Abstract
The purpose of this experiment was to study the effects of fenugreek seed extract (FSE) on the growth performance, intestinal morphology, intestinal immunity and cecal micro-organisms in yellow-feathered broilers. A total of 240 one-day-old male yellow-feathered broilers were selected and randomly assigned to four treatments with 6 replicates per group and ten broilers per replicate. Started from the third day, birds were fed with basal diet (CON group) or basal diet supplemented with 30 mg/kg Zinc bacitracin (ZB group), or basal diet supplemented with 50 (D-FSE group) or 100 (H-FSE group) mg/kg FSE, respectively. The experiment lasted for 56 d. The results showed that dietary FSE supplementation improved average daily weight gain (ADG) and ratio of feed to weight gain (F: G) (P < 0.01), increased intestinal villus height (VH), villus height to crypt depth ratio (V/C) (P < 0.05), serum concentrations of IL-10, and the contents of secretory immunoglobulin A (sIgA) (P < 0.05), as well as decreased the activity of iNOS (P < 0.05). The high-throughput sequencing results showed that dietary FSE supplementation increased the alpha diversity of cecal microbes, and Firmicutes, Bacteroidetes, Verrucomicrobia and Proteobacteria taken up 95% of all phyla detected, FSE significantly reduced Campylobacter, Synergistes, and Lachnoclostridium abundance (P ≤ 0.05). There were significant difference in more than 30 KEGG pathways between FSE added group and control group or ZB group. FSE supplementation, in other words, maintained gut microbiota homeostasis while improving broiler growth performance. As a result, FSE has the potential to replace prophylactic antibiotic use in poultry production system.
Key words: fenugreek seed extracts, anti-inflammatory, intestinal health, growth performance, antibiotics
Introduction
Antibiotics were widely used in livestock and poultry production due to their antibacterial and growth-promoting functions, which has promoted the rapid development of animal husbandry and brought huge economic benefits to human beings (Abd et al., 2019). However, several issues such as bacterial resistance, drug residual effects, endangering human health, environmental pollution, and ecological balance disruption have grown significantly, thus it is critical to produce effective feed additives to replace antibiotics (Bengtsson and Wierup, 2006; Mcewen, 2006). Herb extracts, antibacterial peptides, prebiotics, enzymes, organic acids, and other substances have all recently been studied in this field (Alagawany et al., 2018; Omonijo et al., 2018; Lan and Kim, 2019). Among them, research on pure, natural, nontoxic, and nonresidual Chinese herbal extracts as feed additives has become a focus. Traditional Chinese herbal extract contains not only nutrients such as protein, carbohydrate, mineral element, vitamin, amino acid, etc., but also some bioactive compounds which can improve feed efficiency and growth performance of livestock and poultry production (Chen et al., 2011; Abdallah et al., 2019). Polysaccharides found in Chinese herbal extracts can help boost the body's immunity (Jiang et al., 2010; Xu et al., 2014; Liu et al., 2018). Chinese herbal extracts also contain flavonoids, polyphenols, and other substances that function as antioxidants and improve meat quality (Park et al., 2015; Turgut et al., 2016; Yu et al., 2021). Furthermore, the glycosides, alkaloids, and organic acids included in Chinese herbal extracts can inhibit or kill pathogenic bacteria, control intestinal flora, boost immunity, and have disease prevention and treatment benefits (Zhang et al., 2014). When used as feed additives, Chinese herbal extracts are sourced from natural products and have no drug residual effect nor pollute the environment (Nathiya et al., 2014).
Fenugreek (Trigonella foenum-graecum), an annual leguminous plant which belongs to the family Fabaceae. It is cultivated worldwide as a semiarid crop and was once recognized in China as a homology of food and medicine. Fenugreek seeds are rich in crude protein, dietary fiber, fatty acids, amino acids, and minerals (Pandey and Awasthi, 2015; Nasim et al., 2016). The main chemical components of Fenugreek seed extract (FSE) are polysaccharides, flavones, steroidal saponins and alkaloids. Research evidences proved that FSE owned many positive role in health, such as hypoglycemic (Gad et al., 2006; Lu et al., 2015), hypocholesterolemic (Ramulu et al., 2011; Uemura et al., 2011), anti-oxidation (Abdel-Daim et al., 2015; Goyal et al., 2018), antibacterial (Mozhdeh et al., 2019; Yasmeen and Shashikumar, 2019), anti-inflammatory (Bae et al., 2012; Ahmed et al., 2017), immuno-stimulating activities (Begum et al., 2016; Guardiola et al., 2018) and improve growth performance (Toaha et al., 2016; Covarrubias et al., 2018). FSE enhanced the appetite of broilers through its effects on the nervous system and intestinal flora. The inclusion rate of 3 g/kg FSE could stimulate feed intake in broilers, improve the rate of feed conversion, and increase their live weight (Alloui et al., 2012). However, little is known regarding FSE's possible impact on intestinal health and microbiome composition. As a result, the current study was aimed to explore the effects of dietary FSE supplementation on broiler growth performance, intestinal morphology, intestinal immunology, and cecum microbial composition.
MATERIALS AND METHODS
Fenugreek Seed Extracts
Fenugreek seed extracts (50% fenugreek polysaccharide, 15% saponin, 10% fenugreek flavones, 2% alkaloids) was extracted by water and it was provided by Hunan Geneham Pharmaceutical Co., Ltd (Changsha, China). The product was white powder in appearance.
Animals and Diets
All experimental procedures used in this study were approved by the Institutional Animal Care and Use Committee of Hunan Agricultural University. The male yellow-feathered broilers were all purchased from a nearby commercial hatchery. A total of 240 healthy chicks were chosen at random at 3 d old and allocated to one of 4 treatment groups (6 replicates per group, 10 broilers each replicate). Birds were fed with basal diet (CON group) or basal diet supplemented with 30 mg/kg Zinc bacitracin (ZB group), or basal diet supplemented with 50 (D-FSE group) or 100 (H-FSE group) mg/kg FSE, respectively. The basal diets were prepared in mash according to “Feeding Standard of Chicken (NY/T33-2004)” issued by the Ministry of Agricultural of People's Republic of China (Ministry of Agricultural of the People's Republic of China, 2004). The chemical composition of basal diets was shown in Table 1. All broilers had free access to feed and water and vaccinated according to the vaccination procedure guide for broilers. The trial lasted for 56 d.
Table 1.
Composition and nutrient levels of basal diets (air-dry basis).
| Feed components | 1 to 28 d | 29 to 56 d |
|---|---|---|
| Ingredients | ||
| Corn | 56.55 | 58.65 |
| Soybean meal | 36.10 | 33.40 |
| Soybean oil | 3.00 | 3.50 |
| CaHPO4 | 1.80 | 1.90 |
| Limestone | 1.00 | 1.00 |
| NaCl | 0.30 | 0.30 |
| Cholone chloride | 0.15 | 0.15 |
| DL-Methionine | 0.10 | 0.10 |
| Premix1 | 1.00 | 1.00 |
| Nutrient levels2 | ||
| ME, MJ/kg | 12.20 | 12.46 |
| CP,% | 20.13 | 19.17 |
| Lys,% | 1.01 | 0.97 |
| Met,% | 0.43 | 0.41 |
| Ca,% | 1.09 | 1.11 |
| Available P,% | 0.52 | 0.53 |
The premix provided the following per kg of diets: Cu, 10 mg; Fe, 90 mg; Mn, 90 mg; Zn, 50 mg; I, 0.4 mg; Se, 0.2 mg; Co, 0.4 mg; VA, 5 000 IU; VD, 3 500 IU; VE, 10 IU; VB1, 1.5 mg; VB6, 1.5 mg; VB2, 6.0 mg; VB12, 10 μg; nicotinic acid, 35 mg; folic acid, 0.8 mg; pantothenic acid, 12 mg; biotin, 0.8 mg.
Nutrient levels were all calculated values.
Data and Samples Collection
Growth Performance
Body weight (BW) and feed consumption of each cage were recorded. Then, the average daily feed intake (ADFI), average daily weight gain (ADG), ratio of feed to weight gain (F: G) for entire experimental period was calculated.
Jejunum and Ileum Samples
At d 56, 24 broilers (one broiler per replicate) were euthanized, then approximately 1 to 2 cm segment of intestine at the midpoint of jejunum and ileum (as much as possible in the same position) were cut and isolated. The isolated portions were gently rinsed with 0.9% saline, blotted dry with filter paper, then fixed it in a 4% paraformaldehyde fixing solution for 24 h at 4°C for intestinal tissue morphological analysis.
Jejunal and Ileal Mucosa and Cecum Chyme
About 3 cm segment of intestine at midpoint of jejunum and ileum was cut, rinsed gently with 0.9% saline, blotted dry with filter paper, and scraped using glass slide to isolate the jejunum and ileum mucosa which were wrapped in a tin foil and immediately frozen in liquid nitrogen. Then 24 cecum chyme samples from the euthanized broilers were collected into 2 mL cryogenic vials and quickly frozen in liquid nitrogen. All samples were stored at −80°C until further analyses.
Index Determination Method
Histomorphometry of Jejunum and Ileum
The intestinal segment was removed from 4% paraformaldehyde fixing solution, then these samples were embedded in paraffin. Paraffin sections were made by microtome (Leica microsystems AG, Hessen, Germany), then stained with hematoxylin-eosin (HE staining). Selecting 5 intestine sections with complete morphology and clear vision, the microscope image processing software (Image-Pro Plus 6.0) was used to measure villus height (VH), crypt depth (CD) and ratio of villus height to crypt depth (V/C).
Intestinal Mucosa Cytokines Expression and sIgA Content
After taking out the jejunum and ileum mucosa from −80°C refrigerator, each intestinal mucosa was weighed, diluted with 0.9% phosphate buffer solution (PBS) at a mass-to-volume ratio of 1:9. The mucosa was homogenized at 4°C to make 10% tissue homogenate, and the supernatant was collected into 1.5 mL Eppendorf tubes after the homogenate was centrifuged, stored at 4°C until cytokines and sIgA analyses. Tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-6 (IL-6), interleukin-10 (IL-10), and secretory immunoglobulin A (sIgA) were analyzed by ELISA (Jiangsu Yutong Biological Technology Co. Ltd., Nanjing, China). Inducible nitric oxide synthase (iNOS), nitric oxide (NO) were analyzed by using the kit provided by Nanjing Jiancheng Bioengnieering Institute. All analyses instructions were strictly followed.
Analysis of Cecum Microbe
DNA was extracted from cecum samples using PowerSoil DNA Isolation Kit (MoBio Laboratories, CA) according to the instruction manual. The V3-4 hypervariable region of bacterial 16S rRNA gene were amplified. PCR was performed on the Mastercycler Gradient, with a cycle parameter of 95°C for 5 minutes, followed by 28 cycles (Munyaka et al., 2015). The PCR product was purified using a QIAquick Gel Extraction Kit (QIAGEN, Germany), quantified using Real Time PCR, and sequenced at Allwegene Company, Beijing. Sample-specific barcode sequences were used to separate qualified readings and trimmed with Illumina Analysis Pipeline version 2.6. Then use QIIME (Illumina Miseq PE250, Illumina, Inc., Beijing, China) to analyze the dataset (Schloss et al., 2011; Wang et al., 2012; Edgar, 2013).
Statistical Analysis
Using Excel 2016 to arrange data, One-way ANOVA program of SPSS20.0 statistical software was used for one-way analysis of variance and Duncan method was used for multiple comparison of data. The results were expressed as “mean ± SD,” and significant differences were set at P < 0.05.
RESULTS
Effects on Growth Performance
The performance of broilers was presented in Table 2. The findings revealed that dietary treatments had a significant effect on broiler chicken performance (P < 0.01). The final BW and ADG of broilers supplemented with FSE were significantly greater than those of the CON and ZB groups, with the D-FSE group having the highest ADG. Dietary supplementation with FSE resulted in lower F: G (P < 0.01) than the CON group. The ADFI and mortality rate of broilers were not affected by FSE (P > 0.05).
Table 2.
Effects of FSE on growth performance of broilers.
| Dietary treatment1,2 |
|||||
|---|---|---|---|---|---|
| Items3 | CON | ZB | D-FSE | H-FSE | P-value |
| 3 d Initial BW (g) | 55.25 ± 2.55 | 55.38 ± 2.59 | 55.30 ± 2.53 | 55.30 ± 2.59 | 0.935 |
| 59 d Final BW (g) | 1083.09 ± 43.64b | 1080.9 ± 28.60b | 1199.71 ± 62.52a | 1161.88 ± 50.66a | <0.01 |
| ADFI (g) | 50.48 ± 2.10 | 52.73 ± 1.45 | 52.60 ± 3.80 | 49.90 ± 2.02 | 0.144 |
| ADG (g) | 19.39 ± 0.81b | 19.35 ± 0.49b | 21.59 ± 1.17a | 20.88 ± 0.93a | <0.01 |
| F: G (g:g) | 2.60 ± 0.05a | 2.73 ± 0.02a | 2.44 ± 0.21b | 2.39 ± 0.04b | <0.01 |
| Mortality rate (%) | 8.0 ± 0.08 | 5.0 ± 0.05 | 8.0 ± 0.08 | 8.0 ± 0.10 | 0.841 |
Means with different superscripts within a row differ significantly (P < 0.05).
CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
Data represent mean values of 6 replicate per group.
BW, body weight; ADG, average daily weight gain; ADFI, average daily feed intake; F:G, ratio of feed to weight gain.
Effects of FSE on Intestinal Morphology
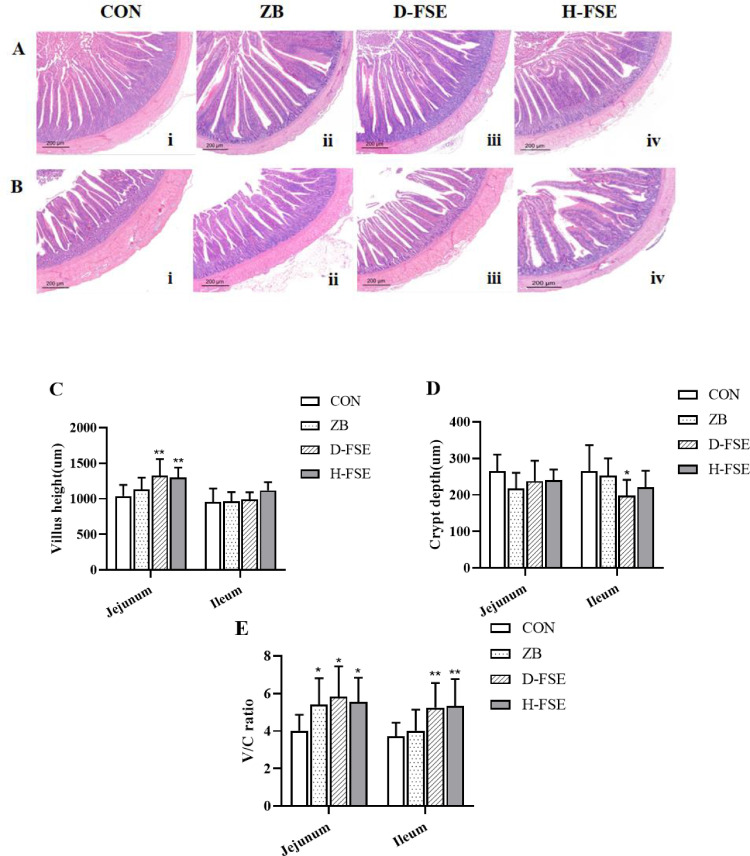
H&E staining revealed alterations in the morphology of the intestinal mucosa in the experimental groups when FSE was added to their diets (Figure 1). Dietary FSE supplementation increased VH (villus height) at jejunum, compared with the CON group (P < 0.01) (Figure 1C), and decreased the CD (crypt depth) at ileum, compared with the ZB group (P < 0.05) (Figure D). The jejunum and ileum V/C were also improved by FSE addition in broiler diet, compared with the CON group (Figure 1E).
Figure 1.
Effects of FSE on intestinal mucosa morphology. Jejunum (A) and ileum (B) stained with H&E (40 ×). (C) The villus height of broilers. (D) The crypt depth of broilers. (E) The ratio of villus height to crypt depth ratio (V/C) of broilers. Data are means ± SD. Bars with different letters are significantly different (P < 0.05). *P < 0.05, **P < 0.01 vs. the control group. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
Effects of FSE on Concentration of Intestinal Mucosa Cytokines
There was a significant difference in the expressions of inflammatory cytokines in the intestinal mucosa (P < 0.01) (Table 3). FSE decreased the expressions of IFN-γ, IL-6, and TNF-α, while increasing the expressions of IL-10 (P < 0.01), as compared to the CON group. At jejunum, the concentration of IL-10 was the highest in the H-FSE group (P < 0.01), while TNF-α and IL-6 were the highest in the ZB group (P < 0.01).
Table 3.
Effect of fenugreek seed extracts on intestinal mucosa cytokines of broilers.
| Dietary treatment |
|||||
|---|---|---|---|---|---|
| Items | CON | ZB | D-FSE | H-FSE | P-value |
| Jejunum | |||||
| TNF-α(pg/mL) | 38.38 ± 7.12b | 54.48 ± 6.00a | 37.87 ± 4.38b | 25.18 ± 7.29c | <0.01 |
| IFN-γ(pg/mL) | 41.10 ± 8.42ab | 48.17 ± 6.28a | 36.56 ± 3.13b | 33.56 ± 7.95b | <0.01 |
| IL-6(pg/mL) | 23.82 ± 1.88b | 28.20 ± 2.94a | 21.37 ± 2.03b | 16.99 ± 0.99c | <0.01 |
| IL-10(pg/mL) | 42.02 ± 5.69b | 35.84 ± 6.67b | 55.95 ± 7.32a | 63.06 ± 7.46a | <0.01 |
| Ileum | |||||
| TNF-α(pg/mL) | 44.72 ± 4.96b | 58.69 ± 7.91a | 44.87 ± 4.63b | 32.15 ± 6.65c | <0.01 |
| IFN-γ(pg/mL) | 51.70 ± 5.47a | 52.12 ± 7.37a | 44.25 ± 5.8b | 39.08 ± 5.0b | <0.01 |
| IL-6(pg/mL) | 25.51 ± 1.68b | 29.76 ± 4.17c | 23.37 ± 1.45b | 21.24 ± 2.52a | <0.01 |
| IL-10(pg/mL) | 53.54 ± 5.12b | 40.81 ± 6.58b | 56.16 ± 5.16a | 66.33 ± 7.14a | <0.01 |
Means with different superscripts within a row differ significantly (P < 0.05).Data are means ± SD, n = 6. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE.
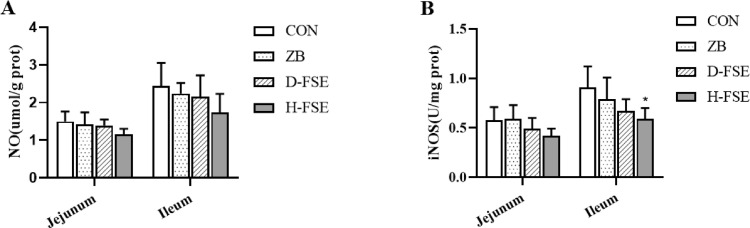
There was no significant difference in the expressions of NO and iNOS at jejunum (P > 0.05) among treatments (Figure 2). However, FSE significantly decreased the activity of iNOS at ileum compared to other groups (P < 0.05).
Figure 2.
Effects of FSE on intestinal mucosa NO, iNOS activity of broilers. (A) Concentration of NO in jejunum and ileum mucosal. (B) Activity of iNOS in jejunum and ileum mucosal. Data are means ± SD. Bars with different letters are significantly different (P < 0.05). *P < 0.05, **P < 0.01 vs. the control group. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
Effects of FSE on Concentration of Intestinal Mucosa sIgA
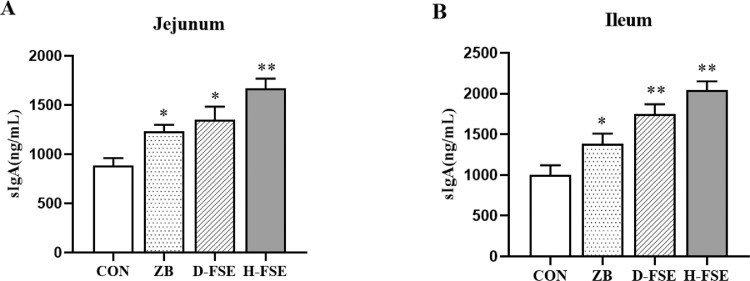
As shown in Figure 3, FSE significantly increased the concentrations of sIgA in the intestinal mucosa of the broilers (P < 0.01). Compared to that of other groups, the H-FSE group had highest concentrations of sIgA at the jejunum (P < 0.01), while CON group had lowest concentrations of sIgA at the ileum (P < 0.01).
Figure 3.
Effects of FSE on concentration of intestinal mucosa sIgA in broilers. (A) (B) Concentration of sIgA in jejunum and ileum mucosal of broilers. Data are means ± SD. Bars with different letters are significantly different (P < 0.05). *P < 0.05, **P < 0.01 vs. the control group. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
Effects of FSE on Cecum Microbial Community Structure
Data Quality Control and OTU Cluster Analysis
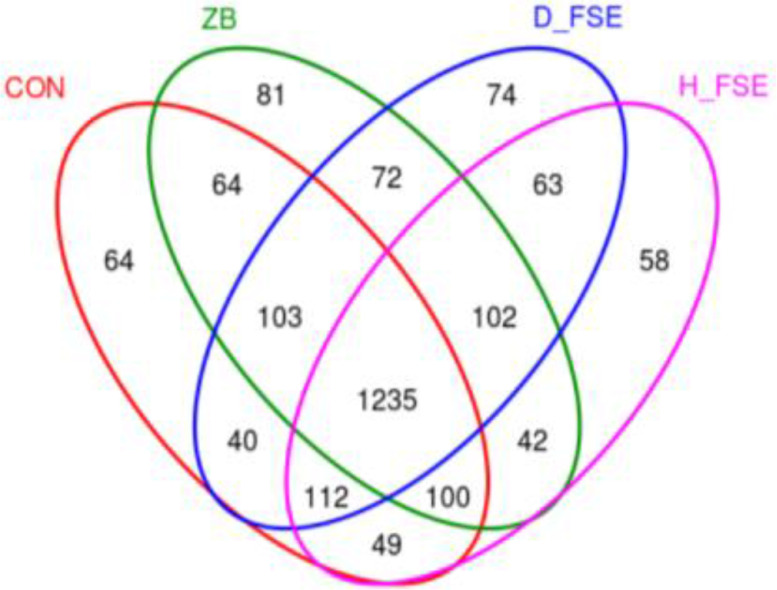
To determine the effect of FSE on cecal microbial community structure of broilers, cecum samples were analyzed by 16s rRNA analyses. From the 24 cecum samples, 1,520,682 high-quality sequences were obtained. Each sample had an average of 30,502 sequences after clustering, and based on 97% sequence similarity, 2,259 operation taxon units (OTUs) were clustered from these sequences. The Venn diagram shows the similarity and overlap of OTUs composition of samples of each group (Figure 4). The figures showed that 1,235 OTUs were shared by all samples, and 64 unique OTUs in CON group, 81 unique OTUs in ZB group, 74 and 58 unique OTUs in the D-FSE and H-FSE groups, respectively.
Figure 4.
Venn diagram of cecum microbial OTUs between different groups of broilers. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
Effects of Dietary Supplementation of FSE on the Cecum Microbial Diversity of Broilers
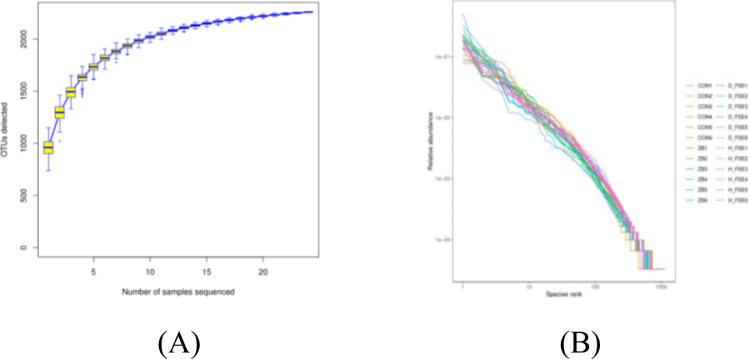
As shown in Figure 5, the species accumulation curve indicated that the samples were reasonable and sample size was enough to reflect the species richness (Figure 5A). The rank abundance curves of the wide and smooth broken lines indicated that there were high species abundance and uniform species distributions of cecum microbes (Figure 5B).
Figure 5.
Species richness and diversity analysis of broilers. (A) Species accumulation curves of cecum. (B) Rank abundance curves of cecum. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
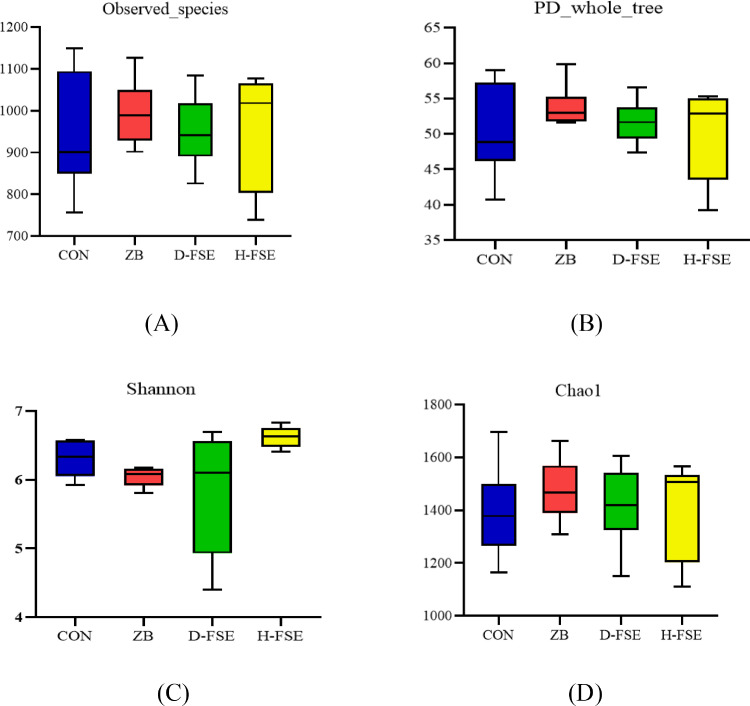
To determine whether FSE changes the species richness, the alpha diversity analysis was used and the alpha diversity index was shown in Figure 6. The observed species and PD whole tree in the CON group were lower than that of other groups (Figure 6A, B). The Shannon index showed great difference between the H-FSE group and the ZB group (P < 0.05) (Figure 6C). The Chao1 index showed a higher richness of microbial community in FSE group compared to that of the CON group (Figure 6D).
Figure 6.
The alpha diversity index of microbial in cecum of broilers. (A) Observed species in cecum. (B) PD whole tree in cecum. (C) Shannon index of cecum microbe. (D) Chao1 index of cecum microbe. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
The Microbial Community Structure in Different Levels
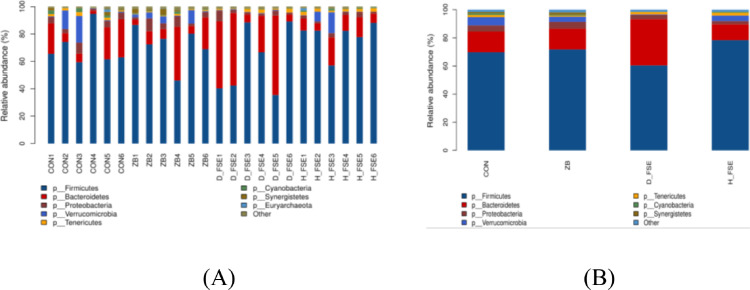
At phylum level, Firmicutes, Bacteroidetes, Verrucomicrobia, and Proteobacteria were the prime phyla in the ceca of broilers. The relative abundance of the 4 phyla reached more than 95% of all phyla detected. The relative abundance of Firmicutes accounting for more than 60% in each group, followed by Bacteroidetes, which the highest relative abundance was up to 32% (Figure 7). The ZB group had the highest abundance of Proteobacteria while the H-FSE group had lowest. The D-FSE and H-FSE groups had the highest abundance of Bacteroidetes, Firmicutes, respectively. Spirochaetes existed in the D-FSE group, Chlamydiae and Chlamydiae were present in the CON group and the ZB group, while the CON group did not have Armatimonadetes.
Figure 7.
The cecum microbe community structure of broilers at phylum level. (A) Comparing microbial differences in all broilers; (B) Comparing the microbial differences in 4 groups. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
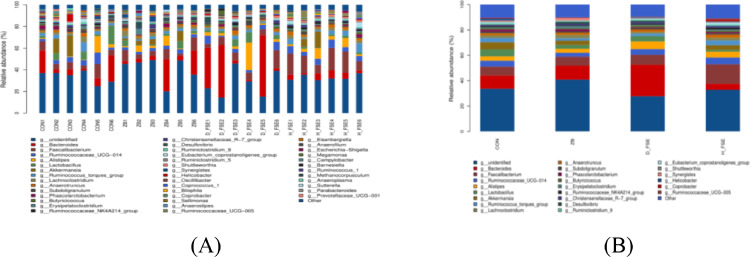
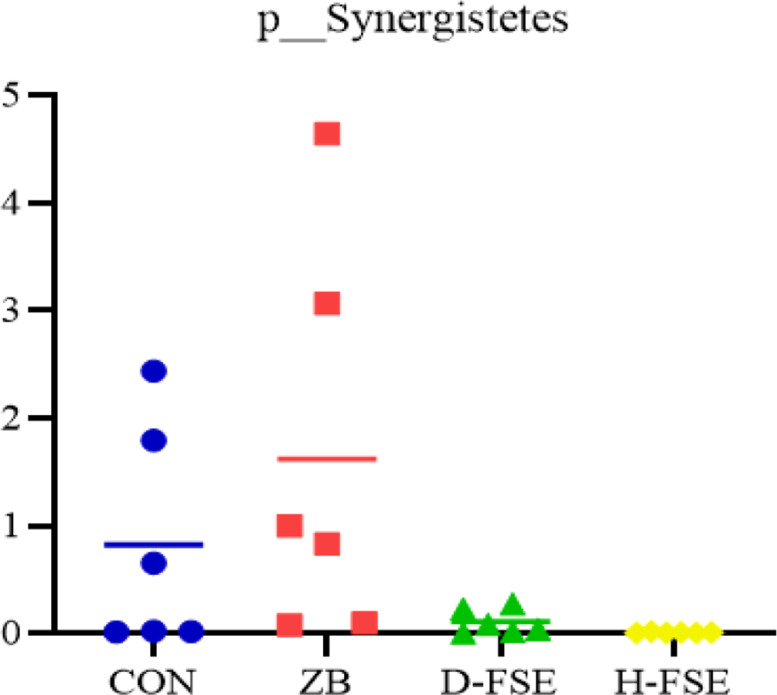
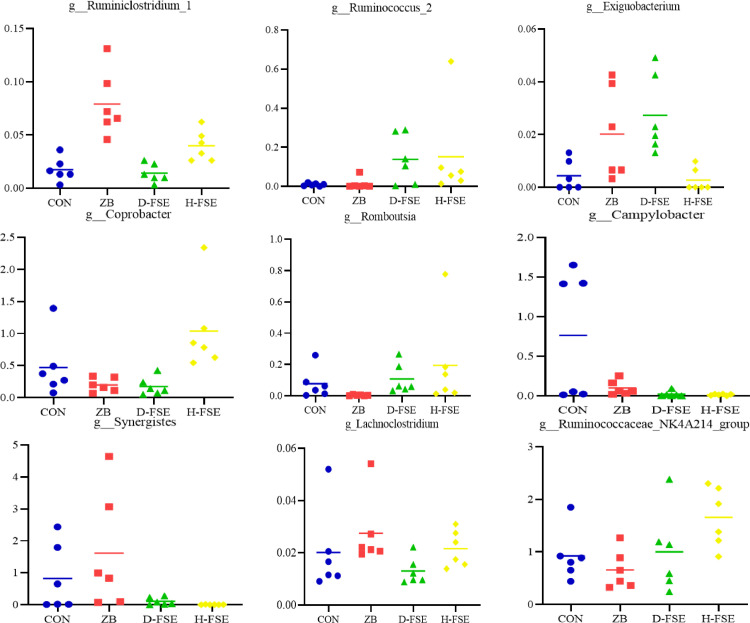
The top 10 genera at the genus level were Bacteroides, Faecalibacterium, Lactobacillus, Akkermansia, Ruminococcaceae_UCG-014, Ruminococcus_torques_group, Alistipes, Lachnoclostridium, Anaerotruncus, Phascolarctobacterium. The Bacteroides, Faecalibacterium, Lactobacillus, and Akkermansia were the main genera in CON group, Bacteroides, Faecalibacterium, Lactobacillus, and Alistipes were prime genera in ZB group, while Bacteroides, Faecalibacterium, Alistipes, and Ruminococcaceae_UCG-014 were main genera in D-FSE group, and Faecalibacterium, Ruminococcaceae_UCG-014, Bacteroides, and Alistipes were detected to dominate the microbial population in H-FSE group (Figure 8). There was changes in the structure of dominant microbial communities in the broilers cecum among treatments. By employing Kruskal-wallis and Metastats analysis, 2 phyla and 21 genera had significant differences among treatments. At phylum level, the relative abundance of Synergistetes in the FSE groups were lower than the ZB group (P < 0.05) (Figure 9). At genus level, Ruminiclostridium_1 was higher in the ZB group (P < 0.05), while Ruminococcus_2 and Exiguobacterium were found to be higher in the D-FSE group. Corprobacter and Ruminococcaceae_NK4A214 in H-FSE group were higher than that of the ZB and CON group (P < 0.05). The relative abundances of Ruminococcaceae_UCG-014, Ruminococcus_2, and Romboutsia in the FSE group were observed to be prominently higher than that of other groups (P < 0.05). Furthermore, FSE significantly reduced Campylobacter, Synergistes, and Lachnoclostridium abundance (P < 0.05) (Figure 10).
Figure 8.
The cecum microbe community structure of broilers at genus level. (A) Comparing the microbial differences in all broilers; (B) Comparing the microbial differences in 4 groups. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
Figure 9.
Comparison of cecum microbial in phylum level of broilers. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
Figure 10.
Comparison of cecal microbial in genus level of broilers. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
The Microbial Community Structure
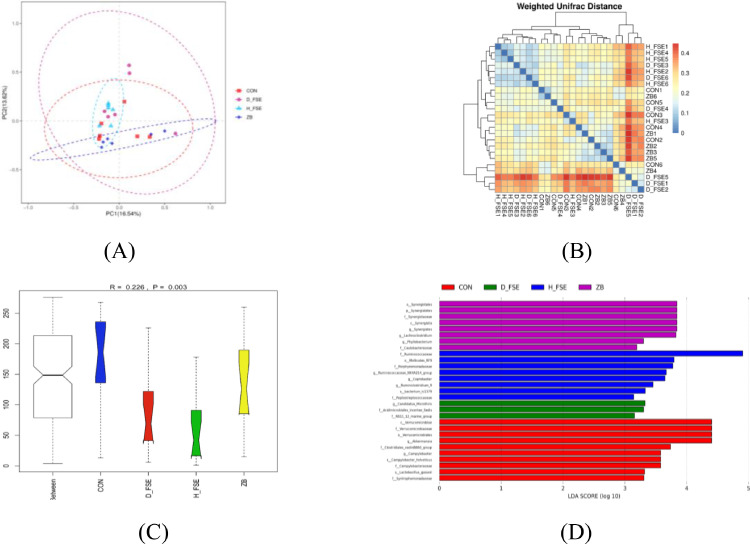
The results of PCA (Figure 11A) and Weighted unifrac distance heat-map (Figure 11B) showed obvious differences of microbial structure in broilers which was confirmed by ANOSIM analysis (Figure 11C) (P < 0.05).
Figure 11.
The microbial community structure in different groups of broilers. (A) Principal Component Analysis (PCA) of cecum microbe. (B) Weighted unifrac distance heat-map of cecum microbe. (C) Anoism analysis of cecum microbe. (D) LDA distribution histogram of cecum microbe. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
LEfSe (Linear Discriminant Analysis [LDA] Effect Size) analysis was carried out to identify taxonomic biomarkers of each group. As shown in Figure 11D, there were 10 genera enriched in the CON group, 8 genera enriched in the ZB group, 3 and 8 genera enriched in the D-FSE group and the H-FSE group, respectively (LDA > 3).
Functional Prediction of Cecum Microbes
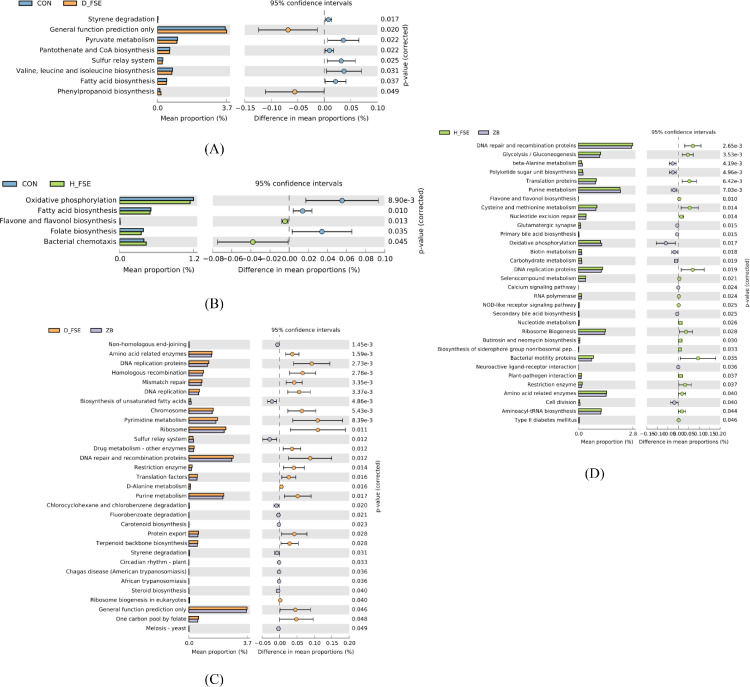
In order to predict the functional alterations of microbes in cecum, PICRUSt was performed to analyze the possible levels of KEGG pathways and its abundances. According to the prediction results, six KEGG pathways were obtained at level 1, with the highest proportion being the metabolism and genetic information processing. Fourty-one KEGG pathways were obtained at level 2, with the highest proportion being the membrane transport, carbohydrate metabolism, amino acid metabolism, replication, and repair. There were significant differences in level 3 KEGG pathway of cecum microbe among treatments (P < 0.05). There were significant difference in 8 KEGG pathways of the D-FSE group (Figure 12A) and 5 KEGG pathways of H-FSE group (Figure 12B) compared to the CON group. Significant difference was identified in 31 KEGG pathways (Figure 12C) between the ZB group and the D-FSE group, and 32 KEGG pathways (Figure 12D) were identified to be significantly different between ZB group and H-FSE group.
Figure 12.
Differences in the predicted functional meta-genomes of the cecum microbiota at KEGG level 3. (A) Comparison of the functional pathways of microbes in the CON and D-FSE groups. (B) Comparison of the functional pathways of microbes in the CON and H-FSE groups. (C) Comparison of the functional pathways of microbes in the ZB and D-FSE groups. (D) Comparison of the functional pathways of microbes in the ZB and H-FSE groups. CON group, basal diet; ZB group, basal diet + 30 mg/kg Zinc bacitracin; D-FSE group, basal diet + 50 mg/kg FSE; H-FSE group, basal diet + 100 mg/kg FSE, n = 6.
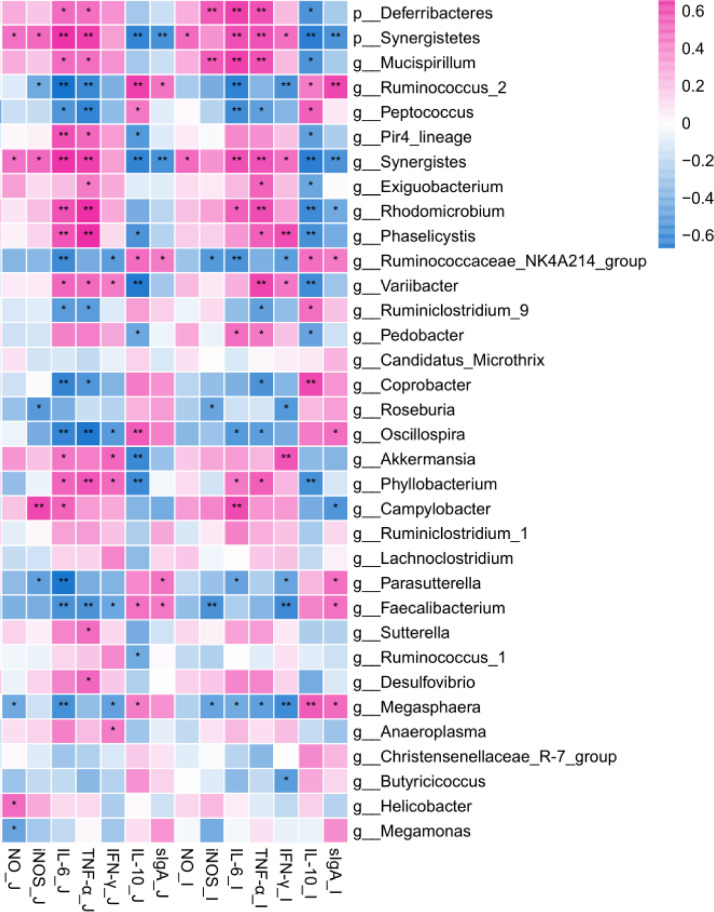
Correlation Between Cecum Microbe and Intestinal Mucosa Immune Indicators of Boilers
The association between cecum microbe and intestinal immune indicators (including intestinal mucosa cytokines and sIgA) of broilers was analyzed by using Spearman's correlation analysis (Figure 13). At the phylum level, Synergistetes was negatively associated with concentrations of IL-10 and sIgA (P < 0.01), while Deferribacteres was positively correlated with concentrations of IL-6 and iNOS (P < 0.01). At the genus level, Megasphaera, Faecalibacterium, Coprobacter, Ruminococcaceae\NK4A214, and Ruminococcus_2 was positively correlated with concentrations of anti-inflammatory cytokines and sIgA (P < 0.01). Furthermore, Campylobacter, Variibacter, Synergistes, and Phyllobacterium were positively correlated with concentrations of pro-inflammatory cytokines, NO and iNOS (P < 0.01). In current study, the data showed that cecum microbe was significantly related to inflammatory response.
Figure 13.
Heat map of cecum microbes and intestine immunity in broilers. *0.01 < P ≤ 0.05, ** meant 0.001 < P ≤ 0.01, respectively, n = 6.
DISCUSSION
The fenugreek seed extracts used in this study showed no signs of toxicity or probable death risk during the initial tissue evaluation and observation. Furthermore, due to its food and medicinal homology, fenugreek could be safely added into broiler diets. This study found that FSE supplementation significantly enhanced ADG and lowered F: G when compared to that of the ZB group and the CON group. These results are congruent with the findings of Weerasingha and Atapattu (2013) and Amein et al. (2019), who found that fenugreek inclusion in the broiler diets could increase feed conversion efficiency, enhance biological and metabolic processes, and optimize nutrients utilization. This observation can be attributed not only to the availability of essential fatty acids and high-quality proteins in fenugreek, but also to the presence of steroid saponins, which activate the hypothalamus gland, enhance food intake, and stimulate the digestive system (Toaha et al., 2016; Covarrubias et al., 2018). The current investigation also found that FSE had a considerable positive effect on the intestinal morphology of broilers. According to Qureshi et al. (2016), fenugreek seeds could increase villus height, decrease crypt depth, and enhance intestinal morphology. Furthermore, the researchers suggested that FSE could minimize pathogenic microorganisms in the gastrointestinal system, hence reducing damage to intestinal epithelial cells and increasing absorptive cells. Moreover, Abdel-Rahman et al. (2014) reported positive correlation between increased intestinal villus height of small intestine of broilers and rate of feed digestion and nutrient absorption. Salehifar et al. (2017) observed that increased villus height and decreased crypt depth provide a larger surface area for nutrient absorption. In the present study, longer villus height, lower crypt depth, and a higher ratio of villus height to crypt depth were observed in FSE groups, indicating that FSE played an important role in improving intestinal morphology, which led to increased absorption of available nutrients, enhanced feed efficiency, and improved broiler growth performance. These findings suggest that FSE is more effective than antibiotics in improving gut morphology.
The action of fenugreek on the intestinal mucosa may change the level of inflammatory cytokine expressions. FSE has been shown to reduce the expression of inflammatory cytokines and exert an anti-inflammatory effect by balancing Th1 cells and lowering the expression of Th2 cytokines in previous researches (Piao et al., 2017; Liu et al., 2019). Also, Sindhu et al. (2012) showed that fenugreek reduced concentrations of IL-6 and TNF-α, inhibit B cells activation, and play an anti-inflammatory role. In this study, broilers fed with FSE had lower levels of expression of TNF-α, IFN-γ, and IL-6, and higher concentrations of IL-10 than broilers fed with antibiotics, which indicated that FSE had an anti-inflammatory function. Moreover, FSE played a significant role in lowering the activities of NO and iNOS compared to the other groups. This observation corroborated the findings of Abdel-Daim et al. (2015), who found that fenugreek oil can lower NO content in liver, kidney, and brain tissues, prevent liver and kidney injury, and avoid inflammatory reaction aggravation. Subsequently, the broilers fed with FSE had the highest concentrations of sIgA among the 4 treatments. Bin-Hafeez et al. (2003) reported that fenugreek has an obvious immune stimulating effect, which are responsible for inducing macrophages, and improve immunity. Also, Motamedi et al. (2014) showed that fenugreek powder can increase antibody titer and IgG content of traits related to immune system, and play an immunomodulatory role in broilers immunity. In our study, FSE attenuated inflammation by decreasing concentration of proinflammatory cytokines, increasing anti-inflammatory cytokines, stimulating the secretion of antibodies, thereby, improving the immune function and disease resistance in broilers. Based on the aforementioned, our study suggested that FSE could be used as an immunopotentiator to replace the use of antibiotics.
In the present study, 16s rRNA gene Illumina MiSeq sequencing was employed to investigate the effect of FSE supplementation on cecal microbiota community composition and their correlations with metabolic pathways in broilers. The results of Venn diagram analysis showed that the FSE group had the highest diversity of cecum microbes, while CON group had the lowest. The index of Shannon in FSE groups was significantly higher than ZB group, and Chao1 index was obviously higher than the CON group which indicated that FSE increased intestinal microbe richness and diversity. This finding was consist with the observation of Bruce-Killer et al. (2020) who revealed robust and significant effects of fenugreek on gut microbiota, with alterations in both alpha and beta diversity. In present investigation, the FSE other than fenugreek seed was used, however both of them contain the main active compounds like polysaccharides and saponins which was considered as probiotic dietary modifiers and was proved to enhance beneficial intestinal bacteria (Chen et al., 2015; Deshmukh et al., 2019). Subsequently, the increased richness and diversity of intestinal microbes was analyzed for the dominating phyla. Many studies have shown that the intestinal domination of Firmicutes and Bacteroidetes phyla has positive correlation with the healthy states of the hosts (Sun et al., 2019; Xiao et al., 2020). In present study, the main structure of cecal microflora was composed of Firmicutes, Bacteroides, Verrucomicrobia, and Proteobacteria, and the relative abundance of the 4 phyla was about 95%. The Firmicutes may serve as a source of energy and have participated in the host's material and energy metabolism cycle (Liu et al., 2018). Meanwhile, the Bacteroides participated in the metabolism of carbohydrate, polysaccharide and other nutrients which promoted nutrients absorption (Ouyang et al., 2018). The Proteobacteria is a gram-negative bacterium that contains a variety of pathogens (Liu et al., 2018). Thus, the symbiotic relationship between Firmicutes and Bacteroides, with a reduced abundance of Proteobacteria, promoted the absorption of nutrients and energy storage in the host which is evident in the increased body weight gain observed in broilers fed diets supplemented with FSE (Wang et al., 2014). Interestingly, the addition of FSE altered the intestinal microbiota community structure, and regulated the intestine microecological balance, which had more beneficial effect than Zinc bacitracin.
The species abundance level of the intestinal microbiota community may be correlated with management of body health, which may be helpful or otherwise for the host animal. In the current study, at genus level, the Synergistes, Campylobacter, Lachnoclostridium, Variibacter, Pir4_lineage was discovered at lower level in FSE groups than the other groups, while the relative abundance of Ruminiclostridium_1, Coprobacter, Romboutsia, Ruminococcus_2, Ruminiclostridium_9, Ruminococcaceae_NK4A214_group were higher in FSE group. From the findings, it showed that the FSE supplementation could reduce pathogenetic genus abundance, thereby reducing intestinal mucosa inflammation, preserving intestinal health, promoting the growth of beneficial bacteria, and increasing the digestion and nutrients availability for absorption. This is because, on the one hand, Campylobacter is associated with acute bacterial enteritis, the Synergistes has been found to be positively correlated with inflammation and increasing levels of Lachnoclostridium may contribute to the susceptibility of intestinal disease (Moore et al., 2005; Hugenholtz et al., 2009; Zhou et al., 2019). Whereas, the Ruminococcaceae are connected to cellulose-degrading ability and Ruminococcus_2 can generate cellulase and have a positive correlation with dietary fiber degradation (Wu et al., 2018; Yang et al., 2019). The Corprobacter, Ruminococcaceae, and Ruminococcus 2 genera, on the other hand, have been reported to produce higher concentrations of succinates and SCFAs due to increased degradation of indigestible macromolecular carbohydrates in the hindgut of broilers, which directly improves broiler growth performance (Shkoporov et al., 2013). These indicated that FSE can promote the growth of beneficial bacteria, inhibit the proliferation of harmful bacteria, maintain intestinal health, improve growth performance of broilers.
PICRUST analysis showed that FSE diet significantly affected the pyruvate metabolism, fatty acid biosynthesis, DNA replication proteins, DNA replication, purine metabolism, and glycolysis/gluconeogenesis, which are probably related to the changes in structure of intestinal microbiota community. Also, FSE increased the functions of KEGG pathways of carbohydrate metabolism and digestive system. These microbial pathways may be related to the increased cellulolytic bacteria such as Ruminococcaceae and Ruminococcus_2. Cellulolytic bacteria degrade undigested crude fibers and carbohydrate, thereby improving the utilization of crude fiber and carbohydrates, and promote nutrients digestion and absorption (Giger-Reverdin et al., 2004). In addition, Zhang et al. (2019) reported that Parasutterella was negatively correlated with pyruvate metabolism pathway, and the Synergistes was negatively correlated with starch and sucrose metabolism, and peptidase pathway. That is, as FSE supplementation decreased the relative abundance of Synergistes and increased the relative abundance of Parasutterella in this study, it depicted that FSE up-regulated the starch and sucrose metabolism, peptidase pathway, and down-regulated pyruvate metabolism pathway. A large amount of glucose is produced through the starch and sucrose metabolism for absorption and utilization by the body, which could provide energy and carbon source for body metabolism (Liu et al., 2020). Overall, the cecal microbial community in broilers has many important physiological functions, and its stability plays a crucial role in their physiology and health.
Spearman's correlation analysis was carried out to explore the correlation between cecal microorganisms of broilers and intestinal immunity. In this study, we measured the expression of inflammatory factors and sIgA in the caecum to explore the potential link between them and the altered genera. We discovered that the Ruminococcaceae_NK4A214 and Ruminococcus_2 was positively related with concentration of sIgA, while Campylobacter, Synergistes were negatively related with concentration of sIgA. We also found that the higher relative abundances of Ruminococcaceae_NK4A214 and Ruminococcus_2 in the FSE groups were positively related with the concentration of sIgA, while the lower relative abundances of Campylobacter and Synergistes in FSE groups were negatively correlated with sIgA compared to the CON and ZB groups. At the same time, the Parasutterella, Peptococcus, and Faecalibacterium were positively correlated with the IL-10 expression, while Phyllobacterium, Phaselicystis, and Variibacter were positively correlated with IL-6, TNF-α, IFN-γ. Our results show that, compared to the other groups, FSE groups had a higher abundance of Parasutterella, Peptococcus, and Faecalibacterium, while Phyllobacterium, Phaselicystis, and Variibacter were lower indicating that increasing abundance of Parasutterella, Peptococcus, and Faecalibacterium would result to increased anti-inflammatory response and enhanced intestinal immunity of the host animal. These observations were in agreement with the findings of Shi et al. (2019) who reported that Faecalibacterium could inhibit inflammatory response by increasing the production of SCFAs and IL-10. Liang et al. (2019) also reported that Peptococcus could ferment proteins, peptides, and amino acids, which induced regulatory T cell differentiation and alleviate inflammatory response. Furthermore, we observed that Megasphaera and Roseburia were negatively correlated with NO, iNOS, while Synergistes were positively correlated with NO, iNOS. Patterson et al. (2017) reported that Roseburia can penetrate mucus layer and attach to host gut epithelial cell surfaces, reverse metabolic disorders, inhibit inflammatory response, and strengthen intestinal barrier function. Zhang et al. (2019) also stated that Megasphaera could downregulate expression of NO, IL-6, IL-12 and other proinflammatory factors. With the above, it indicates that FSE supplementation played an anti-inflammatory role in the intestinal mucosa by inhibiting the expression of inflammatory factors. According to this study, FSE may enhance the relative abundance of anti-inflammatory bacteria, decrease the relative abundance of proinflammatory and pathogenic bacteria, play an anti-inflammatory role, and boost broiler immunity, and thus be employed in poultry production instead of antibiotics.
CONCLUSION
This study demonstrated that dietary FSE supplementation could increase broiler ADG, improve gut health via regulating intestinal microbiota composition and metabolites, inhibiting inflammatory response, and improving body immunity. Furthermore, dietary FSE supplementation could improve the morphology of the jejunum and ileum, increase the abundance of beneficial microorganisms, while decreasing the abundance of harmful microorganisms, preserve gut health, and improve broiler growth performance. In summary, FSE has the potential to replace or partially replace the application of antibiotics in poultry production.
Acknowledgments
ACKNOWLEDGMENTS
This study was supported by National Nature Science Foundation (Grant code 31972600 and 31872991), National Natural Science Foundation of Hunan Province-China (2020JJ4364), Project of science and Technology Department of Hunan Province (2020NK4247), and Cooperation project between Hunan Agricultural University and Hunan Geneham Pharmaceutical Co., Ltd (2019xny-js065). The authors declare that they have no competing interests.
DISCLOSURES
We declare that there's no financial/personal interest or belief that could affect our objectivity, and potential conflicts don't exist.
REFERENCES
- Abd E.S.A., Ghally K.A., Shoulkamy M.O. Effect of fenugreek and yeast additions to Japanese quail diet on digestibility and economical responses. Acta Sci. Nutr. Healthy. 2019;3:78–82. [Google Scholar]
- Abdallah A., Zhang P., Zhong Q.Z., Sun Z.W. Application of Traditional Chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr. Drug Metab. 2019;19:54–64. doi: 10.2174/1389200219666180523102920. [DOI] [PubMed] [Google Scholar]
- Abdel-Daim M.M., Eldaim M.A.A., Hassan A.G.A. Trigonella foenum-graecum ameliorates acrylamide-induced toxicity in rats: roles of oxidative stress, proinflammatory cytokines, and DNA damage. Biochem. Cell Biol. 2015;93:192–198. doi: 10.1139/bcb-2014-0122. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman H.A., Fathallah S.I., Helal M.A., Nafeaa A.A., Zahran I.S. Effect of turmeric (curcuma longa), fenugreek (trigonella foenum-graecum L.) and/or bioflavonoid supplementation to the broiler chicks diet and drinking water on the growth performance and intestinal morphometeric parameters. Global Vet. 2014;12:627–635. [Google Scholar]
- Ahmed E.S., Fatma A., Osama A., Mohamed E.B. Fenugreek (Trigonella foenum graceum) extract mitigates the cyclophosphamide induced immunosuppression, oxidative stress and genotoxicity in rats. Aust Vasc Access Soc. 2017;4:110–132. [Google Scholar]
- Alagawany M., Abd El-Hack M.E., Farag M.R., Sachan S., Karthik K., Dhama K. The use of probiotics as eco-friendly alternatives for antiiotics in poultry nutrition. Environ. Sci. Pollut. Res. 2018;25:10611–10618. doi: 10.1007/s11356-018-1687-x. [DOI] [PubMed] [Google Scholar]
- Alloui N., Aksa S.B., Alloui M.N., Ibrir F. Utilization of fenugreek (Trigonellafoenum-graecum) as growth promoter for broiler chickens. J. World's Poult. Res. 2012;2:25–27. [Google Scholar]
- Amein S.M., Mosaad G.M.M., Hussein M.K. Effect of some medicinal plants as feed additives on growth performance, blood constituents and carcass characteristics of broilers. J. Adv. Vet. Res. 2019;9:170–177. [Google Scholar]
- Bae M.J., Shin H.S., Choi D.W., Shon D.H. Antiallergic effect of Trigonella foenum-graecum L. extracts on allergic skin inflammation induced by trimellitic anhydride in BALB/c mice. J. Ethnopharmacol. 2012;144:514–522. doi: 10.1016/j.jep.2012.09.030. [DOI] [PubMed] [Google Scholar]
- Begum M., Hossain M.M., Kim I.H. Effects of fenugreek seed extract supplementation on growth performance, nutrient digestibility, diarrhoea scores, blood profiles, faecal microflora and faecal noxious gas emission in weanling piglets. J. Anim. Physiol. Anim. Nutr. 2016;100:1121–1129. doi: 10.1111/jpn.12496. [DOI] [PubMed] [Google Scholar]
- Bengtsson B., Wierup M. Antimicrobial resistance in Scandina via after ban of antimicrobial growth promoters. Anim. Biotechnol. 2006;17:147–156. doi: 10.1080/10495390600956920. [DOI] [PubMed] [Google Scholar]
- Bin-Hafeez B., Haque R., Parvez S., Pandey S., Sayeed I., Raisuddin S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int. Immunopharmacol. 2003;3:257–265. doi: 10.1016/S1567-5769(02)00292-8. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller A.J., Richard A.J., Fernandez-Kim S.O., Ribnicky D.M., Salbaum J.M., Newman S., Carmouche R., Stephens J.M. Fenugreek counters the effects of high fat diet on gut microbiota in mice: Links to metabolic benefit. Sci. Rep. 2020;10:1245. doi: 10.1038/s41598-020-58005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Tai C.S., Hsiao W.L. Dietary saponins from four popular herbal tea exert prebiotic-like effects on gut microbiota in C57BL/6 mice. J. Funct. Foods. 2015;;17:892–902. [Google Scholar]
- Chen Q., Zhuying L., Jian-hua H., Yurong Z., Xiaosong W. Achyranthes bidentata polysaccharide enhances growth performance and health status in weaned piglets. Food Agri. Immunol. 2011;22:17–29. [Google Scholar]
- Covarrubias D.A.S., Vezquez H.C., Arevalo J.C.S., Amaya J.C., Martha A.C.V., Luna F.P., El-Sayed A., Castaneda E.S. Effect of feeding Trigonella foenum-graecum on growth performance of broiler chicks. J. Anim. Vet. Adv. 2018;17:39–44. [Google Scholar]
- Deshmukh A.R., Gupta A., Kim B.S. Ultrasound assisted green synthesis of silver and iron oxide nanoparticles using fenugreek seed extract and their enhanced antibacterial and antioxidant activities. BioMed. Res. Int. 2019;2019 doi: 10.1155/2019/1714358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Met. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Gad M., El-Sawalhi M.M., Ismail M.F., El-Tanbouly N.D. Biochemical study of the anti-diabetic action of the Egyptian plants Fenugreek and Balanites. Mol. Cell. Biochem. 2006;281:173–183. doi: 10.1007/s11010-006-0996-4. [DOI] [PubMed] [Google Scholar]
- Giger-Reverdin S., Sauvanr D., Tessierl J., Bertin G., Morand-Fehrl P. Effect of live yeast culture supplementation on rumen fermentation in lactating dairy goats. S. Afr. J. Anim. Sci. 2004;34:59–61. [Google Scholar]
- Goyal S., Gupta N., Kumar A., Chatterjee S. Antibacterial, anticancer and antioxidant potential of silver nanoparticles engineered using Trigonella foenum-graecum seed extract. IET Nanobiotechnol. 2018;12:526–533. doi: 10.1049/iet-nbt.2017.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola F.A., Bahi A., Esteban M.A. Effects of dietary administration of fenugreek seeds on metabolic parameters and immune status of gilthead seabream (Sparus aurata L.) Fish Shellfish Immunol. 2018;74:372–379. doi: 10.1016/j.fsi.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Hugenholtz P., Hooper S.D., Kyrpides N.C. Focus: Synergistetes. Environ. Microbiol. 2009;11:1327–1329. doi: 10.1111/j.1462-2920.2009.01949.x. [DOI] [PubMed] [Google Scholar]
- Jiang M.H., Zhu L., Jiang J.G. Immunoregulatory actions of polysaccharides from Chinese herbal medicine. Expert Opin. Ther. Targets. 2010;14:1367–1402. doi: 10.1517/14728222.2010.531010. [DOI] [PubMed] [Google Scholar]
- Lan R.X., Kim I.H. Effects of Bacillus licheniformis and Bacillus subtilis complex on growth performance and faecal noxious gas emissions in growing-finishing pigs. J. Sci. Food Agri. 2019;99:1554–1560. doi: 10.1002/jsfa.9333. [DOI] [PubMed] [Google Scholar]
- Liang Y.N., Yu J.G., Zhang D.B., Zhang Z., Ren L.L., Li L.H., Wang Z., Tang Z.S. Indigo naturalis ameliorates dextran sulfate sodium-induced colitis in mice by modulating the intestinal microbiota community. Molecular. 2019;24:4086–4103. doi: 10.3390/molecules24224086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.Y., Hsu D.Z., Periasamy S., Chien S.P. Dietary fenugreek attenuates dextran sodium sulfate-induced ulcerative colitis: role of inflammation. Jpn. J. Gastroenterol Hepatol. 2019;1:1–9. [Google Scholar]
- Liu Y., He J.W., Yan Y.T., Liu A.M., Zhang H.Q. Comparative transcriptomic analysis of two rice (oryza sativa L.) male sterile line seed embryos under accelerated aging. Plant Mol. Biol. Rep. 2020;2020:1–12. [Google Scholar]
- Liu Z.Y., Wang X.L., Ou S.Q., Arowolo M.A., Hou D.X., He J.H. Effects of achyranthes bidentata polysaccharides on intestinal morphology, immune response, and gut microbiome in yellow broiler chickens challenged with Escherichia coli K88. Polym. 2018;10:1–15. doi: 10.3390/polym10111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F.R., Cai Q., Zafar M.I., Cai L. 4-hydroelectricity improves hepatic insulin resistance by restoring glycogen synthesis in vitro. Int. J. Clin. Exp. Med. 2015;8:8626–8633. [PMC free article] [PubMed] [Google Scholar]
- Mcewen S.A. Antibiotic use in animal agriculture: what have we learned and where are we going? Anim. Biotechnol. 2006;17:239–250. doi: 10.1080/10495390600957233. [DOI] [PubMed] [Google Scholar]
- Ministry of Agricultural of the People's Republic of China . Chinese Agricultural Press; Beijing: 2004. Feeding Standard of Chicken. [Google Scholar]
- Moore J.E., Corcoran D., Doolegy J.S.G., Fanning S., Lucey B., Matsuda M., McDowell D.A., Mégraud F., Millar B.C., O'Mahony R., O'Riordan R., O'Rourke M., Rao J.R., Rooney P.J., Sails A., Whyte P. Campylobacter. Vet. Res. 2005;36:351–382. doi: 10.1051/vetres:2005012. [DOI] [PubMed] [Google Scholar]
- Mozhdeh S., Mohammadi A., Nabavizadeh S.S., Faridi P., Babaei A.M. Comparison of the efficacy of oral fenugreek seed extract and azithromycin in the treatment of acne vulgaris:a randomized, triple-blind controlled pilot clinical trial. Iranian J. Dermatol. 2019;22:58–64. [Google Scholar]
- Munyaka N., Eissa N., Bernastein C.N., Khafipour E., Ghia J.E. Antepartum antibiotic treatment increases offspring susceptibility to experimental colitis: a role of the gut microbiota. Plos One. 2015;10 doi: 10.1371/journal.pone.0142536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasim, K., Yousefi Asli M., Masoumeh A., Adeli Mirzaie A., Mohammad Mortazavian A. Fenugreek: potential applications as a functional food and nutraceutical. Nutr. Food Sci. Res. 2016;3:3–16. [Google Scholar]
- Nathiya S., Durga M., Devasena T. Therapeutic role of trigonella foenum-graecum (Fenugreek) - a review. Int. J. Pharmac. Sci. Rev. Res. 2014;27:74–80. [Google Scholar]
- Omonijo F.A., Ni L.J., Gong J., Wang Q., Lahaye L., Yang C.B. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018;4:126–136. doi: 10.1016/j.aninu.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang E.M., Lu Y., Ouyang J.T., Wang L.L., Wang X.H. Performance and dynamic characteristics of microbial communities in multi-stage anaerobic reactors treating gibberellin wastewater. J. Biosci. Bioengin. 2018;127:318–325. doi: 10.1016/j.jbiosc.2018.05.017. [DOI] [PubMed] [Google Scholar]
- Pandey H.., Awasthi P. Effect of processing techniques on nutritional composition and antioxidant activity of fenugreek (Trigonella foenum-graecum) seed flour. J. Food Sci. Technol. 2015;52:1054–1060. doi: 10.1007/s13197-013-1057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.H., Sung J.H., Kim E.J., Chung N.H. Berberine induces apoptosis via ROS generation in PANC-1 and MIA-PaCa2 pancreatic cell lines. Braz. J. Med. Bol. Rs. 2015;48:111–119. doi: 10.1590/1414-431X20144293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson A.M., Mulder I.E., Travis A.J., Lan A., Cerf-bensussan N., Gaboriau-routhiau V., Garden K., Logan E., Delday M.I., Coutts A.G.P., Monnais E. Human gut symbiont Roseburia hominis promotes and regulates innate immunity. Front. Immunol. 2017;8:1–14. doi: 10.3389/fimmu.2017.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao C.H., Bui T.T., Song C.H., Shin H.S., Shon D.H., Chai O.H. Trigonella foenum-graecum alleviates airway inflammation of allergic asthma in ovalbumin-induced mouse model. Biochem. Biophys. Res. Commun. 2017;482:1284–1288. doi: 10.1016/j.bbrc.2016.12.029. [DOI] [PubMed] [Google Scholar]
- Qureshi S., Banday M.T., Shakeel I., Adil S., Mir M.S., Beigh Y.A., Amin U. Histomorphological studies of broiler chicken fed diets supplemented with either raw or enzyme treated dandelion leaves and fenugreek seeds. Vet. World. 2016;9:269–275. doi: 10.14202/vetworld.2016.269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramulu P., Giridharan N.V., Udayasekhararao P. Hypolipidemic effect of soluble dietary fiber (galactomannan) isolated from fenugreek seeds in wnin (GR-Ob) obese rats. J. Med. Pant Res. 2011;5:4804–4813. [Google Scholar]
- Salehifar E., Abbasi M., Bahari-Kashani R. Effects of myrtle (myrtus communis) essential oil on growth performance, carcass characteristics, intestinal morphology, immune response and blood parameters in broiler chickens. J. Lives. Sci. 2017;8:63–71. [Google Scholar]
- Schloss P.D., Gevers D., Westcott S.L. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS One. 2011;6:e27310. doi: 10.1371/journal.pone.0027310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z.Y., Qiu Y.H., Wang J.C., Fang Y.H., Zhang Y., Chen H.X., Du Q., Zhao Z.Y., Yan C., Yang M., Zhou H.Y. Dysbiosis of gut microbiota in patients with neuromyelitis optica spectrum disorders: a cross sectional study. J. Neuroimmunol. 2019;339:1–35. doi: 10.1016/j.jneuroim.2019.577126. [DOI] [PubMed] [Google Scholar]
- Shkoporov A.N., Khokhlova E.V., Chaplin A.V., Kafarskaia L.I., Nikolin A.A., Polyakov V.Y., Shcherbakova V.A., Chernaia Z.A., Efimov B.A. Coprobacter fastidiosus gen. nov., sp. nov., a novel member of the family Porphyromonadaceae isolated from infant faeces. Int. J. Syst. Evol. Microbiol. 2013;63:4181–4188. doi: 10.1099/ijs.0.052126-0. [DOI] [PubMed] [Google Scholar]
- Sindhu G., Ratheesh M., Shyni G.L., Nambisan B., Helen A. Anti-inflammatory and antioxidative effects of mucilage of Trigonella foenum graecum (Fenugreek) on adjuvant induced arthritic rats. Int. Immunopharmacol. 2012;12:205–211. doi: 10.1016/j.intimp.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Sun L., Jia H.M., Li J.J., Yu M., Yang Y., Tian D., Zhang H.W., Zou M. Cecal gut microbiota and metabolitesmight contribute to the deversity of acute myocardial ischemia by impacting the intestinal permeability, oxidative stress, and energy metabolism. Front. Microbiol. 2019;10:1–13. doi: 10.3389/fmicb.2019.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toaha S.M., Mollah B.R., Ahammad M.U. Use of dietary fenugreek(Trigonella foenum-graecum L.) seed for the production of safe broiler lean meat. Res. Agri. Livest. Fish. 2016;3:305–314. [Google Scholar]
- Turgut S.S., Soyer A., Isikci F. Effect of pomegranate peel extract on lipid and protein oxidation in beef meatballs during refrigerated storage. Meat Sci. 2016;116:126–132. doi: 10.1016/j.meatsci.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Uemura T., Goto T., Kang M.S., Mizoguchi N. Diosgenin, the main aglycon of fenugreek, inhibits LXRalpha activity in HepG2 cells and decreases plasma and hepatic triglycerides in obese diabetic mice. J. Nutr. 2011;141:17–23. doi: 10.3945/jn.110.125591. [DOI] [PubMed] [Google Scholar]
- Wang J.H., Bose S., Kim G.C., Hong S.U., Kim J.H., Kim J.E., Kim H. Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota. PLoS One. 2014;9:e86117. doi: 10.1371/journal.pone.0086117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Sheng H.F., He Yan., Wu J.Y., Jiang Y.X., Tam N., Fung Y., Zhou H.W. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012;78:8264–8271. doi: 10.1128/AEM.01821-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasingha A.S., Atapattu N.S.B.M. Effects of fenugreek (Trigonella foenum-graecum L.) seed powder on growth performance, visceral organ weight, serum cholesterol levels and the nitrogen retention of broiler chicken. Trop. Agri. Res. 2013;24:289–295. [Google Scholar]
- Wu W., Xiao Z., An W., Dong Y., Zhang B., Smidt H. Dietary sodiumbutyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao E.R., Zhou Y., Xu D., Lu R., Chen Y.H., Zhou Q.H., Wu Z.B. The physiological response of Arundo donax and characteristics of anodic bacterial community in BE-CW systems: effects of the applied voltage. Chem. Eng. J. 2020;380:1–12. [Google Scholar]
- Xu D., Li W., Huang Y., He J., Tian Y. The effect of selenium and Polysaccharide of Atractylodes macrocephala Koidz. (PAMK) on immune response in chicken spleen under heat stress. Biol. Trace Elem. Res. 2014;160:232–237. doi: 10.1007/s12011-014-0056-y. [DOI] [PubMed] [Google Scholar]
- Yang G., Yin Y., Wang J. Microbial community diversity during fermentative hydrogen production inoculating various pretreated cultures. Int. J. Hydrogen Energy. 2019;44:13147–13156. [Google Scholar]
- Yasmeen R., Shashikumar J.N. Fenugreek (Trigonellafoenum-graecum) and its antimicrobial activity -a review. Int. J. Curr. Microbiol. Appl. Sci. 2019;8:710–714. [Google Scholar]
- Yu Q., Fang C., Ma Y., He S., Ajuwon K.M., He J. Dietary resveratrol supplement improves carcass traits and meat quality of Pekin ducks. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.Y., Liu H., Wang S.X., Zhang W., Wang J., Tian H.W., Wang Y.M., Ji H.F. Fecal microbiota and its correlation with fatty acids and free amino acids metabolism in piglets after a Lactobacillus Strain oral administration. Front. Microbiol. 2019;10:785–798. doi: 10.3389/fmicb.2019.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Ding, R.l., Jiang S.X., Ji L.W., Pan M.M., Liu L., Zhang W., Gao X.G., Huang W.G., Zhang G.J., Peng L., Jia H. The adjuvanticity of Ganoderma lucidum polysaccharide for Newcastle disease vaccine. Int. J. Biol. Macromol. 2014;65:431–435. doi: 10.1016/j.ijbiomac.2014.01.067. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Chen L., Sun G.F., Li Y., Huang R.X. Alterations in the gut microbiota of patients with silica-induced pulmonary fibrosis. J. Occup. Med. Toxicol. 2019;14:1–11. doi: 10.1186/s12995-019-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]