill JM. Acyclovir or Aβ42 peptides attenuate HSV-1-induced miRNA-146a levels in human primary brain cells. Neuroreport. 2010;21:922–927. doi: 10.1097/WNR.0b013e32833da51a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Güntert A, Döbeli H, Bohrmann B. High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neuroscience. 2006;143:461–475. doi: 10.1016/j.neuroscience.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 32.White MR, Kandel R, Hsieh IN, De Luna X, Hartshorn KL. Critical role of C-terminal residues of the Alzheimer's associated β-amyloid protein in mediating antiviral activity and modulating viral and bacterial interactions with neutrophils. PLoS One. 2018;13:e0194001. doi: 10.1371/journal.pone.0194001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo Y, Sunderland T, Roth GS, Wolozin B. Physiological levels of beta-amyloid peptide promote PC12 cell proliferation. Neurosci Lett. 1996;217:125–128. doi: 10.1016/0304-3940(96)13087-1. [DOI] [PubMed] [Google Scholar]
- 34.Wiatrak B, Balon K. Protective activity of Aβ on cell cultures (PC12 and THP-1 after differentiation) preincubated with lipopolysaccharide (LPS) Mol Neurobiol. 2021;58:1453–1464. doi: 10.1007/s12035-020-02204-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iqbal UH, Zeng E, Pasinetti GM. The use of antimicrobial and antiviral drugs in Alzheimer's disease. Int J Mol Sci. 2020;21:4920. doi: 10.3390/ijms21144920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spitzer P, Walter M, Göth C, Oberstein TJ, Linning P, Knölker HJ, Kornhuber J, Maler JM. Pharmacological inhibition of amyloidogenic APP processing and knock-down of APP in primary human macrophages impairs the secretion of cytokines. Front Immunol. 2020;11:1967. doi: 10.3389/fimmu.2020.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kucheryavykh LY, Kucheryavykh YV, Washington AV, Inyushin MY. Amyloid beta peptide is released during thrombosis in the skin. Int J Mol Sci. 2018;19:1705. doi: 10.3390/ijms19061705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kucheryavykh LY, Dávila-Rodríguez J, Rivera-Aponte DE, Zueva LV, Washington AV, Sanabria P, Inyushin MY. Platelets are responsible for the accumulation of β-amyloid in blood clots inside and around blood vessels in mouse brain after thrombosis. Brain Res Bull. 2017;128:98–105. doi: 10.1016/j.brainresbull.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai PW, Yang CY, Chang HT, Lan CY. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS One. 2011;6:e17755. doi: 10.1371/journal.pone.0017755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kagan BL, Jang H, Capone R, Teran Arce F, Ramachandran S, Lal R, Nussinov R. Antimicrobial properties of amyloid peptides. Mol Pharm. 2012;9:708–717. doi: 10.1021/mp200419b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inyushin M, Zayas-Santiago A, Rojas L, Kucheryavykh L. On the role of platelet-generated amyloid beta peptides in certain amyloidosis health complications. Front Immunol. 2020;11:571083. doi: 10.3389/fimmu.2020.571083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brothers HM, Gosztyla ML, Robinson SR. The physiological roles of amyloid-β peptide hint at new ways to treat Alzheimer's disease. Front Aging Neurosci. 2018;10:118. doi: 10.3389/fnagi.2018.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morley JE, Farr SA. The role of amyloid-beta in the regulation of memory. Biochem Pharmacol. 2014;88:479–485. doi: 10.1016/j.bcp.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Musardo S, Marcello E. Synaptic dysfunction in Alzheimer's disease: from the role of amyloid β-peptide to the α-secretase ADAM10. Eur J Pharmacol. 2017;817:30–37. doi: 10.1016/j.ejphar.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 45.Lanni C, Fagiani F, Racchi M, Preda S, Pascale A, Grilli M, Allegri N, Govoni S. Beta-amyloid short- and long-term synaptic entanglement. Pharmacol Res. 2018;139:243–260. doi: 10.1016/j.phrs.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 46.Puzzo D, Privitera L, Palmeri A. Hormetic effect of amyloid-β peptide in synaptic plasticity and memory. Neurobiol Aging. 2012;33:1484.e15–e24. doi: 10.1016/j.neurobiolaging.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 47.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 48.Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fagiani F, Lanni C, Racchi M, Pascale A, Govoni S. Amyloid-β and synaptic vesicle dynamics: a cacophonic orchestra. J Alzheimers Dis. 2019;72:1–14. doi: 10.3233/JAD-190771. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Osta A, Alberini CM. Amyloid beta mediates memory formation. Learn Mem. 2009;16:267–272. doi: 10.1101/lm.1310209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morley JE, Farr SA, Banks WA, Johnson SN, Yamada KA, Xu L. A physiological role for amyloid-beta protein: enhancement of learning and memory. J Alzheimers Dis. 2010;19:441–449. doi: 10.3233/JAD-2010-1230. [DOI] [PubMed] [Google Scholar]
- 52.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 53.Puzzo D, Privitera L, Fa' M, Staniszewski A, Hashimoto G, Aziz F, Sakurai M, Ribe EM, Troy CM, Mercken M, Jung SS, Palmeri A, Arancio O. Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory. Ann Neurol. 2011;69:819–830. doi: 10.1002/ana.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flood JF, Roberts E, Sherman MA, Kaplan BE, Morley JE. Topography of a binding site for small amnestic peptides deduced from structure-activity studies: relation to amnestic effect of amyloid beta protein. Proc Natl Acad Sci U S A. 1994;91:380–384. doi: 10.1073/pnas.91.1.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinsson I, Capetillo-Zarate E, Faideau M, Willén K, Esteras N, Frykman S, Tjernberg LO, Gouras GK. APP depletion alters selective pre- and post-synaptic proteins. Mol Cell Neurosci. 2019;95:86–95. doi: 10.1016/j.mcn.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci. 2007;27:14459–14469. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ, 3rd, Kandel ER, Duff K, Kirkwood A, Shen J. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/S0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 58.Seabrook GR, Smith DW, Bowery BJ, Easter A, Reynolds T, Fitzjohn SM, Morton RA, Zheng H, Dawson GR, Sirinathsinghji DJ, Davies CH, Collingridge GL, Hill RG. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology. 1999;38:349–359. doi: 10.1016/S0028-3908(98)00204-4. [DOI] [PubMed] [Google Scholar]
- 59.Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O'Dowd G, Bowery BJ, Boyce S, Trumbauer ME, Chen HY, Van der Ploeg LH, Sirinathsinghji DJ. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience. 1999;90:1–13. doi: 10.1016/S0306-4522(98)00410-2. [DOI] [PubMed] [Google Scholar]
- 60.Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, Borchelt DR, Price DL, Lee HK, Wong PC. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 62.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 63.Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990;248:1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- 64.Pawlik M, Sastre M, Calero M, Mathews PM, Schmidt SD, Nixon RA, Levy E. Overexpression of human cystatin C in transgenic mice does not affect levels of endogenous brain amyloid Beta Peptide. J Mol Neurosci. 2004;22:13–18. doi: 10.1385/JMN:22:1-2:13. [DOI] [PubMed] [Google Scholar]
- 65.Ramsden M, Nyborg AC, Murphy MP, Chang L, Stanczyk FZ, Golde TE, Pike CJ. Androgens modulate beta-amyloid levels in male rat brain. J Neurochem. 2003;87:1052–1055. doi: 10.1046/j.1471-4159.2003.02114.x. [DOI] [PubMed] [Google Scholar]
- 66.Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giedraitis V, Sundelöf J, Irizarry MC, Gårevik N, Hyman BT, Wahlund LO, Ingelsson M, Lannfelt L. The normal equilibrium between CSF and plasma amyloid beta levels is disrupted in Alzheimer's disease. Neurosci Lett. 2007;427:127–131. doi: 10.1016/j.neulet.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 68.Puzzo D, Privitera L, Leznik E, Fà M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 70.Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flood JF, Morley JE, Roberts E. An amyloid beta-protein fragment, A beta[12-28], equipotently impairs post-training memory processing when injected into different limbic system structures. Brain Res. 1994;663:271–276. doi: 10.1016/0006-8993(94)91273-4. [DOI] [PubMed] [Google Scholar]
- 72.Gulisano W, Melone M, Li Puma DD, Tropea MR, Palmeri A, Arancio O, Grassi C, Conti F, Puzzo D. The effect of amyloid-β peptide on synaptic plasticity and memory is influenced by different isoforms, concentrations, and aggregation status. Neurobiol Aging. 2018;71:51–60. doi: 10.1016/j.neurobiolaging.2018.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 74.Iwata N, Mizukami H, Shirotani K, Takaki Y, Muramatsu S, Lu B, Gerard NP, Gerard C, Ozawa K, Saido TC. Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-beta peptide in mouse brain. J Neurosci. 2004;24:991–998. doi: 10.1523/JNEUROSCI.4792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 76.Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- 77.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 79.Radcliffe KA, Dani JA. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J Neurosci. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fodero LR, Mok SS, Losic D, Martin LL, Aguilar MI, Barrow CJ, Livett BG, Small DH. Alpha7-nicotinic acetylcholine receptors mediate an Abeta(1-42)-induced increase in the level of acetylcholinesterase in primary cortical neurones. J Neurochem. 2004;88:1186–1193. doi: 10.1046/j.1471-4159.2003.02296.x. [DOI] [PubMed] [Google Scholar]
- 81.Dineley KT, Bell KA, Bui D, Sweatt JD. beta-Amyloid peptide activates alpha 7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Biol Chem. 2002;277:25056–25061. doi: 10.1074/jbc.M200066200. [DOI] [PubMed] [Google Scholar]
- 82.Sui L, Wang J, Li BM. Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex. Learn Mem. 2008;15:762–776. doi: 10.1101/lm.1067808. [DOI] [PubMed] [Google Scholar]
- 83.Kumar A, Lana E, Kumar R, Lithner CU, Darreh-Shori T. Soluble Aβ42 acts as allosteric activator of the core cholinergic enzyme choline acetyltransferase. Front Mol Neurosci. 2018;11:327. doi: 10.3389/fnmol.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grassi F, Palma E, Tonini R, Amici M, Ballivet M, Eusebi F. Amyloid beta(1-42) peptide alters the gating of human and mouse alpha-bungarotoxin-sensitive nicotinic receptors. J Physiol. 2003;547(Pt 1):147–157. doi: 10.1113/jphysiol.2002.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Small DH, Maksel D, Kerr ML, Ng J, Hou X, Chu C, Mehrani H, Unabia S, Azari MF, Loiacono R, Aguilar MI, Chebib M. The beta-amyloid protein of Alzheimer's disease binds to membrane lipids but does not bind to the alpha7 nicotinic acetylcholine receptor. J Neurochem. 2007;101:1527–1538. doi: 10.1111/j.1471-4159.2006.04444.x. [DOI] [PubMed] [Google Scholar]
- 86.Dougherty JJ, Wu J, Nichols RA. Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. J Neurosci. 2003;23:6740–6747. doi: 10.1523/JNEUROSCI.23-17-06740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang HY, Lee DH, D'Andrea MR, Peterson PA, Shank RP, Reitz AB. beta-Amyloid(1-42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer's disease pathology. J Biol Chem. 2002;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 88.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 89.Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 90.Tu S, Okamoto S, Lipton SA, Xu H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease. Mol Neurodegener. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- 93.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/S0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 94.Fogel H, Frere S, Segev O, Bharill S, Shapira I, Gazit N, O'Malley T, Slomowitz E, Berdichevsky Y, Walsh DM, Isacoff EY, Hirsch JA, Slutsky I. APP homodimers transduce an amyloid-β-mediated increase in release probability at excitatory synapses. Cell Rep. 2014;7:1560–1576. doi: 10.1016/j.celrep.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 95.Giuffrida ML, Tomasello MF, Pandini G, Caraci F, Battaglia G, Busceti C, Di Pietro P, Pappalardo G, Attanasio F, Chiechio S, Bagnoli S, Nacmias B, Sorbi S, Vigneri R, Rizzarelli E, Nicoletti F, Copani A. Monomeric ß-amyloid interacts with type-1 insulin-like growth factor receptors to provide energy supply to neurons. Front Cell Neurosci. 2015;9:297. doi: 10.3389/fncel.2015.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/S0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 98.Alberini CM, Chen DY. Memory enhancement: consolidation, reconsolidation and insulin-like growth factor 2. Trends Neurosci. 2012;35:274–283. doi: 10.1016/j.tins.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zimbone S, Monaco I, Gianì F, Pandini G, Copani AG, Giuffrida ML, Rizzarelli E. Amyloid Beta monomers regulate cyclic adenosine monophosphate response element binding protein functions by activating type-1 insulin-like growth factor receptors in neuronal cells. Aging Cell. 2018;17:e12684. doi: 10.1111/acel.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 101.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y, Li Y, Li X, Zhang S, Zhao J, Zhu X, Tian G. Head injury as a risk factor for dementia and Alzheimer's disease: a systematic review and meta-analysis of 32 observational studies. PLoS One. 2017;12:e0169650. doi: 10.1371/journal.pone.0169650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JC. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/WNL.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 104.Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, Green RC, Sadovnick AD, Duara R, DeCarli C, Johnson K, Go RC, Growdon JH, Haines JL, Kukull WA, Farrer LA. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/WNL.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 105.Mayeux R, Ottman R, Tang MX, Noboa-Bauza L, Marder K, Gurland B, Stern Y. Genetic susceptibility and head injury as risk factors for Alzheimer's disease among community-dwelling elderly persons and their first-degree relatives. Ann Neurol. 1993;33:494–501. doi: 10.1002/ana.410330513. [DOI] [PubMed] [Google Scholar]
- 106.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-β pathology: a link to Alzheimer's disease? Nat Rev Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsitsopoulos PP, Marklund N. Amyloid-β peptides and tau protein as biomarkers in cerebrospinal and interstitial fluid following traumatic brain injury: a review of experimental and clinical studies. Front Neurol. 2013;4:79. doi: 10.3389/fneur.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scott G, Ramlackhansingh AF, Edison P, Hellyer P, Cole J, Veronese M, Leech R, Greenwood RJ, Turkheimer FE, Gentleman SM, Heckemann RA, Matthews PM, Brooks DJ, Sharp DJ. Amyloid pathology and axonal injury after brain trauma. Neurology. 2016;86:821–828. doi: 10.1212/WNL.0000000000002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen XH, Johnson VE, Uryu K, Trojanowski JQ, Smith DH. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol. 2009;19:214–223. doi: 10.1111/j.1750-3639.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Loane DJ, Pocivavsek A, Moussa CE, Thompson R, Matsuoka Y, Faden AI, Rebeck GW, Burns MP. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med. 2009;15:377–379. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pajoohesh-Ganji A, Burns MP, Pal-Ghosh S, Tadvalkar G, Hokenbury NG, Stepp MA, Faden AI. Inhibition of amyloid precursor protein secretases reduces recovery after spinal cord injury. Brain Res. 2014;1560:73–82. doi: 10.1016/j.brainres.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iwata A, Chen XH, McIntosh TK, Browne KD, Smith DH. Long-term accumulation of amyloid-beta in axons following brain trauma without persistent upregulation of amyloid precursor protein genes. J Neuropathol Exp Neurol. 2002;61:1056–1068. doi: 10.1093/jnen/61.12.1056. [DOI] [PubMed] [Google Scholar]
- 113.Tran HT, Sanchez L, Esparza TJ, Brody DL. Distinct temporal and anatomical distributions of amyloid-β and tau abnormalities following controlled cortical impact in transgenic mice. PLoS One. 2011;6:e25475. doi: 10.1371/journal.pone.0025475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tran HT, LaFerla FM, Holtzman DM, Brody DL. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-β accumulation and independently accelerates the development of tau abnormalities. J Neurosci. 2011;31:9513–9525. doi: 10.1523/JNEUROSCI.0858-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abrahamson EE, Ikonomovic MD, Ciallella JR, Hope CE, Paljug WR, Isanski BA, Flood DG, Clark RS, DeKosky ST. Caspase inhibition therapy abolishes brain trauma-induced increases in Abeta peptide: implications for clinical outcome. Exp Neurol. 2006;197:437–450. doi: 10.1016/j.expneurol.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 116.Washington PM, Morffy N, Parsadanian M, Zapple DN, Burns MP. Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer's disease mouse model. J Neurotrauma. 2014;31:125–134. doi: 10.1089/neu.2013.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marklund N, Farrokhnia N, Hånell A, Vanmechelen E, Enblad P, Zetterberg H, Blennow K, Hillered L. Monitoring of β-amyloid dynamics after human traumatic brain injury. J Neurotrauma. 2014;31:42–55. doi: 10.1089/neu.2013.2964. [DOI] [PubMed] [Google Scholar]
- 118.Abu Hamdeh S, Waara ER, Möller C, Söderberg L, Basun H, Alafuzoff I, Hillered L, Lannfelt L, Ingelsson M, Marklund N. Rapid amyloid-β oligomer and protofibril accumulation in traumatic brain injury. Brain Pathol. 2018;28:451–462. doi: 10.1111/bpa.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olsson A, Csajbok L, Ost M, Höglund K, Nylén K, Rosengren L, Nellgård B, Blennow K. Marked increase of beta-amyloid(1-42) and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injury. J Neurol. 2004;251:870–876. doi: 10.1007/s00415-004-0451-y. [DOI] [PubMed] [Google Scholar]
- 120.Raby CA, Morganti-Kossmann MC, Kossmann T, Stahel PF, Watson MD, Evans LM, Mehta PD, Spiegel K, Kuo YM, Roher AE, Emmerling MR. Traumatic brain injury increases beta-amyloid peptide 1-42 in cerebrospinal fluid. J Neurochem. 1998;71:2505–2509. doi: 10.1046/j.1471-4159.1998.71062505.x. [DOI] [PubMed] [Google Scholar]
- 121.Emmerling MR, Morganti-Kossmann MC, Kossmann T, Stahel PF, Watson MD, Evans LM, Mehta PD, Spiegel K, Kuo YM, Roher AE, Raby CA. Traumatic brain injury elevates the Alzheimer's amyloid peptide A beta 42 in human CSF. A possible role for nerve cell injury. Ann N Y Acad Sci. 2000;903:118–122. doi: 10.1111/j.1749-6632.2000.tb06357.x. [DOI] [PubMed] [Google Scholar]
- 122.Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, Zipfel GJ, Holtzman DM. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mannix RC, Zhang J, Park J, Lee C, Whalen MJ. Detrimental effect of genetic inhibition of B-site APP-cleaving enzyme 1 on functional outcome after controlled cortical impact in young adult mice. J Neurotrauma. 2011;28:1855–1861. doi: 10.1089/neu.2011.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miners JS, van Helmond Z, Kehoe PG, Love S. Changes with age in the activities of beta-secretase and the Abeta-degrading enzymes neprilysin, insulin-degrading enzyme and angiotensin-converting enzyme. Brain Pathol. 2010;20:794–802. doi: 10.1111/j.1750-3639.2010.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tokutomi T, Miyagi T, Ogawa T, Ono J, Kawamata T, Sakamoto T, Shigemori M, Nakamura N. Age-associated increases in poor outcomes after traumatic brain injury: a report from the Japan Neurotrauma Data Bank. J Neurotrauma. 2008;25:1407–1414. doi: 10.1089/neu.2008.0577. [DOI] [PubMed] [Google Scholar]
- 126.Mannix RC, Zhang J, Berglass J, Qui J, Whalen MJ. Beneficial effect of amyloid beta after controlled cortical impact. Brain Inj. 2013;27:743–748. doi: 10.3109/02699052.2013.771797. [DOI] [PubMed] [Google Scholar]
- 127.Farah MH, Pan BH, Hoffman PN, Ferraris D, Tsukamoto T, Nguyen T, Wong PC, Price DL, Slusher BS, Griffin JW. Reduced BACE1 activity enhances clearance of myelin debris and regeneration of axons in the injured peripheral nervous system. J Neurosci. 2011;31:5744–5754. doi: 10.1523/JNEUROSCI.6810-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tallon C, Rockenstein E, Masliah E, Farah MH. Increased BACE1 activity inhibits peripheral nerve regeneration after injury. Neurobiol Dis. 2017;106:147–157. doi: 10.1016/j.nbd.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 129.Tallon C, Marshall KL, Kennedy ME, Hyde LA, Farah MH. Pharmacological BACE inhibition improves axonal regeneration in nerve injury and disease models. neurotherapeutics. 2020;17:973–988. doi: 10.1007/s13311-020-00852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, Trapp BD, Yan R. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22:2970–2980. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hu X, Hu J, Dai L, Trapp B, Yan R. Axonal and Schwann cell BACE1 is equally required for remyelination of peripheral nerves. J Neurosci. 2015;35:3806–3814. doi: 10.1523/JNEUROSCI.5207-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baaklini CS, Rawji KS, Duncan GJ, Ho MFS, Plemel JR. Central nervous system remyelination: roles of glia and innate immune cells. Front Mol Neurosci. 2019;12:225. doi: 10.3389/fnmol.2019.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 134.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He W, Hu J, Xia Y, Yan R. β-site amyloid precursor protein cleaving enzyme 1(BACE1) regulates Notch signaling by controlling the cleavage of Jagged 1 (Jag1) and Jagged 2 (Jag2) proteins. J Biol Chem. 2014;289:20630–20637. doi: 10.1074/jbc.M114.579862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jendroska K, Poewe W, Daniel SE, Pluess J, Iwerssen-Schmidt H, Paulsen J, Barthel S, Schelosky L, Cervós-Navarro J, DeArmond SJ. Ischemic stress induces deposition of amyloid beta immunoreactivity in human brain. Acta Neuropathol. 1995;90:461–466. doi: 10.1007/BF00294806. [DOI] [PubMed] [Google Scholar]
- 137.Lee PH, Bang OY, Hwang EM, Lee JS, Joo US, Mook-Jung I, Huh K. Circulating beta amyloid protein is elevated in patients with acute ischemic stroke. J Neural Transm (Vienna) 2005;112:1371–1379. doi: 10.1007/s00702-004-0274-0. [DOI] [PubMed] [Google Scholar]
- 138.Clarke J, Thornell A, Corbett D, Soininen H, Hiltunen M, Jolkkonen J. Overexpression of APP provides neuroprotection in the absence of functional benefit following middle cerebral artery occlusion in rats. Eur J Neurosci. 2007;26:1845–1852. doi: 10.1111/j.1460-9568.2007.05807.x. [DOI] [PubMed] [Google Scholar]
- 139.Grant JL, Ghosn EE, Axtell RC, Herges K, Kuipers HF, Woodling NS, Andreasson K, Herzenberg LA, Herzenberg LA, Steinman L. Reversal of paralysis and reduced inflammation from peripheral administration of β-amyloid in TH1 and TH17 versions of experimental autoimmune encephalomyelitis. Sci Transl Med. 2012;4:145ra105. doi: 10.1126/scitranslmed.3004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maigler KC, Buhr TJ, Park CS, Miller SA, Kozlowski DA, Marr RA. Assessment of the effects of altered amyloid-beta clearance on behavior following repeat closed-head brain injury in amyloid-beta precursor protein humanized mice. J Neurotrauma. 2021;38:665–676. doi: 10.1089/neu.2020.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bird SM, Sohrabi HR, Sutton TA, Weinborn M, Rainey-Smith SR, Brown B, Patterson L, Taddei K, Gupta V, Carruthers M, Lenzo N, Knuckey N, Bucks RS, Verdile G, Martins RN. Cerebral amyloid-β accumulation and deposition following traumatic brain injury--A narrative review and meta-analysis of animal studies. Neurosci Biobehav Rev. 2016;64:215–228. doi: 10.1016/j.neubiorev.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 142.Ueno H, Yamaguchi T, Fukunaga S, Okada Y, Yano Y, Hoshino M, Matsuzaki K. Comparison between the aggregation of human and rodent amyloid β-proteins in GM1 ganglioside clusters. Biochemistry. 2014;53:7523–7530. doi: 10.1021/bi501239q. [DOI] [PubMed] [Google Scholar]
- 143.Götz J, Bodea LG, Goedert M. Rodent models for Alzheimer disease. Nat Rev Neurosci. 2018;19:583–598. doi: 10.1038/s41583-018-0054-8. [DOI] [PubMed] [Google Scholar]
- 144.Otvos L, Jr, Szendrei GI, Lee VM, Mantsch HH. Human and rodent Alzheimer beta-amyloid peptides acquire distinct conformations in membrane-mimicking solvents. Eur J Biochem. 1993;211:249–257. doi: 10.1111/j.1432-1033.1993.tb19893.x. [DOI] [PubMed] [Google Scholar]
- 145.De Gasperi R, Gama Sosa MA, Kim SH, Steele JW, Shaughness MC, Maudlin-Jeronimo E, Hall AA, Dekosky ST, McCarron RM, Nambiar MP, Gandy S, Ahlers ST, Elder GA. Acute blast injury reduces brain abeta in two rodent species. Front Neurol. 2012;3:177. doi: 10.3389/fneur.2012.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dickstein DL, Walsh J, Brautigam H, Stockton SD, Jr, Gandy S, Hof PR. Role of vascular risk factors and vascular dysfunction in Alzheimer's disease. Mt Sinai J Med. 2010;77:82–102. doi: 10.1002/msj.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Biron KE, Dickstein DL, Gopaul R, Jefferies WA. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer's disease. PLoS One. 2011;6:e23789. doi: 10.1371/journal.pone.0023789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jefferies WA, Price KA, Biron KE, Fenninger F, Pfeifer CG, Dickstein DL. Adjusting the compass: new insights into the role of angiogenesis in Alzheimer's disease. Alzheimers Res Ther. 2013;5:64. doi: 10.1186/alzrt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 150.Atwood CS, Bowen RL, Smith MA, Perry G. Cerebrovascular requirement for sealant, anti-coagulant and remodeling molecules that allow for the maintenance of vascular integrity and blood supply. Brain Res Brain Res Rev. 2003;43:164–178. doi: 10.1016/S0165-0173(03)00206-6. [DOI] [PubMed] [Google Scholar]
- 151.Ristori E, Donnini S, Ziche M. New insights into blood-brain barrier maintenance: the homeostatic role of β-amyloid precursor protein in cerebral vasculature. Front Physiol. 2020;11:1056. doi: 10.3389/fphys.2020.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Atwood CS, Bishop GM, Perry G, Smith MA. Amyloid-beta: a vascular sealant that protects against hemorrhage? J Neurosci Res. 2002;70:356. doi: 10.1002/jnr.10388. [DOI] [PubMed] [Google Scholar]
- 153.Buée L, Hof PR, Delacourte A. Brain microvascular changes in Alzheimer's disease and other dementias. Ann N Y Acad Sci. 1997;826:7–24. doi: 10.1111/j.1749-6632.1997.tb48457.x. [DOI] [PubMed] [Google Scholar]
- 154.Xu X, Wang B, Ren C, Hu J, Greenberg DA, Chen T, Xie L, Jin K. Age-related impairment of vascular structure and functions. Aging Dis. 2017;8:590–610. doi: 10.14336/AD.2017.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kalaria RN, Hedera P. Differential degeneration of the cerebral microvasculature in Alzheimer's disease. Neuroreport. 1995;6:477–480. doi: 10.1097/00001756-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 156.Mancardi GL, Perdelli F, Rivano C, Leonardi A, Bugiani O. Thickening of the basement membrane of cortical capillaries in Alzheimer's disease. Acta Neuropathol. 1980;49:79–83. doi: 10.1007/BF00692225. [DOI] [PubMed] [Google Scholar]
- 157.Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology. 2002;58:1629–1634. doi: 10.1212/WNL.58.11.1629. [DOI] [PubMed] [Google Scholar]
- 158.Balin BJ, Gérard HC, Arking EJ, Appelt DM, Branigan PJ, Abrams JT, Whittum-Hudson JA, Hudson AP. Identification and localization of Chlamydia pneumoniae in the Alzheimer's brain. Med Microbiol Immunol. 1998;187:23–42. doi: 10.1007/s004300050071. [DOI] [PubMed] [Google Scholar]
- 159.MacIntyre A, Hammond CJ, Little CS, Appelt DM, Balin BJ. Chlamydia pneumoniae infection alters the junctional complex proteins of human brain microvascular endothelial cells. FEMS Microbiol Lett. 2002;217:167–72. doi: 10.1111/j.1574-6968.2002.tb11470.x. [DOI] [PubMed] [Google Scholar]
- 160.Desai BS, Schneider JA, Li JL, Carvey PM, Hendey B. Evidence of angiogenic vessels in Alzheimer's disease. J Neural Transm (Vienna) 2009;116:587–597. doi: 10.1007/s00702-009-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cantara S, Donnini S, Morbidelli L, Giachetti A, Schulz R, Memo M, Ziche M. Physiological levels of amyloid peptides stimulate the angiogenic response through FGF-2. FASEB J. 2004;18:1943–1945. doi: 10.1096/fj.04-2114fje. [DOI] [PubMed] [Google Scholar]
- 162.Boscolo E, Folin M, Nico B, Grandi C, Mangieri D, Longo V, Scienza R, Zampieri P, Conconi MT, Parnigotto PP, Ribatti D. Beta amyloid angiogenic activity in vitro and in vivo. Int J Mol Med. 2007;19:581–587. doi: 10.3892/ijmm.19.4.581. [DOI] [PubMed] [Google Scholar]
- 163.Donnini S, Solito R, Cetti E, Corti F, Giachetti A, Carra S, Beltrame M, Cotelli F, Ziche M. Abeta peptides accelerate the senescence of endothelial cells in vitro and in vivo, impairing angiogenesis. FASEB J. 2010;24:2385–2395. doi: 10.1096/fj.09-146456. [DOI] [PubMed] [Google Scholar]
- 164.Paris D, Townsend K, Quadros A, Humphrey J, Sun J, Brem S, Wotoczek-Obadia M, DelleDonne A, Patel N, Obregon DF, Crescentini R, Abdullah L, Coppola D, Rojiani AM, Crawford F, Sebti SM, Mullan M. Inhibition of angiogenesis by Abeta peptides. Angiogenesis. 2004;7:75–85. doi: 10.1023/B:AGEN.0000037335.17717.bf. [DOI] [PubMed] [Google Scholar]
- 165.Friesel R, Maciag T. Fibroblast growth factor prototype release and fibroblast growth factor receptor signaling. Thromb Haemost. 1999;82:748–754. doi: 10.1055/s-0037-1615907. [DOI] [PubMed] [Google Scholar]
- 166.Araki S, Shimada Y, Kaji K, Hayashi H. Apoptosis of vascular endothelial cells by fibroblast growth factor deprivation. Biochem Biophys Res Commun. 1990;168:1194–1200. doi: 10.1016/0006-291X(90)91155-L. [DOI] [PubMed] [Google Scholar]
- 167.Cameron DJ, Galvin C, Alkam T, Sidhu H, Ellison J, Luna S, Ethell DW. Alzheimer's-related peptide amyloid-β plays a conserved role in angiogenesis. PLoS One. 2012;7:e39598. doi: 10.1371/journal.pone.0039598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cunvong K, Huffmire D, Ethell DW, Cameron DJ. Amyloid-β increases capillary bed density in the adult zebrafish retina. Invest Ophthalmol Vis Sci. 2013;54:1516–1521. doi: 10.1167/iovs.12-10821. [DOI] [PubMed] [Google Scholar]
- 169.Luna S, Cameron DJ, Ethell DW. Amyloid-β and APP deficiencies cause severe cerebrovascular defects: important work for an old villain. PLoS One. 2013;8:e75052. doi: 10.1371/journal.pone.0075052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Paris D, Ait-Ghezala G, Mathura VS, Patel N, Quadros A, Laporte V, Mullan M. Anti-angiogenic activity of the mutant Dutch A(beta) peptide on human brain microvascular endothelial cells. Brain Res Mol Brain Res. 2005;136:212–230. doi: 10.1016/j.molbrainres.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 171.Patel NS, Quadros A, Brem S, Wotoczek-Obadia M, Mathura VS, Laporte V, Mullan M, Paris D. Potent anti-angiogenic motifs within the Alzheimer beta-amyloid peptide. Amyloid. 2008;15:5–19. doi: 10.1080/13506120701814723. [DOI] [PubMed] [Google Scholar]
- 172.Olofsson A, Sauer-Eriksson AE, Ohman A. The solvent protection of alzheimer amyloid-beta-(1-42) fibrils as determined by solution NMR spectroscopy. J Biol Chem. 2006;281:477–483. doi: 10.1074/jbc.M508962200. [DOI] [PubMed] [Google Scholar]
- 173.Shi HB, Tang B, Liu YW, Wang XF, Chen GJ. Alzheimer disease and cancer risk: a meta-analysis. J Cancer Res Clin Oncol. 2015;141:485–494. doi: 10.1007/s00432-014-1773-5. Erratum in: J Cancer Res Clin Oncol 141: 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shafi O. Inverse relationship between Alzheimer's disease and cancer, and other factors contributing to Alzheimer's disease: a systematic review. BMC Neurol. 2016;16:236. doi: 10.1186/s12883-016-0765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ospina-Romero M, Glymour MM, Hayes-Larson E, Mayeda ER, Graff RE, Brenowitz WD, Ackley SF, Witte JS, Kobayashi LC. Association between Alzheimer disease and cancer with evaluation of study biases: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2025515. doi: 10.1001/jamanetworkopen.2020.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Frain L, Swanson D, Cho K, Gagnon D, Lu KP, Betensky RA, Driver J. Association of cancer and Alzheimer's disease risk in a national cohort of veterans. Alzheimers Dement. 2017;13:1364–1370. doi: 10.1016/j.jalz.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Paris D, Ganey N, Banasiak M, Laporte V, Patel N, Mullan M, Murphy SF, Yee GT, Bachmeier C, Ganey C, Beaulieu-Abdelahad D, Mathura VS, Brem S, Mullan M. Impaired orthotopic glioma growth and vascularization in transgenic mouse models of Alzheimer's disease. J Neurosci. 2010;30:11251–11258. doi: 10.1523/JNEUROSCI.2586-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhao H, Zhu J, Cui K, Xu X, O'Brien M, Wong KK, Kesari S, Xia W, Wong ST. Bioluminescence imaging reveals inhibition of tumor cell proliferation by Alzheimer's amyloid beta protein. Cancer Cell Int. 2009;9:15. doi: 10.1186/1475-2867-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Alves da Costa C, Sunyach C, Pardossi-Piquard R, Sévalle J, Vincent B, Boyer N, Kawarai T, Girardot N, St George-Hyslop P, Checler F. Presenilin-dependent gamma-secretase-mediated control of p53-associated cell death in Alzheimer's disease. J Neurosci. 2006;26:6377–6385. doi: 10.1523/JNEUROSCI.0651-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, Ikezoe K, Furuya H, Kawarabayashi T, Shoji M, Checler F, Iwaki T, Makifuchi T, Takeda K, Kira J, Tabira T. Intracellular Abeta42 activates p53 promoter: a pathway to neurodegeneration in Alzheimer's disease. FASEB J. 2005;19:255–257. doi: 10.1096/fj.04-2637fje. [DOI] [PubMed] [Google Scholar]
- 181.Chaudhary AK, Yadav N, Bhat TA, O'Malley J, Kumar S, Chandra D. A potential role of X-linked inhibitor of apoptosis protein in mitochondrial membrane permeabilization and its implication in cancer therapy. Drug Discov Today. 2016;21:38–47. doi: 10.1016/j.drudis.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yamamori H, Tanaka T, Kudo T, Takeda M. Amyloid-beta down-regulates XIAP expression in human SH-SY5Y neuroblastoma cells. Neuroreport. 2004;15:851–854. doi: 10.1097/00001756-200404090-00023. [DOI] [PubMed] [Google Scholar]
- 183.Paradis E, Douillard H, Koutroumanis M, Goodyer C, LeBlanc A. Amyloid beta peptide of Alzheimer's disease downregulates Bcl-2 and upregulates bax expression in human neurons. J Neurosci. 1996;16:7533–7539. doi: 10.1523/JNEUROSCI.16-23-07533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kirkin V, Joos S, Zörnig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004;1644:229–249. doi: 10.1016/j.bbamcr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 185.Egan MF, Kost J, Tariot PN, Aisen PS, Cummings JL, Vellas B, Sur C, Mukai Y, Voss T, Furtek C, Mahoney E, Harper Mozley L, Vandenberghe R, Mo Y, Michelson D. Randomized trial of verubecestat for mild-to-moderate Alzheimer's disease. N Engl J Med. 2018;378:1691–1703. doi: 10.1056/NEJMoa1706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sur C, Kost J, Scott D, Adamczuk K, Fox NC, Cummings JL, Tariot PN, Aisen PS, Vellas B, Voss T, Mahoney E, Mukai Y, Kennedy ME, Lines C, Michelson D, Egan MF. BACE inhibition causes rapid, regional, and non-progressive volume reduction in Alzheimer's disease brain. Brain. 2020;143:3816–3826. doi: 10.1093/brain/awaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Novak G, Streffer JR, Timmers M, Henley D, Brashear HR, Bogert J, Russu A, Janssens L, Tesseur I, Tritsmans L, Van Nueten L, Engelborghs S. Long-term safety and tolerability of atabecestat (JNJ-54861911), an oral BACE1 inhibitor, in early Alzheimer's disease spectrum patients: a randomized, double-blind, placebo-controlled study and a two-period extension study. Alzheimers Res Ther. 2020;12:58. doi: 10.1186/s13195-020-00614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sperling R, Henley D, Aisen PS, Raman R, Donohue MC, Ernstrom K, Rafii MS, Streffer J, Shi Y, Karcher K, Raghavan N, Tymofyeyev Y, Bogert J, Brashear HR, Novak G, Thipphawong J, Saad ZS, Kolb H, Rofael H, Sanga P, Romano G. Findings of efficacy, safety, and biomarker outcomes of atabecestat in preclinical Alzheimer disease: a truncated randomized phase 2b/3 clinical trial. JAMA Neurol. 2021;78:293–301. doi: 10.1001/jamaneurol.2020.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wessels AM, Tariot PN, Zimmer JA, Selzler KJ, Bragg SM, Andersen SW, Landry J, Krull JH, Downing AM, Willis BA, Shcherbinin S, Mullen J, Barker P, Schumi J, Shering C, Matthews BR, Stern RA, Vellas B, Cohen S, MacSweeney E, Boada M, Sims JR. Efficacy and safety of lanabecestat for treatment of early and mild Alzheimer disease: the AMARANTH and DAYBREAK-ALZ randomized clinical trials. JAMA Neurol. 2020;77:199–209. doi: 10.1001/jamaneurol.2019.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nicolas L, Kammerer KP, Schaible J, Link J, Kleiner O, Borta A, Podhorna J, Scholpp J. P3-283: Pharmacokinetics, pharmacodynamics, and safety of the novel bace inhibitor bi1181181 after oral administration of single ascending doses in healthy subjects. Alzheimer's Dement. 2015;11(7S_Part_16):740–741. doi: 10.1016/j.jalz.2015.06.1656. [DOI] [Google Scholar]
- 191.Lahiri DK, Maloney B, Long JM, Greig NH. Lessons from a BACE1 inhibitor trial: off-site but not off base. Alzheimers Dement. 2014;10(5 Suppl):S411–S419. doi: 10.1016/j.jalz.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yan R. Stepping closer to treating Alzheimer's disease patients with BACE1 inhibitor drugs. Transl Neurodegener. 2016;5:13. doi: 10.1186/s40035-016-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lo AC, Evans CD, Mancini M, Wang H, Shcherbinin S, Lu M, Natanegara F, Willis BA. Phase II (NAVIGATE-AD study) results of LY3202626 effects on patients with mild Alzheimer's disease dementia. J Alzheimers Dis Rep. 2021;5:321–336. doi: 10.3233/ADR-210296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, He F, Sun X, Thomas RG, Aisen PS, Siemers E, Sethuraman G, Mohs R. A phase 3 trial of semagacestat for treatment of Alzheimer's disease. N Engl J Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 195.Coric V, van Dyck CH, Salloway S, Andreasen N, Brody M, Richter RW, Soininen H, Thein S, Shiovitz T, Pilcher G, Colby S, Rollin L, Dockens R, Pachai C, Portelius E, Andreasson U, Blennow K, Soares H, Albright C, Feldman HH, Berman RM. Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol. 2012;69:1430–1440. doi: 10.1001/archneurol.2012.2194. [DOI] [PubMed] [Google Scholar]
- 196.Coric V, Salloway S, van Dyck CH, Dubois B, Andreasen N, Brody M, Curtis C, Soininen H, Thein S, Shiovitz T, Pilcher G, Ferris S, Colby S, Kerselaers W, Dockens R, Soares H, Kaplita S, Luo F, Pachai C, Bracoud L, Mintun M, Grill JD, Marek K, Seibyl J, Cedarbaum JM, Albright C, Feldman HH, Berman RM. Targeting prodromal Alzheimer disease with avagacestat: a randomized clinical trial. JAMA Neurol. 2015;72:1324–1333. doi: 10.1001/jamaneurol.2015.0607. [DOI] [PubMed] [Google Scholar]
- 197.Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, Zavitz KH. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 199.Boche D, Donald J, Love S, Harris S, Neal JW, Holmes C, Nicoll JA. Reduction of aggregated Tau in neuronal processes but not in the cell bodies after Abeta42 immunisation in Alzheimer's disease. Acta Neuropathol. 2010;120:13–20. doi: 10.1007/s00401-010-0705-y. [DOI] [PubMed] [Google Scholar]
- 200.Schneeberger A, Hendrix S, Mandler M, Ellison N, Bürger V, Brunner M, Frölich L, Mimica N, Hort J, Rainer M, Imarhiagbe D, Kurz A, Peters O, Gertz HJ, Tierney L, Mattner F, Schmidt W, Dubois B. Results from a phase II study to assess the clinical and immunological activity of AFFITOPE®AD02 in patients with early Alzheimer's disease. J Prev Alzheimers Dis. 2015;2:103–114. doi: 10.14283/jpad.2015.63. [DOI] [PubMed] [Google Scholar]
- 201.Pasquier F, Sadowsky C, Holstein A, Leterme Gle P, Peng Y, Jackson N, Fox NC, Ketter N, Liu E, Ryan JM. Two phase 2 multiple ascending-dose studies of vanutide cridificar (ACC-001) and QS-21 adjuvant in mild-to-moderate Alzheimer's disease. J Alzheimers Dis. 2016;51:1131–1143. doi: 10.3233/JAD-150376. [DOI] [PubMed] [Google Scholar]
- 202.Vandenberghe R, Riviere ME, Caputo A, Sovago J, Maguire RP, Farlow M, Marotta G, Sanchez-Valle R, Scheltens P, Ryan JM, Graf A. Active Aβ immunotherapy CAD106 in Alzheimer's disease: a phase 2b study. Alzheimers Dement (N Y) 2017;3:10–22. doi: 10.1016/j.trci.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ferrero J, Williams L, Stella H, Leitermann K, Mikulskis A, O'Gorman J, Sevigny J. First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer's disease. Alzheimers Dement (N Y) 2016;2:169–176. doi: 10.1016/j.trci.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O'Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 205.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vandenberghe R, Rinne JO, Boada M, Katayama S, Scheltens P, Vellas B, Tuchman M, Gass A, Fiebach JB, Hill D, Lobello K, Li D, McRae T, Lucas P, Evans I, Booth K, Luscan G, Wyman BT, Hua L, Yang L, Brashear HR, Black RS. Bapineuzumab for mild to moderate Alzheimer's disease in two global, randomized, phase 3 trials. Alzheimers Res Ther. 2016;8:18. doi: 10.1186/s13195-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Abushouk AI, Elmaraezy A, Aglan A, Salama R, Fouda S, Fouda R, AlSafadi AM. Bapineuzumab for mild to moderate Alzheimer's disease: a meta-analysis of randomized controlled trials. BMC Neurol. 2017;17:66. doi: 10.1186/s12883-017-0850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Delnomdedieu M, Duvvuri S, Li DJ, Atassi N, Lu M, Brashear HR, Liu E, Ness S, Kupiec JW. First-In-Human safety and long-term exposure data for AAB-003 (PF-05236812) and biomarkers after intravenous infusions of escalating doses in patients with mild to moderate Alzheimer's disease. Alzheimers Res Ther. 2016;8:12. doi: 10.1186/s13195-016-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T, Ashford E, Retout S, Hofmann C, Delmar P, Klein G, Andjelkovic M, Dubois B, Boada M, Blennow K, Santarelli L, Fontoura P. A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease. Alzheimers Res Ther. 2017;9:95. doi: 10.1186/s13195-017-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 211.Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, Dowsett SA, Pontecorvo MJ, Dean RA, Demattos R. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2016;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 212.Yang T, Dang Y, Ostaszewski B, Mengel D, Steffen V, Rabe C, Bittner T, Walsh DM, Selkoe DJ. Target engagement in an alzheimer trial: Crenezumab lowers amyloid β oligomers in cerebrospinal fluid. Ann Neurol. 2019;86:215–224. doi: 10.1002/ana.25513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Landen JW, Cohen S, Billing CB, Jr, Cronenberger C, Styren S, Burstein AH, Sattler C, Lee JH, Jack CR, Jr, Kantarci K, Schwartz PF, Duggan WT, Zhao Q, Sprenger K, Bednar MM, Binneman B. Multiple-dose ponezumab for mild-to-moderate Alzheimer's disease: safety and efficacy. Alzheimers Dement (N Y) 2017;3:339–347. doi: 10.1016/j.trci.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Relkin NR, Thomas RG, Rissman RA, Brewer JB, Rafii MS, van Dyck CH, Jack CR, Sano M, Knopman DS, Raman R, Szabo P, Gelmont DM, Fritsch S, Aisen PS Alzheimer's Disease Cooperative Study, author. A phase 3 trial of IV immunoglobulin for Alzheimer disease. Neurology. 2017;88:1768–1775. doi: 10.1212/WNL.0000000000003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lowe SL, Duggan Evans C, Shcherbinin S, Cheng YJ, Willis BA, Gueorguieva I, Lo AC, Fleisher AS, Dage JL, Ardayfio P, Aguiar G, Ishibai M, Takaichi G, Chua L, Mullins G, Sims JR. Donanemab (LY3002813) phase 1b study in Alzheimer's disease: rapid and sustained reduction of brain amyloid measured by florbetapir F18 imaging. J Prev Alzheimers Dis. 2021;8:414–424. doi: 10.14283/jpad.2021.56. [DOI] [PubMed] [Google Scholar]
- 216.Ayton S. Brain volume loss due to donanemab. Eur J Neurol. 2021;28:e67–e68. doi: 10.1111/ene.15007. [DOI] [PubMed] [Google Scholar]
- 217.Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RYK, Lannfelt L, Bradley H, Rabe M, Koyama A, Reyderman L, Berry DA, Berry S, Gordon R, Kramer LD, Cummings JL. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther. 2021;13:80. doi: 10.1186/s13195-021-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pradier L, Blanchard-Brégeon V, Bohme A, Debeir T, Menager J, Benoit P, Barneoud P, Taupin V, Bertrand P, Dugay P, Cameron B, Shi Y, Naimi S, Duchesne M, Gagnaire M, Weeden T, Travaline T, Reczek D, Khiroug L, Slaoui M, Brunel P, Fukuyama H, Ravetch J, Canton T, Cohen C. SAR228810: an antibody for protofibrillar amyloid β peptide designed to reduce the risk of amyloid-related imaging abnormalities (ARIA) Alzheimers Res Ther. 2018;10:117. doi: 10.1186/s13195-018-0447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Leyhe T, Andreasen N, Simeoni M, Reich A, von Arnim CA, Tong X, Yeo A, Khan S, Loercher A, Chalker M, Hottenstein C, Zetterberg H, Hilpert J, Mistry P. Modulation of β-amyloid by a single dose of GSK933776 in patients with mild Alzheimer's disease: a phase I study. Alzheimers Res Ther. 2014;6:19. doi: 10.1186/alzrt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 221.Wang CY, Wang PN, Chiu MJ, Finstad CL, Lin F, Lynn S, Tai YH, De Fang X, Zhao K, Hung CH, Tseng Y, Peng WJ, Wang J, Yu CC, Kuo BS, Frohna PA. UB-311, a novel UBITh®amyloid β peptide vaccine for mild Alzheimer's disease. Alzheimers Dement (N Y) 2017;3:262–272. doi: 10.1016/j.trci.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lacosta AM, Pascual-Lucas M, Pesini P, Casabona D, Pérez-Grijalba V, Marcos-Campos I, Sarasa L, Canudas J, Badi H, Monleón I, San-José I, Munuera J, Rodríguez-Gómez O, Abdelnour C, Lafuente A, Buendía M, Boada M, Tárraga L, Ruiz A, Sarasa M. Safety, tolerability and immunogenicity of an active anti-Aβ40 vaccine (ABvac40) in patients with Alzheimer's disease: a randomised, double-blind, placebo-controlled, phase I trial. Alzheimers Res Ther. 2018;10:12. doi: 10.1186/s13195-018-0340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Aisen PS, Saumier D, Briand R, Laurin J, Gervais F, Tremblay P, Garceau D. A phase II study targeting amyloid-beta with 3APS in mild-to-moderate Alzheimer disease. Neurology. 2006;67:1757–1763. doi: 10.1212/01.wnl.0000244346.08950.64. [DOI] [PubMed] [Google Scholar]
- 224.Gauthier S, Aisen PS, Ferris SH, Saumier D, Duong A, Haine D, Garceau D, Suhy J, Oh J, Lau W, Sampalis J. Effect of tramiprosate in patients with mild-to-moderate Alzheimer's disease: exploratory analyses of the MRI sub-group of the Alphase study. J Nutr Health Aging. 2009;13:550–557. doi: 10.1007/s12603-009-0106-x. [DOI] [PubMed] [Google Scholar]
- 225.Salloway S, Sperling R, Keren R, Porsteinsson AP, van Dyck CH, Tariot PN, Gilman S, Arnold D, Abushakra S, Hernandez C, Crans G, Liang E, Quinn G, Bairu M, Pastrak A, Cedarbaum JM ELND005-AD201 Investigators, author. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology. 2011;77:1253–1262. doi: 10.1212/WNL.0b013e3182309fa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sampson EL, Jenagaratnam L, McShane R. Metal protein attenuating compounds for the treatment of Alzheimer's dementia. Cochrane Database Syst Rev. 2014;2:CD005380. doi: 10.1002/14651858.CD005380.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW PBT2-201-EURO Study Group, author. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7:779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 228.Xiao S, Chan P, Wang T, Hong Z, Wang S, Kuang W, He J, Pan X, Zhou Y, Ji Y, Wang L, Cheng Y, Peng Y, Ye Q, Wang X, Wu Y, Qu Q, Chen S, Li S, Chen W, Xu J, Peng D, Zhao Z, Li Y, Zhang J, Du Y, Chen W, Fan D, Yan Y, Liu X, Zhang W, Luo B, Wu W, Shen L, Liu C, Mao P, Wang Q, Zhao Q, Guo Q, Zhou Y, Li Y, Jiang L, Ren W, Ouyang Y, Wang Y, Liu S, Jia J, Zhang N, Liu Z, He R, Feng T, Lu W, Tang H, Gao P, Zhang Y, Chen L, Wang L, Yin Y, Xu Q, Xiao J, Cong L, Cheng X, Zhang H, Gao D, Xia M, Lian T, Peng G, Zhang X, Jiao B, Hu H, Chen X, Guan Y, Cui R, Huang Q, Xin X, Chen H, Ding Y, Zhang J, Feng T, Cantillon M, Chen K, Cummings JL, Ding J, Geng M, Zhang Z. A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer's dementia. Alzheimers Res Ther. 2021;13:62. doi: 10.1186/s13195-021-00795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Glynn-Servedio BE, Ranola TS. AChE inhibitors and NMDA receptor antagonists in advanced Alzheimer's disease. Consult Pharm. 2017;32:511–518. doi: 10.4140/TCP.n.2017.511. [DOI] [PubMed] [Google Scholar]
- 230.Henley D, Raghavan N, Sperling R, Aisen P, Raman R, Romano G. Preliminary results of a trial of atabecestat in preclinical Alzheimer's disease. N Engl J Med. 2019;380:1483–1485. doi: 10.1056/NEJMc1813435. [DOI] [PubMed] [Google Scholar]
- 231.Penninkilampi R, Brothers HM, Eslick GD. Pharmacological agents targeting γ-secretase increase risk of cancer and cognitive decline in Alzheimer's disease patients: a systematic review and meta-analysis. J Alzheimers Dis. 2016;53:1395–1404. doi: 10.3233/JAD-160275. [DOI] [PubMed] [Google Scholar]
- 232.Lannfelt L, Relkin NR, Siemers ER. Amyloid-ß-directed immunotherapy for Alzheimer's disease. J Intern Med. 2014;275:284–295. doi: 10.1111/joim.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, Vlachouli C, Wilkinson D, Bayer A, Games D, Seubert P, Schenk D, Holmes C. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 234.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.WNL.0000073623.84147.A8. [DOI] [PubMed] [Google Scholar]
- 235.Mahase E. Three FDA advisory panel members resign over approval of Alzheimer's drug. BMJ. 2021;373:n1503. doi: 10.1136/bmj.n1503. [DOI] [PubMed] [Google Scholar]
- 236.Alexander GC, Emerson S, Kesselheim AS. Evaluation of aducanumab for Alzheimer disease: scientific evidence and regulatory review involving efficacy, safety, and futility. JAMA. 2021;325:1717–1718. doi: 10.1001/jama.2021.3854. [DOI] [PubMed] [Google Scholar]
- 237.Knopman DS, Jones DT, Greicius MD. Failure to demonstrate efficacy of aducanumab: an analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimers Dement. 2021;17:696–701. doi: 10.1002/alz.12213. [DOI] [PubMed] [Google Scholar]
- 238.Abi-Saab D, Andjelkovic M, Delmar P, Voyle N, Esau N, Lasser RA. [P1-045]: The effect of 6 months' dosing on the rate of amyloid-related imaging abnormalities (aria) in the marguerite road study. Alzheimers Dement. 2017;13(7 Suppl Pt 5):P252–P253. doi: 10.1016/j.jalz.2017.06.112. [DOI] [Google Scholar]
- 239.DiFrancesco JC, Longoni M, Piazza F. Anti-Aβ autoantibodies in amyloid related imaging abnormalities (ARIA): candidate biomarker for immunotherapy in Alzheimer's disease and cerebral amyloid angiopathy. Front Neurol. 2015;6:207. doi: 10.3389/fneur.2015.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sperling RA, Jack CR, Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P, Carrillo MC, Thies W, Bednar MM, Black RS, Brashear HR, Grundman M, Siemers ER, Feldman HH, Schindler RJ. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, Wilkinson D, Holmes C, Nicoll JA. Consequence of Abeta immunization on the vasculature of human Alzheimer's disease brain. Brain. 2008;131(Pt 12):3299–3310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- 242.Barakos J, Sperling R, Salloway S, Jack C, Gass A, Fiebach JB, Tampieri D, Melançon D, Miaux Y, Rippon G, Black R, Lu Y, Brashear HR, Arrighi HM, Morris KA, Grundman M. MR imaging features of amyloid-related imaging abnormalities. AJNR Am J Neuroradiol. 2013;34:1958–1965. doi: 10.3174/ajnr.A3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Selkoe DJ. Alzheimer disease and aducanumab: adjusting our approach. Nat Rev Neurol. 2019;15:365–366. doi: 10.1038/s41582-019-0205-1. [DOI] [PubMed] [Google Scholar]
- 244.Sakono M, Zako T. Amyloid oligomers: formation and toxicity of Abeta oligomers. FEBS J. 2010;277:1348–1358. doi: 10.1111/j.1742-4658.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- 245.Ackley SF, Zimmerman SC, Brenowitz WD, Tchetgen Tchetgen EJ, Gold AL, Manly JJ, Mayeda ER, Filshtein TJ, Power MC, Elahi FM, Brickman AM, Glymour MM. Effect of reductions in amyloid levels on cognitive change in randomized trials: instrumental variable meta-analysis. BMJ. 2021;372:n156. doi: 10.1136/bmj.n156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Schilling S, Rahfeld JU, Lues I, Lemere CA. Passive Aβ immunotherapy: current achievements and future perspectives. Molecules. 2018;23:1068. doi: 10.3390/molecules23051068. [DOI] [PMC free article] [PubMed] [Google Scholar]