Abstract
Objectives:
We describe the methodology of Post–Acute Pancreatitis Pancreatic Exocrine Insufficiency (PAPPEI), a prospective, observational, multicenter cohort study. The objectives of PAPPEI are to estimate the incidence rate of post–acute pancreatitis (AP) pancreatic exocrine insufficiency (PEI), define factors that determine the development of post-AP PEI, and evaluate the impact of post-AP PEI on nutritional status and quality of life.
Methods:
Enrollment started in June 2017 in 3 expert academic centers in the United States. Data were collected during hospitalization (baseline) at 3 and 12 months after enrollment. Fecal elastase-1 was used to assess PEI. Study questionnaires are completed by patient interview and review of electronic medical records. Blood is obtained to evaluate vitamin deficiencies and nutritional markers.
Results:
As of August 2020, 77 subjects have completed the baseline evaluation. The median agewas 58 years (interquartile range, 39–67 years), 38% were male, and 90% were white. The etiology of AP was biliary in 39 subjects (51%), and 51 subjects (66%) had mild AP. Three- and 12-month follow-up data have been collected in 29 and 13 subjects, respectively.
Conclusion:
The PAPPEI study aims to expand our understanding of post-AP PEI incidence, including its impact on nutritional status and quality of life.
Keywords: acute pancreatitis, malnutrition, pancreatic exocrine insufficiency, steatorrhea
Acute pancreatitis (AP) is a common inflammatory gastrointestinal disease, which accounts for an annual global incidence of 34 cases per 100,000 population and imposes a high financial burden on the health care system.1,2 While the majority of AP subjects experience a mild clinical course, approximately 20% develop local or systemic complications.3,4 Recent trends demonstrate a reduction in the case fatality of AP, likely as a consequence of evolved knowledge in fluid therapy, nutritional support, critical care management of organ failure, and step-up approaches in necrotizing pancreatitis.5,6 As more patients with AP survive, it is essential to understand the long-term complications of the disease and accordingly construct secondary and tertiary prevention strategies to reduce their impact.
Pancreatic exocrine insufficiency (PEI) is a sequela of AP, which may develop from direct tissue injury or impaired regulation of pancreatic enzymes secretion.7 Recent meta-analyses of more than 30 retrospective and prospective studies estimated that the prevalence of PEI during an AP attack was approximately 60%, and at 3 years after recovery, the prevalence decreased to 30%.8-10 Although these meta-analyses have importantly contributed to our understanding of the development of post-AP PEI, they have noted a high level of heterogeneity among existing studies, as a result of variable diagnostic methods, suboptimal data quality, and inconsistent follow-up strategies. Furthermore, the vast majority of the included studies had small sample sizes and were conducted in single centers from Europe or Asia, with limited generalizability to North America. In addition, the majority of studies reported only point prevalence estimates of PEI after AP but did not perform longitudinal PEI assessments to assess the trajectory of PEI after AP.
Previous studies have shown that alcoholic etiology, pancreatic necrosis, and disease severity are associated with a higher risk of post-AP PEI development; however, the role of other clinical factors and therapies used for AP needs further evaluation.8,9 Determination of risk and protective factors may allow targeted screening interventions and preventive strategies for AP subpopulations at higher risk of PEI development. A recent single-center prospective study demonstrated that subjects who recover from AP have impaired quality of life (QOL); however, it is unknown whether the development of post-AP PEI has an impact on QOL.11 Furthermore, the degree to which PEI may impact patient symptoms or nutritional status after AP has not been well studied.12,13
The Post–Acute Pancreatitis Pancreatic Exocrine Insufficiency (PAPPEI) study has been designed with the following aims: (a) determine the incidence rate of PEI at 3 and 12 months post-AP, (b) define risk and protective factors associated with the development of post-AP PEI, and (c) measure the impact of post-AP PEI on clinical symptoms, nutritional deficiencies, and QOL.
MATERIALS AND METHODS
Study Organization and Participating Sites
This is a multicenter, prospective, cohort study, which was proposed to and accepted by Abbvie's investigator-initiated research program in 2016. Institutional review board (IRB) of the University of Pittsburgh Medical Center (UPMC) approved the study (IRB no. 19080096). Recruitment began at UPMC in July 2017 at the UPMC. In 2019, the study was expanded to include the Ohio State University and Johns Hopkins University Medical Center to increase recruitment and generalizability across AP populations. All 3 participating sites are high-volume referral centers with expertise in pancreatic disorders. The additional sites received approval from their local IRBs (Ohio State University 2018H0398, and Johns Hopkins University Medical Center IRB no. 000179109). The study has been registered in Clinicaltrials.gov (NCT03063398). Investigators from all 3 sites and the study sponsors meet monthly via teleconference directed by the principal investigator for study updates and management. Figure 1 summarizes the development of PAPPEI study.
FIGURE 1.
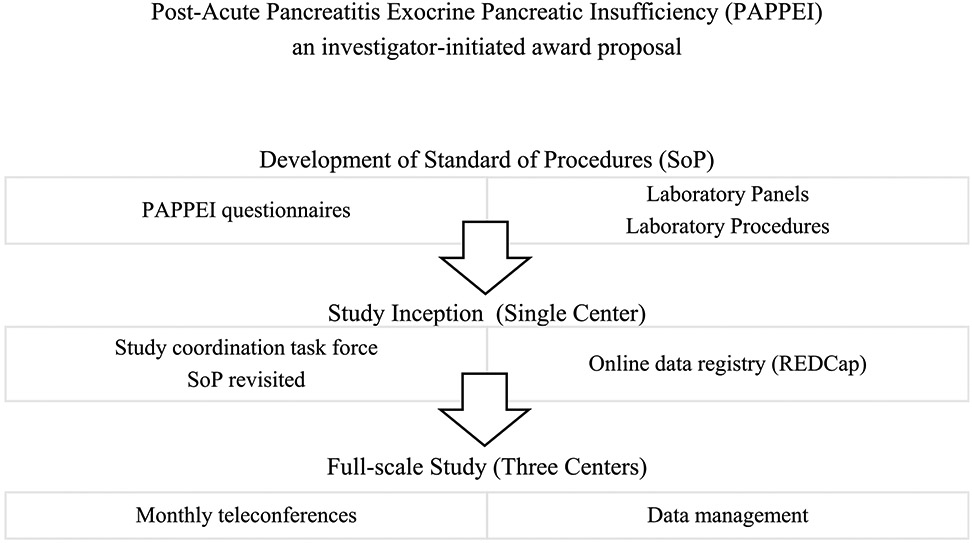
Development and organization of the PAPPEI study.
Study Subjects
Eligible subjects were 18 years or older, admitted to the inpatient hospital setting with an AP attack defined as the presence of 2 or more of the following criteria: (i) abdominal pain consistent with the disease, (ii) serum amylase/lipase greater than 3 times the upper limit of normal, or (iii) characteristic findings from abdominal imaging.14 The exclusion criteria, confirmed by history, are as follows: (1) preexisting diagnosis of chronic pancreatitis (CP), (2) preexisting PEI, (3) history of gastric bypass, (4) pancreatic resection, (5) history of small bowel disease with risk of related malabsorption including celiac disease and inflammatory bowel disease, (6) pancreatic cancer, (7) history of gastroparesis, and (8) cystic fibrosis.
Study Workflow
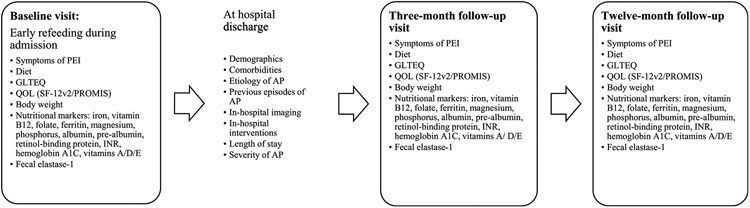
Figure 2 depicts the study workflow process. Potential study participants were identified by screening of daily laboratory alerts, received for patients with elevated serum amylase/lipase levels, and by daily communication with the inpatient gastroenterology consulting service. Eligibility was assessed through a personal interview of potential study participants. Eligible subjects were introduced to the study by the research team and were invited to participate. When enrolled, biospecimen collection was done after the reinitiation of oral feeding. Participants were scheduled for 2 follow-up visits at 3 months (window: 2–5 months) and 12 months (window: 10–16 months).
FIGURE 2.
The PAPPEI study flowchart. SF-12v2 indicates SF-12 version 2.
Data were collected via case report forms that were filled out by research coordinators obtaining information in 3 different ways: (1) face-to-face interview of the patient to collect medical history, social history, exercise, dietary habits, and PEI symptoms; (2) review of the electronic medical record to obtain AP etiology, history of previous AP episodes, and the clinical course during the hospital admission; and (3) direct written responses from study subjects who independently complete QOL assessments via validated questionnaires.
Baseline Assessment
The baseline questionnaire (see Supplemental Digital Content 1, http://links.lww.com/MPA/A847) captured demographics, anthropometric measurements, medical history, social history, AP phenotype, and medication use during the 2 months preceding admission. The study baseline case report forms were modeled after the APPRENTICE (Acute Pancreatitis Patient Registry to Examine Novel Therapies in Clinical Experience) study15 with modifications in the wording of questions as needed to improve clarity and granularity of information collected. Questions not included in APPRENTICE were education, employment, and marital status.
Post-AP PEI evaluates physical activity with the Godin Leisure-Time Exercise Questionnaire (GLTEQ).16 Godin Leisure-Time Exercise Questionnaire is a concise 4-item instrument that captures discretionary time each subject partakes in physical exercise that increases energy expenditure. Compared with occupational, household, or commuting physical activities, GLTEQ is more likely to reflect high-intensity and volitional activity in our AP cohort.17 The dietary habits questionnaire clarified the subject's consumption pattern of prebiotics/probiotics, fat, starch, meat, dairy products, fruits or vegetables, and fluids.
Quality of life was evaluated by the Short Form-12 questionnaire (SF-12) version 218 and the Patient-Reported Outcomes Measurement Information System (PROMIS) Global Health questionnaire version 1.1.19 The Short Form-12 questionnaire consists of 12 questions, which are summarized into physical component and mental component summaries. This questionnaire has been previously applied and validated in QOL studies in AP, recurrent AP, and CP.11,20,21 The PROMIS questionnaire was created by the National Institutes of Health as a concentrated effort to standardize patient-reported outcomes measurement using a single system.22 The PROMIS global health scale includes 5 primary domains of physical function, pain, emotional distress, fatigue, and social health.23 The PROMIS instrument is currently being utilized by another longitudinal study of subjects with AP, recurrent AP, and CP, the PROCEED study24; thus, its adoption by PAPPEI will permit direct QOL comparisons with other stages of pancreatitis.
Follow-up Assessment
All subjects' weights were measured at 3- and 12-month follow-up timepoints. During follow-up assessments, subjects were questioned about any AP-related hospital readmissions or emergency visits since the last study contact. In patients with pancreatitis-related readmissions, length of hospital stay and admission to the intensive care unit were recorded. In subjects who died during the follow-up, the cause of death was classified as either related or unrelated to AP by the principal investigator. Any newly diagnosed comorbid condition including diabetes mellitus (DM) was recorded based on patient report. The date of DM diagnosis was recorded and classified as “before,” “during,” or “after” the index AP attack. Diabetic management was categorized into “diet only,” “pills,” “insulin,” or “uncontrolled.”
Subjects were asked at each timepoint whether they were diagnosed with PEI (yes, no, unsure). The PEI diagnostic methods were categorized as “by clinical signs (steatorrhea),” “quantitative stool fat excretion,” “fecal elastase-1 (FE-1)” or “unknown.” A section of the questionnaire was focused on the symptoms of PEI as shown in Section 2.5.3 of Supplemental Digital Content 2 (http://links.lww.com/MPA/A847). This questionnaire interrogates the defecation pattern and altered daily activity due to gastrointestinal symptoms in the 2 weeks preceding the follow-up visit. If applicable, data related to enzyme supplements were collected under the pancreatic enzyme replacement therapy (PERT) section. The PERT questionnaire records the daily dosage and timing of administration (before, during, and after meals).
Similar to the baseline visit, GLTEQ, dietary habits, SF-12 version 2, and PROMIS QOL questionnaires were administered. The follow-up dietary instruments included 3 extra questions to track changes in the dietary pattern during the follow-up course. The subjects were questioned about changes in calories, fat content, or fruit/vegetable intake since the last study contact.
The pain pattern was assessed according to patterns originally identified by Ammann and Muellhaupt25 and previously used for both AP and CP.26,27 Finally, employment and disability status were checked at a 3- and 12-month follow-up visits.
Laboratory Panel
At each timepoint, a stool sample was collected. Fecal elastase-1 was measured via enzyme-linked immunosorbent assay quantifying CELA2/CELA3 isoforms. To reduce false-positive FE-1 results, all subjects were instructed to collect formed stool samples if able.28 Fecal elastase-1 level greater than 200 μg/g stool was considered normal.29 Pancreatic exocrine insufficiency was stratified as mild to moderate (FE-1: 100–200 μg/g stool) or severe (FE-1 ≤100 μg/g stool).
Additionally, a blood sample was collected during each follow-up. Glycemic status was evaluated by glycated hemoglobin. To address vitamin deficiencies, vitamins B12, A, D, and E and folic acid levels were measured. Additional nutritional markers included iron, ferritin, total iron-binding capacity, magnesium, phosphorus, albumin/prealbumin, retinol-binding protein, prothrombin time, and international normalized ratio. The complete laboratory procedures of PAPPEI study are summarized in Supplemental Digital Content 3 (http://links.lww.com/MPA/A847).
Data Management
The information collected in the paper-form PAPPEI questionnaires was subsequently entered into REDCap (Research Electronic Data Capture)30 by the research coordinator. The coordinating center (UPMC) manages access to the REDCap accounts centrally. A data management team monitored the quality of data entry, identified discrepancies, and provided guidance to participating sites. The coordinating center conducted data quality teleconferences monthly with the participating sites. The leading site presented a regular monthly report of the enrollment.
Statistical Considerations
Based on the available literature, we hypothesize that the incidence of PEI at 1 year following an AP attack is 25%.10,12,31 To achieve a study power of 80% and assuming a type I error of 0.05, we required a total of 128 subjects: 32 with PEI, and 96 without PEI. With an estimated dropout rate of 25%, we anticipated that 171 subjects would need to be enrolled in PAPPEI to meet the required power.
RESULTS
A total number of 77 AP subjects have been enrolled in PAPPEI study. The median age was 58 years (interquartile range [IQR], 39–67 years), 29 subjects (37.6%) were male, and 69 (89.6%) were White. Median body mass index in the baseline cohort was 30.2 kg/m2 (IQR, 25.2–37.5 kg/m2). The study population included 46 transferred subjects (59.7%), and 42 subjects (54.5%) were enrolled during the first attack of AP. History of diabetes was noted in 20 subjects (26.0%). Gallstone was the leading cause of AP noted in 39 subjects (50.6%), followed by idiopathic AP in 24 subjects (31.2%). Regarding the severity of AP, 3 subjects (3.9%) experienced severe, 23 subjects (29.9%) moderately severe AP, and the remaining 51 (66.2%) had mild disease. The median length of hospital stay was 6 days (IQR, 4–8 days), and 6 subjects (7.8%) needed intensive care unit admission. The 3- and 12-month follow-up data have been completed in 29 and 13 subjects, respectively. Overall, 10 subjects (13%) were lost to follow-up at the 3-month assessment and 18 subjects (23%) at the 12-month assessment. Table 1 summarizes the demographics and clinical features of this cohort.
TABLE 1.
Baseline Demographics and Baseline Clinical Characteristics of the PAPPEI Study Population
| Variables | Enrolled Subjects (n = 77) |
|---|---|
| Age, median (IQR), y | 58 (39–67) |
| Sex, male, n (%) | 29 (37.6) |
| Race, n (%) | |
| White | 69 (89.6) |
| Black | 7 (9.1) |
| Ethnicity, n (%) | |
| Non-Hispanic/Latino | 77 (100.0) |
| BMI, median (IQR), kg/m2 | 30.2 (25.2–37.5) |
| BMI, n (%) | |
| Underweight | 2 (2.6) |
| Normal | 10 (13.0) |
| Overweight | 21 (27.3) |
| Obese | 44 (57.1) |
| Current smoking, n (%) | 12 (15.6) |
| Current alcohol consumption, n (%) | 21 (27.3) |
| Transferred, n (%) | 46 (59.7) |
| Prior AP, n (%) | 35 (45.4) |
| Preexisting DM, n (%) | 20 (26.0) |
| Etiology, n (%) | |
| Biliary | 39 (50.6) |
| Idiopathic | 24 (31.2) |
| Alcoholic | 7 (9.1) |
| HTG | 4 (5.2) |
| Post-ERCP | 3 (3.9) |
| Severity of AP, n (%) | |
| Mild | 51 (66.2) |
| Moderately severe | 23 (29.9) |
| Severe | 3 (3.9) |
BMI indicates body mass index; ERCP, endoscopic retrograde cholangiopancreatography; HTG, hypertriglyceridemia.
DISCUSSION
Post-AP PEI represents an ongoing multicenter, prospective, cohort study of post-AP PEI in the United States. To address the methodological limitations of previous studies, PAPPEI proposes a rigorous, harmonized, 12-month plan to monitor the natural course of post-AP PEI in 3 discrete timepoints. This approach will allow us to accurately estimate the incidence of post-AP PEI and to assess the longitudinal trajectory of the pancreatic exocrine function following the resolution of the inflammatory component of AP. Another innovative aspect of this study includes the comprehensive collection of clinical data, nutritional markers, and QOL indicators. This can enhance our understanding of potential risk factors that determine the development of PEI and its impact on clinical parameters, nutrition, and QOL. This study can also serve as a platform for future clinical and translational research endeavors focusing on observing and/or managing long-term complications of AP.
The effectiveness of routine screening for PEI after an episode of AP has not been directly studied. This would require large cost-effectiveness or efficacy trials comparing screening interventions versus standard of care (no screening), which would be expensive and labor-intensive. The fact that post-AP PEI is fairly common, can be early detected through simple tests (FE-1), and can be treated effectively makes PEI screening after AP theoretically justified.8,9,32 However, previous studies estimating the burden of post-AP PEI were prompted to methodological issues and biases and had limited generalizability to the United States.8,9 Estimation of incidence rates using a prospective cohort design with follow-up in predetermined time intervals, such as the ones used in PAPPEI, may translate into better informed post-AP screening practices and society guidelines. The evaluation of multiple risk factors in PAPPEI may assist in targeting screening to high-risk subgroups of AP patients screening efforts should be directed to, or whether all patients should be screened. Testing FE-1 longitudinally can also help elucidate when is a more appropriate time to perform PEI screening, either at 3 or 12 months.
Mild PEI is usually asymptomatic, whereas patients with moderate and severe PEI often complain of abdominal pain, steatorrhea, bloating, cramping, and increased flatulence.33 A large proportion of patients with post-AP PEI remain underdiagnosed because of lack of physician awareness, erroneous diagnosis, or absence of symptoms. Routine screening for post-AP PEI may reduce underdiagnosis and avoid delayed initiation of PERT. This may translate into multiple health benefits, even in subjects who are asymptomatic. Untreated PEI results in maldigestion, malabsorption, and various nutritional deficiencies; however, this has not been well studied after AP.12,34 Therefore, we will evaluate any association of post-AP PEI with longitudinally collected clinical data, weight measurements, and key nutritional blood markers.
Evaluating potential associations of post-AP PEI with other long-term consequences of AP such as impaired QOL, pain, and disability will likely result in additional health benefits of post-AP PEI screening.11,26 The impact of post-AP PEI and PERT on these long-term outcomes is not well understood. In a small clinical trial, oral PERT during the refeeding period significantly improved the QOL of AP patients with PEI.35 However, this trial assessed only early treatment during a limited refeeding period. Data generated by PAPPEI will help us assess whether PEI is an independent modifiable risk factor that predisposes subjects following an AP attack to have lower QOL. In addition, any treatments, including PERT and nutrition supplements, are recorded in parallel with PEI symptoms and QOL metrics in subjects enrolled in PAPPEI. These data may help us elucidate the impact of PERT and other nutrition patterns, in PEI manifestations and QOL after AP.
The selection of the FE-1 assay as the test to diagnose PEI in our study can be critiqued because direct pancreatic function tests and the coefficient of fat absorption perform better than FE-1 to diagnose PEI.36,37 However, those tests are time-consuming, cumbersome, and not widely performed in clinical practice. Therefore, we opted for a more convenient, noninvasive, cost-effective, and relatively accurate diagnostic approach.38 The FE-1 assay measures elastase-1, a proteolytic enzyme that is produced by pancreatic acinar cells and passes through the gut with minimal degradation. Elastase-1 is highly stable in feces for up to 1 week at room temperature and up to 1 month when stored at 4°C, which makes FE-1 an extremely convenient test.39 Fecal elastase-1 testing is widely available in the United States. Another advantage of FE-1 is that the levels are not affected by PERT, so participants with overt PEI symptoms are not required to interrupt therapy. However, various other conditions can cause low FE-1 levels (eg, inflammatory bowel disease, celiac sprue, pancreatic cancer). Furthermore, the FE-1 assay has a high negative predictive value but is only moderately sensitive in diagnosing mild clinical forms of PEI. For all these reasons, we have selected FE-1 as the screening test for PEI in our study with the understanding that this assay may result in a slight underestimation of the post-AP PEI incidence estimates provided in the study.
The PAPPEI study will improve our understanding of the incidence and risk factors of post-AP PEI. Moreover, this study will divulge the impact of PEI on clinical symptoms, nutritional status, and QOL after an episode of AP. The results of this study will likely inform screening practices and future guideline recommendations on post-AP PEI. Our findings will also provide the basis for future clinical trials that assess the long-term effects of PERT in post-AP PEI.
Supplementary Material
Footnotes
Financial support was provided by AbbVie Pharmaceuticals.
The authors declare no conflict of interest.
REFERENCES
- 1.Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55. [DOI] [PubMed] [Google Scholar]
- 2.Wadhwa V, Patwardhan S, Garg SK, et al. Health care utilization and costs associated with acute pancreatitis. Pancreas. 2017;46:410–415. [DOI] [PubMed] [Google Scholar]
- 3.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. [DOI] [PubMed] [Google Scholar]
- 4.Matta B, Gougol A, Gao X, et al. Worldwide variations in demographics, management, and outcomes of acute pancreatitis. Clin Gastroenterol Hepatol. 2020;18:1567–1575.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munigala S, Yadav D. Case-fatality from acute pancreatitis is decreasing but its population mortality shows little change. Pancreatology. 2016;16: 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479–496. [DOI] [PubMed] [Google Scholar]
- 7.Murtaugh LC, Keefe MD. Regeneration and repair of the exocrine pancreas. Annu Rev Physiol. 2015;77:229–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W, de la Iglesia-García D, Baston-Rey I, et al. Exocrine pancreatic insufficiency following acute pancreatitis: systematic review and meta-analysis. Dig Dis Sci. 2019;64:1985–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollemans RA, Hallensleben NDL, Mager DJ, et al. Pancreatic exocrine insufficiency following acute pancreatitis: systematic review and study level meta-analysis. Pancreatology. 2018;18:253–262. [DOI] [PubMed] [Google Scholar]
- 10.Das SL, Kennedy JI, Murphy R, et al. Relationship between the exocrine and endocrine pancreas after acute pancreatitis. World J Gastroenterol. 2014;20:17196–17205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machicado JD, Gougol A, Stello K, et al. Acute pancreatitis has a long-term deleterious effect on physical health related quality of life. Clin Gastroenterol Hepatol. 2017;15:1435–1443.e2. [DOI] [PubMed] [Google Scholar]
- 12.Vujasinovic M, Tepes B, Makuc J, et al. Pancreatic exocrine insufficiency, diabetes mellitus and serum nutritional markers after acute pancreatitis. World J Gastroenterol. 2014;20:18432–18438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pendharkar SA, Salt K, Plank LD, et al. Quality of life after acute pancreatitis: a systematic review and meta-analysis. Pancreas. 2014;43:1194–1200. [DOI] [PubMed] [Google Scholar]
- 14.Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013; 108:1400–1415; 1416. [DOI] [PubMed] [Google Scholar]
- 15.Papachristou GI, Machicado JD, Stevens T, et al. Acute Pancreatitis Patient Registry to Examine Novel Therapies in Clinical Experience (APPRENTICE): an international, multicenter consortium for the study of acute pancreatitis. Ann Gastroenterol. 2017;30:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 17.Amireault S, Godin G, Lacombe J, et al. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Methodol. 2015;15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware J Jr., Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 19.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007; 45(5 Suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machicado JD, Amann ST, Anderson MA, et al. Quality of life in chronic pancreatitis is determined by constant pain, disability/unemployment, current smoking, and associated co-morbidities. Am J Gastroenterol. 2017; 112:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coté GA, Yadav D, Abberbock JA, et al. Recurrent acute pancreatitis significantly reduces quality of life even in the absence of overt chronic pancreatitis. Am J Gastroenterol. 2018;113:906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumhauer JF. Patient-reported outcomes - are they living up to their potential? N Engl J Med. 2017;377:6–9. [DOI] [PubMed] [Google Scholar]
- 23.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav D, Park WG, Fogel EL, et al. PROspective evaluation of chronic pancreatitis for EpidEmiologic and translational StuDies: rationale and study design for PROCEED from the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas. 2018;47:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology. 1999;116:1132–1140. [DOI] [PubMed] [Google Scholar]
- 26.Gougol A, Machicado JD, Matta B, et al. Prevalence and associated factors of abdominal pain and disability at 1-year follow-up after an attack of acute pancreatitis. Pancreas. 2019;48:1348–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullady DK, Yadav D, Amann ST, et al. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart PA, Conwell DL. Challenges and updates in the management of exocrine pancreatic insufficiency. Pancreas. 2016;45:1–4. [DOI] [PubMed] [Google Scholar]
- 29.Sziegoleit A, Krause E, Klör HU, et al. Elastase 1 and chymotrypsin B in pancreatic juice and feces. Clin Biochem. 1989;22:85–89. [DOI] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bavare C, Prabhu R, Supe A. Early morphological and functional changes in pancreas following necrosectomy for acute severe necrotizing pancreatitis. Indian J Gastroenterol. 2004;23:203–205. [PubMed] [Google Scholar]
- 32.Tu J, Zhang J, Ke L, et al. Endocrine and exocrine pancreatic insufficiency after acute pancreatitis: long-term follow-up study. BMC Gastroenterol. 2017;17:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288:813–815. [DOI] [PubMed] [Google Scholar]
- 34.Andersson B, Pendse ML, Andersson R. Pancreatic function, quality of life and costs at long-term follow-up after acute pancreatitis. World J Gastroenterol. 2010;16:4944–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahl S, Schütte K, Glasbrenner B, et al. The effect of oral pancreatic enzyme supplementation on the course and outcome of acute pancreatitis: a randomized, double-blind parallel-group study. JOP. 2014;15:165–174. [DOI] [PubMed] [Google Scholar]
- 36.Forsmark CE. Diagnosis and management of exocrine pancreatic insufficiency. Curr Treat Options Gastroenterol. 2018;16:306–315. [DOI] [PubMed] [Google Scholar]
- 37.Hart PA, Conwell DL. Diagnosis of exocrine pancreatic insufficiency. Curr Treat Options Gastroenterol. 2015;13:347–353. [DOI] [PubMed] [Google Scholar]
- 38.Vanga RR, Tansel A, Sidiq S, et al. Diagnostic performance of measurement of fecal elastase-1 in detection of exocrine pancreatic insufficiency: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;1:1220–1228.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Domínguez-Muñoz JE, Hardt PD, Lerch MM, et al. Potential for screening for pancreatic exocrine insufficiency using the fecal elastase-1 test. Dig Dis Sci. 2017;62:1119–1130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.