Abstract
Purpose
Results from adjuvant trials evaluating 6 cycles of epirubicin-based chemotherapy regimens suggested these programs may be more effective than 4 cycles of doxorubicin-based chemotherapy.
Method
NSABP B-36 was a phase III clinical trial originally designed as a 2 × 2 factorial study comparing 6 cycles of 5-FU, epirubicin, and cyclophosphamide (FEC-100) to 4 cycles of conventional doxorubicin and cyclophosphamide (AC) with celecoxib or placebo. Shortly after activation, concerns regarding increased cardiovascular risks among selective COX-2 inhibitors resulted in a decision to remove the celecoxib/placebo from the trial. Women with histologically node-negative invasive breast cancer who had undergone primary surgery with a lumpectomy or total mastectomy were eligible. Primary endpoint was disease-free survival (DFS).
Results
Between May 2004 and July 2008, 2722 patients were enrolled. Administration of FEC-100 did not result in improvement in DFS compared to AC (HR 1.09; 95% CI 0.92–1.29, p value = 0.31). The effect of FEC-100 compared to AC on DFS was significantly different for receptor-positive (HR 1.32, 95% CI 1.05–1.66) compared to receptor-negative patients (HR 0.86, 95% CI 0.66–1.11) (treatment-by-receptor status interaction p value = 0.02). There was no statistically significant difference in the effect of treatment on overall survival (OS) with FEC-100 compared to AC (HR 1.06; 95% CI 0.84–1.35, p value = 0.61). Overall, Grade 3 and 4 adverse events were more frequent in the FEC-100 group.
Conclusion
The results of B-36 do not support use of six-cycle anthracycline-based regimens in node-negative breast cancer. Prolongation of anthracycline-based therapy with FEC-100 does not improve DFS or OS, relative to AC for 4 cycles, and was associated with expected increases in toxicity. A statistically significant interaction between treatment and hormone receptor status favoring AC in hormone-receptor-positive breast cancers is consistent with the hypothesis that optimal duration of chemotherapy may be four cycles in these patients. Late cardiac events and deaths prior to recurrence or second cancer were infrequent on both arms, but slightly higher with FEC-100.
Trial registration
Keywords: Node-negative breast cancer, Anthracyclines, Duration of therapy
Introduction
Prior to the development of taxanes as a component of adjuvant chemotherapy of early breast cancer, the National Surgical Adjuvant Breast and Bowel Project (NSABP) conducted a series of trials in patients with node-positive and node-negative breast cancer comparing four cycles of doxorubicin and cyclophosphamide (AC) to six cycles of cyclophosphamide, methotrexate, 5-FU (CMF), which established AC as an alternative standard regimen to CMF. [1, 2]
A series of other studies attempting to improve outcomes relative to CMF were conducted in North America and France. The SWOG 8897 study [3] evaluated standard six-cycle schedules of cyclophosphamide, doxorubicin, 5-FU (CAF) compared to CMF in patients with high-risk, node-negative breast cancer. The regimen of CAF had similar disease-free survival (DFS) outcomes compared to CMF and only slightly better overall survival (OS) outcomes with greater toxicity. The NCIC MA5 trial [4] compared CMF to six cycles of 5-FU, epirubicin, and cyclophosphamide (FEC-120) in premenopausal women with node-positive breast cancer. There was an improvement in 10-year recurrence-free survival (RFS) with intensified FEC-120 with a trend toward improved survival, but at a cost of increased toxicity.
The French Adjuvant Study Group (FASG) 01 trial [5] demonstrated improved outcomes with six cycles of FEC-50 compared to three cycles of FEC-50 in premenopausal women with node-positive breast cancer. The subsequent FASG 05 trial [6] showed that six cycles of FEC-100 was more effective than six cycles of FEC-50 in women with node-positive breast cancer, without substantially increasing toxicity. These results suggested six cycles of anthracycline-based chemotherapy might improve outcomes relative to the shorter-duration four-cycle AC regimen. A trial comparing four versus six cycles of AC was considered but was not developed due to concerns regarding increasing risks of cardiotoxicity with cumulative doses of 360 mg/m2 in women with node-negative breast cancer.
As an alternative, based on the activity and favorable toxicity profile of the six-cycle FEC-100 program, B-36 was designed to determine whether six cycles of FEC-100 would be superior to four cycles of AC for DFS, without substantially increased toxicity in the adjuvant setting in women with node-negative breast cancer. To better understand the relative impact of the two regimens on patients we incorporated a behavioral and health outcomes (BAHO) sub-study within the B-36 clinical trial, focusing on symptoms and quality of life. We also explored if post-treatment amenorrhea would influence outcomes and assessed the impact of the two regimens on cardiac function as measured by MUGA scan and self-report measures in a sub-study.
B-36 was originally designed as a 2 × 2 factorial study, which also randomly assigned patients to receive celecoxib or placebo for three years. Shortly after activation, concerns regarding increased cardiovascular risks among selective COX-2 inhibitors resulted in a decision to remove the celecoxib/placebo from the trial, so the original rationale for addressing the activity of celecoxib is not provided in this background but is available in the Background section of the protocol included in the Supplemental Appendix.
Patients and methods
Trial design
NSABP Protocol B-36 was a phase III trial that opened for accrual May 20, 2004, as a 2 × 2 factorial study to compare six cycles of 5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2 every 3 weeks (FEC-100) with four cycles of doxorubicin 60 mg/ m2 and cyclophosphamide 600 mg/m2 every 3 weeks (AC) with celecoxib 400 mg po BID or placebo administered for three years. Suspension of accrual occurred on December 17, 2004, due to uncertainty regarding cardiovascular risks of COX-2 inhibitors identified in ongoing chemoprevention trials after enrollment of 327 patients. The trial reopened for accrual on April 25, 2005, as a two-arm study of FEC-100 vs AC following removal of celecoxib/placebo from the trial. The protocol was subsequently amended on November 22, 2005, to allow sequential administration of trastuzumab after chemotherapy for patients with HER2-positive breast cancer at investigator’s discretion. The study was closed to accrual on July 25, 2008, after 2722 women were enrolled.
Women treated with lumpectomy were required to receive breast radiotherapy after completion of their assigned chemotherapy. Administration of post-mastectomy chest wall radiotherapy was permitted at the discretion of the investigator; however, regional nodal radiotherapy was prohibited. Women with estrogen receptor-positive or progesterone receptor-positive tumors received endocrine therapy for a minimum of five years after the completion of chemotherapy. The specific agents used for endocrine therapy were left to investigator discretion.
Eligibility
Enrollment required a pathologic diagnosis of invasive adenocarcinoma of the breast. Women with histologically node-negative invasive breast cancer who had undergone primary surgery with a lumpectomy or total mastectomy were eligible. Patients were required to have undergone axillary staging procedures with sentinel node biopsy or an axillary node dissection. Patients with hormone receptor-positive or -negative disease were eligible. Staging criteria for enrollment included T1-3 by clinical and pathological evaluation. Lymph nodes obtained from axillary staging procedures were required to be pN0 according to pathological staging criteria of the AJCC Cancer Staging Manual, 6th edition. Other criteria included adequate hematological, hepatic, and renal function. Baseline left ventricular ejection fraction had to be equal to or greater than the lower limit of normal at the facility. Exclusion criteria included definitive evidence of metastatic disease, T4 tumors, bilateral breast cancer, or prior history of breast cancer (including DCIS). Patients with pure tubular or mucinous adenocarcinomas were not eligible.
Study oversight
The NSABP B-36 protocol was approved by the Central Institutional Review Board of the National Cancer Institute (NCI) and by the human investigations committee or institutional review board at each participating site, each of which has approval for human subjects research from the Department of Health and Human Services. Written informed consent was obtained from all participants.
Random assignment and stratification
Patients were randomly assigned in a 1:1 ratio to receive either six cycles of FEC-100 or four cycles of AC and were stratified by hormone receptor status (ER or PgR positive, ER and PgR negative) and type of surgery (mastectomy, lumpectomy). Assignment to the two treatment groups was balanced with respect to the stratification factors. Random assignment was based on using a biased-coin minimization algorithm [7].
Endpoints
The primary endpoint was disease-free survival (DFS), defined as time from random assignment to first local, regional, or distant recurrence, contralateral breast cancer, second primary cancer (other than squamous or basal cell carcinoma of the skin, melanoma in situ, carcinoma in situ of the cervix, or lobular carcinoma in situ of the breast), or death from any cause prior to recurrence or second primary. Secondary endpoints included OS, defined as time from random assignment to death from any cause, recurrence-free interval (RFI) defined as time from random assignment until first local, regional, or distant recurrence, and distant recurrence-free interval (DRFI) defined as time from random assignment until distant disease recurrence. Patients otherwise event-free were censored at the time of their last follow-up. Clinical assessment was required for determining patients' status for all endpoints except OS. Toxicities between the two regimens of chemotherapy FEC-100 and AC were also compared. Other secondary endpoints were components of the BAHO sub-study, including quality of life and symptoms, development of post-chemotherapy amenorrhea, and a cardiac toxicity/cardiac function [8]. Methods and outcomes of the analyses have been previously reported.
Sample Size
Following the early modification from the original 2 × 2 trial to a two-arm study, B-36 was designed to have 80% power to detect a 25% reduction in rate of DFS events with administration of FEC-100, using a 0.05 two-sided significance level. A total of 2700 patients were to be enrolled to the study. Three formal interim analyses were planned with the final definitive analyses to be performed after the report of the 385th DFS event on both treatment arms combined.
Statistical methods
Differences between the two treatment groups were evaluated by means of the log-rank test stratified for receptor status and type of surgery. The stratified Cox proportional-hazards model was used to estimate the hazard ratios and corresponding 95% CIs. The proportional-hazards assumption for Cox’s model was assessed by the method developed by Lin et al. [9]. The Cox proportional-hazards model was used to estimate and control for the effects of additional prognostic variables and treatment-by-covariate interactions were investigated.
The Kaplan–Meier method was used to estimate rates of DFS and OS. Cumulative proportions of RFI and DRFI events were estimated using the competing risks analysis based on the cumulative incidence function. Death as a first event was considered as a competing event for RFI and DRFI. The analyses reported here are based on the intent-to-treat principle, and thus include all patients with follow-up, regardless of eligibility status and of the treatment received. Patients who experienced their first non-death event within 30 days from randomization were considered not to be at risk for the primary endpoint and were excluded. All P-values for the analyses were evaluated as significant at the two-sided 0.05 level. All statistical analyses were done in SAS, v9.4. The primary analyses were presented previously in an oral session at SABCS 2014 [10]. Analyses reported here include all data received as of May 31, 2016, which was the study closure date.
Results
Patients
Between May 2004 and July 2008, 2722 patients were randomly assigned to the AC group (1361) or the FEC-100 group (1361) (Fig. 1). Patient characteristics were well balanced between the two treatment groups (Table 1). Most of all randomized patients were ≥ 50 years (60%), had lumpectomy as definitive surgery (68%), and had ER and/or PgR positive tumors (65%). Trastuzumab was administered following completion of chemotherapy to 11% of patients. Approximately 60% of patients had a primary tumor of size ≤ 2 cm and 48% of tumors had either low or intermediate tumor grade. Patients randomized to celecoxib/placebo were included in the analyses. In total, 52 patients were identified as ineligible (25 on AC and 27 on FEC-100). Ninety patients (40 on AC and 50 on FEC-100) withdrew consent to be followed while on study. There were 20 patients (5 in AC and 15 in FEC-100) without follow-up and one patient from the FEC-100 group who was considered as being not at risk for the primary endpoint excluded from the analyses. The median follow-up time for 2701 patients included in the efficacy analyses was 9.1 years.
Figure 1.
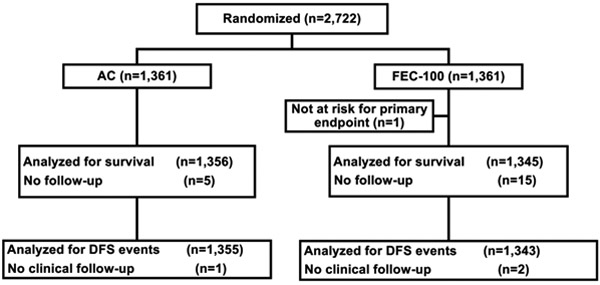
CONSORT diagram. AC, doxorubicin and cyclophosphamide; FEC-100, 5-fluorouracil, epirubicin and cyclophosphamide.
Table 1.
Patient and tumor characteristics of patients enrolled in NSABPB-36
| Characteristic | AC (n=1361) |
FEC-100 (n=1361) |
||
|---|---|---|---|---|
| n | % | n | % | |
| Age (years) | ||||
| < 50 | 548 | 40.3 | 550 | 40.4 |
| ≥ 50 | 813 | 59.7 | 811 | 59.6 |
| Race | ||||
| White | 1135 | 83.4 | 1162 | 85.4 |
| Black | 141 | 10.4 | 116 | 8.5 |
| Other | 57 | 4.2 | 60 | 4.4 |
| Unknown | 28 | 2.1 | 23 | 1.7 |
| Ethnic origin | ||||
| Hispanic | 83 | 6.1 | 97 | 7.1 |
| Non-Hispanic | 1194 | 87.7 | 1185 | 87.1 |
| Unknown | 84 | 6.2 | 79 | 5.8 |
| Hormone receptor status | ||||
| ER− and PgR− | 472 | 34.7 | 470 | 34.5 |
| ER+ and/or PgR+ | 889 | 65.3 | 891 | 65.5 |
| Surgery type | ||||
| Lumpectomy | 919 | 67.5 | 924 | 67.9 |
| Mastectomy | 442 | 32.5 | 437 | 32.1 |
| Size of primary tumor | ||||
| ≤ 2 cm | 812 | 59.7 | 833 | 61.2 |
| 2.1–5 cm | 528 | 38.8 | 501 | 36.8 |
| >5 cm | 20 | 1.5 | 26 | 1.9 |
| Unknown | 0 | 0.1 | 1 | 0.1 |
| Tumor grade | ||||
| Low | 150 | 11.0 | 136 | 10.0 |
| Intermediate | 514 | 37.8 | 513 | 37.7 |
| High | 691 | 50.8 | 704 | 51.7 |
| Unknown | 6 | 0.4 | 8 | 0.6 |
| Trastzumab therapy* | ||||
| Yes | 160 | 11.8 | 151 | 11.1 |
| No | 1195 | 87.8 | 1193 | 87.7 |
| Unknown | 6 | 0.4 | 17 | 1.2 |
AC doxorubicin and cyclophosphamide, ER estrogen receptor, FEC-100 5-fluorouracil, epirubicin, and cyclophosphamde, PgR progesterone receptor
Before the first evnet
Treatment
Among 2701 patients eligible for the efficacy analyses, 7 (0.5%) did not initiate AC and 13 (1.0%) did not initiate FEC-100. Information on treatment initiation was not available from one AC patient and two FEC-100 patients. Overall, 1315 (97%) AC patients and 1193 (88.7%) FEC-100 patients completed planned protocol treatment. There were 16 AC patients (1.2%) and 47 FEC-100 (3.5%) patients who discontinued protocol therapy early due to adverse events (AEs). In addition, 11 patients (0.8%) from the AC group and 75 (5.6%) from the FEC-100 treatment group discontinued protocol therapy because of patient withdrawal or refusal. There were 21 patients who discontinued the protocol therapy due to other reasons (AC: 6 [0.4%]; FEC-100: 15 [1.1%]).
Efficacy
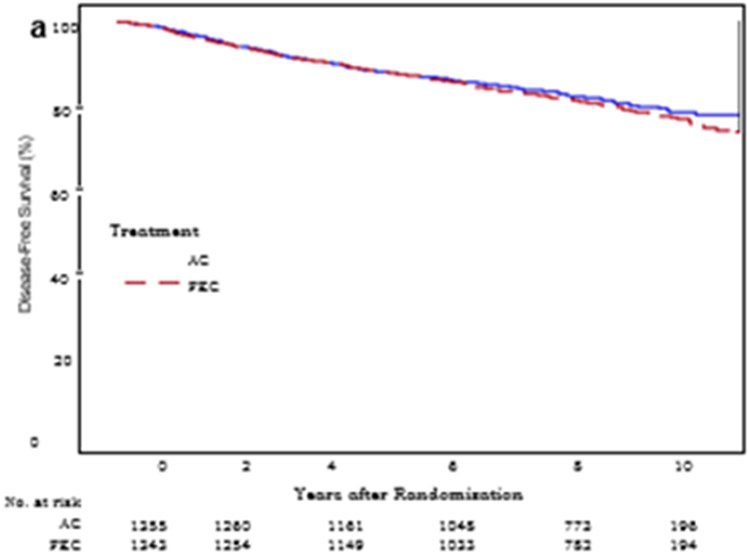
Since study entry, 521 (19.3%) women had a DFS event (251 in the AC group and 270 in the FEC-100 group) (Table 2). Administration of FEC-100 did not result in improvement in DFS compared to AC (HR 1.09; 95% CI 0.92–1.29, p value = 0.31). The type of first DFS events by treatment group are shown in Table 2. The 9-year DFS point estimates for AC and FEC-100 were 80.1% and 79.4%, correspondingly (Fig. 2a).
Table 2.
Type of first events by treatment group: NSABPB-36
| First event | AC (n=1355) |
FEC-100 (n=1343) |
||
|---|---|---|---|---|
| # | % | # | % | |
| Distant recurrence | 76 | 5.6 | 70 | 5.2 |
| Local-regional recurrence | 51 | 3.8 | 61 | 4.5 |
| Contralateral breast cancer | 37 | 2.7 | 37 | 2.8 |
| Second non-breast primary cancer | 62 | 4.6 | 68 | 5.1 |
| Death | 25 | 1.8 | 34 | 2.5 |
| Total first event | 251 | 18.5 | 270 | 20.1 |
| Alive, event free | 11.4 | 81.5 | 1073 | 79.9 |
AC doxorubicin and cyclophosphamide, FEC-100 5-fluorouracil, epirubicin, and cyclophosphamide
Figure 2.
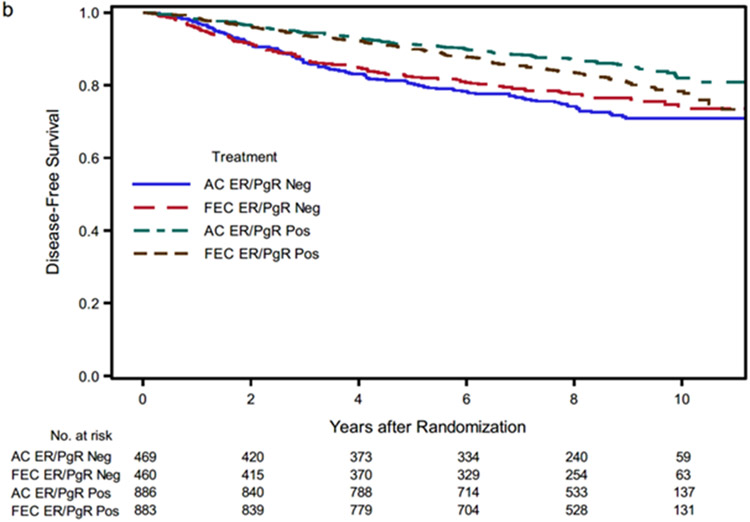
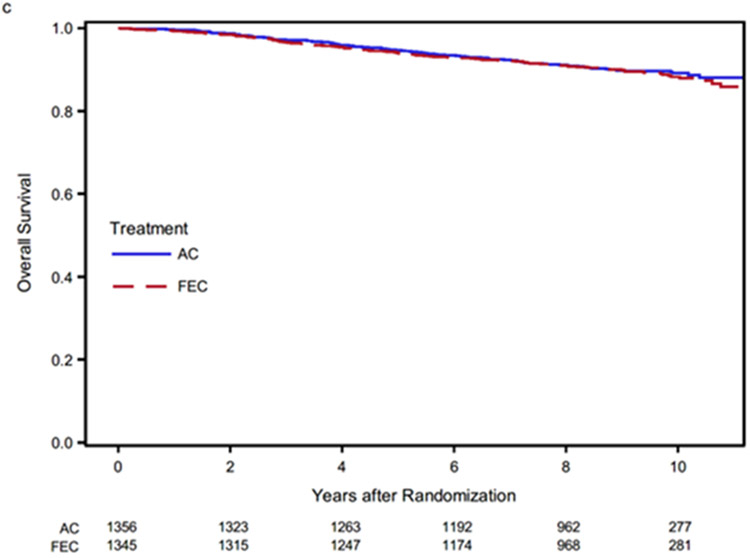
Kaplan-Meier estimates of disease-free survival. AC, doxorubicin and cyclophosphamide; FEC-100, 5-fluorouracil, epirubicin and cyclophosphamide.
The effect of FEC-100 compared to AC on DFS was significantly different for receptor-positive (HR 1.32, 95% CI 1.05–1.66) compared to receptor-negative disease (HR 0.86, 95% CI 0.66–1.11) (interaction between treatment and hormone receptor status; p value = 0.02) but was not different based on type of surgery. Additional analyses by histologic grade, tumor size, and age, also failed to demonstrate differences in DFS between FEC-100 and AC. (Supplemental Fig S1). Kaplan–Meier curves by treatment and hormone receptor status are shown in Fig. 2b. The nine-year DFS point estimates for AC and FEC-100 were 85.0% and 80.8%, respectively, in hormone receptor-positive disease and 70.9% and 76.5% in hormone receptor-negative disease. To further explore the effect of treatment according to hormone receptor status, sites of first events were tabulated by treatment and receptor status (Supplemental Table S1). Finally, the Cox model analysis demonstrated that histologic grade (low vs intermediate and high), tumor size (≤ 2 cm vs > 2 cm), and race (White vs Black) were independently associated with DFS (Table 3).
Table 3.
Results of multivariable analyese of DFS: NSABP-36
| Characteristic | No. of patients* (n=2636) |
No. (%) of DFS events | Hazards ratio (95% CI) | p value | |
|---|---|---|---|---|---|
| Hormone receptor status† | 0.02† | ||||
| ER− and PgR− | AC | 455 | 122 (26.8) | — | |
| FEC-100 | 454 | 104 (22.9) | 0.87 (0.67, 1.13) | ||
| ER+ and/or PgR+ | AC | 868 | 123 (14.2) | — | |
| FEC-100 | 859 | 161 (18.7) | 1.37 (1.07, 1.71) | ||
| Size of primary tumor | < 0.001 | ||||
| ≤2 cm | 1598 | 268 (16.8) | — | ||
| > 2 cm | 1038 | 242 (2.3) | 1.39 (1.17, 1.66) | ||
| Tumor grade | 0.01 | ||||
| Low | 278 | 31 (11.2) | — | ||
| Intermediate | 1003 | 173 (17.2) | 1.56 (1.06, 2.29) | ||
| High | 1355 | 306 (22.6) | 1.80 (1.22, 2.65) | ||
| Race | < 0.001 | ||||
| White | 2267 | 413 (18.2) | — | ||
| Black | 252 | 77 (30.6) | 1.66 (1.30, 2.13) | ||
| Other | 117 | 20 (17.1) | 0.99 (0.63, 1.55) |
AC doxorubicin and cyclophosphamide, DFS disease-free survival, ER estrogen receptor, FEC-100 5-fluorouracil, epirubicin, and cyclophosphamide, PgR progresterone receptor
62 patients were excluded due to either unknown race, histologic grade, or tumor size
P-value for the interaction between treatment and receptor status
For AC treatment group, ER+ and/or PgR+ compared to ER− and PgR−, HR 0.57, 95% CI 0.43–0.75; for FEC-100 treatment group, ER+ and/ or PgR+ compared to ER− and PgR−, HR 0.88, 95% CI 0.67–1.14
Analysis of secondary endpoints demonstrated a total of 276 RFI events (140 in the AC group and 136 in the FEC-100 group) and 201 distant recurrences (107 in the AC group and 94 in the FEC-100 group). There was no statistically significant difference in the effect of FEC-100 compared to AC on RFI (HR 0.98; 95% CI 0.78–1.24, p value = 0.88) and DRFI (HR 0.89; 95% CI 0.68–1.18, p value = 0.41). The cumulative incidence of RFI events through nine years was 10.8% in patients who received AC and 10.4% in patients who received FEC-100. The cumulative incidence of distant recurrences through nine years was 8.3% in patients who received AC and 7.2% in patients who received FEC-100.
There were 132 and 139 cumulative deaths observed in the AC and FEC-100 treatment groups, correspondingly. There was no statistically significant difference in the effect of treatment on OS with FEC-100 compared to AC (HR 1.06; 95% CI 0.84–1.35, p value = 0.61). The 9-year OS point estimates for AC and FEC-100 were 89.8% and 89.9%, correspondingly (Fig. 2c). Analyses of these endpoints by treatment arm and hormone receptor status are provided in Supplemental Table S2. Interaction tests were not statistically significant for these endpoints.
Adverse events
Among 2701 patients eligible for the efficacy analyses, information on AEs was available for 2682 (99%) patients (1349 AC; 1333 FEC-100) (Table 4). Overall, Grade 3 and 4 AEs were more frequent in the FEC-100 treatment group. There were 51 patients (4%) on the AC regimen and 98 (7%) on the FEC-100 regimen with reported Grade 4 event as their highest. For 11 patients, a Grade 5 AE was reported as the highest (3 AC; 8 FEC-100). There were seven cases of myeloid leukemia (5 AC; 2 FEC-100) and two cases of lymphoid leukemias (1 AC; 1 FEC-100) reported (among these nine patients, six were reported to have died while in follow-up).
Table 4.
Most common toxicities reported on NSABPB-36–greatest toxicity grade per patient
| Term | AC (n=1349) | FEC-100 (n=1333) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade <2 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade <2 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Overall* | 964 (71.5%) | 79 (5.9%) | 252 (18.7%) | 51 (3.8%) | 3 (0.2%) | 733 (55%) | 67 (5%) | 427 (32%) | 98 (7.4%) | 8 (0.6%) |
| Febrille neutropenia | 1330 (96.4%) | 0 (0%) | 45 (3.3%) | 4 (0.3%) | 0 (0%) | 1206 (90.5%) | 0 (0%) | 113 (8.5%) | 13 (1%) | 1 (0.1%) |
| Fatigue | 1213 (91.3%) | 70 (5.2%) | 48 (3.6%) | 0 (0%) | 0 (0%) | 1170 (87.8%) | 51 (3.8%) | 112 (8.4%) | 0 (0%) | 0 (0%) |
| Vomiting | 1287 (95.4%) | 21 (1.6%) | 41 (3%) | 0 (0%) | 0 (0%) | 1236 (92.7%) | 30 (2.3%) | 67 (5%) | 0 (0%) | 0 (0%) |
| Neutrophil count decreased | 1285 (95.3%) | 14 (1%) | 21 (1.6%) | 29 (2.1%) | 0 (0%) | 1255 (94.1%) | 20 (1.5%) | 24 (1.8%) | 34 (2.6%) | 0 (0%) |
| Nausea | 1256 (93.1%) | 58 (4.3%) | 35 (2.6%) | 0 (0%) | 0 (0%) | 1213 (91.0%) | 61 (4.6%) | 59 (4.4%) | 0 (0%) | 0 (0%) |
| Platelet count decreased | 1338 (99.2%) | 1 (0.1%) | 4 (0.3%) | 6 (0.4%) | 0 (0%) | 1270 (95.3%) | 3 (0.2%) | 30 (2.3%) | 30 (2.3%) | 0 (0%) |
| Infection and infestations–Other, specify | 1314 (97.4%) | 9 (0.7%) | 26 (1.9%) | 0 (0%) | 0 (0%) | 1276 (95.7%) | 14 (1.1%) | 41 (3.1%) | 2 (0.2%) | 0 (0%) |
| Thromboembolic event | 1326 (98.3%) | 0 (0%) | 19 (1.4%) | 4 (0.3%) | 0 (0%) | 1288 (96.6%) | 0 (0%) | 38 (2.9%) | 7 (0.5%) | 0 (0%) |
| Anemia | 1315 (97.5%) | 12 (0.9%) | 21 (1.6%) | 1 (0.1%) | 0 (0%) | 1261 (94.6%) | 28 (2.1%) | 37 (2.8%) | 7 (0.5%) | 0 (0%) |
| Mucositis oral | 1309 (97%) | 31 (2.3%) | 9 (0.7%) | 0 (0%) | 0 (0%) | 1253 (94%) | 36 (2.7%) | 43 (3.2%) | 1 (0.1%) | 0 (0%) |
| Dehydration | 1333 (98.8%) | 5 (0.4%) | 11 (0.8%) | 0 (0%) | 0 (0%) | 1288 (96.6%) | 12 (0.9%) | 33 (2.5%) | 0 (0%) | 0 (0%) |
| Diarrhea | 1327 (98.4%) | 11 (0.8%) | 11 (0.8%) | 0 (0%) | 0 (0%) | 1289 (96.7%) | 14 (1.1%) | 30 (2.3%) | 0 (0%) | 0 (0%) |
| Hyperglycemia | 1332 (98.7%) | 3 (0.2%) | 13 (1%) | 1 (0.1%) | 0 (0%) | 1307 (98%) | 2 (0.2%) | 23 (1.7%) | 1 (0.1%) | 0 (0%) |
| Syncope | 1341 (99.4%) | 0 (0%) | 8 (0.6%) | 0 (0%) | 0 (0%) | 1309 (98.2%) | 0 (0%) | 24 (1.8%) | 0 (0%) | 0 (0%) |
| Headache | 1324 (98.1%) | 14 (1%) | 11 (0.8%) | 0 (0%) | 0 (0%) | 1302 (97.7%) | 15 (1.1%) | 16 (1.2%) | 0 (0%) | 0 (0%) |
| Hypokalemia | 1337 (99.1%) | 0 (0%) | 12 (0.9%) | 0 (0%) | 0 (0%) | 1319 (98.9%) | 0 (0%) | 14 (1.1%) | 0 (0%) | 0 (0%) |
Adverse events occurring in ≥ of patients and all Grade 5–5 events occurring in ≥ of patients are presented. Per protocol, adverse events were to be assessed and reported at the end of each chemotherapy cycle; in addition, unexpected with hospitalization Grade 3 adverse events, unexpected Grade 4 and all Grade 5 that occur > 30 days after the last dose of study therapy with attribution of possible-, probable-, or definite-to-study therapy required reporting
AC doxorubicin and cyclophosphamide, FEC-100 5-flourouracil, epirubicin, and cyclophosphamide, NOS not otherwise specified
There were three Grade 5 adverse events in the AC treatment group (Sudden death NOS, Enterocolitis infectious, Leukemia secondary to oncology chemotherapy) audi eight in the FEC-100 treatment group (two cases of Sudden death NOS, Leukemia secondary to oncology, Death NOS, Febrile neutropenia, Left ventricular systolic, dysfunction (LVSD), Endocarditis infective, and Heart failure)
Late cardiac AEs reported after completion of the protocol treatment and before cancer recurrence, which were considered possibly related to chemotherapy per investigator, were recorded for 30 patients (8 AC; 22 FEC-100). Among them, the initiation of trastuzumab before the late cardiac event had been reported for nine patients (3 AC; 6 FEC-100) (Supplemental Table S3).
Deaths prior to recurrence or second primary tumors occurred in 25/1355 (1.8%) patients in the AC arm and 34/1343 (2.5%) in the FEC-100 arm. Etiologies of the deaths reported by investigators are shown in Supplemental Table S4.
Discussion
The Consensus Conference on Adjuvant Therapy of Breast Cancer held in November of 2000 had stressed the need for clinical trials to specifically address the issue of cycle duration. [11] NSABP B-36 was designed to determine whether six cycles of an anthracycline-based chemotherapy regimen were better than four cycles of conventional AC in a node-negative patient population. Prolongation of anthracycline-based therapy with FEC-100 for six cycles did not improve DFS, RFI, DRFI, or OS, relative to AC for four cycles, and was associated with expected increases in toxicity, particularly febrile neutropenia and mucositis. Higher histologic grade, larger tumor size, and Black vs White race were independently associated with increased risk for DFS events. Symptomatic CHF was fortunately rare in both groups with nine years of follow-up. Rates of treatment-related leukemia were also infrequent, though they were numerically higher in the AC arm.
The results of this study clearly do not support use of six-cycle anthracycline-based regimens in node-negative breast cancer. Since this trial completed accrual, management of adjuvant therapy in node-negative breast cancer has substantially evolved. In hormone receptor-positive breast cancer, multigene expression assays such as the Oncotype DX Recurrence Score [12, 13] and the MammaPrint [14] are used to identify patients who do not benefit from chemotherapy and can receive endocrine therapy alone. When chemotherapy is indicated for hormone receptor-positive, node-negative patients, four cycles of docetaxel and cyclophosphamide (TC) have been demonstrated to be superior to four cycles of AC, providing improved outcomes without exposure to the potential cardiotoxicity of anthracyclines. In hormone receptor-negative, node-negative patients, options include sequential administration of four cycles of AC and a taxane or four cycles of TC, depending on tumor size and grade, patient preference, and co-morbidity. Thus, AC alone for four cycles has largely been eliminated as a regimen for node-negative breast cancer.
The statistically significant interaction between treatment and hormone receptor status is noteworthy. In the two-thirds of patients with hormone receptor-positive breast cancer, nine-year DFS was numerically lower with FEC-100 (80.8%) vs. AC (85.0%), with an HR of 1.32 (95% CI 1.05–1.66). In contrast, in the one third of patients with hormone receptor-nega-tive disease, nine-year DFS was higher with FEC-100 (76.5%) vs. AC (70.9%), with an HR of 0.86 (95% CI 0.66–1.11). Although not conclusive, the data are consistent with a hypothesis that in hormone receptor-positive breast cancers, optimal duration of chemotherapy may be four cycles and that longer regimens, which result in a more extended delay in initiation of endocrine therapy, may be detrimental in some patients with hormone therapy responsive, node-negative breast cancers.
Supplementary Material
Acknowledgements
The authors would also like to acknowledge John Wilson, PhD (retired), who was the original Protocol Statistician for the study and was an invaluable part of the protocol team. The authors acknowledge the contributions of Barbara C. Good, PhD, Director of Scientific Publications, Christine I. Rudock, Publications and Graphics Specialist, and Wendy L. Rea, BA, Editorial Associate, all of whom are employees of NSABP. They were not compensated beyond their normal salaries for this work.
Funding
This work was supported by the National Institutes of Health grants U10-CA-180868, -189867, -180822, and -44066-26; and Pharmacia & Upjohn Company, a subsidiary of Pfizer, Inc.
Footnotes
Conflict of interest Charles E. Geyer, Jr.—Grants, non-financial support and other from Genentech/Roche, Daiichi/Sankyo, and AstraZeneca, during the conduct of the study, and personal fees from Exact Sciences and Athenex, outside the submitted work. Priya Rastogi: Genentech/Roche—Unpaid advisory boards, travel, accommodations; Lilly—Travel, accommodations; Astra/Zeneca— Travel, accommodations. Johnathan Polikoff – Consultant, Natera. Louise Provencher: Consulting or Advisory Role: Lilly, Pfizer, Roche, Novartis; Research Funding: Pfizer, Roche, Novartis, Merck, GlaxoSmithKline, Odonate Therapeutics Sandra M. Swain: Personal Fees for consulting/advisory services/nonpromotional speaking: Astra-Zeneca, Athenex, Daiichi-Sankyo, Genentech/Roche, Exact Sciences (Genomic Health), Eli Lilly and Company, Bejing Medical Award Association, Merck, Natera, Molecular Templates, Silverback Therapeutics; Non-financial support (i.e., travel, accommodations, and/or food & beverage); Genentech/Roche: Research support (to institution): Genentech/Roche, Kailos Genetics; Other support: AstraZeneca (member, independent data monitoring committee) and Genentech/Roche (third-party writing assistance). Eleftherios P. Mamounas: Genentech/Roche, Exact Sciences: Consultant (Advisory Board) and Speaker’s Bureau. Biotheranostics, Daiichi Sankyo, Puma Biotechnology, Merck: Consultant (Advisory Board).
Supplementary Information The online version contains supplemen-tary material available at https://doi.org/10.1007/s10549-021-06417-y.
References
- 1.Fisher B, Brown AM, Dimitrov NV et al. (1990) Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol 8(9):1483–1496 [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Tan-Chiu E et al. (2001) Tamoxifen and chemotherapy for axillary node-negative, estrogen receptor-negative breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-23. J Clin Oncol 19(4):931–942. 10.1200/JCO.2001.19.4.931 [DOI] [PubMed] [Google Scholar]
- 3.Hutchins LF, Green SJ, Ravdin PM et al. (2005) Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup Protocol INT-0102. J Clin Oncol 23(33):8313–8321 [DOI] [PubMed] [Google Scholar]
- 4.Levine MN, Pritchard KI, Bramwell VH et al. (2005) Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol 23(22):5166–5170 [DOI] [PubMed] [Google Scholar]
- 5.Fumoleau P, Kerbrat P, Romestaing P et al. (2003) Randomized trial comparing six versus three cycles of epirubicin-based adjuvant chemotherapy in premenopausal, node-positive breast cancer patients: 10-year follow-up results of the French Adjuvant Study Group 01 trial. J Clin Oncol 21(2):298–305 [DOI] [PubMed] [Google Scholar]
- 6.Bonneterre J, Roché H, Kerbrat P et al. (2005) Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-year follow up results of the French Adjuvant Study Group 05 randomized trial. J Clin Oncol 23(12):2686–2693 [DOI] [PubMed] [Google Scholar]
- 7.White SJ, Freedman LS (1978) Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer 37:849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganz PA, Bandos H, Geyer CE Jr, et al. (2022) Behavioral and health outcomes from the NRG Oncology/NSABP B-36 trial comparing two different adjuvant therapy regimens for early-stage node-negative breast cancer. Breast Cancer Res Treat. 192(1):153–161. 10.1007/s10549-021-06475-2. Epub 2022 Feb 3. PMID: 35112166. https://pubmed.ncbi.nlm.nih.gov/35112166/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin DY, Wei LJ, Ying Z (1993) Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 80:557–572 [Google Scholar]
- 10.Jacobs SA, Wilson JW, Bandos H, et al. NSABP B-36: A randomized phase III trial comparing six cycles of 5-fluorouracil (5-FU), epirubicin, and cyclophosphamide (FEC) to four cycles of adriamycin and cyclophosphamide (AC) in patients (pts) with node-negative breast cancer. Cancer Res 75 (9); SABCS; 2014. December 9–13, 2015; San Antonio, TX. Abstr S3–02. [Google Scholar]
- 11.McNeil C (2000) Consensus panel endorses range of adjuvant therapies for breast cancer. J Natl Cancer Inst 92(23):1870. 10.1093/jnci/92.23.1870 [DOI] [PubMed] [Google Scholar]
- 12.Paik S, Tang G, Shak S et al. (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726–3734 [DOI] [PubMed] [Google Scholar]
- 13.Sparano JA, Gray RJ, Makower DF et al. (2018) Adjuvant chemotherapy guided by a 21-Gene Expression Assay in breast cancer. N Engl J Med 379:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardoso F, van't Veer LJ (2016) Bogaerts J (2016) MINDACT Investigators: 70-Gene Signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375:717–729 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.