Abstract
Twenty-five unique CfoI-generated whole-cell DNA profiles were identified in a study of 30 Paenibacillus alvei isolates cultured from honey and diseased larvae collected from honeybee (Apis mellifera) colonies in geographically diverse areas in Australia. The fingerprint patterns were highly variable and readily discernible from one another, which highlighted the potential of this method for tracing the movement of isolates in epidemiological studies. 16S rRNA gene fragments (length, 1,416 bp) for all 30 isolates were enzymatically amplified by PCR and subjected to restriction analysis with DraI, HinfI, CfoI, AluI, FokI, and RsaI. With each enzyme the restriction profiles of the 16S rRNA genes from all 30 isolates were identical (one restriction fragment length polymorphism [RFLP] was observed in the HinfI profile of the 16S rRNA gene from isolate 17), which confirmed that the isolates belonged to the same species. The restriction profiles generated by using DraI, FokI, and HinfI differentiated P. alvei from the phylogenetically closely related species Paenibacillus macerans and Paenibacillus macquariensis. Alveolysin gene fragments (length, 1,555 bp) were enzymatically amplified from some of the P. alvei isolates (19 of 30 isolates), and RFLP were detected by using the enzymes CfoI, Sau3AI, and RsaI. Extrachromosomal DNA ranging in size from 1 to 10 kb was detected in 17 of 30 (57%) P. alvei whole-cell DNA profiles. Extensive biochemical heterogeneity was observed among the 28 P. alvei isolates examined with the API 50CHB system. All of these isolates were catalase, oxidase, and Voges-Proskauer positive and nitrate negative, and all produced acid when glycerol, esculin, and maltose were added. The isolates produced variable results for 16 of the 49 biochemical tests; negative reactions were recorded in the remaining 30 assays. The genetic and biochemical heterogeneity in P. alvei isolates may be a reflection of adaptation to the special habitats in which they originated.
Paenibacillus alvei is a spore-forming bacterium that swarms vigorously on routine culture media. P. alvei, Enterococcus faecalis, Enterococcus faecium, and Achromobacter eurydice are often recovered from diseased larvae obtained from honeybee (Apis mellifera) colonies infected with Melissococcus pluton, the causative agent of European foulbrood (EFB) (3). In Australia, P. alvei is the third-most common bacterium detected in honeybee colonies, and E. faecalis, E. faecium, and A. eurydice are rarely recovered from EFB-affected colonies (17, 18). P. alvei can produce signs in larvae that are similar to the signs produced by Paenibacillus larvae subsp. larvae, which causes American foulbrood, the other major bacterial disease of honeybees.
Several studies based on comparative analyses of 16S rRNA gene sequences of different Bacillus species revealed five phylogenetically distinct clusters (groups 1 through 5), which confirmed that this genus is genetically heterogeneous and in need of extensive taxonomic revision (1, 27). Furthermore, the authors suggested that P. alvei (formerly Bacillus alvei) belongs in group 3, which comprises 10 species. Members of group 3 exhibited comparatively low levels of sequence homology with the other Bacillus groups and constituted a distinct lineage, and these organisms were transferred to a new genus, the genus Paenibacillus (2).
Unlike the pathogens M. pluton and P. larvae subsp. larvae, which are found only in association with honeybees, P. alvei occupies many environmental niches, including the soil (30), milk (24), mosquito larvae (4), the wax moth (11), and humans (26). P. alvei produces alveolysin, a thiol-activated toxin that is highly homologous to listeriolysin O, perfringolysin O, pneumolysin, and streptolysin O (10). The role of P. alvei in the microbiology and ecology of honeybees has received comparatively little attention. When restriction endonuclease analysis (REA) and immunoblot analysis were used, high levels of genetic and antigenic homogeneity were observed among geographically diverse Australian isolates of the primary honeybee pathogens P. larvae subsp. larvae (7, 16) and M. pluton (9). There have been no reports of the levels of genetic heterogeneity among geographically diverse isolates of P. alvei. In this study, amplified ribosomal DNA restriction analysis (ARDRA) of the 16S rRNA gene was used to investigate the species identities of a collection of isolates of P. alvei recovered from foulbrood-affected honeybee colonies in the eastern states of Australia. Whole-cell DNA fingerprint profiles obtained by using restriction enzyme CfoI provided a way to investigate the amount of genetic heterogeneity in P. alvei and to study the utility of this method for tracing the movement of P. alvei isolates in epidemiological studies. Moreover, the API 50CHB system was used to rapidly confirm the identities of P. alvei isolates and to biochemically characterize the isolates.
MATERIALS AND METHODS
P. alvei strains from larvae.
Thirty P. alvei isolates from different geographic regions in the eastern half of Australia were cultured from honey and honeybee larval samples suspected of having EFB (Table 1). Smears of diseased larvae were submitted by apiarists or state apiary officers. The methods used to culture P. alvei have been described previously (19). A mixture of brood material and saline was streaked onto sheep blood agar, which contained blood agar base no. 2 (Oxoid, Basingstoke, United Kingdom) supplemented with 7% citrated ovine blood. Plates were incubated at 37°C for 2 days in air containing 10% CO2.
TABLE 1.
Biochemical data obtained by using the API 50CHB system for 28 geographically diverse Australian isolates of P. alvei
| Isolate
|
Test results
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Designation | Salicin | Ribose | Adonitol | Galactose | Glucose | Mannose | N-Acetylglucosamine | Amygdalin | Arbutin | Cellobiose | Melibiose | Sucrose | Trehalose | Raffinose | Starch | Gentiobiose |
| New South Wales isolates | |||||||||||||||||
| 1 | 97/1133/J1 | − | + | − | − | + | − | + | − | − | + | − | − | + | − | + | − |
| 2 | 97/895/4 | + | + | + | − | + | − | + | − | + | + | − | − | + | − | + | − |
| 3 | 97/1004/6N | + | + | + | − | + | − | + | + | − | + | − | − | + | + | + | + |
| 4 | 97/1004/IN | + | + | + | − | + | − | + | + | + | + | − | − | + | − | + | − |
| 5 | 97/1133/E3 | + | + | + | + | + | − | + | + | + | + | + | + | − | + | + | − |
| 7 | 97/1004/3N | + | + | + | − | + | − | + | − | − | + | − | − | + | − | + | − |
| 8 | 97/1004/5N | + | + | + | − | + | − | + | − | − | + | − | − | + | − | + | − |
| 14 | 97/895/3 | + | + | + | − | + | − | + | − | + | + | − | − | + | − | − | − |
| 6 | 97/895/2 | + | + | + | − | + | − | + | − | − | + | − | − | + | − | + | − |
| Tasmania isolates | |||||||||||||||||
| 9 | 97/331/D2 | + | + | − | + | + | − | + | − | + | + | − | − | − | − | + | + |
| 11 | 97/331/C2 | + | + | + | + | + | + | + | + | + | + | − | − | + | − | + | + |
| 12 | 97/331/C1 | + | + | − | + | + | + | − | − | + | − | − | − | − | − | + | + |
| 13 | 97/331/B1 | + | + | − | − | + | − | + | − | − | − | − | − | + | − | + | + |
| 15 | 97/331/1A | + | + | + | + | + | − | + | + | + | + | − | − | + | − | − | + |
| Victoria isolates | |||||||||||||||||
| 16 | 96/C245/3 | + | + | − | − | + | − | + | − | + | + | − | − | − | − | + | + |
| 17 | 96/B852/2 | + | + | + | − | + | − | + | − | + | + | − | − | − | − | + | + |
| 18 | 96/B852/4 | + | + | − | + | + | − | + | − | + | + | − | − | − | − | + | + |
| 19 | 96/C245/24A | − | + | − | − | + | − | + | − | + | + | − | − | − | − | + | + |
| 20 | 96/C245/12B | − | + | + | − | + | − | + | − | + | + | − | − | − | − | + | + |
| Queensland isolates | |||||||||||||||||
| 21 | 96/5742 | + | + | + | − | + | − | + | − | + | − | − | − | + | − | − | − |
| 22 | 96/A345 | + | + | + | + | + | − | + | + | + | + | − | + | + | − | − | − |
| South Australia isolates | |||||||||||||||||
| 23 | K2217 | + | − | − | − | − | + | + | + | − | + | − | − | + | − | − | − |
| 24 | K7245/1 | + | + | − | + | + | + | + | + | − | + | − | − | − | − | − | − |
| 25 | K7156 | + | + | − | + | + | − | + | + | + | + | − | − | − | − | − | − |
| 26 | K6227 | + | + | − | + | + | − | + | + | − | + | + | + | − | + | + | − |
| 27 | 7666/52R | + | + | − | + | + | − | + | + | + | + | + | + | − | − | − | − |
| 28 | K7463/L8 | + | + | − | + | + | − | + | + | + | − | − | − | − | − | − | − |
| 29 | K8103 | + | − | − | − | + | − | + | + | + | + | − | − | + | − | − | − |
| Isolates of unknown origin | |||||||||||||||||
| 30 | A673/2 | ||||||||||||||||
| 31 | A294 | ||||||||||||||||
P. alvei strains from honey.
An aliquot (75 ml) of honey was mixed with 75 ml of phosphate-buffered saline (pH 7.2) and centrifuged for 45 min at 3,000 × g, and most of the supernatant was removed; about 1.5 ml of fluid was left, which was mixed with the sediment in the bottle. Each sample was streaked onto sheep blood agar and incubated as described above.
Identification of P. alvei.
Swarming colonies were considered to be P. alvei colonies if (i) smears prepared from the colonies and stained by the Gram method consisted of gram-positive rods that were 2 to 5 μm long and 0.5 to 0.8 μm wide, (ii) the organisms produced oval spores, and (iii) the organisms were Voges-Proskaure and oxidase positive. Other non-carbohydrate-based tests (catalase, nitrate, urease, and indole tests), carried out by using the procedures described by Cowan and Steel (5), were also used to further characterize the isolates. ARDRA was also used to confirm the identities of the P. alvei isolates.
Carbohydrate acidification.
The biochemical characteristics of P. alvei were determined with the API 50CHB system, which was used as recommended by the manufacturer. A dendrogram based on the API 50CHB biochemical reactions of P. alvei isolates was produced with the computer package GENSTAT by using the average linkage of cluster analysis applied to similarities based on the matching coefficient.
DNA isolation.
Total cellular DNA of 30 P. alvei isolates was extracted and purified by using the procedure developed for isolation of DNA from M. pluton and other gram-positive bacteria (7, 8).
REA of P. alvei DNA.
Preliminary experiments revealed that P. alvei DNA digested with CfoI produced clearly resolved DNA profiles when the DNA was separated with 3.5% polyacrylamide gels stained with silver. The methods used for electrophoresis and silver staining of DNA fragments have been described previously (6). Gels were loaded with 1 to 2 μg of restricted DNA, and fragments were separated by electrophoresis (35 V per gel, 17 to 21 h). Isolates were considered clonal if their restriction profiles exhibited a degree of similarity (DOS) greater than 92% when the following formula was used: DOS = 100% − (Nd × 100)/Ns, where Ns is the total number of bands of two fingerprints being compared and Nd is an estimate of the number of bands not shared by the two fingerprints (21, 25).
Extrachromosomal DNA analysis.
Total-cell DNA (3 to 5 μg) prepared as described above was loaded onto 1.0% agarose gels and separated for 5 h (50 V) in 0.5× TBE buffer (45 mM Tris-HCl, 45 mM borate, 0.01 mM EDTA; pH 8.0) containing ethidium bromide (0.5 μg ml−1).
PCR amplification and restriction fragment length polymorphism (RFLP) analysis of the alveolysin gene.
Alveolysin-specific PCR primers ALV100 (5′ TAAAAAGGGGATGACTGTAT 3′; positions 1 to 20) and ALV101 (5′ AATGAGGAGATGTTCATACA 3′; positions 1555 to 1536) were designed by aligning the DNA sequences of the thiol-activated toxins alveolysin (EMBL accession no. M62709), listeriolysin O (X15127), perfringolysin O (M81080), pneumolysin (X52474), and streptolysin O (M18638). The sequences were retrieved from EMBL and were aligned by using the Pileup program (Genetics Computer Group [GCG], University of Wisconsin, Madison, Wis.) accessed via the Australian National Genome Information Service (University of Sydney, Sydney, Australia). The primers aligned with alveolysin-specific sequences and facilitated amplification of a 1,555-bp product by PCR. Reactions were performed in 0.2-ml tubes, and each 50-μl reaction mixture consisted of 20 pmol of each primer, 200 μM dTTP, 200 μM dCTP, 200 μM dATP, 200 μM dGTP, 1× PCR buffer (10 mM Tris-HCl, 50 mM KCl; pH 8.3), 0.2 U of Taq polymerase, 1.5 mM magnesium chloride, and 50 to 100 ng of P. alvei total-cell DNA. Amplifications were performed by using Corbett Research thermocyclers (models FTS-960 and PC-960). After an initial denaturation cycle consisting of 93°C for 3 min, the following conditions were used. A denaturation temperature of 93°C for 30 s and an extension temperature of 72°C for 2.5 min were used throughout. A touchdown protocol consisting of 10 cycles at 51°C for 1 min followed by 5 cycles at 50°C for 1 min, 5 cycles at 49°C for 1 min, 5 cycles at 48°C for 1 min, and then 10 cycles at 47°C for 1 min was used. A final cycle consisting of 72°C for 3 min completed the PCR.
Amplification products were separated by electrophoresis through 1.0% agarose. The identify of the 1,555-bp amplicon was confirmed by performing a heminested PCR with forward primer ALV102 (5′ CTTGAAAGGAAGGAAAGTAC 3′; positions 39 to 58) and reverse primer ALV101. The conditions used for the PCR were exactly the same as the conditions used for the reaction described above. The heminested reaction amplified a 1,517-bp fragment, which was separately digested overnight with CfoI, RsaI, and Sau3A1 in a preliminary study to investigate the intragenic genetic heterogeneity in the alveolysin gene.
ARDRA.
16S rRNA genes from representative strains belonging to the Paenibacillus cluster (2, 14), including strains of Paenibacillus polymyxa (accession no. X60636 and X57308), Paenibacillus macerans (X60624 and X57306), Paenibacillus macquariensis (X60625 and X57305), P. larvae (X60619), Paenibacillus pulvifaciens (X60636), Paenibacillus amylolyticus (X60606), Paenibacillus pabuli (X60630), Paenibacillus gordonae (X60617), Paenibacillus peoriae (D78476), Paenibacillus lautus (D78473), and P. alvei (X60604, X57304, and M62709), as well as strains of several Bacillus species, including Bacillus laterosporus (X57307) and Bacillus stearothermophilus (X57309), were aligned by using the Pileup program (GCG, University of Wisconsin) accessed through the Australian National Genome Information Service. Based on predicted cleavage sites derived by using the mapplot program (GCG, University of Wisconsin), a panel of six enzymes (FokI, RsaI, CfoI, DraI, HinfI, and AluI) was chosen and used to differentiate among these phylogenetically related species. Primers ALV16SF (5′ CCTGGCTCAGGACGAACGCT 3′; positions 18 to 37) and ALV16SR (5′ TTGTAAACTCTCGTGGTGTGACGG 3′; positions 1437 to 1414), which exhibited 100% homology to the 16S rRNA gene of P. alvei (accession no. X57304), were designed to facilitate amplification of a 1,416-bp fragment by PCR. Each 50-μl reaction mixture consisted of 20 pmol of each primer, 200 μM dTTP, 200 μM dCTP, 200 μM dATP, 200 μM dGTP, 1× PCR buffer, 1 U of Taq polymerase, P. alvei DNA (0.1 to 0.5 μg), and 1.5 mM magnesium chloride. Sterile pure water (Milli Q) was used to bring the final volume to 50 μl. After an initial denaturation cycle (94°C, 3 min), 35 cycles consisting of 94°C for 30 s, 56°C for 45 s, and 72°C for 1.5 min followed by a final extension cycle consisting of 72°C for 2 min completed the PCR. Amplification products were separately digested with each of the six restriction enzymes, and the products of digestion were separated by agarose gel electrophoresis.
RESULTS
Identification of P. alvei.
All isolates were identified as P. alvei isolates based on their Gram staining characteristics and biochemical reactions. The ARDRA results for all of the isolates were consistent with a P. alvei 16S rRNA gene profile (see below).
To eliminate the possibility that isolates comprised mixed cultures of P. alvei, CfoI-generated restriction endonuclease fragment profiles (REFPs) of whole-cell DNA from several different sites of the advancing front of a swarming colony (produced separately by a subset of five isolates) were analyzed. These profiles were indistinguishable from one another and from the profile generated when DNA from the original DNA extraction was used (data not shown), which confirmed the homogeneous population structure of the original isolates.
API 50CHB tests.
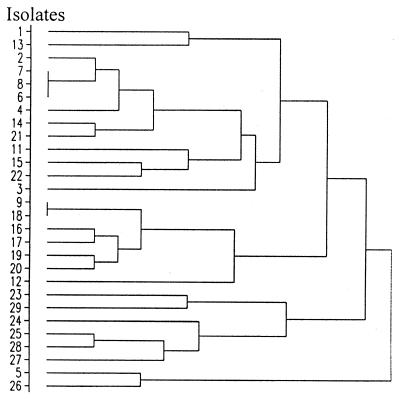
Twenty-eight of the 30 isolates were tested with the API 50CHB system; (isolates 30 and 31 were used only for the genetic studies). All of the isolates were positive for glycerol, esculin, and maltose reactions and negative for erythritol, d-arabinose, l-arabinose, d-xylose, l-xylose, α-methylxyloside, d-fructose, l-sorbose, rhamnose, dulcitol, inositol, mannitol, sorbitol, α-methyl-d-mannoside, α-methyl-d-glucoside, lactose, inulin, melezitose, glycogen, xylitol, d-turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, gluconate, 2-ketogluconate, and 5-ketogluconate reactions (Table 1). There were 16 biochemical tests in the API 50CHB system for which P. alvei strains gave variable results. These were the salicin, ribose, adonitol, galactose, glucose, mannose, N-acetylglucosamine, amygdalin, arbutin, cellobiose, melibiose, sucrose, d-trehalose, raffinose, starch, and gentiobiose tests. The profiles for each isolate are shown in Table 1. A dendrogram summarizing the biochemical relationships among the 28 isolates is shown in Fig. 1.
FIG. 1.
Dendrogram showing the levels of similarity between P. alvei isolates based on their API 50CHB biochemical reactions.
Using API tests, we identified 22 isolates as P. alvei at a confidence level greater than 99% and two isolates (97/1133/J1 and 96/A964/K7156) as P. alvei at a confidence level greater than 89%. Four isolates (97/331/C1, 96/B852/2, K2217, and K8103) produced carbohydrate profiles that were more like the carbohydrate profiles of Bacillus species. However, these identities that were determined tentatively could be ruled out based on a combination of the ability of the organisms to swarm on laboratory medium, spore morphology, and supplementary biochemical test results (see below). Isolates 6, 7, and 8 (Table 1) were identical based on the API 50CHB biochemical reactions, as were isolates 9 and 18. The final amalgamation in the dendrogram (Fig. 1) occurred at a similarity level of 54%.
Supplementary biochemical assays.
All P. alvei isolates were catalase positive and nitrate negative. All of the isolates except three Victoria isolates (96/B852/4, 96/C245/24A, and 96/C245/12B) and one New South Wales isolate (97/1133/J1) were urease positive. Most of the isolates were indole positive; the only exceptions were four Tasmania isolates (97/331/B2, 97/331/C1, 97/331/C2, and 97/331/B1). Most isolates were o-nitrophenyl-β-d-galactopyranoside positive; the only exceptions were three New South Wales isolates (97/C245/3, 97/1133/J1, and 97/1133/E3), one Tasmania isolate (97/331/C1), and one Victoria isolate (96/B852/2).
ARDRA.
Previous studies have shown that ARDRA is a simple and reliable tool for identifying bacterial species (13–15). DNA of all 30 P. alvei isolates was used as templates for amplification of a 1,416-bp portion of the 16S rRNA gene in which primers ALV16SF and ALV16SR were used. The 1,416-bp amplicon from each of the 30 isolates was separately digested with RsaI, CfoI, FokI, HinfI, AluI, and DraI, and the restriction profiles which are representative of each enzyme are shown in Fig. 2. The 16S rRNA gene restriction profiles obtained with each enzyme were identical, which confirmed that all of the isolates belonged to the same species. The 16S rRNA gene fragment from isolate 17 produced a slightly different HinfI profile (Fig. 2, lane 6) compared to the HinfI profile of the remaining 29 isolates (Fig. 2, lane 5). Restriction endonucleases DraI and HinfI produced RFLPs that were identical to the RFLPs predicted when the mapplot program (GCG, University of Wisconsin) was used, and the recognition sites of these two enzymes spanned nucleotide sequences which varied in virtually all of the species in the group 3 cluster, including species that were less closely related phylogenetically (B. stearothermophilus and B. laterosporus). Only Paenibacillus azotofixans could not be reliably differentiated from P. alvei with the panel of six enzymes used in this study. However, P. azotofixans could easily be differentiated from P. alvei based on its inability to swarm on laboratory agar and its fermentation pattern when API 50CHB tests were performed (Table 2) (28). Collectively, our data confirmed that all 30 isolates were P. alvei isolates. Several restriction endonucleases (RsaI, CfoI, and AluI) generated RFLP profiles that were not similar to profiles predicted with the mapplot program. The presence of undesignated nucleotides in the 16S rRNA gene sequences deposited in GenBank (accession no. X57304, X60604, and D78317) and minor sequence variations are the most likely explanations for these profile variations.
FIG. 2.
Restriction endonuclease profiles of amplified regions of the 16S rRNA and alveolysin genes of P. alvei. 16S rRNA gene fragments (length, 1,416 bp) were separately amplified by PCR and digested with CfoI, RsaI, FokI, HinfI, DraI, and AluI. Representative profiles generated by using CfoI (lane 2), RsaI (lane 3), FokI (lane 4), HinfI (lanes 5 and 6), DraI (lane 7), and AluI (lane 8) are shown. The profiles were generated after digestion of the 16S rRNA gene fragment derived from isolate 17 (except in lane 6, which shows the HinfI profile of the 16S rRNA gene fragment derived from isolate 18). CfoI profiles of the alveolysin gene fragment derived from P. alvei isolates 23, 14, and 19 are shown in lanes 9 through 11, respectively. RsaI profiles of the alveolysin gene fragment derived from P. alvei isolates 30, 29, and 17 are shown in lanes 12 through 14, respectively. The molecular size markers used included a 100-bp ladder (lanes 1 and 15) and lambda DNA digested with HindIII (lane 16). Restriction fragments were separated by using 1% (wt/vol) agarose and were stained with ethidium bromide.
TABLE 2.
Comparison of API 50CHB test results for P. alvei, P. azotofixans, P. macerans, P. polymyxa, P. larvae subsp. larvae, P. peoriae, and P. lautus isolates
| Test | % Positive
|
||||||
|---|---|---|---|---|---|---|---|
| P. alveia | P. azotofixansb | P. maceransc | P. polymyxac | P. larvae subsp. larvaed | P. peoriaee | P. lautus | |
| Glycerol | 100 | 0 | 100 | 100 | 100 | vf | 100 |
| Esculin | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Maltose | 100 | 100 | 100 | 100 | 0 | 100 | 100 |
| d-Arabinose | 0 | 0 | 93 | 7 | 0 | 100 | 100 |
| l-Arabinose | 0 | 0 | 100 | 100 | 0 | 0 | 100 |
| Ribose | 93 | 0 | 100 | 100 | 100 | 100 | 100 |
| d-Xylose | 0 | 0 | 100 | 100 | 0 | 100 | 100 |
| α-Methylxyloside | 0 | 0 | 100 | 100 | 0 | 100 | 100 |
| Rhamnose | 0 | 0 | 100 | 33 | 0 | v | 0 |
| Dulcitol | 0 | 47 | 0 | 0 | 0 | 0 | 0 |
| Inositol | 0 | 0 | 40 | 0 | 0 | 0 | 0 |
| Sorbitol | 0 | 0 | 73 | 0 | 0 | 0 | 0 |
| 1-Methyl-d-mannoside | 0 | 9 | 100 | 40 | 0 | 100 | 0 |
| Arbutin | 68 | 67 | 100 | 100 | 0 | 100 | 100 |
| Salicin | 89 | 73 | 100 | 100 | v | 100 | 100 |
| Lactose | 0 | 0 | 100 | 100 | 0 | 100 | 100 |
| Inulin | 0 | 100 | 100 | 87 | 0 | 0 | v |
| Melezitose | 0 | 100 | 100 | 40 | 0 | 0 | v |
| Starch | 64 | 32 | 100 | 100 | 0 | 100 | 100 |
| Glycogen | 0 | 32 | 100 | 100 | 0 | 100 | 100 |
| d-Tagatose | 0 | 41 | 0 | 0 | v | 0 | 0 |
| l-Fucose | 0 | 0 | 87 | 0 | 0 | 0 | v |
| d-Arabitol | 0 | 0 | 80 | 7 | 0 | 0 | 0 |
| Gluconate | 0 | 9 | 93 | 80 | 0 | v | v |
| 5-Ketogluconate | 0 | 14 | 93 | 53 | 0 | 0 | 0 |
| 2-Ketogluconate | 0 | 9 | 0 | 0 | 0 | 0 | 0 |
| Gentiobiose | 39 | 100 | 100 | 100 | 0 | 100 | 100 |
| Raffinose | 11 | 100 | 100 | 100 | 0 | 100 | 100 |
| Trehalose | 54 | 100 | 100 | 100 | 100 | 0 | 100 |
| Sucrose | 14 | 100 | 100 | 100 | 0 | 100 | 100 |
| Melibiose | 11 | 100 | 100 | 100 | 0 | 100 | 100 |
| Cellobiose | 86 | 47 | 100 | 100 | 0 | 100 | 100 |
| Amygdalin | 46 | >90 | 100 | 100 | 0 | 100 | 100 |
| N-Acetylglucosamine | 96 | 15 | 20 | 20 | 100 | v | 100 |
| Mannose | 14 | 100 | 100 | 100 | 100 | 100 | 100 |
| Glucose | 96 | 100 | 100 | 100 | 100 | 100 | 100 |
| Galactose | 43 | 100 | 100 | 100 | v | 100 | 100 |
| Adonitol | 50 | 0 | 100 | 100 | 0 | 0 | 0 |
| d-Fucose | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
PCR and RFLP analyses of the alveolysin gene.
When primers ALV100 and ALV101 were used, a 1,555-bp fragment representing virtually all of the alveolysin gene was amplified from 19 of the 30 P. alvei isolates. The identity of the amplification product was confirmed by performing a heminested PCR with a second internal primer (ALV102), which amplified a 1,517-bp portion of the alveolysin gene. Primers ALV100 and ALV101 or ALV102 did not amplify an alveolysin gene fragment when DNA from P. alvei isolates 5, 9, 11 through 13, 16, 22, 24, and 26 through 28 were used. Digestion of the 1,517-bp portion of the alveolysin gene with CfoI resulted in identification of three fragments whose approximate molecular sizes were 730, 310, and 270 (doublet) bp for all P. alvei isolates (Fig. 2, lanes 9 and 11). A fourth fragment at 195 bp (Fig. 2, lane 10) was observed in profiles representing isolates 7, 8, 1, and 14. Five fragments (400, 315, 300, 265, and 240 bp) were predicted by digestion of the alveolysin gene (accession no. M62709) with CfoI.
Digestion of the 1,517-bp alveolysin gene fragment with RsaI produced four fragments whose approximate sizes were 650, 370 (doublet), and 125 bp (Fig. 2, lane 12) for all but three isolates. RsaI-generated RPLPs were observed in alveolysin gene fragments from isolate 17 (630, 420, and 300 bp) (Fig. 2, lane 14) and from isolates 29 and 23 (650, 370, 300, and 125 bp) (Fig. 2, lane 13). Digestion of the alveolysin gene fragment with Sau3AI generated four fragments whose approximate sizes were 880, 250 (doublet), and 120 bp (data not shown). These data suggest that there is sequence variability among alveolysin genes in different isolates of P. alvei.
DNA restriction endonuclease profiles.
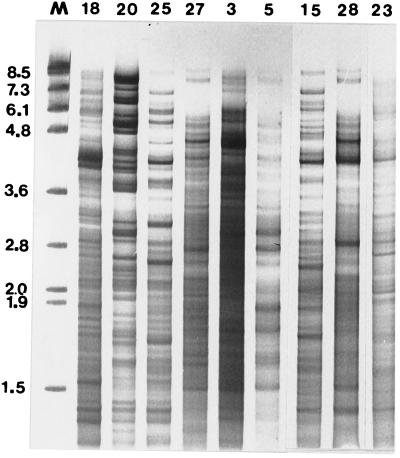
Digestion of P. alvei DNA with CfoI generated fragments whose molecular sizes ranged from approximately 8.5 to 0.3 kb, and these fragments were clearly resolved by using 3.5% polyacrylamide gels stained with silver (Fig. 3). The REFPs of 25 of the 30 P. alvei isolates obtained from five eastern states of Australia could be readily distinguished from one another. Profile variations were often evident throughout the molecular size range (8.5 to 0.3 kb). DNA from isolates 27 (South Australia) and 16 (Victoria) produced indistinguishable CfoI-generated REFPs (Fig. 3, lanes 6 and 7) that were clearly different from the CfoI-generated patterns of the other isolates. DNA from isolates 5 (New South Wales) and 9 (Tasmania) (Fig. 3, lanes 2 and 3) produced similar REFPs, although some differences were evident. The CfoI-generated patterns of isolates 19 and 20 (both from Victoria) were also very similar but could be distinguished from one another (Fig. 3, lanes 13 and 14), and these two isolates were considered to be clonal. The appearance of darkly staining fragments suggested that reiterated sequences and/or extrachromosomal DNA was present in some isolates.
FIG. 3.
Silver-stained 3.5% polyacrylamide gel of CfoI-generated REFPs of geographically diverse Australian isolates of P. alvei. The gel was electrophoresed for 15 h and shows DNA fragments between 0.45 and 8.5 kb long. The lanes show the DNA profiles for New South Wales isolate 5, Tasmania isolates 9 and 12, South Australia isolates 25 and 27, Victoria isolates 16 through 18, South Australia isolates 29 and 28, Tasmania isolate 15, Victoria isolates 19 and 20, and Queensland isolate 21; isolate numbers are indicated at the top. Lane M contained molecular size markers (lambda DNA digested with HindIII).
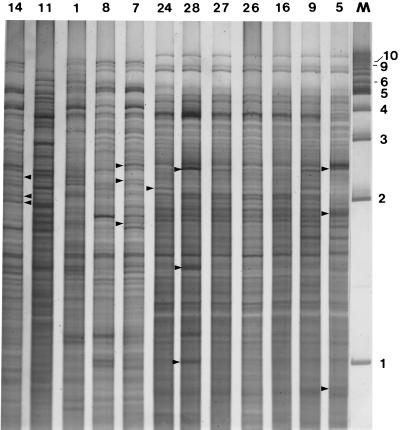
A longer electrophoretic time (21 h) highlighted the genetic heterogeneity of the P. alvei isolates, particularly in the high-molecular-weight region (7 to 9 kb) (Fig. 4 and 5). The similarity in the CfoI-generated profiles of isolates 19 and 20 was reinforced when the longer electrophoretic time was used (Fig. 4A, lanes 5 and 8). The CfoI profiles of DNA from Tasmania isolates 12 and 13 (Fig. 4B, lanes 3 and 4) were indistinguishable, and these isolates were considered to be clonal. The CfoI-derived REFPs in the high-molecular-weight region (7 to 9 kb) for isolates 28, 26, 27, and 24 (South Australia), isolate 16 (Victoria), isolate 9 (Tasmania), and isolate 5 (New South Wales) were identical with respect to three fragments (Fig. 3 through 5). However, a comparison of the CfoI profiles throughout the entire molecular size range (8.5 to 0.3 kb) for isolates 28 and 24 revealed RFLPs which distinguished these profiles from one another and from the profiles obtained for isolates 27, 26, and 16. The CfoI patterns of isolates 5 and 9 were very similar to the CfoI pattern obtained for isolates 27, 26, and 16 (Fig. 3 through 5). The close genetic relationships of the members of this subgroup of seven P. alvei isolates was highlighted when the CfoI-generated DNA profiles were compared on the same polyacrylamide gel (Fig. 6). The CfoI profiles of isolates 16, 26, and 27 were indistinguishable, while the profiles of isolates of 28, 24, and 9 were identical except for the presence of several heavily stained fragments. The CfoI profile of isolate 5 was also very similar to the profiles of the other six isolates, although there were several more distinguishing features (Fig. 6, lane 12). The high level of genetic similarity among these seven geographically diverse isolates suggested that they are clonally related. The CfoI profiles of DNA from New South Wales isolates 14, 8, and 7 (Fig. 6, lanes 1, 4, and 5) had many similarities throughout the entire molecular size range, although numerous RFLPs made it easier to rapidly distinguish between the profiles. The profile of isolate 1 (New South Wales) exhibited some minor similarities to the profiles of the other three New South Wales isolates (isolates 14, 7, and 8) but was more distantly related. The CfoI pattern of Victoria isolate 11 was clearly different from the other 11 patterns shown in Fig. 6. The presence of numerous RFLPs in the remaining isolates suggests that P. alvei is a genetically heterogeneous species.
FIG. 4.
CfoI-generated REFPs of P. alvei DNA recovered from geographically diverse Australian isolates. Gels (3.5% polyacrylamide) were electrophoresed for 21 h to separate large molecular fragments and were stained with silver. (A) REFPs for isolates 30 and 31 (origin unknown), New South Wales isolates 4 and 6, and Victoria isolates 19, 17, 18, and 20. Isolate numbers are indicated at the top. The positions of molecular size markers (lambda DNA digested with HindIII) are indicated on the left. (B) REFPs for Queensland isolates 22 and 21, Tasmania isolates 12 and 13, South Australia isolates 29 and 24, New South Wales isolate 2, Tasmania isolate 15, and South Australia isolate 28. Isolate numbers are indicated at the top.
FIG. 5.
CfoI profiles of P. alvei DNA isolated from eastern and southern states of Australia. The lanes show the profiles for Victoria isolates 18 and 20, South Australia isolates 25 and 27, New South Wales isolates 3 and 5, Tasmania isolate 15, and South Australia isolates 28 and 23. Isolate numbers are indicated at the top. Lane M contained molecular size markers (phage SPPI digested with EcoRI).
FIG. 6.
CfoI profiles of a subset of genetically related P. alvei isolates. The lanes show the profiles for New South Wales isolate 14, Tasmania isolate 11, New South Wales isolates 1, 8, and 7, South Australia isolates 24, 28, 27, and 26, Victoria isolate 16, Tasmania isolate 9, and New South Wales isolate 5. Isolate numbers are indicated at the top. Lane M contained a 1-kb ladder (Bio-Rad).
Compared with the restriction endonuclease patterns, the biochemical profiles were unreliable indicators of similarity between isolates. Clonal isolates 9 and 16 produced almost identical fermentation patterns for the 49 carbohydrates examined (isolate 9 fermented galactose, but isolate 16 did not ferment galactose), as did genetically indistinguishable isolates 19 and 20 (isolate 20 fermented adonitol, but isolate 19 did not ferment adonitol). However, a comparison of clonal isolate 16 with isolates 26 and 27 revealed seven (galactose, amygdalin, arbutin, melibiose, sucrose, raffinose, and gentibiose) and six (galactose, amygdalin, melibiose, sucrose, starch, and raffinose) pattern differences, respectively. Isolates that had very dissimilar CfoI profiles and originated from geographically separated regions of Australia, such as isolates 22 (Queensland) and 12 (Tasmania), exhibited nine differences in their carbohydrate patterns, while isolates 6 (New South Wales) and 13 (Tasmania) exhibited only three differences.
Extrachromosomal DNA.
Extrachromosomal DNA elements were observed in the whole-cell DNA (undigested) profiles of 17 of the 30 P. alvei isolates (isolates 1, 2, 7 through 9, 13, 14, 17 through 21, 25, and 28 through 31). The molecular sizes of these elements ranged from 1 to 15 kb (Fig. 7). Some isolates produced multiple bands, suggesting that more than one extrachromosomal element may be present in these isolates.
FIG. 7.
Whole-cell DNA profiles of P. alvei isolates on 1.0% (wt/vol) agarose gels. The lane designations correspond to isolate numbers. Extrachromosomal elements (arrowheads) were observed in the whole-cell DNA profiles of 17 isolates. Lane M contained a 1-kb ladder.
DISCUSSION
Although P. alvei was one of the first species described in the genus Bacillus, comparatively little is known about this organism. Using REA and a range of biochemical tests, we demonstrated that geographically diverse Australian isolates of P. alvei are genetically and biochemically heterogeneous, although seven isolates (isolates 5, 9, 16, 24, and 26 through 28) that were obtained from four states of Australia were considered to be clonal. Isolates 19 and 20 (both from Victoria) were considered to be clonal, as were Tasmanian isolates 12 and 13. However, the CfoI patterns that were representative of each of the clonal clusters were clearly different from one another and from the patterns of the remaining P. alvei isolates. Twenty-five different CfoI-generated profiles were discerned when 30 Australian isolates were examined, suggesting that REA may be a useful tool for tracing the movement of P. alvei isolates in epidemiological studies. While 17 of the 30 P. alvei isolates contained extrachromosomal nucleic acid species which may have contributed to differences in the restriction patterns, isolates which did not contain extrachromosomal elements also exhibited marked genetic heterogeneity. The alveolysin gene was amplified by PCR from only 19 of the 30 isolates, and RFLPs in the alveolysin gene were revealed by using the enzymes CfoI, RsaI, and Sau3AI. Southern hybridization and immunoblot studies should be performed to confirm the presence of the alveolysin gene and to determine if this gene is actively expressed in these and other isolates of P. alvei derived from ecologically diverse habitats.
The genetic heterogeneity of Australian P. alvei isolates revealed by REA contrasts with the intragenic homogeneity of the REFPs of the 16S rRNA gene. The restriction endonuclease profiles of the 16S rRNA gene amplified from DNA of the 30 isolates were remarkably consistent for each of six enzymes, which confirmed that these isolates were P. alvei isolates. Ash et al. (1) placed P. alvei together with nine other species in a separate phylogroup (group 3) based on a distance matrix analysis of 16S rRNA gene sequence comparisons and split the genus Bacillus into five major clusters representing separate genera. Rössler et al. (27) showed that P. alvei was most closely related to Bacillus macerans, Bacillus macquariensis, and Bacillus polymyxa, and the levels of 16S rRNA gene sequence similarity ranged from 93 to 94%. The results of RFLP analyses of the 16S rRNA gene performed with DraI and HinfI clearly differentiated P. alvei from these phylogenetically related species.
Morphology (swarming ability and spore morphology) and biochemical tests (API 50CHB tests, as well as several supplementary tests) were sufficiently discriminatory to confirm the genus and species identities of the isolates (Table 2). The API 50CHB system was most useful for biochemical characterization of the species and revealed considerable variability among the 28 Australian isolates tested. Logan and Berkeley (22) used a number of morphological and biochemical tests (including API 50CHB tests) to characterize 1,075 strains representing the genus Bacillus. A comparison of the results obtained for 12 P. alvei strains in their study with the results for the 28 strains used in our study revealed many similarities. Variations were observed for 14 carbohydrates (ribose, lactose, melezitose, starch, glycogen, d-turanose, l-fucose, adonitol, d-glucose, inositol, gluconate, α-methyl-d-glucoside, N-acetylglucosamine and 5-ketogluconate). The major differences involved the ability of the majority of the isolates in the study of Logan and Berkeley to ferment α-methyl-d-glucoside, d-turanose, glycogen, and inositol; none of the Australian isolates fermented these carbohydrates. The remaining differences were minor and involved variability in the percentage of isolates that were able to ferment some carbohydrates (ribose, N-acetylglucosamine, adonitol, and glucose). In addition, a small percentage of isolates (less than 25%) in the study of Logan and Berkeley fermented lactose, melezitose, gluconate, d-fucose, and 5-ketogluconate; these carbohydrates were not fermented by any of the Australian isolates. The sources and geographic locations of the P. alvei isolates used in the study of Logan and Berkeley were not reported, and these isolates may not have originated from honeybee colonies. Collectively, the data described above confirm the biochemical heterogeneity of P. alvei.
A polyphasic reassessment of the genus Paenibacillus revealed limited heterogeneity among seven P. alvei isolates (14). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, API test, and pyrolysis mass spectrometry results did not group the P. alvei isolates into a single cluster, even though the organisms appeared to be members of a phylogenetically homogeneous species when ARDRA was used. Among Paenibacillus species, the ability to produce indole and the ability to generate acid via fermentation of adonitol are features that distinguish P. alvei (14). Of the 28 Australian P. alvei isolates used in our study, only 50% fermented adonitol, while 24 (86%) produced indole. Some of the Australian isolates (11 of 28 isolates) fermented gentiobiose, while all seven European isolates fermented this sugar (14). The ability of all 28 Australian isolates to ferment maltose and glycerol, the ability of some isolates to ferment salicin and d-trehalose, and the inability of the isolates to ferment xylose, arabinose, α-methyxyloside, d-fructose, mannitol, and methyl-d-mannoside are consistent with the findings of Heyndrickx et al. (14).
While morphologic and biochemical analyses may be informative for identification of P. alvei, there was biochemical variability even among clonal isolates that had very similar DNA profiles. This is perhaps not unexpected since the ability to ferment a carbohydrate often depends on expression of several functional genes, including genes involved in an enzymatic reaction(s) and in transport of the carbohydrate across the cell membrane. Genetic homogeneity demonstrated by fingerprint profiles is largely a measure of genome stability and may not reflect the presence of point mutations, deletions, and small sequence rearrangements which fail to dramatically alter fingerprint profiles but which may have profound consequences for transcription and translation. Chromosomal instability through genetic rearrangement is the likely source of the heterogeneity observed in the restriction patterns of bacteria (12). Invertrons, insertion elements, transposons, and conjugative transposons contribute to site-specific recombination events which generate heterogeneity in genomes, and consequently, genetic heterogeneity is a feature that is common in bacterial genomes (20).
Genetic diversity is considered essential for bacterial adaptation to fluctuating environments (31). Investigations of genetic heterogeneity among strains of P. polymyxa and P. azotofixans, which are phylogenetically closely related to P. alvei, have been described previously (23, 29). A cluster analysis of phenotypic features revealed that all strains of P. polymyxa exhibited a high degree of similarity but that strain diversity depended on strain origin. Strains recovered from the rhizoplane (a close association with wheat roots) exhibited the smallest amount of variability and clustered into one phenotypic group, while strains recovered further from the rhizoplane exhibited greater genetic, serological, and phenotypic diversity. Strains that were firmly attached to wheat roots fermented sorbitol and were genetically homogeneous (23). Similarly, the genetic diversity within populations of P. azotofixans was affected by the soil type. Seldin et al. (29) showed that strains recovered from different soil types generally belonged to different but related groups which could be distinguished on the basis of their ability to ferment different carbohydrates in API 50CHB tests.
Unlike P. azotofixans and P. polymyxa, which form close associations with roots of grain crops, P. alvei not only is recovered from honeybee colonies with EFB but also is commonly isolated from very different environmental niches, including soil, milk, various insect species, and humans (4, 11, 24, 26, 30). The high degree of genetic heterogeneity revealed by genomic profiles and the biochemical variability of this species support the hypothesis that P. alvei is not a primary honeybee pathogen (3). The two primary bacterial pathogens of honeybee colonies (M. pluton and P. larvae subsp. larvae) exhibit marked genetic, protein, and antigenic homogeneity, as determined by REA, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblot analyses (7, 9, 16), which suggests that these organisms evolved so that they form close host-parasite relationships with honeybee larvae. Although isolates 5, 9, 16, 24, and 26 through 28, which represent 23% of our isolates, were clonally related, biochemically they were among the most diverse isolates. They were not amalgamated into a single group until the lowest similarity value for the whole group of isolates (54%) (Fig. 1). Further studies will be necessary to determine if the disproportionately high occurrence of this genotype represents the emergence of a predominant clone or if a much simpler explanation (e.g., migratory patterns of Australian beekeepers) is responsible for this observation.
ACKNOWLEDGMENTS
This work was funded in part by the Australian Honey Bee Research and Development Committee.
We acknowledge the efforts of Liz Summerell, who provided technical assistance for the culture of P. alvei. The expert photographic skills of Lowan Turton and the assistance of Paul Nicholls in preparing the dendrogram are gratefully acknowledged.
REFERENCES
- 1.Ash C, Farrow J A E, Wallbanks S, Collins M D. Phylogenetic heterogeneity of the genus Bacillus revealed by comparative analysis of small-subunit-ribosomal RNA sequences. Lett Appl Microbiol. 1991;13:202–206. [Google Scholar]
- 2.Ash C, Priest F G, Collins M D. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Antonie Leewenhoek J Microbiol. 1993;64:253–260. doi: 10.1007/BF00873085. [DOI] [PubMed] [Google Scholar]
- 3.Bailey L, Ball B V. Honey bee pathology. 2nd ed. Sidcup, United Kingdom: Harcourt Brace Jovanovich; 1991. pp. 36–41. [Google Scholar]
- 4.Balaraman K, Rao U S B, Rajagopalan P K. Bacterial pathogens of mosquito larvae—Bacillus alvei (Cheshire and Cheyene) and Bacillus brevis (Migula) isolated in Pondicherry. Indian J Med Res. 1979;70:615–619. [PubMed] [Google Scholar]
- 5.Cowan S T, Steele K J. Appendix C: characterisation tests. In: Barrow G I, Feltham R K A, editors. Manual for the identification of medical bacteria. Cambridge, United Kingdom: Cambridge University Press; 1993. pp. 219–238. [Google Scholar]
- 6.Djordjevic S P, Eamens G J, Ha H, Walker M J, Chin J C. Demonstration that Australian Pasteurella multocida isolates from sporadic outbreaks of porcine pneumonia are non-toxigenic (toxA−) and display heterogeneous DNA restriction endonuclease profiles compared with toxigenic isolates from herds with progressive atrophic rhinitis. J Med Microbiol. 1998;47:679–688. doi: 10.1099/00222615-47-8-679. [DOI] [PubMed] [Google Scholar]
- 7.Djordjevic S P, HoShon M, Hornitzky M A Z. DNA restriction endonuclease profiles and typing of geographically diverse isolates of Bacillus larvae. J Apic Res. 1994;33:95–103. [Google Scholar]
- 8.Djordjevic S P, Noone K, Smith L, Hornitzky M A Z. Development of a hemi-nested PCR assay for the specific detection of Melissococcus pluton. J Apic Res. 1998;37:165–173. [Google Scholar]
- 9.Djordjevic S P, Smith L A, Forbes W A, Hornitzky M A Z. Geographically diverse Australian isolates of Melissococcus pluton exhibit minimal genotypic diversity by restriction endonuclease analysis. FEMS Microbiol Lett. 1999;173:311–318. doi: 10.1111/j.1574-6968.1999.tb13519.x. [DOI] [PubMed] [Google Scholar]
- 10.Geoffroy C, Mengaud J, Alouf J E, Cossart P. Alveolysin, the thiol-activated toxin of Bacillus alvei, is homologous to listeriolysin O, perfringolysin O, pneumolysin, and streptolysin O and contains a single cysteine. J Bacteriol. 1990;172:7301–7305. doi: 10.1128/jb.172.12.7301-7305.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillam M. Microbes from apiarian sources: Bacillus spp. in fras of the greater wax moth. J Invertebr Pathol. 1985;45:218–224. [Google Scholar]
- 12.Hall L M C. Are point mutations or DNA rearrangements responsible for the restriction fragment length polymorphisms that are used to type bacteria? Microbiology. 1994;140:197–204. doi: 10.1099/13500872-140-1-197. [DOI] [PubMed] [Google Scholar]
- 13.Heyndrickx M, Vandemeulebroecke K, Hoste B, Janssen P, Kersters K, De Vos P, Logan N A, Ali N, Berkeley R C W. Reclassification of Paenibacillus (formerly Bacillus) pulvifaciens (Nakamura 1984) Ash et al. 1994, a later subjective synonym of Paenibacillus (formerly Bacillus) larvae (White 1906) Ash et al. 1994, as a subspecies of P. larvae, with emended descriptions of P. larvae as P. larvae subsp. larvae and P. larvae subsp. pulvifaciens. Int J Syst Bacteriol. 1996;46:270–279. doi: 10.1099/00207713-46-1-270. [DOI] [PubMed] [Google Scholar]
- 14.Heyndrickx M, Vandemeulebroecke K, Scheldeman P, Kersters K, De Vos P, Logan N A, Aziz A M, Ali N, Berkeley R C W. A polyphasic study of the genus Paenibacillus, reclassification of Bacillus lautus (Nakamura 1984) as Paenibacillus lautus comb. nov. and of Bacillus peoriae (Montefusco et al. 1993) as Paenibacillus peoriae comb. nov., and emended descriptions of P. lautus and P. peoriae. Int J Syst Bacteriol. 1996;46:988–1003. doi: 10.1099/00207713-46-4-988. [DOI] [PubMed] [Google Scholar]
- 15.Heyndrickx M, Vauterin L, Vandamme P, Kersters K, De Vos P. Applicability of combined amplified ribosomal DNA restriction analysis (ARDRA) patterns in bacterial phylogeny and taxonomy. J Microbiol Methods. 1996;26:247–259. [Google Scholar]
- 16.Hornitzky M A Z, Djordjevic S P. Sodium dodecyl sulfate polyacrylamide gel electrophoresis profiles and western blots of Bacillus larvae. J Apic Res. 1992;31:47–49. [Google Scholar]
- 17.Hornitzky M A Z, Karlovskis S. A culture technique for the detection of Bacillus larvae in honey bees. J Apic Res. 1989;28:118–120. [Google Scholar]
- 18.Hornitzky M A Z, Smith L. Procedures for the culture of Melissococcus pluton from diseased brood and bulked honey samples. J Apic Res. 1998;37:292–294. [Google Scholar]
- 19.Hornitzky M A Z, Wilson S C. A system for the diagnosis of the major bacterial brood diseases of honey bees. J Apic Res. 1989;28:191–195. [Google Scholar]
- 20.Kolstø A-B. Dynamic bacterial genome organization. Mol Microbiol. 1997;24:241–248. doi: 10.1046/j.1365-2958.1997.3501715.x. [DOI] [PubMed] [Google Scholar]
- 21.Kristansen B E, Sorensen B, Simonsen T, Spanne O, Lund V, Bjorvant B. Isolates of Neisseria meningitidis from different sites in the same patient: phenotypic and genomic studies, with special reference to adherence, piliation, and DNA restriction endonuclease pattern. J Infect Dis. 1984;150:389–396. doi: 10.1093/infdis/150.3.389. [DOI] [PubMed] [Google Scholar]
- 22.Logan N A, Berkeley R C W. Identification of Bacillus strains using the API system. J Gen Microbiol. 1984;130:1871–1882. doi: 10.1099/00221287-130-7-1871. [DOI] [PubMed] [Google Scholar]
- 23.Mavingui P, Laguerre G, Berge O, Heulin T. Genetic and phenotypic diversity of Bacillus polymyxa in soil and in wheat rhizosphere. Appl Environ Microbiol. 1992;58:1894–1903. doi: 10.1128/aem.58.6.1894-1903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukundan M, Subrahmanyam M, Parameswaran M N. Studies on microflora in boiled milk. J Vet Sci. 1979;10:234–239. [Google Scholar]
- 25.Orskov F, Orskov I. From the National Institutes of Health. Summary of a workshop on the clone concept in the epidemiology, taxonomy, and evolution of the Enterobacteriaceae and other bacteria. J Infect Dis. 1983;148:346–357. doi: 10.1093/infdis/148.2.346. [DOI] [PubMed] [Google Scholar]
- 26.Reboli A C, Bryan C S, Farrar W E. Bacteremia and infection of a hip prosthesis caused by Bacillus alvei. J Clin Microbiol. 1989;27:1395–1396. doi: 10.1128/jcm.27.6.1395-1396.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rössler D, Wolfgang L, Schleifer K, Lin C, McGill T, Wisotzkey J, Jurtshuk P, Jr, Fox G. Phylogenetic diversity in the genus Bacillus as seen by 16S rRNA sequencing studies. Syst Appl Microbiol. 1991;14:266–269. doi: 10.1016/S0723-2020(11)80379-6. [DOI] [PubMed] [Google Scholar]
- 28.Seldin L, Penido E G C. Identification of Bacillus azotofixans using API tests. Antonie Leewenhoek J Microbiol. 1986;52:403–409. doi: 10.1007/BF00393468. [DOI] [PubMed] [Google Scholar]
- 29.Seldin L, Rosado A S, da Cruz D W, Nobrega A, van Elsas J D, Paiva E. Comparison of Paenibacillus azotofixans strains isolated from rhizoplane, rhizosphere, and non-root-associated soil from maize planted in two different brazilian soils. Appl Environ Microbiol. 1998;64:3860–3868. doi: 10.1128/aem.64.10.3860-3868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smirnov A M. Disinfection of the soil of apiaries infected with the bacteria of bee diseases. Veterinariya (Moscow) 1976;6:32–37. [Google Scholar]
- 31.Torsvik V, Goksoyr J, Daae F L. High diversity in DNA of soil bacteria. Appl Environ Microbiol. 1990;56:782–787. doi: 10.1128/aem.56.3.782-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]