Abstract
Chagas disease vector control relies on prompt, accurate identification of houses infested with triatomine bugs for targeted insecticide spraying. However, most current detection methods are laborious, lack standardization, have substantial operational costs and limited sensitivity, especially when triatomine bug densities are low or highly focal. We evaluated the use of FTA cards or cotton-tipped swabs to develop a low-technology, non-invasive method of detecting environmental DNA (eDNA) from both triatomine bugs and Trypanosoma cruzi for use in household surveillance in eastern Colombia, an endemic region for Chagas disease. Study findings demonstrated that Rhodnius prolixus eDNA, collected on FTA cards, can be detected at temperatures between 21 and 32 °C, when deposited by individual, recently blood-fed nymphs. Additionally, cotton-tipped swabs are a feasible tool for field sampling of both T. cruzi and R. prolixus eDNA in infested households and may be preferable due to their lower cost. eDNA detection should not yet replace current surveillance tools, but instead be evaluated in parallel as a more sensitive, higher-throughput, lower cost alternative. eDNA collection requires virtually no skills or resources in situ and therefore has the potential to be implemented in endemic communities as part of citizen science initiatives to control Chagas disease transmission.
Subject terms: Molecular biology, Environmental sciences
Introduction
Chagas disease remains the most important parasitic infection in Latin America, responsible for the loss of 275,000 disability-adjusted life years (DALYs) in 20191. The geographical range of the aetiological agent, Trypanosoma cruzi, extends from the southern USA to Argentinean Patagonia, where it is transmitted by triatomine bugs (Hemiptera: Reduviidae: Triatominae) to at least eight orders of domestic, synanthropic and sylvatic mammalian hosts2,3. Human disease occurs when infected triatomine faeces enter through intact mucosa or abraded skin, causing an initial asymptomatic or non-specific self-limiting febrile illness, followed by life-long infection and potentially fatal cardiomyopathy (30–40% of infected individuals)4. Without a vaccine or highly efficacious treatment options for adults5,6, accurate detection of triatomine-infested houses and residual insecticide spraying of domestic and peri-domestic structures are crucial to prevent new cases7–9. Domiciliary populations of triatomine bugs have been successfully controlled by spraying of pyrethroid insecticides across large parts of Latin America, contributing to reductions in their distribution from an estimated 6.28 million km2 in the 1960s to less than 1 million km2 today10. Despite these achievements, the success of contemporary Chagas disease vector control programmes is threatened by persistent peri-domestic foci11,12, the emergence of insecticide resistance13–15 and household invasion from sylvatic triatomine bug populations16–20.
Chagas disease vector surveillance typically relies on identification of infested houses using timed-manual collections (TMCs), conducted by skilled personnel, with or without a dislodging spray21,22. However, this methodology is laborious and suffers from several other drawbacks, including lack of standardization, substantial operational costs and limited sensitivity, especially when infestation is highly focal and/or triatomine bug densities are very low after a recent insecticide spraying campaign23–25. Community-based bug collections or bug notifications (with or without proof of collection) performed by householders have reported similar or sometimes superior levels of sensitivity to TMCs26–28, but are prone to variability due to changes in motivation and skills of local voluntary residents23,26. Additional passive devices, including double-sided sticky traps and artificial shelter units, often supplemented with semiochemical attractants, have shown more promising results in some endemic communities29–31. Following triatomine bug collection, further incrimination of risk of T. cruzi infection requires triatomine hindgut dissection and microscopic parasite visualization, parasite isolation by haemoculturing, or direct DNA extraction from faeces and PCR-based detection methods32. In some remote endemic areas, these procedures are not feasible, resulting in an inadequate understanding of triatomine bug infection rates.
Environmental DNA (eDNA) refers to genetic material sampled from the environment rather than the organism itself33. This technique can offer a non-invasive, highly sensitive alternative for surveillance, particularly of low levels of target organisms or new invasive species found in complex environments (e.g. water, soil or air)34–36. However, the sensitivity of eDNA detection is influenced by changes in temperature, rate of DNA degradation, target species density and ultraviolet light exposure37–39. Flinders Technology Associates (FTA) cards consist of filter paper impregnated with a proprietary chemical mixture that lyses cells, inhibits overgrowth of bacteria and other microorganisms, denatures proteins and immobilises nucleic acids in a matrix, designed for long-term storage at room temperature40. In the context of vector-borne diseases, FTA cards have been used for successful preservation of different pathogens, including arboviruses41–44, Plasmodium falciparum, P. vivax and P. berghei41,45,46, Mansonella ozzardi47 Theileria pava48, Trypanosoma brucei s.l.49 and T. cruzi50,51, as well as infected vector species, such as Aedes (Ae.) aegypti52, Ae. albopictus53, Culex species54 and Forcipomyia (Lasiohelea)55. To date, the feasibility of using FTA cards for detection of triatomine bug or T. cruzi eDNA has not been assessed. Therefore, this study evaluated the use of FTA cards and cotton-tipped swabs as low-technology, cost-effective tools for simultaneous surveillance of triatomine bug and T. cruzi eDNA in the laboratory and field.
Results
This study had two components: (1) laboratory evaluation and optimisation of FTA cards to detect triatomine eDNA; (2) detection of triatomine and T. cruzi eDNA in household samples from an endemic region in eastern Colombia.
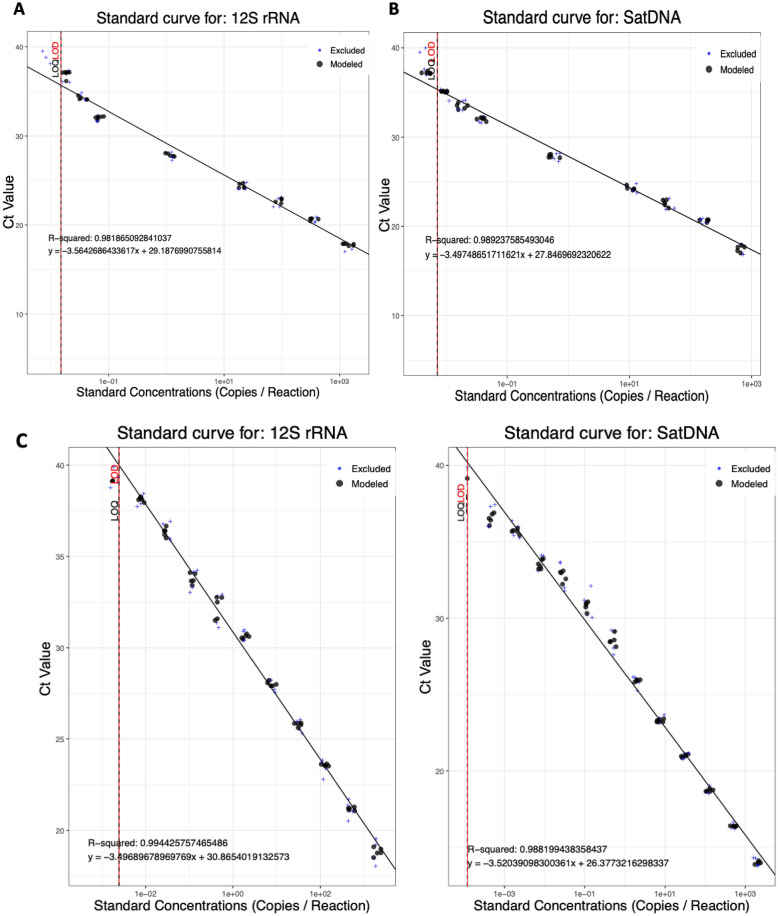
R. prolixus and T. cruzi gDNA LoD / LoQ
To estimate the analytical sensitivity, linearity and dynamic range of the two qPCR assays for eDNA detection, serial dilutions of R. prolixus and T. cruzi gDNA were tested in three independent assays, with technical triplicates per assay. Both qPCR assays produced good linearity (R2 = 0.98 and 0.99 for R. prolixus and T. cruzi assays, respectively) and efficiencies of 90.94% and 93.07%, respectively (Fig. 1A,B). The LoD/LoQs were determined to be 1.5 × 10–2 and 7.1 × 10–3 copies/reaction for R. prolixus and T. cruzi assays, respectively (Fig. 1). Next, the analytical sensitivity, linearity and dynamic range of the multiplex qPCR assay was evaluated using R. prolixus and T. cruzi gDNA serially diluted in equal proportions. Similarly, the multiplex assay was highly efficient (93.07% and 92.35% for R. prolixus and T. cruzi, respectively) with good linearities (R2 = 0.99 for detection of both R. prolixus and T. cruzi gDNA). The LoD/LoQs were determined to be 2.4 × 10–3 and 1.19 × 10–4 copies/reaction for R. prolixus and T. cruzi assays, respectively (Fig. 1C).
Figure 1.
Standard curves for individual detection of R. prolixus 12S rRNA (A), T. cruzi satellite DNA (B) and simultaneous detection of R. prolixus 12S rRNA and T. cruzi satellite DNA (C), across serial gDNA dilution series.
The R. prolixus probe was highly specific to triatomine DNA; no amplification was detected with gDNA from other vector species: Ae. aegypti, Ae. albopictus, Cx. quinquefasciatus, An. arabiensis, An. gambiae s.s., An. coluzzii, An. stephensi, An. funestus s.s., L. longipalpis and P. papatasi. Low levels of cross-reactivity (i.e. high/borderline Ct values) were observed for T. infestans and T. dimidiata gDNA. All qPCR data are reported in Supplementary file S1.
Triatomine eDNA environmental variables
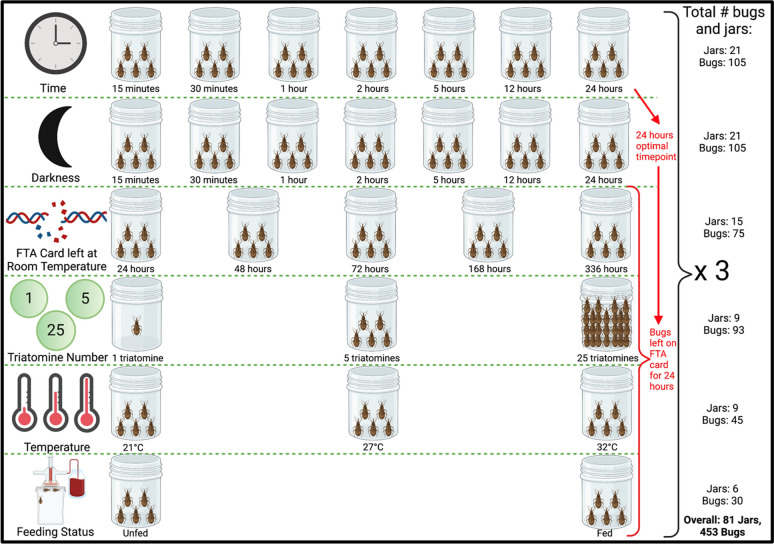
The effects of altering five different environmental variables (darkness, triatomine number, temperature, feeding status and degradation at ambient temperature) on detection of R. prolixus eDNA from FTA cards were investigated (Fig. 2).
Figure 2.
Design of experiments to investigate the impact of different environmental conditions on R. prolixus eDNA detection, including variables altered, number of jars and number of triatomines used. Figure created with BioRender.com.
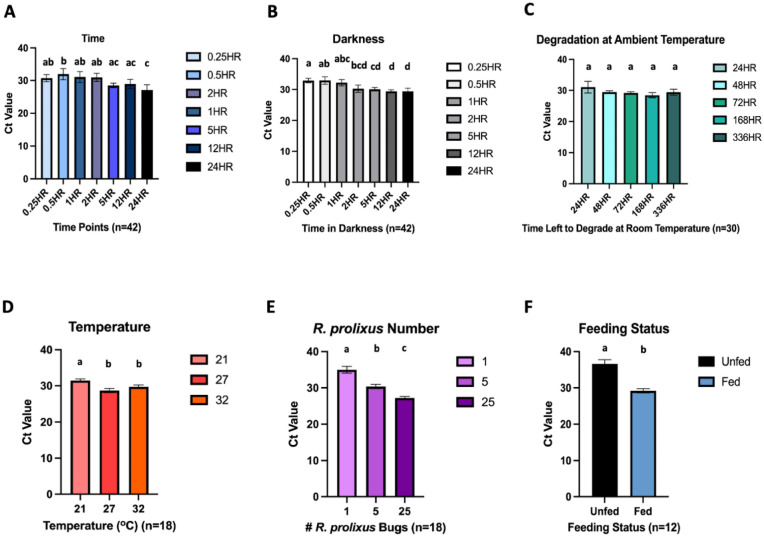
In artificial light, varying the amount of time R. prolixus 3rd/4th instars had to walk/urinate/defecate on FTA cards from 15 min to 24 h, did not substantially affect eDNA detection levels, with qPCR amplification observed at all timepoints (Fig. 3A). In general, with increasing time, Ct values decreased significantly (Fig. 3A). The average Ct values for each time point were 15 min: 30.72 [95% CI 29.66–31.78]; 30 min: 31.96 [95% CI 30.25–33.67]; 1 h: 31.05 [95% CI 29.40–32.70]; 2 h: 30.97 [95% CI 29.74–32.19]; 5 h: 28.49 [95% CI 27.80–29.17]; 12 h: 28.94 [95% CI 27.54–30.35]; and 24 h: 27.10 [95% CI 25.52–28.68]. Results from a parallel experiment, conducted in darkness, demonstrated comparable Ct values (Fig. 3B), indicating that light/darkness did not impact R. prolixus eDNA detection. The average Ct values for the darkness experiment were 15 min: 32.89 [95% CI 32.16–33.61]; 30 min: 32.92 [95% CI 31.72–34.12]; 1 h: 32.20 [95% CI 31.17–33.23]; 2 h: 30.26 [95% CI 29.09–31.42]; 5 h: 30.08 [95% CI 29.52–30.64]; 12 h: 29.39 [95% CI 28.94–29.85]; and 24 h: 29.37 [95% CI 28.34–30.41]. With the lowest Ct values, 24 h was chosen for subsequent experiments in which other environmental variables were altered.
Figure 3.
Detection of R. prolixus eDNA from FTA cards under different environmental conditions. (A) Time; (B) darkness; (C) degradation at ambient temperature; (D) temperature; (E) R. prolixus number; (F) feeding status. N = number of separate DNA extractions performed. qPCR detection for all extractions were run in technical triplicate. Conditions sharing a superscript do not differ significantly (Dunn’s multiple comparisons test, p > 0.05). Error bars indicate 95% confidence intervals (CIs).
A series of FTA cards were held at ambient temperature (mean temperature of 19.6 °C [95% CI 19.52–19.71 °C] and mean relative humidity of 62.3% [95% CI 62.08–62.48%]), once R. prolixus 3rd/4th instars had walked/urinated/defecated on them for 24 h, to determine how long triatomine eDNA takes to potentially degrade. R. prolixus eDNA was still detectable after 2 weeks, with no significant decline in detection level (Fig. 3C). The average Ct values were 24 h: 31.05 [95% CI 29.18–32.92]; 48 h: 29.44 [95% CI 29.00–29.87]; 72 h: 29.21 [95% CI 28.82–29.59]; 1 week (168 h): 28.44 [95% CI 27.60–29.28]; and 2 weeks (336 h): 29.40 [95% CI 28.44–30.37]. However, by 8 weeks R. prolixus eDNA was no longer detectable (all six DNA extractions failed to amplify during all three technical qPCR replicates).
To simulate a range of household temperatures in Chagas endemic regions, R. prolixus 3rd/4th instars were allowed to walk/urinate/defecate on FTA cards for 24 h at 21 °C, 27 °C and 32 °C (Fig. 3D), with significantly lower Ct values detected at 27 °C (average Ct value: 28.69 [95% CI 28.11–29.27]) and 32 °C (average Ct value: 29.72 [95% CI 29.20–30.24]), compared to 21 °C (average Ct value: 31.45 [95% CI 31.03–31.88]) (Kruskal–Wallis test, p < 0.0001).
A strong inverse relationship between number of R. prolixus 3rd/4th instars allowed to walk/urinate/defecate on FTA cards for 24 h was also apparent (Fig. 3E). The average Ct values for this experiment were 1 bug: 34.99 [95% CI 34.06–35.92]; 5 bugs: 30.36 [95% CI 29.77–30.95]; and 25 bugs: 27.22 [95% CI 26.77–27.66], with significantly greater levels of detection with increasing triatomine density (Kruskal–Wallis test, p < 0.0001).
Finally, R. prolixus physiological status also had a significant impact on eDNA detection, with significantly higher levels of sensitivity observed for recently blood-fed bugs (Fig. 3F). The average Ct values were unfed: 36.59 [95% CI 35.39–37.80] and fed: 29.20 [95% CI 28.60–29.79] (Mann Whitney test, p < 0.0001). Furthermore, the rate of qPCR non-amplification was higher among the unfed bug group, with three out of six DNA extractions failing to amplify during all three technical replicates; one extraction amplified once, and two extractions amplified twice.
Triatomine and T. cruzi eDNA detection from field specimens
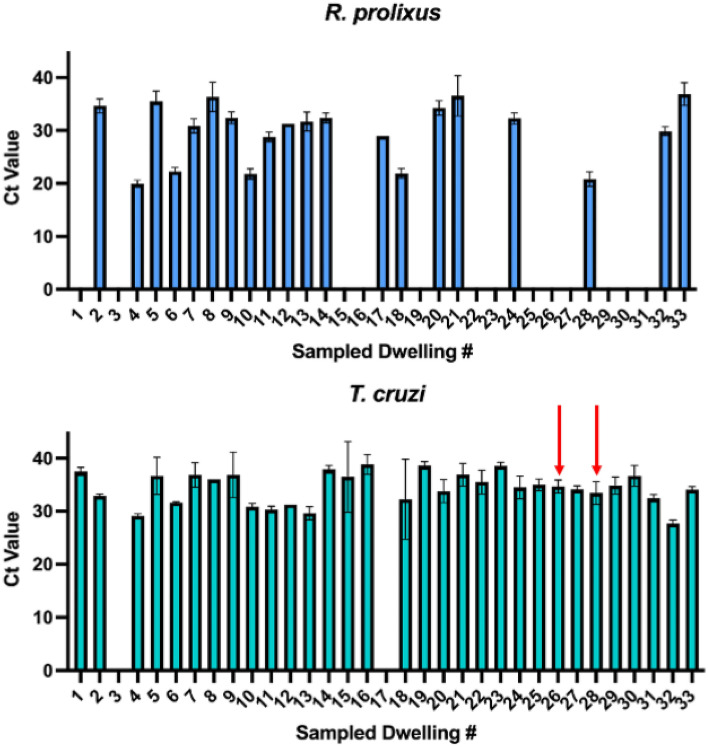
In Colombia, sixty-three houses were sampled in Prado Veraniego (n = 30), Bogotá (non-endemic for Chagas disease), and Agualinda (n = 14) and San Isidro (n = 19), Casanare (endemic for Chagas disease), for R. prolixus/T. cruzi eDNA using cotton-tipped swabs. Samples were collected from a range of domestic and peridomestic locations, including from living room walls, living room ceilings, TV room walls, kitchen walls, dining room walls, stables and hen nests (Supplementary file S2). All houses in Agualinda and San Isidro were confirmed as infested with triatomine bugs by self-reporting householders, while all houses in Prado Veraniego were reported as uninfested. Only two households in San Isidro reported human T. cruzi infection.
Among 33 households in Agualinda and San Isidro with confirmed triatomine infestations, R. prolixus eDNA was detected in 20, giving an estimated sensitivity of 60.6% (Fig. 4); average Ct value was 29.56 [95% CI 28.20–30.91]. In these same houses, T. cruzi eDNA was detected in 31, including the two houses with reported human infections (houses #26 and 28; Fig. 4); average Ct value was 34.17 [95% CI 33.56–34.78]. The average eDNA concentration for positive house swabs was 3.18 ng/μL [95% CI 1.18–5.19 ng/μL] Among 30 households in Prado Veraniego, which were negative for triatomine infestation, no R. prolixus or T. cruzi eDNA was detected, giving an estimated specificity of 100%. Finally, no R. prolixus or T. cruzi eDNA was amplified from 15 cotton-tipped swabs sampled from walls in the UK.
Figure 4.
Detection of R. prolixus and T. cruzi eDNA from cotton swabs
taken from 33 house with confirmed triatomine infestations in Casanare, Colombia. Red arrows indicate houses with reported human T. cruzi infection.
Discussion
Accurate surveillance of triatomine household infestation is crucial for Chagas disease vector control. However, no gold standard vector detection method with high levels of sensitivity or specificity is currently available. Given there are several intrinsic features of triatomine bug behaviour and the lifecycle of T. cruzi which lead to deposition of pathogen and vector eDNA in infested houses, this study evaluated the use of FTA cards or cotton-tipped swabs for simultaneous, non-invasive parasitological and entomological surveillance in eastern Colombia, an endemic region for Chagas disease.
In the proof-of-concept laboratory experiments, R. prolixus eDNA from five 3rd/4th instar nymphs was successfully isolated and amplified from FTA cards after as little as 15 min of contact time under standard insectary conditions. This indicates that FTA cards may be able to detect transient triatomine movements and lighter triatomine infestations comprising of immature vector stages; these are features of new infestations and/or vector population recovery post insecticide spraying, and are notoriously harder to measure with conventional TMCs26. While altering contact time (up to 24 h), and light/dark cycles, did not significantly improve R. prolixus eDNA detection, increasing temperature from 21 to 27–32 °C and triatomine bug density from 1 to 25 bugs, were both correlated with levels of qPCR amplification. Due to the laboratory conditions being highly artificial, the sensitivity of the FTA cards may have been overestimated by placing triatomines in smaller confined spaces than their colony jars and altering their light:dark cycles, causing them to move or defecate more due to stress. R. prolixus physiological status also impacted eDNA detection, with significantly higher levels of sensitivity observed for recently blood-fed bugs, compared to those starved for 19 weeks, suggesting that the majority of detectable eDNA is likely derived from triatomine faeces/urine compared to tarsal deposition upon contact. These findings are consistent with FTA card evaluations for other vectors and pathogens of public health importance, demonstrating direct dose responses between detection levels and target organism density41,43. Under simulated conditions of degradation, R. prolixus eDNA was shown to be stable on FTA cards for at least two weeks at room temperature, which aligns with previous studies reporting successful pathogen isolation from cards stored for several weeks under similar conditions; published observations indicate longer-term stability can be achieved when FTA cards are stored at 4 °C40.
In the field specimens, R. prolixus eDNA was detected from infested houses with an estimated sensitivity of 60.6% which is comparable with reported detection levels for TMCs, householder collections and other trapping techniques26,29,56. Given that household triatomine presence was self-reported, one explanation for the number of false negative houses may be inaccuracies in resident reports, infestation with other local triatomine species (ten triatomine species, including R. prolixus, Triatoma maculata and Panstrongylus geniculatus, have been identified in Casanare57), relative sensitivity of using cotton-tipped swabs to detect eDNA (these are not specifically optimised for DNA capture like FTA cards), or the LoD of our qPCR assay. More importantly, this technique was shown to be 100% specific in this particular field setting; false-positives in triatomine vector surveys arise due to taxonomic errors (i.e. mis-identifying non-triatomine reduviid nymphs or non-vector species), use of indirect proxies of vector infestation (e.g. triatomine bug faecal streaks which may be confused with those of other arthropods58) or when householders report vector presence without visual confirmation23. In Casanare (eastern Colombia), traditional methods are still used for entomological surveillance of R. prolixus and T. cruzi. Together these observations highlight the potential for this technique to accurately identify foci of residual triatomine infestation, which are important sources of operational failure of current Chagas disease vector control programmes59,60. In terms of implementation, the COVID-19 pandemic increased the molecular diagnostic capabilities of several laboratories nationwide, including the Casanare department (Secretaria Departamental de Salud y Unitropico). These laboratories oversee monitoring for SARS-CoV-2 and other vector borne diseases that are endemic in this department, with the potential to support implementation of the detection tools develop herein, for accurate and feasible field surveillance of Chagas disease in the region.
Interestingly, T. cruzi eDNA was amplified from 93.9% of infested houses, when only 6.06% reported human infection. We excluded possible laboratory contamination as a confounder by processing known negative controls (cotton-tipped swabs wiped on house walls in the UK) in parallel with field specimens at every analytical step. Instead, this discordance may reflect differences in relative amounts of T. cruzi and R. prolixus eDNA deposition/differential rates of eDNA degradation between species, or the gene copy number between the two targets used for qPCR detection; in our assay the LoD for the T. cruzi satellite DNA was an order of magnitude more sensitive compared to the 12S rRNA in R. prolixus. Alternatively, these findings may be indicative of active infected vectors in these houses; parasite transmission is known to be highly precarious and inefficient, requiring an estimated 900–4000 infected contacts per case61 and previous studies in Casanare have identified houses where 100% of collected Rhodnius are positive for T. cruzi62. While human infection was only self-reported in two houses, T. cruzi transmission in this area is also under-diagnosed due to substantial heterogeneity in acute symptomology57 and other significant barriers to adequate healthcare, including lack of diagnostics, infrastructure and financial investment, and limited physician awareness63. Regardless, the potential presence of residual parasite eDNA in these houses requires further investigation, including confirmation of infected vectors using a second entomological surveillance method and serodiagnosis of householders.
Findings from this study provide insights into the feasibility of using FTA cards or cotton-tipped swabs for community-level surveillance. Based on laboratory results, FTA cards could be taped to house walls for several weeks at temperatures between 21 and 32 °C and potentially detect eDNA deposited by individual, recently blood-fed R. prolixus nymphs. Field specimens further confirmed our ability to amplify R. prolixus/T. cruzi eDNA deposited on house walls in Casanare using a cheaper, lower-technology tool. By comparison to TMCs and other trapping techniques, these methodologies require virtually no skills or training and do not involve residents actively exposing themselves to potentially infectious triatomines. This surveillance strategy could be integrated into newly developed citizen science initiatives for Chagas disease, which have used social media applications and behavioural design frameworks to improve community disease awareness and reporting of house infestation64–66. In addition to field standardization, longitudinal evaluations of this methodology are needed alongside parallel TMCs and community serosurveys, to quantitatively evaluate this technique, to establish the true LoD of parasite/vector eDNA in the field using both FTA cards and cotton-tipped swabs, to determine how seasonal changes in triatomine population dynamics affect eDNA degradation and to optimise timing and logistics of eDNA wall sampling. While this study only investigated presence/absence of T. cruzi and R. prolixus, further research is warranted to assess the possibility of using recovered eDNA for community-wide blood-meal analysis, surveillance of molecular insecticide resistance and characterization of parasite and vector population genetic structures, including reinfestation dynamics after insecticide spraying campaigns13,17,67. These aspects are pivotal to the development of effective vector control programmes in Chagas disease endemic regions.
Conclusions
This study validated the use of FTA cards and cotton-tipped swabs for simultaneous entomological and parasitological Chagas disease surveillance. Study findings demonstrated that R. prolixus eDNA, collected on FTA cards, can be detected at temperatures between 21 and 32 °C, when deposited by individual, recently blood-fed 3rd/4th instar nymphs. Additionally, cotton-tipped swabs are feasible for field sampling of both T. cruzi and R. prolixus eDNA in situ and are arguably more preferable due to their lower cost. eDNA detection should not yet supplant current methods such as TMCs, but instead be evaluated alongside them as a more sensitive, higher-throughput, lower cost prospective alternative. eDNA collection can be implemented in local endemic communities as part of citizen science initiatives to monitor and control Chagas disease transmission. Further studies are needed to investigate the feasibility of using recovered eDNA for exploratory genetic analyses.
Methods
Triatomine colony maintenance
The insectary at the London School of Hygiene and Tropical Medicine (LSHTM) provided the R. prolixus for this study. They were maintained at 25 °C ± 60–80% relative humidity with 12 h:12 h light:dark cycles. This colony was derived from material sent to LSHTM from Venezuela in 1927. All R. prolixus individuals used in this study were 3rd or 4th instar nymphs. During the study period, triatomines were blood-fed with equine blood warmed through a Hemotek feeder, used for experimentation, returned to the colony, and then re-used for experimentation once every 6 weeks.
Optimising triatomine eDNA sampling
Before altering any environmental variables, which might have affected triatomine eDNA detection, an initial experiment was conducted to determine the optimal time for eDNA sampling. One hundred and five 3rd/4th instar R. prolixus were blood-fed then immediately placed in different glass jars (dimensions: opening diameter 6.5 cm; base diameter 9.5 cm; height 14 cm) in groups of 5 under standard insectary conditions (25.1–26.2 °C, 68–74% relative humidity). For eDNA sampling, QIAcard FTA classic cards (Qiagen, UK) were used. These are cards treated with FTA, a chemical which causes cell lysis and DNA immobilisation to isolate pure DNA. These were stapled to plain A4 paper and secured over the jar openings with an elastic band (Fig. 5A). The jar was then inverted so that the R. prolixus were walking, urinating, and defecating on the card. Triatomines in the 21 jars were left on the FTA cards for 7 time points: 15 min, 30 min, 1 h, 2 h, 5 h, 12 h and 24 h (Fig. 5E). Each time point was trialled in biological triplicate, i.e. three independent jars were used per time point. After each time point, the glass jars were reverted (Fig. 5C), R. prolixus returned to the colony, and the FTA cards removed and stored at − 20 °C. eDNA extraction, amplification, and detection (described below) indicated that 24 h was the optimal time point to leave the triatomines on the FTA cards to detect their eDNA. This time point was used in subsequent experiments.
Figure 5.
Photos of experimental set-ups. (A) QIAcard FTA classic card, stapled to plain A4 paper, to be secured over a glass jar opening with an elastic band; (B) bug number experiment with 25 blood-fed 3rd/4th instar R. prolixus; (C) process to invert glass jars; (D) aggregating behaviour of 3rd/4th instar R. prolixus; (E) time point assay experiment; (F) temperature experiment with incubator set to 32 °C for 24 h; (G) darkness experiment with blackout blanket over inverted jars; (H) blood-feeding the triatomines with Hemotek feeder using equine blood; (I) appearance of fed (left) vs. unfed bugs (right) in feeding status experiment.
Altering triatomine eDNA environmental variables
Next a series of experiments investigating the impact of five different environmental variables (darkness, triatomine number, temperature, feeding status and degradation at ambient temperature) on triatomine eDNA detection were performed (Fig. 2). All experiments were performed in biological triplicate. Negative controls were run concurrently in all experiments by placing FTA cards over empty glass jars. Temperature and humidity were consistently controlled in all experiments, except when temperature was the independent variable under evaluation. All experiments were performed consistently in 24 h of artificial light, except when light was the independent variable under evaluation.
Darkness
After blood-feeding 105 3rd/4th instar R. prolixus and placing 5 in each jar, the jars were inverted onto FTA cards for one time point per group: 15 min, 30 min, 1 h, 2 h, 5 h, 12 h, and 24 h, and covered by a blackout blanket to measure the effect of darkness on the quantity of eDNA sampled (Figs. 2 and 5G). Using all time points allowed us to compare whether the triatomines, which are nocturnal, would be more active in the dark than in artificial light and therefore deposit more eDNA. At the end of each time limit, bugs were returned to the colony and the FTA cards stored at − 20 °C.
Triatomine number
After blood-feeding 93 3rd/4th instar R. prolixus and placing the following numbers in each glass jar: 1, 5 and 25 (Figs. 2 and 5B,D,H), the jars were inverted onto FTA cards for 24 h. After this time, bugs were returned to the colony and FTA cards stored at − 20 °C.
Temperature
After blood-feeding 45 3rd/4th instar R. prolixus and placing 5 in each glass jar, the jars were inverted onto FTA cards for 24 h. Each of the jars were placed in different temperatures in incubators for this duration: 21 °C, 27 °C, and 32 °C (Figs. 2 and 5F). After 24 h, bugs were returned to the colony and the FTA cards stored at − 20 °C.
Feeding status
Fifteen 3rd/4th instar R. prolixus that had not blood-fed for 19 weeks were removed from the colony and 5 placed into each jar. An FTA card was secured to the opening and the jar inverted, and the bugs left for 24 h. The same was done with 15 3rd/4th instar R. prolixus which had been fed just before the FTA card was secured to the jar (Figs. 2 and 5H,I). After 24 h, bugs were returned to the colony and the FTA cards were stored at − 20 °C.
Degradation at ambient temperature
After blood-feeding 105 3rd/4th instar R. prolixus and placing 5 in each jar, the jars were inverted onto FTA card for 24 h. After this point, bugs were returned to the colony and FTA cards detached from jars and left at room temperature for the following time points: 24 h, 48 h, 72 h, 1 week, 2 weeks and 8 weeks to measure the effect of time at ambient conditions on eDNA degradation (Fig. 2). Ambient temperature and humidity were recorded hourly using an EL-USB-2 RH/temperature data logger (Lascar Electronics, UK). The FTA cards were then stored at − 20 °C until eDNA extraction.
Triatomine eDNA extraction from laboratory specimens
FTA cards from each different condition were cut in half and then into 1 × 2 cm strips using scissors sterilised between samples with 10% (v/v) bleach and 70% (v/v) ethanol. Two independent eDNA extractions were performed per FTA card. Strips from each half FTA card were placed into individual sterile 50 mL Falcon tubes (Fisher Scientific, UK), immersed in 5400 μL ATL buffer and 600 μL proteinase K (Qiagen, UK) and incubated overnight at 56 °C. eDNA was extracted from 6 mL of sample using Qiagen DNeasy 96 Blood and Tissue kits (Qiagen, UK), according to the manufacturer’s protocol.
Triatomine eDNA detection from laboratory specimens
Triatomine eDNA was detected using qPCR to amplify a fragment of the R. prolixus 12S rRNA gene. A standard curve of Ct values for this assay was generated using a tenfold serial dilution of control R. prolixus gDNA (extracted from individual colony adults), to assess PCR efficiency. Genomic DNA concentration was determined using the Qubit 4 fluorometer 1X dsDNA HS assay (Invitrogen, UK).
Standard curve reactions were performed in a final volume of 10 μL containing 2 × PrimeTime Gene Expression Master Mix (IDT, USA), 250 nM of forward (P2B 5ʹ-AAAGAATTTCCGGGTAATTTAGTCT-3ʹ) and reverse (P6R 5ʹ-GCTGCACCTTGACCTGACATT-3ʹ) primers, 150 nM of Triat probe (5ʹ-/56FAM/ TCAGAGGAA/ZEN/CCTGCCCTGTA/3IABkFQ/-3ʹ) and 2 μL genomic DNA (adapted from68). Reactions were run on a Stratagene Mx3005P Real-Time PCR system (Agilent Technologies, UK) at 95 °C for 3 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. All assays were run in technical triplicate alongside PCR no-template controls (NTCs). The maximum Ct cut-off was 40.
To confirm primer and probe specificity to R. prolixus, the TaqMan assay was used to assess amplification of available stock gDNA from Triatoma (T.) infestans, T. dimidiata, Ae. aegypti, Ae. albopictus, Cx. quinquefasciatus, Anopheles (An.) arabiensis, An. gambiae s.s., An. coluzzii, An. stephensi, An. funestus s.s., Lutzomyia (L.) longipalpis and Phlebotomus (P.) papatasi, using the same reaction conditions as the standard curve experiment.
eDNA detection in laboratory samples was performed in a final volume of 10 μL containing 2 × PrimeTime Gene Expression Master Mix (IDT, USA), 250 nM of forward and reverse primers, 150 nM of probe and 4.35 μL eDNA. Reactions were run on a Stratagene Mx3005P Real-Time PCR system (Agilent Technologies, UK) at 95 °C for 3 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. All assays were run in technical triplicate alongside PCR NTCs and R. prolixus positive controls. The maximum Ct cut-off was 40.
Household eDNA sampling and extraction
Due to the SARS-CoV-2 pandemic, we were unable to ship FTA cards to field collaborators in Colombia. Instead, between June–September 2021, cotton-tipped swabs (Guangzhou Improve Medical Instruments Co., Ltd, China) were used to sample triatomine and T. cruzi eDNA from different surfaces in houses and peri-domestic structures in Agualinda and San Isidro, municipality of Pore, Department of Casanare, Colombia (endemic for Chagas disease) and Prado Veraniego, municipality of Bogotá (non-endemic for Chagas disease) (Fig. 6; Supplementary file S2). Each dry swab was used to wipe one random 10 cm2 surface area of a selected part of the house/peri-domestic structure; roofs were not sampled because in Casanare, Rhodnius are preferentially found in the walls62. Householders completed a short questionnaire to confirm triatomine infestation and T. cruzi infection. Field workers changed gloves between each sample and individual cotton swabs were packaged in sterile, small plastic bags filled with silica gel and shipped to the LSHTM at room temperature. In addition, a series of negative control cotton-tipped swabs, collected by sampling house walls in the United Kingdom, were prepared and processed in parallel to field specimens.
Figure 6.
Houses and peri-domestic sites where eDNA was collected in Casanare Department, Colombia.
To extract eDNA, swabs were placed into individual sterile 50 mL Falcon tubes (Fisher Scientific, UK), immersed in 900 μL ATL buffer and 100 μL proteinase K (Qiagen, UK) and incubated overnight at 56 °C. eDNA was extracted from the entire sample using Qiagen DNeasy 96 Blood and Tissue kits (Qiagen, UK), according to the manufacturer’s protocol.
Triatomine and T. cruzi eDNA detection from field specimens
Parasite eDNA was detected using qPCR to amplify a fragment of the T. cruzi satellite DNA69. A standard curve of Ct values for this assay was generated using a tenfold serial dilution of control T. cruzi gDNA (strain SMA6: TcI70), to assess PCR efficiency. Genomic DNA concentration was determined using the Qubit 4 fluorometer 1X dsDNA HS assay (Invitrogen, UK).
Standard curve reactions for T. cruzi qPCR were performed in a final volume of 10 μL containing 2 × PrimeTime Gene Expression Master Mix (IDT, USA), 250 nM of forward (Cruzi 1 5ʹ-ASTCGGCTGATCGTTTTCGA-3ʹ) and reverse (Cruzi 2 5ʹ-AATTCCTCCAAGCAGCGGATA-3ʹ) primers, 150 nM of Cruzi 3 probe (5ʹ-/5HEX/TTGGTGTCC/ZEN/AGTGTGTG/3IABkFQ-3ʹ) and 2 μL genomic DNA . Reactions were run on a Stratagene Mx3005P Real-Time PCR system (Agilent Technologies, UK) at 95 °C for 3 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. All assays were run in technical triplicate alongside PCR NTCs. The maximum Ct cut-off was 40.
Standard curve reactions for simultaneous detection of R. prolixus and T. cruzi gDNA were generated using a 13-fold serial dilution of equal proportions of R. prolixus and T. cruzi gDNA, to assess PCR efficiency. Genomic DNA concentration was determined using the Qubit 4 fluorometer 1X dsDNA HS assay (Invitrogen, UK). Reactions were performed in a final volume of 10 μL containing 2 × PrimeTime Gene Expression Master Mix (IDT, USA), 250 nM of P2B, 250 nM of P6R, 150 nM of Triat probe, 250 nM of Cruzi 1, 250 nM of Cruzi 2, 150 nM of Cruzi 3 probe and 2 μL genomic DNA . Reactions were run on a Stratagene Mx3005P Real-Time PCR system (Agilent Technologies, UK) at 95 °C for 3 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. All assays were run in technical triplicate alongside PCR NTCs. The maximum Ct cut-off was 40.
Simultaneous detection of R. prolixus and T. cruzi eDNA in field samples was performed in a final volume of 10 μL containing 2 × PrimeTime Gene Expression Master Mix (IDT, USA), 250 nM of P2B, 250 nM of P6R, 150 nM of Triat probe, 250 nM of Cruzi 1, 250 nM of Cruzi 2, 150 nM of Cruzi 3 probe and 3.65 μL eDNA. Reactions were run on a Stratagene Mx3005P Real-Time PCR system (Agilent Technologies, UK) at 95 °C for 3 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. All assays were run in technical triplicate alongside PCR NTCs and R. prolixus and T. cruzi positive controls. The maximum Ct cut-off was 40. Environmental DNA concentration was determined using the Qubit 4 fluorometer 1X dsDNA HS assay (Invitrogen, UK).
Data analysis
Stratagene MxPro qPCR software (Agilent Technologies, UK) was used to produce qPCR standard curves. qPCR assay limits of detection (LoD) and limits of quantification (LoQ) were determined using the “Generic qPCR LoD/LoQ calculator”34, implemented in R version 4.0.271. All other statistical analyses were conducted in GraphPad Prism v9.2.0.
Ethics approval and consent to participate
Ethical approval for the study was obtained from the London School of Hygiene and Tropical Medicine (LSHTM; ref#25638) and the Universidad del Rosario (“Genómica, evolución y biogeografía de especies del género Rhodnius: vectores de la enfermedad de Chagas” act number 007/2016) and all study procedures were performed in accordance with relevant guidelines and regulations.
Supplementary Information
Acknowledgements
Study funding was provided by London School of Hygiene and Tropical Medicine MSc bench fees awarded to GG and a Wellcome Trust/Royal Society Sir Henry Dale Fellowship awarded to TW (101285/Z/13/Z): https://wellcome.org and https://royalsociety.org. The authors would like to thank Bethanie Pelloquin for assistance with DNA extraction.
Author contributions
G.G., P.U., L.B.G., S.B., J.D. and L.A.M. designed the study. G.G. led the laboratory experiments and performed the molecular analysis, under the supervision of L.A.M. L.B.G. and S.B. maintained the triatomine colonies and participated in data collection. P.U. and J.D. led the entomology field activities and participated in data collection. M.K. and T.W. provided laboratory resources and participated in data analysis and interpretation. L.A.M. drafted the manuscript, which was revised by co-authors. All authors read and approved the final manuscript.
Data availability
The datasets generated and/or analysed during the current study are contained within the supplementary files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-14051-x.
References
- 1.Chagas disease - Level 3 cause: Institute of Health Metrics and Evaluation. http://www.healthdata.org/results/gbd_summaries/2019/chagas-disease-level-3-cause (2019).
- 2.Vieira C, et al. Triatomines: trypanosomatids, bacteria, and viruses potential vectors? Front. Cell. Infect. Microbiol. 2018;8:405. doi: 10.3389/fcimb.2018.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen, A. M., das Chagas Xavier, S. C. & Roque, A. L. R. Landmarks of the Knowledge and Trypanosoma cruzi Biology in the Wild Environment. Front. Cell. Infect. Microbiol.10, 10 (2020). [DOI] [PMC free article] [PubMed]
- 4.Rassi AJ, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 5.Morillo CA, et al. Randomized trial of benznidazole for chronic chagas’ cardiomyopathy. N. Engl. J. Med. 2015;373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 6.Bivona, A. E., Sanchez Alberti, A., Cerny, N., Trinitario, S. N. & Malchiodi, E. L. Chagas disease vaccine design: the search for an efficient Trypanosoma cruzi immune-mediated control. Biochim. Biophys. Acta (BBA) Mol. Basis Dis.1866, 165658 (2020). [DOI] [PubMed]
- 7.World Health Organization . Integrating neglected tropical diseases. World Health Organization; 2017. [Google Scholar]
- 8.Sosa-Estani S, Segura EL. Integrated control of Chagas disease for its elimination as public health problem. Mem. Inst. Oswaldo Cruz. 2015;110:289–298. doi: 10.1590/0074-02760140408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gürtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16194–16199. doi: 10.1073/pnas.0700863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Cecere MC, Vázquez-Prokopec GM, Gürtler RE, Kitron U. Spatio-temporal analysis of reinfestation by Triatoma infestans (Hemiptera: Reduviidae) following insecticide spraying in a rural community in northwestern Argentina. Am. J. Trop. Med. Hyg. 2004;71:803–810. doi: 10.4269/ajtmh.2004.71.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurevitz JM, et al. Intensified surveillance and insecticide-based control of the Chagas disease vector Triatoma infestans in the Argentinean Chaco. PLoS Negl. Trop. Dis. 2013;7:e2158. doi: 10.1371/journal.pntd.0002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcet P, Santo-Orihuela P, Messenger LA, Vassena CV. Insights into the evolution and dispersion of pyrethroid resistance among sylvatic Andean Triatoma infestans from Bolivia. Infect. Genet. Evol. 2021;90:104759. doi: 10.1016/j.meegid.2021.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Depickere S, et al. Susceptibility and resistance to deltamethrin of wild and domestic populations of Triatoma infestans (Reduviidae: Triatominae) in Bolivia: New discoveries. Mem. Inst. Oswaldo Cruz. 2012;107:1042–1047. doi: 10.1590/S0074-02762012000800013. [DOI] [PubMed] [Google Scholar]
- 15.Piccinali RV, Fronza G, Mougabure-Cueto GA, Toloza AC. Genetic structure of deltamethrin-resistant populations of Triatoma infestans (Hemiptera: Reduviidae) in the Gran Chaco. Parasitol. Res. 2020;119:3305–3313. doi: 10.1007/s00436-020-06789-y. [DOI] [PubMed] [Google Scholar]
- 16.Ceballos LA, et al. Hidden Sylvatic foci of the main vector of chagas disease Triatoma infestans: Threats to the vector elimination campaign? PLoS Negl. Trop. Dis. 2011;5:e1365. doi: 10.1371/journal.pntd.0001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breniere SF, et al. Wild populations of Triatoma infestans are highly connected to intra-peridomestic conspecific populations in the Bolivian Andes. PLoS ONE. 2013;8:e80786. doi: 10.1371/journal.pone.0080786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breniere SF, et al. Wild populations of Triatoma infestans: Compilation of positive sites and comparison of their ecological niche with domestic population niche. Acta Trop. 2017;176:228–235. doi: 10.1016/j.actatropica.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Noireau F, Cortez MGR, Monteiro FA, Jansen AM, Torrico F. Can wild Triatoma infestans foci in Bolivia jeopardize Chagas disease control efforts? Trends Parasitol. 2005;21:7–10. doi: 10.1016/j.pt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Stevens L, et al. Migration and gene flow among domestic populations of the chagas insect vector Triatoma dimidiata (Hemiptera: Reduviidae) detected by Microsatellite Loci. J. Med. Entomol. 2015;52:419–428. doi: 10.1093/jme/tjv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schofield CJ. A comparison of sampling techniques for domestic populations of Triatominae. Trans. R. Soc. Trop. Med. Hyg. 1978;72:449–455. doi: 10.1016/0035-9203(78)90160-8. [DOI] [PubMed] [Google Scholar]
- 22.Rabinovich JE, Gürtler RE, Leal JA, Feliciangeli D. Density estimates of the domestic vector of Chagas disease, Rhodnius prolixus Stål (Hemiptera: Reduviidae), in rural houses in Venezuela. Bull. World Health Organ. 1995;73:347–357. [PMC free article] [PubMed] [Google Scholar]
- 23.Abad-Franch F, Valenca-Barbosa C, Sarquis O, Lima MM. All that glisters is not gold: Sampling-process uncertainty in disease-vector surveys with false-negative and false-positive detections. PLoS Negl. Trop. Dis. 2014;8:e3187. doi: 10.1371/journal.pntd.0003187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gürtler RE, Vázquez-Prokopec GM, Ceballos LA, Petersen CL, Salomon OD. Comparison between two artificial shelter units and timed manual collections for detecting peridomestic Triatoma infestans (Hemiptera: Reduviidae) in rural northwestern Argentina. J. Med. Entomol. 2001;38:429–436. doi: 10.1603/0022-2585-38.3.429. [DOI] [PubMed] [Google Scholar]
- 25.Gürtler RE, et al. Monitoring house reinfestation by vectors of Chagas disease: A comparative trial of detection methods during a four-year follow-up. Acta Trop. 1999;72:213–234. doi: 10.1016/S0001-706X(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 26.Abad-Franch F, Vega MC, Rolon MS, Santos WS, de Arias AR. Community participation in Chagas disease vector surveillance: systematic review. PLoS Negl. Trop. Dis. 2011;5:e1207. doi: 10.1371/journal.pntd.0001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cecere MC, Rodriguez-Planes LI, Vázquez-Prokopec GM, Kitron U, Gürtler RE. Community-based surveillance and control of chagas disease vectors in remote rural areas of the Argentine Chaco: A five-year follow-up. Acta Trop. 2019;191:108–115. doi: 10.1016/j.actatropica.2018.12.038. [DOI] [PubMed] [Google Scholar]
- 28.Dumonteil E, Ramirez-Sierra MJ, Ferral J, Euan-Garcia M, Chavez-Nuñez L. Usefulness of community participation for the fine temporal monitoring of house infestation by non-domiciliated triatomines. J. Parasitol. 2009;95:469–471. doi: 10.1645/GE-1712.1. [DOI] [PubMed] [Google Scholar]
- 29.Enriquez GF, et al. Improved detection of house infestations with triatomines using sticky traps: A paired-comparison trial in the Argentine Chaco. Parasit. Vectors. 2020;13:26. doi: 10.1186/s13071-020-3891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Arias AR, et al. Post-control surveillance of triatoma infestans and triatoma sordida with chemically-baited sticky traps. PLoS Negl. Trop. Dis. 2012;6:e1822. doi: 10.1371/journal.pntd.0001822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abrahan LB, Gorla DE, Catalá SS. Dispersal of Triatoma infestans and other Triatominae species in the arid Chaco of Argentina: Flying, walking or passive carriage? The importance of walking females. Mem. Inst. Oswaldo Cruz. 2011;106:232–239. doi: 10.1590/S0074-02762011000200019. [DOI] [PubMed] [Google Scholar]
- 32.Messenger LA, Yeo M, Lewis MD, Llewellyn MS, Miles MA. Molecular genotyping of Trypanosoma cruzi for lineage assignment and population genetics. Methods Mol. Biol. 2015;1201:297–337. doi: 10.1007/978-1-4939-1438-8_19. [DOI] [PubMed] [Google Scholar]
- 33.Thomsen PF, Willerslev E. Environmental DNA – An emerging tool in conservation for monitoring past and present biodiversity. Biol. Cons. 2015;183:4–18. doi: 10.1016/j.biocon.2014.11.019. [DOI] [Google Scholar]
- 34.Merkes C, et al. Reporting the limits of detection (LOD) and quantification (LOQ) for environmental DNA assays. Environ. DNA. 2020;2:271–282. doi: 10.5066/P9AKHU1R. [DOI] [Google Scholar]
- 35.Odero J, Gomes B, Fillinger U, Weetman D. Detection and quantification of Anopheles gambiae sensu lato mosquito larvae in experimental aquatic habitats using environmental DNA (eDNA) Wellcome Open Res. 2018;3:26. doi: 10.12688/wellcomeopenres.14193.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider J, et al. Detection of invasive mosquito vectors using environmental DNA (eDNA) from water samples. PLoS ONE. 2016;11:e0162493. doi: 10.1371/journal.pone.0162493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robson HL, et al. Fine-tuning for the tropics: application of eDNA technology for invasive fish detection in tropical freshwater ecosystems. Mol. Ecol. Resour. 2016;16:922–932. doi: 10.1111/1755-0998.12505. [DOI] [PubMed] [Google Scholar]
- 38.Barnes MA, et al. Environmental conditions influence eDNA persistence in aquatic systems. Environ. Sci. Technol. 2014;48:1819–1827. doi: 10.1021/es404734p. [DOI] [PubMed] [Google Scholar]
- 39.Pilliod DS, Goldberg CS, Arkle RS, Waits LP. Factors influencing detection of eDNA from a stream-dwelling amphibian. Mol. Ecol. Resour. 2014;14:109–116. doi: 10.1111/1755-0998.12159. [DOI] [PubMed] [Google Scholar]
- 40.Cardona-Ospina JA, Villalba-Miranda MF, Palechor-Ocampo LA, Mancilla LI, Sepulveda-Arias JC. A systematic review of FTA cards® as a tool for viral RNA preservation in fieldwork: Are they safe and effective? Prev. Vet. Med. 2019;172:104772. doi: 10.1016/j.prevetmed.2019.104772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melanson VR, et al. Improving vector-borne pathogen surveillance: A laboratory-based study exploring the potential to detect dengue virus and malaria parasites in mosquito saliva. J. Vector Borne Dis. 2017;54:301–310. doi: 10.4103/0972-9062.225834. [DOI] [PubMed] [Google Scholar]
- 42.Flies EJ, Toi C, Weinstein P, Doggett SL, Williams CR. Converting mosquito surveillance to arbovirus surveillance with honey-baited nucleic acid preservation cards. Vector Borne Zoon. Dis. 2015;15:397–403. doi: 10.1089/vbz.2014.1759. [DOI] [PubMed] [Google Scholar]
- 43.Foss L, Reisen WK, Fang Y, Kramer V, Padgett K. Evaluation of nucleic acid preservation cards for West Nile virus testing in dead birds. PLoS ONE. 2016;11:e0157555. doi: 10.1371/journal.pone.0157555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fynmore N, et al. Rapid assessment of West Nile virus circulation in a German zoo based on honey-baited FTA cards in combination with box gravid traps. Parasit. Vectors. 2021;14:449. doi: 10.1186/s13071-021-04951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hassanpour G, et al. Simplified pan-species real-time PCR-based detection of Plasmodium Spp blood smear. Iran. J. Parasitol. 2016;11:463–470. [PMC free article] [PubMed] [Google Scholar]
- 46.Brugman VA, et al. Detection of malaria sporozoites expelled during mosquito sugar feeding. Sci. Rep. 2018;8:7545. doi: 10.1038/s41598-018-26010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medeiros, J. F. et al. A field trial of a PCR-based Mansonella ozzardi diagnosis assay detects high-levels of submicroscopic M. ozzardi infections in both venous blood samples and FTA card dried blood spots. Parasit. Vectors8, 280 (2015). [DOI] [PMC free article] [PubMed]
- 48.Uchida L, et al. FTA-Sodium hydroxide-based polymerase chain reaction (PCR): An efficient and cheaper option for Theileria parva detection in dairy cattle in Mbarara Uganda. J. Vet. Med. Sci. 2020;82:188–192. doi: 10.1292/jvms.19-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed HA, MacLeod ET, Hide G, Welburn SC, Picozzi K. The best practice for preparation of samples from FTA®cards for diagnosis of blood borne infections using African trypanosomes as a model system. Parasit. Vectors. 2011;4:68. doi: 10.1186/1756-3305-4-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ballering, G. et al. in XXX Reunión Anual de la Sociedad Argentina de Protozoologia (Sociedad Argentina de Protozoologia, Resistencia, Chaco, 2018).
- 51.Aysanoa E, et al. Molecular epidemiology of trypanosomatids and trypanosoma cruzi in primates from Peru. EcoHealth. 2017;14:732–742. doi: 10.1007/s10393-017-1271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall-Mendelin S, et al. FTA cards facilitate storage, shipment, and detection of arboviruses in infected Aedes aegypti collected in adult mosquito traps. Am. J. Trop. Med. Hyg. 2017;96:1241–1243. doi: 10.4269/ajtmh.16-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honório, N. A., Câmara, D. C. P., Wiggins, K., Eastmond, B. & Alto, B. W. High-Throughput Method for Detection of Arbovirus Infection of Saliva in Mosquitoes Aedes aegypti and Ae. albopictus. Viruses12, 1343 (2020). [DOI] [PMC free article] [PubMed]
- 54.Wipf NC, et al. Evaluation of honey-baited FTA cards in combination with different mosquito traps in an area of low arbovirus prevalence. Parasit. Vectors. 2019;12:554. doi: 10.1186/s13071-019-3798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panahi E, et al. Utilising a novel surveillance system to investigate species of Forcipomyia (Lasiohelea) (Diptera: Ceratopogonidae) as the suspected vectors of Leishmania macropodum (Kinetoplastida: Trypanosomatidae) in the Darwin region of Australia. Int. J. Parasitol. Parasites Wildl. 2020;12:192–198. doi: 10.1016/j.ijppaw.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weeks ENI, et al. Community-based sampling methods for surveillance of the Chagas disease vector, Triatoma dimidiata (Hemiptera: Reduviidae: Triatominae) J. Med. Entomol. 2014;51:1035–1042. doi: 10.1603/ME14033. [DOI] [PubMed] [Google Scholar]
- 57.Rincón-Acevedo CY, et al. Clinical and epidemiological characterization of acute Chagas disease in Casanare, Eastern Colombia, 2012–2020. Front. Med. 2021;8:681635. doi: 10.3389/fmed.2021.681635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gürtler RE, Oneto ML, Cecere MC, Castañera MB, Canale DM. A simple method to identify triatomine (Hemiptera: Reduviidae) feces in sensing devices used in vector surveillance programs. J. Med. Entomol. 2001;38:147–152. doi: 10.1603/0022-2585-38.2.147. [DOI] [PubMed] [Google Scholar]
- 59.Gaspe MS, et al. Improved vector control of Triatoma infestans limited by emerging pyrethroid resistance across an urban-to-rural gradient in the Argentine Chaco. Parasit. Vectors. 2021;14:437. doi: 10.1186/s13071-021-04942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barbu CM, et al. Residual infestation and recolonization during urban Triatoma infestans bug control campaign Peru. Emerg. Infect. Dis. 2014;20:2055–2063. doi: 10.3201/eid2012.131820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nouvellet P, Dumonteil E, Gourbiere S. The improbable transmission of Trypanosoma cruzi to human: The missing link in the dynamics and control of Chagas disease. PLoS Negl. Trop. Dis. 2013;7:e2505. doi: 10.1371/journal.pntd.0002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rincon-Galvis HJ, Urbano P, Hernandez C, Ramirez JD. Temporal variation of the presence of Rhodnius prolixus (Hemiptera: Reduviidae) into rural dwellings in the Department of Casanare, eastern Colombia. J. Med. Entomol. 2020;57:173–180. doi: 10.1093/jme/tjz162. [DOI] [PubMed] [Google Scholar]
- 63.Olivera MJ, Porras Villamil JF, Toquica Gahona CC, Rodríguez Hernández JM. Barriers to diagnosis access for chagas disease in Colombia. J. Parasitol. Res. 2018;2018:4940796. doi: 10.1155/2018/4940796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Curtis-Robles R, Wozniak EJ, Auckland LD, Hamer GL, Hamer SA. Combining public health education and disease ecology research: Using citizen science to assess chagas disease entomological risk in Texas. PLoS Negl. Trop. Dis. 2015;9:e0004235. doi: 10.1371/journal.pntd.0004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buttenheim AM, et al. A behavioral design approach to improving a Chagas disease vector control campaign in Peru. BMC Public Health. 2019;19:1272. doi: 10.1186/s12889-019-7525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Otieno AW, et al. The kiss of death - unearthing conversations surrounding Chagas disease on YouTube. Cogent Soc. Sci. 2020;7:1858561. [Google Scholar]
- 67.Murillo-Solano C, et al. Diversity and interactions among triatomine bugs, their blood feeding sources, gut microbiota and Trypanosoma cruzi in the Sierra Nevada de Santa Marta in Colombia. Sci. Rep. 2021;11:12306. doi: 10.1038/s41598-021-91783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moreira OC, et al. Development of conventional and real-time multiplex PCR-based assays for estimation of natural infection rates and Trypanosoma cruzi load in triatomine vectors. Parasit. Vectors. 2017;10:404. doi: 10.1186/s13071-017-2343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piron M, et al. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop. 2007;103:195–200. doi: 10.1016/j.actatropica.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 70.Messenger LA, et al. Ecological host fitting of Trypanosoma cruzi TcI in Bolivia: mosaic population structure, hybridization and a role for humans in Andean parasite dispersal. Mol. Ecol. 2015;24:2406–2422. doi: 10.1111/mec.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.R Core Team (2021). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are contained within the supplementary files.