Abstract
Primary liver cancer includes hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA). Incidence of liver cancer has been increasing in recent years, and the 5-year survival is <20%. HCC and CCA are often accompanied with a dense stroma coupled with infiltrated immune cells, which is referred to as the tumor microenvironment. Populations of specific immune cells, such as high density of CD163+ macrophages and low density of CD8+ T cells, are associated with prognosis and survival rates in both HCC and CCA. Immune cells in the tumor microenvironment can be a therapeutic target for liver cancer treatments. Previous studies have introduced immunotherapy using immune checkpoint inhibitors, pulsed dendritic cells, or transduced T cells, to enhance cytotoxicity of immune cells and inhibit tumor growth. This review summarizes current understanding of the roles of immune cells in primary liver cancer covering HCC and CCA.
Liver cancer includes hepatocellular carcinoma (HCC), which is the most common primary liver cancer, and relatively rare biliary cancer, cholangiocarcinoma (CCA).1,2 Liver cancer is the second most common death-causing tumor after pancreatic cancer, and 5-year survival is still <20% because of challenging early diagnosis and limited treatment options, especially for CCA.3 Liver cancer is heterogeneous, and in some cases, liver tumors display characteristics of both HCC and CCA, which is referred to as combined hepatocellular cholangiocarcinoma (cHCC-CCA).4 Pathologic characteristics of cHCC-CCA are similar to those in HCC, but not CCA, although further studies are required to establish diagnostic testing and specific treatment options.5 Incidence of HCC, CCA, and cHCC-CCA is increasing in recent years; therefore, more attention needs to be given to liver cancer.1,2,6 Various risk factors have been identified for liver cancer. Viral infections, such as hepatitis B virus and hepatitis C virus, are a common risk factor for both HCC and CCA.1,2 Accumulating evidence suggests that fatty liver disease is linked to liver cancer, and the increasing incidence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis may promote liver cancer development via lipid metabolism and generation of reactive oxygen species.1,2 Increased reactive oxygen species production causes accumulation of oncogenic alterations in hepatocytes and can induce endoplasmic reticulum stress in hepatocytes, which induces inflammatory responses and proinflammatory cytokine production, leading to HCC development.1
The tumor microenvironment of HCC and CCA is a dense stromal tissues containing extracellular matrix components secreted predominantly from cancer-associated fibroblasts (CAFs) and adjacent hepatic cells.7 CAFs and other cells populating the tumor microenvironment cross talk with tumor cells to promote progression and invasion. For instance, CAFs cross talk with CCA cells and secrete not only extracellular matrix components, but also growth factors such as epidermal growth factor–like growth factor, leading to CCA growth and progression.2 Angiogenesis in endothelial cells influences the liver microenvironment and is mediated by vascular endothelial growth factors, which promote tumor growth and metastasis.1,2 The tumor microenvironment contains various infiltrating immune cells, and accumulation of extracellular matrix components along with these immune cells plays a vital role in the pathophysiology of liver cancer.7 Immune cells, such as tumor-associated macrophages (TAMs), secrete various cytokines and chemokines that promote immune cell infiltration, inflammation, and fibrogenesis, thereby promoting tumor progression and metastasis.8 Regulation of immune cell cytotoxic clearance of tumor cells is an essential cellular event modulating tumor growth and progression; therefore, immune cell functions are primary targets for immunotherapy, such as treatments with immune checkpoint inhibitors.9 This review summarizes the functional roles of immune cells and their therapeutic potentials in HCC, CCA, and cHCC-CCA.
Populations of Immune Cells in CCA Microenvironment
Populations of immune cells, such as TAMs, tumor-associated neutrophils (TANs), dendritic cells (DCs), and T cells, including regulatory T cells (Tregs), are altered in the tumor microenvironment. A previous study using immunohistochemistry to determine populations of specific cell types in the tumor area of CCA patients found a high population of CD66b+ TANs, CD163+ TAMs, and forkhead box P3 (FOXP3)+ Tregs and a low population of CD8+ T cells that were associated with poor survival.10 Another immunohistochemical analysis using 40 CCA tissues identified decreased populations of CD4+, CD8+, and CD31+ cells in metastatic CCA tumors, indicating the association of T-cell activity/population with CCA metastases.11 Infiltration of T cells, natural killer (NK) cells, and DCs is associated with improved prognosis, whereas high numbers of Tregs and myeloid-derived suppressor cells (MDSCs) are linked to poor prognosis in HCC.12 Low T-cell populations and high Treg populations were identified in cHCC-CCA patients, demonstrating that the immune cell population is critical to the pathophysiology of liver cancer.13
Ratios of certain cell populations can be a useful indicator for liver cancer prognosis. A previous study demonstrated that CCA patients with ≥3.0 neutrophil/lymphocyte ratio (NLR) showed a lower 5-year overall survival and recurrence-free survival compared with CCA patients with <3.0 NLR.14 Another study showed that NLR >2.0 was an independent risk factor for postoperative complications for CCA patients.15 NLR is negatively associated with survival in HCC patients and may be a better prognostic tool compared with α-fetoprotein.16 High NLR (>2.75) was associated with poor survival rates in patients with cHCC-CCA.17 Furthermore, high NLR and high platelet/lymphocyte ratio are associated with poor prognosis in patients with CCA.18 These studies suggest the strong association of immune cell populations with liver cancer progression and demonstrate the potentials of NLR and platelet/lymphocyte ratio as prognosis predictors.
The Roles and Therapeutic Potentials of Immune Cells in Liver Cancer
Neutrophils
High NLR is associated with poor prognosis and survival rates in liver cancer, indicating that high infiltration of neutrophils or TANs into the tumor area may play a critical role in tumor progression and metastasis. Immunohistochemical analysis for the neutrophil marker CD66b performed on 245 biliary tract tumors, including 71 CCA patients, found that patients with high tumoral neutrophil infiltration showed poor overall survival compared with patients with low infiltration.19 Neutrophil populations are negatively correlated with CD8+ T-cell populations, and low numbers of CD8+ T cells are associated with poor survival.19 Accumulation of neutrophils promotes inflammatory responses, leading to HCC progression and decreased survival rates.20 Neutrophil infiltration is mediated by various chemokines, and CXCL5 is one of the most well-known chemokines promoting neutrophil infiltration in the tumor area of liver cancer.21 CXCL5 promotes neutrophil infiltration via AKT/extracellular signal-regulated kinase 1/2 signaling, leading to proliferation, migration, and invasion of tumor cells.21 CXCL1–CXC motif chemokine receptor 2 (CXCR2) signaling also promotes neutrophil infiltration into HCC tumor tissues and is associated with overall survival.22 Hepatic cells communicate with one another to regulate cellular functions in liver diseases and cancer.23 CAFs secrete cardiotrophin-like cytokine factor 1, which induces secretion of CXCL6 and transforming growth factor-β (TGF-β) in HCC tumor cells.24 Tumor-derived CXCL6 and TGF-β promote neutrophil infiltration into the tumor microenvironment and enhance cardiotrophin-like cytokine factor 1 production in CAFs, implicating cellular cross talk in HCC progression.24 Tumor-infiltrating monocytes produce CXCL2 and CXCL8, which promote neutrophil recruitment into the HCC microenvironment.25 Monocyte-derived CXCL2 and CXCL8 inhibit apoptosis in neutrophils, resulting in TAN accumulation.25 Monocytes also up-regulate glycolysis, which leads to CXCL2 and CXCL8 production in infiltrating monocytes via 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3–NF-κB signaling.25 Monocytes differentiate into macrophages, and neutrophils are closely associated with macrophage recruitment and functions in liver cancer. TANs in HCC promote the recruitment of macrophages and Tregs via secretion of C-C motif ligand 2 (CCL2) and CCL17.26 In CCA tumor tissues, CD66b+ neutrophils/TANs and CD68+ macrophages/TAMs were adjacent to one another, indicating a close relationship between TANs and TAMs.27 A combination of TAN and TAM infiltration promotes CCA cell proliferation compared with TANs or TAMs alone, which is mediated by activation of STAT3 signaling in CCA cells.27 Neutrophils/TANs can be a therapeutic target to inhibit tumor growth and progression. Inhibition of CXCL1 signaling decreases neutrophil infiltration in the liver and attenuates liver damage and inflammation in a mouse model of steatohepatitis.28 Combination of neutrophil depletion using anti–lymphocyte antigen 6 complex locus G and sorafenib decreased tumor size compared with sorafenib treatment alone.26 Blockade of chemokine signaling and neutrophil infiltration may have therapeutic potential against liver cancer, although further studies are required.
Macrophages
Neutrophil infiltration is correlated with macrophage infiltration in the CCA microenvironment, indicating the pathologic roles of elevated macrophage populations in liver cancer.27 A high population of CD163+ TAMs was identified in CCA tumor tissues and was associated with poor survival rates.10 Macrophages in the liver include liver-resident Kupffer cells and bone marrow–derived macrophages that infiltrate following liver injury, and these macrophages can be classified into M1 and M2 subsets.29 A previous study showed that most TAMs populating the CCA microenvironment were bone marrow–derived macrophages with M2 phenotypes,30 although other studies demonstrated that Kupffer cells were part of the population of TAMs in the HCC microenvironment,31 and high-density CD68+ macrophages were associated with large tumor size and metastasis in HCC patients.32 A subset of M2 macrophages expressing high levels of CCL18 is enriched in advanced HCC patients, which is associated with tumor progression and poor survival.33 In HCC, NADPH oxidase 2–dependent reactive oxygen species generation causes HCC-induced autophagy, leading to down-regulation of NF-κB and up-regulation of M2 macrophage polarization. Immunohistochemical analysis for macrophage subsets in CCA samples found that low CD86+ M1 macrophage populations and high CD206+ M2 macrophage populations were associated with worse overall and recurrence-free survival, whereas data for CD68+ macrophages did not show a significant difference.34 CD68 and F4/80 are commonly used as pan-macrophage markers, but markers to identify specific macrophage subsets, such as inducible nitric oxide synthase, CD80, and CD86 for M1 and CD163, CD204, and CD206 for M2, may be required to elucidate the functional roles of TAMs in liver cancer.30 TAMs could be targeted via inhibition of monocyte recruitment, depletion of TAMs, influencing TAM phenotypes, or neutralizing TAM products, such as IL-6.35 Some candidates, such as anti–glypican-3 antibodies, which inhibit M2 TAM recruitment, have proceeded to ongoing clinical trials.35
Inflammation promotes and exacerbates liver cancer malignancy, and TAMs secrete various cytokines and chemokines that induce inflammatory responses in macrophages and other hepatic cells.36 IL-6 is a major proinflammatory cytokine that mediates responses in various cells. IL-6 expression is elevated in CCA tumor tissues and cell lines, indicating the association between IL-6 and CCA.37 IL-6 promotes hepatocyte proliferation and initiates HCC development via STAT3/extracellular signal-regulated kinase pathways.38 IL-6 activates STAT3 signaling through IL-6 receptor, which leads to neuroblastoma RAS viral oncogene homolog activation, resulting in elevated cell proliferation via the extracellular signal-regulated kinase–dependent pathway.39 IL-1β is a proinflammatory cytokine up-regulated during liver inflammation. M1 macrophages induce programmed death-ligand 1 expression in HCC cells through IL-1β signaling, which mediates immune escape and tumor growth.40 Specific genotypes of IL-1β are associated with HCC prognosis, indicating the pathologic role of IL-1β in liver cancer, although further studies are needed to elucidate the detailed mechanisms.41 Tumor necrosis factor-α (TNF-α) is mainly produced by macrophages and strongly related to inflammation-associated HCC development.42 TNF-α elevates the expression of activation-induced cytidine deaminase, a mutation-generating enzyme, leading to higher mutation numbers in tumor suppressor genes, such as p16 and p53, leading to CCA development.43 Previous studies suggest that specific polymorphisms in the TNF-α gene contribute to the development of CCA,44 indicating the association of TNF-α with liver cancer. CCL2 binds to CCR2 and functions as a proinflammatory cytokine as well as a chemokine promoting macrophage infiltration. CCL2/CCR2 signaling mediates tumor cell invasion and progression via hedgehog signaling in HCC.45 CCL2 is highly expressed in patients with liver cancer, and blocking CCL2/CCR2 signaling can inhibit tumor growth via activating T-cell antitumor responses.46 IL-10 is secreted from M2 macrophages and functions as an immunosuppressive cytokine. CCA cells induce M2 macrophage polarization and IL-10 production via STAT3 signaling, which promotes malignant properties of tumor cells, indicating the critical roles of macrophage-derived cytokines in liver cancer.47
M1 and M2 macrophages are referred to as inflammatory and anti-inflammatory phenotypes, respectively.29 During liver injury, M1 subsets promote hepatic inflammation and immune responses, whereas M2 subsets have anti-inflammatory functions, leading to wound repair.29 Macrophage differentiation into specific phenotypes is promoted by cytokines, such as IL-1β and IL-6 for M1 and IL-4 and IL-10 for M2.29 As mentioned, hepatic inflammation and proinflammatory cytokines, including IL-1β and IL-6, contribute to liver cancer development and progression; however, most identified macrophage subsets in the tumor microenvironment are M2 polarized, and M2-derived IL-10, which is an anti-inflammatory cytokine, functions as an immunosuppressive cytokine, thereby promoting cancer progression.1,2,47 Further studies are required to elucidate the functional changes of macrophages and the balance of proinflammatory and anti-inflammatory cytokines in liver cancer.
Myeloid-Derived Suppressor Cells
MDSCs are cell types originating from myeloid progenitors with immunosuppressive activity. MDSCs inhibit T-cell activation via generation of reactive nitrogen species followed by lymphocyte-specific protein tyrosine kinase nitration. A previous study identified increased MDSC numbers in the blood samples of CCA patients compared with those of healthy individuals, and high MDSC populations were correlated with clinical cancer stage.48 A meta-analysis found that HCC patients had a higher proportion of MDSCs compared with healthy individuals and patients with chronic liver disease, and a high MDSC population correlated with poor overall survival and recurrence-free survival.49 High percentages of MDSCs in HCC patients are correlated with monocyte counts, suggesting that MDSCs may be involved in immune cell infiltration, although detailed mechanisms are still undefined.50 MDSCs are heterogeneous and can be classified into two subsets: monocytic MDSCs, with monocyte-like morphologic characteristics, and polymorphonuclear MDSCs (PMN-MDSCs), showing morphologic characteristics of neutrophils.51 MDSCs can be a therapeutic target by depletion of MDSCs, inhibition of MDSC trafficking, or blocking the immunosuppressive function of MDSCs.51 A recent study has demonstrated that the gut microbiome drives hepatocyte CXCL1 expression, leading to accumulation of CXCR2+ PMN-MDSCs, and blocking CXCL1 expression and depletion of PMN-MDSCs inhibited CCA growth in animal models.52 Drugs targeting immune checkpoints, such as programmed death-1 (PD-1) and programmed death-ligand 1, show anti-cancer effects and can be a promising treatment option for liver cancer.53 TAMs express high levels of programmed death-ligand 1 in CCA, and depletion of both TAMs and PMN-MDSCs using anti–colony-stimulating factor 1 receptor antibody and anti–lymphocyte antigen 6 complex locus G antibody increased the therapeutic effects of anti–PD-1 immunotherapy in CCA mouse models.54 Blocking CXCR2-mediated PMN-MDSC trafficking using anti-CXCR2 monoclonal antibody increases the anti-cancer effects of anti–PD-1 immunotherapy.55 These studies indicate that MDSCs are negatively correlated with liver cancer via immunosuppressive properties; however, a study analyzed MDSC populations in peripheral blood mononuclear cells of patients with various liver cancers, including HCC and CCA, and found that the percentage of MDSCs was significantly increased in HCC patients, but not in CCA patients, compared with healthy donors.56 Further studies are required to elucidate the functional roles of MDSCs in liver cancer.
Dendritic Cells
DCs are phagocytic antigen-presenting cells that activate T cells and induce immune responses. CCA tumor tissues have high populations of activated DCs compared with normal tissues, indicating the association of DCs with liver cancer.57 Although DCs are heterogeneous with various subsets, most DC phenotypes include myeloid DCs and plasmacytoid DCs. T cells are activated within lymph nodes, and mature myeloid DCs are decreased and plasmacytoid DCs are significantly increased in hepatic lymph nodes of HCC patients, suggesting functional differences depending on DC subsets in liver cancer.58 High populations of plasmacytoid DCs in the peritumoral area were associated with tumor size, lymphatic metastasis, and poor prognosis in CCA patients, whereas populations of intratumoral plasmacytoid DCs were not, indicating that the functions of DCs may differ depending on the residing location.59 Because DCs regulate the cytotoxic activity of T cells against tumor cells, DCs can be a therapeutic target for liver cancer. Human DCs were generated from peripheral blood mononuclear cells isolated from healthy donors and were pulsed with cell lysate or total RNAs of human CCA cell lines.60 Lymphocytes co-cultured with pulsed DCs showed higher anti-tumor effects against CCA cell lines compared with those with control DCs, indicating the therapeutic potential of pulsed DCs.60 DCs fused with HCC cells, CD90+ HepG2, showed a greater capacity to activate proliferation of lymphocytes, and cytotoxic T lymphocytes activated by fused DCs showed a specific killing activity against CD90+ HepG2 cells.61 DC vaccine immunotherapy increases cytotoxicity of immune cells, leading to anti-cancer effects, and significantly improves survival rates of HCC patients.62 DC vaccine using DCs pulsed by tumor antigens enhanced anti-tumor immune responses, leading to significant delays in tumor progression in HCC patients, showing promising potentials of immunotherapy using DCs.63 Functions of DCs are dependent on immunosuppressive cytokines, TGF-β and IL-10, and a previous study generated DCs from peripheral blood monocytes with knocked down expression of TGF-β or IL-10 receptors.64 DCs were pulsed with protein lysate of CCA cell lines, and pulsed DCs with TGF-β or IL-10 receptor knockdown increased cytotoxicity of effector T cells against CCA cell lines compared with control DCs.64 CD40/CD40 ligand (CD40L) interaction between antigen-presenting cells and T cells is important for activation and cytotoxicity of T cells, and a recent study showed that low CD40 expression in CCA tissues was associated with poor survival rates of patients.65 Treatments using anti–PD-1 antibody and anti-CD40 agonistic antibody significantly inhibited tumor growth compared with IgG control or anti–PD-1 or anti-CD40 alone in mouse models.65 Anti–PD-1/CD40 treatments increased populations of CD4+ T cells, CD8+ T cells, NK cells, macrophages, and DCs in CCA tissues.65 Depletion of these immune cells abrogated treatment efficacy of anti–PD-1/CD40 in vivo, indicating the critical role of immune cell infiltration in CCA via PD-1/programmed death-ligand 1 and CD40/CD40L signaling.65 A previous study isolated human DCs from buffy coats of healthy donors, pulsed them with CCA cell lysates, and transduced them with adenovirus for human CD40L.66 CD40L expression in DCs induced CD40/CD40L interaction between DCs, leading to formation of cell aggregates, and CD40L-expressing DCs activated co-cultured cytokine-induced killer (CIK) cells, leading to elevated anti-cancer effects against CCA cells compared with non-transduced or mock-transduced DCs.66 These studies suggest that specific immune cells, such as DCs, can be a therapeutic tool for the treatment of liver cancer by enhancing cytotoxicity of T cells against tumor cells.
Natural Killer Cells
NK cells are a type of effector lymphocyte with cytotoxicity. Decreased populations of NK cells were identified in human CCA tumor microenvironment, and increased NK cell populations by anti–PD-1/CD40 treatments inhibited CCA tumor growth in mouse models.65 NK cells produce various cytokines and chemokines, particularly interferon-γ, promoting the defense against tumor surveillance. It is known that NK cell functions are dysregulated in HCC, and low populations of interferon-γ–producing NK cells are correlated with advanced stages of HCC.67 Low numbers of interferon-γ–producing NK cells are also associated with higher recurrence, indicating the protective roles of NK functions in liver cancer.67 CXCL9 is a chemokine that induces infiltration of T cells and NK cells, and a previous study using resected CCA samples found that low CXCL9 expression correlated with low NK cell infiltration and poor overall and recurrence-free survival.68 Knockdown of CXCL9 decreased NK cell infiltration and increased tumor volume compared with control in murine CCA models.68 Because NK cells have cytotoxicity against tumor cells, injection of NK cells might be a therapeutic approach for liver cancer. Tail vein injection of human NK cells, which were isolated and expanded from healthy donors, induced NK cell infiltration in the tumor area and inhibited tumor growth compared with saline injection in xenograft CCA mouse models established by HuCCT-1 transplantation.69 Injection of expanded NK cells decreased tumor growth and improved survival in HCC mouse models.70 Allogenic NK cell immunotherapy also enhanced the clinical effectiveness of irreversible electroporation, leading to better overall survival in HCC patients.71 Activation of NK group 2 member D (NKG2D) signaling by its ligands is critical for NK cell activation and functions. NKG2D ligand expression is positively correlated with NKG2D expression, and low expression of NKG2D ligands is associated with poor overall survival in CCA patients.72 NKG2D activation is also essential for anti-HCC activity, and enhanced NKG2D signaling increased the anti-cancer effects of expanded NK cells in HCC mouse models.70 CIK cells are a heterogeneous subset of effector NK cells with anti-tumor activity. Similar to NK cell immunotherapy, CIK cell immunotherapy can be another treatment option for liver cancer. Immunotherapy with DCs, CIK cells, or the combination of DCs and CIK cells significantly improved overall survival of HCC patients.62 Adjuvant CIK cell immunotherapy for stage I or II HCC patients with curative surgical resection or radiofrequency ablation significantly improved recurrence-free survival.73 A meta-analysis showed that CIK cell immunotherapy decreased 1- and 3-year recurrence rates and improved 5-year overall survival for HCC patients.74 These studies support the promising potential of immunotherapy using NK cells or CIK cells for liver cancer.
Tumor-Infiltrating Lymphocytes
Tumor-infiltrating lymphocytes include CD4+ T cells, CD8+ T cells, Tregs, and B cells. Immunohistochemical analyses identified a low population of CD4+ or CD8+ T cells and B cells, as well as a high population of FOXP3+ Tregs, in CCA and cHCC-CCA tissues, which is associated with poor survival.10,13 B cells, or B lymphocytes, are a type of white blood cells associated with adaptive immunity by mediating antigen-specific Ig production. Numbers of infiltrated B cells in CCA tissues are positively correlated with better overall survival.75 High populations of infiltrating CD20+ B cells, naïve B cells, IgM+ memory B cells, and isotype-switched memory B cells were associated with better survival, showing characteristics as prognostic factors for HCC patients.76 Infiltration of CD20+ or CD79a+ B cells in the tumor microenvironment was positively correlated with prolonged HCC patient survival.77 Tumor-infiltrating T cells and B cells are closely localized in the HCC microenvironment, and B cells promote T-cell activation via interaction, leading to anti-tumor activities, indicating the vital role of B cells in liver cancer.78
Similar to B cells, T cells populating the tumor microenvironment contribute to better prognosis in liver cancer. Low populations of CD3+ T cells and CD8+ T cells are associated with poor survival rates in CCA patients.79 Infiltration of cytotoxic CD4+ and CD8+ T cells is impaired in HCC tumors.80 High densities of CD3+ and CD8+ T cells in the tumor microenvironment are associated with low recurrence rates and prolonged recurrence-free survival in HCC patients.81 High infiltration levels of CD8+PD-1+CD161+ T cells are also correlated with better prognosis in HCC.82 Infiltration of CD8+CXCR5+ T cells promotes IgG production in B cells via IL-21, leading to better prognosis.83 Similar to pulsed DCs that increase the cytotoxic ability of T cells against tumor cells, transduction of tumor antigens into T cells can enhance their cytotoxicity against liver cancer cells. CCA tumors had high expression levels of mucin 1, and T cells generated from peripheral blood mononuclear cells and transduced for the anti–mucin 1 single-chain variable fragment showed elevated cytotoxic functions against human CCA cell lines compared with non-transduced T cells, indicating the therapeutic potential of modified T cells in liver cancer.84 This approach is referred to as chimeric antigen receptor T cell therapy, and a previous study has demonstrated that T cells expressing chimeric α-fetoprotein lysed cancer cells can inhibit tumor growth in HCC mouse models.85 CD133 is highly expressed in various cancer cells, and a previous phase 1 study generated chimeric antigen receptor T cells transduced for CD133, and these cells showed anti-tumor activity against CD133+ cancer cells.86
Elevated populations of FOXP3+ Tregs were identified in CCA and HCC tumors, which correlated with poor prognosis.10,12 A single-cell RNA-sequencing analysis using CCA and adjacent tissues has demonstrated that Tregs have high expression of immunosuppression markers, including cytotoxic T-lymphocyte–associated protein 4 and glucocorticoid-induced TNF receptor-related protein, indicating the pathologic roles of Tregs in liver cancer.87 FOXP3+ Tregs are increased in HCC, contributing to immunosuppression and poor survival, and FOXP3+ Treg/CD4+ T cell ratio acts as a prognostic factor for overall survival.88 HCC tumor cells secrete TGF-β1, and inhibition of TGF-β1 reduces Treg numbers and metastatic nodules in mouse models.89 Inhibition of Treg production/infiltration may be a novel therapeutic approach for liver cancer. A recent study analyzing Treg populations in HCC patients with transarterial chemoembolization using gelatin sponge microparticles found that transarterial chemoembolization using gelatin sponge microparticles significantly decreased Treg populations postoperatively, showing regulatory effects on the anti-cancer immune functions, although further studies are required to elucidate the mechanisms and confirm the efficacy.90
Type 17 helper T (Th17) cells secrete a proinflammatory cytokine IL-17, and the association of IL-17 with CCA was suggested by up-regulated serum IL-17A levels in patients with CCA.91 In HCC, increased numbers of Tregs and Th17 cells were identified, and high percentages of Tregs and Th17 cells were closely correlated to the tumor stage and size.92 Treg/Th17 cell ratio or balance may be important for HCC progression and treatments. A previous study performed thermal ablation treatments in an HCC mouse model and found that thermal ablation decreased frequencies of Th17 cells but increased Tregs.93 Expression levels of IL-17 were decreased, but FOXP3 was increased, in murine HCC tissues.93 Although the roles of Treg/Th17 cell ratio in the pathophysiology of liver cancer are undefined and further studies are required, functions of Tregs and Th17 cells may be closely related, and increased Tregs and Th17 cell levels may be required for HCC growth.
Mast Cells
Mast cells are derived from myeloid progenitors and mediate allergic responses by secretion of histamine. Immunohistochemistry identified elevated mast cell numbers in CCA by detecting mast cell marker receptor tyrosine kinase and the enzymes chymase and tryptase.94 Regulation of mast cell activities and histamine secretion by cromolyn sodium inhibited CCA tumor growth in xenograft mouse models, indicating the pathologic involvement of mast cells in CCA.94 A previous study has demonstrated that histamine receptors H1HR and H2HR are up-regulated in CCA tumor tissues, and serum histamine levels are significantly elevated in CCA patients compared with control individuals.95 Administration of H1HR or H2HR antagonists inhibited tumor growth in CCA xenograft mice in vivo, as well as cell proliferation and invasion of human CCA cells.95 In contrast, the up-regulation of H3HR attenuated the growth of CCA by activating the inositol triphosphate/Ca2+/protein kinase C-α signaling.96 Moreover, the H4 histamine receptor agonist, clobenpropit, suppresses human CCA progression in a Ca2+-dependent manner.97 Prognostic roles of mast cells are also controversial in HCC. A previous study demonstrated that tryptase+ mast cell infiltration significantly decreased overall survival in patients with solid tumors, including HCC, showing the association of mast cells with poor prognosis.98 However, another study showed that high density of mast cells in the HCC microenvironment correlated with a better prognosis in HCC patients with orthotopic liver transplantation. Patients lacking intratumoral mast cells showed larger tumors and higher recurrence rates, indicating the potential roles of mast cells in anti-cancer immunity.99 Further studies are required to elucidate the roles of mast cells in the pathophysiology of liver cancer, which is important to develop novel therapeutic strategies targeting mast cells.
Conclusion and Future Perspectives
Figure 1 summarizes our current understanding of the roles of immune cells in liver cancer, and Table 1 lists markers and associated functions of immune cells in liver cancer. Although studies have mainly focused on the association of immune cells with liver cancer (eg, high NLR associated with poor prognosis), some recent studies elucidated the detailed mechanisms of the pathophysiology of immune cells and cytokines in liver cancer (eg, NKG2D signaling in NK cells). Future studies may develop novel therapeutic approaches targeting these candidate pathways. Immunotherapy, not only immune checkpoint blockade but also allogenic cell vaccination using DCs, NK cells, CIK cells, or T cells, shows promising results in animal models, as well as human patients. Modification of cells for vaccination, such as pulsed DCs and transduced T cells, even enhances the anti-cancer effects of cell vaccine. Although further studies and optimization are required, current studies provide supportive and promising data, and various clinical trials are ongoing for immunotherapy against liver cancer. Some studies show controversial results for the roles of immune cells (eg, mast cells). A possible cause of inconsistent studies may include the location of the cells and their phenotypes. For example, close localization of T cells and B cells in HCC microenvironment is required to initiate T-cell activation and anti-cancer activities,78 meaning that not only cell numbers but also cell localization should be considered to reveal the functional roles of immune cells. Immune cells can infiltrate the tumor or surrounding area or can circulate in the blood stream. Cell functions could differ depending on the location of the cells and relationship with tumor cells. In addition, immune cells are heterogeneous and can have various phenotypes expressing different cytokines and membrane proteins. Although two major subsets of macrophages are M1 and M2, a previous study identified CD11b−CD68+, CD11b+CD68−, and CD11b+CD68+ subsets of Kupffer cells, and CD11b+ subsets are functionally different from CD68+ subsets.100 Immune cells may function differently depending on their phenotypes, and multiple membrane markers may need to be analyzed to identify specific phenotypes and elucidate the functional roles. In conclusion, immune cells play a vital role in the pathophysiology of liver cancer and can be promising therapeutic targets for novel treatment options or tools for immunotherapy. Because immune cells are heterogeneous and functions may vary depending on phenotypes, further studies are required to determine the roles of immune cells with specific phenotypes at specific locations in liver cancer.
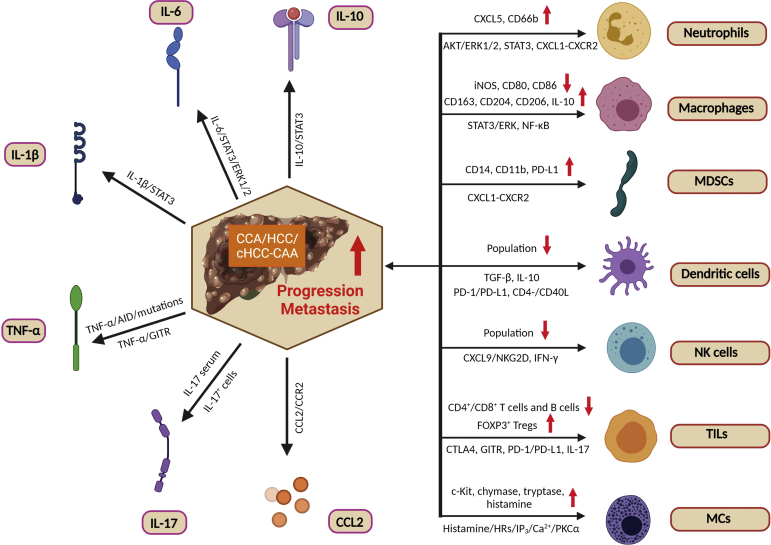
Figure 1.
The interplay between immune cells, inflammatory cytokines, and liver cancer. Although the detailed mechanisms of hepatic carcinogenesis in hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), and combined hepatocellular cholangiocarcinoma (cHCC-CCA) remain elusive, multiple immune cells [neutrophils, macrophages, myeloid-derived suppressor cells (MDSCs), dendritic cells, natural killer (NK) cells, tumor-infiltrating lymphocytes (TILs), and mast cells (MCs)], inflammatory mediators [IL-6, IL-1β, tumor necrosis factor (TNF)-α, C-C motif ligand 2 (CCL2), IL-10, and IL-17], and their combination may be responsible for biliary carcinogenesis and CCA metastases. The up-regulation of inflammatory cytokines in the tumor microenvironment and the association between immune cells and CCA are mediated through a diverse range of signaling pathways. Red arrows indicate up-regulation/down-regulation of cell population and protein expression associated with liver cancer and poor prognosis. However, further studies are needed to elucidate these complicated relationships. This figure was generated with BioRender.com (Toronto, ON, Canada). AID, activation-induced cytidine deaminase; CD40L, CD40 ligand; c-Kit, receptor tyrosine kinase; CTLA4, cytotoxic T-lymphocyte–associated protein 4; CXCR, CXC motif chemokine receptor; ERK, extracellular signal-regulated kinase; FOXP3, forkhead box P3; GITR, glucocorticoid-induced TNF receptor-related protein; HR, histamine receptor; IFN-γ, interferon-γ; iNOS, inducible nitric oxide synthase; IP3, inositol triphosphate; NKG2D, natural killer group 2 member D; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; PKC, protein kinase C; TGF-β, transforming growth factor-β; Treg, regulatory T cell.
Table 1.
Major Immune Cells Associated with Liver Cancer
| Immune cells | Common markers for identification | Association with poor prognosis | Functions associated with liver cancer |
|---|---|---|---|
| Neutrophils/TANs | CD11b, CD66b, Ly6G | High population10 | Inflammatory responses,20 macrophage recruitment26 |
| Macrophages/TAMs | iNOS, CD80, and CD86 for M1 subsets and CD163, CD204, and CD206 for M2 | High population10 | Inflammatory responses and cytokine secretion26,27,35,36 |
| MDSCs | CD33, CD45 | High population48,49 | Monocyte recruitment,50 immunosuppression51 |
| DCs | HLA-DR | May differ depending on activation or localization57,59 | Regulation of T-cell cytotoxic activity60,61 |
| NK cells | CD27, NKG2D | Low population68 | IFN-γ production,67 anti-tumor cytotoxicity69,70 |
| B cells | CD20, CD79a | Low population75 | Promotion of T-cell activation78 |
| T cells | CD3, CD4, CD8 | Low population79,80 | Cytotoxic activity against tumor cells79, 80, 81 |
| Tregs | FOXP3 | High population10 | Immunosuppression87 |
| Th17 cells | CD3+CD4+CD8− | High population92 | Suggested close relationship with Tregs92,93 |
| Mast cells | c-Kit, chymase, tryptase | Controversial98,99 | Histamine secretion95 |
c-Kit, receptor tyrosine kinase; DC, dendritic cell; FOXP3, forkhead box P3; HLA-DR, major histocompatibility complex class II DR; IFN, interferon; iNOS, inducible nitric oxide synthase; Ly6G, lymphocyte antigen 6 complex locus G; MDSC, myeloid-derived suppressor cell; NK, natural killer; NKG2D, NK group 2 member D; TAM, tumor-associated macrophage; TAN, tumor-associated neutrophil; Th17, type 17 helper T; Treg, regulatory T cell.
Footnotes
Supported by the Hickam Endowed Chair, Gastroenterology, Medicine, Indiana University, the Indiana University Health–Indiana University School of Medicine Strategic Research Initiative, the Veterans Affairs Merit awards 5I01BX000574 (G.A.) and 1I01BX003031 (H.F.), Career Development Award-2 1IK2BX005306 (L.K.) from the US Department of Veteran's Affairs, Biomedical Laboratory Research and Development Service, NIH grants DK108959 and DK119421 (H.F.) and DK054811, DK115184, DK076898, DK107310, DK110035, DK062975, and AA028711 (G.A. and S.G.), the PSC Partners Seeking a Cure (G.A.), and Cancer Prevention and Research Institute of Texas (CPRIT) RP210213 (S.C.). Portions of these studies were supported by resources at Richard L. Roudebush VA Medical Center, Indianapolis, IN, and Medical Physiology, Medical Research Building, Temple, TX.
Disclosures: None declared.
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Author Contributions
L.P. designed the study and wrote the first draft; K.K., T.Z., L.C., and L.B. critically reviewed and edited the manuscript; L.K., S.C., S.G., H.F., and G.A. critically reviewed and edited the manuscript, and obtained funding; and K.S. designed the study and finalized the manuscript.
References
- 1.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., Lencioni R., Koike K., Zucman-Rossi J., Finn R.S. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 2.Banales J.M., Marin J.J.G., Lamarca A., Rodrigues P.M., Khan S.A., Roberts L.R., Cardinale V., Carpino G., Andersen J.B., Braconi C., Calvisi D.F., Perugorria M.J., Fabris L., Boulter L., Macias R.I.R., Gaudio E., Alvaro D., Gradilone S.A., Strazzabosco M., Marzioni M., Coulouarn C., Fouassier L., Raggi C., Invernizzi P., Mertens J.C., Moncsek A., Rizvi S., Heimbach J., Koerkamp B.G., Bruix J., Forner A., Bridgewater J., Valle J.W., Gores G.J. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato K., Baiocchi L., Kennedy L., Zhang W., Ekser B., Glaser S., Francis H., Alpini G. Current advances in basic and translational research of cholangiocarcinoma. Cancers (Basel) 2021;13:3307. doi: 10.3390/cancers13133307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunt E., Aishima S., Clavien P.A., Fowler K., Goodman Z., Gores G., Gouw A., Kagen A., Klimstra D., Komuta M., Kondo F., Miksad R., Nakano M., Nakanuma Y., Ng I., Paradis V., Nyun Park Y., Quaglia A., Roncalli M., Roskams T., Sakamoto M., Saxena R., Sempoux C., Sirlin C., Stueck A., Thung S., Tsui W.M.S., Wang X.W., Wee A., Yano H., Yeh M., Zen Y., Zucman-Rossi J., Theise N. cHCC-CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68:113–126. doi: 10.1002/hep.29789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin C.W., Wu T.C., Lin H.Y., Hung C.M., Hsieh P.M., Yeh J.H., Hsiao P., Huang Y.L., Li Y.C., Wang Y.C., Shu C.W., Chen Y.S. Clinical features and outcomes of combined hepatocellular carcinoma and cholangiocarcinoma versus hepatocellular carcinoma versus cholangiocarcinoma after surgical resection: a propensity score matching analysis. BMC Gastroenterol. 2021;21:20. doi: 10.1186/s12876-020-01586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., Li E., Yang H., Wu J., Lu H.C., Yi C., Lei J., Liao W., Wu L. Combined hepatocellular-cholangiocarcinoma: a population level analysis of incidence and mortality trends. World J Surg Oncol. 2019;17:43. doi: 10.1186/s12957-019-1586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabris L., Sato K., Alpini G., Strazzabosco M. The tumor microenvironment in cholangiocarcinoma progression. Hepatology. 2021;73(Suppl 1):75–85. doi: 10.1002/hep.31410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y., Ge W., Zhou J., Gao B., Qian X., Wang W. The role of tumor associated macrophages in hepatocellular carcinoma. J Cancer. 2021;12:1284–1294. doi: 10.7150/jca.51346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangro B., Sarobe P., Hervas-Stubbs S., Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525–543. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitano Y., Okabe H., Yamashita Y.I., Nakagawa S., Saito Y., Umezaki N., Tsukamoto M., Yamao T., Yamamura K., Arima K., Kaida T., Miyata T., Mima K., Imai K., Hashimoto D., Komohara Y., Chikamoto A., Ishiko T., Baba H. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. 2018;118:171–180. doi: 10.1038/bjc.2017.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamma R., Annese T., Ruggieri S., Brunetti O., Longo V., Cascardi E., Mastropasqua M.G., Maiorano E., Silvestris N., Ribatti D. Inflammatory cells infiltrate and angiogenesis in locally advanced and metastatic cholangiocarcinoma. Eur J Clin Invest. 2019;49:e13087. doi: 10.1111/eci.13087. [DOI] [PubMed] [Google Scholar]
- 12.Sachdeva M., Arora S.K. Prognostic role of immune cells in hepatocellular carcinoma. EXCLI J. 2020;19:718–733. doi: 10.17179/excli2020-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng B.H., Ma J.Q., Tian L.Y., Dong L.Q., Song G.H., Pan J.M., Liu Y.M., Yang S.X., Wang X.Y., Zhang X.M., Zhou J., Fan J., Shi J.Y., Gao Q. The distribution of immune cells within combined hepatocellular carcinoma and cholangiocarcinoma predicts clinical outcome. Clin Transl Med. 2020;10:45–56. doi: 10.1002/ctm2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omichi K., Cloyd J.M., Yamashita S., Tzeng C.D., Conrad C., Chun Y.S., Aloia T.A., Vauthey J.N. Neutrophil-to-lymphocyte ratio predicts prognosis after neoadjuvant chemotherapy and resection of intrahepatic cholangiocarcinoma. Surgery. 2017;162:752–765. doi: 10.1016/j.surg.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Kumamoto Y., Kaizu T., Tajima H., Nishizawa N., Ei S., Igarashi K., Watanabe M. Neutrophil-to-lymphocyte ratio as a predictor of postoperative morbidity in patients with distal cholangiocarcinoma. Mol Clin Oncol. 2018;9:362–368. doi: 10.3892/mco.2018.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bannaga A., Arasaradnam R.P. Neutrophil to lymphocyte ratio and albumin bilirubin grade in hepatocellular carcinoma: a systematic review. World J Gastroenterol. 2020;26:5022–5049. doi: 10.3748/wjg.v26.i33.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He C., Mao Y., Lao X., Li S., Lin X. Neutrophil-to-lymphocyte ratio predicts overall survival of patients with combined hepatocellular cholangiocarcinoma. Oncol Lett. 2018;15:4262–4268. doi: 10.3892/ol.2018.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshimoto S., Hishinuma S., Shirakawa H., Tomikawa M., Ozawa I., Ogata Y. Association of preoperative platelet-to-lymphocyte ratio with poor outcome in patients with distal cholangiocarcinoma. Oncology. 2019;96:290–298. doi: 10.1159/000499050. [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Bo X., Suo T., Liu H., Ni X., Shen S., Li M., Xu J., Liu H., Wang Y. Tumor-infiltrating neutrophils predict prognosis and adjuvant chemotherapeutic benefit in patients with biliary cancer. Cancer Sci. 2018;109:2266–2274. doi: 10.1111/cas.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuang D.M., Zhao Q., Wu Y., Peng C., Wang J., Xu Z., Yin X.Y., Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948–955. doi: 10.1016/j.jhep.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S.L., Dai Z., Zhou Z.J., Chen Q., Wang Z., Xiao Y.S., Hu Z.Q., Huang X.Y., Yang G.H., Shi Y.H., Qiu S.J., Fan J., Zhou J. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis. 2014;35:597–605. doi: 10.1093/carcin/bgt397. [DOI] [PubMed] [Google Scholar]
- 22.Li L., Xu L., Yan J., Zhen Z.J., Ji Y., Liu C.Q., Lau W.Y., Zheng L., Xu J. CXCR2-CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:129. doi: 10.1186/s13046-015-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato K., Kennedy L., Liangpunsakul S., Kusumanchi P., Yang Z., Meng F., Glaser S., Francis H., Alpini G. Intercellular communication between hepatic cells in liver diseases. Int J Mol Sci. 2019;20:2180. doi: 10.3390/ijms20092180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song M., He J., Pan Q.Z., Yang J., Zhao J., Zhang Y.J., Huang Y., Tang Y., Wang Q., He J., Gu J., Li Y., Chen S., Zeng J., Zhou Z.Q., Yang C., Han Y., Chen H., Xiang T., Weng D.S., Xia J.C. Cancer-associated fibroblast-mediated cellular crosstalk supports hepatocellular carcinoma progression. Hepatology. 2021;73:1717–1735. doi: 10.1002/hep.31792. [DOI] [PubMed] [Google Scholar]
- 25.Peng Z.P., Jiang Z.Z., Guo H.F., Zhou M.M., Huang Y.F., Ning W.R., Huang J.H., Zheng L., Wu Y. Glycolytic activation of monocytes regulates the accumulation and function of neutrophils in human hepatocellular carcinoma. J Hepatol. 2020;73:906–917. doi: 10.1016/j.jhep.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Zhou S.L., Zhou Z.J., Hu Z.Q., Huang X.W., Wang Z., Chen E.B., Fan J., Cao Y., Dai Z., Zhou J. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150:1646–1658.e17. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z., Wang P., Sun R., Li J., Hu Z., Xin H., Luo C., Zhou J., Fan J., Zhou S. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. J Immunother Cancer. 2021;9:e001946. doi: 10.1136/jitc-2020-001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Z., Xu M.J., Cai Y., Wang W., Jiang J.X., Varga Z.V., Feng D., Pacher P., Kunos G., Torok N.J., Gao B. Neutrophil-hepatic stellate cell interactions promote fibrosis in experimental steatohepatitis. Cell Mol Gastroenterol Hepatol. 2018;5:399–413. doi: 10.1016/j.jcmgh.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato K., Hall C., Glaser S., Francis H., Meng F., Alpini G. Pathogenesis of Kupffer cells in cholestatic liver injury. Am J Pathol. 2016;186:2238–2247. doi: 10.1016/j.ajpath.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou M., Wang C., Lu S., Xu Y., Li Z., Jiang H., Ma Y. Tumor-associated macrophages in cholangiocarcinoma: complex interplay and potential therapeutic target. EBioMedicine. 2021;67:103375. doi: 10.1016/j.ebiom.2021.103375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degroote H., Lefere S., Vandierendonck A., Vanderborght B., Meese T., Van Nieuwerburgh F., Verhelst X., Geerts A., Van Vlierberghe H., Devisscher L. Characterization of the inflammatory microenvironment and hepatic macrophage subsets in experimental hepatocellular carcinoma models. Oncotarget. 2021;12:562–577. doi: 10.18632/oncotarget.27906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding W., Tan Y., Qian Y., Xue W., Wang Y., Jiang P., Xu X. Clinicopathologic and prognostic significance of tumor-associated macrophages in patients with hepatocellular carcinoma: a meta-analysis. PLoS One. 2019;14:e0223971. doi: 10.1371/journal.pone.0223971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Song G., Shi Y., Zhang M., Goswami S., Afridi S., Meng L., Ma J., Chen Y., Lin Y., Zhang J., Liu Y., Jin Z., Yang S., Rao D., Zhang S., Ke A., Wang X., Cao Y., Zhou J., Fan J., Zhang X., Xi R., Gao Q. Global immune characterization of HBV/HCV-related hepatocellular carcinoma identifies macrophage and T-cell subsets associated with disease progression. Cell Discov. 2020;6:90. doi: 10.1038/s41421-020-00214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun D., Luo T., Dong P., Zhang N., Chen J., Zhang S., Liu L., Dong L., Zhang S. CD86+/CD206+ tumor-associated macrophages predict prognosis of patients with intrahepatic cholangiocarcinoma. PeerJ. 2020;8:e8458. doi: 10.7717/peerj.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian Z., Hou X., Liu W., Han Z., Wei L. Macrophages and hepatocellular carcinoma. Cell Biosci. 2019;9:79. doi: 10.1186/s13578-019-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol. 2014;816:401–435. doi: 10.1007/978-3-0348-0837-8_16. [DOI] [PubMed] [Google Scholar]
- 37.Sugawara H., Yasoshima M., Katayanagi K., Kono N., Watanabe Y., Harada K., Nakanuma Y. Relationship between interleukin-6 and proliferation and differentiation in cholangiocarcinoma. Histopathology. 1998;33:145–153. doi: 10.1046/j.1365-2559.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- 38.Maeda S., Kamata H., Luo J.L., Leffert H., Karin M. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 39.O'Hara S.P., Splinter P.L., Trussoni C.E., Gajdos G.B., Lineswala P.N., LaRusso N.F. Cholangiocyte N-Ras protein mediates lipopolysaccharide-induced interleukin 6 secretion and proliferation. J Biol Chem. 2011;286:30352–30360. doi: 10.1074/jbc.M111.269464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zong Z., Zou J., Mao R., Ma C., Li N., Wang J., Wang X., Zhou H., Zhang L., Shi Y. M1 macrophages induce PD-L1 expression in hepatocellular carcinoma cells through IL-1β signaling. Front Immunol. 2019;10:1643. doi: 10.3389/fimmu.2019.01643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamoto K., Ishida C., Ikebuchi Y., Mandai M., Mimura K., Murawaki Y., Yuasa I. The genotypes of IL-1 beta and MMP-3 are associated with the prognosis of HCV-related hepatocellular carcinoma. Intern Med. 2010;49:887–895. doi: 10.2169/internalmedicine.49.3268. [DOI] [PubMed] [Google Scholar]
- 42.Pikarsky E., Porat R.M., Stein I., Abramovitch R., Amit S., Kasem S., Gutkovich-Pyest E., Urieli-Shoval S., Galun E., Ben-Neriah Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 43.Komori J., Marusawa H., Machimoto T., Endo Y., Kinoshita K., Kou T., Haga H., Ikai I., Uemoto S., Chiba T. Activation-induced cytidine deaminase links bile duct inflammation to human cholangiocarcinoma. Hepatology. 2008;47:888–896. doi: 10.1002/hep.22125. [DOI] [PubMed] [Google Scholar]
- 44.Promthet S., Songserm N., Woradet S., Pientong C., Ekalaksananan T., Wiangnon S., Ali A. Opisthorchiasis with proinflammatory cytokines (IL-1β and TNF-α) polymorphisms influence risk of intrahepatic cholangiocarcinoma in Thailand: a nested case-control study. BMC Cancer. 2018;18:846. doi: 10.1186/s12885-018-4751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhuang H., Cao G., Kou C., Liu T. CCL2/CCR2 axis induces hepatocellular carcinoma invasion and epithelial-mesenchymal transition in vitro through activation of the Hedgehog pathway. Oncol Rep. 2018;39:21–30. doi: 10.3892/or.2017.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X., Yao W., Yuan Y., Chen P., Li B., Li J., Chu R., Song H., Xie D., Jiang X., Wang H. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157–167. doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 47.Yuan H., Lin Z., Liu Y., Jiang Y., Liu K., Tu M., Yao N., Qu C., Hong J. Intrahepatic cholangiocarcinoma induced M2-polarized tumor-associated macrophages facilitate tumor growth and invasiveness. Cancer Cell Int. 2020;20:586. doi: 10.1186/s12935-020-01687-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu X.D., Hu J., Wang M., Peng F., Tian R., Guo X.J., Xie Y., Qin R.Y. Circulating myeloid-derived suppressor cells in patients with pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2016;15:99–105. doi: 10.1016/s1499-3872(15)60413-1. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X., Fu X., Li T., Yan H. The prognostic value of myeloid derived suppressor cell level in hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One. 2019;14:e0225327. doi: 10.1371/journal.pone.0225327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li T., Zhang X., Lv Z., Gao L., Yan H. Increased expression of myeloid-derived suppressor cells in patients with HBV-related hepatocellular carcinoma. Biomed Res Int. 2020;2020:6527192. doi: 10.1155/2020/6527192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu L.C., Chang C.J., Hsu C.H. Targeting myeloid-derived suppressor cells in the treatment of hepatocellular carcinoma: current state and future perspectives. J Hepatocell Carcinoma. 2019;6:71–84. doi: 10.2147/JHC.S159693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q., Ma C., Duan Y., Heinrich B., Rosato U., Diggs L.P., Ma L., Roy S., Fu Q., Brown Z.J., Wabitsch S., Thovarai V., Fu J., Feng D., Ruf B., Cui L.L., Subramanyam V., Frank K.M., Wang S., Kleiner D.E., Ritz T., Rupp C., Gao B., Longerich T., Kroemer A., Wang X.W., Ruchirawat M., Korangy F., Schnabl B., Trinchieri G., Greten T.F. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. 2021;11:1248–1267. doi: 10.1158/2159-8290.CD-20-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo X., Shen W. Latest evidence on immunotherapy for cholangiocarcinoma. Oncol Lett. 2020;20:381. doi: 10.3892/ol.2020.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loeuillard E., Yang J., Buckarma E., Wang J., Liu Y., Conboy C., Pavelko K.D., Li Y., O'Brien D., Wang C., Graham R.P., Smoot R.L., Dong H., Ilyas S. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest. 2020;130:5380–5396. doi: 10.1172/JCI137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Highfill S.L., Cui Y., Giles A.J., Smith J.P., Zhang H., Morse E., Kaplan R.N., Mackall C.L. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bayik D., Lauko A.J., Roversi G.A., Serbinowski E., Acevedo-Moreno L.A., Lanigan C., Orujov M., Lo A., Alban T.J., Kim A., Silver D.J., Nagy L.E., Brown J.M., Allende D.S., Aucejo F.N., Lathia J.D. Hepatobiliary malignancies have distinct peripheral myeloid-derived suppressor cell signatures and tumor myeloid cell profiles. Sci Rep. 2020;10:18848. doi: 10.1038/s41598-020-75881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y., Chen S., Li J., Dai W., Qian Y. Immune infiltrating cells in cholangiocarcinoma may become clinical diagnostic markers: based on bioinformatics analysis. World J Surg Oncol. 2021;19:59. doi: 10.1186/s12957-021-02168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang T.J., Vukosavljevic D., Janssen H.L., Binda R.S., Mancham S., Tilanus H.W., Ijzermans J.N., Drexhage H., Kwekkeboom J. Aberrant composition of the dendritic cell population in hepatic lymph nodes of patients with hepatocellular carcinoma. Hum Pathol. 2006;37:332–338. doi: 10.1016/j.humpath.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Hu Z.Q., Zhou Z.J., Luo C.B., Xin H.Y., Li J., Yu S.Y., Zhou S.L. Peritumoral plasmacytoid dendritic cells predict a poor prognosis for intrahepatic cholangiocarcinoma after curative resection. Cancer Cell Int. 2020;20:582. doi: 10.1186/s12935-020-01676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Junking M., Grainok J., Thepmalee C., Wongkham S., Yenchitsomanus P.T. Enhanced cytotoxic activity of effector T-cells against cholangiocarcinoma by dendritic cells pulsed with pooled mRNA. Tumour Biol. 2017;39 doi: 10.1177/1010428317733367. 1010428317733367. [DOI] [PubMed] [Google Scholar]
- 61.Pang Y.B., He J., Cui B.Y., Xu S., Li X.L., Wu M.Y., Liang R., Feng Y., Guo X., Zhang X.H., Luo X.L. A potential antitumor effect of dendritic cells fused with cancer stem cells in hepatocellular carcinoma. Stem Cells Int. 2019;2019:5680327. doi: 10.1155/2019/5680327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao J., Kong F.H., Liu X., Wang X.B. Immunotherapy with dendritic cells and cytokine-induced killer cells for hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2019;25:3649–3663. doi: 10.3748/wjg.v25.i27.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee J.H., Lee Y., Lee M., Heo M.K., Song J.S., Kim K.H., Lee H., Yi N.J., Lee K.W., Suh K.S., Bae Y.S., Kim Y.J. A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Br J Cancer. 2015;113:1666–1676. doi: 10.1038/bjc.2015.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thepmalee C., Panya A., Sujjitjoon J., Sawasdee N., Poungvarin N., Junking M., Yenchitsomanus P.T. Suppression of TGF-β and IL-10 receptors on self-differentiated dendritic cells by short-hairpin RNAs enhanced activation of effector T-cells against cholangiocarcinoma cells. Hum Vaccin Immunother. 2020;16:2318–2327. doi: 10.1080/21645515.2019.1701913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diggs L.P., Ruf B., Ma C., Heinrich B., Cui L., Zhang Q., McVey J.C., Wabitsch S., Heinrich S., Rosato U., Lai W., Subramanyam V., Longerich T., Loosen S.H., Luedde T., Neumann U.P., Desar S., Kleiner D., Gores G., Wang X.W., Greten T.F. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J Hepatol. 2021;74:1145–1154. doi: 10.1016/j.jhep.2020.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sadeghlar F., Vogt A., Mohr R.U., Mahn R., van Beekum K., Kornek M., Weismuller T.J., Branchi V., Matthaei H., Toma M., Schmidt-Wolf I.G.H., Kalff J.C., Strassburg C.P., Gonzalez-Carmona M.A. Induction of cytotoxic effector cells towards cholangiocellular, pancreatic, and colorectal tumor cells by activation of the immune checkpoint CD40/CD40L on dendritic cells. Cancer Immunol Immunother. 2021;70:1451–1464. doi: 10.1007/s00262-020-02746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee H.A., Goh H.G., Lee Y.S., Jung Y.K., Kim J.H., Yim H.J., Lee M.G., An H., Jeen Y.T., Yeon J.E., Byun K.S., Seo Y.S. Natural killer cell activity is a risk factor for the recurrence risk after curative treatment of hepatocellular carcinoma. BMC Gastroenterol. 2021;21:258. doi: 10.1186/s12876-021-01833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fukuda Y., Asaoka T., Eguchi H., Yokota Y., Kubo M., Kinoshita M., Urakawa S., Iwagami Y., Tomimaru Y., Akita H., Noda T., Gotoh K., Kobayashi S., Hirata M., Wada H., Mori M., Doki Y. Endogenous CXCL9 affects prognosis by regulating tumor-infiltrating natural killer cells in intrahepatic cholangiocarcinoma. Cancer Sci. 2020;111:323–333. doi: 10.1111/cas.14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jung I.H., Kim D.H., Yoo D.K., Baek S.Y., Jeong S.H., Jung D.E., Park S.W., Chung Y.Y. In vivo study of natural killer (NK) cell cytotoxicity against cholangiocarcinoma in a nude mouse model. In Vivo. 2018;32:771–781. doi: 10.21873/invivo.112307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamiya T., Chang Y.H., Campana D. Expanded and activated natural killer cells for immunotherapy of hepatocellular carcinoma. Cancer Immunol Res. 2016;4:574–581. doi: 10.1158/2326-6066.CIR-15-0229. [DOI] [PubMed] [Google Scholar]
- 71.Alnaggar M., Lin M., Mesmar A., Liang S., Qaid A., Xu K., Chen J., Niu L., Yin Z. Allogenic natural killer cell immunotherapy combined with irreversible electroporation for stage IV hepatocellular carcinoma: survival outcome. Cell Physiol Biochem. 2018;48:1882–1893. doi: 10.1159/000492509. [DOI] [PubMed] [Google Scholar]
- 72.Tsukagoshi M., Wada S., Yokobori T., Altan B., Ishii N., Watanabe A., Kubo N., Saito F., Araki K., Suzuki H., Hosouchi Y., Kuwano H. Overexpression of natural killer group 2 member D ligands predicts favorable prognosis in cholangiocarcinoma. Cancer Sci. 2016;107:116–122. doi: 10.1111/cas.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoon J.S., Song B.G., Lee J.H., Lee H.Y., Kim S.W., Chang Y., Lee Y.B., Cho E.J., Yu S.J., Sinn D.H., Kim Y.J., Lee J.H., Yoon J.H. Adjuvant cytokine-induced killer cell immunotherapy for hepatocellular carcinoma: a propensity score-matched analysis of real-world data. BMC Cancer. 2019;19:523. doi: 10.1186/s12885-019-5740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J., Shen T., Wang Q., Zhang T., Li L., Wang Y., Fang Y. The long-term efficacy of cytokine-induced killer cellular therapy for hepatocellular carcinoma: a meta-analysis. Immunotherapy. 2019;11:1325–1335. doi: 10.2217/imt-2019-0079. [DOI] [PubMed] [Google Scholar]
- 75.Chen Z., Yu M., Yan J., Guo L., Zhang B., Liu S., Lei J., Zhang W., Zhou B., Gao J., Yang Z., Li X., Zhou J., Fan J., Ye Q., Li H., Xu Y., Xiao Y. PNOC expressed by B cells in cholangiocarcinoma was survival related and LAIR2 could be a T cell exhaustion biomarker in tumor microenvironment: characterization of immune microenvironment combining single-cell and bulk sequencing technology. Front Immunol. 2021;12:647209. doi: 10.3389/fimmu.2021.647209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z., Ma L., Goswami S., Ma J., Zheng B., Duan M., Liu L., Zhang L., Shi J., Dong L., Sun Y., Tian L., Gao Q., Zhang X. Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma. Oncoimmunology. 2019;8:e1571388. doi: 10.1080/2162402X.2019.1571388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brunner S.M., Itzel T., Rubner C., Kesselring R., Griesshammer E., Evert M., Teufel A., Schlitt H.J., Fichtner-Feigl S. Tumor-infiltrating B cells producing antitumor active immunoglobulins in resected HCC prolong patient survival. Oncotarget. 2017;8:71002–71011. doi: 10.18632/oncotarget.20238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garnelo M., Tan A., Her Z., Yeong J., Lim C.J., Chen J., Lim K.H., Weber A., Chow P., Chung A., Ooi L.L., Toh H.C., Heikenwalder M., Ng I.O., Nardin A., Chen Q., Abastado J.P., Chew V. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66:342–351. doi: 10.1136/gutjnl-2015-310814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vigano L., Soldani C., Franceschini B., Cimino M., Lleo A., Donadon M., Roncalli M., Aghemo A., Di Tommaso L., Torzilli G. Tumor-infiltrating lymphocytes and macrophages in intrahepatic cholangiocellular carcinoma: impact on prognosis after complete surgery. J Gastrointest Surg. 2019;23:2216–2224. doi: 10.1007/s11605-019-04111-5. [DOI] [PubMed] [Google Scholar]
- 80.Chaoul N., Mancarella S., Lupo L., Giannelli G., Dituri F. Impaired anti-tumor T cell response in hepatocellular carcinoma. Cancers (Basel) 2020;12:627. doi: 10.3390/cancers12030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gabrielson A., Wu Y., Wang H., Jiang J., Kallakury B., Gatalica Z., Reddy S., Kleiner D., Fishbein T., Johnson L., Island E., Satoskar R., Banovac F., Jha R., Kachhela J., Feng P., Zhang T., Tesfaye A., Prins P., Loffredo C., Marshall J., Weiner L., Atkins M., He A.R. Intratumoral CD3 and CD8 T-cell densities associated with relapse-free survival in HCC. Cancer Immunol Res. 2016;4:419–430. doi: 10.1158/2326-6066.CIR-15-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Z., Zheng B., Qiu X., Wu R., Wu T., Yang S., Zhu Y., Wu X., Wang S., Gu Z., Shen S., Wu M., Wang H., Chen L. The identification and functional analysis of CD8+PD-1+CD161+ T cells in hepatocellular carcinoma. NPJ Precis Oncol. 2020;4:28. doi: 10.1038/s41698-020-00133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye L., Li Y., Tang H., Liu W., Chen Y., Dai T., Liang R., Shi M., Yi S., Chen G., Yang Y. CD8+CXCR5+T cells infiltrating hepatocellular carcinomas are activated and predictive of a better prognosis. Aging (Albany NY) 2019;11:8879–8891. doi: 10.18632/aging.102308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Supimon K., Sangsuwannukul T., Sujjitjoon J., Phanthaphol N., Chieochansin T., Poungvarin N., Wongkham S., Junking M., Yenchitsomanus P.T. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci Rep. 2021;11:6276. doi: 10.1038/s41598-021-85747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu H., Xu Y., Xiang J., Long L., Green S., Yang Z., Zimdahl B., Lu J., Cheng N., Horan L.H., Liu B., Yan S., Wang P., Diaz J., Jin L., Nakano Y., Morales J.F., Zhang P., Liu L.X., Staley B.K., Priceman S.J., Brown C.E., Forman S.J., Chan V.W., Liu C. Targeting alpha-fetoprotein (AFP)-MHC complex with CAR T-cell therapy for liver cancer. Clin Cancer Res. 2017;23:478–488. doi: 10.1158/1078-0432.CCR-16-1203. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y., Chen M., Wu Z., Tong C., Dai H., Guo Y., Liu Y., Huang J., Lv H., Luo C., Feng K.C., Yang Q.M., Li X.L., Han W. CD133-directed CAR T cells for advanced metastasis malignancies: a phase I trial. Oncoimmunology. 2018;7:e1440169. doi: 10.1080/2162402X.2018.1440169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang M., Yang H., Wan L., Wang Z., Wang H., Ge C., Liu Y., Hao Y., Zhang D., Shi G., Gong Y., Ni Y., Wang C., Zhang Y., Xi J., Wang S., Shi L., Zhang L., Yue W., Pei X., Liu B., Yan X. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol. 2020;73:1118–1130. doi: 10.1016/j.jhep.2020.05.039. [DOI] [PubMed] [Google Scholar]
- 88.Tu J.F., Ding Y.H., Ying X.H., Wu F.Z., Zhou X.M., Zhang D.K., Zou H., Ji J.S. Regulatory T cells, especially ICOS+ FOXP3+ regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci Rep. 2016;6:35056. doi: 10.1038/srep35056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y., Liu T., Tang W., Deng B., Chen Y., Zhu J., Shen X. Hepatocellular carcinoma cells induce regulatory T cells and lead to poor prognosis via production of transforming growth factor-β1. Cell Physiol Biochem. 2016;38:306–318. doi: 10.1159/000438631. [DOI] [PubMed] [Google Scholar]
- 90.Ren Z., Yue Y., Zhang Y., Dong J., Liu Y., Yang X., Lin X., Zhao X., Wei Z., Zheng Y., Wang T. Changes in the peripheral blood Treg cell proportion in hepatocellular carcinoma patients after transarterial chemoembolization with microparticles. Front Immunol. 2021;12:624789. doi: 10.3389/fimmu.2021.624789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Su S.B., Zhang J.F., Huang F.F., Cen Y., Jiang H.X. Large numbers of interleukins-22- and -17A-producing T helper cells in cholangiocarcinoma related to liver fluke infection. Microbiol Immunol. 2017;61:345–354. doi: 10.1111/1348-0421.12500. [DOI] [PubMed] [Google Scholar]
- 92.Lan Y.T., Fan X.P., Fan Y.C., Zhao J., Wang K. Change in the Treg/Th17 cell imbalance in hepatocellular carcinoma patients and its clinical value. Medicine (Baltimore) 2017;96:e7704. doi: 10.1097/MD.0000000000007704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang S., Qu N., Men Y., Liu Z. Effects of thermal ablation on Treg/Th17 in hepatocellular carcinoma of mice. Eur J Inflamm. 2019;17:1–9. [Google Scholar]
- 94.Johnson C., Huynh V., Hargrove L., Kennedy L., Graf-Eaton A., Owens J., Trzeciakowski J.P., Hodges K., DeMorrow S., Han Y., Wong L., Alpini G., Francis H. Inhibition of mast cell-derived histamine decreases human cholangiocarcinoma growth and differentiation via c-Kit/stem cell factor-dependent signaling. Am J Pathol. 2016;186:123–133. doi: 10.1016/j.ajpath.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kennedy L., Hargrove L., Demieville J., Karstens W., Jones H., DeMorrow S., Meng F., Invernizzi P., Bernuzzi F., Alpini G., Smith S., Akers A., Meadows V., Francis H. Blocking H1/H2 histamine receptors inhibits damage/fibrosis in Mdr2-/- mice and human cholangiocarcinoma tumorigenesis. Hepatology. 2018;68:1042–1056. doi: 10.1002/hep.29898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Francis H., Onori P., Gaudio E., Franchitto A., DeMorrow S., Venter J., Kopriva S., Carpino G., Mancinelli R., White M., Meng F., Vetuschi A., Sferra R., Alpini G. H3 histamine receptor-mediated activation of protein kinase Cα inhibits the growth of cholangiocarcinoma in vitro and in vivo. Mol Cancer Res. 2009;7:1704–1713. doi: 10.1158/1541-7786.MCR-09-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meng F., Han Y., Staloch D., Francis T., Stokes A., Francis H. The H4 histamine receptor agonist, clobenpropit, suppresses human cholangiocarcinoma progression by disruption of epithelial mesenchymal transition and tumor metastasis. Hepatology. 2011;54:1718–1728. doi: 10.1002/hep.24573. [DOI] [PubMed] [Google Scholar]
- 98.Hu G., Wang S., Cheng P. Tumor-infiltrating tryptase+ mast cells predict unfavorable clinical outcome in solid tumors. Int J Cancer. 2018;142:813–821. doi: 10.1002/ijc.31099. [DOI] [PubMed] [Google Scholar]
- 99.Rohr-Udilova N., Tsuchiya K., Timelthaler G., Salzmann M., Meischl T., Woran K., Stift J., Herac M., Schulte-Hermann R., Peck-Radosavljevic M., Sieghart W., Eferl R., Jensen-Jarolim E., Trauner M., Pinter M. Morphometric analysis of mast cells in tumor predicts recurrence of hepatocellular carcinoma after liver transplantation. Hepatol Commun. 2021;5:1939–1952. doi: 10.1002/hep4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kinoshita M., Uchida T., Sato A., Nakashima M., Nakashima H., Shono S., Habu Y., Miyazaki H., Hiroi S., Seki S. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J Hepatol. 2010;53:903–910. doi: 10.1016/j.jhep.2010.04.037. [DOI] [PubMed] [Google Scholar]