Abstract
Idiopathic pulmonary fibrosis (IPF) is a dramatic disease without cure. The US Food and Drug Administration–approved drugs, pirfenidone and nintedanib, only slow disease progression. The clinical investigation of novel therapeutic approaches for IPF is an unmet clinical need. Nucleotide-binding oligomerization domain-like receptor or NOD-like receptors are pattern recognition receptors capable of binding a large variety of stress factors. NLR family pyrin domain-containing protein 3 (NLRP3), once activated, promotes IL-1β, IL-18 production, and innate immune responses. Multiple reports indicate that the inflammasome NLRP3 is overactivated in IPF patients, leading to increased production of class I IL and collagens. Similarly, data from animal models of pulmonary fibrosis confirm the role of NLRP3 in the development of chronic lung injury and pulmonary fibrosis. This report provides a review of the evidence of NLRP3 activation in IPF and of NLRP3 inhibition in different animal models of fibrosis, and highlights the recent advances in direct and indirect NLRP3 inhibitors.
More than 40% of all deaths recorded globally can be attributed to increased accumulation of collagen in organs and tissues, culminating in fibrotic disease.1 Idiopathic pulmonary fibrosis (IPF), the most common interstitial lung disease, has increasing trends and poor outcomes, with 3 to 5 years of life expectancy after diagnosis.2,3 Pirfenidone and nintedanib, the only US Food and Drug Administration–approved drugs, improve IPF patients' quality of life and slow disease progression, but a cure is still missing.4,5 Although the exact cause of IPF is unknown, most theories suggest dysfunction in the wound-healing response,6 genetic predisposition,7 endoplasmic reticulum (ER) stress,8 and/or exaggerated immune responses with fibroblast activation.9
Multiple reports underline the role of the inflammasomes in IPF, suggesting that their activity may be a driving factor for the development of fibrosis and a potential target for new therapies. Inflammasomes are pattern recognition receptors (PRRs)—nucleotide-binding oligomerization domain-like receptors or NOD-like receptors (NLRs)—capable of binding a large number of molecular motifs of microorganisms, as well as alarm signals produced by immune cells (pathogen-associated molecular patterns and damage-associated molecular patterns).10 The discovery of the inflammasome in early 2000 also revealed its essential role in augmenting the innate immune response in toll-like receptor–mediated signaling.11 The most studied of the inflammasome family, NLR family pyrin domain-containing protein 3 (NLRP3), is encoded by the gene NLRP3 on the long arm of chromosome 1.12 NLRP3 was initially found in macrophages,13 but later reports detected high levels in multiple other cells, including epithelial and endothelial cells.14,15 It participates in the innate immune response via the secretion of IL-1 family cytokines. Under basal conditions, NLRP3 resides in the mitochondria and endoplasmic reticulum, but during stress it migrates to perinuclear regions.16
The inflammasome differs from other PRRs as it displays the rare feature of recognizing a wide range of unrelated bacterial, viral, and fungal pathogen-associated molecular patterns as well as endogenous damage-associated molecular patterns in sterile inflammation or following exposure to environmental irritants. Indeed, NLRP3 is activated by multiple stimuli, including mitochondrial, lysosomal, ER, and oxidative stresses, as well as ion flux and DNA damage.17
Various animal models have underlined NLRP3’s role in the fibrotic process,18, 19, 20 and clinical investigations have identified the NLRP3/IL-1 pathway in chronic lung diseases.21,22 These proinflammatory cytokines participate in epithelial-to-mesenchymal transformation and in the production and deposition of collagen.23
Thus, the investigation of direct and indirect NLRP3 inhibitors for lung disease represents an unmet clinical need. This review highlights NLRP3 involvement in lung fibrosis, collagen deposition, and mesenchymal transformation, and summarize the therapeutic advances in the development of NLRP3 inhibitors.
NLRP3 Structure and Assembly
NLRP3 is a PRR located in the cytoplasm and is able to sense microbes and other danger signals. NLRP3 exists as inactive monomers expressed in the cytoplasm or on the surface of mitochondria and ER, as aggregated, active oligomers. The exact mechanism that leads to NLRP3 assembly remains unclear. However, recent reports have provided a deeper understanding of the activation process.
The active inflammasome consists of a detector (NLRP3), an adaptor (ASC; part of apoptosis-associated speck-like protein), and an effector, caspase 1.
The detector NLRP3 is a pyrin-like protein, with an amino-terminal pyrin domain (PYD), a central nucleotide binding site domain, and a carboxy-terminal leucin-rich repeat motif. The adaptor ASC and its caspase activation and recruitment domain moderate the interaction of NLRP3 with other proteins, and the formation of large complexes, among which is its crucial binding with the amino-terminal domain of caspases.24 Caspase 1, or IL-1 converting enzyme—the effector—is a highly conserved enzyme that cleaves precursors of IL-1β, IL-18, and gasdermin D into their activated forms.25
The domains of NLRP3 possess self-regulated activity that coordinates protein oligomerization and structural rearrangements required for its functionality. The central part on NLRP3 is divided into the nucleotide binding site and NACHT [neuronal apoptosis inhibitor proteins (NAIP), class II transactivator (CIITA), HET-E, and topoisomerase 1 (TP-1)] domains, the latter exerting both apoptotic and antiapoptotic ATPase activity.26 The third domain, leucin-rich repeat, senses danger signals and is similarly expressed by multiple other innate immune receptors. The ATPase activity of the central domain promotes its assembly, whereas the leucin-rich repeat is capable of folding toward the central domain, interfering with this process.27
During stress, the central domain (NACHT) mediates the oligomerization of multiple NLRP3 proteins. ASC is recruited, and after its link with the PYD domains, forms helical ASC filaments. Finally, ASC filaments unite into a singular macromolecule capable of recruiting inactive caspase-1, which in this bound conformation self-cleaves, releases p20-p10 fragments, and acquires its active enzymatic form.28
NLRP3 Regulation
The assembly and activation of NLRP3 is highly regulated. Indeed, NLRP3 activation requires a two-step process: first priming and later activation. Priming is necessary for two main functions; the first one is to increase the expression of its components, NLRP3, caspase-1, and pro–IL-1β. The transcription of these proteins is up-regulated by toll-like receptors, by NOD2 receptors, or through cytokines tumor necrosis factor-α and IL-1β that promote NF-κB activation and gene transcription.29 The second is for NLRP3 post-translational modifications, which maintain NLRP3 into an autosuppressed and inactive, but signal competent, state. These post-translational modifications include ubiquitylation, phosphorylation, and sumoylation. While priming guarantees the proper cellular microenvironment to sustain NLRP3 function, activation occurs through the recognition of molecular patterns. As mentioned, an incredibly large number of pathogen-associated molecular patterns, damage-associated molecular patterns, and chemical signals can promote NLRP3 activation. As a result, NLRP3 is involved in the response to insults and in the cellular stress response. However, the mechanism by which the NLRP3 inflammasome senses stress is not completely understood. In addition, because many overlapping and interrelated pathways culminate in NLRP3 activation, a unique consensus is missing. Indeed, several cellular processes lead to NLRP3 activation, such as ionic imbalances (K+ efflux, Cl− efflux, and Ca2+ flux), lysosomal disruption, mitochondrial dysfunction, metabolic changes, endoplasmic reticulum stress, and others.30 It is clear then, that in contrast to most PRRs, which promote transcription of inflammatory mediators, NLRP3 participates in the post-transcriptional activation of the IL-1 class family, providing a last checkpoint before the inflammatory response.
NLRP3 in the Lung
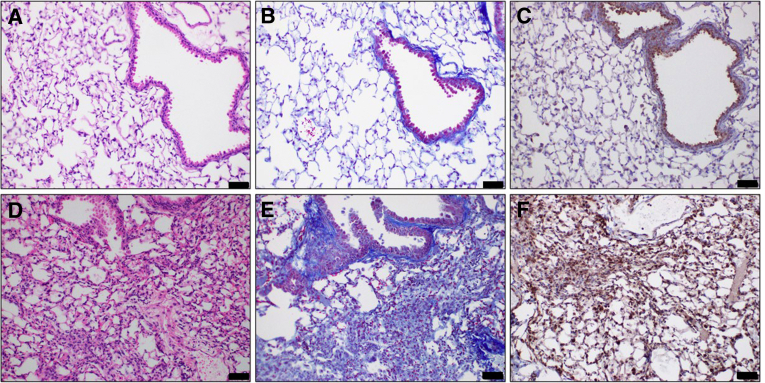
Lungs are constantly exposed to inhaled microbes, particulates, and host-derived danger signals, thus requiring the presence of an active, but regulated, innate immune response, able to guarantee protection from disease. The lung contains various families of innate immune PPRs, like toll-like receptor, RIG-I–like receptors, and NOD-like receptors (NLRs), that mediate the initial signaling and regulation of inflammatory mediators.31 Alveolar macrophages, as well as alveolar epithelial and endothelial cells, express high levels of NLRP3 mRNA.32, 33, 34 Thus, the whole alveolocapillary structure shares a common defense mechanism that is highly conserved against multiple infective and noninfective stimuli (Figure 1).
Figure 1.
Inflammasome NLR family pyrin domain-containing protein 3 (NLRP3) expression in the lungs. Sections from mouse lungs 30 days after intratracheal instillation of 0.1N hydrochloric acid. Hematoxylin and eosin staining, depicting increased alveolar thickness, white blood cell infiltration, and hyaline membranes in HCl-instilled animals (D) compared with controls (A). HCl-instilled animals, stained for Masson trichrome, display loss of alveolar architecture and increased deposition of collagen (E) compared with controls (B). Immunohistochemistry for NLRP3: under normal conditions, NLRP3 is expressed in the bronchial and alveolar surfaces (C), but in fibrosis, its expression is increased dramatically in the parenchyma and the interstitial space (F). Immunohistochemistry for NLRP3 was performed with NLRP3 antibody (Novusbio, Denver, CO; catalog number NBP2-12446). All animal studies have been approved by the Old Dominion University Institutional Animal Care and Use Committee under protocol 19-014. Scale bars = 50 μm (A–F).
NLRP3 is activated in animal models of silica/asbestos exposure; mice lacking either NLRP3 or ASC do not display increased IL-1β, inflammatory cells in the bronchoalveolar lavage fluid (BALF), or collagen deposition.35,36 Thus, it is hypothesized that a dysregulated activation of NLRP3 may affect the progression of chronic pulmonary diseases, such as IPF, asthma, and chronic obstructive pulmonary disease.37 This hypothesis is supported by the high levels of NLRP3 found in clinical samples, evidence from animal studies in lung fibrosis, as well as results from the use of NLRP3 inhibitors in in vitro and in vivo studies.
IL-1 Family of Cytokines in IPF
The IL-1 superfamily is a group of 11 cytokines that play a central role in the regulation of immune and inflammatory processes in response to a large group of stimuli. These cytokines are crucial in the innate immune response, induction of hyperpyrexia, expression of adhesion molecules, as well as in mediating hyperalgesia, vasodilation, and hypotension.
Nine of these cytokines occur in a single cluster of chromosome 2, probably originating from gene duplications of an IL-1β ligand of ancestral origin (IL-1α, IL-1β, IL-36α, IL-36β, IL-36γ, IL-36Ra, IL37, IL-38, and IL-1Ra).38 IL-1α, Il-1β, and IL-1Ra (the competing receptor antagonist) bind the IL-1R receptor, whose signaling is mediated initially by toll- and IL-1R–like domains on adaptor proteins, and later by phosphorylation of myeloid differentiation primary response gene 88 and IL-1 receptor-activated protein kinases.39 Alternatively, IL-18 and IL-33 may originate from another genetic locus, but share high structural similarity with typical IL-1 isoforms, are internalized by IL-1 receptor accessory protein (coreceptor or IL-1R), and, thus, have been classified as members of the same family.
Myeloid differentiation primary response gene 88 and IL-1 receptor-activated protein kinases interact with tumor necrosis factor-α receptor-associated factor 6, transforming growth factor-β–activated protein kinase, and mitogen activated protein kinase 3 (MAPK3) and lead to the transcription of NF-κB, activator protein-1 (AP-1), c-Jun N-terminal kinase, and p38.40
IL-18 and IL-18Ra expression is increased in patients with IPF, and experimental administration of IL-18 in mice augments the progressive deposition of extracellular matrix and the development of fibrosis, underlining the important role of IL-18 in lung pathology.23 Also, IL-18 triggers fibroblast senescence, and its secretory phenotype (senescence-associated secretory phenotype) is associated with worse outcomes in lung fibrosis.41 Similarly, levels of IL-1 cytokines are chronically overexpressed in patients with chronic obstructive pulmonary disease, asthma, and IPF.42,43 In the lung, IL-1β participates in inflammation, white blood cell migration, disruption of elastin fibers, and collagen deposition.44 These effects are in part mediated by IL-17A pathways.45,46
However, some evidence indicates that IL-1β can exert both synergistic and antagonist effects on transforming growth factor-β function, epithelial-to-mesenchymal transformation, and collagen deposition.47,48 Lung fibroblasts, but not dermal fibroblasts, display a reduced transforming growth factor-β–mediated production of collagen when treated with IL-1β and IL-1, produced by the airway epithelium, which similarly modulates fibroblast activity.49 It is possible that IL-1 family cytokines—products of NLRP3 activation—not only participate as mediators of inflammation, but also act as regulators of their intrinsic network, with cell, and organ, specificity. This mechanism could promote balanced inflammatory responses to a wide range of stressors able to activate NLRP3.
NLRP3 in IPF
The development of an animal model of a disease with idiopathic (ie, unknown) etiology is at best challenging. Intratracheal instillation of bleomycin (BLM) is the most commonly used animal model of IPF, which, however, remains suboptimal because of the unknown origin of IPF.50 BLM induces DNA damage via oxidative stress, and inflammation, repair, and fibrotic responses are mediated by IL-1β and the IL-R1/myeloid differentiation primary response gene 88 signaling pathway.51 NLRP3−/− mice display much lower levels of NLRP3 activation, IL-1β production, and fibrosis compared with wild-type animals.52 The activation of the inflammasome NLRP3 in IPF has been ascribed to multiple interrelated as well as distinct signaling mechanisms.
Extracellular ATP
Purine nucleotides and nucleosides are critical structures for eukaryotic systems. ATP represents the fundamental energy exchange utilized by cells, but when released in the extracellular space in response to cell injury, acts as damage-associated molecular patterns and, depending on metabolic processing and receptor binding, exerts proinflammatory or anti-inflammatory effects.
The BALF of IPF patients displays increased levels of extracellular ATP and UDP, which increases during disease flare-ups.53,54 The expression of the purinergic receptor, P2Y2, is increased on macrophages and neutrophils, whereas alveolar epithelial cells display mostly P2Y6 receptors.55 Accordingly, high levels of extracellular ATP exist in BLM-instilled fibrotic mice,54 and genetic exclusion of either P2Y2 or P2Y6 genes resulted in mice expressing lower inflammation and white blood cell recruitment. In IPF, extracellular ATP is probably released as a result of continuous micro-injuries, airway remodeling, and disorganized and excessive angiogenesis with copious extracellular matrix deposition and mesenchymal transition. Extracellular ATPs are a well-known mechanism of inflammasome activation, and BALF cells of IPF patients display higher sensitivity to NLRP3 assembly and IL-1β production, on ATP challenge than BALF cells from healthy donors.22 However, more data are needed to understand the contribution of extracellular ATP–mediated NLRP3 activation in IPF.
Endoplasmic Reticulum Stress
The ER is a specialized organelle that hosts proteins during their folding and quality control phases, and guarantees proteomic homeostasis (ie, proteostasis). Disruption of protein processing results in ER stress, which is followed by the unfolded protein response, a cell mechanism that aims to restore proteostasis or, if ER stress is prolonged or irreversible, mediate cell death.8 Because of the dysregulated large amounts of ECM proteins and collagens deposited during fibrosis, signs of ER stress are a common finding in IPF patients.56 In addition, mutation in surfactant protein C, observed in patients with familial IPF, is associated with accumulation of mutant proteins in the ER and consequent ER stress.57
The unfolded protein response consists of three mediators: BiP (IgH chain-binding protein), PKR-like ER-resistant kinase, and inositol-requiring enzyme 1α. Inositol-requiring enzyme 1α promotes nuclear transcription of NF-κB and AP-1, via tumor necrosis factor-α receptor-associated factor signaling either in a NOD1/2- or receptor interacting serine/threonine protein kinase 1 (RIPK1)–dependent cascade.58 As a result, the phenotype of immune cells changes to limited M1 macrophage polarization and increased number of M2.59
Evidence of an interplay between ER stress and NLRP3 activation suggests that C/EPB homologous protein (CHOP) overexpression—mediated by the sensors inositol-requiring enzyme 1α and PKR-like ER-resistant kinase—promotes IL-1β and pyroptosis, whereas the ER stress inhibitor tauroursodeoxycholic acid (TUDCA) prevents caspase-1 and caspase-11, release of IL-1β, and cell death.60 Another ER stress inhibitor, farnesoid X receptor, prevents ER stress-related NLRP3 assembly, whereas farnesoid X receptor deficiency promotes unfolded protein response–mediated activation of NLRP3.61 Additional hypotheses for a link between NLRP3 and ER stress include unfolded protein response–mediated cytokinemia, angiotensin II signaling, RIP1 phosphorylation, and oxidative stress.62,63
Mitochondrial and Oxidative Stress
In unstimulated conditions, NLRP3 relies on mitochondria that act as docking sites for NLRP3 assembly, through binding of cardiolipin, mitochondrial antiviral signaling protein, and mitofusin 2.64, 65, 66 Disruption in mitochondrial membrane and polarity, resulting from increased production of mitochondrial reactive oxygen species, releases the inflammasome,16,67 suggesting that mitophagy is an important regulator of NLRP3.68 Another mechanism by which NLRP3 senses mitochondrial stress is by nuclear factor erythroid 2–related factor 2. Nuclear factor erythroid 2–related factor 2 regulates the expression of antioxidant genes to guarantee cell survival, reducing mitochondrial reactive oxygen species production, attenuating NF-κB transcription, and modulating the priming and the activation of NLRP3.69 Finally, circulating oxidized forms of mitochondrial DNA participate in the activation pathways of NLRP3.70
Impaired mitochondrial homeostasis represents an established hallmark of aging, and recently, a feature of lung disease and progression. Alveolar macrophages of IPF patients display mitochondrial defects, reduced homeostasis, and higher mitochondrial reactive oxygen species levels when compared with controls.71 Mitochondrial reactive oxygen species activate NLRP3 and interferon inducible protein AIM2 inflammasomes and produce high levels of IL-1β.72
The hypothesis that aged mitochondria contribute to IPF progression is confirmed by animal studies, where NLRP3 activation and BLM-induced fibrosis were stronger in old compared with young mice and prevented in NLRP3(−/−) null mice.19 Age dependency of NLRP3 activation was also evaluated in p24 mice, indicating that age-dependent mechanism of inflammasome activation may be more complex during growth.18
Pyroptosis
In addition to the production of IL-1 family cytokines, NLRP3 leads to pyroptosis, a mechanism of lytic programed cell death, mediated by gasdermin D. Caspase 1 cleaves gasdermin D into its activated form, which migrates to the cell inner membrane, oligomerizes, forms pores of 10- to 14-nm diameter, and provokes free passage of ions and cell death from within.73 Gasdermin D further promotes the nonconventional release of IL-1β and IL-18, as pyroptosis induces the secretion of full-length and calpain-processed IL-1α.74
Mice instilled with BLM display NLRP3 activation and pyroptosis, which is inhibited by lycorine, an alkaloid able to disrupt the interaction of NLRP3 with the adaptor ASC, by targeting its PYD domain in Leu 6, Leu 50, and Thr 53.75 Senescent fibroblasts, associated with persistent matrix production and IPF progression, display increased levels of fragmented gasdermin D, indicating pyroptosis as a mechanism of cell death in IPF.76 Aggregation of gasdermin D in macrophages, and subsequent pyroptosis, is blocked by andrographolide, and may be useful in radiation-induced inflammation and fibrosis in the lung.77
NLRP3 Inhibitors
Multiple drugs have been developed to interfere with the NLRP3 machinery, and the knockout of the inflammasome NLRP3 has been investigated in different animal models of pulmonary fibrosis. The mechanical stretch in the pulmonary fibrosis model exhibited significantly lower levels of cleaved caspase-1 and IL-1β in lungs obtained from NLRP3-knockout mice compared with wild-type mice.78 Aged NLRP3 null mice, challenged with BLM, also showed a reduction of lung fibrosis compared with their wild-type age-matched counterparts.19 However, NLRP3-deficient mice, infected with influenza virus, demonstrated increased mortality compared with healthy mice, in spite of lower IL-1β and IL-18 levels and less white blood cell amount in BALF.37 Thus, the investigation of NLRP3 inhibitors is sustained by direct beneficial effects observed in knockout mice.
Agents that directly inhibit the inflammasome NLRP3 include MCC950, 3,4-methylenedioxy-β-nitrostyrene, tranilast, type I interferon (IFN; IFN-α and IFN-β), and CY-09 (Table 1).
Table 1.
Inhibitors of the NLRP3 Inflammasome and Their Mechanism of Action
| Inhibitor | Main target | Mechanism |
|||
|---|---|---|---|---|---|
| ATPase domain | IL-1β | IL-18 | Caspase-1 | ||
| MCC950 | NLRP3 inflammasome inhibitor | Decrease | Decrease | Decrease | |
| MNS | Tyrosine kinase inhibitor | Decrease | — | ||
| TR | Antihistamine | — | Decrease | Decrease | |
| IFN type I | Immunomodulator | — | Decrease | Decrease | Decrease |
| CY-09 | NLRP3 inflammasome inhibitor | Decrease | — | — | Decrease |
| Glyburide | Sulfonylurea | Decrease | Decrease | — | — |
| EC144 | HSP90 inhibitor | — | Decrease | — | — |
| 17-DMAG | HSP90 inhibitor | Decrease | Decrease | Decrease | Decrease |
| 17-AAG | HSP90 inhibitor | Decrease | Decrease | Decrease | Decrease |
| Carnosol | HSP90 inhibitor | Decrease | — | — | — |
Decrease indicates inhibition of respective pathway.
17-AAG, 17-allylamino-17-demethoxygeldanamycin; 17-DMAG, 17-dimethylaminoethylamino-17-demethoxygeldanamycin; HSP90, heat shock protein 90; IFN, interferon; MNS, 3,4-methylenedioxy-β-nitrostyrene; NLRP3, NLR family pyrin domain-containing protein 3; TR, tranilast [N-(3′,4′-dimethoxycinnamoyl)-anthranilic acid].
MCC950 (CP-456,773 or CRID3) is one of the most studied direct inhibitors of the NLRP3 pathway, but its molecular target remains unknown. The small molecule, containing diarylsulfonylurea, reduces expression of interleukins IL-1β and IL-18, ameliorating the severity of experimental autoimmune encephalomyelitis and cryopyrin-associated periodic fever syndrome in mice.79 MCC950 acts jointly with walker B, to block hydrolysis of ATP and reduce the NLRP3-mediated inflammation.80 MCC950 improves spinal cord edema and hind limb movements in mice with spinal cord injury, through blocking both NLRP3–caspase-1 and excretion of IL-1β, IL-18, and tumor necrosis factor-α.81 Overexpression of cytokines, but not transforming growth factor-β, was suppressed by MCC950 in mice with cardiac arrest.82 MCC950 ameliorates cholestatic liver injury and liver fibrosis by blocking NLRP3 activation and inhibiting neutrophil infiltration.83 Specific NLRP3 inhibition in mouse lungs with cystic fibrosis by MCC950 blocks IL-1β, resulting in reduced airway inflammation.84
3,4-Methylenedioxy-β-nitrostyrene is another direct potent inhibitor of NLRP3 inflammasome activation. 3,4-Methylenedioxy-β-nitrostyrene does not affect activation of AIM2 or NLR family CARD domain containing 4 (NLRC4), but suppresses the ATPase activity of NLRP3 inflammasome through the NACHT and leucin-rich repeat domains.85
Tranilast [N-(3′,4′-dimethoxycinnamoyl)-anthranilic acid], a tryptophan metabolite analog, is an anti-anaphylactic drug in clinical use. Tranilast directly binds to NLRP3 and inhibits assembly of NLRP3 inflammasome and the subsequent caspase-1 activation and IL-1β production.86 It does not affect NLRC4 or AIM2 inflammasomes. Tranilast destroys the endogenous NLRP3-ASC interaction but does not affect the NLRP3-NEK7 [never-in-mitosis A (NIMA)–related kinase] bond. The drug binds the NLRP3 NACHT domain and prevents NLRP3-NLRP3 interaction.87 Because of its lack of severe adverse effects in clinic use, it has been proposed as a good treatment for NLRP3-related diseases.88
Type I interferon (IFN-α and IFN-β) has been used as treatment of multiple sclerosis.89 Type I IFNs are produced by macrophages and dendritic cells in response to bacteria or viruses.90 It is not clear how type I IFNs affect NLRP3 inflammasome and its production of IL-1β and IL-18.91 IFNs repress the activity of the NLRP1 and NLRP3 inflammasomes via the STAT1 transcription factor, thereby suppressing caspase-1–dependent IL-1β maturation.91 However, in one report, IFN-α activated the inflammasome in human intestinal mucosa.92
CY-09 is a direct inhibitor of inflammasome NLRP3 in mice and human cells. CY-09 directly binds to the ATP-binding motif of NLRP3 NACHT domain and inhibits NLRP3 ATPase activation. Because of the binding of NACHT domain, it blocks NLRP3 inflammasome formation and activation.93 In bone marrow–derived macrophages challenged with lipopolysaccharide, CY-09 significantly reduced activation of ATP and caspase-1. CY-09 reduces inflammation and pain via inhibiting transient receptor potential cation channel subfamily A member 1 (TRPA1)–mediated activation of NLRP3 inflammasome in a mouse pain model.94
In addition, there are multiple drugs that, through diverse mechanisms of action, inhibit the inflammasome NLRP3 (Table 1).
Glyburide is a sulfonylurea drug that is used to control high blood glucose in people with type 2 diabetes. The drug works by inhibiting ATP-sensitive K+ATP channels and results in blockage of NLRP3 activation.95 However, it does not prevent IL-1β expression from activated NLRC4 or NLRP1 pathways and does not block caspase-1 in bone marrow–derived macrophages infected with Salmonella typhimurium,96 but in diabetic mice it decreases the expression of IFN-γ, tumor necrosis factor-α, and IL-6.97
HSP90 Inhibitors
Several studies have suggested that heat shock protein 90 (HSP90) inhibitors ameliorate inflammation partly through NLRP3 inhibition. NLRP3, especially in the inactive but signal competent state, is regulated by the cochaperones HSP90 and Sgt1, whose interaction is critical for NLRP3 activation.98 Geldanamycin, the first-generation HSP90 inhibitor, prevents the activation of the inflammasome in human retinal pigment epithelial cells.99 EC144 is a selective synthetic HSP90 inhibitor that recently completed phase 2 clinical trials.100 This drug inhibits inflammation, including priming and activation of NLRP3 inflammasome in vitro and in vivo. Interestingly, in vivo EC144 completely inhibits inflammasome-dependent IL-1β release, much more effectively than MCC950.101 17-Dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a second-generation HSP90 inhibitor, significantly reduced NLRP3 inflammasome-mediated activation of caspase-1 and cytokine secretion in a murine model of acute and chronic alcoholic liver injury.99 Another second-generation HSP90 inhibitor, 17-allylamino-17-demethoxygeldanamycin (17-AAG), down-regulated the levels of NLRP3, caspase-1, IL-1β, and HSP90 in a subarachnoid hemorrhage mouse model.102 The rosemary and sage polyphenol, carnosol, inhibits NLRP3 inflammasome activation by directly interacting with HSP90 and blocking its ATPase activity, in lipopolysaccharide-induced septic mice. Moreover, the protective effect of carnosol against lipopolysaccharide-induced lethality was similar to that of MCC950.103
Conclusions
The inflammasome NLRP3 is a highly regulated and conserved machinery for the post-translational expression of ancestral IL-1 class cytokines. Although a PRR, its complex interactions with cytoplasm, cellular organelles, and electrochemical balance produce proinflammatory cell responses or pyroptosis, and have positioned NLRP3 as a master regulator of innate inflammation. NLRP3's role in innate immune responses, its contribution to epithelial-to-mesenchymal transformation and tissue reorganization, and its contribution to ER stress suggest its involvement at multiple levels of the pathophysiology of IPF, providing a direct mechanistic rationale for its investigation as a potentially useful drug target. Data from preclinical studies suggest that NLRPP3 inhibition could offer a promising approach against the fibrotic process in the lungs. Although multiple direct and indirect NLRP3 inhibitors are available, further investigations are required to establish their proper use.
Acknowledgments
We thank the Eastern Virginia Medical School Department of Anatomy and Pathology Histology Laboratory for lung tissue processing and staining; and Betsy Gregory and Tierney Day for outstanding technical support.
Footnotes
Supported by the Old Dominion Research Foundation and the CounterACT Program (J.D.C.), NIH Office of the Director, and the National Institute of Environmental Health Sciences grant UO1ES030674.
Disclosures: None declared.
References
- 1.Wynn T.A. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomeer M.J., Costabe U., Rizzato G., Poletti V., Demedts M. Comparison of registries of interstitial lung diseases in three European countries. Eur Respir J Suppl. 2001;32:114s–118s. [PubMed] [Google Scholar]
- 3.Olson A.L., Swigris J.J., Lezotte D.C., Norris J.M., Wilson C.G., Brown K.K. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 4.Bonella F., Stowasser S., Wollin L. Idiopathic pulmonary fibrosis: current treatment options and critical appraisal of nintedanib. Drug Des Devel Ther. 2015;9:6407–6419. doi: 10.2147/DDDT.S76648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albera C., Costabel U., Fagan E.A., Glassberg M.K., Gorina E., Lancaster L., Lederer D.J., Nathan S.D., Spirig D., Swigris J.J. Efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis with more preserved lung function. Eur Respir J. 2016;48:843–851. doi: 10.1183/13993003.01966-2015. [DOI] [PubMed] [Google Scholar]
- 6.Chambers R.C. Abnormal wound healing responses in pulmonary fibrosis: focus on coagulation signalling. Eur Respir Rev. 2008;17:130. [Google Scholar]
- 7.Kropski J.A., Blackwell T.S., Loyd J.E. The genetic basis of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1717. doi: 10.1183/09031936.00163814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burman A., Tanjore H., Blackwell T.S. Endoplasmic reticulum stress in pulmonary fibrosis. Matrix Biol. 2018;68-69:355–365. doi: 10.1016/j.matbio.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai O., Winkler J., Minasyan M., Herzog E.L. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front Med. 2018;5:43. doi: 10.3389/fmed.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinon F., Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Tschopp J., Martinon F., Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman H.M., Wright F.A., Broide D.H., Wanderer A.A., Kolodner R.D. Identification of a locus on chromosome 1q44 for familial cold urticaria. Am J Human Genetics. 2000;66:1693–1698. doi: 10.1086/302874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keyel P.A., Heid M.E., Salter R.D. Macrophage responses to bacterial toxins: a balance between activation and suppression. Immunol Res. 2011;50:118–123. doi: 10.1007/s12026-011-8212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai B., Yang Y., Wang Q., Li M., Tian C., Liu Y., Aung L.H.H., Li P-f, Yu T., Chu X-m. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11:776. doi: 10.1038/s41419-020-02985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kostadinova E., Chaput C., Gutbier B., Lippmann J., Sander L.E., Mitchell T.J., Suttorp N., Witzenrath M., Opitz B. NLRP3 protects alveolar barrier integrity by an inflammasome-independent increase of epithelial cell adherence. Sci Rep. 2016;6:30943. doi: 10.1038/srep30943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 17.Chen X., Guo X., Ge Q., Zhao Y., Mu H., Zhang J. ER stress activates the NLRP3 inflammasome: a novel mechanism of atherosclerosis. Oxid Med Cell Longev. 2019;2019:3462530. doi: 10.1155/2019/3462530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colunga Biancatelli R.M.L., Solopov P., Dimitropoulou C., Catravas J.D. Age-dependent chronic lung injury and pulmonary fibrosis following single exposure to hydrochloric acid. Int J Mol Sci. 2021;22:8833. doi: 10.3390/ijms22168833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stout-Delgado H.W., Cho S.J., Chu S.G., Mitzel D.N., Villalba J., El-Chemaly S., Ryter S.W., Choi A.M.K., Rosas I.O. Age-dependent susceptibility to pulmonary fibrosis is associated with NLRP3 inflammasome activation. Am J Respir Cell Mol Biol. 2016;55:252–263. doi: 10.1165/rcmb.2015-0222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y., Li H., Liu S., Pan P., Su X., Tan H., Wu D., Zhang L., Song C., Dai M., Li Q., Mao Z., Long Y., Hu Y., Hu C. Pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activation. Mol Immunol. 2018;99:134–144. doi: 10.1016/j.molimm.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Lasithiotaki I., Giannarakis I., Tsitoura E., Samara K.D., Margaritopoulos G.A., Choulaki C., Vasarmidi E., Tzanakis N., Voloudaki A., Sidiropoulos P., Siafakas N.M., Antoniou K.M. NLRP3 inflammasome expression in idiopathic pulmonary fibrosis and rheumatoid lung. Eur Respir J. 2016;47:910. doi: 10.1183/13993003.00564-2015. [DOI] [PubMed] [Google Scholar]
- 22.Jäger B., Seeliger B., Terwolbeck O., Warnecke G., Welte T., Müller M., Bode C., Prasse A. The NLRP3-inflammasome-caspase-1 pathway is upregulated in idiopathic pulmonary fibrosis and acute exacerbations and is inducible by apoptotic A549 cells. Front Immunol. 2021;12:642855. doi: 10.3389/fimmu.2021.642855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitasato Y., Hoshino T., Okamoto M., Kato S., Koda Y., Nagata N., Kinoshita M., Koga H., Yoon D.Y., Asao H., Ohmoto H., Koga T., Rikimaru T., Aizawa H. Enhanced expression of interleukin-18 and its receptor in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2004;31:619–625. doi: 10.1165/rcmb.2003-0306OC. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasula S.M., Poyet J.-L., Razmara M., Datta P., Zhang Z., Alnemri E.S. The pyrin-card protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 25.Franchi L., Eigenbrod T., Muñoz-Planillo R., Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koonin E.V., Aravind L. The NACHT family – a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem Sci. 2000;25:223–224. doi: 10.1016/s0968-0004(00)01577-2. [DOI] [PubMed] [Google Scholar]
- 27.Duncan J.A., Bergstralh D.T., Wang Y., Willingham S.B., Ye Z., Zimmermann A.G., Ting J.P.-Y. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci U S A. 2007;104:8041. doi: 10.1073/pnas.0611496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boucher D., Monteleone M., Coll R.C., Chen K.W., Ross C.M., Teo J.L., Gomez G.A., Holley C.L., Bierschenk D., Stacey K.J., Yap A.S., Bezbradica J.S., Schroder K. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med. 2018;215:827–840. doi: 10.1084/jem.20172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B.G., Fitzgerald K.A., Hornung V., Latz E. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swanson K.V., Deng M., Ting J.P.Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang M., Fan J. Pattern recognition receptor-dependent mechanisms of acute lung injury. Mol Med. 2010;16:69–82. doi: 10.2119/molmed.2009.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peeters P.M., Perkins T.N., Wouters E.F., Mossman B.T., Reynaert N.L. Silica induces NLRP3 inflammasome activation in human lung epithelial cells. Part Fibre Toxicol. 2013;10:3. doi: 10.1186/1743-8977-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito H., Kimura H., Karasawa T., Hisata S., Sadatomo A., Inoue Y., Yamada N., Aizawa E., Hishida E., Kamata R., Komada T., Watanabe S., Kasahara T., Suzuki T., Horie H., Kitayama J., Sata N., Yamaji-Kegan K., Takahashi M. NLRP3 inflammasome activation in lung vascular endothelial cells contributes to intestinal ischemia/reperfusion-induced acute lung injury. J Immunol. 2020;205:1393–1405. doi: 10.4049/jimmunol.2000217. [DOI] [PubMed] [Google Scholar]
- 34.Gwyer Findlay E., Hussell T. Macrophage-mediated inflammation and disease: a focus on the lung. Mediators Inflamm. 2012;2012:140937. doi: 10.1155/2012/140937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassel S.L., Eisenbarth S.C., Iyer S.S., Sadler J.J., Colegio O.R., Tephly L.A., Carter A.B., Rothman P.B., Flavell R.A., Sutterwala F.S. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dostert C., Pétrilli V., Van Bruggen R., Steele C., Mossman B.T., Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Nardo D., De Nardo C.M., Latz E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am J Pathol. 2014;184:42–54. doi: 10.1016/j.ajpath.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivers-Auty J., Daniels M.J.D., Colliver I., Robertson D.L., Brough D. Redefining the ancestral origins of the interleukin-1 superfamily. Nat Commun. 2018;9:1156. doi: 10.1038/s41467-018-03362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber A., Wasiliew P., Kracht M. Interleukin-1 (IL-1) pathway. Sci Signaling. 2010;3:cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 40.Fields J.K., Günther S., Sundberg E.J. Structural basis of IL-1 family cytokine signaling. Front Immunol. 2019;10:1412. doi: 10.3389/fimmu.2019.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L.M., Zhang J., Zhang Y., Fei C., Wang L., Yi Z.W., Zhang Z.Q. Interleukin-18 promotes fibroblast senescence in pulmonary fibrosis through down-regulating Klotho expression. Biomed Pharmacother. 2019;113:108756. doi: 10.1016/j.biopha.2019.108756. [DOI] [PubMed] [Google Scholar]
- 42.Osei E.T., Brandsma C.-A., Timens W., Heijink I.H., Hackett T.-L. Current perspectives on the role of interleukin-1 signaling in the pathogenesis of asthma and COPD. Eur Respir J. 2019;55:1900563. doi: 10.1183/13993003.00563-2019. [DOI] [PubMed] [Google Scholar]
- 43.Smith D.R., Kunkel S.L., Standiford T.J., Rolfe M.W., Lynch J.P., 3rd, Arenberg D.A., Wilke C.A., Burdick M.D., Martinez F.J., Hampton J.N. Increased interleukin-1 receptor antagonist in idiopathic pulmonary fibrosis: a compartmental analysis. Am J Respir Crit Care Med. 1995;151:1965–1973. doi: 10.1164/ajrccm.151.6.7767546. [DOI] [PubMed] [Google Scholar]
- 44.Lappalainen U., Whitsett J.A., Wert S.E., Tichelaar J.W., Bry K. Interleukin-1β causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 45.Wilson M.S., Madala S.K., Ramalingam T.R., Gochuico B.R., Rosas I.O., Cheever A.W., Wynn T.A. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207:535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park M.-J., Moon S.-J., Lee E.-J., Jung K.-A., Kim E.-K., Kim D.-S., Lee J.-H., Kwok S.-K., Min J.-K., Park S.-H., Cho M.-L. IL-1-IL-17 signaling axis contributes to fibrosis and inflammation in two different murine models of systemic sclerosis. Front Immunol. 2018;9:1611. doi: 10.3389/fimmu.2018.01611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masola V., Carraro A., Granata S., Signorini L., Bellin G., Violi P., Lupo A., Tedeschi U., Onisto M., Gambaro G., Zaza G. In vitro effects of interleukin (IL)-1 beta inhibition on the epithelial-to-mesenchymal transition (EMT) of renal tubular and hepatic stellate cells. J Translational Med. 2019;17:12. doi: 10.1186/s12967-019-1770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mia M.M., Boersema M., Bank R.A. Interleukin-1β attenuates myofibroblast formation and extracellular matrix production in dermal and lung fibroblasts exposed to transforming growth factor-β1. PLoS One. 2014;9:e91559. doi: 10.1371/journal.pone.0091559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osei E.T., Mostaço-Guidolin L.B., Hsieh A., Warner S.M., Al-Fouadi M., Wang M., Cole D.J., Maksym G.N., Hallstrand T.S., Timens W., Brandsma C.-A., Heijink I.H., Hackett T.-L. Epithelial-interleukin-1 inhibits collagen formation by airway fibroblasts: implications for asthma. Sci Rep. 2020;10:8721. doi: 10.1038/s41598-020-65567-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenkins R.G., Moore B.B., Chambers R.C., Eickelberg O., Königshoff M., Kolb M., Laurent G.J., Nanthakumar C.B., Olman M.A., Pardo A., Selman M., Sheppard D., Sime P.J., Tager A.M., Tatler A.L., Thannickal V.J., White E.S., ATSAoR Cell and B Molecular An official American Thoracic Society workshop report: use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am J Respir Cell Mol Biol. 2017;56:667–679. doi: 10.1165/rcmb.2017-0096ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gasse P., Mary C., Guenon I., Noulin N., Charron S., Schnyder-Candrian S., Schnyder B., Akira S., Quesniaux V.F., Lagente V., Ryffel B., Couillin I. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007;117:3786–3799. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gasse P., Riteau N., Charron S., Girre S., Fick L., Pétrilli V., Tschopp J., Lagente V., Quesniaux V.F., Ryffel B., Couillin I. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 53.Müller T., Fay S., Vieira R.P., Karmouty-Quintana H., Cicko S., Ayata K., Zissel G., Goldmann T., Lungarella G., Ferrari D., Di Virgilio F., Robaye B., Boeynaems J.M., Blackburn M.R., Idzko M. The purinergic receptor subtype P2Y2 mediates chemotaxis of neutrophils and fibroblasts in fibrotic lung disease. Oncotarget. 2017;8:35962–35972. doi: 10.18632/oncotarget.16414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riteau N., Gasse P., Fauconnier L., Gombault A., Couegnat M., Fick L., Kanellopoulos J., Quesniaux V.F., Marchand-Adam S., Crestani B., Ryffel B., Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 55.Müller T., Fay S., Vieira R.P., Karmouty-Quintana H., Cicko S., Ayata C.K., Zissel G., Goldmann T., Lungarella G., Ferrari D., Di Virgilio F., Robaye B., Boeynaems J.-M., Lazarowski E.R., Blackburn M.R., Idzko M. P2Y6 receptor activation promotes inflammation and tissue remodeling in pulmonary fibrosis. Front Immunol. 2017;8:1028. doi: 10.3389/fimmu.2017.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanjore H., Blackwell T.S., Lawson W.E. Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;302:L721–L729. doi: 10.1152/ajplung.00410.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maguire J.A., Mulugeta S., Beers M.F. Endoplasmic reticulum stress induced by surfactant protein C BRICHOS mutants promotes proinflammatory signaling by epithelial cells. Am J Respir Cell Mol Biol. 2011;44:404–414. doi: 10.1165/rcmb.2009-0382OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hollien J., Lin J.H., Li H., Stevens N., Walter P., Weissman J.S. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shan B., Wang X., Wu Y., Xu C., Xia Z., Dai J., Shao M., Zhao F., He S., Yang L., Zhang M., Nan F., Li J., Liu J., Liu J., Jia W., Qiu Y., Song B., Han J.-D.J., Rui L., Duan S.-Z., Liu Y. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol. 2017;18:519–529. doi: 10.1038/ni.3709. [DOI] [PubMed] [Google Scholar]
- 60.Lebeaupin C., Proics E., de Bieville C.H.D., Rousseau D., Bonnafous S., Patouraux S., Adam G., Lavallard V.J., Rovere C., Le Thuc O., Saint-Paul M.C., Anty R., Schneck A.S., Iannelli A., Gugenheim J., Tran A., Gual P., Bailly-Maitre B. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015;6:e1879. doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han C.Y., Rho H.S., Kim A., Kim T.H., Jang K., Jun D.W., Kim J.W., Kim B., Kim S.G. FXR inhibits endoplasmic reticulum stress-induced NLRP3 inflammasome in hepatocytes and ameliorates liver injury. Cell Rep. 2018;24:2985–2999. doi: 10.1016/j.celrep.2018.07.068. [DOI] [PubMed] [Google Scholar]
- 62.Chong W.C., Shastri M.D., Peterson G.M., Patel R.P., Pathinayake P.S., Dua K., Hansbro N.G., Hsu A.C., Wark P.A., Shukla S.D., Johansen M.D., Schroder K., Hansbro P.M. The complex interplay between endoplasmic reticulum stress and the NLRP3 inflammasome: a potential therapeutic target for inflammatory disorders. Clin Translational Immunol. 2021;10:e1247. doi: 10.1002/cti2.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gargioli C., Turturici G., Barreca M.M., Spinello W., Fuoco C., Testa S., Feo S., Cannata S.M., Cossu G., Sconzo G., Geraci F. Oxidative stress preconditioning of mouse perivascular myogenic progenitors selects a subpopulation of cells with a distinct survival advantage in vitro and in vivo. Cell Death Dis. 2018;9:1. doi: 10.1038/s41419-017-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ichinohe T., Yamazaki T., Koshiba T., Yanagi Y. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc Natl Acad Sci U S A. 2013;110:17963–17968. doi: 10.1073/pnas.1312571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Subramanian N., Natarajan K., Clatworthy M.R., Wang Z., Germain R.N. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153:348–361. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park S., Juliana C., Hong S., Datta P., Hwang I., Fernandes-Alnemri T., Yu J.W., Alnemri E.S. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J Immunol. 2013;191:4358–4366. doi: 10.4049/jimmunol.1301170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cruz C.M., Rinna A., Forman H.J., Ventura A.L., Persechini P.M., Ojcius D.M. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang N.-P., Liu X.-J., Xie L., Shen X.-Z., Wu J. Impaired mitophagy triggers NLRP3 inflammasome activation during the progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis. Lab Invest. 2019;99:749–763. doi: 10.1038/s41374-018-0177-6. [DOI] [PubMed] [Google Scholar]
- 69.Liu X., Zhang X., Ding Y., Zhou W., Tao L., Lu P., Wang Y., Hu R. Nuclear factor E2-related factor-2 negatively regulates NLRP3 inflammasome activity by inhibiting reactive oxygen species-induced NLRP3 priming. Antioxid Redox Signal. 2017;26:28–43. doi: 10.1089/ars.2015.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong Z., Liang S., Sanchez-Lopez E., He F., Shalapour S., Lin X-j, Wong J., Ding S., Seki E., Schnabl B., Hevener A.L., Greenberg H.B., Kisseleva T., Karin M. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. 2018;560:198–203. doi: 10.1038/s41586-018-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsitoura E., Vasarmidi E., Bibaki E., Trachalaki A., Koutoulaki C., Papastratigakis G., Papadogiorgaki S., Chalepakis G., Tzanakis N., Antoniou K.M. Accumulation of damaged mitochondria in alveolar macrophages with reduced OXPHOS related gene expression in IPF. Respir Res. 2019;20:264. doi: 10.1186/s12931-019-1196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trachalaki A., Tsitoura E., Mastrodimou S., Invernizzi R., Vasarmidi E., Bibaki E., Tzanakis N., Molyneaux P.L., Maher T.M., Antoniou K. Enhanced IL-1β release following NLRP3 and AIM2 inflammasome stimulation is linked to mtROS in airway macrophages in pulmonary fibrosis. Front Immunol. 2021;12:661811. doi: 10.3389/fimmu.2021.661811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding J., Wang K., Liu W., She Y., Sun Q., Shi J., Sun H., Wang D.C., Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 74.Gross O., Yazdi A.S., Thomas C.J., Masin M., Heinz L.X., Guarda G., Quadroni M., Drexler S.K., Tschopp J. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 75.Liang Q., Cai W., Zhao Y., Xu H., Tang H., Chen D., Qian F., Sun L. Lycorine ameliorates bleomycin-induced pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and pyroptosis. Pharmacol Res. 2020;158:104884. doi: 10.1016/j.phrs.2020.104884. [DOI] [PubMed] [Google Scholar]
- 76.Garcia S., Hohmann M., Espindola M.S., Hogaboam C.M. American Thoracic Society; New York, NY: 2020. Senolytic Drug-Induced Fibroblast Death Involves Apoptosis, Necroptosis, and Pyroptosis in IPF. B64: Mechanistic Advances in Lung Fibrosis. A4056. [Google Scholar]
- 77.Gao J., Peng S., Shan X., Deng G., Shen L., Sun J., Jiang C., Yang X., Chang Z., Sun X., Feng F., Kong L., Gu Y., Guo W., Xu Q., Sun Y. Inhibition of AIM2 inflammasome-mediated pyroptosis by andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis. Cell Death Dis. 2019;10:957. doi: 10.1038/s41419-019-2195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lv Z., Wang Y., Liu Y.J., Mao Y.F., Dong W.W., Ding Z.N., Meng G.X., Jiang L., Zhu X.Y. NLRP3 inflammasome activation contributes to mechanical stretch-induced endothelial-mesenchymal transition and pulmonary fibrosis. Crit Care Med. 2018;46:e49–e58. doi: 10.1097/CCM.0000000000002799. [DOI] [PubMed] [Google Scholar]
- 79.Coll R.C., Robertson A.A., Chae J.J., Higgins S.C., Muñoz-Planillo R., Inserra M.C., Vetter I., Dungan L.S., Monks B.G., Stutz A., Croker D.E., Butler M.S., Haneklaus M., Sutton C.E., Núñez G., Latz E., Kastner D.L., Mills K.H., Masters S.L., Schroder K., Cooper M.A., O'Neill L.A. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coll R.C., Hill J.R., Day C.J., Zamoshnikova A., Boucher D., Massey N.L., Chitty J.L., Fraser J.A., Jennings M.P., Robertson A.A.B., Schroder K. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol. 2019;15:556–559. doi: 10.1038/s41589-019-0277-7. [DOI] [PubMed] [Google Scholar]
- 81.Jiao J., Zhao G., Wang Y., Ren P., Wu M. MCC950, a selective inhibitor of NLRP3 inflammasome, reduces the inflammatory response and improves neurological outcomes in mice model of spinal cord injury. Front Mol Biosci. 2020;7:37. doi: 10.3389/fmolb.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang M., Li R., Lyu J., Li X., Wang W., Wang Z., Sheng H., Zhang W., Karhausen J., Yang W. MCC950, a selective NLPR3 inflammasome inhibitor, improves neurologic function and survival after cardiac arrest and resuscitation. J Neuroinflammation. 2020;17:256. doi: 10.1186/s12974-020-01933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qu J., Yuan Z., Wang G., Wang X., Li K. The selective NLRP3 inflammasome inhibitor MCC950 alleviates cholestatic liver injury and fibrosis in mice. Int Immunopharmacol. 2019;70:147–155. doi: 10.1016/j.intimp.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 84.McElvaney O.J., Zaslona Z., Becker-Flegler K., Palsson-McDermott E.M., Boland F., Gunaratnam C., Gulbins E., O'Neill L.A., Reeves E.P., McElvaney N.G. Specific inhibition of the NLRP3 inflammasome as an antiinflammatory strategy in cystic fibrosis. Am J Respir Crit Care Med. 2019;200:1381–1391. doi: 10.1164/rccm.201905-1013OC. [DOI] [PubMed] [Google Scholar]
- 85.He Y., Varadarajan S., Muñoz-Planillo R., Burberry A., Nakamura Y., Núñez G. 3,4-Methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem. 2014;289:1142–1150. doi: 10.1074/jbc.M113.515080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang Y., Jiang H., Chen Y., Wang X., Yang Y., Tao J., Deng X., Liang G., Zhang H., Jiang W., Zhou R. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol Med. 2018;10:e8689. doi: 10.15252/emmm.201708689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aroor A.R., Shukla S.D. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–2364. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 88.Zahid A., Li B., Kombe A.J.K., Jin T., Tao J. Pharmacological inhibitors of the NLRP3 inflammasome. 2019;10:2538. doi: 10.3389/fimmu.2019.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shao B.-Z., Xu Z.-Q., Han B.-Z., Su D.-F., Liu C. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol. 2015;6:262. doi: 10.3389/fphar.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 91.Guarda G., Braun M., Staehli F., Tardivel A., Mattmann C., Förster I., Farlik M., Decker T., Du Pasquier R.A., Romero P., Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 92.Jarry A., Malard F., Bou-Hanna C., Meurette G., Mohty M., Mosnier J.-F., Laboisse C.L., Bossard C. Interferon-alpha promotes Th1 response and epithelial apoptosis via inflammasome activation in human intestinal mucosa. Cell Mol Gastroenterol Hepatol. 2016;3:72–81. doi: 10.1016/j.jcmgh.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang H., He H., Chen Y., Huang W., Cheng J., Ye J., Wang A., Tao J., Wang C., Liu Q., Jin T., Jiang W., Deng X., Zhou R. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med. 2017;214:3219–3238. doi: 10.1084/jem.20171419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fan Y., Xue G., Chen Q., Lu Y., Dong R., Yuan H. CY-09 inhibits NLRP3 inflammasome activation to relieve pain via TRPA1. Comput Math Methods Med. 2021;2021:9806690. doi: 10.1155/2021/9806690. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Lamkanfi M., Mueller J.L., Vitari A.C., Misaghi S., Fedorova A., Deshayes K., Lee W.P., Hoffman H.M., Dixit V.M. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mariathasan S., Newton K., Monack D.M., Vucic D., French D.M., Lee W.P., Roose-Girma M., Erickson S., Dixit V.M. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 97.Yang X., Qu C., Jia J., Zhan Y. NLRP3 inflammasome inhibitor glyburide expedites diabetic-induced impaired fracture healing. Immunobiology. 2019;224:786–791. doi: 10.1016/j.imbio.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 98.Mayor A., Martinon F., De Smedt T., Pétrilli V., Tschopp J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol. 2007;8:497–503. doi: 10.1038/ni1459. [DOI] [PubMed] [Google Scholar]
- 99.Piippo N., Korhonen E., Hytti M., Skottman H., Kinnunen K., Josifovska N., Petrovski G., Kaarniranta K., Kauppinen A. Hsp90 inhibition as a means to inhibit activation of the NLRP3 inflammasome. Sci Rep. 2018;8:6720. doi: 10.1038/s41598-018-25123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shi J., Van de Water R., Hong K., Lamer R.B., Weichert K.W., Sandoval C.M., Kasibhatla S.R., Boehm M.F., Chao J., Lundgren K., Timple N., Lough R., Ibanez G., Boykin C., Burrows F.J., Kehry M.R., Yun T.J., Harning E.K., Ambrose C., Thompson J., Bixler S.A., Dunah A., Snodgrass-Belt P., Arndt J., Enyedy I.J., Li P., Hong V.S., McKenzie A., Biamonte M.A. EC144 is a potent inhibitor of the heat shock protein 90. J Med Chem. 2012;55:7786–7795. doi: 10.1021/jm300810x. [DOI] [PubMed] [Google Scholar]
- 101.Nizami S., Arunasalam K., Green J., Cook J., Lawrence C.B., Zarganes-Tzitzikas T., Davis J.B., Di Daniel E., Brough D. Inhibition of the NLRP3 inflammasome by HSP90 inhibitors. Immunology. 2021;162:84–91. doi: 10.1111/imm.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zuo Y., Wang J., Liao F., Yan X., Li J., Huang L., Liu F. Inhibition of heat shock protein 90 by 17-AAG reduces inflammation via P2X7 receptor/NLRP3 inflammasome pathway and increases neurogenesis after subarachnoid hemorrhage in mice. 2018;11:401. doi: 10.3389/fnmol.2018.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi W., Xu G., Zhan X., Gao Y., Wang Z., Fu S., Qin N., Hou X., Ai Y., Wang C., He T., Liu H., Chen Y., Liu Y., Wang J., Niu M., Guo Y., Xiao X., Bai Z. Carnosol inhibits inflammasome activation by directly targeting HSP90 to treat inflammasome-mediated diseases. Cell Death Dis. 2020;11:252. doi: 10.1038/s41419-020-2460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]