Highlights
-
•
We explore compulsivity and social disinhibition as subcomponents of disinhibition.
-
•
An original behavioural approach in a semi-ecological situation was used.
-
•
Different neural networks are implicated in compulsivity and social disinhibition.
Keywords: Compulsivity, Diffusion tensor imaging, Semi-ecological situation, Social disinhibition, Voxel-based morphometry
Abbreviations: AF, Arcuate Fasciculus; AD, Axial Diffusivity; bvFTD, behavioural variant frontotemporal dementia; CST, Corticospinal Tract; DTI, Diffusion Tensor Imaging; DWI, Diffusion weighted images; FA, Fractional Anisotropy; FAB, Frontal Assessment Battery; FM, Forceps Minor; FTD, frontotemporal Dementia; GM, Grey Matter; HC, Healthy Controls; ILF, Inferior Longitudinal Fasciculus; MD, Mean Diffusivity; mini-SEA, mini-Social cognition & Emotional Assessment; MMSE, Mini-Mental State Examination; OCD, Obsessive-Compulsive Disorders; OFC, Orbitofrontal Cortex; RD, Radial Diffusivity; ROI, Region Of Interest; SAN, Semantic Appraisal Network; SN, Salience Network; SN-F, Salience Network Frontal; T1w, T1-weighted; UF, Uncinate Fasciculus; VBM, Voxel-Based Morphometry; WM, White Matter
Abstract
Disinhibition is a core symptom of many neurodegenerative diseases, particularly frontotemporal dementia, and is a major cause of stress for caregivers. While a distinction between behavioural and cognitive disinhibition is common, an operational definition of behavioural disinhibition is still missing. Furthermore, conventional assessment of behavioural disinhibition, based on questionnaires completed by the caregivers, often lacks ecological validity. Therefore, their neuroanatomical correlates are non-univocal.
In the present work, we used an original behavioural approach in a semi-ecological situation to assess two specific dimensions of behavioural disinhibition: compulsivity and social disinhibition. First, we investigated disinhibition profile in patients compared to controls. Then, to validate our approach, compulsivity and social disinhibition scores were correlated with classic cognitive tests measuring disinhibition (Hayling Test) and social cognition (mini-Social cognition & Emotional Assessment). Finally, we disentangled the anatomical networks underlying these two subtypes of behavioural disinhibition, taking in account the grey (voxel-based morphometry) and white matter (diffusion tensor imaging tractography). We included 17 behavioural variant frontotemporal dementia patients and 18 healthy controls.
We identified patients as more compulsive and socially disinhibited than controls. We found that behavioural metrics in the semi-ecological task were related to cognitive performance: compulsivity correlated with the Hayling test and both compulsivity and social disinhibition were associated with the emotion recognition test. Based on voxel-based morphometry and tractography, compulsivity correlated with atrophy in the bilateral orbitofrontal cortex, the right temporal region and subcortical structures, as well as with alterations of the bilateral cingulum and uncinate fasciculus, the right inferior longitudinal fasciculus and the right arcuate fasciculus. Thus, the network of regions related to compulsivity matched the “semantic appraisal” network. Social disinhibition was associated with bilateral frontal atrophy and impairments in the forceps minor, the bilateral cingulum and the left uncinate fasciculus, regions corresponding to the frontal component of the “salience” network.
Summarizing, this study validates our semi-ecological approach, through the identification of two subtypes of behavioural disinhibition, and highlights different neural networks underlying compulsivity and social disinhibition. Taken together, these findings are promising for clinical practice by providing a better characterisation of inhibition disorders, promoting their detection and consequently a more adapted management of patients.
1. Introduction
Frontotemporal dementia (FTD) is the second most common form of early-onset dementia after Alzheimer’s disease and involves the degeneration of the frontal and temporal lobes, causing dysfunction in these regions (Miller and Llibre Guerra, 2019). Particularly, patients with behavioural variant of frontotemporal dementia (bvFTD) show progressive cognitive and behavioural changes (Rascovsky et al., 2011). Among these symptoms, disinhibition is a clinical core feature and one of the major causes of caregiver distress (Davis and Tremont, 2007, Cheng, 2017).
Defining disinhibition is a complex task, which argues in favour of a multifactorial model of disinhibition. Two types of inhibition are commonly distinguished: cognitive and behavioural inhibition (Aron, 2007). Cognitive inhibition consists in the ability to resist exogenous or endogenous interference, to inhibit previously activated cognitive contents and to suppress inappropriate or irrelevant responses (Wilson and Kipp, 1998). Distinct from this, behavioural inhibition refers to the control of emotional and social behaviours in a social context, the ability to adapt actions to environmental changes, to suppress impulses which violate norms and to control impulsive actions (Harnishfeger, 1995).
In a recent review, we summarised the main tests available to assess cognitive or behavioural inhibition in neurodegenerative diseases. The tools currently used to measure disinhibition are often incomplete and do not show sufficient psychometric properties (sensitivity, specificity) for the bvFTD population (Migliaccio et al., 2020). Current tests are most often developed in a patient population other than FTD, such as Alzheimer's disease (see for example the Mattis Dementia Rating Scale, Mattis DRS) (Mattis, 1976), or patients with frontal lesions (see for example the Hayling Sentence Completion Test, HSCT) (Burgess and Shallice, 1996), and are therefore less adapted to other disorders. In addition, these tests usually involve language, and even when norms are established in other populations, they do not always sufficiently account for language and cultural differences. Behavioural disinhibition is usually well assessed with the classical questionnaires administered to caregivers but the severity and frequency of symptoms are greatly affected by the perception of the latter. Moreover, these tools were not developed to allow the differentiation of different types of behavioural disinhibition, which is a crucial aspect due to the possibility these may require different forms of management. Indeed, differentiating disinhibition subtypes would allow to refine the diagnosis and to improve the management of the patients. Behavioural disinhibition encompasses a broad spectrum of behaviours that are likely to require tailored approaches. More generally, complex behaviours and their disorders are inherently difficult to capture through questionnaires and assessment scales, and their assessment could therefore benefit from an ecological observation approach.
Moreover, the neural bases of behavioural disinhibition are still debated. Brain-behavioural correlation studies in bvFTD report behavioural disinhibition (mostly assessed by questionnaires) as arising from different patterns of grey matter damage globally involving the orbitofrontal cortex (OFC) (Peters et al., 2006, Massimo et al., 2009, Krueger et al., 2011), the temporal lobe (Massimo et al., 2009, Zamboni et al., 2008, Paholpak et al., 2016, O'Connor et al., 2017), the cingulate cortex (Krueger et al., 2011, Rosen et al., 2005) and subcortical structures (Zamboni et al., 2008, García et al., 2015). White matter tracts studies, which are less common, also reported heterogeneous results showing alterations of uncinate fasciculus (Hornberger et al., 2011, Santillo et al., 2016), right cingulum (Santillo et al., 2016), superior longitudinal fasciculus (Borroni et al., 2007, Sheelakumari et al., 2020) and right corona radiata (Powers et al., 2014). These correlates suggest that a large network, or more probably, several discrete networks underlie disinhibition.
Taking these limitations and considerations into account, we recently proposed a behavioural study using an ecological paradigm mimicking a real-life situation, under controlled conditions and a structured scenario (ECOCAPTURE, FRONTlab, ICM), to identify objectively and quantify behavioural disorders, such as apathy and disinhibition (Batrancourt et al., 2019, Godefroy et al., 2021). We have previously proposed a precise description of behavioural disinhibition in bvFTD based on the literature review (Paholpak et al., 2016, Rascovsky et al., 20112011, Snowden et al., 2001) which involves a list of 16 behaviours of interest potentially observable in the context of the ECOCAPTURE scenario. In this previous study, we distinguished three disinhibition categories: compulsivity, impulsivity and social disinhibition (Godefroy et al., 2021). We found that a bivariate model fit the data from our bvFTD patients well, defining a two-dimensional structure for the classification of behaviours related to inhibition deficits. This model segregated compulsivity and social disinhibition, while impulsivity was shared between the two extracted dimensions. This previous result is in line with a study by Snowden et al. (Snowden et al., 2001) which suggested that behavioural profiles of patients with FTD follow two principal dimensions, possibly corresponding to compulsivity and social disinhibition.
Thus, based on the literature and our previous work (Godefroy et al., 2021), we decided for the present study to focus on these two subtypes of inhibition deficits, which seem particularly relevant for the exploration and characterization of disinhibition in bvFTD patients. Compulsivity involves dysfunctional inhibition of thoughts and behaviour and could be defined as “actions inappropriate to the situation which persist, have no obvious relationship to the overall goal and which often result in undesired consequences” (Dalley et al., 2011), and it often results in an inability to flexibly adapt behaviours and/or switch attention between stimuli (Fineberg et al., 2014, Fineberg et al., 20182018). These behaviours range from simple repetitive movements to more complex ritualistic behaviours or stereotypies (Rascovsky et al., 20112011, Rosso et al., 2001). Social disinhibition is defined as behaviours violating social norms and social graces (Rascovsky et al., 2011). These major symptoms are respectively reported in 71% to 80% (Miller and Llibre Guerra, 2019, Seeley, 2019) and 65% to 98% (Bang et al., 2015, Desmarais et al., 2018) of bvFTD patients.
In this work, we wanted to demonstrate the validity of our semi-ecological approach firstly by investigating behavioural disinhibition and its subtypes in bvFTD and in healthy controls (HC), and then by correlating behavioural scores with traditional paper-and-pencil neuropsychological tests. Finally, we wanted to identify the neuroanatomical correlates of these two different subtypes of disinhibition. We have correlated disinhibition scores, obtained in our semi-ecological setting, with grey and white matter damage indexes using voxel-based morphometry (VBM) and white matter tractography. The study of white matter tracts provides an overview of the structural connectivity, a complementary approach to the classical tools of cortical analysis, which help to fully describe the neural bases of behavioural disinhibition.
We hypothesised that: 1) our semi-ecological approach would be able to differentiate bvFTD patients and HC on the basis of two different subtypes of behavioural disinhibition previously established (Godefroy et al., 2021), 2) these subtypes would be associated with distinct cognitive measures of inhibition deficits; and 3) these subtypes would be related to different neural networks, in term of grey and white matter.
2. Materials and methods
2.1. Participants
A total of 17 bvFTD patients were recruited to the clinical observational study (ECOCAPTURE, Clinicaltrials.gov: NCT03272230) from an AP-HP (Paris Public Hospitals) expert clinical site (the “National reference centre on FTD”, “Institut de la Mémoire et de la Maladie d’Alzheimer”-IM2A) at the Pitié-Salpêtrière Hospital. Inclusions were carried out between September 2017 and January 2021. Patients were diagnosed according to the International Consensus Diagnostic Criteria (Rascovsky et al., 2011). Eighteen HC were recruited by public announcement. HC subjects were matched to bvFTD patients for age, gender and education level. All participants underwent the same cognitive and behavioural assessments, as well as the same MRI protocol for the study. The demographic characteristics, cognitive tests and behavioural disinhibition scores of bvFTD patients and HC are described in Table 1. Table 1 also reports tests performed in clinical practice and available only for patients. Among bvFTD patients, two patients were taking medications related to bvFTD: one took cyproterone acetate to manage sexual compulsions, and the other took risperidone to limit aggressivity.
Table 1.
Demographic, cognitive and behavioural characterisation of patients and healthy controls.
| bvFTD (n = 17) | HC (n = 18) | p-value | |
|---|---|---|---|
| Women/Men | 5/12 | 10/8 | 0.12a |
| Age (years) | 64.4 (8.3) | 62.6 (7.2) | 0.42b |
| Disease duration (years) | 4.4 (2.3) | – | |
| Education (years) | 14.3 (4.9) | 13.8 (2.2) | 0.69.c |
| Cognitive tests (/max score) | |||
| MMSE (/30) | 23.7 (2.6) | 29.4 (0.8) | ***b |
| FAB (/18) | 12.5 (3.4) | 17.3 (0.8) | ***b |
| Mattis DRS (/144) | 119.2 (9.0) | 142.2 (1.3) | *** c |
| Picture naming task (/40) | 36.2 (3.6) | 39.4 (0.9) | *** b |
| Verbal semantic matching task (/40) | 36.5 (3.6) | 39.2 (1.1) | ** b |
| Direct verbal span | 5.3 (1.7) | – | |
| Indirect verbal span | 3.5 (1.2) | – | |
| Category fluency | 13.8 (7.3) | – | |
| Phonemic fluency | 8 (5.1) | – | |
| Hayling error score (/42) | 20.1 (15.1) | 3.1 (2.6) | ***b |
| Hayling time B-A | 119.4 (114.4) | 37 (17.9) | **c |
| Mini-SEA faux-pas (/15) | 8.3 (3.5) | 13.5 (1.1) | ***c |
| Mini-SEA recognition (/15) | 9.0 (2.5) | 13.0 (0.9) | ***c |
| Behavioural metrics | |||
| Compulsivity | 10.7 (17.3) | 0.2 (0.9) | ** b |
| Social disinhibition | 8.7 (6.9) | 3.3 (5.0) | ** b |
Data are given as mean (SD).
*P < 0.05; **P < 0.01; ***P < 0.001.
Abbreviations = bvFTD: behavioural variant frontotemporal dementia; FAB: frontal assessment battery; Hayling time B-A: time to complete part B of the test minus time to complete part A of the test. Hayling error score: total error score on the Hayling Test; Mattis DRS: Mattis Dementia Rating Scale; Mini-SEA: mini- social & emotional assessment; MMSE: mini-mental state examination; n.s. = non-significant.
Chi-square test.
Wilcoxon test.
Aspin Welch test.
2.2. General cognitive assessment
All patients underwent a general cognitive battery including several neuropsychological tests. The Mini-Mental State Examination (MMSE) and the Mattis Dementia Rating Scale (Mattis DRS) (Foss et al., 2013), a widely used dementia screening instrument, exploring attention, initiation, perseveration, construction, conceptualization, and memory, were administered to assess overall cognitive performance. Executive functions were evaluated with the Frontal Assessment Battery (FAB) (Dubois et al., 2000). Language skills and semantic memory were assessed using the GRECO semantic battery (BECS-GRECO) (Merck et al., 2011), that included a picture naming task (DENO 40), and a verbal semantic matching task. Additionally, verbal working memory was assessed using a verbal span test (direct and indirect orders) (Wechsler, 1981). Finally, a verbal fluency test measuring category (semantic) and phonemic (lexical) fluency (Cardebat et al., 1990) explored the integrity of lexical and semantic representations, as well as executive functions.
2.3. Cognitive assessment of disinhibition
All participants, patients and HCs underwent two specific tests to investigate cognitive inhibition deficits as well as social cognition disorders. Cognitive inhibition was assessed through the Hayling Test (Burgess and Shallice, 1996). Participants were asked to complete 15 sentences using the appropriate word (automatic condition, part A), and 15 sentences using a completely unconnected word (inhibition condition, part B), as quickly as possible. Two outcome measures were used: the Hayling time B-A (total time to complete sentences in part A subtracted from the total time to complete sentences in part B) and the Hayling error score (reflecting errors in part B). It has been demonstrated that this test is a reliable measure of cognitive inhibition impairments in bvFTD (since the earliest stage in pre-symptomatic C9orf72 mutation carriers) (Montembeault et al., 2020). To evaluate the cognitive aspect of social disorders, participants performed the mini-Social cognition & Emotional Assessment (mini-SEA) orbitofrontal battery, composed of a reduced version of the faux-pas test, assessing theory of mind deficits, and a facial emotion recognition test using 35 Ekman faces, assessing the ability to recognise emotions (Funkiewiez et al., 2012). The faux-pas test data was missing for one patient.
2.4. Behavioural assessment of disinhibition
2.4.1. ECOCAPTURE protocol
This study is part of the ECOCAPTURE protocol designed to obtain objective measures of behavioural syndromes, such as apathy or disinhibition in participants undergoing a 45-minute structured scenario (Batrancourt et al., 2019, Godefroy et al., 2021). The ECOCAPTURE paradigm mimics a naturalistic situation (i.e., waiting comfortably in a waiting room) under controlled conditions. Experiments took place into a functional exploration platform (PRISME, ICM core facility, Salpêtrière hospital, Paris, France) transformed into a fully furnished waiting room (Supplementary Fig. 1).
Participants were asked to wait in the room prior to the following cognitive tests, with their instructions being to make themselves comfortable and to enjoy the room. This was to promote the ecological validity of the context in which their behaviour was recorded. The room contained specific objects that provided opportunities for subjects to interact with their environment and pass the time (games, magazines, food and drink, furniture such as a sofa, chairs, tables, etc). The scenario was divided in five different phases: 1) a freely moving phase (7 min) during which the participant is explicitly encouraged to explore the room; 2) a freely moving phase with eye-tracking glasses (7 min); 3) a positive or negative stimulation phase (playing pleasant music or a crackling noise, 7 min); 4) an externally guided phase consisting of filling out a questionnaire (e.g., questions about items present in or absent from the room) using pens of different colours which had to be found in the room, making the subject interact with the environment (10 min); and 5) a second stimulation phase (negative if the first one was positive, and vice versa, 7 min). Between each phase, the investigator entered the room to interact with the subject and provide him/her instruction for the next phase of the scenario.
The PRISME platform is equipped with a six-camera system covering the entire waiting room, which allows the direct observation of the subject and video recording for subsequent analysis.
2.4.2. Disinhibition behaviours studied
In a previous study, (Godefroy et al., 2021) we had defined a list of behaviours related to disinhibition potentially observable in the context of the ECOCAPTURE scenario, according to the definitions of symptoms by Rascovsky et al. (Rascovsky et al., 2011) and to previous relevant studies in the field (Krueger et al., 2011, Dubois et al., 2000). Based on symptom descriptions and classification in these works, we had proposed a description of behavioural disinhibition by distinguishing three concepts related to inhibition troubles: compulsivity, impulsivity and social disinhibition. By using an exploratory factor analysis, we identified two patterns of inhibition deficits: compulsivity and social disinhibition.
Within this framework, 11 behaviours extracted from the literature and our previous experience were organised into these two categories: compulsivity (e.g. repetitive movements, perseveration) and social disinhibition (e.g. unwarranted or excessive familiar behaviour towards the investigator, lack of manners). The detailed ECOCAPTURE ethogram with some examples for each behaviour is shown in Table 2. See the complete ECOCAPTURE ethogram at Mendeley Data (Batrancourt et al., 2022).
Table 2.
Ethogram listing the 11 disinhibited behaviours and their definition.
| Behaviour label | Definition | Example |
|---|---|---|
| Compulsivity | ||
| Utilisation behaviour (Snowden et al., 2002) | Grasping and touching objects of the environment without any contextual reason | Opening and closing the window without any real purpose |
| Perseveration (Snowden et al., 2002) | Difficulty in shifting mental set and behavioural perseveration | Keep trying to open the tap unsuccessfully (no running water in the room) |
| Repetitive movements (Rascovsky et al., 2011) |
Repeating stereotyped, compulsive/ritualistic behaviours | Rubbing hands |
| Compulsive eating (Rascovsky et al., 2011) | Eating excessive amounts of food in the absence of real hunger and/or inappropriate foods in the specific context | Eating canned sardines just after breakfast |
| Social Disinhibition | ||
| Aggressive behaviour towards investigator (Rascovsky et al., 2011) | Showing hostility, verbal or physical aggressiveness towards the investigator | Angrily yelling “Come in” when the investigator knocks repeatedly at the door |
| Familiar behaviour towards investigator (Rascovsky et al., 2011) | Showing inappropriate familiarity towards the investigator | Speaking in inappropriately colloquial language |
| Nudity (Rascovsky et al., 2011) | Exposing inappropriate parts of one's body | Removing one's pants |
| Harsh handling of objects (Rascovsky et al., 2011) | Handling an object of the room in a way which may cause potential damage, thus showing lack of respect for other people’s possessions | Trying to break a locker box instead of searching for the key |
| Inappropriate gesture or posture (Rascovsky et al., 2011) | Impolite, inappropriate physical behaviour in a social context | Picking one’s nose/teeth |
| Lack of decorum (Rascovsky et al., 2011) | Failing to respect cultural norms of politeness | Yawning, sneezing or coughing without covering one’s mouth |
| Disregards for rules or investigator | Lack of response to social cues, ignoring instructions given by the investigator | Not answering the investigator’s questions |
Behavioural coding data were collected through the continuous sampling method (all occurrences of behaviours were recorded) using The Observer XT (Noldus). Behavioural coding was conducted based on the definitions of this ethogram. The number of occurrences of each disinhibited behaviour was summed for each participant, and these 11 sub-scores were then summed within each behavioural category to obtain a global scores of compulsivity and social disinhibition, specific to each individual.
Thus, for each subject, behavioural data was obtained by behavioural coding from 45-minute footage, using a manual video annotation tool (The Observer XT®, Noldus) (DT). Behavioural coding data were collected through the continuous sampling method (all occurrences of behaviours were recorded) and conducted based on the ECOCAPTURE ethogram (Table 2). The number of times a behaviour of interest occurs per video during the 45-minute sample session was counted in each individual. These behaviours are instantaneous events without an appreciable duration, and such behaviours can be scored as present, and reported as occurrences. Thus, this analysis yielded a set of 11 metrics (one per behaviour) measuring the number of occurrences of each behaviour, in each participant. These 11 sub-scores were then summed together within each behavioural category to obtain two global scores, one for compulsivity and one for social disinhibition, specific to each individual. If one cannot deny the part of subjectivity inherent to the coder, behavioural coding was conducted according to the already established ethogram (an objective and detailed resource) with specific guidelines. In addition, among the 35 subjects, eight videos were coded by deux independent coders (DT, VG) to assess the intercoder reliability through the calculation of the intraclass correlation coefficient. The calculated intraclass correlation coefficients were all between 0.80 and 1, indicating very high reliability.
2.5. Behavioural and cognitive analyses
All statistical analyses on behavioural and neurocognitive data were performed using RStudio 1.2.5033. with p-values under 0.05 considered statistically significant. Chi-squared tests were used for gender comparisons, Shapiro-Wilk tests were used to test data normality and Fisher’s test for variances equality. For data normally distributed with or without equal variance, Student’s t-tests and Aspin-Welch tests were used respectively. For not normally distributed data, Mann-Whitney U-tests were used. In this study we were interested in the difference between bvFTD patients and HCs on neuropsychological scores and ECOCAPTURE behavioural disinhibition metrics. We calculated the effect size (Cohen's d) of the population. The effect sizes were large (range 0.71–0.88) for the cognitive and executive scores (MMSE, FAB, Mattis DRS, mini-SEA), ranged from medium to large (0.47–0.63) for the cognitive disinhibition scores (Hayling B-A, Hayling error score) and the semantic battery outcomes (direct verbal span, indirect verbal span), and were medium for the ECOCAPTURE behavioural disinhibition metrics (0.4).
2.6. MRI acquisition
Structural MRI acquisitions were performed at CENIR (Human MRI Neuroimaging core facility, ICM, Salpêtrière hospital, Paris, France) using a 3 T Siemens MRI scanner 64-channel TIM system. The brain MRI protocol included a 3D T1 scan allowing the study of structural abnormalities. Diffusion weighted imaging (DWI) data was acquired using a single-shot spin-echo sequence with 60 directions, covering the whole head with a posterior-anterior phase acquisition (b0 = 0 s.mm−2, b = 2000 s.mm−2, TE = 75 ms, TR = 3500 ms, flip angle = 90 degrees, field of view = 208 mm2, voxel size = 1.8 × 1.8 × 1.8 mm3).
2.7. Voxel-based morphometry analysis
Images were processed using VBM implemented in Statistical Parametric Mapping (SPM12) running under ®MATLAB R2017b (Mathworks, Natick, MA).
2.7.1. Pre-processing
T1-weighted images were segmented to generate the roughly aligned grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) tissue probability maps in the Montreal Neurological Institute (MNI) space for each subject. Affine registered tissue segments were then used to create a custom template using the DARTEL (diffeomorphic anatomical registration using exponentiated Lie algebra) approach (Ashburner, 2007). For each participant, the flow fields were calculated during template creation, describing the transformation from each native GM image to the template. These were then applied to each participant’s GM image, to warp them to the common DARTEL space. The VBM analysis was based on modulated GM images, where the GM for each voxel was multiplied by the Jacobian determinant derived from spatial normalisation to preserve the total amount of GM from the original images (Ashburner and Friston, 2000). Since the DARTEL process warps to a common space that is smaller than the MNI space, we performed an additional transformation as follows: the modulated images from DARTEL were normalized to the MNI template using an affine transformation estimated from the DARTEL GM template and the a priori GM probability map without resampling (http://brainmap.wisc.edu/normalizeDARTELtoMNI). The resulting modulated and normalised images were then smoothed using a 3D Gaussian filter of 8 mm full-width-half-max. These smoothed GM images were used for statistical analysis.
2.7.2. Statistical analyses
Whole brain statistical comparison between groups (patients vs controls) were performed using a two-sample t-test with age, gender and total intracranial volume (TIV) as nuisance covariates.
The relationship between neuropsychological scores, behavioural data and grey matter atrophy was tested by fitting multiple regression statistical models. Specific matrices were designed for each score of interest, with age, gender and TIV as nuisance covariates. These correlations were performed on the combined bvFTD patients and HC groups in order to provide greater variance in scores and to increase the statistical power to detect relationships with GM, in line with previous studies (Sollberger et al., 2009, Strikwerda-Brown et al., 20212021). In order to clarify the correlations obtained with bvFTD patients and HC groups combined, scatter plots representing each subject related to the global maxima (main site of atrophy associated with compulsivity and social disinhibition) are provided in Fig. 3. To visualise whether HC and bvFTD groups differ, partial regression plots of residual GM volume associated with compulsivity/social disinhibition, influenced by the covariates (age, sex and TIV) in bvFTD and HC were also added in Fig. 3. As additional information, correlations performed on the bvFTD patient group only are available in Fig. 3 and Supplementary Table 2.
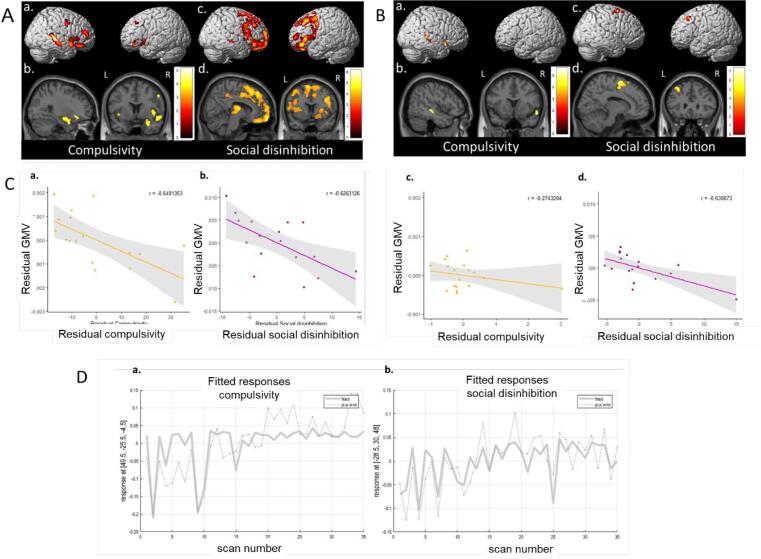
Fig. 3.
Regions of significant grey matter atrophy associated with compulsivity (a,b) and social disinhibition (c,d) in behavioural variant frontotemporal dementia patients and healthy controls (A) and in behavioural variant frontotemporal dementia patients only (B). Results are displayed in neurological convention on the 3-dimensional brain template and on axial and sagittal slices of the MNI standard brain (P < 0.001 uncorrected). L: left; R: right. (C) Partial regression plots of residual grey matter volume associated with residual compulsivity (a,c) and residual social disinhibition (b,d) influenced by the covariates age, sex and total intracranial volume in behavioural variant frontotemporal dementia patients (a,d) and healthy controls (c,d). Grey matter volumes were extracted from all clusters of significant grey matter atrophy associated with compulsivity and social disinhibition. (D) Scatter plots with marked subjects related to the global maxima of atrophy associated with compulsivity (a) and social disinhibition (b) in behavioural variant frontotemporal dementia patients and healthy controls. The 17 first scans are bvFTD patients, the 18 lasts scans are HC.
The brain-behaviour correlation was tested using [-1] or a [+1] t-contrast, assuming that higher or lower scores respectively, indicating poor performances, would be associated with decreased GM volumes.
The relationship between performance and grey matter intensity was considered significant at P < 0.001 corrected at the cluster level. The highlighted anatomical regions were identified using the automated anatomical labelling atlas 3 (aal3) software (Rolls et al., 2020).
2.8. Diffusion tensor imaging analysis
Fibre tracking was performed using 3D Slicer (https://www.slicer.org). Three patients were removed from the analysis as they did not undergo the complete MRI protocol and therefore had no DWI acquisition. One HC presenting an abnormal acquisition was also removed from the analysis. WM analysis was thus conducted on 14 bvFTD patients and 17 HC.
2.8.1. Pre-processing
T1-weighted (T1w) images were denoised using the non-local means algorithm included in the Dipy library (Descoteaux et al., 2008) and the inhomogeneity correction was performed by means of the N4 bias field correction algorithm from the Advanced Normalization Tools (Tustison et al., 2010). The ROBEX brain extraction tool (Iglesias et al., 2011) was then used to extract the brain mask, used in the subsequent co-registration steps. DWI were denoised, Gibbs ringing artifacts were removed and intensity inhomogeneities were corrected using the MRtrix3 suite (Tustison et al., 2010, Tournier et al., 2019). Then, a tensor model was fit to the DWI image intensities using the weighted least squares method included in SlicerDMRI (Norton et al., 2017, Zhang et al., 2020) to generate diffusion tensor images (DTI). Additional metrics were extracted from the DTI: Fractional Anisotropy (FA), Mean Diffusivity (MD), Radial Diffusivity (RD) and Axial Diffusivity (AD) maps.
A deformable registration of the b0 DWI with the T1w structural image was calculated for each subject using the BRAINSFit tool from 3D Slicer (Johnson et al., 2007). The DTI was then resampled in 3D Slicer with preservation of the principal direction using the transformation previously calculated to be finally co-registered with the T1w (Alexander et al., 2001).
2.8.2. Fiber-tracking
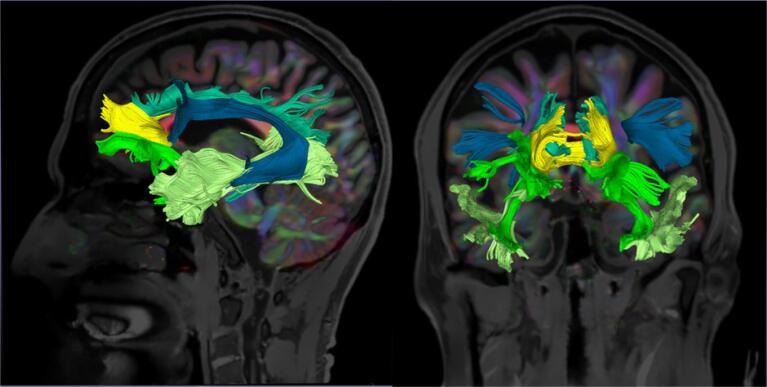
Taking in account previous knowledge about fibres bundles damage and disinhibition (Hornberger et al., 2011, Santillo et al., 2016, Borroni et al., 2007, Sheelakumari et al., 2020), we dissected five pathways: the cingulum, the forceps minor (FM), the uncinate fasciculus (UF), the inferior longitudinal fasciculus (ILF), and the arcuate fasciculus (AF) (Fig. 1). We also dissected the corticospinal tract (CST) as a control bundle. In order to isolate these tracts in the DTI data set, deterministic seeded tractography was used with defined “start/first” and “end/second” regions of interest (ROIs), as follows. The T1w structural images were segmented via registration to a pre-annotated in-house anatomical template (Haegelen et al., 2013). ROIs were manually drawn on the axial, coronal, and sagittal FA images onto this template before being deformably registered to the subject T1w images using the BRAINSFit tool from 3D Slicer (Johnson et al., 2007).
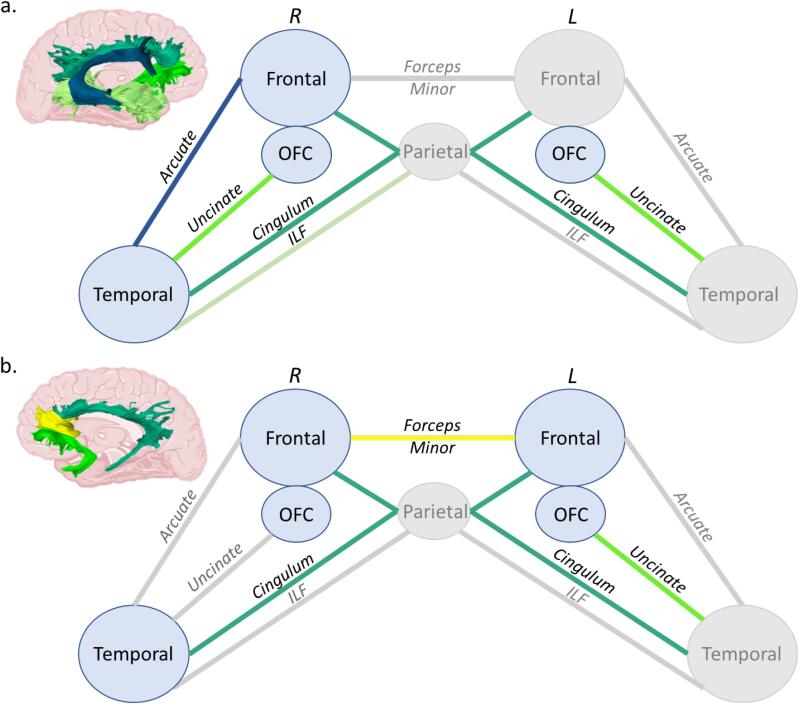
Fig. 1.
Illustrative reconstruction of the tracts of interest in a sagittal (left) and a coronal (right) view. The cingulum (light blue), the forceps minor (yellow), the uncinate fasciculus (neon green), the inferior longitudinal fasciculus (light green) and the arcuate fasciculus (dark blue) are displayed. These data are from a behavioural variant frontotemporal dementia patient (64 years old, male): the tracts of interest are derived from 3D Slicer and overlaid on the T1-weighted image associated to the fractional anisotropy map.
ROIs were delineated based on previous tractography works (Wakana et al., 2007, Catani and Thiebautdeschotten, 2008), and detailed in supplementary material.
A deformable registration of the template with the T1w image was computed for each subject and the ROIs were resampled using the resulting transformation by means of the BRAINS Resample module in 3D Slicer. The purpose of this registration was to obtain an approximate localisation of the ROIs on each patient T1w image to serve as seeding points for the tractography algorithm. The ROIs placements were visually inspected and manually corrected if required using the DTI and T1w images as references. Deterministic region-based tractography was performed using ROIs previously computed with an FA threshold of 0.15 and an angular threshold of 45 degrees, as these parameters provided convenient results in previous studies in neurodegenerative diseases (Migliaccio et al., 2012, Forkel et al., 2020). The ILF and AF were more difficult to track and for them a specific visualisation and tracking in native space for each subject was performed.
All tracts were successfully identified in all subjects, except for the FM which was missing in two patients, the right UF missing in one patient and the right AF missing in one patient and two HC.
The average FA, MD, RD, and AD values along each track were computed using 3D Slicer, allowing for the distribution of these diffusion characteristics (reflecting the WM integrity) to be collected for each of the fibres of interest.
2.8.3. Statistical analyses
The comparison of diffusion metrics values between bvFTD patients and HC were analysed through a mixed linear model with group as fixed factor, age and gender as random factors (using packages {lme4} (Bates et al., 2015) and {car} (Fox and Sanford, 2019) in RStudio 1.2.5033). The relationship between neuropsychological scores, behavioural data and DTI metrics of tracts were examined using Spearman correlations and linear regression models. Influence of age and gender on these variables was previously controlled. Model assumptions were checked and the logarithm of the dependent variable was used to achieve normality if needed.
2.9. Ethical statement
This study is part of clinical trial C16-87 sponsored by INSERM. It was granted approval by the local Ethics Committee, or “Comité de Protection des Personnes,” on May 17, 2017 and registered in a public clinical trial registry (clinicaltrials.gov: NCT03272230). All study participants gave their written informed consent to participate, in line with French ethical guidelines.
2.10. Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request, while respecting the anonymity of the participants. The neuropsychological data and the ECOCAPTURE behavioural metrics are available on Mendeley Data (Tanguy et al., 2022). The ECOCAPTURE ethograms (coding scheme) used for behavioural coding from video are available on Mendeley Data (Batrancourt et al., 2022).
3. Results
3.1. Demographic and neuropsychological subjects’ characteristics
Demographic and neuropsychological characteristics of all participants are shown in Table 1. BvFTD patients did not differ in terms of age, gender and education in comparison to HC. As expected, bvFTD patients presented lower scores than HC on MMSE, FAB, on both scores of the Hayling test and on the faux-pas and emotion recognition subtests of the mini-SEA.
3.2. Behavioural disinhibition: Group comparison
BvFTD patients showed higher compulsivity (W = 219.5, p = 0.0046) and social disinhibition (W = 239.5, p = 0.0042) than HC (Table 1 and Supplementary Fig. 2).
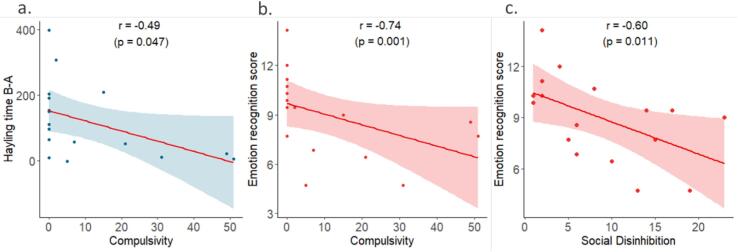
3.3. Correlations between cognitive and behavioural disinhibition
Within the patient group, we found a correlation between the Hayling time B-A and the compulsivity score (r = -0.49, p = 0.047) but not with the social disinhibition score. We did not find any correlation between the Hayling error score and behavioural disinhibition. Both compulsivity and social disinhibition scores were correlated to the emotion recognition score of the mini-SEA (r = -0.74, p = 0.001 and r = -0.6, p = 0.011 respectively) (Fig. 2). We did not find any correlation between cognitive and behavioural disinhibition in the HC group.
Fig. 2.
Significant correlations between the Hayling time B-A and compulsivity (a) and between the emotion recognition score and compulsivity (b) and social disinhibition (c). r: Spearman’s correlation coefficient, p: p-value derived from the Spearman’s correlation.
3.4. Voxel-based morphometry: groups’ comparison
In comparison to HC, bvFTD patients showed a large area of GM atrophy including bilateral frontal and temporal lobes, the middle cingulate, as well as in bilateral insula and right thalamus (P < 0.05, FWE corrected, Supplementary Fig. 3 and Supplementary Table 1).
3.5. Voxel-based morphometry: Correlations with behaviours
Results of multiple regression analysis relating scores of compulsivity and social disinhibition to GM atrophy are presented in Fig. 3 (P < 0.001 uncorrected with a cluster-extent threshold of 174 and 166 -expected number of voxels per cluster- for compulsivity and social disinhibition respectively) and Table 3. Compulsivity correlated with reduced GM volume in bilateral orbitofrontal cortices, middle frontal gyri, right superior and middle temporal gyri, precentral gyrus, thalamus, amygdala and bilateral insula. Social disinhibition correlated with a large pattern of GM loss including bilateral frontal regions such as the OFC and the cingulate cortex, the bilateral thalamus, the left insula as well as with the right superior temporal gyrus and putamen.
Table 3.
Voxel-based morphometry results showing regions with significant grey matter decrease significantly correlating with behavioural disinhibition scores in behavioural variant frontotemporal dementia patients and healthy controls.
|
Region |
Hemisphere |
MNI coordinates |
t-score |
||
|---|---|---|---|---|---|
| x | y | z | |||
| Compulsivity | |||||
| Middle frontal gyrus | Left | −27 | 27 | 41 | 3.89 |
| Right | 35 | 53 | 21 | 4.50 | |
| Orbitofrontal cortex | Left | −53 | 36 | −6 | 3.77 |
| Right | 42 | 32 | −21 | 4.15 | |
| Superior temporal gyrus | Right | 50 | −26 | −5 | 5.51 |
| Middle temporal gyrus | Right | 51 | −21 | −12 | 5.30 |
| Superior temporal pole | Right | 57 | 14 | −8 | 4.92 |
| Precentral gyrus | Right | 54 | 0 | 39 | 4.22 |
| Thalamus | Right | 9 | −24 | 15 | 4.38 |
| Amygdala | Right | 27 | 3 | −17 | 4.22 |
| Insula | Right | 44 | 9 | 0 | 4.16 |
| Left | −35 | 12 | −17 | 3.80 | |
| Social Disinhibition | |||||
| Superior frontal gyrus | Left | −18 | 32 | 38 | 3.79 |
| −17 | 63 | 21 | 4.00 | ||
| Right | 17 | 66 | 18 | 6.32 | |
| 23 | 41 | 36 | 3.48 | ||
| Middle frontal gyrus | Left | −29 | 30 | 48 | 6.36 |
| −30 | 56 | 5 | 4.51 | ||
| Right | 45 | 27 | 42 | 4.50 | |
| 38 | 36 | 38 | 4.42 | ||
| Inferior frontal gyrus, pars opercularis | Left | −39 | 17 | 11 | 4.11 |
| Right | 42 | 14 | 36 | 4.84 | |
| Inferior frontal gyrus, pars triangularis | Left | −38 | 20 | 8 | 4.54 |
| Right | 56 | 26 | 6 | 4.38 | |
| Orbitofrontal cortex | Left | −36 | 41 | −17 | 4.94 |
| −8 | 30 | −26 | 4.49 | ||
| Right | 41 | 53 | −15 | 4.34 | |
| 8 | 63 | −21 | 5.88 | ||
| Superior temporal gyrus | Right | 60 | −21 | −6 | 4.09 |
| Anterior cingulate gyrus | Left | −5 | 36 | 27 | 4.36 |
| Right | 9 | 32 | 29 | 4.09 | |
| Middle cingulate gyrus | Left | −6 | −41 | 53 | 4.13 |
| Right | 5 | −36 | 47 | 3.97 | |
| Thalamus | Left | −11 | −9 | 11 | 4.74 |
| Right | 15 | −12 | 12 | 5.15 | |
| Putamen | Left | −29 | 6 | 11 | 3.94 |
| Right | 29 | 9 | 9 | 4.14 | |
| 23 | 6 | −11 | 3.67 | ||
| Insula | Left | −36 | 20 | 6 | 4.51 |
Additional correlation analyses with traditional paper-and-pencil tests for cognitive inhibition and social cognition showed that the Hayling error score correlated with GM atrophy in the bilateral frontal lobes, orbitofrontal cortex and thalamus; the emotion recognition score correlated with atrophy in the frontal, orbitofrontal, temporal and cingulate cortices as well as with the thalamus and putamen (Supplementary Table 2). No correlation was found for the Hayling time B-A.
3.6. Diffusion tensor imaging: groups’ comparison
We investigated DTI metrics values in five white matter tracts connecting grey matter regions previously found to correlate with disinhibition: cingulum, FM, UF, ILF and AF. Mixed linear models with age and sex as random effects showed significant white matter damage involving all these tracts in bvFTD patients compared to HC. More specifically, bvFTD patients had lower FA and higher MD, RD and AD in the right UF compared with HC. They also had lower FA and higher MD and RD in the right cingulum and AF, the FM and the left UF. Relative to HC, bvFTD patients showed significantly lower FA and higher RD in the left cingulum and AF. They also showed higher MD, RD and AD in the right ILF and higher MD and RD in the left ILF. There was no difference for the CST between bvFTD patients and HC. A summary table of group comparisons for each DTI metric in the six bundles is available in Supplementary Table 3.
3.7. Diffusion tensor imaging: Correlations with behaviours
By using a step wise regression method, we first found no influence of age or sex on DTI metrics and disinhibition scores. We then used linear regression models to describe the relationship between DTI metrics and disinhibition scores.
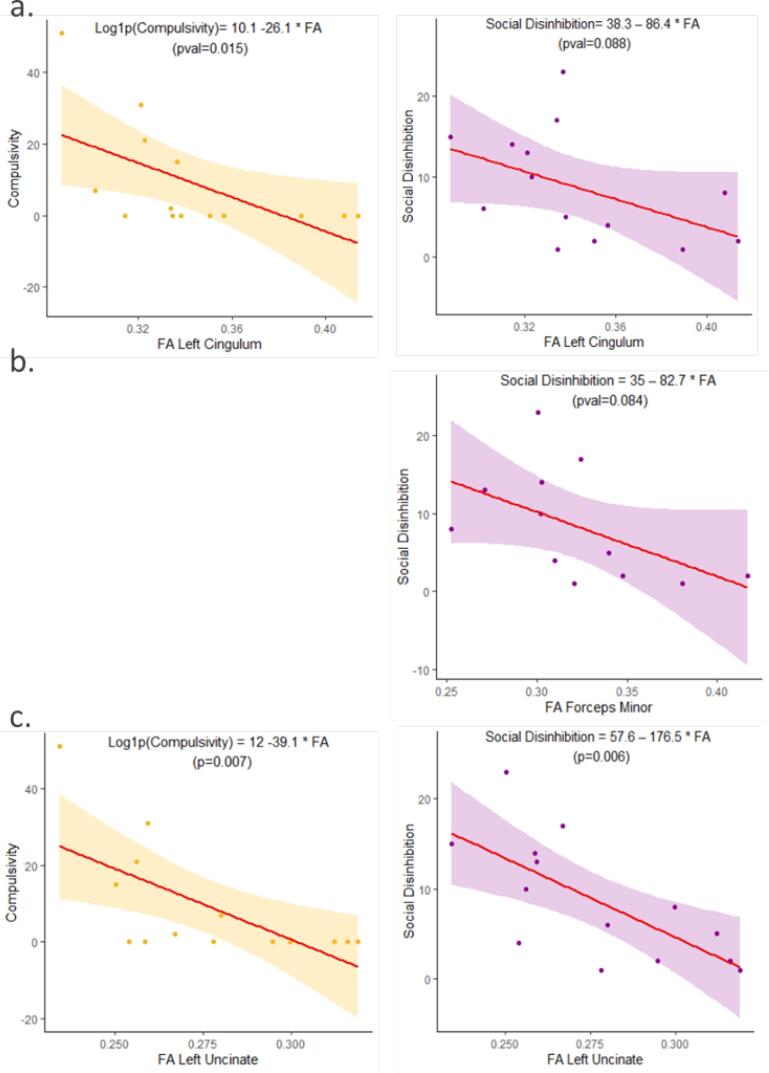
The compulsivity score was negatively correlated with WM integrity (decreased FA or increased MD, RD or AD) in the bilateral cingulum, the bilateral UF, the right ILF and the right AF. Social disinhibition was negatively correlated with WM integrity of the FM, the left cingulum and the left UF. There was also a trend for social disinhibition to positively correlate with RD in the right cingulum (r = 0.53; p = 0.053). Linear regression models describing the relationship between behavioural disinhibition and FA with a significant p-value are displayed in Fig. 4.
Fig. 4.
Behavioural disinhibition and fractional anisotropy of white matter tracts. Linear regression model and scatter plots of compulsivity and social disinhibition scores and fractional anisotropy in the left cingulum (a), the forceps minor (b) and the left uncinate fasciculus (c) in behavioural variant frontotemporal dementia patients. Equations and p-values are derived from the linear regression model.
Correlation analysis with the cognitive tests showed that the Hayling error score correlated with alteration of the right cingulum, right AF and the left UF; the emotion recognition score correlated with impairments in the bilateral cingulum and the left UF and AF. As illustration, linear regression models describing the relationship between cognitive tests and FA with a significant p-value in the left cingulum and the left UF are displayed in Supplementary Fig. 4. No correlation was found for the Hayling time B-A. All linear regression models and Spearman’s correlation coefficients describing the correlation of disinhibition with DTI metrics extracted from bundles of interest are available in Supplementary Table 4.
4. Discussion
In the present work, we used an original semi-ecological approach to identify and assess two specific dimensions of behavioural disinhibition, compulsivity and social disinhibition, their association with classic cognitive tests, as well as their neuroanatomical correlates. As expected, the observation in our semi-ecological setting was able to characterise patients as more compulsive and socially disinhibited than HC. We found that the behavioural metrics extracted from the semi-ecological task were correlated with cognitive performance: compulsivity correlated with Hayling test, and both compulsivity and social disinhibition correlated with mini-SEA emotion recognition subtest. Finally, compulsivity and social disinhibition correlated with distinct patterns of grey matter atrophy and white matter bundles impairments, suggesting that these two forms of disinhibition are underpinned by different brain networks. Compulsivity mainly correlated with a right-sided brain network, including the right temporal regions and subcortical structures, the right inferior longitudinal and arcuate fasciculi, but also bilateral cingulum and uncinate fasciculus. Social disinhibition was associated with a large anterior brain network, including bilateral frontal, orbitofrontal and cingulate regions, as well as subcortical structures, forceps minor, bilateral cingulum and the left uncinate fasciculus.
It is interesting to note that correlations performed within bvFTD patients only still suggest the involvement of the right temporal lobe for compulsivity, and bilateral involvement of the medial and dorsolateral frontal cortices for social disinhibition, highlighting clearly separate areas. We discuss these results in light of the literature relating compulsivity and social disinhibition in FTD and other brain disorders to classical cognitive testing, and we interpret neuroimaging findings within the framework of known brain networks.
4.1. Behavioural observation and cognitive testing
One of the aims of this work was to estimate the correspondence between our observational approach and classic cognitive tests routinely used in clinical practice.
Direct behavioural observations in the natural environment or in a simulated setting under controlled conditions, associated or not with a structured scenario, are promising methods of exploration of human behavior (Burgess and Stuss, 2017). However, we acknowledge that such ecological methods, using naturalistic paradigms, require sophisticated training and are not yet easily applicable in a clinical context. In addition, the classification of disinhibited behaviours in different categories is arbitrary in some way, although it was based on the literature (Paholpak et al., 2016, Rascovsky et al., 20112011, Snowden et al., 2002) and our previous study. To check the reliability of our novel approach, we analysed the correlations of the observed behaviours with classical cognitive assessment.
First, we found that the compulsivity score was negatively correlated to the Hayling time B-A. The shorter the reaction time, the more compulsive the patient was. The production of compulsive behaviours was thus associated to difficulties in inhibiting the expected response, these subjects giving a hasty answer which was usually incorrect. Indeed, we found a significant correlation between time B-A and the number of errors: patients who answered faster were also those who produced more errors. To our knowledge, this is the first time that this association has been reported, because usually, in pathological conditions altering inhibitory control, there is a lengthening of the response times. It is possible that this paradoxically shortened response time is unique to bvFTD, cognitive inhibition skills being so altered that patients are unable to initiate the inhibition process in order to find an inappropriate word. This element should probably be added to the Hayling test evaluation, as a parameter of compulsivity. Surprisingly, we did not find a correlation between measures of grey matter and white matter damage and the Hayling time B-A. Given the performances of the patients while they were asked to complete sentences with unrelated words, we suggest that the Hayling time B-A can be differently impaired for distinct reasons: lengthened time B-A could reflect difficulties in inhibiting the dominant response, while shortened time B-A may reflect compulsivity and cause more errors. Hence, we suppose that the relationship between pathology and Hayling time B-A is not linear, but instead follows a U-shaped curve, which makes it difficult for linear regression models to relate brain atrophy to this score.
Secondly, the social disinhibition score negatively correlated with the emotion recognition score. The emotion recognition subtest measures the ability to identify the basic facial emotions. The less the patient recognised facial emotions, the more socially disinhibited the patient was. Emotion recognition is a central element of non-verbal communication. The ability to express emotions allows an individual to give information to others who can then adjust their behaviour appropriately (Désiré et al., 2002). Thus, the ability to share and infer emotions and mental states with others is a basis for the development of social behaviour. Misidentification, misrecognition or misinterpretation of others' emotions may lead to socially inappropriate behaviours in everyday social life. In patients with traumatic brain injury, emotion recognition has been associated with difficulties in social behaviour (Milders et al., 2008, May et al., 2017). In the same vein, patients with orbitofrontal lesions show an impairment in recognition of emotions associated with inappropriate social behaviour (Jonker et al., 2015). In schizophrenia, relationships between altered emotion recognition and poor social skills have also been demonstrated (Hooker and Park, 2002, Fett et al., 2011, Pelletier et al., 2013, Horton et al., 2017). Thus, socially inappropriate behaviours appear to be trans-nosologically related to emotion recognition impairments.
In the same prospective, the compulsivity score was also associated with difficulties to recognise emotions in the mini-SEA. This finding is consistent with previous results on schizophrenia patients in which the patients’ performance on the emotion recognition task was negatively correlated with the number of perseverative errors in the Wisconsin card sorting test, a marker of cognitive inhibition disorders (Brüne, 2005).
Overall, our innovative semi-ecological approach is consistent with results obtained with cognitive tests already widely used in clinical practice and research. If behavioural and cognitive inhibition are commonly distinguished in clinical practice, these two components are intrinsically linked and rely on strong mutual interactions and influences. This distinction makes it possible to use two complementary approaches, including a more objective and direct semi-ecological approach that allows for the differentiation of several subtypes. We previously found that the stratification of patients based on these subtypes suggests different clinico-anatomical profiles of bvFTD patients (Godefroy et al., 2021). The current results reinforce our distinction between subtypes of inhibition deficits and show that they could improve the characterisation of the deficit, ultimately leading to a more appropriate clinical management of behavioural disorders, and improving patients' care. A direct application following these results could be the implementation of more adapted and relevant non-pharmacological intervention, based on a better knowledge of the underlying mechanisms of behavioural symptoms.
4.2. Brain networks
The literature regarding the brain-behaviour relationships regarding behavioural disinhibition remains at least in part non-univocal. This lack of consensus could be partially explained by the high variability in disinhibition assessment, mainly based on the use of different questionnaires with very low specificity sub-scores, and by the use of disparate neuroimaging methods. The current findings help to reconcile previous findings by pointing to compulsivity and social disinhibition as emerging from the dysfunction of different distributed network centred on the anterior brain regions and their white matter connections.
In the present study, compulsivity correlated with bilateral frontal and orbitofrontal cortices and right lateralized brain structures, including temporal cortices, as well as thalamus and amygdala and white matter bundles connecting frontal and temporal regions (Fig. 5a).
Fig. 5.
Schematic representation of the cortical regions and white matter tracts involved in compulsivity (a) and social disinhibition (b). Each white matter bundle associated with a deficit is displayed in a specific colour as in Fig. 1. Non-significant bundles are shown in grey. See also Supplementary Table 4 for statistical significance. Abbreviations = ILF: Inferior longitudinal fasciculus; L: Left; OFC; Orbitofrontal cortex; R: Right.
Frontal and orbitofrontal atrophy associated with compulsivity is in agreement with previous studies of compulsivity, showing the role of the fronto-striatal loop in the emergence of disinhibited behaviours (Cagnin et al., 2014, Whiteside et al., 2004, Robbins et al., 2019, Mendez et al., 1997, Maltête, 2016). Overall, although compulsivity may be usually seen as a loss of the fronto-striatal circuits to implement effective goal-directed behaviours and inhibit inappropriate behaviours, we also show that the temporal cortex is more involved than initially thought in compulsive behaviours.
Previous studies have demonstrated the role of the temporal lobe and its anatomical connections in compulsive behaviours of FTD (Snowden et al., 2001, Rosso et al., 2001, Cagnin et al., 2014, McMurtray et al., 2006, Perry et al., 2012). Interestingly, patients affected by right temporal variant of FTD frequently present compulsive behaviours (Ulugut Erkoyun et al., 2020) while right lateralised semantic dementia patients are described as obsessive, rigid and irritable (Kamminga et al., 2015). In the same vein, semantic dementia patients with a greater right temporal damage tend to show more obsessive behaviours than semantic dementia patients with a left lateralized brain atrophy (Pozueta et al., 2019).
More generally, the involvement of the temporal cortex has been already widely suggested in obsessive–compulsive disorders (OCD) (Choi et al., 2006), as well as in patients affected by temporal lobe epilepsy who have a high prevalence of obsessive–compulsive symptoms compared to the general population (Isaacs et al., 2004, Monaco et al., 2005). Patients with right hemisphere seizure tend to score higher than left hemisphere patients on the Obsessive-Compulsive Inventory in temporal lobe epilepsy (Isaacs et al., 2004), while right-sided temporal lobe lesions from haemorrhage or brain infarctions have been associated with the acquisition of OCD (Figee et al., 2013). Furthermore, in stroke, right sided lesions are also more prone to produce stereotypies than left sided lesions (Shukla and Pandey, 2020). Concerning the structural connectivity, a recent review on OCD reported the association between compulsivity and white matter changes within the temporal lobe (Robbins et al., 2019). These reported changes are consistent with the present brain correlates of compulsivity, that include white matter alteration of the uncinate, arcuate and inferior longitudinal fasciculi, bundles connecting the temporal lobes with frontal and more posterior brain areas.
Finally, among the sub-cortical structures, we identified a tight correlation between compulsivity and amygdala and thalamic atrophy. In this vein, more recent neuroimaging evidence in OCD have shown the critical involvement of amygdalo-cortical circuitry, in addition to classic cortico-striatal circuitry, in the pathophysiology of compulsive disorders (Milad and Rauch, 2012).
The thalamus also may contribute to a variety of inappropriate behaviours and plays a key role in OCD (Burguière et al., 2015). However, the direct link between thalamus and social disinhibition is less known. Thalamus and subthalamus are connected to the frontal and temporal lobes, which in turn regulate behavioural inhibition (Massimo et al., 2009, Peters et al., 2006, Sheelakumari et al., 2020, Zamboni et al., 2008); . Very recently, Scarioni et al. (2022) found positive correlations between behavioural disinhibition in FTD and tau burden in the thalamus and with both TDP-43 and amyloid-beta burden in the subthalamus (Scarioni et al., 2022). These results show that the core criteria symptoms of bvFTD are not only linked to cortical pathology, but also to subcortical regions such as the thalamus/subthalamus.
In a network framework, the temporal lobe is the epicentre of a recently described functional neural network, called limbic/semantic-appraisal network (SAN). This network includes bilateral cortical structures such as the temporal poles, the subgenual cingulate cortex, but also subcortical structures such as the caudate, the nucleus accumbens, and the amygdala (Guo et al., 20132013, Ranasinghe et al., 2016, Seeley et al., 2009). Its role, which is not completely clear, is at least in part to elaborate semantically driven personal evaluation. Interestingly, Magrath Guimet et al. pointed out in a recent review that the SAN, the salience network (SN) and all the networks involved in task control are particularly vulnerable in FTD, and are therefore central to understand the phenomenon of behavioural disinhibition (Magrath Guimet et al., 2021). In a recent work from Ranasinghe et al. (Ranasinghe et al., 2016), bvFTD patients were stratified on the basis of their patterns of brain atrophy. The group defined as having a prevalent damage of SAN, centred on right temporal lobe, showed higher behavioural disinhibition and obsessive behaviours, highlighting the centrality of right SAN contributions to socioemotional sensitivity. Indeed, the SAN is related to the socio-emotional context, with a role in understanding emotions and assigning an emotional valence to a stimulus, whose personal salience the SN can then recognise (Rankin, 2020). Compulsive behaviours reported in our “observational” scenario range from clear compulsive and repetitive acts (i.e., utilisation behaviour) possibly corresponding to obsessive behaviours reported above, to more complex actions (i.e., eating excessively or eating inappropriate food), that could be also considered as general disinhibited behaviours. Hence, compulsivity could be the result of an error in valence attribution within a given context, resulting in errors in the evaluation of what to do in relation to the context. This confusion in valence allocation might be attributable to the loss of personal semantic references.
Social disinhibition, as evaluated in the present work, was correlated with brain structures within a large cortico-subcortical circuit including frontal, temporal, and cingulate cortices, and thalamus and putamen, as well as WM bundles connected with frontal areas, as forceps minor, cingulum, and uncinate fasciculus (Fig. 5b).
Interestingly, these structures are similar to those reported as being correlated with a poor score in the emotion recognition test. This result is consistent with the previous correlation found between the cognitive and behavioural scores of social deficits: patients with socially inappropriate behaviours also show social cognition impairments and these two types of social deficits involve common cerebral structures.
To our knowledge, only a few studies have focused on social behaviour deficits associated with bvFTD. Most of the literature has investigated social cognition (see review by Christidi et al.) (Christidi et al., 2018) or drawn conclusions about disinhibited social behaviour from questionnaires associating both social and generalised disinhibition such as the NPI (Peters et al., 2006, Krueger et al., 2011). Our results focusing specifically on socially inappropriate behaviours are in line with a previous study distinguishing the NPI questions related to person-based disinhibition and generalised-impulsivity in bvFTD and Alzheimer’s disease patients (Paholpak et al., 2016). The authors indeed found a correlation between person-based disinhibition severity and the left anterior superior temporal sulcus, in line with our results highlighting the left temporal white matter alteration. Besides, impairments of the orbitofrontal cortex were already reported in altered social behaviour following traumatic brain injuries (Osborne-Crowley and McDonald, 2018). Globally, our results are in agreement with previous studies conducted on anatomical correlates of social disinhibition in various disorders, highlighting the importance of prefrontal (Bertoux et al., 2012) and cingulate cortices (Adolphs, 2001, O’Callaghan et al., 2016), the orbitofrontal cortex (Krueger et al., 2011), as well as the cingulum (Santillo et al., 2016, Herbet et al., 2014, Herbet et al., 2015), the uncinate fasciculus (Santillo et al., 2016, Samson et al., 2016) and the forceps minor (Santillo et al., 2016) in impaired social skills.
Functionally, these structures are part of the Salience Network-Frontal (SN-F), a subcomponent of SN recently described by Ranasinghe et al. (Ranasinghe et al., 2016). In their work, the SN-F group had extensive frontal atrophy and showed diminished interpersonal warmth and impairment of complex social cognition. In the same vein, a previous study showed that bvFTD patients with atrophy involving left-lateralised salience network structures have reduced pro-social behaviours (Sturm et al., 2018). Also, FTD patients show deficits in activating complex motions such as embarrassment (Sturm et al., 2006), which could explain why these patients are more likely to violate social norms. Finally, O’Callaghan et al. (O’Callaghan et al., 2016) applied a neuroeconomic task assessing specifically fairness, prosocial and punishing behaviours to investigate social norm compliance in bvFTD. They demonstrated that more complex normative behaviours (prosociality, punishment) require integration of contextual social information and are associated with atrophy in key fronto-striatal regions, such as bilateral orbitofrontal, anterior cingulate, right inferior frontal gyrus, left anterior insula as well as bilateral putamen. These are the same regions we have identified in the present work.
Within this framework, social disinhibition would be due to an inability to generate complex social reasoning, including the adherence to social rules when interacting with others.
Focusing on studies targeting FTD patients, using specific measures of disinhibition and advanced imaging analysis methods, the orbitofrontal and insula cortices seem to be the most important regions implicated in disinhibition (Peters et al., 2006, Massimo et al., 2009, Santillo et al., 2016, Seeley, 2010, Farb et al., 2013). Farb et al. identified in patients with bvFTD elevated prefrontal connectivity which was in turn associated with more severe behavioural dysfunction (Farb et al., 2013). In particular brain activity at the level of left insula correlated with disinhibition, as measured by the Frontal Behavioral Inventory scale (Farb et al., 2013). Moreover, in a study which classified bvFTD patients based on their behaviour (apathetic vs disinhibited) multivariate analyses showed that orbitofrontal, but also dorsolateral prefrontal, and caudate nucleus were sufficient to identify the disinhibited subgroup (Santamaría-García et al., 2016).
4.3. Limitations
The present study has some limitations. Firstly, we acknowledge the small sample size of bvFTD patients, the difficulty of recruitment being partly explained by our highly-demanding protocol (two days of experimental protocol including extensive neuropsychological testing, behavioural assessment and MRI acquisition). In addition, we applied highly selective inclusion criteria (e.g., MMSE score > 20) due to the need to include patients at a very early stage, which reduced the number of patients available for the study. At the same time, this allowed us to explore in details patients in the early stages of the disease while avoiding the confounding effect of advanced neurodegeneration on behaviour. This limit about the small sample size also extends to the anatomical results, which nevertheless seem to be consistent with the current literature on the networks underlying behavioural disorders in bvFTD. Another limitation concerns the subjectivity of the behavioural evaluation since the design involves a non-blinded encoding of the videos. However, this was controlled by the high level of intercoder reliability and clear guidelines for the rating.
Concerning the cognitive assessment, as expected, we found that bvFTD patients and HC were significantly different in the language battery, which is a limitation for the use of some tests, such as the Hayling test. However, such language disorders were not relevant: patients endured a demanding protocol, with mostly linguistic tasks and showing no comprehension problems. Moreover, for the Hayling test, we administered part B only if part A had been well understood and performed, and focused on the time B-A, hence controlling for basic semantic or task understanding difficulties.
Finally, we did not investigate WM pathways connecting cortical and subcortical structures (ex. cortico-striatal connections), due to limits in the reconstruction of WM tracts with highly crossing fibres. WM imaging of these pathways would probably constitute an interesting complement to our present models (Fig. 5).
In conclusion, by combining an ecologically valid behavioural approach with the study of neuroanatomy, we explored the multifaceted nature of disinhibition and found different networks implicated in two main categories of inhibition deficits. We showed that compulsivity is mostly related to the integrity of bilateral orbitofrontal cortex and the right temporal lobe, and thalamus and amygdala sub-cortical structures, and their connections with frontal lobe through the cingulum, the UF, the AF and the ILF. These regions and connections seem to correspond to the semantic appraisal network. Compulsivity may result from an evaluation error of what to do in relation to the context. We found that social disinhibition related to the integrity of bilateral frontal, temporal and cingulate regions, and their connections through the forceps minor, the UF and cingulum. These regions and connections seem to correspond to the frontal component of salience network. Within this framework, social disinhibition can be resumed as the inability to generate socially complex reasoning. Our findings suggest that differences between compulsivity and social disinhibition networks would be linked to very distinct networks. Overall, an added value of this study is a better understanding of the mechanisms underlying disinhibition, on the basis of behaviour. Present results will help, for example, in driving tailored non-pharmacological interventions. Thus, assessment and management (pharmacological or non-pharmacological) will benefit from the enlightenment of these mechanisms. These results could also motivate and encourage future studies to look at finer categories of disinhibition in the study of neurodegenerative diseases. Finally, our semi-ecological approach could be further used to explore the context of occurrence of these troubles and promises great implications in clinical practice to improve the detection and the management of these disorders in dementia.
CRediT authorship contribution statement
Delphine Tanguy: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Bénédicte Batrancourt: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Alfonso Estudillo-Romero: Formal analysis, Software, Writing – review & editing. John S.H. Baxter: Formal analysis, Software, Writing – review & editing. Isabelle Le Ber: Writing – review & editing. Arabella Bouzigues: Writing – review & editing. Valérie Godefroy: Validation, Writing – review & editing. Aurélie Funkiewiez: Resources. Céline Chamayou: Resources. Emmanuelle Volle: Resources, Writing – review & editing. Dario Saracino: Writing – review & editing. Armelle Rametti-Lacroux: Investigation, Resources. Xavier Morandi: Supervision, Writing – review & editing. Pierre Jannin: Supervision, Writing – review & editing. Richard Levy: Funding acquisition, Investigation, Resources, Writing – review & editing. Raffaella Migliaccio: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Delphine Tanguy is supported by École Normale Supérieure Paris-Saclay. Raffaella Migliaccio is supported by France Alzheimer, Fondation Recherche Alzheimer, and Philippe Chatrier Foundations, and by Rosita Gomez association.
We acknowledge Maxime Montembeault for his valuable suggestions.
We sincerely acknowledge the participants and caregivers for their involvement in this study.
Funding
This study was funded by the program “Investissements d’avenir” ANR-10-IAIHU-06; the “Fondation pour la recherche médicale”, FRM DEQ20150331725, frm.org; and by ENEDIS, enedis.fr.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103079.
Contributor Information
Delphine Tanguy, Email: delphine.tanguy@ens-paris-saclay.fr.
Raffaella Migliaccio, Email: lara.migliaccio@icm-institute.org.
Appendix 1.
Collaborators.
ECOCAPTURE study group: Bénédicte Batrancourt, Carole Azuar, Bruno Dubois, Karen Lecouturier, Carla Matos Araujo, Estelle Janvier, Aline Jourdain, Armelle Rametti-Lacroux, Sophie Coriou, Vanessa Batista Brochard, Cécile Gaudebout, Johan Ferrand-Verdejo, Louis Bonnefous, Flore Pochan-Leva, Lucie Jeanne, Mathilde Joulié, Myriam Provost, Rozenn Renaud, Sarah Hachemi, Vincent Guillemot, David Bendetowicz, Guilhem Carle, Julie Socha, Fanny Pineau, Frédéric Marin, Yongjian Liu, Pierre Mullot, Aymen Mousli, Armelle Blossier, Giulia Visentin, Delphine Tanguy, Valérie Godefroy, Idil Sezer, Daphné Tessereau-Barbot, Anaïs Raud, Emmanuel Cognat, Manon Le Bozec, Arabella Bouzigues, Vincent Le Du, Stéphanie Bombois, Camille Simard, Paolo Fulcheri, Hortense Guitton, Caroline Peltier, François-Xavier Lejeune, Lars Jorgensen, Isabelle Le Ber, Louise-Laure Mariani, Jean-Christophe Corvol, Raffaella Migliaccio, Richard Levy.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Miller, B., Llibre Guerra JJ. Frontotemporal dementia. In: Handbook of Clinical Neurology. Vol 165. Elsevier; 2019:33-45. doi:10.1016/B978-0-444-64012-3.00003-4. [DOI] [PubMed]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G.P., Onyike C.U., Hillis A.E., Josephs K.A., Boeve B.F., Kertesz A., Seeley W.W., Rankin K.P., Johnson J.K., Gorno-Tempini M.-L., Rosen H., Prioleau-Latham C.E., Lee A., Kipps C.M., Lillo P., Piguet O., Rohrer J.D., Rossor M.N., Warren J.D., Fox N.C., Galasko D., Salmon D.P., Black S.E., Mesulam M., Weintraub S., Dickerson B.C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T.W., Manes F., Grafman J., Cappa S.F., Freedman M., Grossman M., Miller B.L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.D., Tremont G. Impact of frontal systems behavioral functioning in dementia on caregiver burden. J. Neuropsychiatry Clin. Neurosci. 2007;19(1):43–49. doi: 10.1176/appi.neuropsych.19.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.T. Dementia Caregiver Burden: a Research Update and Critical Analysis. Curr Psychiatry Rep. 2017;19(9) doi: 10.1007/s11920-017-0818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13(3):214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Wilson S.P., Kipp K. The Development of Efficient Inhibition: Evidence from Directed-Forgetting Tasks. Dev. Rev. 1998;18(1):86–123. doi: 10.1006/drev.1997.0445. [DOI] [Google Scholar]
- Harnishfeger, K.K. The development of cognitive inhibition. In: Interference and Inhibition in Cognition. Elsevier; 1995:175-204. doi:10.1016/B978-012208930-5/50007-6.
- Migliaccio R., Tanguy D., Bouzigues A., Sezer I., Dubois B., Le Ber I., Batrancourt B., Godefroy V., Levy R. Cognitive and behavioural inhibition deficits in neurodegenerative dementias. Cortex. 2020;131:265–283. doi: 10.1016/j.cortex.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis, S. Mental status examination for organic mental syndrome in the elderly patient. In: Geriatric Psychiatry: A Handbook for Psychiatrists and Primary Care Physicians. New York: Grune and Stratton. Bellack L, Larasu TB; 1976:p77-121.
- Burgess P.W., Shallice T. Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia. 1996;34(4):263–272. doi: 10.1016/0028-3932(95)00104-2. [DOI] [PubMed] [Google Scholar]
- Peters F., Perani D., Herholz K., Holthoff V., Beuthien-Baumann B., Sorbi S., Pupi A., Degueldre C., Lemaire C., Collette F., Salmon E. Orbitofrontal Dysfunction Related to Both Apathy and Disinhibition in Frontotemporal Dementia. DEM. 2006;21(5-6):373–379. doi: 10.1159/000091898. [DOI] [PubMed] [Google Scholar]
- Massimo L., Powers C., Moore P., Vesely L., Avants B., Gee J., Libon D.J., Grossman M. Neuroanatomy of Apathy and Disinhibition in Frontotemporal Lobar Degeneration. Dement Geriatr Cogn Disord. 2009;27(1):96–104. doi: 10.1159/000194658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger C.E., Laluz V., Rosen H.J., Neuhaus J.M., Miller B.L., Kramer J.H. Double dissociation in the anatomy of socioemotional disinhibition and executive functioning in dementia. Neuropsychology. 2011;25(2):249–259. doi: 10.1037/a0021681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni G., Huey E.D., Krueger F., Nichelli P.F., Grafman J. Apathy and disinhibition in frontotemporal dementia: Insights into their neural correlates. Neurology. 2008;71(10):736–742. doi: 10.1212/01.wnl.0000324920.96835.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paholpak P., Carr A.R., Barsuglia J.P., Barrows R.J., Jimenez E., Lee G.J., Mendez M.F. Person-based versus generalized impulsivity disinhibition in frontotemporal dementia and alzheimer disease. J. Geriatr. Psychiatry Neurol. 2016;29(6):344–351. doi: 10.1177/0891988716666377. [DOI] [PubMed] [Google Scholar]
- O'Connor C.M., Landin-Romero R., Clemson L., Kaizik C., Daveson N., Hodges J.R., Hsieh S., Piguet O., Mioshi E. Behavioral-variant frontotemporal dementia. Neurology. 2017;89(6):570–577. doi: 10.1212/WNL.0000000000004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H.J., Allison S.C., Schauer G.F., Gorno-Tempini M.L., Weiner M.W., Miller B.L. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128(11):2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García H.S., Reyes P., Santacruz J., Baez S., Ibañez A., Matallana D. Clinical, neuropsychological and neural correlates underlying the first symptoms in Behavioral Variant of Fronto Temporal Dementia (bvFTD) J. Neurol. Sci. 2015;357:e12–e13. doi: 10.1016/j.jns.2015.08.116. [DOI] [Google Scholar]
- Hornberger M., Geng J., Hodges J.R. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134(Pt 9):2502–2512. doi: 10.1093/brain/awr173. [DOI] [PubMed] [Google Scholar]
- Santillo A.F., Lundblad K., Nilsson M., Landqvist Waldö M., van Westen D., Lätt J., Blennow Nordström E., Vestberg S., Lindberg O., Nilsson C., Kobeissy F.H. Grey and White Matter Clinico-Anatomical correlates of disinhibition in neurodegenerative disease. PLoS ONE. 2016;11(10):e0164122. doi: 10.1371/journal.pone.0164122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B., Brambati S.M., Agosti C., Gipponi S., Bellelli G., Gasparotti R., Garibotto V., Di Luca M., Scifo P., Perani D., Padovani A. Evidence of white matter changes on diffusion tensor imaging in frontotemporal dementia. Arch. Neurol. 2007;64(2):246. doi: 10.1001/archneur.64.2.246. [DOI] [PubMed] [Google Scholar]
- Sheelakumari R., Bineesh C., Varghese T., Kesavadas C., Verghese J., Mathuranath P.S. Neuroanatomical correlates of apathy and disinhibition in behavioural variant frontotemporal dementia. Brain Imaging and Behavior. 2020;14(5):2004–2011. doi: 10.1007/s11682-019-00150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J.P., Massimo L., McMillan C.T., Yushkevich P.A., Zhang H., Gee J.C., Grossman M. White matter disease contributes to apathy and disinhibition in behavioral variant frontotemporal dementia. Cogn Behav Neurol. 2014;27(4):206–214. doi: 10.1097/WNN.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrancourt B., Lecouturier K., Ferrand-Verdejo J., Guillemot V., Azuar C., Bendetowicz D., Migliaccio R., Rametti-Lacroux A., Dubois B., Levy R. Exploration deficits under ecological conditions as a marker of apathy in frontotemporal dementia. Front Neurol. 2019;10 doi: 10.3389/fneur.2019.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy V., Tanguy D., Bouzigues A., Sezer I., Ferrand‐Verdejo J., Azuar C., Bendetowicz D., Carle G., Rametti‐Lacroux A., Bombois S., Cognat E., Jannin P., Morandi X., Ber I.L., Levy R., Batrancourt B., Migliaccio R. Frontotemporal dementia subtypes based on behavioral inhibition deficits. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2021;13(1):e12178. doi: 10.1002/dad2.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden J., Bathgate D., Varma A., Blackshaw A., Gibbons Z., Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J. Neurol. Neurosurg Psychiatry. 2001;70(3):323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley J.W., Everitt B.J., Robbins T.W. Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron. 2011;69(4):680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Fineberg N.A., Chamberlain S.R., Goudriaan A.E., Stein D.J., Vanderschuren L.J.M.J., Gillan C.M., Shekar S., Gorwood P.A.P.M., Voon V., Morein-Zamir S., Denys D., Sahakian B.J., Moeller F.G., Robbins T.W., Potenza M.N. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr. 2014;19(1):69–89. doi: 10.1017/S1092852913000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg N.A., Apergis-Schoute A.M., Vaghi M.M., Banca P., Gillan C.M., Voon V., Chamberlain S.R., Cinosi E., Reid J., Shahper S., Bullmore E.T., Sahakian B.J., Robbins T.W. Mapping Compulsivity in the DSM-5 Obsessive Compulsive and Related Disorders: Cognitive Domains, Neural Circuitry, and Treatment. Int. J. Neuropsychopharmacol. 2018;21(1):42–58. doi: 10.1093/ijnp/pyx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso S.M., Roks G., Stevens M., de Koning I., Tanghe H.L.J., Kamphorst W., Ravid R., Niermeijer M.F., van Swieten J.C. Complex compulsive behaviour in the temporal variant of frontotemporal dementia. J. Neurol. 2001;248(11):965–970. doi: 10.1007/s004150170049. [DOI] [PubMed] [Google Scholar]
- Seeley W.W. Behavioral Variant Frontotemporal Dementia: CONTINUUM: Lifelong Learning in Neurology. 2019;25(1):76–100. doi: 10.1212/CON.0000000000000698. [DOI] [PubMed] [Google Scholar]
- Bang J., Spina S., Miller B.L. Frontotemporal dementia. The Lancet. 2015;386(10004):1672–1682. doi: 10.1016/S0140-6736(15)00461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarais P., Lanctôt K.L., Masellis M., Black S.E., Herrmann N. Social inappropriateness in neurodegenerative disorders. Int. Psychogeriatr. 2018;30(2):197–207. doi: 10.1017/S1041610217001260. [DOI] [PubMed] [Google Scholar]
- Foss M.P., Carvalho V.A.d., Machado T.H., Reis G.C.D., Tumas V., Caramelli P., Nitrini R., Porto C.S. Mattis Dementia Rating Scale (DRS): Normative data for the Brazilian middle-age and elderly populations. Dement neuropsychol. 2013;7(4):374–379. doi: 10.1590/S1980-57642013DN74000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B., Slachevsky A., Litvan I., Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55(11):1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Merck, C., Charnallet, A., Auriacombe S, et al. The GRECO neuropsychological semantic battery (BECS GRECO): Validation and normative data. Revue de neuropsychologie. 2011;3(4):235-255. Accessed May 19, 2022. https://www.cairn.info/revue-de-neuropsychologie-2011-4-page-235.htm.
- Wechsler D.-W.-A.-I.-S.-R. Manual : Wechsler Adult Intelligence Scale-Revised. Harcourt Brace Jovanovich [for] Psychological Corp. 1981 [Google Scholar]
- Cardebat D., Doyon B., Puel M., Goulet P., Joanette Y. Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg. 1990;90(4):207–217. [PubMed] [Google Scholar]
- Montembeault M., Sayah S., Rinaldi D., Le Toullec B., Bertrand A., Funkiewiez A., Saracino D., Camuzat A., Couratier P., Chouly M., Hannequin D., Aubier-Girard C., Pasquier F., Delbeuck X., Colliot O., Batrancourt B., Azuar C., Lévy R., Dubois B., Le Ber I., Migliaccio R. Cognitive inhibition impairments in presymptomatic C9orf72 carriers. J. Neurol. Neurosurg Psychiatry. 2020;91(4):366–372. doi: 10.1136/jnnp-2019-322242. [DOI] [PubMed] [Google Scholar]
- Funkiewiez A., Bertoux M., de Souza L.C., Lévy R., Dubois B. The SEA (Social Cognition and Emotional Assessment): A clinical neuropsychological tool for early diagnosis of frontal variant of frontotemporal lobar degeneration. Neuropsychology. 2012;26(1):81–90. doi: 10.1037/a0025318. [DOI] [PubMed] [Google Scholar]
- Snowden J.S., Neary D., Mann D.M. Frontotemporal dementia. The British journal of psychiatry. 2002;180(2):140–143. doi: 10.1192/bjp.180.2.140. [DOI] [PubMed] [Google Scholar]
- Batrancourt, B., Migliaccio, R., (Lara), Tanguy, D., Sezer, I., Godefroy, V., Bouzigues, A. The ECOCAPTURE ethograms: apathy ethogram and disinhibition ethogram. 2022;2. doi:10.17632/mv8hndcd95.2.
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Sollberger M., Stanley C.M., Wilson S.M., Gyurak A., Beckman V., Growdon M., Jang J., Weiner M.W., Miller B.L., Rankin K.P. Neural Basis of Interpersonal Traits in Neurodegenerative Diseases. Neuropsychologia. 2009;47(13):2812–2827. doi: 10.1016/j.neuropsychologia.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strikwerda-Brown C., Ramanan S., Goldberg Z.-L., Mothakunnel A., Hodges J.R., Ahmed R.M., Piguet O., Irish M. The interplay of emotional and social conceptual processes during moral reasoning in frontotemporal dementia. Brain. 2021;144(3):938–952. doi: 10.1093/brain/awaa435. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Huang C.C., Lin C.P., Feng J., Joliot M. Automated anatomical labelling atlas 3. NeuroImage. 2020;206 doi: 10.1016/j.neuroimage.2019.116189. [DOI] [PubMed] [Google Scholar]
- Descoteaux M., Wiest-Daesslé N., Prima S., Barillot C., Deriche R. Impact of Rician adapted Non-Local Means filtering on HARDI. Med. Image Comput. Comput Assist Interv. 2008;11(Pt 2):122–130. doi: 10.1007/978-3-540-85990-1_15. [DOI] [PubMed] [Google Scholar]
- Tustison N.J., Avants B.B., Cook P.A., Yuanjie Zheng, Egan A., Yushkevich P.A., Gee J.C. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J.E., Liu C.Y., Thompson P.M., Tu Z. Robust brain extraction across datasets and comparison with publicly available methods. IEEE Trans. Med. Imaging. 2011;30(9):1617–1634. doi: 10.1109/TMI.2011.2138152. [DOI] [PubMed] [Google Scholar]
- Tournier J.-D., Smith R., Raffelt D., Tabbara R., Dhollander T., Pietsch M., Christiaens D., Jeurissen B., Yeh C.-H., Connelly A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137. doi: 10.1016/j.neuroimage.2019.116137. [DOI] [PubMed] [Google Scholar]
- Norton I., Essayed W.I., Zhang F., Pujol S., Yarmarkovich A., Golby A.J., Kindlmann G., Wassermann D., Estepar R.S.J., Rathi Y., Pieper S., Kikinis R., Johnson H.J., Westin C.-F., O'Donnell L.J. SlicerDMRI: Open Source Diffusion MRI Software for Brain Cancer Research. Cancer Res. 2017;77(21):e101–e103. doi: 10.1158/0008-5472.CAN-17-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Noh T., Juvekar P., Frisken S.F., Rigolo L., Norton I., Kapur T., Pujol S., Wells W., Yarmarkovich A., Kindlmann G., Wassermann D., San Jose Estepar R., Rathi Y., Kikinis R., Johnson H.J., Westin C.-F., Pieper S., Golby A.J., O’Donnell L.J. SlicerDMRI: Diffusion MRI and Tractography Research Software for Brain Cancer Surgery Planning and Visualization. JCO Clin Cancer Inform. 2020;(4):299–309. doi: 10.1200/CCI.19.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, H., Harris, G., Williams, K. BRAINSFit: Mutual Information Rigid Registrations of Whole-Brain 3D Images, Using the Insight Toolkit. Published online 2007:11.
- Alexander D.C., Pierpaoli C., Basser P.J., Gee J.C. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med. Imaging. 2001;20(11):1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Haegelen C., Coupé P., Fonov V., Guizard N., Jannin P., Morandi X., Collins D.L. Automated segmentation of basal ganglia and deep brain structures in MRI of Parkinson’s disease. Int J Comput Assist Radiol Surg. 2013;8(1):99–110. doi: 10.1007/s11548-012-0675-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Caprihan A., Panzenboeck M.M., Fallon J.H., Perry M., Gollub R.L., Hua K., Zhang J., Jiang H., Dubey P., Blitz A., van Zijl P., Mori S. Reproducibility of Quantitative Tractography Methods Applied to Cerebral White Matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Thiebautdeschotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Migliaccio R., Agosta F., Scola E., Magnani G., Cappa S.F., Pagani E., Canu E., Comi G., Falini A., Gorno-Tempini M.L., Bartolomeo P., Filippi M. Ventral and dorsal visual streams in posterior cortical atrophy: A DT MRI study. Neurobiol. Aging. 2012;33(11):2572–2584. doi: 10.1016/j.neurobiolaging.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkel S.J., Rogalski E., Drossinos Sancho N., D'Anna L., Luque Laguna P., Sridhar J., Dell'Acqua F., Weintraub S., Thompson C., Mesulam M.-M., Catani M. Anatomical evidence of an indirect pathway for word repetition. Neurology. 2020;94(6):e594–e606. doi: 10.1212/WNL.0000000000008746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Fox, J., Sanford W. An {R} Companion to Applied Regression. Third Edition. Thousand Oaks CA: Sage; 2019. https://socialsciences.mcmaster.ca/jfox/Books/Companion/.
- Tanguy, D., Batrancourt, B., Migliaccio, R., (Lara), Godefroy, V., Guignebert, A., Chamayou, C. ECOCAPTURE Dataset - Behavioral coding from video - 17 bvFTD and 18 HC - 28.04.2022. 2022;1. doi:10.17632/vdrstwp5xx.1.
- Burgess P.W., Stuss D.T. Fifty years of prefrontal cortex research: impact on assessment. J. Int. Neuropsychol. Soc. 2017;23(9–10):755–767. doi: 10.1017/S1355617717000704. [DOI] [PubMed] [Google Scholar]
- Désiré L., Boissy A., Veissier I. Emotions in farm animals: Behav. Process. 2002;60(2):165–180. doi: 10.1016/s0376-6357(02)00081-5. [DOI] [PubMed] [Google Scholar]
- Milders M., Ietswaart M., Crawford J.R., Currie D. Social behavior following traumatic brain injury and its association with emotion recognition, understanding of intentions, and cognitive flexibility. J Inter Neuropsych Soc. 2008;14(02) doi: 10.1017/S1355617708080351. [DOI] [PubMed] [Google Scholar]
- May M., Milders M., Downey B., Whyte M., Higgins V., Wojcik Z., Amin S., O’Rourke S. Social Behavior and Impairments in Social Cognition Following Traumatic Brain Injury. J Int Neuropsychol Soc. 2017;23(5):400–411. doi: 10.1017/S1355617717000182. [DOI] [PubMed] [Google Scholar]
- Jonker F.A., Jonker C., Scheltens P., Scherder E.J.A. The role of the orbitofrontal cortex in cognition and behavior. Rev. Neurosci. 2015;26(1):1–11. doi: 10.1515/revneuro-2014-0043. [DOI] [PubMed] [Google Scholar]
- Hooker C., Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 2002;112(1):41–50. doi: 10.1016/S0165-1781(02)00177-4. [DOI] [PubMed] [Google Scholar]
- Fett A.-K., Viechtbauer W., Dominguez M.-d.-G., Penn D.L., van Os J., Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Pelletier A.L., Dean D.J., Lunsford-Avery J.R., Smith A.K., Orr J.M., Gupta T., Millman Z.B., Mittal V.A. Emotion recognition and social/role dysfunction in non-clinical psychosis. Schizophr. Res. 2013;143(1):70–73. doi: 10.1016/j.schres.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton L.E., Bridgwater M.A., Haas G.L. Emotion recognition and social skills in child and adolescent offspring of parents with schizophrenia. Cognitive Neuropsychiatry. 2017;22(3):175–185. doi: 10.1080/13546805.2017.1297223. [DOI] [PubMed] [Google Scholar]
- Brüne M. Emotion recognition, ‘theory of mind’, and social behavior in schizophrenia. Psychiatry Res. 2005;133(2–3):135–147. doi: 10.1016/j.psychres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Burguière Eric, Monteiro Patricia, Mallet Luc, Feng Guoping, Graybiel Ann M. Striatal circuits, habits, and implications for obsessive–compulsive disorder. Curr. Opin. Neurobiol. 2015;30:59–65. doi: 10.1016/j.conb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnin A., Formentin C., Pompanin S., Zarantonello G., Jelcic N., Venneri A., Ermani M. Simple motor stereotypies are not specific features of behavioural frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry. 2014;85(8):945–946. doi: 10.1136/jnnp-2013-307471. [DOI] [PubMed] [Google Scholar]
- Whiteside S.P., Port J.D., Abramowitz J.S. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Res. 2004;132(1):69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Robbins T.W., Vaghi M.M., Banca P. Obsessive-Compulsive Disorder: Puzzles and Prospects. Neuron. 2019;102(1):27–47. doi: 10.1016/j.neuron.2019.01.046. [DOI] [PubMed] [Google Scholar]
- Mendez M.F., Perryman K.M., Miller B.L., Swartz J.R., Cummings J.L. Compulsive Behaviors as Presenting Symptoms of Frontotemporal Dementia. J Geriatr Psychiatry Neurol. 1997;10(4):154–157. doi: 10.1177/089198879701000405. [DOI] [PubMed] [Google Scholar]
- Maltête D. Adult-onset stereotypical motor behaviors. Revue Neurologique. 2016;172(8–9):477–482. doi: 10.1016/j.neurol.2016.07.002. [DOI] [PubMed] [Google Scholar]
- McMurtray A.M., Chen A.K., Shapira J.S., Chow T.W., Mishkin F., Miller B.L., Mendez M.F. Variations in regional SPECT hypoperfusion and clinical features in frontotemporal dementia. Neurology. 2006;66(4):517–522. doi: 10.1212/01.wnl.0000197983.39436.e7. [DOI] [PubMed] [Google Scholar]
- Perry D.C., Whitwell J.L., Boeve B.F., Pankratz V.S., Knopman D.S., Petersen R.C., Jack C.R., Josephs K.A. Voxel-based morphometry in patients with obsessive-compulsive behaviors in behavioral variant frontotemporal dementia. Eur J Neurol. 2012;19(6):911–917. doi: 10.1111/j.1468-1331.2011.03656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulugut Erkoyun H., Groot C., Heilbron R., Nelissen A., van Rossum J., Jutten R., Koene T., van der Flier W.M., Wattjes M.P., Scheltens P., Ossenkoppele R., Barkhof F., Pijnenburg Y. A clinical-radiological framework of the right temporal variant of frontotemporal dementia. Brain. 2020;143(9):2831–2843. doi: 10.1093/brain/awaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamminga J., Kumfor F., Burrell J.R., Piguet O., Hodges J.R., Irish M. Differentiating between right-lateralised semantic dementia and behavioural-variant frontotemporal dementia: an examination of clinical characteristics and emotion processing. J Neurol Neurosurg Psychiatry. 2015;86(10):1082–1088. doi: 10.1136/jnnp-2014-309120. [DOI] [PubMed] [Google Scholar]
- Pozueta A., Lage C., García-Martínez M., Kazimierczak M., Bravo M., López-García S., Riancho J., González-Suarez A., Vázquez-Higuera J.L., de Arcocha-Torres M., Banzo I., Jiménez-Bonilla J., Berciano J., Rodríguez-Rodríguez E., Sánchez-Juan P. Cognitive and Behavioral Profiles of Left and Right Semantic Dementia: Differential Diagnosis with Behavioral Variant Frontotemporal Dementia and Alzheimer’s Disease. JAD. 2019;72(4):1129–1144. doi: 10.3233/JAD-190877. [DOI] [PubMed] [Google Scholar]
- Choi J.-S., Kim H.-S., Yoo S.Y., Ha T.-H., Chang J.-H., Kim Y.Y., Shin Y.-W., Kwon J.S. Morphometric alterations of anterior superior temporal cortex in obsessive–compulsive disorder. Depression and Anxiety. 2006;23(5):290–296. doi: 10.1002/da.20171. [DOI] [PubMed] [Google Scholar]
- Isaacs K.L., Philbeck J.W., Barr W.B., Devinsky O., Alper K. Obsessive-compulsive symptoms in patients with temporal lobe epilepsy. Epilepsy Behav. 2004;5(4):569–574. doi: 10.1016/j.yebeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Monaco F., Cavanna A., Magli E., Barbagli D., Collimedaglia L., Cantello R., Mula M. Obsessionality, obsessive–compulsive disorder, and temporal lobe epilepsy. Epilepsy Behav. 2005;7(3):491–496. doi: 10.1016/j.yebeh.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Figee M., Wielaard I., Mazaheri A., Denys D. Neurosurgical targets for compulsivity: What can we learn from acquired brain lesions? Neurosci. Biobehav. Rev. 2013;37(3):328–339. doi: 10.1016/j.neubiorev.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Shukla T., Pandey S. Stereotypies in adults: a systematic review. Neurol. Neurochir. Pol. 2020;54(4):11. doi: 10.5603/PJNNS.a2020.0058. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Rauch S.L. Obsessive Compulsive Disorder: Beyond Segregated Cortico-striatal Pathways. Trends Cogn Sci. 2012;16(1):43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C.C., Gorno-Tempini M.L., Gesierich B., Henry M., Trujillo A., Shany-Ur T., Jovicich J., Robinson S.D., Kramer J.H., Rankin K.P., Miller B.L., Seeley W.W. Anterior temporal lobe degeneration produces widespread network-driven dysfunction. Brain. 2013;136(10):2979–2991. doi: 10.1093/brain/awt222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe K.G., Rankin K.P., Lobach I.V., Kramer J.H., Sturm V.E., Bettcher B.M., Possin K., Christine You S., Lamarre A.K., Shany-Ur T., Stephens M.L., Perry D.C., Lee S.E., Miller Z.A., Gorno-Tempini M.L., Rosen H.J., Boxer A., Seeley W.W., Rabinovici G.D., Vossel K.A., Miller B.L. Cognition and neuropsychiatry in behavioral variant frontotemporal dementia by disease stage. Neurology. 2016;86(7):600–610. doi: 10.1212/WNL.0000000000002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrath Guimet N., Miller B.L., Allegri R.F., Rankin K.P. What Do We Mean by Behavioral Disinhibition in Frontotemporal Dementia? Front Neurol. 2021;12 doi: 10.3389/fneur.2021.707799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin K.P. Brain Networks Supporting Social Cognition in Dementia. Curr Behav Neurosci Rep. 2020;7(4):203–211. doi: 10.1007/s40473-020-00224-3. [DOI] [Google Scholar]
- Christidi F., Migliaccio R., Santamaría-García H., Santangelo G., Trojsi F. Social Cognition Dysfunctions in Neurodegenerative Diseases: Neuroanatomical Correlates and Clinical Implications. Behav. Neurol. 2018;2018:1–18. doi: 10.1155/2018/1849794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne-Crowley K., McDonald S. A review of social disinhibition after traumatic brain injury. Journal of Neuropsychology. 2018;12(2):176–199. doi: 10.1111/jnp.12113. [DOI] [PubMed] [Google Scholar]
- Bertoux M., Volle E., Funkiewiez A., Cruz de Souza L., Leclercq D., Dubois B. Social Cognition and Emotional Assessment (SEA) is a Marker of Medial and Orbital Frontal Functions: A Voxel-Based Morphometry Study in Behavioral Variant of Frontotemporal Degeneration. Journal of the International Neuropsychological Society. JINS. 2012;18:972–985. doi: 10.1017/S1355617712001300. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001;11(2):231–239. doi: 10.1016/S0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- O’Callaghan C., Bertoux M., Irish M., Shine J.M., Wong S., Spiliopoulos L., Hodges J.R., Hornberger M. Fair play: social norm compliance failures in behavioural variant frontotemporal dementia. Brain. 2016;139(1):204–216. doi: 10.1093/brain/awv315. [DOI] [PubMed] [Google Scholar]
- Herbet G., Lafargue G., Bonnetblanc F., Moritz-Gasser S., Menjot de Champfleur N., Duffau H. Inferring a dual-stream model of mentalizing from associative white matter fibres disconnection. Brain. 2014;137(Pt 3):944–959. doi: 10.1093/brain/awt370. [DOI] [PubMed] [Google Scholar]
- Herbet G., Lafargue G., Moritz-Gasser S., Menjot de Champfleur N., Costi E., Bonnetblanc F., Duffau H. A disconnection account of subjective empathy impairments in diffuse low-grade glioma patients. Neuropsychologia. 2015;70:165–176. doi: 10.1016/j.neuropsychologia.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Samson A.C., Dougherty R.F., Lee I.A., Phillips J.M., Gross J.J., Hardan A.Y. White matter structure in the uncinate fasciculus: Implications for socio-affective deficits in Autism Spectrum Disorder. Psychiatry Research: Neuroimaging. 2016;255:66–74. doi: 10.1016/j.pscychresns.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Sturm V.E., Sible I.J., Datta S., Hua A.Y., Perry D.C., Kramer J.H., Miller B.L., Seeley W.W., Rosen H.J. Resting parasympathetic dysfunction predicts prosocial helping deficits in behavioral variant frontotemporal dementia. Cortex. 2018;109:141–155. doi: 10.1016/j.cortex.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm V.E., Rosen H.J., Allison S., Miller B.L., Levenson R.W. Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain. 2006;129(Pt 9):2508–2516. doi: 10.1093/brain/awl145. [DOI] [PubMed] [Google Scholar]
- Scarioni Marta, Gami-Patel Priya, Peeters Carel F.W., de Koning Florianne, Seelaar Harro, O. Mol Merel, van Swieten John C., Rozemuller Annemieke J.M., Hoozemans Jeroen J.M., Pijnenburg Yolande A.L., Dijkstra Anke A., Netherlands Brain Bank Psychiatric symptoms of frontotemporal dementia and subcortical (co--)pathology burden: new insights. Brain. 2022 doi: 10.1093/brain/awac043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W. Anterior insula degeneration in frontotemporal dementia. Brain Struct Funct. 2010;214(5):465–475. doi: 10.1007/s00429-010-0263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb N.A.S., Grady C.L., Strother S., Tang-Wai D.F., Masellis M., Black S., Freedman M., Pollock B.G., Campbell K.L., Hasher L., Chow T.W. Abnormal network connectivity in frontotemporal dementia: Evidence for prefrontal isolation. Cortex. 2013;49(7):1856–1873. doi: 10.1016/j.cortex.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Santamaría-García H., Reyes P., García A., Baéz S., Martinez A., Santacruz J.M., Slachevsky A., Sigman M., Matallana D., Ibañez A. First Symptoms and Neurocognitive Correlates of Behavioral Variant Frontotemporal Dementia. Journal of Alzheimer’s Disease. 2016;54(3):957–970. doi: 10.3233/JAD-160501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request, while respecting the anonymity of the participants. The neuropsychological data and the ECOCAPTURE behavioural metrics are available on Mendeley Data (Tanguy et al., 2022). The ECOCAPTURE ethograms (coding scheme) used for behavioural coding from video are available on Mendeley Data (Batrancourt et al., 2022).