Abstract
In the last decade, the development of messenger RNA (mRNA) therapeutics by lipid nanoparticles (LNP) leads to facilitate clinical trial recruitment, which improves the efficacy of treatment modality to a large extent. Although mRNA-LNP vaccine platforms for the COVID-19 pandemic demonstrated high efficiency, safety and adverse effects challenges due to the uncontrolled immune responses and inappropriate pharmacological interventions could limit this tremendous efficacy. The current study reveals the interplay of immune responses with LNP compositions and characterization and clarifies the interaction of mRNA-LNP therapeutics with dendritic, macrophages, neutrophile cells, and complement. Then, pharmacological profiles for mRNA-LNP delivery, including pharmacokinetics and cellular trafficking, were discussed in detail in cancer types and infectious diseases. This review study opens a new and vital landscape to improve multidisciplinary therapeutics on mRNA-LNP through modulation of immunopharmacological responses in clinical trials.
Graphical Abstract
Keywords: Lipid nanoparticles, mRNA delivery, Immunogenicity, Pharmacologic response, Dendritic cell, Immune system, Toll-like receptor
Introduction
Therapeutics based on RNA holds great promise to expand new drug candidates into developing of translational medicine [1, 2]. This proof of concept study has indicated the broad potential application of cancer and disease treatment and encompassed more than a dozen RNA therapies being tested in clinical trials [3, 4]. ONPATTRO™ (patisiran) is the first of short interfering RNA (siRNA)-based therapeutics and the only FDA-approved RNA interference (RNAi) drug, which has been administrated for the treatment of the transthyretin induced amyloidosis in adult patients [5]. siRNA interferes with specific genes expression through degrading messenger RNA (mRNA) after transcription to prevent the translation [6]. In contrast, mRNA has been utilized in the systemic administration of genetic vaccines in cancer [7] and infectious disease [8] and loading and activation of dendritic cells (DC) for the cellular vaccines [9, 10]. In other words, applications of mRNA have encompassed protein replacement therapy, cancer immunotherapy, vaccines, cellular reprogramming, and gene editing. Usage of aberrant protein for muscular dystrophy demonstrated necessitates mRNA for protein replacement [11] due to mRNA as a single molecule creates many copies of a protein during a short period. Compared to traditional vaccination, mRNA-based vaccines were efficient alternatives for personalized and prophylactic trials such as influenza, human immunodeficiency viruses (HIV), Zika, Cytomegalovirus3 (CMV) 3, and rabies [8, 11–13]. In cancer immunotherapy, in addition to mRNA encodes tumor-associated peptide antigens (Ags) to induce prophylactic immunity like vaccines [14, 15] mRNA modifies T cells with chimeric antigen receptor (CAR) T cell [16, 17]. Gene editing uses mRNA encoding to deliver clustered regularly interspaced short palindromic repeats (CRISPR) transcription activator-like effector nuclease (TALEN) and zinc-finger nucleases (ZFNs) through genome cut-and-paste mechanism for the lasting cure for genetic diseases [18]. The last application of mRNA is cellular reprogramming, which involves programming cell fate and function through tissue engineering and regenerative medicine, for instance, usage four using factors to induce pluripotent stem cells (iPSCs) stem lead to Nobel Prize-winning discovery in 2012 [19].
mRNA-based cancer therapy requires the transfection of antigen-presenting cells (APCs). To develop these vaccines, the transfection of APCs, such as DCs, is required to generate a cytotoxic T-cell response [9]. mRNA does not need any required nuclear localization or transcription but also includes at least a possible section of genomic integration for transfected sequences [20]. Although the mRNA therapeutics have been clinically advanced due to the design and manufacture of mRNA, the broad application of mRNA to express the desired protein is due to in vivo delivery challenges such as nucleases degradation, lack of stability, endosomal trapping, and immunotoxicity responses of mRNA are hindered. Among them, the critical factors of mRNA therapeutic becoming a viable clinical option based on dispelling mRNA instability and nucleases degradation and reducing endosomal trapping result in ineffective delivery to specific target cells through specific-purpose vectors [21–23]. In the last decade, the application and development of non-viral or synthetic and viral or natural delivery systems as the most promising strategies for gene manipulation to produce a human protein with higher expression efficiency and lower immunogenicity has attracted enormous interest [24, 25]. Immunogenicity and cytotoxicity concerns, the risks of reverse genome insertion, transient gene expression control, and vector-size limitations of viral constructs restricted their utilization for long-term therapeutics despite the obvious advantage of efficient delivery due to high-efficiency transfection of host cells and low and off-target expression [26, 27].
In contrast, non-viral vectors reduce the main drawbacks of using a viral vector, including innate immune stimulation and cytotoxicity concerns. In addition, non-viral vectors prevent nonspecific interactions with proteins or non-target cells and promote efficient transport to multiple tissues through targeted delivery [28–30]. More recently, numerous non-viral vectors, including polymer [31–33], hybrid [34–36], and even scaffold-mediated [37] nanoparticles (NPs), have been used and fostered for mRNA delivery. Recent studies have demonstrated that lipid-based NPs as the most frequently used carrier for mRNA delivery, are promising to treat cancer and disease disorders [11, 14, 38–40]. The first-time liposome was used for the mRNA delivery system in 1978 [41], and following that, the efforts were gradually continued up to now [42–44]. Finally, lipid nanoparticles (LNP) were developed for mRNA delivery in 2015 to decrease toxicity and immunogenicity concerns and also achieve high in vivo transfection [42].
LNP have mainly consisted of four lipid components such as phospholipids, including especially 2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) or 1,2-Dioleoyl-sn-Glycero-3 Phosphatidylethanolamine (DOPE) and helper lipids such as ionizable amine-containing lipidoid, cholesterol, and polyethylene glycol (PEG) [45–48]. LNP holds tremendous potential for mRNA delivery due to protection from nucleases, avoiding the mononuclear phagocyte system (MPS), low immunogenicity and endosomal trapping, and facilitating cellular uptake [26, 49]. However, LNP loaded with mRNA faced many challenges, including immune interaction (innate and adaptive immunity) such as interplay with LNP components [50]. On the other hand, the LNP compositions indicated a critical challenge for the cytosolic delivery of mRNA to target tissues in the clinic. In some cases, they could enhance DC uptake of the vaccine and prevent the mRNA from interacting with non-APCs, consequently hinder undesirable side effects [51, 52].
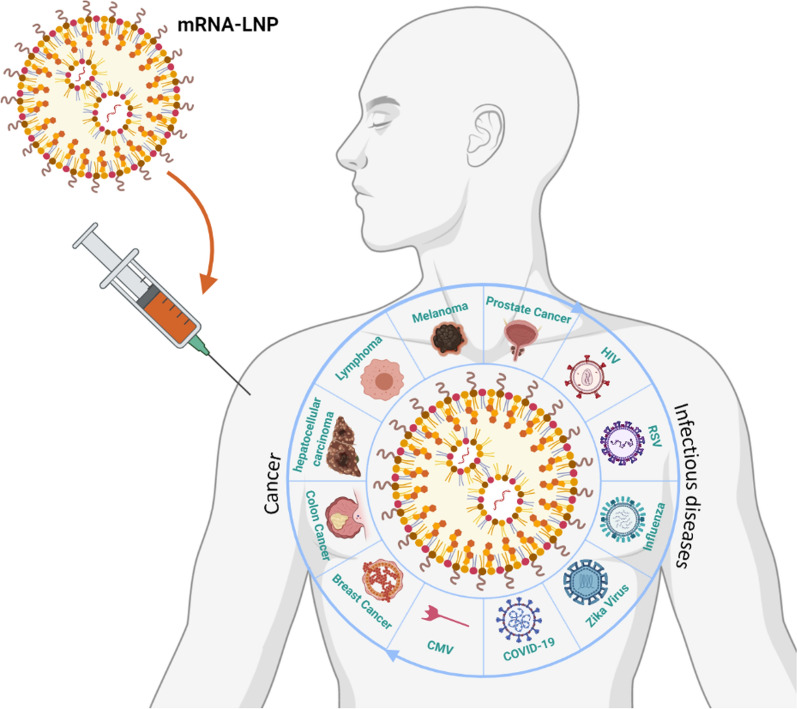
Following the onset of the ongoing global coronavirus disease 2019 (COVID-19) pandemic and vaccination in 2020, two mRNA-LNP vaccine platforms from Pfizer/BioNTech, and Moderna were approved and administered worldwide. The lack of explanatory data analysis of the immune responses and pharmacological interplay between LNP used in mRNA-based therapeutics and the target site [53–55], the necessity to evaluate an immunopharmacological profile of LNP have pointed out. This review study discusses the interplay between immune responses and LNP compositions and the profile of immune and pharmacologic responses to delivery mRNA through LNP. In order to analyze the immune responses, the interaction of LNP with neutrophil (Neut), macrophage (MQ), DC, complement, and adaptive immune system will be evaluated, then immunogenicity of mRNA delivery will be studied. Furthermore, pharmacological aspects of mRNA delivery using LNP containing pharmacokinetics (PK) and pharmacodynamics (PD) studies and the role of pattern recognition receptor (PRR) as the most important player in the common cancer cases including prostate, melanoma, lymphoma, hepatocellular carcinoma, colon cancer, and breast cancer will be discussed in detail.
Interplay of immune responses with compositions of LNP
LNP compositions consisted of cationic or ionizable lipids and helper lipids such as DSPC, DOPE, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), cholesterol, and PEG lipids. The cationic lipid in liposomal formulation for spontaneous negatively charged mRNA encapsulation was utilized by a combination of attractive electrostatic and hydrophobic interactions [56, 57]. Cholesterol as neutral lipid not only enhances particle stability and membrane fusion [58] but also promotes transfection efficiency in vitro [59] and in vivo [60, 61]. Although, it has been demonstrated that lipoplexes dissociate rapidly by substituting cholesterol with DOPE due to serum binding [58, 59]. Moreover, cholesterol can improve CL-complexed mRNA [62] and enhance transfection efficiency of mRNA delivery on account of the physical stability of the colloidal dispersion [63–66] in contrast, excess cholesterol destructs liposome preparation [62]. Using cationic lipids for in vivo administration demonstrated relative toxic effects due to high positive charge and poor endosomal escape [67]. Furthermore, they act to trigger an immunological response through the antibodies production. Forasmuch as helper lipids can shield the positive charge of lipoplexes, decrease accumulation in the lung, shifting in other organs specific the liver [68]. Furthermore, helper lipids can induce systemic inflammation due to their uptake by the liver kupffer cells [30, 42, 69]. DSPC, which is substrates for phospholipases A2 (PLA2s), plays a vital role in releasing eicosanoids (proinflammatory mediator) and produces allergens that induce inflammatory cytokines. Moreover, it is reported that the lysosomal PLA2s and cytosolic PLA2s may cause complement activation and anaphylaxis, respectively [70, 71]. To bypass these problems, reduce positive charge, overcome endosomal escape, and decrease immune and inflammatory responses, ionizable lipids for mRNA delivery were developed [72–74].
Despite common lipid materials that reactive T cells through the restriction CD1 family of APCs by the T cells [75, 76], phospholipids (PLs) generally do not incorporate reactive T cells [77]. Following in vivo administration of the first dose of phosphatidylcholine (PC) lipids, B-1 cells directly activate. For this reason, they induce natural Immunoglobulin M (IgM). Following repeated dosing, delivery to APC, including follicular DCs and marginal zone (MZ) B cells, was pushed due to anti-PC IgMs binding to LNP [78]. Cruz-Leal et al. showed the rational evidence for interaction between B-1 cells and dipalmitoyl-phosphatidylcholine (DPPC)-liposomes with ovalbumin (OVA) entrapped. This liposomal formulation modulates the immune response to OVA, which plays an antigen role in evaluating the character of B-1 cells in humoral immunity [78]. Furthermore, they demonstrated that following immunization of BALB/c mice with DPPC-liposomes containing OVA, B-1 cells secrete DPPC-specific IgM independently of the presence of OVA [79].
In 1989, one year after that, Felgner et al. introduced LNP, including 1,2-di-O-octadecenyl-3-trimethylammonium (DOTMA) and DOPE for nucleic acid delivery transfection; LNP was assessed with luciferase mRNA for in vivo analysis (human, mouse, rat, drosophila, and Xenopus) [80, 81]. DOPE, which is synthetic, unsaturated phosphatidylethanolamine (PE) as a common class of membrane or neutral lipid, increases the efficiency of LNP considerably. In contrast to other neutral PLs of the same acyl chain composition, such as even dioleoylphosphatidylcholine (DOPC), with the most similar in structure, DOPE serves to promote intracellular delivery of RNA [82–84] due to an endosmotic or fusogenic role, similar to the adenovirus [85] and fusion peptides [86]. This unique performance is relevant to the known relation between unsaturated PE, non-bilayer lipid structures, and membrane fusion [87]. Based on rational connectedness conjecture, DOPE is responsible for enabling LNP/RNA cargo to fuse with either the plasma or endosomal membrane after phagocytosis [88]. First-time, Zuhorn et al. indicated the major entry pathway to transfect lipoplexes (SAINT-2/DOPE) was clathrin-mediated endocytosis (CME) [89]. DOPE disrupts lipid bilayers to non-bilayer hexagonal (HII) at acidic pH because of its geometry with two bulky and unsaturated oleoyl chains, which make a cone-like shape [90, 91]. Moreover, the enriched level of DOPE could destabilize the endosome membrane; thus, the LNP could release mRNA into the cytosol after the fusion [90]. To that end, excessive fusogenicity leads to an enhanced interaction with serum proteins [88].
In this study, mRNA encoding the HIV-1 antigen gag with LNP (1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)/DOPE) could be delivered to DCs for immunization shows antigen-specific responses which display immune-activating properties such as secretion of type I interferon (IFN). IFN inhibits DOTAP/DOPE loaded antigen-encoding mRNA expression and results in the induction of antigen-specific immune responses [43]. On the other hand, although some studies showed DC maturation and Toll-like receptor (TLR) agonists restrict mRNA transfection, therefore decreased overall expression could be happened [92, 93]. To evaluate the effect on co-delivery of modified mRNA with nucleoside and TLR agonists to induce effective antigen-specific T cell immunity for improving mRNA vaccination efficiency was studied, and cholesterol was replaced by DOPE in lipoplex formulation. In what follows, the stability and transfection of APCs by DOTAP-cholesterol mRNA lipoplexes was successful; however, the mRNA expression was hindered by induction of cellular entry type I IFNs through unmodified mRNA. In contrast, modified mRNA with nucleoside declines the intracellular immune recognition and release of type I IFN [94].
In order to remove intolerable side effects of cationic lipids such as pro-inflammatory responses, toxicity, and immunogenicity following systemic administration, PEGylated PLs such as methoxy-PEG-distearoyl phosphoethanolamine (mPEG-DSPE) and dimyristoyl-rac-glycero-3- methoxy-PEG (DMG-mPEG) have been added to the membrane of the cationic lipids on the LNP surface. Moreover, PEGylation of LNP due to high water uptake creates a steric barrier around the LNP, which prevents the aggregation of LNP and inhibits LNP from reticuloendothelial system (RES) recognition. Despite these advantages, PEG plays a double-edged sword role. It inhibits the conversion of the bilayer to hexagonal form and eliminates targeted cellular uptake due to biodistribution reduction. Thus, PEGylation prolongs the circulation half-life, improves transfection efficiency and bioavailability, and decreases MQ uptake of LNP [95–98]. Following the first administration, the secretion of anti-PEG-IgM, which is responsible for the accelerated blood clearance (ABC), results in the more rapid clearance of LNP [99, 100]. Similarly to Cruz-Leal group researches which PC lipids had directly activated B-1 cells to induce natural IgM [78, 79], recently it demonstrated that interaction between B-1 cells and LNP leads to anti-PEG IgM response, both humoral responses being critical in driving the clearance of the LNP from the blood and into the MPS of the lymphoid (and other) tissues. Extending these findings to other delivery technologies that might similarly be subject to natural IgM opsonization is tempting. For instance, gene therapies often rely on viral vectors that include repeating PC-like epitopes and other oligonucleotide therapeutics that use LNP like those described in this study [15, 40].
Impact of LNP characterization and immune responses
Some physical and chemical properties of LNP such as size, polydispersity index (PDI), surface chemistry, charge and molar ratio of components affect their interaction with the immune system and biodistribution [2, 101]. Few studies have shown the impact of LNP characterization and immune responses up to now. In 2021, Hassett et al. investigated the effect of various biophysical factors on the immunogenicity of the CMV mRNA-LNP vaccine through the determination of antibody titer. Among the different parameters, LNP size showed the highest correlation with immunogenicity. It was observed that in mice, there is a positive relationship between size and immunogenicity; as the LNP size increases (until 100 nm), the antibody titer increases. While in non-human primates (NHPs), immunogenicity is independent of the size of the LNP, and the robust immune response is generated in all particle sizes studied [102]. Brewer et al. evaluated the antibody response to OVA-loaded liposomes with different sizes (100, 155, 225, and 560 nm) in mice. Significantly higher IgG2a and more significant IFN-γ generation were obtained for larger vesicles, while all other vesicles elicited anti-OVA IgG1 [103]. Similar observations were obtained in another study in which liposomes of either 250 or 980 nm in size were loaded with influenza A hemagglutinin [104]. Liposome size also affects the T-helper 1/T-helper 2 (Th1/Th2) ratio. So that vesicles with a size of 250–750 nm induce more potent Th1 responses compared to larger or smaller vesicles. Multilamellar vesicles, on the other hand, may induce more Th2 responses [103, 104].
In addition, the surface charge of LNP has been shown to affect their ability to stimulate the immune system. For instance, vesicles with a positive or negative charge induce antibody-neutralizing responses greater than neutral vesicles [105]. Cationic lipids increase the immunogenicity of LNP-formulated mRNA vaccines due to their ability to interact with the components of the innate immune system, which in turn leads to acceptable therapeutic efficacy [106]. For instance, cationic lipid dimethyl-dioctadecyl ammonium (DDA) acts as an adjuvant in mRNA-based vaccines due to its ability to induce ionic interactions with Ags [107–109]. Korsholm et al. demonstrated that the absorption of Ags onto DDA liposomes leads to enhanced immunogenicity due to an increase in its uptake by APCs as well as promotion of expression of maturation markers in APCs [109, 110]. Likewise, LNP containing DOTAP and DOTMA have been shown to activate TLRs and NLRP3 inflammasome pathways [111].
Functionalization of LNP is another factor that affects their immunogenicity. For instance, T N Pham et al. demonstrated that surface functionalization of LNP with different lipid-anchored gadolinium chelates leads to complement system activation initiated by IgM antibodies [112]. Moreover, self-amplifying mRNA (SAM) vaccines encapsulated in mannosylated LNP exhibited enhanced antibody levels and Ag-specific CD8+ T responses compared to unglycosylated LNP [113]. Another study demonstrated that incorporating ester prodrug of anti-inflammatory steroids such as rofleponide and budesonide to LNP could decrease dose-dependent inflammatory responses induced in the case of non-functionalized mRNA-LNP formulations [114]. In addition, as shown in the case of two COVID-19 mRNA-LNP vaccines, mRNA-1273 (from Moderna) (lipid composition: SM-102, [PEG-2000]-dimyristoyl glycerol [DMG], 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC] and cholesterol), and BNT162b2 (from Pfizer/BioNTech) (lipid composition: ALC-0315: (4 hydroxybutyl) azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), ALC-0159: 2[PEG-2000]-N,N-ditetradecylacetamide, 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) and cholesterol), the introduction of PEG into the LNP results in the formation of anti-PEG antibodies, followed by activation of the complement system, so their use in individuals with allergic reactions is challenging. Moreover, complement system activation leads to ABC phenomenon, which results in the poor therapeutic efficacy of mRNA-LNP-based vaccines [115, 116]. Likewise, Carreño et. al. observed the same pattern of anti-PEG antibodies in irrelevant LNPs from an influenza virus mRNA vaccine and demonstrated that reactivity of such anti-PEG antibodies correlated with antibody levels against low and high MW PEG. In addition, they demonstrated that the difference in the pattern of anti-PEG antibodies in serum of participants who received mRNA-1273 and BNT162b2 was not related to the difference in the structure of PEG in the two vaccines, but due to the higher dose of PEGylated lipid in mRNA-1273 compared to BNT162b2 and also how the PEG is presented by the carrier [117]. Biodegradable and non-toxic polymers such as polysarcosin, which have similar physicochemical properties to PEG, are less immunogenic and can be used as an alternative to PEG in LNP-mediated mRNA delivery [118].
mRNA-LNP delivery and innate immune system
The most essential function of the immune system is discrimination between self and non-self, which is coordinated by numerous and diverse cells in a unique and harmonic network. The immune system has encountered different substances and molecular patterns in various sizes, forms, and surface charges [119]. Therefore, the pharmaceutical NPs may elicit innate and adaptive immune responses and alter nanomedicines' PK and safety properties. Accordingly, keeping away from the immune response induced by NPs is the rapidly growing area in the delivery of nanomedicines into tumor cells, and for access to desirable results, the immunotoxicity tests of NPs are approved by regulatory agencies in the research and development of nanomedicines [120]. Nevertheless, the assessment of the sterility of manufactured NPs is necessary. Because bacterial endotoxins contamination may cause the formation of biomolecular corona on the surface of NPs under non-sterile conditions. The contamination of NPs by bacterial endotoxins or lipopolysaccharides (LPS) could evoke an immune response when intravenously administrated via activation of TLRs which express on the monocytes, MQs, DCs, and B lymphocytes [121]. Although excipients (such as Cremophor EL, polysorbate 80) and linkers (such as PEG) which are used in the synthesis of some NPs, are immunostimulants [122].
The initial barrier of the immune system is the innate cells which rapidly recognize distinct molecular patterns such as microbial agents, molecular apoptotic alarmins, and misfolded biomolecules [123]. Recognition of molecular patterns results in the initiation of signaling cascades that could subsequently activate the adaptive immune system via soluble factors or cell-to-cell contact [124]. The innate system consists of different cellular and soluble elements such as polymorphonuclear leukocytes (as Neut), monocytes, resident leukocytes (as MQ), professional APCs (as DC), natural killer cells (NK), eosinophils, basophils, mast cells, and distinct plasma proteins (complement system) [125].
mRNA-LNP delivery and neutrophils
Neuts, as predominant white blood cells, are polymorphonuclear cells that have an important role in acute inflammation and are the earliest immune cells recruited into tissue during acute inflammation [126]. Hwang et al. [127] demonstrated that cationic solid lipid NPs stimulated lactate dehydrogenase (LDH) release from Neuts, a biomarker for cytotoxicity. On the other hand, neutral solid lipid NPs were safe with no adverse effect on releasing LDH. They also showed that cationic solid LNP, unlike neutral solid LNP, could degranulate Neuts by phosphorylation of Janus N kinase (JNK) and led to elastase and superoxide release anion. Eventually, unlike neutral solid LNP, cationic solid LNP could stimulate NETosis, a biomarker of activated Neuts, and increase inflammation. The activation of Neuts by cationic solid LNP may be related to the cationic surfactant that is incorporated into solid LNP. In another study, Hwang et al. [128] investigated the interplay between cationic liposomes and human Neuts. They used two different cationic surfactants, cetyltrimethylammonium bromide (CTAB) and soyaethyl morpholinium ethosulfate (SME), for the synthesis of cationic liposomes. Firstly, they showed that CTAB-LNP dramatically decreased the cell viability of Neuts. The cytotoxicity of SME-LNP was concentration-dependent and less than CTAB-LNP and conventional liposomes, had no significant toxic effect on Neuts. They also indicated that CTAB-LNP could increase superoxide anion production while SME-LNP and simple liposomes had no significant impact on the production of superoxide anion. Activation of the NETosis also effective in CTAB-LNP, while SME-LNP could increase NET release less than CTAB-LNP. It may be related to the more critical positive zeta potential of CTAB-LNP (+ 45.6 mV) instead of SME-LNP (+ 27.9 mV).
mRNA-LNP delivery and macrophages
MQs are mononuclear phagocytic cells originating from fetal myeloid progenitors or are frequently derived from circulating monocytes. During tissue inflammation, monocytes are recruited and migrated into inflamed tissue under the extravasation mechanism which is controlled by inflammatory mediators such as cytokines, chemokines, and complement fragments [129]. Resident classical MQs are professional phagocytes that, similar to other innate immune cells, have PRRs such as pathogen-associated molecular pattern (PAMP) and damage-associated molecular pattern (DAMP). They are connected with microbial pathogens, apoptotic cells, or various chemicals [130]. In the concept of nano-immunology, NPs could as molecular patterns are known as nanoparticle-associated molecular patterns (NAMPs) [120]. PRRs could be categorized into two groups: membrane PRRs be composed of some TLRs, scavenger receptors, and C-type lectin receptor (CLR), and cytoplasmic PRRs consist of NOD-like receptors (NLR), RIG-1-like receptor (RLR), and endosomal TLRs [131]. Recognition of ligand by PRRs could enhance the overexpression of inflammatory mediators (such as cytokines and chemokines), antimicrobial peptides (such as defensins and cathelicidin), enzymes (such as lysozyme), endothelial adhesion molecules (such as selectins) as well as costimulatory molecules (such as CD80 and 86) [132]. Cytokines such as IL-1β, IL-6, IL-12, and TNF could trigger inflammation and evoke adaptive immune responses. Chemokines such as CCL2 and CXCL8 (also called IL-8) could recruit monocytes and Neuts, respectively [133]. The avoidance of the uptake of NPs by MQs and other phagocytes is the most critical criterion in designing nanomedicines. The route of NPs administration is determinant in NPs encountering with resident classical MQ [134]. For instance, intravenously administrated NPs must be resistant to engulfing and uptaking by blood monocytes or liver, spleen, and lung MQs. Also, the oral NPs must be resistant to MQs in lamina properia and Peyer's patches. MQs and other phagocytes also express complement receptors that bind to complement fragments (or opsonins) such as C3b and iC3b. Nevertheless, MQs express receptors for the Fc region of immunoglobulins (FcRs), and immunoglobulin G (IgG) antibodies could act as an opsonin. Opsonization facilitates the phagocytosis of particles and activates MQs and other phagocytes. Therefore, the nanopharmaceutical strategies have been designed to decrease immune-mediated recognition and activation of NPs [119, 135]. The size and surface charge of NPs are other considerable criteria in recognizing and eliminating NPs by MQs. For instance, NPs larger than 100 nm are phagocyted by liver kupffer cells and splenic red pulp MQs [136]. Also, cationic LNP, which are acceptable carriers of therapeutic nucleic acids (such as DNA, mRNA, and siRNA) are immune-cytotoxic and stimulate the production of inflammatory cytokines from phagocytes [51, 137]. Furthermore, therapeutic nucleic acids may elicit immune responses via intracellular PRRs of innate immune cells.
mRNA-LNP delivery and dendritic cells
DCs are professional APCs that express different extracellular and intracellular receptors for PAMPs, DAMPs, and NAMPs. They are sentinel cells distributed throughout epithelial surfaces such as skin (Langerhans cells), gastrointestinal, respiratory, and urogenital tract. Ags are captured, processed, and then presented by mature DCs to naive T cells in lymph nodes. It could elicit the T cell-mediated adaptive response. Therefore, MQs and DCs as the earlier immune cells encountered with NPs that administrated through intradermal, subcutaneous, and intralymphatic routes. DCs could evoke an immune response that leads to inflammation [138].
On the other hand, NPs may be applied in cancer nano-immunotherapy via delivering different pharmaceutical biomolecules such as mRNA, siRNA, or other genes into DCs. They will discuss more in the next section. Here we direct immunotoxicity of NPs concerning DCs. The frequency of mature DCs in peripheral blood is low (around 0.2% of human blood mononuclear cells) as well as the population of DCs is heterogeneous. Accordingly, isolation of DCs from blood is complex, and ex vivo manufacturing of bone marrow-derived DCs (BMDCs) or circulating monocytes may be applied to evaluate NPs immunotoxicity [139]. Barbosa et al. [140] investigated that LNP could be a suitable carrier for delivering resveratrol into DCs without toxicity. The size of LNP was below 200 nm with a highly negative zeta potential (up to − 30 mV). They showed that LNP was internalized by naïve and stimulated DCs in a dose-dependent manner. In addition, the induction of the cell response by LNP-mRNA platforms depends on the PRR pathways and DCs engaged, so the introduction of PRR ligands or mRNAs encoding T cell-polarizing cytokines into the LNP leads to the induction of Th cell subsets by these vaccines. Also, it has been suggested that one of the important strategies to overcome the inflammation caused by the LNP content of the mRNA-LNP vaccines is targeting those lacking cationic/ionizable lipids to a subset of DCs capable of eliciting an antibody response in the absence of inflammatory agents [141].
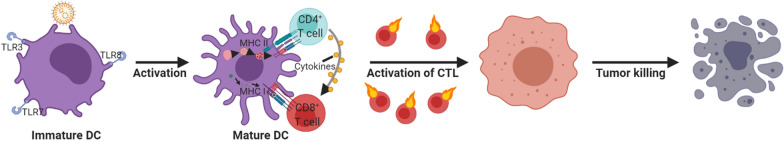
Furthermore, the immunotoxicity of LNP on DCs investigated by detection of apoptosis as well as morphological changes without any remarkable cytotoxicity. Finally, they showed that the immunomodulatory effects of resveratrol on DCs in the presence of inflammatory cytokine tumor necrosis factor-alpha (TNF-α) were improved by LNP. The mechanism of tumor cell killing through DCs maturation by LNP assisted mRNA delivery is represented in Fig. 1.
Fig. 1.
Schematic representation of the mechanism of tumor cell killing through DCs maturation by LNP assisted mRNA delivery
mRNA-LNP delivery and complement
The complement system is one of the noticeable parts of the innate immune system that collaborates with the adaptive immune system. It comprises more than 30 proteins synthesized by the liver and released into the serum as precursor proteins or pro-enzymes (also known as zymogens) and sequentially activates each other in a cascade [142]. The main result of the complement activation is triggering inflammation, stimulating phagocytosis, and forming a membrane attack complex that leads to the elimination of pathogens. The complement system has three pathways: the classical pathway, the alternative pathway, and the lectin pathway. All pathways form a C3 convertase that cleaves C3 into C3b and C3a. C3b may bound to the microbial surface and stimulate the phagocytosis or form the membrane attack complex (C3b-9), which disregards the cell membrane and causes cell lysis. C3a (and C5a) are anaphylatoxins that recruit phagocytes such as Neuts, MQs, and eosinophils and promote inflammation [143]. Anaphylatoxins in large amounts cause a systemic allergic reaction that is mediated by Th2 cells and immunoglobulin E (IgE) antibodies. It may be referred to as complement activation-related pseudoallergy (CARPA) or non-immune allergy in the nanomedical literature [144]. Intravenously administrated NPs must be avoided from the attachment of C3b and indeed activation of the alternative pathway. For instance, there are some clinical findings of the complement activation due to the liposomal doxorubicin (Doxil®) [145–147]. Szebeni J listed the liposomal nanomedicines such as Amphotericin B formulations (Abelcet®, AmBisome®, and Amphotec/Amphocyl®), Daunorubicin (DaunoXome®), Doxorubicin (Doxil®, and Myocet®), and verteporfin (Visudyne®) which induced CARPA [144]. On the other hand, NPs in the tumor microenvironment can trigger the release of C5a and afterward cause the recruitment of regulatory and immunosuppressive immune cells such as T regulatory (Treg) cells which suppress the anti-tumoral immune responses [121]. Recently, Sabnis et al. [148] in Moderna Therapeutics developed a novel amino lipid series for improving PD and immunotoxicity of mRNA-based LNP. They showed for the first time that this novel amino-LNP could be tolerable at doses up to 1 mg kg−1 with no complement activation in both rats and primates. They also found that novel amino-LNP, compared to the conventional LNP, efficiently escaped from endosomes and released their mRNA contents into the cytosol.
mRNA-LNP delivery and adaptive immune system
The recognition of Ags in the adaptive immune system is performed by specific T or B cell receptors. Accordingly, T cells recognize immunogenic Ags, which are presented by APCs such as DCs, MQs, and also B lymphocytes. The recognition of Ags is mediated by surface immunoglobulins of B cells and then presented by major histocompatibility complex (MHC) molecules to T cells [149]. Furthermore, the pro-inflammatory mediators such as IL-1, TNF, and IL-12 are other activatory factors of adaptive immune cells.IL-1 and IL-1ra act critical regulators of the inflammatory response to RNA vaccines. Unlike humans, murine leukocytes respond to RNA vaccines by upregulating anti-inflammatory IL-1ra relative to IL-1 (predominantly IL-1α), protecting mice from cytokinemediated toxicities at >1,000-fold higher vaccine doses. to this end, the IL-1 was unexpectedly amplified by lipid components in the formulations incorporating N1-methyl-pseudouridine-modified RNA to alleviate activation of TLR. Indeed, recognition of specific Ags by T and B lymphocytes triggers the clonal expansion of activated and memory cells. The particular traits of adaptive immune cells are specificity and memory [152]. NPs that APCs present could elicit hypersensitivities such as allergic or autoimmune responses. Attachment of LNP to proteins in blood or biological fluids and corona proteins can activate T and B lymphocytes. DCs may take up corona proteins, and these cells present the peptides to T cells. It may lead to hypersensitivity reactions [153]. In some circumstances, the chemical structure of liposomes could trigger the production of antibodies by B lymphocytes. For example, the lipid A of liposomes could produce antibodies against PC and PE in animal models. However, the titers of antibodies were not as much as the levels that cause autoimmune reactions. Moreover, repeated administration of PEGylated liposomes could induce B cells and produce anti-PEG antibodies such as IgM that accelerate their blood clearance [154].
mRNA-LNP delivery and immunogenicity
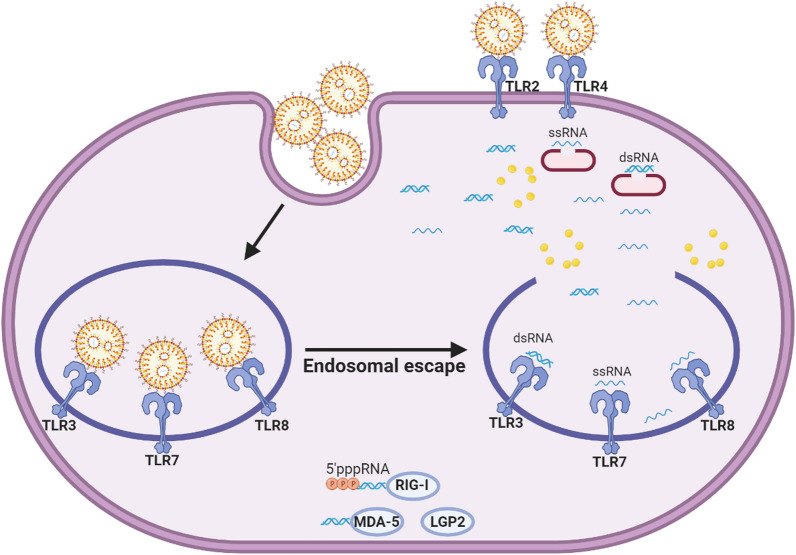
The mRNA-based therapeutics open new avenues in molecular medicine, especially cancer immunotherapy. Besides biochemical instability, the immunogenicity of mRNA is the main challenge in its drug ability. Moreover, in the NPs-mediated delivery of mRNA, the escaping from endosomal degradation and releasing into the cytosol in the proper biomolecular conformation is another critical obstacle [155]. The RNA sensors of innate immune cells are endosomal PRRs and cytosolic retinoic acid-inducible RLRs. Endosomal PRRs belong to the TLRs family and include TLR 3, 7, and 8. TLR3 is a sensor for double-stranded RNA (dsRNA), and TLR 7 and 8 recognize single-stranded RNA (ssRNA). Activation of endosomal TLRs is the main initiator of overexpression of the type 1 interferons (IFN α/β), IL-1, IL-6, as well as TNF [133]. On the other hand, RLRs are retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene-5 (MDA-5) that recognize viral RNAs. RIG-I recognizes a 5′-triphosphate moiety of RNA molecules, as well as MDA-5, which recognizes long double-stranded RNAs (dsRNAs) [156]. Figure 2 depicts the PRRs reorganization for mRNA and LNP in intracellular and extracellular pathways through TLRs, RLRs, and NLRs. In the extracellular pathway, TLRs as endosomal receptors are expressed by immune cells. dsRNA, which is a secondary structure form of mRNA, is detected by TLR3, and ssRNA form of mRNA is also detected by TLR7 and TLR8. The RLRs represent recognizing dsRNA through the unique interaction of uncapped dsRNA with RIG-I, binding long dsRNA with MDA-5, and binding or regulatory effects in RNA detection with LGP2. NLRP3 and NOD2 from NLRs family members detect dsRNA and ssRNA, respectively. In the intracellular pathway, cationic lipids compositions of LNP are mainly recognized by extracellular TLR2 and TLR4 and intracellular NLRP3.
Fig. 2.
PRRs recognize both mRNA and LNP in both intracellular and extracellular pathways through TLRs, RLRs, and NLRs
Eventually, RLRs could activate the expression of IFN α/β and also, viral inhibitory proteins [157]. Therefore, the conformational modification of mRNA is one of the approaches for declining the innate immune response [158]. For instance, Kormann et al. [159] showed that partial substitution of uridine and cytidine with 2-thiouridine and 5-methyl-cytidine decreased the innate immune activation via recognition by endosomal TLRs and cytosolic RIG-1. They also found that increasing the stability of mRNA could improve the productivity of protein expression in vitro and in vivo. Besides mRNA modification, designing an accurate delivery platform is essential in avoiding an immune encounter. On the other hand, in the cancer vaccine concept, the delivery platform is designed to deliver antigen-encoded mRNA into APCs such as DCs and MQs. In this regard, using cationic LNP is applicable, but due to their possible interaction with serum opsonins, it must be considered to decline the formation of protein corona and destroyed by phagocytes. Lastly, cationic LNP may facilitate mRNA evasion from endosomes and reduce its endosomal degradation or recognition by endosomal TLRs [30]. In addition, mRNA-LNP (1273) COVID-19 vaccine, which induces a protective immune profile including induction of humoral immunity, is attended to spike-specific T follicular helper (Tfh), CD4+ Th1, and CD8+ T cells, and generation of spike-binding germinal center B cells and neutralizing antibodies [160].
In addition to mRNA, LNP used as delivery systems have been shown to cause inflammation in preclinical studies. In a study by Ndeupen et al., both intradermal and intranasal administration of LNP were shown to activate various inflammatory pathways.likewise, they produce inflammatory cytokines, resulting in a severe inflammatory response and high mortality rate in mice [161]. The cationic/ionizable lipid content of LNP is also cytotoxic and causes inflammatory responses through activating TLRs [162]. In addition, Pfizer/BioNTech and Moderna mRNA-LNP vaccines caused fever, pain, and swelling in humans [163, 164]. As a result, a balance should be struck between positive adjuvant and negative inflammatory properties of LNP in mRNA-LNP vaccines.
Pharmacological study of mRNA-LNP delivery
The process of protein expression is managed by mRNA, which is naturally dynamic in the cytoplasm. In addition to endogenous mRNA, the exogenous synthetic mRNA can be delivered to the target cells to initiate the desired protein(s) production. The PK barriers against exogenous mRNA delivery in vitro or in vivo include degrading ubiquitous RNases in all extracellular environments and the negative charge of the cellular membrane, which repels mRNA. Both barriers reduce exogenous mRNA bioavailability and the production of the desired protein(s) [165]. Moreover, in vitro transfection of naked exogenous mRNA to humans, mice, and DCs did not represent a significant protein expression effect compared to material-mediated mRNA delivery. In the same study, the responses achieved via in vivo transfected naked exogenous mRNA were highly related to the route of administration, where the SC injection has been reported as the successful route [166]. Also, locally in vivo injection could not significantly increase the activated T-cells level [167]. Therefore, the need for improving the efficiency of in vitro and in vivo exogenous mRNA transfection has become a challenging issue. Many investigations have reported different solutions to this issue through material-free mRNA transfection methods, but the better results seem to be represented via material-mediated mRNA delivery [168]. The efficient delivery of material-mediated mRNA to DCs has been described both in vitro and in vivo subcutaneously, intravenously, and intranasally [32]. To confirm more, Pardi et al. showed significant more protein expression kinetics in mice through various intravenous (IV), intraperitoneal (IP), subcutaneous (SC), intramuscular (IM), intratumoral (IT), and intradermal (ID) routes of administration with the application of LNP for delivery of exogenous mRNA [169]. Ionizable lipid-mediated LNP not only can improve cellular uptake but also can optimize the endosomal release of the carried mRNA. In 2018, Moderna Therapeutics Company improved the pharmacological impact of mRNA-LNP with lipid manipulation in the LNP development through the synthesis of a new amino ionizable lipid, which was also utilized in their COVID-19 vaccine manufacture. Reduced endosomal trapping and sustained pharmacology and achieved optimal safety using ionizable LNP formulation in rodent and primate models [148].
mRNA-LNP delivery in cancer types
Many investigations on cancer have recently demonstrated the benefits of mRNA-based therapies in vitro and in vivo on activating innate and adaptive immune responses, as discussed in "Impact of LNP characterization and immune responses and mRNA-LNP delivery and innate immune system" section. Here we review the impact of mRNA-based interventions on different types of cancers, including melanoma, lymphoma, hepatocellular carcinoma, colon cancer, breast cancer, and prostate cancer (Table 1).
Table 1.
mRNA-LNP delivery in cancer types
| LNP name | mRNA | Cancer type | Pharmacological effects and feedback | Refs. |
|---|---|---|---|---|
| Cationic LNP | OVA and the control EGFP mRNA | Lymphoma |
Significant increase in expression of CD80 and CD86 in the splenic CD11+ T-lymphocytes Increased CD69+ T-cells of the spleen and the lymph nodes was the other sign of activation of T-cell responses |
[174] |
| Ionizable lipid-based NPs (OF-Deg-Lin) | Human factor VIII (hFVIII) mRNA | Successful induction of protein expression and production in the B-cells of the spleen | [184] | |
| LNP | IL-12A mRNA | Hepatocellular carcinoma | Enhancement of activated CD44+ immune cells such as CD3+ and CD4+ Th cells following IL-12 expression, shrinkage of tumor size, and increased survival in mice | [185] |
| cKK-E12 LNP | in vitro-transcribed mRNA was designed and formulated by cKK-E12 LNP to produce trastuzumab, an anti-HER2 monoclonal antibody | Breast cancer | In vivo PK characteristics of trastuzumab mRNA vs. synthetic trastuzumab (Herceptin®) in C57BL/6 mice: the significant higher serum level of trastuzumab, and interestingly the used dosage of this system was lower than Herceptin®, the higher morbidity-free survival in HER2+ mice and the lower average tumor volume via trastuzumab mRNA | [180] |
| PEG-coated hybrid of a cationic lipid-like compound (G0-C14) and poly lactic-co-glycolic acid (PLGA) | PTEN mRNA | Prostate cancer | Decrease in tumor size and weight with the least adverse effects on body weight increased apoptosis and declined survival of tumor cells | [36] |
As cancer with high somatic mutations, melanoma has been the area of interest for mRNA-based LNP-assisted investigations [170]. The mRNA encoding the human melanoma-associated antigen recognized by T cells 1 (MART1), a specific tumor antigen, mRNA encoding Lysosome Associated Membrane Protein-1 (LAMP1), and Bax-mRNA have been used for immunization of the mice model of melanoma using different LNP. Modified liposomes such as histidylatedor mannosylated lipopolyplexes carrying mRNA vs. sugar-free lipopolyplexes lead to enhanced antitumor immune responses and increased survival rate [52, 171]. Moreover, cationic liposomes containing Bax-mRNA vs. Bax-plasmid cationic liposomes demonstrated more efficient anti-tumor effects in vitro and in vivo [172]. Oberli et al. [14] used OVA mRNA-lipid based formulation as a vaccine system to stimulate the immune system via improved CD8+ T-cell responses against mice models of melanoma. The usage of 3β-[N-(N′, N′-dimethylaminoethane)-carbamoyl] cholesterol hydrochloride in substitution for cholesterol did not preferably stimulate CD8+ T-cell toxicity. Also, the pegylated lipids with the smallest size stimulated the most clonal expansion of CD8+ T-cells.
On the other hand, the addition of sodium lauryl sulfate improved the performance efficacy of the LNP. The detailed characteristic properties of the LNP formulations that caused higher clonal expansion and function of the CD8+ T-lymphocytes included the lower molar composition of the ionizable lipid and LNP with zeta potentials of − 15 to − 3 mV. The delivery of Cre-recombinase-mRNA through the final ideal LNP to Ai14D reporter mice clarified the protein expression rate to DCs, Neuts, MQs, and B cells, respectively. Moreover, unmodified vs. modified OVA mRNA denoted a better CD8+ T cell production profile primarily through induction of IFN when assessed on the 7th day of the injection. In another study, OVA mRNA modified liposomes that contained α-Galactosylceramide as an immune adjuvant and agonist for invariant NKs, showed a significant reduction of tumor growth in B16-OVA melanoma model; however the survival rate was moderate [173].
Non-Hodgkin’s lymphoma is known as cancer with dysregulation of B cells, which negatively affects the activation and function of T-cells. Therefore, targeting the lymphocytes with the delivery of specific mRNAs to them could be a reasonable approach for treating this cancer. Furthermore, mRNA delivery to the spleen and lymphoid glands, as the organs of lymphocytes’ activation site, might be other targeting points [174]. Some of the studies that investigated the effects of LNP-mediated mRNA delivery in lymphoma are listed in Table 1.
An ideal anti-cancer against hepatocellular carcinoma should pass the tests for the effective anti-tumoral characteristics and have enough hepatic safety to be approved for clinical usage. LNP-mediated mRNA delivery in transgenic mice, which is the animal model of hepatocellular carcinoma with overexpressed MYC oncogene, led to shrinkage of tumor size and increased survival in these mice suggesting the possible effectiveness of mRNA with LNP. Moreover, the other important point of the mentioned delivery system was the lowest toxicity in normal cells compared to other similar works [175].
An attempt to improve mRNA delivery by LNP from PD or PK aspects is slight modifications of LNP. In some investigations on colon cancer, the complexation of protamine to liposome increased mRNA condensing to the liposome, lowered enzymatic degradation, and improved delivery efficiency. Moreover, higher immune safety and better anti-tumor activity in all IP, IT, and IV administration routes, especially the IV administration of mRNA protamine-liposome, was obtained for mRNA delivery system in comparison to DNA plasmid [176, 177].
One of the vital standard medications for treating specific breast cancers is the application of synthetic antibodies as targeted therapy. Although this treatment is an approved method, the high costs of pure antibody production, the challenging production process, low bioavailability, and high adverse reactions led the researchers to substitute the antibody synthesis with in vivo production of antibodies through the delivery of specific in vitro-transcribed mRNA [178, 179]. In vivo evaluation of PK characteristics of this system vs. synthetic trastuzumab (Herceptin®) in C57BL/6 mice revealed a significantly higher serum level of trastuzumab, and interestingly the used dosage of this system was lower than Herceptin®. Moreover, the higher morbidity-free survival in HER2+ mice, the lower average tumor volume, and no significant toxic effects was observed via trastuzumab mRNA in comparison with Herceptin® [180, 181].
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is a well-known tumor suppressor whose non-functionality in several cancers, such as prostate cancer, has been recognized. This event leads to dysregulation of the phosphoinositide 3-kinase (PI3K) signaling pathway, increases survival, angiogenesis, and metastasis, and declines apoptosis [182]. Therefore, PTEN mRNA delivery to prostate cancer cells could effectively increase tumor suppression potential by impacting various cell behaviors. It has been demonstrated that PEG-coated polymer-lipid hybrid NPs can be used as stable non-immunogen systems for in vitro and in vivo delivery of modified PTEN mRNA, increasing PTEN expression and early apoptosis and also inhibition of cell viability and PI3K pathway activity. Also, the PC3 tumor-bearing nude mice could achieve this delivery system intravenously with acceptable serum stability and PK properties such as significant tumor accumulation. Pharmacodynamically, this delivery system could decrease tumor size and weight with the least adverse effects on body weight, reflecting increased apoptosis and declined survival of tumor cells [36, 183].
mRNA-LNP delivery in infectious disease
mRNA vaccines have been developed as a new technology for combating infectious diseases due to their advantages, such as ease of manufacturing, acceptable immunogenicity, and good safety profile. Moreover, mRNA vaccines are more appropriate for targeting diseases via high genetic instability and infectivity than other vaccines. mRNA-based vaccines were first used as a therapeutic drug in 1989 [186, 187]. It has been proven that the encapsulation of mRNA in LNP facilitates the control of biodistribution as well as cell targeting. Recently, six COVID-19 vaccines based on mRNA encapsulated in LNP have entered clinical trials, and mRNA-1273 (Moderna vaccine) and BNT162b2; Comirnaty (Pfizer/BioNTech vaccine) were given FDA emergency use authorization (EUA) in 2020 [188]. In a longitudinal cohort, the T cell responses were evaluated following the administration of the SARS-CoV-2 mRNA vaccine in naive and recovered individuals, and it was observed that vaccine-induced CD4+ and CD8+ T cells were similar to those of the baseline in natural infection with SARS-CoV-2. In addition, it was shown that the CD4+ T cell responses were rapidly induced in naive subjects following the administration of the first dose of the vaccine, and robust changes in CD4+ T cell response followed by the administration of the second dose, while the second dose appears to have minimal effect on the CD4+ and CD8+ T cell responses in recovered individuals [189]. In addition to SARS-CoV-2, mRNA-LNP vaccine candidates for various viruses, including HIV, seasonal influenza, Zika virus, respiratory syncytial virus (RSV), and Epstein-Barr virus (EBV), are also in the preclinical and clinical stages (Table 2) [190]. For example, several studies demonstrated that mRNA-based influenza vaccines elicit a significantly protective immune response against heterosubtypic and homologous influenza viruses [8, 191]. In addition to being utilized as a vaccine, mRNA could also be used for therapeutic goals. For instance, Pardi et al. demonstrated that the administration of mRNA encoding the anti-HIV antibody encapsulated in LNP represents passive immunotherapy against HIV-1 [192]. It should be noted that the type of mRNA delivery system as well as the administration route affect its in vivo biodistribution and, consequently its efficacy. In a study, mRNA delivery to the APCs was performed using two types of delivery systems, mRNA-LNP, and DC-mRNA, and it was observed that after IV administration, DC-mRNA has the highest accumulation in the lungs, while mRNA-LNP accumulated in the spleen, indicating its rapid passage through the lungs and further uptake by the APCs [193]. In another study, Zhang et al., evaluated the biodistribution of FLuc mRNA-LNP in mice inoculated via IM, SC, or intranasal routes. Following IM and SC injection, robust mRNA expression was observed in the upper abdomen and the injection site, while no signal was detected in route [194]. In another study, the effect of LNP content on pharmacological parameters of H10N8 mRNA formulated via the five ionizable lipids as LNP was investigated by determining lipid levels after IM administration. These ionizable lipids showed faster degradation in muscle, liver, and spleen compared to MC3. There was also a slight correlation in LNP performance between the IM and IV routes, which is related to the differences in the optimal physical or chemical properties of the two routes [195].
Table 2.
mRNA-LNP delivery in infectious disease
| LNP components | mRNA | Infection | Pharmacological effects and feedback | Refs. |
|---|---|---|---|---|
| Ionizable lipid: DSPC: cholesterol: PEG-lipid | mRNA encoding prM-E genes | Zika virus infection | High neutralizing antibody titers which conferred sterilizing immunity | [11] |
| Ionizable cationic lipid/phosphatidylcholine/cholesterol/PEG-lipid | mRNAs encoding the broadly neutralizing anti-HIV-1 antibody VRC01 | HIV-1 infection |
Increase in VRC01 antibody concentrations in the mice plasma 24 h after IV injection No increase in pro-inflammatory cytokine and type I IFN production |
[192] |
| Ionizable cationic lipid (proprietary to Acuitas Therapeutics)/phosphatidylcholine/cholesterol/PEG-lipid | mRNAs encoding HIV-1 envelope, ZIKV prM-E, and influenza virus hemagglutinin (HA) | HIV, influenza, Zika viruses |
Induction of high levels of Tfh cells and germinal center (GC) B cells Induction of potent antigen-specific CD4+ T cell responses and neutralizing antibody responses in nonhuman primates and mice |
[196] |
| Ionizable lipid: DSPC: cholesterol: PEG-lipid | RSV F-expressing mRNAs | RSV infection |
Induction of potent neutralizing antibody responses in both cotton rats and mice Induction of strong CD4+ and CD8+ T cell responses in mice |
[197] |
| Ionizable cationic lipid (proprietary to Acuitas Therapeutics)/phosphatidylcholine/cholesterol/PEG-lipid | mRNA encoding envelope or HIV-1 Env gp160 | HIV-1 infection | Induction of durable antibody responses | [198] |
| Ionizable cationic lipid (proprietary to Acuitas Therapeutics)/phosphatidylcholine/cholesterol/PEG-lipid | mRNA encoding the clade C transmitted/founder 1086C HIV-1 Env | HIV infection | Induction of high levels of gp120-specific antibodies in rhesus macaques and rabbits | [199] |
| Ionizable lipid: structural lipid: sterol: PEG-lipid | mRNA encoding the POWV prM and E genes | Powassan virus (POWV), an emerging tick-borne flavivirus | Induction of high levels of neutralizing antibodies and sterilizing immunity | [200] |
Some of the clinical studies that used mRNA-LNP -based vaccines for cancer and infectious disease are summarized in Table 3.
Table 3.
mRNA-LNP-based vaccines for cancer and infectious disease in clinical trials
| Name | Condition | Clinical phase | Status | NCT No |
|---|---|---|---|---|
| mRNA-1345 | RSV | Phase I | Recruiting | NCT04528719 |
| Rabipur® | Rabies virus | Phase I | Active, not recruiting | NCT03713086 |
|
mRNA-1325 mRNA-1893 |
Zika virus |
Phase I Phase I |
Completed Completed |
|
| VAL-506440; mRNA-1440 | Influenza A virus [H10N8] | Phase I | Completed | NCT03076385 |
| VAL-339851; mRNA-1851 | Influenza A virus [H7N9] | Phase I | Completed | NCT03345043 |
| mRNA-1647 and mRNA-1443 | CMV | Phase I | – | NCT03382405 |
| SAM-LNP- Spike | COVID-19 | Phase I | Recruiting | NCT04776317 |
| SAM-LNP- Spike | COVID-19 | Phase I | Active, not recruiting | NCT04758962 |
| ChulaCov19 mRNA vaccine | COVID-19 | Phase I | Not yet recruiting | NCT04566276 |
| SARS-CoV-2 mRNA vaccine | COVID-19 | Phase III | Not yet recruiting | NCT04847102 |
| TAA mRNA | Melanoma | Phase I | Recruiting | NCT02410733 |
| TAA and neo‑Ag mRNA | Breast cancer | Phase I | Recruiting | NCT02316457 |
| mRNA-2752 | Solid tumors | Phase 1 | Recruiting | NCT03739931 |
| mRNA-2416 | Solid tumor, Lymphoma, and Ovarian | Phase I-II | Recruiting | NCT03323398 |
| mRNA-4157 | Bladder carcinoma and NSCLC | Phase I | Recruiting | NCT03313778 |
| mRNA-4157 | Melanoma | Phase II | Recruiting | NCT03897881 |
Conclusion
As seen in the recent pandemic, LNP with dual adjuvant and carrier properties can provide mRNA vaccine candidates with the beneficial aspects of increased stability, cellular uptake, and desired therapeutic effects in solid tumors and infectious diseases. Likewise, two mRNA-LNP COVID-19 vaccine platforms from Pfizer/BioNTech and Moderna were approved and administered worldwide. Although, the possible immune responses against LNP is one of the major criteria in the PK and safety properties of nanomedicine. The chemical compositions and possible microbial contamination of LNP, as well as using of immunostimulants such as excipients and route of in vivo administration, are major determinants in immunogenicity and the PK and PD yield of mRNA delivery. Furthermore, the immunogenicity of mRNA via activation of cytoplasmic RNA sensors such as TLRs and RLRs is the main challenge in its drug ability. Therefore, the conformational modification of mRNAs is one of the approaches to the decline of immune responses. Taken together, it seems likely that further research between immunology and pharmacology areas and LNP compositions will illuminate the dark side of LNP for safe and efficient mRNA delivery. Consequently, thoroughgoing studies in immunopharmacological responses of LNP for therapeutic delivery of mRNA would cause a dramatic evolution in cancer and infectious disease treatment.
Acknowledgements
Not applicable.
Abbreviations
- ABC
Accelerated blood clearance
- Ags
Antigens
- APCs
Antigen-presenting cells
- CAR
Chimeric antigen receptor
- CARPA
Complement activation-related pseudoallergy
- CLR
C-type lectin receptor
- CME
Clathrin-mediated endocytosis
- CRISPR
Clustered regularly interspaced short palindromic repeats
- CTAB
Cetyltrimethylammonium bromide
- DAMP
Damage-associated molecular pattern
- DC
Dendritic cell
- DOPC
Dioleoylphosphatidylcholine
- DOPE
1,2-Dioleoyl-sn-Glycero-3 phosphatidylethanolamine
- DMG-mPEG
Dimyristoyl-rac-glycero-3-methoxy-PEG
- DOTAP
1,2-Dioleoyl-3-trimethylammonium-propane) DPPC: Dipalmitoyl-phosphatidylcholine
- DSPC
2-Distearoyl-sn-glycero-3-phosphocholine
- dsRNAs
Double-stranded RNAs
- EGFP
Enhanced green fluorescent protein
- ID
Intradermal
- IFN
Interferon
- IgG
Immunoglobulin G
- IgE
Immunoglobulin E
- IgM
Immunoglobulin
- IM
Intramuscular
- IP
Intraperitoneal
- iPSCs
Induce pluripotent stem cells
- IT
Intratumoral
- IV
Intravenous
- JNK
Janus N kinase
- LDH
Lactate dehydrogenase
- LNP
Lipid nanoparticles
- LPS
Lipopolysaccharides
- MART1
Melanoma associated antigen recognized by T cells 1
- MDA-5
Melanoma differentiation-associated gene-5
- MHC
Major histocompatibility complex
- mPEG-DSPE
Methoxy-PEG-distearoyl phosphoethanolamine
- MPS
Mononuclear phagocyte system
- mRNA
Messenger RNA
- MQ
Macrophage
- MZ
Marginal zone
- NAMP
Nanoparticle-associated molecular pattern
- MBL
Mannose-binding lectin
- NET
Neutrophil extracellular traps
- Neut
Neutrophil
- NK
Natural killer cell
- NLR
NOD-like receptor
- NPs
Nanoparticles
- OVA
Ovalbumin
- PAMP
Pathogen-associated molecular pattern
- PD
Pharmacodynamics
- PE
Phosphatidylethanolamine
- PEG
Polyethylene glycol
- PI3K
Phosphoinositide 3-kinase
- PK
Pharmacokinetics
- PLGA
Poly lactic-co-glycolic acid
- PRR
Pattern recognition receptor
- PTEN
Phosphatase and tensin homologue deleted on chromosome 10
- RNAi
RNA interference
- RLR
RIG-1-like receptor
- RIG-I
Retinoic acid-inducible gene I
- siRNA
Short interfering RNA
- ssRNA
Single-stranded RNA
- SME
Soyaethyl morpholinium ethosulfate
- SC
Subcutaneous
- TALEN
Transcription activator-like effector nuclease
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor alpha
- Th2
T-helper 2
- Treg
T regulatory
- ZFNs
Zinc-finger nucleases
Author contributions
SHK and RJ accomplished the conception of the research work. SHK, NMZ, AA, and RB carried out data collection and analysis. SHK and NMZ Draft the article manuscript. AA and SHK design and generation of the figures. FK and HV do the critical revision of the article. RJ and FK did final approval of the version to be published. All authors read and approved the final manuscript.
Authors' information
Seyed Hossein Kiaie, Senior Formulation Scientist at ReNAP (mRNA)Therapeutics and PhD Candidate of Pharmaceutical Nanotechnology at Department of Pharmaceutics, Tabriz University of Medical Sciences, Tabriz, Iran.
Naime Majidi Zolbanin, Assistant Professor of Pharmacology (PhD) at Department of Pharmacology, Urmia University of Medical Sciences, Urmia, Iran.
Armin Ahmadi, PhD Candidate of Mechanical Engineering at Chemical and Materials Engineering, University of Alabama in Huntsville, USA.
Rafieh Bagherifar, PhD Candidate of Pharmaceutical Nanotechnology at Department of Pharmaceutics, Tabriz University of Medical Sciences, Tabriz, Iran.
Hadi Valizadeh, Professor of Pharmaceutics (Pharm. D & PhD) at Department of Pharmaceutics, Tabriz University of Medical Sciences.
Fatah Kashanchi, Professor of Virology (PhD) and Director of the Laboratory of Molecular Virology at George Mason University, USA.
Reza Jafari, Assistant professor of immunology (PhD) at Solid Tumor Research Center, Cellular and Molecular Medicine Institute, Urmia University of Medical Sciences, Urmia, Iran.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors give consent for the publication of manuscript in Journal of Nanobiotechnology.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fatah Kashanchi, Email: fkashanc@gmu.edu.
Reza Jafari, Email: rezajaafary@yahoo.com.
References
- 1.Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects. Chem Biol. 2012;19(1):60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah RR, Taccone M, Monaci E, Brito LA, Bonci A, O’Hagan DT, et al. The droplet size of emulsion adjuvants has significant impact on their potency, due to differences in immune cell-recruitment and-activation. Sci Rep. 2019;9(1):1–9. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaczmarek JC, Kowalski PS, Anderson DG. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome medicine. 2017;9(1):60. doi: 10.1186/s13073-017-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou L-Y, Qin Z, Zhu Y-H, He Z-Y, Xu T. Current RNA-based therapeutics in clinical trials. Curr Gene Ther. 2019;19(3):172–196. doi: 10.2174/1566523219666190719100526. [DOI] [PubMed] [Google Scholar]
- 5.Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14(12):1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RC, Doudna JA. Molecular mechanisms of RNA interference. Annu Rev Biophys. 2013;42:217–239. doi: 10.1146/annurev-biophys-083012-130404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiedler K, Lazzaro S, Lutz J, Rauch S, Heidenreich R. mRNA cancer vaccines. Current Strategies in Cancer Gene Therapy: Springer; 2016. p. 61–85. [DOI] [PubMed]
- 8.Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benteyn D, Heirman C, Bonehill A, Thielemans K, Breckpot K. mRNA-based dendritic cell vaccines. Expert Rev Vaccines. 2015;14(2):161–176. doi: 10.1586/14760584.2014.957684. [DOI] [PubMed] [Google Scholar]
- 10.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199(1):251–263. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 11.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, et al. Modified mRNA vaccines protect against Zika virus infection. Cell. 2017;168(6):1114–25. e10. [DOI] [PMC free article] [PubMed]
- 12.John S, Yuzhakov O, Woods A, Deterling J, Hassett K, Shaw CA, et al. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine. 2018;36(12):1689–1699. doi: 10.1016/j.vaccine.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Armbruster N, Jasny E, Petsch B. Advances in RNA vaccines for preventive indications: a case study of a vaccine against rabies. Vaccines. 2019;7(4):132. doi: 10.3390/vaccines7040132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17(3):1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broos K, Van der Jeught K, Puttemans J, Goyvaerts C, Heirman C, Dewitte H, et al. Particle-mediated intravenous delivery of antigen mRNA results in strong antigen-specific T-cell responses despite the induction of type I interferon. Mol Therapy-Nucleic Acids. 2016;5:e326. doi: 10.1038/mtna.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39(1):49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiesinger M, März J, Kummer M, Schuler G, Dörrie J, Schuler-Thurner B, et al. Clinical-scale production of CAR-T cells for the treatment of melanoma patients by mRNA transfection of a CSPG4-Specific CAR under full GMP compliance. Cancers. 2019;11(8):1198. doi: 10.3390/cancers11081198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H-X, Zhang Y, Yin H. Genome editing with mRNA encoding ZFN, TALEN, and Cas9. Mol Ther. 2019;27(4):735–746. doi: 10.1016/j.ymthe.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. cell. 2006;126(4):663–76. [DOI] [PubMed]
- 20.McIvor RS. Therapeutic delivery of mRNA: the medium is the message. Mol Ther. 2011;19(5):822–823. doi: 10.1038/mt.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowdy SF. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol. 2017;35(3):222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]
- 22.Radoshevich L, Dussurget O. Cytosolic innate immune sensing and signaling upon infection. Front Microbiol. 2016;7:313. doi: 10.3389/fmicb.2016.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanton MG, Murphy-Benenato KE. Messenger RNA as a novel therapeutic approach. RNA Therapeutics: Springer; 2017. pp. 237–253. [Google Scholar]
- 24.Schott WJ, Galla M, Godinho T, Baum C, Schambach A. Viral and non-viral approaches for transient delivery of mRNA and proteins. Curr Gene Ther. 2011;11(5):382–398. doi: 10.2174/156652311797415872. [DOI] [PubMed] [Google Scholar]
- 25.Schott JW, Morgan M, Galla M, Schambach A. Viral and synthetic RNA vector technologies and applications. Mol Ther. 2016;24(9):1513–1527. doi: 10.1038/mt.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 27.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 28.Uludag H, Ubeda A, Ansari A. At the intersection of biomaterials and gene therapy: progress in non-viral delivery of nucleic acids. Front Bioeng Biotechnol. 2019;7:131. doi: 10.3389/fbioe.2019.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajj KA, Whitehead KA. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat Rev Mater. 2017;2(10):1–17. doi: 10.1038/natrevmats.2017.56. [DOI] [Google Scholar]
- 30.Granot Y, Peer D, editors. Delivering the right message: Challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics—An innate immune system standpoint. Seminars in immunology; 2017: Elsevier. [DOI] [PubMed]
- 31.Dong Y, Dorkin JR, Wang W, Chang PH, Webber MJ, Tang BC, et al. Poly (glycoamidoamine) brushes formulated nanomaterials for systemic siRNA and mRNA delivery in vivo. Nano Lett. 2016;16(2):842–848. doi: 10.1021/acs.nanolett.5b02428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lungwitz U, Breunig M, Blunk T, Göpferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60(2):247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Sharifnia Z, Bandehpour M, Hamishehkar H, Mosaffa N, Kazemi B, Zarghami N. In-vitro Transcribed mRNA Delivery Using PLGA/PEI Nanoparticles into Human Monocyte-derived Dendritic Cells. Iranian J Pharmaceut Res. 2019;18(4):1659. doi: 10.22037/ijpr.2019.1100872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao W, Zhang C, Li B, Zhang X, Luo X, Zeng C, et al. Lipid polymer hybrid nanomaterials for mRNA delivery. Cell Mol Bioeng. 2018;11(5):397–406. doi: 10.1007/s12195-018-0536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaczmarek JC, Patel AK, Kauffman KJ, Fenton OS, Webber MJ, Heartlein MW, et al. Polymer–lipid nanoparticles for systemic delivery of mRNA to the lungs. Angew Chem. 2016;128(44):14012–14016. doi: 10.1002/ange.201608450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Islam MA, Xu Y, Tao W, Ubellacker JM, Lim M, Aum D, et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat Biomed Eng. 2018;2(11):850–864. doi: 10.1038/s41551-018-0284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen R, Zhang H, Yan J, Bryers JD. Scaffold-mediated delivery for non-viral mRNA vaccines. Gene Ther. 2018;25(8):556–567. doi: 10.1038/s41434-018-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kose N, Fox JM, Sapparapu G, Bombardi R, Tennekoon RN, De Silva AD, et al. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Science immunology. 2019;4(35):6647. doi: 10.1126/sciimmunol.aaw6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truong B, Allegri G, Liu X-B, Burke KE, Zhu X, Cederbaum SD, et al. Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc Natl Acad Sci. 2019;116(42):21150–21159. doi: 10.1073/pnas.1906182116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.An D, Schneller JL, Frassetto A, Liang S, Zhu X, Park J-S, et al. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2017;21(12):3548–3558. doi: 10.1016/j.celrep.2017.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dimitriadis GJ. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature. 1978;274(5674):923–924. doi: 10.1038/274923a0. [DOI] [PubMed] [Google Scholar]
- 42.Michel T, Luft D, Abraham M-K, Reinhardt S, Medina MLS, Kurz J, et al. Cationic nanoliposomes meet mRNA: Efficient delivery of modified mRNA using hemocompatible and stable vectors for therapeutic applications. Molecular Therapy-Nucleic Acids. 2017;8:459–468. doi: 10.1016/j.omtn.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollard C, Rejman J, De Haes W, Verrier B, Van Gulck E, Naessens T, et al. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol Ther. 2013;21(1):251–259. doi: 10.1038/mt.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F, Xiao W, Elbahnasawy MA, Bao X, Zheng Q, Gong L, et al. Optimization of the linker length of mannose-cholesterol conjugates for enhanced mRNA delivery to dendritic cells by liposomes. Front Pharmacol. 2018;9:980. doi: 10.3389/fphar.2018.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28(2):172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 46.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 47.Dong Y, Love KT, Dorkin JR, Sirirungruang S, Zhang Y, Chen D, et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc Natl Acad Sci. 2014;111(11):3955–3960. doi: 10.1073/pnas.1322937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen D, Love KT, Chen Y, Eltoukhy AA, Kastrup C, Sahay G, et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J Am Chem Soc. 2012;134(16):6948–6951. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- 49.Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18(7):1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Najafi-Hajivar S, Zakeri-Milani P, Mohammadi H, Niazi M, Soleymani-Goloujeh M, Baradaran B, et al. Overview on experimental models of interactions between nanoparticles and the immune system. Biomed Pharmacother. 2016;83:1365–1378. doi: 10.1016/j.biopha.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 51.Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7(5):319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perche F, Benvegnu T, Berchel M, Lebegue L, Pichon C, Jaffrès PA, et al. Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomed Nanotechnol Biol Med. 2011;7(4):445–453. doi: 10.1016/j.nano.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Hoerr I, Obst R, Rammensee HG, Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol. 2000;30(1):1–7. doi: 10.1002/1521-4141(200001)30:1<1::AID-IMMU1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 54.Scheel B, Aulwurm S, Probst J, Stitz L, Hoerr I, Rammensee HG, et al. Therapeutic anti-tumor immunity triggered by injections of immunostimulating single-stranded RNA. Eur J Immunol. 2006;36(10):2807–2816. doi: 10.1002/eji.200635910. [DOI] [PubMed] [Google Scholar]
- 55.Lu D, Benjamin R, Kim M, Conry R, Curiel D. Optimization of methods to achieve mRNA-mediated transfection of tumor cells in vitro and in vivo employing cationic liposome vectors. Cancer Gene Ther. 1994;1(4):245–252. [PubMed] [Google Scholar]
- 56.Cullis PR, Hope MJ. Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017;25(7):1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin Q, Chen J, Zhang Z, Zheng G. Lipid-based nanoparticles in the systemic delivery of siRNA. Nanomedicine. 2014;9(1):105–120. doi: 10.2217/nnm.13.192. [DOI] [PubMed] [Google Scholar]
- 58.Cheng X, Lee RJ. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv Drug Deliv Rev. 2016;99:129–137. doi: 10.1016/j.addr.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Anchordoquy TJ. The role of lipid charge density in the serum stability of cationic lipid/DNA complexes. Biochim Biophys Acta. 2004;1663(1–2):143–157. doi: 10.1016/j.bbamem.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Hong K, Zheng W, Baker A, Papahadjopoulos D. Stabilization of cationic liposome-plasmid DNA complexes by polyamines and poly (ethylene glycol)-phospholipid conjugates for efficient in vivo gene delivery. FEBS Lett. 1997;400(2):233–237. doi: 10.1016/S0014-5793(96)01397-X. [DOI] [PubMed] [Google Scholar]
- 61.Li S, Rizzo M, Bhattacharya S, Huang L. Characterization of cationic lipid-protamine–DNA (LPD) complexes for intravenous gene delivery. Gene Ther. 1998;5(7):930–937. doi: 10.1038/sj.gt.3300683. [DOI] [PubMed] [Google Scholar]
- 62.Khatri N, Baradia D, Vhora I, Rathi M, Misra A. Development and characterization of siRNA lipoplexes: effect of different lipids, in vitro evaluation in cancerous cell lines and in vivo toxicity study. AAPS PharmSciTech. 2014;15(6):1630–1643. doi: 10.1208/s12249-014-0193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao X, Huang L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem Biophys Res Commun. 1991;179(1):280–285. doi: 10.1016/0006-291X(91)91366-K. [DOI] [PubMed] [Google Scholar]
- 64.De Rosa G, De Stefano D, Laguardia V, Arpicco S, Simeon V, Carnuccio R, et al. Novel cationic liposome formulation for the delivery of an oligonucleotide decoy to NF-κB into activated macrophages. Eur J Pharm Biopharm. 2008;70(1):7–18. doi: 10.1016/j.ejpb.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Hattori Y, Nakamura A, Arai S, Kawano K, Maitani Y, Yonemochi E. siRNA delivery to lung-metastasized tumor by systemic injection with cationic liposomes. J Liposome Res. 2015;25(4):279–286. doi: 10.3109/08982104.2014.992024. [DOI] [PubMed] [Google Scholar]
- 66.Hafez I, Maurer N, Cullis P. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001;8(15):1188–1196. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- 67.Kedmi R, Ben-Arie N, Peer D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials. 2010;31(26):6867–6875. doi: 10.1016/j.biomaterials.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 68.Wang X, Yu B, Ren W, Mo X, Zhou C, He H, et al. Enhanced hepatic delivery of siRNA and microRNA using oleic acid based lipid nanoparticle formulations. J Control Release. 2013;172(3):690–698. doi: 10.1016/j.jconrel.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Filion MC, Phillips NC. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochimica et Biophysica Acta (BBA) Biomembranes. 1997;1329(2):345–56. [DOI] [PubMed]
- 70.Pniewska E, Pawliczak R. The involvement of phospholipases A2 in asthma and chronic obstructive pulmonary disease. Mediat Inflammat. 2013;2013:793505. doi: 10.1155/2013/793505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hatziantoniou S, Maltezou HC, Tsakris A, Poland GA, Anastassopoulou C. Anaphylactic reactions to mRNA COVID-19 vaccines: a call for further study. Vaccine. 2021;39(19):2605. doi: 10.1016/j.vaccine.2021.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Habrant D, Peuziat P, Colombani T, Dallet L, Gehin J, Goudeau E, et al. Design of ionizable lipids to overcome the limiting step of endosomal escape: application in the intracellular delivery of mRNA, DNA, and siRNA. J Med Chem. 2016;59(7):3046–3062. doi: 10.1021/acs.jmedchem.5b01679. [DOI] [PubMed] [Google Scholar]
- 73.Guimaraes PP, Zhang R, Spektor R, Tan M, Chung A, Billingsley MM, et al. Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening. J Control Release. 2019;316:404–417. doi: 10.1016/j.jconrel.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Billingsley MM, Singh N, Ravikumar P, Zhang R, June CH, Mitchell MJ. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20(3):1578–1589. doi: 10.1021/acs.nanolett.9b04246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12(12):845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Rhijn I, Godfrey DI, Rossjohn J, Moody DB. Lipid and small-molecule display by CD1 and MR1. Nat Rev Immunol. 2015;15(10):643–654. doi: 10.1038/nri3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Donnell VB, Rossjohn J, Wakelam MJ. Phospholipid signaling in innate immune cells. J Clin Investig. 2019;128(7):2670–2679. doi: 10.1172/JCI97944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cruz-Leal Y, Machado Y, López-Requena A, Canet L, Laborde R, Alvares AM, et al. Role of B-1 cells in the immune response against an antigen encapsulated into phosphatidylcholine-containing liposomes. Int Immunol. 2014;26(8):427–437. doi: 10.1093/intimm/dxu042. [DOI] [PubMed] [Google Scholar]
- 79.Cruz-Leal Y, López-Requena A, Lopetegui-González I, Machado Y, Alvarez C, Pérez R, et al. Phosphocholine-specific antibodies improve T-dependent antibody responses against OVA encapsulated into phosphatidylcholine-containing liposomes. Front Immunol. 2016;7:374. doi: 10.3389/fimmu.2016.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci. 1987;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dwarki V, Malone RW, Verma IM. [43] Cationic liposome-mediated RNA transfection. Methods in Enzymology. 217: Elsevier; 1993. p. 644–54. [DOI] [PubMed]
- 82.Legendre J-Y, Szoka FC., Jr Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: comparison with cationic liposomes. Pharm Res. 1992;9(10):1235–1242. doi: 10.1023/A:1015836829670. [DOI] [PubMed] [Google Scholar]
- 83.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114(1):100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 84.Felgner JH, Kumar R, Sridhar C, Wheeler CJ, Tsai YJ, Border R, et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994;269(4):2550–2561. doi: 10.1016/S0021-9258(17)41980-6. [DOI] [PubMed] [Google Scholar]
- 85.Curiel DT, Agarwal S, Wagner E, Cotten M. Adenovirus enhancement of transferrin-polylysine-mediated gene delivery. Proc Natl Acad Sci. 1991;88(19):8850–8854. doi: 10.1073/pnas.88.19.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Plank C, Oberhauser B, Mechtler K, Koch C, Wagner E. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J Biol Chem. 1994;269(17):12918–12924. doi: 10.1016/S0021-9258(18)99963-1. [DOI] [PubMed] [Google Scholar]
- 87.Cullis PR, Hope MJ, Tilcock CP. Lipid polymorphism and the roles of lipids in membranes. Chem Phys Lipid. 1986;40(2–4):127–144. doi: 10.1016/0009-3084(86)90067-8. [DOI] [PubMed] [Google Scholar]
- 88.Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochimica et Biophysica Acta (BBA) Biomembranes. 1995;1235(2):289–95. [DOI] [PubMed]
- 89.Zuhorn IS, Kalicharan R, Hoekstra D. Lipoplex-mediated transfection of mammalian cells occurs through the cholesterol-dependent clathrin-mediated pathway of endocytosis. J Biol Chem. 2002;277(20):18021–18028. doi: 10.1074/jbc.M111257200. [DOI] [PubMed] [Google Scholar]
- 90.Litzinger DC, Huang L. Phosphatodylethanolamine liposomes: drug delivery, gene transfer and immunodiagnostic applications. Biochimica et Biophysica Acta (BBA) Reviews On Biomembranes. 1992;1113(2):201–27. [DOI] [PubMed]
- 91.Hattori Y, Suzuki S, Kawakami S, Yamashita F, Hashida M. The role of dioleoylphosphatidylethanolamine (DOPE) in targeted gene delivery with mannosylated cationic liposomes via intravenous route. J Control Release. 2005;108(2–3):484–495. doi: 10.1016/j.jconrel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 92.Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RA, et al. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc Natl Acad Sci. 2010;107(9):4287–4292. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Diken M, Kreiter S, Selmi A, Britten C, Huber C, Türeci Ö, et al. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 2011;18(7):702–708. doi: 10.1038/gt.2011.17. [DOI] [PubMed] [Google Scholar]
- 94.Verbeke R, Lentacker I, Wayteck L, Breckpot K, Van Bockstal M, Descamps B, et al. Co-delivery of nucleoside-modified mRNA and TLR agonists for cancer immunotherapy: restoring the immunogenicity of immunosilent mRNA. J Control Release. 2017;266:287–300. doi: 10.1016/j.jconrel.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 95.Yi Xue H, Guo P, Wen W-C, Lun WH. Lipid-based nanocarriers for RNA delivery. Curr Pharm Des. 2015;21(22):3140–3147. doi: 10.2174/1381612821666150531164540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balazs DA, Godbey W. Liposomes for use in gene delivery. Journal of drug delivery. 2011;2011. [DOI] [PMC free article] [PubMed]
- 97.Campani V, Salzano G, Lusa S, De Rosa G. Lipid nanovectors to deliver RNA oligonucleotides in cancer. Nanomaterials. 2016;6(7):131. doi: 10.3390/nano6070131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83(3):97–112. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 99.Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, Van der Meer JW, et al. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther. 2000;292(3):1071–1079. [PubMed] [Google Scholar]
- 100.Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, et al. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Control Release. 2006;112(1):15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 101.Howard GP, Verma G, Ke X, Thayer WM, Hamerly T, Baxter VK, et al. Critical size limit of biodegradable nanoparticles for enhanced lymph node trafficking and paracortex penetration. Nano Res. 2019;12(4):837–844. doi: 10.1007/s12274-019-2301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hassett KJ, Higgins J, Woods A, Levy B, Xia Y, Hsiao CJ, et al. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J Control Release. 2021;335:237–246. doi: 10.1016/j.jconrel.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 103.Brewer JM, Tetley L, Richmond J, Liew FY, Alexander J. Lipid vesicle size determines the Th1 or Th2 response to entrapped antigen. J Immunol. 1998;161(8):4000–4007. [PubMed] [Google Scholar]
- 104.Mann JF, Shakir E, Carter KC, Mullen AB, Alexander J, Ferro VA. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine. 2009;27(27):3643–3649. doi: 10.1016/j.vaccine.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 105.Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, et al. Positively charged liposome functions as an efficient immunoadjuvant in inducing immune responses to soluble proteins. Biochem Biophys Res Commun. 1997;240(3):793–797. doi: 10.1006/bbrc.1997.7749. [DOI] [PubMed] [Google Scholar]
- 106.Guevara ML, Persano F, Persano S. Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Front Chem. 2020;8:963. doi: 10.3389/fchem.2020.589959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lou G, Anderluzzi G, Schmidt ST, Woods S, Gallorini S, Brazzoli M, et al. Delivery of self-amplifying mRNA vaccines by cationic lipid nanoparticles: the impact of cationic lipid selection. J Control Release. 2020;325:370–379. doi: 10.1016/j.jconrel.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 108.Alfagih IM, Aldosari B, AlQuadeib B, Almurshedi A, Alfagih MM. Nanoparticles as adjuvants and nanodelivery systems for mRNA-based vaccines. Pharmaceutics. 2020;13(1):45. doi: 10.3390/pharmaceutics13010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith Korsholm K, Agger EM, Foged C, Christensen D, Dietrich J, Andersen CS, et al. The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology. 2007;121(2):216–226. doi: 10.1111/j.1365-2567.2007.02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blakney AK, McKay PF, Yus BI, Aldon Y, Shattock RJ. Inside out: optimization of lipid nanoparticle formulations for exterior complexation and in vivo delivery of saRNA. Gene Ther. 2019;26(9):363–372. doi: 10.1038/s41434-019-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lonez C, Bessodes M, Scherman D, Vandenbranden M, Escriou V, Ruysschaert J-M. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomed Nanotechnol Biol Med. 2014;10(4):775–782. doi: 10.1016/j.nano.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 112.Pham CT, Mitchell LM, Huang JL, Lubniewski CM, Schall OF, Killgore JK, et al. Variable antibody-dependent activation of complement by functionalized phospholipid nanoparticle surfaces. J Biol Chem. 2011;286(1):123–130. doi: 10.1074/jbc.M110.180760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goswami R, Chatzikleanthous D, Lou G, Giusti F, Bonci A, Taccone M, et al. Mannosylation of LNP results in improved potency for self-amplifying RNA (SAM) vaccines. ACS Infect Dis. 2019;5(9):1546–1558. doi: 10.1021/acsinfecdis.9b00084. [DOI] [PubMed] [Google Scholar]
- 114.Davies N, Hovdal D, Edmunds N, Nordberg P, Dahlén A, Dabkowska A, et al. Functionalized lipid nanoparticles for subcutaneous administration of mRNA to achieve systemic exposures of a therapeutic protein. Molecular Therapy-Nucleic Acids. 2021;24:369–384. doi: 10.1016/j.omtn.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Vrieze J. Pfizer's vaccine raises allergy concerns. American Association for the Advancement of Science; 2021. [DOI] [PubMed]
- 116.Kozma G, Shimizu T, Ishida T, Szebeni J. Anti-PEG antibodies: Properties, formation and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv Drug Del Rev. 2020;154:163–175. doi: 10.1016/j.addr.2020.07.024. [DOI] [PubMed] [Google Scholar]
- 117.Carreño JM, Singh G, Tcheou J, Srivastava K, Gleason C, Muramatsu H, et al. mRNA-1273 but not BNT162b2 induces antibodies against polyethylene glycol (PEG) contained in mRNA-based vaccine formulations. medRxiv. 2022:2022.04.15.22273914. [DOI] [PMC free article] [PubMed]
- 118.Nogueira SS, Schlegel A, Maxeiner K, Weber B, Barz M, Schroer MA, et al. Polysarcosine-functionalized lipid nanoparticles for therapeutic mRNA delivery. ACS Appl Nano Mater. 2020;3(11):10634–10645. doi: 10.1021/acsanm.0c01834. [DOI] [Google Scholar]
- 119.Smith DM, Simon JK, Baker JR., Jr Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13(8):592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fadeel B. Clear and present danger? Engineered nanoparticles and the immune system. Swiss medical weekly. 2012;142(2526). [DOI] [PubMed]
- 121.Boraschi D, Italiani P, Palomba R, Decuzzi P, Duschl A, Fadeel B, et al., editors. Nanoparticles and innate immunity: new perspectives on host defence. Seminars in Immunology; 2017: Elsevier. [DOI] [PubMed]
- 122.Dobrovolskaia MA, Shurin M, Shvedova AA. Current understanding of interactions between nanoparticles and the immune system. Toxicol Appl Pharmacol. 2016;299:78–89. doi: 10.1016/j.taap.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6(1):10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stuart LM, Paquette N, Boyer L. Effector-triggered versus pattern-triggered immunity: how animals sense pathogens. Nat Rev Immunol. 2013;13(3):199–206. doi: 10.1038/nri3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20(1):197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 126.Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Frontiers in physiology. 2018: 113. [DOI] [PMC free article] [PubMed]
- 127.Hwang T-L, Aljuffali IA, Hung C-F, Chen C-H, Fang J-Y. The impact of cationic solid lipid nanoparticles on human neutrophil activation and formation of neutrophil extracellular traps (NETs) Chem Biol Interact. 2015;235:106–114. doi: 10.1016/j.cbi.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 128.Hwang T-L, Hsu C-Y, Aljuffali IA, Chen C-H, Chang Y-T, Fang J-Y. Cationic liposomes evoke proinflammatory mediator release and neutrophil extracellular traps (NETs) toward human neutrophils. Colloids Surf, B. 2015;128:119–126. doi: 10.1016/j.colsurfb.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 129.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10(6):427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 130.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 131.Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 2021;6(1):1–24. doi: 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 134.Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today. 2015;10(4):487–510. doi: 10.1016/j.nantod.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J Control Release. 2005;107(2):215–228. doi: 10.1016/j.jconrel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 136.Liu Y, Hardie J, Zhang X, Rotello VM, editors. Effects of engineered nanoparticles on the innate immune system. Seminars in immunology; 2017: Elsevier. [DOI] [PMC free article] [PubMed]
- 137.Dow SW, Fradkin LG, Liggitt DH, Willson AP, Heath TD, Potter TA. Lipid-DNA complexes induce potent activation of innate immune responses and antitumor activity when administered intravenously. J Immunol. 1999;163(3):1552–1561. [PubMed] [Google Scholar]
- 138.Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154(1):3–20. doi: 10.1111/imm.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nair S, Archer GE, Tedder TF. Isolation and generation of human dendritic cells. Current protocols in immunology. 2012;Chapter 7:Unit7.32. [DOI] [PMC free article] [PubMed]
- 140.Barbosa JP, Neves AR, Silva AM, Barbosa MA, Reis MS, Santos SG. Nanostructured lipid carriers loaded with resveratrol modulate human dendritic cells. Int J Nanomed. 2016;11:3501. doi: 10.2147/IJN.S108694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Igyártó BZ, Jacobsen S, Ndeupen S. Future considerations for the mRNA-lipid nanoparticle vaccine platform. Curr Opin Virol. 2021;48:65–72. doi: 10.1016/j.coviro.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343(1):227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Janeway Jr CA, Travers P, Walport M, Shlomchik MJ. The complement system and innate immunity. Immunobiology: The Immune System in Health and Disease 5th edition: Garland Science; 2001.
- 144.Dézsi L, Fülöp T, Mészáros T, Szénási G, Urbanics R, Vázsonyi C, et al. Features of complement activation-related pseudoallergy to liposomes with different surface charge and PEGylation: comparison of the porcine and rat responses. J Control Release. 2014;195:2–10. doi: 10.1016/j.jconrel.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 145.Uziely B, Jeffers S, Isacson R, Kutsch K, Wei-Tsao D, Yehoshua Z, et al. Liposomal doxorubicin: antitumor activity and unique toxicities during two complementary phase I studies. J Clin Oncol. 1995;13(7):1777–1785. doi: 10.1200/JCO.1995.13.7.1777. [DOI] [PubMed] [Google Scholar]
- 146.Muggia FM, Hainsworth JD, Jeffers S, Miller P, Groshen S, Tan M, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J Clin Oncol. 1997;15(3):987–993. doi: 10.1200/JCO.1997.15.3.987. [DOI] [PubMed] [Google Scholar]
- 147.Muggia F, Hamilton A. Phase III data on Caelyx® in ovarian cancer. Eur J Cancer. 2001;37:15–18. doi: 10.1016/S0959-8049(01)00330-6. [DOI] [PubMed] [Google Scholar]
- 148.Sabnis S, Kumarasinghe ES, Salerno T, Mihai C, Ketova T, Senn JJ, et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol Ther. 2018;26(6):1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bianca C, Brézin L. Modeling the antigen recognition by B-cell and T-cell receptors through thermostatted kinetic theory methods. Int J Biomath. 2017;10(05):1750072. doi: 10.1142/S1793524517500723. [DOI] [Google Scholar]
- 150.Tahtinen S, Tong AJ, Himmels P, Oh J, Paler-Martinez A, Kim L, Wichner S, Oei Y, McCarron MJ, Freund EC, Amir ZA. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nature Immunology. 2022;23(4):532-42. [DOI] [PubMed]
- 151.Mellman I, Tahtinen S, Tong AJ, Himmels P, Oh J, Paler-Martinez A, Kim L, Wichner S, Oei Y, McCarron M, Freund E. IL-1β and IL-1ra are key regulators of the inflammatory response to RNA vaccines. [DOI] [PubMed]
- 152.Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S33–40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 153.Neagu M, Piperigkou Z, Karamanou K, Engin AB, Docea AO, Constantin C, et al. Protein bio-corona: critical issue in immune nanotoxicology. Arch Toxicol. 2017;91(3):1031–1048. doi: 10.1007/s00204-016-1797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kohli AG, Kierstead PH, Venditto VJ, Walsh CL, Szoka FC. Designer lipids for drug delivery: from heads to tails. J Control Release. 2014;190:274–287. doi: 10.1016/j.jconrel.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27(4):710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rehwinkel J, Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. 2020;20(9):537–551. doi: 10.1038/s41577-020-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jiang F, Ramanathan A, Miller MT, Tang G-Q, Gale M, Patel SS, et al. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature. 2011;479(7373):423–427. doi: 10.1038/nature10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stuart LM. In Gratitude for mRNA Vaccines. New England Journal of Medicine. 2021. [DOI] [PubMed]
- 159.Kormann MS, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S, et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat Biotechnol. 2011;29(2):154–157. doi: 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- 160.DiPiazza AT, Leist SR, Abiona OM, Moliva JI, Werner A, Minai M, et al. COVID-19 vaccine mRNA-1273 elicits a protective immune profile in mice that is not associated with vaccine-enhanced disease upon SARS-CoV-2 challenge. Immunity. 2021;54:1869–1882. doi: 10.1016/j.immuni.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ndeupen S, Qin Z, Jacobsen S, Estanbouli H, Bouteau A, Igyártó BZ. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. Biorxiv. 2021;24:103479. doi: 10.1016/j.isci.2021.103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Samaridou E, Heyes J, Lutwyche P. Lipid nanoparticles for nucleic acid delivery: current perspectives. Adv Drug Deliv Rev. 2020;154:37–63. doi: 10.1016/j.addr.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 163.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. New England J Med. 2020. [DOI] [PMC free article] [PubMed]
- 164.McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. Npj Vaccines. 2021;6(1):1–14. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics—developing a new class of drugs. Nat Rev Drug Discovery. 2014;13(10):759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 166.Phua KK, Leong KW, Nair SK. Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format. J Control Release. 2013;166(3):227–233. doi: 10.1016/j.jconrel.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Phua KK, Nair SK, Leong KW. Messenger RNA (mRNA) nanoparticle tumour vaccination. Nanoscale. 2014;6(14):7715–7729. doi: 10.1039/C4NR01346H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kauffman KJ, Webber MJ, Anderson DG. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J Control Release. 2016;240:227–234. doi: 10.1016/j.jconrel.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 169.Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dana N, Vaseghi G, Javanmard SH. Activation of PPARγ Inhibits TLR4 signal transduction pathway in melanoma cancer in vitro. Adv Pharma Bulletin. 2020;10(3):458–463. doi: 10.34172/apb.2020.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mockey M, Bourseau E, Chandrashekhar V, Chaudhuri A, Lafosse S, Le Cam E, et al. mRNA-based cancer vaccine: prevention of B16 melanoma progression and metastasis by systemic injection of MART1 mRNA histidylated lipopolyplexes. Cancer Gene Ther. 2007;14(9):802–814. doi: 10.1038/sj.cgt.7701072. [DOI] [PubMed] [Google Scholar]
- 172.Okumura K, Nakase M, Inui M, Nakamura S, Watanabe Y, Tagawa T. Bax mRNA therapy using cationic liposomes for human malignant melanoma. J Gene Med. 2008;10(8):910–917. doi: 10.1002/jgm.1214. [DOI] [PubMed] [Google Scholar]
- 173.Verbeke R, Lentacker I, Breckpot K, Janssens J, Van Calenbergh S, De Smedt SC, et al. Broadening the message: a nanovaccine co-loaded with messenger RNA and α-GalCer induces antitumor immunity through conventional and natural killer T cells. ACS Nano. 2019;13(2):1655–1669. doi: 10.1021/acsnano.8b07660. [DOI] [PubMed] [Google Scholar]
- 174.Fan Y-N, Li M, Luo Y-L, Chen Q, Wang L, Zhang H-B, et al. Cationic lipid-assisted nanoparticles for delivery of mRNA cancer vaccine. Biomater Sci. 2018;6(11):3009–3018. doi: 10.1039/C8BM00908B. [DOI] [PubMed] [Google Scholar]
- 175.Lai I, Swaminathan S, Baylot V, Mosley A, Dhanasekaran R, Gabay M, et al. Lipid nanoparticles that deliver IL-12 messenger RNA suppress tumorigenesis in MYC oncogene-driven hepatocellular carcinoma. J Immunother Cancer. 2018;6(1):125. doi: 10.1186/s40425-018-0431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang R, Men K, Zhang X, Huang R, Tian Y, Zhou B, et al. Delivery of a modified mRNA encoding IL-22 binding protein (IL-22BP) for colon cancer gene therapy. J Biomed Nanotechnol. 2018;14(7):1239–1251. doi: 10.1166/jbn.2018.2577. [DOI] [PubMed] [Google Scholar]
- 177.Zhang X, Men K, Zhang Y, Zhang R, Yang L, Duan X. Local and systemic delivery of mRNA encoding survivin-T34A by lipoplex for efficient colon cancer gene therapy. Int J Nanomed. 2019;14:2733. doi: 10.2147/IJN.S198747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Karikó K. In vitro-transcribed mRNA therapeutics: out of the shadows and into the spotlight. Mol Ther. 2019;27(4):691–692. doi: 10.1016/j.ymthe.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Van Hoecke L, Roose K. How mRNA therapeutics are entering the monoclonal antibody field. J Transl Med. 2019;17(1):1–14. doi: 10.1186/s12967-018-1762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rybakova Y, Kowalski PS, Huang Y, Gonzalez JT, Heartlein MW, DeRosa F, et al. mRNA delivery for therapeutic anti-HER2 antibody expression in vivo. Mol Ther. 2019;27(8):1415–1423. doi: 10.1016/j.ymthe.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kariko K, Kuo A, Barnathan E. Overexpression of urokinase receptor in mammalian cells following administration of the in vitro transcribed encoding mRNA. Gene Ther. 1999;6(6):1092–1100. doi: 10.1038/sj.gt.3300930. [DOI] [PubMed] [Google Scholar]
- 182.Wise HM, Hermida MA, Leslie NR. Prostate cancer, PI3K, PTEN and prognosis. Clin Sci. 2017;131(3):197–210. doi: 10.1042/CS20160026. [DOI] [PubMed] [Google Scholar]
- 183.Islam MA, Xu Y, Zope H, Cao W, Mahmoudi M, Langer R, et al. Restoration of tumor suppression in vivo by systemic delivery of chemically-modified PTEN mRNA nanoparticles. American Society of Clinical Oncology; 2017.
- 184.Fenton OS, Kauffman KJ, Kaczmarek JC, McClellan RL, Jhunjhunwala S, Tibbitt MW, et al. Synthesis and biological evaluation of ionizable lipid materials for the in vivo delivery of messenger RNA to B lymphocytes. Adv Mater. 2017;29(33):1606944. doi: 10.1002/adma.201606944. [DOI] [PubMed] [Google Scholar]
- 185.Lai I, Swaminathan S, Baylot V, Mosley A, Dhanasekaran R, Gabay M, et al. Lipid nanoparticles that deliver IL-12 messenger RNA suppress tumorigenesis in MYC oncogene-driven hepatocellular carcinoma. J Immunother Cancer. 2018;6(1):1–11. doi: 10.1186/s40425-018-0431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cai X, Li JJ, Liu T, Brian O, Li J. Infectious disease mRNA vaccines and a review on epitope prediction for vaccine design. Briefings Funct Genomics. 2021;20:289–303. doi: 10.1093/bfgp/elab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Verbeke R, Lentacker I, De Smedt SC, Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 2019;28:100766. doi: 10.1016/j.nantod.2019.100766. [DOI] [Google Scholar]
- 188.Food U, Administration D. Vaccines and Related Biological Products Advisory Committee meeting—December 17, 2020—FDA briefing document—Moderna COVID-19 vaccine.
- 189.Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immune responses to SARS-CoV-2 mRNA vaccination. Immunity. 2021;54:2133–2142. doi: 10.1016/j.immuni.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pilkington EH, Suys EJ, Trevaskis NL, Wheatley AK, Zukancic D, Algarni A, et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021;131:16–40. doi: 10.1016/j.actbio.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Feldman RA, Fuhr R, Smolenov I, Ribeiro AM, Panther L, Watson M, et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37(25):3326–3334. doi: 10.1016/j.vaccine.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 192.Pardi N, Secreto AJ, Shan X, Debonera F, Glover J, Yi Y, et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat Commun. 2017;8(1):1–8. doi: 10.1038/ncomms14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Firdessa-Fite R, Creusot RJ. Nanoparticles versus dendritic cells as vehicles to deliver mRNA encoding multiple epitopes for immunotherapy. Mol Therapy-Methods Clin Devel. 2020;16:50–62. doi: 10.1016/j.omtm.2019.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang N-N, Li X-F, Deng Y-Q, Zhao H, Huang Y-J, Yang G, et al. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182(5):1271–83. e16. doi: 10.1016/j.cell.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hassett KJ, Benenato KE, Jacquinet E, Lee A, Woods A, Yuzhakov O, et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol Therapy Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pardi N, Hogan MJ, Naradikian MS, Parkhouse K, Cain DW, Jones L, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215(6):1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Espeseth AS, Cejas PJ, Citron MP, Wang D, DiStefano DJ, Callahan C, et al. Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. Npj Vaccines. 2020;5(1):1–14. doi: 10.1038/s41541-020-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Saunders KO, Pardi N, Parks R, Santra S, Mu Z, Sutherland L, et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. NPJ vaccines. 2021;6(1):1–14. doi: 10.1038/s41541-020-00265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pardi N, LaBranche CC, Ferrari G, Cain DW, Tombácz I, Parks RJ, et al. Characterization of HIV-1 nucleoside-modified mRNA vaccines in rabbits and rhesus macaques. Mol Therapy-Nucleic Acids. 2019;15:36–47. doi: 10.1016/j.omtn.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.VanBlargan LA, Himansu S, Foreman BM, Ebel GD, Pierson TC, Diamond MS. An mRNA vaccine protects mice against multiple tick-transmitted flavivirus infections. Cell Rep. 2018;25(12):3382–3923. doi: 10.1016/j.celrep.2018.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.