Abstract
Objective
To evaluate the dose–response, efficacy and safety of dapirolizumab pegol (DZP) in patients with SLE.
Methods
Adults with moderately to severely active SLE (SLEDAI-2K score ≥6 and ≥1 BILAG A or ≥2 BILAG B domain scores), receiving stable CS (≤40 mg/day prednisone-equivalent), antimalarial or immunosuppressant drugs were included. Patients with stable LN (proteinuria ≤2 g/day) not receiving high-dose CS or CYC were permitted entry. Randomized patients received placebo or i.v. DZP (6/24/45 mg/kg) and standard-of-care (SOC) treatment every 4 weeks to week 24, after which patients received only SOC to week 48. The primary objective was to establish a dose–response relationship based on week 24 BILAG-Based Composite Lupus Assessment (BICLA) responder rates.
Results
All DZP groups exhibited improvements in clinical and immunological outcomes vs placebo at week 24; however, BICLA responder rates did not fit pre-specified dose–response models [best-fitting model (Emax): P = 0.07]. Incidences of serious treatment-emergent adverse events across DZP groups were low and similar to placebo. Following DZP withdrawal, SLEDAI-2K, physician’s global assessment (PGA), BILAG, and Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) scores stabilized; BICLA and SLE Responder Index (SRI-4) responder rates declined (likely due to interventions with disallowed escape medications), BILAG flares increased, and immunologic parameters returned towards baseline.
Conclusions
Although the primary objective was not met, DZP appeared to be well tolerated, and patients exhibited improvements across multiple clinical and immunological measures of disease activity after 24 weeks relative to placebo. The potential clinical benefit of DZP warrants further investigation.
Keywords: systemic lupus erythematosus, SLE, lupus, dapirolizumab pegol, CD40 ligand
Rheumatology key messages.
Despite not meeting the primary objective (selecting a dose–response), dapirolizumab pegol was well tolerated.
Improvements in disease activity (clinical and immunological) were observed after 24 weeks, vs placebo.
The potential clinical benefit of dapirolizumab pegol warrants further investigation.
Introduction
SLE, a chronic, multi-system autoimmune disease, is characterized by chronic inflammation and the accrual of organ damage either from the disease itself or the medications used to treat SLE [1–4]. The prevalence, which varies according to ethnicity [5], gender [6, 7] and age [8], ranges from 30 to 100 cases per 100,000 people [8–13].
Glucocorticoids, antimalarial drugs, and immunosuppressives have been the mainstay of SLE therapy for several decades now [14]. However, long-term glucocorticoid therapy is associated with severe side effects, including increased risk for infections, cardiovascular events, metabolic syndrome, cognitive impairment, osteonecrosis, and osteoporosis [15, 16]. With just one biologic, the anti-BLyS mAb, belimumab, approved for the treatment of SLE, there remains a significant unmet clinical need for additional treatment options [17, 18].
Interactions between CD40 ligand (CD40L, CD154; mainly expressed on activated T cells and platelets) and the CD40 receptor (expressed on a variety of cells, including antigen-presenting cells and B cells) play a key role in adaptive immune activation and drive pathological processes in SLE, including B cell differentiation and proliferation [19, 20]. Inhibiting the interaction between CD40L and the CD40 receptor has been efficacious in animal models of several autoimmune diseases, including SLE [20–24]. As such, CD40L has long been an attractive therapeutic target in human SLE. However, early SLE studies with BG9588, an anti-CD40L antibody, were suspended due to an increased rate of thromboembolic events [25]. This may have been a result of platelet activation and aggregation arising from BG9588 fragment crystallizable (Fc)-mediated cross-linking [26].
Nevertheless, CD40L remains a target of interest for SLE drug development given the significant reduction of DNA antibody-forming cells observed with BG9588 in patients with LN [23]. DZP, a polyethylene glycol-conjugated antigen-binding (Fab′) fragment, which targets CD40L, but lacks a functional Fc domain, was constructed to mitigate the potential for platelet activation and aggregation. DZP was shown to have high affinity for CD40L in cell-based assays, with a dissociation constant (Kd) of 7.9 pM [27]. In a preclinical study in rhesus macaques, histopathological data revealed no increase in thromboembolic events upon administration of DZP compared with placebo, unlike the widespread pulmonary thrombi that were previously observed with BG9588 [27].
DZP has since been investigated in two phase 1 clinical studies. The first was a double-blind, dose-escalation study (NCT01093911), in which healthy volunteers and patients with SLE received single i.v. doses of DZP or placebo [28]. Rates of adverse events were comparable between the DZP and placebo groups, and no thromboembolic events were reported [28]. The second study was a double-blind, placebo-controlled study (NCT01764594), in which 24 patients with SLE received 30 mg/kg DZP i.v., followed by 15 mg/kg DZP every 2 weeks for a 10-week period [29]. Multiple doses of DZP were well-tolerated, and there were no thromboembolic events during the study [29].
Herein, we report outcomes from a phase 2b, placebo-controlled study of DZP in adult patients with SLE (RISE; NCT02804763). The aims were to establish a dose–response relationship, and evaluate the efficacy and safety of DZP compared with SOC treatment, and to assess the durability of response following study drug withdrawal.
Methods
Patients
Eligible patients were adults (aged ≥18 years) with SLE diagnosed by a physician and confirmed using the SLICC classification criteria. Patients had one or more of the following immunologic criteria (confirmed by the central laboratory): anti-dsDNA antibodies (Farr assay); low complement (C3, C4 or both); and/or an ANA titre ≥1:80, in combination with historical positivity for anti-dsDNA and/or positivity for anti-ENA [anti-Smith antibody (anti-Sm), anti-SSA, anti-SSB or anti-RNP].
Patients were recruited from Europe, Latin America, and North America between 2 June 2016 and 19 November 2018. Patients had moderate-to-severe disease activity determined by: BILAG 2004 A score in one or more domain at screening or BILAG 2004 B scores in two or more domains (if a BILAG 2004 A domain score was absent); SLEDAI-2K total score ≥6 at screening, and a SLEDAI-2K score excluding points from laboratory values ≥4 at the baseline visit. Patients were required to remain on stable doses of SOC medications [at least 2 weeks prior to screening, 4 and 8 weeks prior to baseline for CS, antimalarials, and immunosuppressants (e.g. AZA, MTX, MMF), respectively]. Antimalarial and immunosuppressant doses could not be changed until week 24 unless toxicity or tolerability issues arose. When a dose was increased due to worsening disease activity, the patient was considered a nonresponder for the primary analysis.
Exclusion criteria included pregnancy or breastfeeding; a history of malignancy (except for treated cervical carcinoma in situ, basal cell carcinoma, or dermatological squamous cell carcinoma); a clinically relevant, recurrent infection (three or more times per year); and severe neuropsychiatric SLE, or other neurological symptoms that might have prevented the patient from completing the required procedures and assessments. Prior biologics were permitted provided that a wash-out period was undertaken prior to screening (2- to 12-month wash-out, depending on the biologic).
Patients with stable LN were permitted entry, excluding those with new or worsening International Society of Nephrology/Renal Pathology Society class III or IV LN, or with chronic kidney disease stage 3b (estimated glomerular filtration rate <45 ml/min/1.73 m2, serum creatinine >2.5 mg/dl, proteinuria >2 g/day, or protein:creatinine ratio >226 mg/mmol).
Ethics approval and consent for publication
The study protocol, amendments, and patient informed consent were reviewed by a national, regional, or Independent Ethics Committee or Institutional Review Board. This study was conducted in accordance with the current version of the applicable regulatory and International Council for Harmonisation-Good Clinical Practice requirements, the ethical principles that have their origin in the principles of the declaration of Helsinki, and the local laws of the countries involved. All the results presented in this article are in aggregate form, and no personally identifiable information was used for this study.
Study design
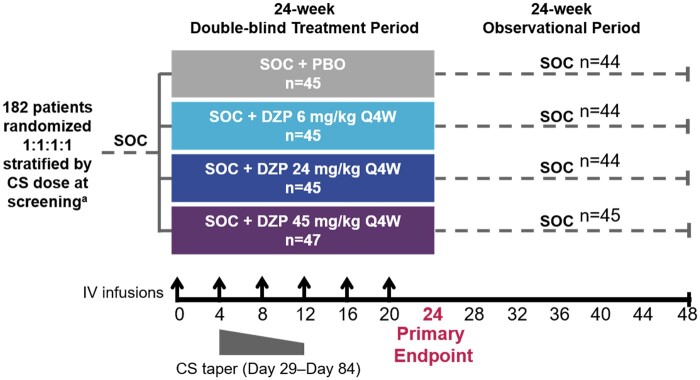
This phase 2b study began with a 24-week, double-blind, placebo-controlled period, during which patients received study drug and SOC. After the double-blind period, the study drug was withdrawn, and patients were observed for 24 weeks during which they received only SOC to evaluate the duration of the treatment effect (Fig. 1). Patients were randomized 1:1:1:1 via an interactive voice/web response system to receive placebo or intravenous DZP at 6, 24, or 45 mg/kg (all with concomitant SOC) every 4 weeks, up to and including week 20 (Fig. 1). Three dose levels of DZP were selected to explore the dose–response relationship for DZP in SLE: 45 mg/kg every 4 weeks, as it was determined to be the equivalent dose to that used in the initial dose repeating study [29], 24 mg/kg, as it was expected to be equivalent to the dose for which reduction in anti-dsDNA antibodies were observed in previous studies [25], and 6 mg/kg, as this was the lowest dose that showed an effect in preclinical analyses. The patient’s body weight at baseline was used to determine the individual dose. Randomization was stratified by CS dose at screening (≤10 mg/day or >10 mg/day prednisone-equivalent).
Fig. 1.
Study design
aPatients stratified by CS dose ≤10 mg/day or >10 mg/day prednisone equivalent. CS: corticosteroids; DZP: dapirolizumab pegol; IV: intravenous; PBO: placebo; Q4W: every 4 weeks; SOC: standard-of-care, including CS, immunosuppressants and/or antimalarials.
Patients receiving concomitant CS doses between 10–40 mg/day prednisone-equivalent were required to initiate a protocol-specified taper 4 weeks after the first study drug infusion (Fig. 1). The tapering regimen aimed to reduce the daily dose to ≤7.5 mg/day by week 12, with no requirement to reduce beyond this dose. Slower tapers were permitted. Patients who could not adhere to the tapering regimen remained in the study and were not considered nonresponders for the primary endpoint unless their CS dose exceeded the baseline prednisone-equivalent dose.
Primary efficacy endpoint
The primary objective was to identify a dose–response relationship, based on the primary efficacy variable of week 24 BICLA responder rates, across three doses of DZP and placebo, simultaneously. Continuous dose–response models were evaluated in order to facilitate the selection of suitable doses between those tested in the study that provided evidence of efficacy. Four different dose–response models were selected to be tested using one-sided Multiple Comparison Procedure-Modelling methodology [30] to control for multiplicity: a linear model, a logistic model, and two Emax models (all for dose vs BICLA response at week 24).
BICLA response is defined as: (i) BILAG 2004 improvement without worsening (defined as all BILAG A domain scores at baseline improved to B/C/D, all BILAG B domain scores at baseline improved to C/D, no new BILAG A domain scores and no more than one new BILAG B domain score compared with baseline); (ii) no worsening in SLEDAI-2K score compared with baseline; (iii) no worsening in PGA compared with baseline (<10 mm increase on a 100 mm visual analogue scale); and (iv) no increase in concomitant SLE medications (no increase or addition of immunosuppressant or antimalarial medication, and no increase in CS dose over baseline).
Secondary efficacy endpoints
Secondary efficacy endpoints included the BICLA responder rate in individual dose groups at week 24. Other secondary endpoints included SRI-4 response rates, changes from baseline in SLEDAI-2K, PGA, CLASI scores, swollen and tender joint counts, cumulative number of severe BILAG flares, and changes from baseline in immunologic parameters (anti-dsDNA and aPL, and complement C3/C4 levels).
Adverse events [AEs, including serious AEs (SAEs) and AEs of interest (AEOIs)] were collected throughout the study. SAEs were defined as those that resulted in death or inpatient hospitalization (or prolongation of existing hospitalization), were life-threatening or medically important events, were congenital anomalies, or caused persistent or significant disability. AEOIs, regardless of severity, were defined as moderate-to-severe infections, infusion reactions, hypersensitivity reactions, thromboembolic events, or prespecified neurological events. AEs were classified as treatment-emergent (TEAEs) if the onset occurred at any time at or after the first administration of study drug, and up to 12 weeks (84 days) after the last dose. All AEs were coded using MedDRA version 19.1.
Statistical analysis
Based upon prior evidence in SLE, a placebo response rate of 25% was anticipated at week 24 [31–33]. A power calculation determined that a total of 112 patients were required to complete the 24-week double-blind period; this would provide 80% power of detecting a clinical effect of 29% above placebo (one-sided type-1 error of 5% and equally-sized treatment groups).
Efficacy outcomes were determined in the full analysis set, which comprised all patients who received one or more full dose of study drug and had one or more post-baseline efficacy measurement. All patients who received at least one dose (any dose) of study drug were in the safety set, and safety and immunologic outcomes were analyzed.
Immunologic outcomes are reported as the mean (s.d.) change from baseline, or median (min–max) change from baseline if data are not normally distributed. Efficacy outcomes are reported as the percentage of responders for binary outcomes (BICLA/SRI-4 responder rates), or mean (s.d.) change from baseline for continuous outcomes (SLEDAI-2K/PGA score). For continuous outcomes, baseline values were included as covariates. Where P-values are reported, reference is made to either odds ratios (BICLA/SRI-4 responder rate) or least squares mean differences (SLEDAI-2K/PGA score) between DZP and placebo. These P-values were not adjusted for multiplicity. Analyses of the primary efficacy variable are described in detail in the Supplementary Methods, available at Rheumatology online.
Results
Patient disposition and baseline characteristics
Of 182 randomized patients, 178 (97.8%) completed the double-blind period, of whom 11 permanently discontinued study treatment prior to the end of the double-blind period. Ninety percent of patients [164/182 (90.1%)] completed the observational period (supplementary Fig. S1, available at Rheumatology online).
Baseline demographics and clinical characteristics were similar across treatment groups. All treatment groups were represented by a high proportion of White patients, though this proportion was greater in the DZP 24 mg/kg group (Table 1). There was a slightly lower mean SLEDAI-2K score in the DZP 24 mg/kg group, and a longer time to diagnosis in the DZP 45 mg/kg group (Table 1). Mucocutaneous and musculoskeletal BILAG domains were the predominant domains that were present at baseline in this patient population (supplementary Table S1, available at Rheumatology online). More than half of the patients had baseline CS doses ≥10 mg/day; less than one-quarter of patients had baseline doses ≥20 mg/day (Table 1). Across all groups, the majority of patients were able to taper their CS doses to ≤7.5 mg/day by week 24 (83.7, 86.0, 79.5, and 80.4% of the placebo and DZP 6, 24, and 45 mg/kg groups, respectively).
Table 1.
Baseline demographics and clinical characteristics
| Mean (s.d.), unless otherwise stated | SOC + PBO (n = 43) | SOC + DZP 6 mg/kg (n = 43) | SOC + DZP 24 mg/kg (n = 44) | SOC + DZP 45 mg/kg (n = 46) |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, mean (s.d.) | 42.7 (12.5) | 40.5 (11.7) | 42.6 (10.5) | 39.0 (13.1) |
| Female, n (%) | 39 (90.7) | 40 (93.0) | 39 (88.6) | 42 (91.3) |
| BMI, kg/m2, mean (s.d.) | 25.6 (4.2) | 26.1 (5.0) | 26.0 (4.4) | 25.3 (4.8) |
| Racial group, n (%) | ||||
| American Indian/Alaskan native | 2 (4.7) | 1 (2.3) | 1 (2.3) | 1 (2.2) |
| Asian | 1 (2.3) | 0 | 1 (2.3) | 0 |
| Black | 1 (2.3) | 4 (9.3) | 1 (2.3) | 5 (10.9) |
| White | 25 (58.1) | 24 (55.8) | 32 (72.7) | 27 (58.7) |
| Other/mixed | 14 (32.6) | 14 (32.6) | 9 (20.5) | 13 (28.3) |
| Clinical characteristics | ||||
| Time since diagnosis, years, median (min–max) | 5.4 (0.1–30.0) | 5.0 (0.2–27.8) | 5.1 (0.3–27.0) | 8.2 (0.3–25.0) |
| BILAG 2004 total score, mean (s.d.) | 18.6 (3.7) | 19.1 (4.1) | 18.6 (3.9) | 19.8 (5.5) |
| SLEDAI-2K total scorea, mean (s.d.) | 10.7 (3.4) | 11.4 (2.4) | 9.9 (2.5) | 11.1 (3.4) |
| CLASI activity score, mean (s.d.) | 7.8 (6.1) | 7.5 (6.2) | 7.0 (6.4) | 8.6 (6.2) |
| Swollen joint count, mean (s.d.) | 7.7 (5.9) | 7.9 (5.4) | 6.0 (4.7) | 6.7 (4.5) |
| Tender joint count, mean (s.d.), (n)b | 9.3 (5.9) (n = 41) | 9.7 (6.3) (n = 43) | 9.1 (5.6) (n = 41) | 10.7 (7.2) (n = 44) |
| ANA ≥1:80, n (%) | 43 (100.0) | 41 (95.3) | 42 (95.5) | 41 (89.1) |
| Anti-dsDNA >10 IU, n (%)c | 17 (39.5) | 24 (55.8) | 18 (40.9) | 21 (45.7) |
| Low C3 or C4, n (%) | 23 (53.5) | 25 (58.1) | 26 (59.1) | 26 (56.5) |
| Medications at baseline | ||||
| CS at baseline, n (%) | 38 (88.4) | 40 (93.0) | 39 (88.6) | 36 (78.3) |
| Dose at baseline, mg/day, median (min–max) | 10.0 (0.0–40.0) | 10.0 (0.0–25.0) | 10.0 (0.0–20.0) | 10.0 (0.0–30.0) |
| ≥10 mg/day, n (%) | 27 (62.8) | 28 (65.1) | 24 (54.5) | 24 (52.2) |
| ≥20 mg/day, n (%) | 10 (23.3) | 6 (14.0) | 4 (9.1) | 8 (17.4) |
| Immunosuppressants, n (%) | 22 (51.2) | 25 (58.1) | 25 (56.8) | 26 (56.5) |
| SLE-related immunosuppressants, n (%)d | 22 (48.9) | 25 (55.6) | 25 (55.6) | 26 (55.3) |
| AZA, n (%) | 14 (31.1) | 9 (20.0) | 15 (33.3) | 12 (25.5) |
| MTX, n (%) | 6 (13.3) | 11 (24.4) | 6 (13.3) | 7 (14.9) |
| MTX sodium, n (%) | 0 | 0 | 0 | 1 (2.1) |
| MMF, n (%) | 2 (4.4) | 6 (13.3) | 5 (11.1) | 7 (14.9) |
| Mycophenolate sodium, n (%) | 0 | 0 | 0 | 1 (2.1) |
| LEF, n (%) | 1 (2.2) | 0 | 0 | 0 |
| Antimalarials, n (%) | 29 (67.4) | 30 (69.8) | 33 (75.0) | 28 (60.9) |
| Prior SLE-related medicationsd,e | ||||
| CS, n (%) | 20 (44.4) | 27 (60.0) | 20 (44.4) | 24 (51.1) |
| Immunosuppressants, n (%) | 12 (26.7) | 21 (46.7) | 11 (24.4) | 12 (25.5) |
| AZA, n (%) | 2 (4.4) | 6 (13.3) | 3 (6.7) | 5 (10.6) |
| MMF, n (%) | 3 (6.7) | 6 (13.3) | 3 (6.7) | 1 (2.1) |
| Belimumab, n (%) | 5 (11.1) | 4 (8.9) | 1 (2.2) | 1 (2.1) |
| MTX, n (%) | 1 (2.2) | 5 (11.1) | 3 (6.7) | 2 (4.3) |
| Anifrolumab, n (%) | 1 (2.2) | 4 (8.9) | 1 (2.2) | 2 (4.3) |
| Blisibimod, n (%) | 0 | 1 (2.2) | 0 | 1 (2.1) |
| Mycophenolate sodium, n (%) | 0 | 0 | 0 | 2 (4.3) |
| Ustekinumab, n (%) | 1 (2.2) | 0 | 1 (2.2) | 1 (2.1) |
| MTX sodium, n (%) | 0 | 1 (2.2) | 0 | 0 |
| Lulizumab pegol, n (%) | 0 | 0 | 1 (2.2) | 0 |
| Antiprotozoals, n (%)f | 4 (8.9) | 9 (20.0) | 4 (8.9) | 9 (19.1) |
| mAbs, n (%) | 4 (8.9) | 4 (8.9) | 3 (6.7) | 4 (8.5) |
Full analysis set, unless otherwise stated. aSLEDAI-2K total score calculated using anti-dsDNA positive if >10 IU; bsubjects had arthritis at baseline; cFarr assay; ddata shown for the safety set; ea prior medication is any medication with an end date and time before the date of first administration of the study drug; fincluding antimalarials. Combinations of immunosuppressants with antimalarials and/or CS are listed in supplementary Table S5, available at Rheumatology online. ANA: anti-nuclear antibody; AZA: azathioprine; BILAG: British Isles Lupus Assessment Group; BMI: body mass index; C3/C4: complement C3/C4; CLASI: Cutaneous Lupus Erythematosus Disease Area and Severity Index; CS: corticosteroids; dsDNA: double-stranded DNA; DZP: dapirolizumab pegol; IU: International Units; LEF: leflunomide; mAb: monoclonal antibody; MMF: mycophenolate mofetil; MTX: methotrexate; PBO: placebo; S.D.: standard deviation; SLE: systemic lupus erythematosus; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000; SOC: standard-of-care.
Clinical and immunologic outcomes (double-blind period)
As no pre-specified dose–response relationship model fit the observed BICLA response rates at week 24 with statistical significance [best-fitting model (Emax): P = 0.07], the primary endpoint was not met (supplementary Fig. S2, available at Rheumatology online). The study was stratified for baseline CS dose, but this was not found to be a significant factor in this study. It was, however, kept in all relevant planned models as a covariate.
Clinical measures of disease activity, including BICLA, SRI-4, SLEDAI-2K, PGA, and BILAG total scores, generally improved from baseline in DZP-treated patients vs placebo (Fig. 2A–E). Moreover, the cumulative numbers of BILAG severe flares at week 24 were numerically lower in patients treated with DZP 6, 24, or 45 mg/kg (4, 0, and 1, respectively) compared with placebo (7) (Fig. 2F). Additionally, greater improvements from baseline in CLASI activity scores were observed in DZP-treated patients vs placebo (supplementary Table S2, available at Rheumatology online).
Fig. 2.
Clinical outcomes. (A) BICLAa and (B) SRI-4a responder rates, change from baseline in (C) SLEDAI-2Kb, (D) PGAb and (E) BILAG 2004 total scorec, and (F) cumulative number of severe BILAG flaresd
*P < 0.05 for the odds ratio between DZP and PBO (A and B), or for the least squares mean differences between DZP and PBO (C, D, and E). aFull analysis set, modified nonresponder imputation using logistic regression; bCompleter set, observed case using mixed model with repeated measures; cFull analysis set, observed cases using ANCOVA; dFull analysis set, observed case, BILAG severe flare: new Grade A since the previous visit. Multiple flares that may have occurred in a single patient were recorded separately. BICLA: BILAG-Based Composite Lupus Assessment; BILAG: British Isles Lupus Assessment; DZP: dapirolizumab pegol; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000; SRI-4: SLE Responder Index; PBO: placebo; PGA: physician’s global assessment; SOC: standard-of-care.
Compared with placebo, DZP-treated patients had greater reductions from baseline in anti-dsDNA levels and greater increases from baseline in C3 and C4 levels throughout the double-blind period (Fig. 3). Of the patients with either low C3 or C4 levels at baseline, more DZP-treated patients achieved normal levels by week 24 [16/27 (59.3%), 19/27 (70.4%), and 12/26 (46.2%) of the DZP 6, 24, and 45 mg/kg groups, respectively], compared with placebo-treated patients [9/24 (37.5%)]. Levels of IgG and IgM beta-2 glycoprotein I and aCL also declined to a greater extent in DZP-treated patients with elevated baseline levels (supplementary Table S2, available at Rheumatology online).
Fig. 3.
Immunologic outcomes. Change from baseline in (A) Anti-dsDNAa, (B) Complement C3b, and (C) Complement C4c
Safety set. aIn patients with <10 IU at baseline (measured using the Farr assay); bin patients with complement C3 <LLN at baseline; cin patients with complement C4 <LLN at baseline. dsDNA: double-stranded DNA (Farr assay); DZP: dapirolizumab pegol; IU: international units; LLN: lower limit of normal; PBO: placebo; SOC: standard of care.
Clinical and immunologic outcomes (observational period)
Following study drug withdrawal, 5, 8, 5, and 4 severe BILAG flares occurred in the placebo and DZP 6, 24, and 45 mg/kg groups, respectively, compared with 7, 4, 0, and 1 severe flares that occurred in the placebo and DZP 6, 24, and 45 mg/kg groups, respectively, during the double-blind period. However, the cumulative total number of flares (12, 12, 5, and 5 in the placebo and DZP 6, 24, and 45 mg/kg groups, respectively) that occurred during the 48-week study period was higher in the placebo and DZP 6 mg/kg groups than in the DZP 24 and 45 mg/kg groups (Fig. 2F). SLEDAI-2K, PGA and BILAG total scores stabilized across treatment groups after study drug withdrawal, whereas BICLA and SRI-4 response rates were generally lower at week 48 than at week 24 in all groups (Fig. 2A–E). For BICLA, the reductions ranged from 11.6 to 18.1% across DZP groups, and in the placebo group, the decrease was 11.6%. For SRI-4, the decline ranged from 9.1 to 13.9% across DZP groups and was 14.0% in the placebo group (Fig. 2A and B). The decreases in BICLA and SRI-4 response rates were most often due to interventions with escape medicines during this period, which per protocol led to nonresponder classification. This was the case in 7 (16.3%), 14 (32.6%), 14 (31.8%), and 11 (23.9%) of the placebo and DZP 6, 24, and 45 mg/kg groups, respectively (supplementary Table S3, available at Rheumatology online). Following study drug withdrawal, serologies generally worsened and returned towards baseline (Fig. 3;supplementary Table S2, available at Rheumatology online).
Safety
There was a higher incidence of TEAEs in the treatment groups compared with placebo, and a greater number of severe TEAES occurred in the DZP 45 mg/kg group than in other groups; however, serious TEAEs were generally low and similar across treatment groups (Table 2). In addition, the incidence of TEAEs related to abnormal laboratory values was low and similar across treatment groups, and no safety concerns for abnormal vital signs or ECG findings associated with DZP were identified (data not shown). Rates of infection (particularly upper respiratory tract infections) were greater among DZP-treated patients vs placebo (Table 2). Herpes zoster infections occurred in the DZP 6 mg/kg group more than in other groups; however, the incidence of herpes zoster infection overall was low and similar across treatment groups. Most of the upper respiratory tract infections were mild or moderate in intensity, and none led to discontinuations of the study drug. One bacteraemia caused by Pseudomonas infection in the DZP 24 mg/kg group led to permanent discontinuation of study drug. Four thromboembolic TEAEs were observed during the double-blind period: three in the placebo group and one in a patient receiving DZP 24 mg/kg (supplementary Table S4, available at Rheumatology online).
Table 2.
Safety outcomes in the double-blind treatment period (safety set)
| n (%) | SOC + PBO (n = 45) | SOC + DZP 6 mg/kg (n = 45) | SOC + DZP 24 mg/kg (n = 45) | SOC + DZP 45 mg/kg (n = 47) |
|---|---|---|---|---|
| Mean (s.d.) duration of exposure (days) | 216.7 (27.0) | 224.0 (4.7) | 215.6 (29.6) | 212.1 (38.5) |
| Any TEAEa | 28 (62.2) [90] | 29 (64.4) [130] | 35 (77.8) [116] | 34 (72.3) [84] |
| Infections and infestationsa | 15 (33.3) [21] | 21 (46.7) [43] | 26 (57.8) [40] | 22 (46.8) [29] |
| Mild | 9 (20.0) | 10 (22.2) | 12 (26.7) | 13 (27.7) |
| Moderate | 5 (11.1) | 11 (24.4) | 13 (28.9) | 6 (12.8) |
| Severe | 1 (2.2) | 0 | 1 (2.2) | 3 (6.4) |
| Herpes viral infectionsa | 1 (2.2) [1] | 5 (11.1) [5] | 2 (4.4) [2] | 0 |
| Herpes zostera | 1 (2.2) [1] | 3 (6.7) [3] | 2 (4.4) [2] | 0 |
| Oral herpesa | 0 | 2 (4.4) [2] | 0 | 0 |
| Upper respiratory tract infectionsa | 6 (13.3) [8] | 12 (26.7) [14] | 15 (33.3) [20] | 13 (27.7) [15] |
| Infusion reactionsa | 0 | 0 | 0 | 1 (2.1) [1] |
| Thromboembolic events | 3 (6.7) | 0 | 1 (2.2) | 0 |
| TEAEs of interesta | 9 (20.0) [11] | 11 (24.4) [18] | 13 (28.9) [18] | 10 (21.3) [12] |
| Serious TEAEsa | 5 (11.1) [6] | 2 (4.4) [2] | 4 (8.9) [4] | 5 (10.6) [6] |
| Study discontinuation due to TEAEsa | 1 (2.2) [1] | 0 | 0 | 0 |
| Permanent withdrawal of study drug due to TEAEsa | 4 (8.9) [4] | 0 | 2 (4.4) [2] | 2 (4.3) [2] |
| Severe TEAEsa | 3 (6.7) [4] | 1 (2.2) [1] | 3 (6.7) [3] | 7 (14.9) [8] |
| Deaths | 0 | 0 | 0 | 0 |
Data presented as n (%) [ER per 100 patient-years]. TEAEs were those with onset at the time of, or after, the first dose of study drug, until 12 weeks after the last dose. Patients who withdrew from the study early (during the double-blind treatment period) entered a safety follow-up period, which ended 12 weeks after the final dose of study drug. DZP: dapirolizumab pegol; ER: event rate; PBO: placebo; TEAE: treatment-emergent adverse event; S.D.: standard deviation; SOC: standard of care.
Discussion
The aim of this phase 2b double-blind placebo-controlled trial was to establish a dose–response relationship and evaluate the efficacy and safety of DZP in patients with active SLE compared with SOC treatment. This study also assessed the durability of response following study drug withdrawal through a biphasic study design, in which patients received double-blind treatment with DZP or placebo (and SOC) for 24 weeks, followed by a 24-week observational period during which patients received SOC only. The decision to withdraw study drug at week 24 and incorporate an observational follow-up period was based on research in animal transplant models that reported sustained responses even after withdrawal of CD40L antagonist [24].
Multiple Comparison Procedure-Modelling methodology was used to assess the dose–response relationship of DZP based on week 24 BICLA responder rates (the primary efficacy variable). Continuous dose–response models were selected as these can facilitate the selection of suitable doses between those that demonstrate efficacy in the study. Since the BICLA responder rates at week 24 did not fit any of the pre-specified dose–response models with statistical significance [best-fitting model (Emax): P = 0.07], the primary objective was not met. DZP-treated patients did show consistent numerical improvements across multiple clinical measures of disease activity; however, as these were not part of the primary endpoint it is not appropriate to discuss the significance of these outcomes as the study was not designed or powered to determine this. Furthermore, DZP treatment resulted in improvements relative to placebo across multiple pharmacodynamic biomarkers, indicating a biologic effect.
Upon withdrawal of DZP, immunologic parameters generally worsened and returned to baseline levels, providing further evidence of DZP biologic activity, but challenging the capacity of DZP to induce tolerance. While BILAG, SLEDAI, and PGA scores stabilized across treatment groups, BICLA and SRI-4 response rates declined. This was most likely due to interventions with escape medicines used during this period, which automatically led to nonresponder classification.
DZP appeared to have an acceptable safety profile and was generally well tolerated. The risk of thromboembolic events in the anti-CD40L drug class should be acknowledged and while a total of four thromboembolic events were reported during the double-blind period, three occurred in patients receiving placebo. For one DZP-treated patient (24 mg/kg) a deep vein thrombosis of the left upper extremity (subclavian axillary, associated with a peripherally inserted central catheter line inserted for antibiotic treatment due to cellulitis) was reported, which started 74 days after the last infusion of DZP, and was deemed unrelated to the study drug. The findings indicate that DZP treatment may provide a smaller risk of thromboembolism in comparison with other anti-CD40L treatments. These outcomes are similar to those of preclinical and phase 1 DZP studies, which revealed low rates of thromboembolic events during DZP treatment compared with placebo [27–29].
Although the present study was limited by its relatively short duration and the small sample size, which prevented analyses being conducted within specific racial groups, high retention rates were observed (>95% for the 24-week double-blind period and >90% for the full 48-week study). As a consequence of high retention rates, missing data imputation was minimized. Therefore, these results are highly representative of the study population as estimation bias is reduced. The positive results from this study are also limited by the failure to meet the primary endpoint. This should be recognized as such but highlights the need for future studies of DZP to demonstrate the efficacy and safety of DZP within a larger study population and for a longer duration, using carefully selected endpoints.
Treatment options for SLE are limited at the time of publication, with only one biologic, belimumab, approved by regulatory authorities for the treatment of SLE [34, 35], while many others have failed in clinical development [17, 18]. Glucocorticoids are frequently used for their potent anti-inflammatory and immunosuppressive properties, but their chronic use is associated with severe adverse events [16]. As such, there is a need to widen the biologic repertoire available for the treatment of SLE patients to reduce disease activity, prevent flares, and restrict accrual of organ damage.
These data indicate DZP may be efficacious, with an acceptable safety profile, in adult patients with moderately to severely active SLE. The potential clinical benefit of DZP warrants further investigation in a larger study.
Supplementary Material
Acknowledgements
The authors thank the patients, the investigators and their teams who took part in this study. The authors also acknowledge Jacob Boyle and JoAnn Gorden, for data and statistical programming; Simone Emanuele Auteri, PhD (UCB Pharma, Brussels, Belgium) for publication coordination; and Sam Fraser, PhD and Alyssa McVey, PhD (Costello Medical, Cambridge, UK) for medical writing and editorial assistance based on the authors’ input and direction. I.N.B. is a National Institute for Health Research (NIHR) Senior Investigator and is funded by Versus Arthritis and the NIHR Manchester Biomedical Research Centre; the views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. This study was funded by UCB Pharma and Biogen Inc. Substantial contributions to study conception and design were made by R.A.F., I.N.B., T.D., M.G.L., P.L., M.U., B.H., T.J., C.Brittain, J.L., C.Barbey and C.S.; substantial contributions to analysis and interpretation of the data were made by R.A.F., I.N.B., T.D., M.G.L., P.L., M.U., B.H., T.J., C.Brittain, J.L., C.Barbey and C.S.; drafting the article or revising it critically for important intellectual content was carried out by R.A.F., I.N.B., T.D., M.G.L., P.L., M.U., B.H., T.J., C.Brittain, J.L., C.Barbey and C.S.; and final approval of the version of the article to be published was given by R.A.F., I.N.B., T.D., M.G.L., P.L., M.U., B.H., T.J., C.Brittain, J.L., C.Barbey and C.S.
Funding: This work was supported by UCB Pharma and Biogen Inc. Support for third-party writing assistance for this article, provided by Costello Medical Consulting, was funded by UCB Pharma in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Disclosure statement: R.A.F. received research grants to conduct this study, and has received consulting fees from UCB Pharma and Biogen; I.N.B. received consulting fees from UCB Pharma, Astra Zeneca, Merck Serono and Eli Lilly, and has received grants from Genzyme Sanofi and GlaxoSmithKline; T.D. received research grants to conduct this study, and has received consulting fees from UCB Pharma; M.G.L. and P.L., none declared; M.U. is Chair of the Data Safety Monitoring Committee; B.H., T.J., C.Brittain and C.S. are employees and stockholders of UCB Pharma; J.L. and C.Barbey are employees and stockholders of Biogen.
Data availability statement
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient-level data and redacted trial documents, which may include: analysis-ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password-protected portal.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Dema B, Charles N.. Autoantibodies in SLE: specificities, isotypes and receptors. Antibodies 2016;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rahman A, Isenberg DA.. Systemic lupus erythematosus. N Engl J Med 2008;358:929–39. [DOI] [PubMed] [Google Scholar]
- 3. Ünlü O, Zuily S, Erkan D.. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol 2016;3:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bruce IN, O’Keeffe AG, Farewell V. et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drenkard C, Lim SS.. Update on lupus epidemiology: advancing health disparities research through the study of minority populations. Curr Opin Rheumatol 2019;31:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Margery-Muir AA, Bundell C, Nelson D, Groth DM, Wetherall JD.. Gender balance in patients with systemic lupus erythematosus. Autoimmun Rev 2017;16:258–68. [DOI] [PubMed] [Google Scholar]
- 7. Rider V, Abdou NI, Kimler BF. et al. Gender bias in human systemic lupus erythematosus: a problem of steroid receptor action? Front Immunol 2018;9:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rees F, Doherty M, Grainge M. et al. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999–2012. Ann Rheum Dis 2016;75:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al Dhanhani AM, Agarwal M, Othman YS, Bakoush O.. Incidence and prevalence of systemic lupus erythematosus among the native Arab population in UAE. Lupus 2017;26:664–9. [DOI] [PubMed] [Google Scholar]
- 10. Dall'era M, Cisternas MG, Snipes K. et al. The incidence and prevalence of systemic lupus erythematosus in San Francisco County, California: the California Lupus Surveillance Project. Arthritis Rheumatol 2017;69:1996–2005. [DOI] [PubMed] [Google Scholar]
- 11. Fatoye F, Gebrye T, Svenson LW.. Real-world incidence and prevalence of systemic lupus erythematosus in Alberta, Canada. Rheumatol Int 2018;38:1721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Osio-Salido E, Manapat-Reyes H.. Epidemiology of systemic lupus erythematosus in Asia. Lupus 2010;19:1365–73. [DOI] [PubMed] [Google Scholar]
- 13. Zou YF, Feng CC, Zhu JM. et al. Prevalence of systemic lupus erythematosus and risk factors in rural areas of Anhui Province. Rheumatol Int 2014;34:347–56. [DOI] [PubMed] [Google Scholar]
- 14. Mosca M, Tani C, Carli L, Bombardieri S.. Glucocorticoids in systemic lupus erythematosus. Clin Exp Rheumatol 2011;29:S126–9. [PubMed] [Google Scholar]
- 15. Judd LL, Schettler PJ, Brown ES. et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry 2014;171:1045–51. [DOI] [PubMed] [Google Scholar]
- 16. Ruiz-Irastorza G, Danza A, Khamashta M.. Glucocorticoid use and abuse in SLE. Rheumatology (Oxford) 2012;51:1145–53. [DOI] [PubMed] [Google Scholar]
- 17. Dall’era M, Bruce IN, Gordon C. et al. Current challenges in the development of new treatments for lupus. Ann Rheum Dis 2019;78:729–35. [DOI] [PubMed] [Google Scholar]
- 18. Mahieu MA, Strand V, Simon LS, Lipsky PE, Ramsey-Goldman R.. A critical review of clinical trials in systemic lupus erythematosus. Lupus 2016;25:1122–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yazdany J, Davis J.. The role of CD40 ligand in systemic lupus erythematosus. Lupus 2004;13:377–80. [DOI] [PubMed] [Google Scholar]
- 20. Daoussis D, Andonopoulos AP, Liossis S-NC.. Targeting CD40L: a promising therapeutic approach. Clin Diagn Lab Immunol 2004;11:635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Comte D, Karampetsou MP, Tsokos GC.. T cells as a therapeutic target in SLE. Lupus 2015;24:351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grammer AC, Slota R, Fischer R. et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest 2003;112:1506–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang W, Sinha J, Newman J. et al. The effect of anti-CD40 ligand antibody on B cells in human systemic lupus erythematosus. Arthritis Rheum 2002;46:1554–62. [DOI] [PubMed] [Google Scholar]
- 24. Karnell JL, Rieder SA, Ettinger R, Kolbeck R.. Targeting the CD40-CD40L pathway in autoimmune diseases: humoral immunity and beyond. Adv Drug Deliv Rev 2019;141:92–103. [DOI] [PubMed] [Google Scholar]
- 25. Boumpas DT, Furie R, Manzi S. et al. ; on behalf of the BG9588 Lupus Nephritis Trial Group. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum 2003;48:719–27. [DOI] [PubMed] [Google Scholar]
- 26. Robles-Carrillo L, Meyer T, Hatfield M. et al. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol 2010;185:1577–83. [DOI] [PubMed] [Google Scholar]
- 27. Shock A, Burkly L, Wakefield I. et al. CDP7657, an anti-CD40L antibody lacking an Fc domain, inhibits CD40L-dependent immune responses without thrombotic complications: an in vivo study. Arthritis Res Ther 2015;17:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tocoian A, Buchan P, Kirby H. et al. First-in-human trial of the safety, pharmacokinetics and immunogenicity of a PEGylated anti-CD40L antibody fragment (CDP7657) in healthy individuals and patients with systemic lupus erythematosus. Lupus 2015;24:1045–56. [DOI] [PubMed] [Google Scholar]
- 29. Chamberlain C, Colman PJ, Ranger AM. et al. Repeated administration of dapirolizumab pegol in a randomised phase I study is well tolerated and accompanied by improvements in several composite measures of systemic lupus erythematosus disease activity and changes in whole blood transcriptomic profiles. Ann Rheum Dis 2017;76:1837–44. [DOI] [PubMed] [Google Scholar]
- 30. Bretz F, , Pinheiro JC, , Branson M. Combining multiple comparisons and modeling techniques in dose-response studies. Biometrics 2005;61:738–48. [DOI] [PubMed] [Google Scholar]
- 31. Furie RA, Petri MA, Wallace DJ. et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum 2009;61:1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merrill JT, Van Vollenhoven RF, Buyon JP. et al. Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2016;75:332–40. [DOI] [PubMed] [Google Scholar]
- 33. Wallace DJ, Strand V, Merrill JT. et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: a phase II dose-ranging randomised controlled trial. Ann Rheum Dis 2017;76:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Furie R, Petri M, Zamani O. et al. ; BLISS-76 Study Group. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Navarra SV, Guzmán RM, Gallacher AE. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient-level data and redacted trial documents, which may include: analysis-ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password-protected portal.