Abstract
Background:
Management of type 1 diabetes (T1D) is challenging. We compared outcomes using a commercially-available hybrid closed loop system versus a new investigational system with features potentially useful for adolescents and young adults with T1D.
Methods:
In this open-label, multi-national, seven center, randomised crossover trial, participants 14 to 29 years old, with T1D and HbA1c 7·0% to 11·0% (53 mmol/mol to 97 mmol/mol), used a Medtronic MiniMed™ 670G hybrid closed-loop system (670G) during one 12-week period and a Medtronic advanced hybrid closed-loop system (AHCL) during the other 12-week period. In an intention-to-treat analysis, co-primary outcomes, measured with continuous glucose monitoring, were percent time >180 mg/dL (>10·0 mmol/L) from 6AM to 11:59PM tested for superiority and time <54 mg/dL (<3·0 mmol/L) calculated over the full 24-hours tested for non-inferiority (non-inferiority margin 2%). The trial is registered with Clinicaltrials.gov: NCT03040414.
Findings:
From June 3, 2019 to August 22, 2019, 113 individuals entered the trial. Mean percentage of time with daytime glucose levels >180 mg/dL (>10·0 mmol/L) was 42±13% at baseline, 37±9% during the 670G arm, and 34±9% during the AHCL arm (mean difference [AHCL minus 670G] = −3%; 95% CI −4% to −2%, P<0·001). Mean 24-hour percentage of time with glucose levels <54 mg/dL (<3·0 mmol/L) was 0·46%±0·42% at baseline, 0·50±0·35% during the 670G arm and 0·46±0·33% in the AHCL arm (mean difference [AHCL minus 670G] = −0·06%; 95% CI −0·11% to −0·02%; P<0·001 for non-inferiority). One severe hypoglycaemia event occurred in the AHCL arm and none in the 670G arm.
Interpretation:
Hyperglycaemia was reduced without increasing hypoglycaemia in adolescents and young adults with type 1 diabetes using the investigational AHCL system compared with the commercially-available MiniMed 670G system.
INTRODUCTION
Use of technology in type 1 diabetes management is becoming an important component of care, often with a continuous glucose monitor first1 and an insulin pump as needed, and most recently systems combining the two to automate insulin delivery2. The systems currently available in the U.S., the Medtronic MiniMed™ 670G hybrid closed-loop and the Tandem Control-IQ systems, are considered hybrid closed-loop since their intended function requires user input to enter carbohydrate intake before each meal, enabling determination of the appropriate meal-time insulin bolus. These systems have been demonstrated to reduce both hyperglycaemia and hypoglycaemia3,4. However, use of these systems only achieves ~70% time in the target range of 70–180 mg/dL (3·9–10·0 mmol/L) on average, reflecting more than 6 hours per day with blood glucose concentrations >180 mg/dL (>10·0 mmol/L)3–5. The percent time in the target range is even lower in adolescents and young adults than in older adults, especially during the daytime,6,7 reflecting the need for algorithmic advances to reduce blood glucose excursions after meals.
The MiniMed 670G system was the first system granted regulatory approval, employing a conservative approach to glucose control focused on modifying the basal insulin delivery rate in response to the glucose levels. A new investigational system, the Medtronic Advanced Hybrid Closed-Loop (AHCL) system, has been developed that includes several algorithm enhancements and features of the MD-Logic artificial pancreas algorithm8–10 (DreaMed Diabetes, Ltd, Israel); including automated-correction boluses delivered up to every 5 minutes, two target setpoints of 100 mg/dL (5·5 mmol/L) and 120 mg/dL (6·7 mmol/L); and an updated controller that includes a modified integral action and new controller gains, a modified insulin feedback module, a modified adaptation method that ensures a more robust personalization of the therapy and increases time in closed loop, and a meal detection module which, if triggered, can potentially let the system deliver more aggressive auto-correction boluses.
This randomized crossover trial compares the existing MiniMed 670G system to the AHCL system in adolescents and young adults with type 1 diabetes, many of whom were not using continuous glucose monitoring and/or an insulin pump.
RESEARCH DESIGN AND METHODS
Study Design
This randomised open-label two-period crossover trial was conducted at 7 academic-based endocrinology practices (4 in the United States and 1 each in Germany, Israel, and Slovenia). The protocol and informed consent/assent forms were approved by the appropriate institutional review boards and ethics committees, and regulatory approval to conduct the study was obtained in all four countries. The protocol is available at https://public.jaeb.org/datasets.
Participants
Major eligibility criteria included clinical diagnosis of type 1 diabetes, age 14 to 29 years, diabetes duration ≥1 year, use of either an insulin pump or multiple daily insulin injections, and hemoglobin A1c (HbA1c) 7·0% to 11·0% (53 mmol/mol to 97 mmol/mol) inclusive (see Supplemental Table 1 for complete listing of inclusion and exclusion criteria). Written informed consent was obtained from each participant 18 years or older or the parent/legal guardian for younger participants. Additionally, participants less than 18 years old signed an assent form.
Randomization and Masking
During each of two 12-week periods, each participant used one of the two closed-loop systems: the MiniMed 670G system (670G) or the AHCL system, assigned in random order on the study website using a computer-generated sequence created by the study statistician, with a permuted block design (block sizes of 2 and 4), stratified by baseline HbA1c (≤8·5% and >8·5%, ≤69 mmol/mol and >69 mmol/mol) and by use of a personal MiniMed 670G system at enrollment. Participants and site investigators and staff were not masked to the intervention being used during each period; differences in the pump user interface between systems precluded the ability to mask.
Procedures
Prior to randomization, each participant was trained to use the study pump (without automated insulin delivery) and the continuous glucose monitor. Training was customized according to prior device experience since many had not used an insulin pump and or a continuous glucose monitor before. Following the training period, participation in the randomized trial required that the participant have continuous glucose monitoring data for at least 80% of the possible time over 14 days and an average of at least three blood glucose meter tests per day.
Both the MiniMed 670G and AHCL systems consisted of the same Medtronic 670G insulin pump and Guardian Sensor 3 continuous glucose monitor, with only the software differing between systems (Supplemental Table 2). The time during which the system is active is referred to as “Auto Mode”.
At the beginning of each period, participants and a parent/guardian where applicable were trained on use of the closed-loop system. After 12 weeks, each participant switched to the other closed-loop system, with no washout period in between. In each period, the closed-loop system initially was used with Auto Mode deactivated for the first 6–10 days (except for experienced 670G system users who could activate Auto Mode from the start when using the MiniMed 670G system). The AHCL system was started with an Auto Mode target glucose set point of 120 mg/dL (6·7 mmol/L). At the Auto Mode initiation contact, participants were reminded to obtain an overnight fingerstick blood glucose measurement (between 2–3AM) for 2–3 nights following Auto Mode initiation and if the glucose level was <70 mg/dL (<3·9 mmol/L) to treat with carbohydrate, discontinue Auto Mode, and notify the investigator or designee the next day for advice. During the 2-week assessment visit, the target glucose set point could be lowered to 100 mg/dL (5·5 mmol/L) if the participant had no severe hypoglycaemia and met the following additional, pre-specified criteria (1) no more than 1% of sensor glucose readings <54 mg/dL (<3·0 mmol/L) for the 24-hour time period with no sensor glucose readings less than 54 mg/dL (3·0 mmol/L) from midnight to 6AM, or (2) no more than 3% of sensor glucose readings less than 70 mg/dL (3·9 mmol/L) for 24 hours. If the participant did not meet these criteria, the setpoint remained at 120 mg/dL (6·7 mmol/L) and the participant was reevaluated later to see if the setpoint could be lowered. In the MiniMed 670G system, the glucose set point is fixed at 120 mg/dL (6·7 mmol/L). The active insulin time in the AHCL system was adjustable and recommended to start at 3·0 to 4·0 hours. The active insulin time determines the aggressiveness of correction and meal boluses. Shorter active insulin times result in more aggressive dosing; longer active insulin times lead to less aggressive dosing. The active insulin time could be adjusted in 30-minute increments following review of continuous glucose monitoring data every 2 weeks based on investigator judgment in reviewing the glucose patterns and assessing the amount of time in hyperglycaemia and hypoglycaemia, in consultation with the participant.
Blood glucose testing was performed with a study-provided Contour Next Link 2·4 blood glucose meter for calibration of the sensor and in accordance with the labeling of the continuous glucose monitoring device. Per protocol, there was a minimum of three blood glucose tests per day recommended. Blood ketone testing was performed with a study-provided Abbott Precision Xtra blood ketone meter whenever Auto Mode was operating and the participant’s continuous glucose monitoring readings were >300 md/dL (>16·7 mmol/L) for more than 1-hour or anytime the continuous glucose monitoring reading was ≥400 mg/dL (≥22·2 mmol/L).
During both periods, there were phone contacts at 1 and 5 days and 4 and 9 weeks, and clinic visits at 2, 6, and 12 weeks timed from the start of Auto Mode. The final study visit for 1 participant was completed remotely due to restrictions related to Covid-19. Participants were asked to upload data before each contact and at least every two weeks for review during visits and phone contacts. HbA1c was measured at randomization and at the end of each period by a central laboratory at the University of Minnesota Advanced Research and Diagnostic Laboratory using the Tosoh G8 HPLC system.
Adverse events were recorded throughout the course of the trial. Reportable adverse events included serious adverse events, adverse events occurring in association with a study device or procedure, severe hypoglycaemia (defined as hypoglycaemia requiring assistance due to altered consciousness), diabetic ketoacidosis as defined by the Diabetes Control and Complications Trial11, or hyperglycaemia with ketonemia for which a health care provider was contacted.
Outcomes
Primary Outcomes
The trial had co-primary outcomes, measured with continuous glucose monitoring, of percent time >180 mg/dL (>10·0 mmol/L) from 6AM to 11:59PM tested for superiority and percent time <54 mg/dL (<3·0 mmol/L) calculated over the full 24-hours of the day tested for non-inferiority.
Pre-Specified Secondary Outcomes
Secondary outcomes calculated for the full 24-hour day, daytime (6AM-11:59PM) and nighttime (12AM-5:59AM) included mean glucose concentration, coefficient of variation (standard deviation of mean glucose divided by mean glucose), percentage of glucose values <70 mg/dL (<3·9 mmol/L), 70 to 180 mg/dL (3·9–10·0 mmol/L) and 70 to 140 mg/dL (3·9–7·8 mmol/L); percentage of glucose values >250 mg/dL (>13·9 mmol/L), and percentage of glucose values >180 mg/dL (>10·0 mmol/L) (for 24-hour period and nighttime). Additional secondary outcomes included basal, bolus (including auto-correction bolus insulin), and total daily insulin; percent time in Auto Mode and number of exits from Auto Mode per week; HbA1c, and body mass index. Other pre-specified outcomes are listed in the Statistical Analysis Plan for tabulation without statistical testing. This included the international consensus outcome of target time in range >70% combined with <1% time <54 mg/dL (<3·0 mmol/L)12. Analyses of continuous glucose monitoring metrics during the post-meal periods will be reported separately. The proportion of participants with an increase in time in range 70 to 180 mg/dL (3·9–10·0 mmol/L) of 5% or more and the number of alarms per week were added as post-hoc outcomes.
Continuous glucose monitoring outcomes were calculated over 84 days starting with the time Auto Mode was initiated. A minimum of 72 hours of continuous glucose monitoring data was pre-specified to be required for a participant’s data in that period to be included in the analyses. The percentage of time using the continuous glucose monitor was calculated by dividing the total number of available continuous glucose monitor readings by the maximum possible number of readings based on the length of the study period; starting with the time Auto Mode was first turned on and ending after 84 days or at 11:59 p.m. on the day prior to the date of the 12-week visit, whichever came first. The percentage of time using Auto Mode was computed by summing the gaps between segments when Auto Mode was active and dividing by the study period duration. Gaps longer than 90 minutes where Auto Mode was active at the start and end of the gap were not counted in the numerator because it was possible the system may not have been on during those periods.
The Glucose Monitoring Satisfaction Survey was completed at screening and at the end of each period. The survey consists of 15 items on a 5-point Likert scale that assesses treatment satisfaction and burden13; higher scores indicate greater satisfaction. Broader quality of life questionnaires were completed at screening and at the end of each period and will be reported separately.
Safety
Safety outcomes included the frequency of severe hypoglycaemia, diabetic ketoacidosis, and other serious adverse events.
Statistical Methods
The total sample size was computed to be 65 based on the following assumptions (1) 90% power, with adjustment to account for the two co-primary analyses; (2) a 5% absolute reduction in time >180 mg/dL (>10·0 mmol/L) with a standard deviation of paired differences of 12%, and a 2-sided type 1 error rate of 5%; and (3) a non-inferiority limit of 2% (selected based on clinical opinion) for the treatment group comparison of time <54 mg/dL (<3·0 mmol/L) with an standard deviation of paired differences of 1·3% and 1-sided type 1 error of 2·5%. The sample size was increased to 112 so that at least 100 would complete the trial to provide increased precision for safety analyses and subgroup analyses. With a sample size of 100 participants completing the trial, statistical power was 98% for the primary efficacy analysis.
Statistical analyses were performed on an intention-to-treat basis, and all participants were included in the primary and all secondary analyses except for the per-protocol analysis. For the co-primary outcome analyses, repeated measures least squares regression models with an unstructured covariance structure were fit to compare the two intervention arms adjusting for period, pre-study MiniMed 670G system use, and HbA1c at randomization as fixed effects. Analyses for all secondary and exploratory outcomes paralleled the primary analysis. Modification of the treatment effect (subgroup analyses) on the two primary outcomes was assessed in exploratory analyses by including an interaction term in the models described above. The per-protocol analysis included participants who used the continuous glucose monitor and Auto Mode at least 80% of the time in both periods. Missing data were handled by means of direct likelihood analyses and not imputed for any analyses.
For the two primary analyses, since the intervention was to be deemed effective only if the null hypotheses for both co-primary outcomes were rejected, the type 1 error was not inflated and there was no correction for multiple comparisons. For secondary analyses, the false discovery rate was controlled using the adaptive two stage Benjamini-Hochberg procedure14 with <0·05 as the threshold for statistical significance.
All p-values are two-tailed. Analyses were performed using SAS 9·4. The trial is registered with Clinicaltrials.gov: NCT03040414. An independent Data and Safety Monitoring Board provided trial oversight.
Role of the Funding Source
Funding was provided by the National Institute of Diabetes and Digestive and Kidney Diseases, which had membership on the study’s Steering Committee that approved the study design and writing of the report but was not involved in the data collection or data analysis. Medtronic MiniMed Inc (Northridge, CA) provided the closed-loop systems, provided technical expertise related to device issues, and reviewed the manuscript prior to publication but was not otherwise involved in trial design, conduct, or analysis. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Between June 3, 2019 and August 22, 2019, 126 adolescents and young adults with type 1 diabetes were screened and 113 entered the randomised trial (Supplemental Figures 1 and 2). The number per site in the randomised trial ranged from 10 to 23 (see number for each site in the Acknowledgements section). Participants ranged in age from 14 to 29 years and HbA1c ranged from 7·0% to 10·9% (53 mmol/mol to 96 mmol/mol) at screening; 23 (20%) were not using pumps, 43 (38%) were not using continuous glucose monitors, and 14 (12%) were using neither a pump nor a continuous glucose monitor (Table 1). Participant characteristics according to the randomized intervention assignment for period one are provided in Supplemental Table 3.
Table 1.
Baseline Characteristics
| Overall N=113 | Age 14–20 N=73 | Age 21–29 N=40 | |
|---|---|---|---|
|
| |||
| Age – Mean ± SD years | 19 ± 4 | 17 ± 2 | 24 ± 3 |
| Median Duration of Diabetes (IQR) - years | 12 (7, 15) | 10 (6, 13) | 15 (13, 18) |
| Insulin Delivery N (%) | |||
| Insulin Pump | 90 (80%) | 59 (81%) | 31 (78%) |
| Multiple Daily Injections | 23 (20%) | 14 (19%) | 9 (23%) |
| Use of a Continuous Glucose Monitor at Enrollment – N (%) | 70 (62%) | 47 (64%) | 23 (58%) |
| Use of MiniMed 670G in Auto Mode at Enrollment – N (%) | 15 (13%) | 8 (11%) | 7 (18%) |
| Body Mass Index – median (IQR) | |||
| Participants ≥18 years old kg/m2 | 26 (23, 29) | ||
| Participants <18 years old percentile | 81% (56%,90%) | ||
| Female Sex – N (%) | 70 (62%) | 42 (58%) | 28 (70%) |
| Race – N (%) | |||
| White | 104 (92%) | 68 (93%) | 36 (90%) |
| Black/African American | 3 (3%) | 1 (1%) | 2 (5%) |
| Hispanic or Latino | 5 (4%) | 3 (4%) | 2 (5%) |
| More than One Race | 1 (<1%) | 1 (1%) | 0 (0%) |
| Highest Education Levela | |||
| ≤ H.S. diploma | 20 (18%) | 16 (22%) | 4 (10%) |
| Associates Degree/Some College but no Degree | 40 (35%) | 22 (30%) | 18 (45%) |
| Bachelor’s Degree | 34 (30%) | 20 (27%) | 14 (35%) |
| Master’s Degree | 12 (11%) | 9 (12%) | 3 (8%) |
| Doctoral or Prof Degree | 7 (6%) | 6 (8%) | 1 (3%) |
| HbA1c at Screening – N (%) | |||
| ≤8·5% (≤69 mmol/mol) | 83 (73%) | 51 (70%) | 32 (80%) |
| ≥8·6% (≥70 mmol/mol) | 30 (27%) | 22 (30%) | 8 (20%) |
| mean ± SD % (mmol/mol) | 8·1 ± 0·8 (65±9) | 8·2 ± 0·8 (66±9) | 7·9 ± 0·7 (63±8) |
| HbA1c at Randomization – N (%) | |||
| ≤8·5% (≤69 mmol/mol) | 97 (86%) | 60 (82%) | 37 (93%) |
| ≥8·6% (≥70 mmol/mol) | 16 (14%) | 13 (18%) | 3 (8%) |
| mean ± SD % (mmol/mol) | 7·9 ± 0·7 (63±8) | 8·0 ± 0·7 (64±8) | 7·7 ± 0·6 (61±6) |
Highest level completed by participant, or by primary caregiver if participant <18 years old.
For period one, 57 participants were assigned to use 670G and 56 were assigned to use AHCL. All participants completed both arms except for two (one in each arm) who dropped out during period 1; both were included in the analysis for the partial period they completed. Among the 111 participants completing both arms, visit and phone contact completion rates were 100% in each arm. There were 113 unscheduled visits during the 670G arm and 119 during the AHCL arm (1·0 and 1·1 per participant, respectively).
Mean percentages of time between 6AM and 11:59PM with glucose levels >180 mg/dL (>10·0 mmol/L) were 42±13% at baseline, 37±9% during the 670G arm and 34±9% during the AHCL arm (mean difference [AHCL minus 670G] = −3%, 95% CI −4% to −2%, P<0·001 for superiority; Table 2). Mean percentages of time with glucose levels <54 mg/dL (<3·0 mmol/L) were 0·46±0·42% at baseline, 0·50±0·35% during the 670G arm, and 0·46±0·33% during the AHCL arm (mean difference [AHCL minus 670G] = −0·06%, 95% CI −0·11% to −0·02%, P<0·001 for non-inferiority). Results of the per-protocol analysis were similar to the primary intent-to-treat analysis (Supplemental Table 4). Results for the co-primary outcomes were consistent in subgroups based on age, baseline HbA1c, prior device use, clinical site, and other factors (Supplemental Tables 5 and 6).
Table 2.
Primary and Key Secondary Efficacy Outcomes
| Baseline N=113 | 670G Arm N=112 | AHCL Arm N=112 | ||
|---|---|---|---|---|
|
| ||||
| Hours of Data | ||||
| Mean ± SD | 319 ± 29 | 1604 ± 339 | 1652 ± 251 | |
| Percentage of Time above 180 mg/dL (10·0 mmol/L) Daytime (6:00 AM – 11:59 PM) | ||||
| Mean ± SD | 42% ± 13% | 37% ± 9% | 34% ± 9% | |
| Mean Adjusted Difference [95% CI] [P-Value] a | −3% [−4%, −2%] [<0·001] | |||
| Percentage of Time below 54 mg/dL (3·0 mmol/L)b (Over the Full 24 Hours of the Day) | ||||
| Mean ± SD | 0·46% ± 0·42% | 0·50% ± 0·35% | 0·46% ± 0·33% | |
| Mean Adjusted Difference [95% CI] [P-Value] a | −0·06% [−0·11%, −0·02%] [<0·001]c | |||
|
| ||||
| Keyt Secondary Outcomes | ||||
| Continuous Glucose Monitoring Outcomes (Over the Full 24 Hours of the Day) Mean ± SD | P-Value d | |||
| Mean Glucose mg/dL (mmol/L) | 173 ± 19 (9·6 ± 1·1) | 166 ± 13 (9·2 ± 0·7) | 159 ± 13 (8·8 ± 0·7) | <0·001 |
| Time in the Target Range (70–180 mg/dL, 3·9–10·0 mmol/L) | 57% ± 12% | 63% ± 8% | 67% ± 8% | <0·001 |
| Time in the Tight Target Range (70–140 mg/dL, 3·9–7·8 mmol/L) | 34% ± 11% | 40% ± 7% | 44% ± 7% | <0·001 |
| Time >180 mg/dL(10·0 mmol/L) | 41% ± 13% | 34% ± 8% | 31% ± 8% | <0·001 |
| Time >250 mg/dL(13·9 mmol/L) | 13% ± 8% | 10% ± 6% | 9% ± 5% | <0·001 |
| Time <70 mg/dL (3·9 mmol/L) | 2·3% ± 1·8% | 2·1% ± 1·4% | 2·1% ± 1·2% | 0·42 |
| Coefficient of Variation | 36·1% ± 4·2% | 36·5% ± 3·9% | 37·2% ± 3·3% | 0·001 |
| HbA1c Mean±SD % d (mmol/mol) | 7·9 ± 0·7 (63±8) | 7·6±0·6 (59±7) | 7·4±0·8 (57±9) | 0·03 |
Based on a repeated measures least squares regression model comparing the two treatment arms adjusting for period, pre-study MiniMed 670G use, and HbA1c at randomization as fixed effects. The model includes 3 time points: (1) baseline, (2) period 1 outcome, and (3) period 2 outcome. Carryover effect p-value was 0·93 for daytime time above 180 mg/dL (10·0 mmol/L) and 0·19 for time below 54 mg/dL (3·0 mmol/L).
Time < 54 mg/dL (3·0 mmol/L) was winsorized at the 10th and 90th percentiles to account for skewness.
P-value for non-inferiority (Non-inferiority limit = 2%).
Values for 670G and AHCL arms represent the end of period 1 only. Baseline mean and standard deviation were the same for each treatment arm. P-value calculated using a repeated measures least squares regression model comparing the two treatment arms adjusting for period and pre-study MiniMed 670G use as fixed effects. The model includes 2 time points: (1) baseline and (2) period 1 outcome.
Over the full 24-hour period, time in target range 70–180 mg/dL (3·9–10·0 mmol/L) was 57±12% at baseline, 63±8% during the 670G arm and 67±8% during the AHCL arm (P<0·001 comparing 670G arm versus AHCL arm; Table 2, Figure 1). Time in target range was 56±12% at baseline, 61±9% during the 670G arm, and 64±9% during the AHCL arm during daytime (6AM-11:59PM; P<0·001 comparing 670G arm versus AHCL arm) and 58±17%, 70±12%, and 74±11%, respectively overnight (12AM-5:59AM; P<0·001 comparing 670G arm versus AHCL arm, Supplemental Table 7) and is depicted over 24 hours in Figure 2. The glucose profile over 24 hours showed a consistently lower mean glucose for AHCL vs. 670G (Supplemental Figure 3). The percentages of participants achieving the combined outcome of achieving both a target time in range of >70% and time <54 mg/dL (<3·0 mmol/L) of <1% were 12% at baseline, 21% during the 670G arm, and 30% during the AHCL arm, and the percentages achieving a 5% or more improvement in time in range from baseline were 53% in the 670G arm and 65% in the AHCL arm (Supplemental Table 8). Mean HbA1c was 7·9±0·7% (63±8 mmol/mol) at randomization and at the end of period one was 7·6±0·6% (59±7 mmol/mol) in the 670G arm and 7·4±0·8% (57±9 mmol/mol) in the AHCL arm (P=0·03; Table 2 and Supplemental Table 9). HbA1c level <7% (<53 mmol/mol) was 4% at baseline, 13% at the end of the 670G arm, and 24% at the end of AHCL arm (Supplemental Table 8).
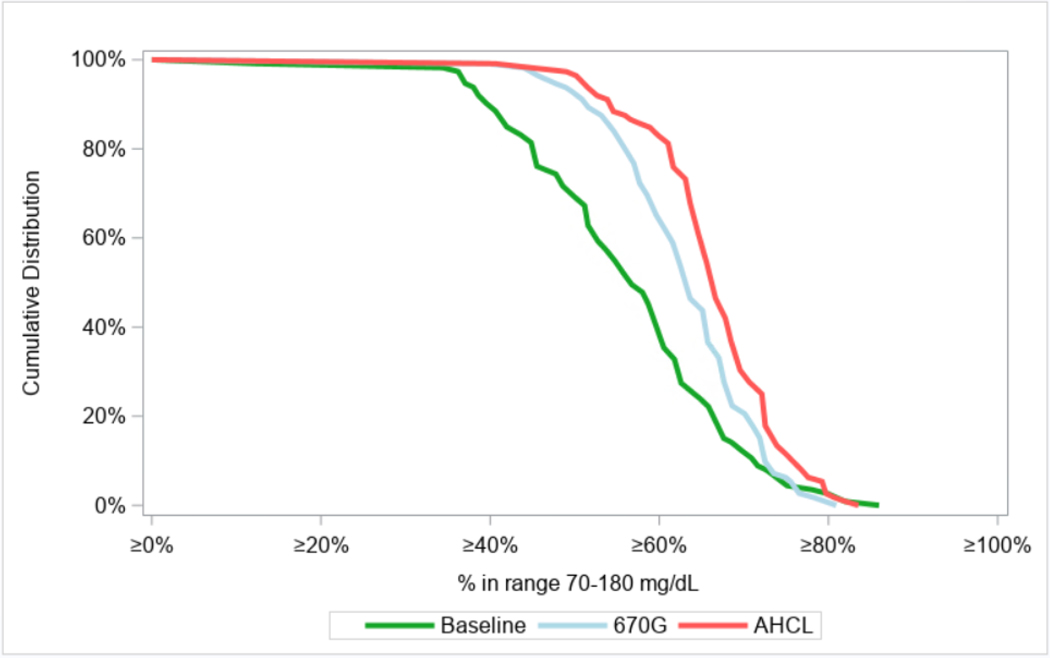
Figure 1. Cumulative Distribution of Time in Range 70–180 mg/dL (3·9–10·0 mmol/L).
Figure 1 shows the cumulative distribution of the percentage of time that the glucose level was within the range of 70 to 180 mg per dL (3·9 to 10·0 mmol per liter), as measured by continuous glucose monitoring, for baseline and during each treatment arm.
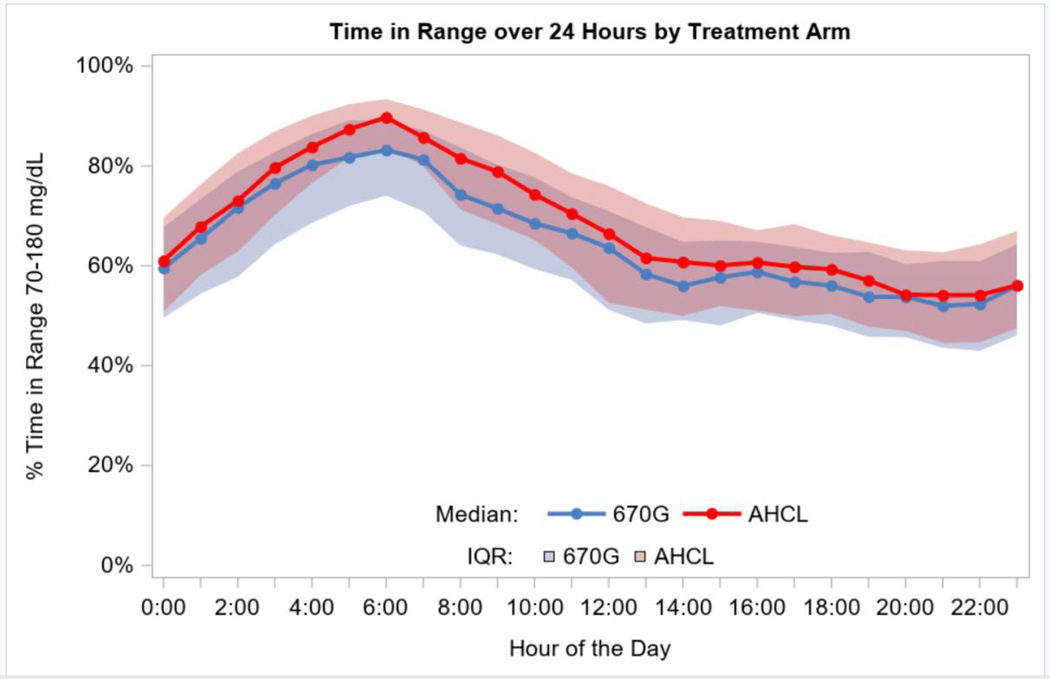
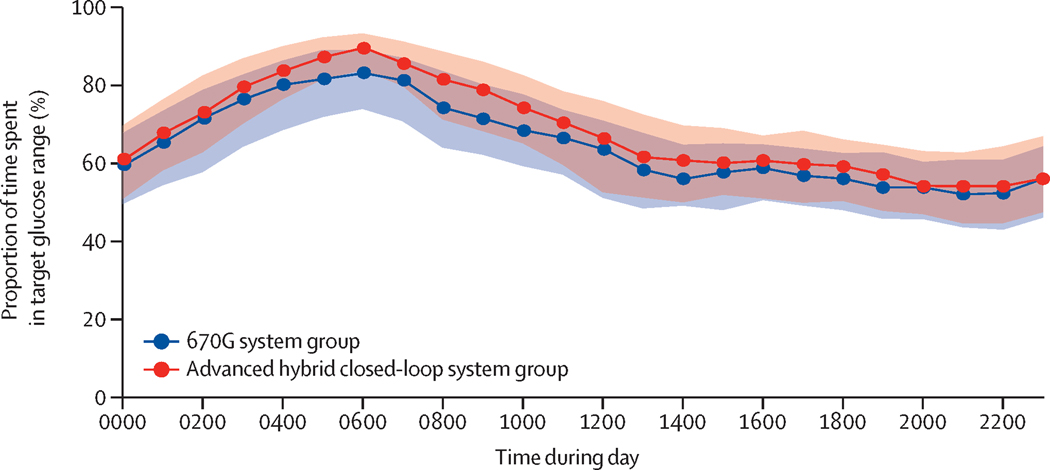
Figure 2. Time in Range 70–180 mg/dL (3·9–10·0 mmol/L) By Hour Over 24 Hours.
Figure 2 shows an envelope plot of the percentage of time that the glucose level was within the range of 70 to 180 mg per dL (3·9 to 10·0 mmol per liter), as measured by continuous glucose monitoring, according to the time of day. Symbols denote the hourly median values, and the shaded regions are defined by the 25th and 75th percentiles.
Mean total daily insulin dose was 50±21 units in the 670G arm, with on average 51% of the insulin delivery as basal and 49% as bolus; and 55±22 units in the AHCL arm, with on average 36% as basal and 64% as bolus (of which 36% of bolus insulin was delivered by auto-correction bolus, Supplemental Table 10). During daytime the mean basal-bolus ratios were 45%/55% in the 670G arm and 31%/69% (32% of bolus was auto-correction bolus) in the AHCL arm, and during nighttime the mean ratios were 87%/13% and 61%/39% (80% of bolus was auto-correction bolus), respectively. The average amount of insulin delivered per hour of the day as automated basal, user-initiated bolus, and automated-bolus is shown in Figure 3. Active insulin time was 2 hours in 14 (13%) participants, 2.5–3 hours in 88 (79%), and 3.5–4 hours in 10 (9%) at the end of the 670G arm; and 6 (5%), 79 (71%), and 27 (24%), respectively at the end of the AHCL arm.
Figure 3. Average Insulin Delivery By Hour Over 24 Hours.
Figure 3 shows a stacked bar chart with bars representing the average insulin delivered during each hour of the day. The blue regions show the units of automated basal insulin delivered, the red regions show user-initiated bolus delivered, and the green regions show auto-correction bolus delivered.
Body mass index data at the beginning and end of each period in each arm are shown in Supplemental Table 11 and blood glucose meter testing in Supplemental Table 12.
At the end of each treatment arm, all participants who completed the arm were using the assigned closed-loop system. Median continuous glucose monitoring usage was 85% (IQR 77%–91%) in the 670G arm and 86% (76%–93%) in the AHCL arm (P=0·006; Supplemental Table 13). Median percentage of time the system was in Auto Mode was 75% (64%–83%) during the 670G arm and 86% (77%–91%) during the AHCL arm (P<0·001; Supplemental Table 14). During the AHCL arm, 103 of 112 (92%) were switched from the starting set point of 120 mg/dL (6·7 mmol/L) to 100 mg/dL (5·6 mmol/L) at some point during the 12-week period; 80% of the 103 within the first 4 weeks. At the end of the AHCL arm, the set point was 100 mg/dL in 93 (83%) of participants and 120 mg/dL in 19 (17%).
The number of alarms averaged 102±30 per week during the 670G arm and 75±26 per week during the AHCL arm (P<0·001). Exits from Auto Mode averaged 5·7±2·0 times/week for 670G compared with 1·7±0·9 times/week for AHCL. Continuous glucose monitor and Auto Mode use by period are shown in Supplemental Table 15. Reported device problems with use of each system are summarized in Supplemental Table 16. Mean scores on the Glucose Monitoring Satisfaction Survey were 2·76±0·52 at screening, 2·65±0·63 at the end of the 670G arm, and 2·80±0·55 at the completion of the AHCL arm (P=0·003 comparing 670G and AHCL, Supplemental Table 17).
Seven adverse events were reported for 7 (6·2%) participants during the 670G arm and 6 events for 6 (5·3%) participants during the AHCL (Table 3). Severe hypoglycaemia occurred in one participant during the AHCL arm and none during the 670G arm. There were no cases of diabetic ketoacidosis during either arm.
Table 3.
Safety Outcomes
| During 670G Arm (N=112) | During AHCL Arm (N=112) | |
|---|---|---|
|
| ||
| Any Reportable Adverse Event a | ||
| Number of Events | 7 | 6 |
| Participants with ≥ 1 Event | 7 (6·2%) | 6 (5·3%) |
| Specific Events – # Participants [# Events] | ||
| Severe Hypoglycemia b | 0 [0] | 1 [1] |
| Diabetic Ketoacidosis | 0 [0] | 0 [0] |
| Other Serious Adverse Events c | 2 [2] | 0 [0] |
| Hyperglycemia or Ketosis Related to Insulin Pump Problem (without diabetic ketoacidosis) | 3 [3] | 2 [2] |
| Hyperglycemia or Ketosis Events not Related to Insulin Pump Problem (without diabetic ketoacidosis) | 1 [1] | 0 [0] |
| Other Reportable Events Related to Study Device d | 1 [1] | 2 [2] |
| Other Reportable Events Not Related to Study Device e | 0 [0] | 1 [1] |
Reportable adverse events included serious adverse events, adverse device effects, an adverse event occurring in association with study procedure, an adverse event which led to the discontinuation of a study device for two or more hours, hypoglycemia meeting the definition of severe hypoglycemia, and diabetic ketoacidosis
The severe hypoglycemia event in the AHCL arm occurred in a 14 year old participant who forgot to give a manual bolus for dinner bolus but then gave the bolus about an hour after dinner. This was followed by another bolus for a late night snack about one to two hours later. The closed loop system functioned as intended and it is presumed that the event occurred due to the amount of insulin onboard from the boluses.
Hospitalizations due to suicidal tendencies and due to ruptured appendix.
Skin reaction at infusion site (1) and skin reaction around the continuous glucose monitor sensor site (2).
Gastroenteritis
DISCUSSION
In this crossover trial of adolescents and young adults with type 1 diabetes, who had varying degrees of prior use of diabetes devices, the investigational AHCL system was demonstrated to have a greater reduction in hyperglycaemia during the day without an increase in hypoglycaemia compared with the MiniMed 670G system. The effects of the enhancements in the algorithm in the investigational system were evident in the greater time spent in Auto Mode, resulting in an increase in time in range (70–180 mg/dL, 3·9–10·0 mmol/L). The modification of the algorithm also was evident in the way insulin was delivered, as evidenced by a marked shift in the basal to bolus ratio of insulin delivery from being about 1:1 with the MiniMed 670G system to 1:2 with the AHCL system, with just over one-third of boluses delivered as auto-correction boluses.
We selected daytime hyperglycaemia as the primary efficacy outcome as we expected the algorithmic changes to have a greater effect on this outcome than 24-hour glucose control. Thus, we were surprised that the difference in time-in-range and hyperglycaemia between arms appeared greatest from about 5am, which we presume was pre-breakfast on most days, through about 10am; thereafter, the difference between arms appeared to be smaller through the rest of the day and night. The explanation may be that the auto-correction bolus function was activated not only at mealtimes during the day, if needed, but also overnight during periods of significant hyperglycaemia and was more effective combined with the automated basal insulin delivery than the automated basal function alone.
Early real-world use of the first commercially approved hybrid closed-loop system (Medtronic 670G) exposed issues around usability. Real-world use studies of the 670G system have indicated a high discontinuation rate, particularly in adolescents and young adults15–19. Factors influencing discontinuation include continuous glucose monitor issues (e.g., calibrations), number of alarms and efforts to limit Auto Mode exits17,19. In this regard, the frequency of alarms and frequency of exits from auto-mode were substantially lower with the AHCL system than the 670G system. This likely explains at least in part the greater user satisfaction with the AHCL system compared with the 670G system on the Glucose Monitoring Satisfaction Survey. Presumably, greater user satisfaction will result in a lower discontinuation rate of the AHCL system when it is commercially-available compared with the 670G system. Although it was encouraging that use of the AHCL system remained consistent and reasonably high over the three months, the need for a minimum of twice daily fingerstick calibrations of the continuous glucose monitor sensor is a limitation compared with other systems that utilize a factory-calibrated sensor.
Time in range (70–180 mg/dL, 3·9–10·0 mmol/L) increased from 57% to 67% in the AHCL arm compared with 57% to 63% in the 670G arm. The latter is similar to the amount of improvement in the subgroup of 30 adolescents 14 to 21 years old with type 1 diabetes in the 3-month Medtronic 670G single-arm study in whom time in range improved from 60% at baseline to 67% during follow up.6 The 4% increase in mean time-in-range comparing AHCL to 670G represents close to one hour per day with glucose levels between 70 and 180 mg/dL. This mean increase has clinical relevance in that it corresponded with an improvement, shown in a post-hoc analysis, in time in range ≥5% of 65% versus 53%, a relative 23% increase. Time-in-range improvement ≥5% has been cited as clinically relevant12, in part due to an analysis of data from the Diabetes Control and Complications Trial, using standardized blood glucose measurements on one day every 3 months, showing that for every 5% higher time-in-range, the risk of retinopathy was reduced by 28% and microalbuminuria by 18%20.
Two other studies have used the same AHCL investigational system. In a single-arm study of 157 individuals ≥14 years old with type 1 diabetes, mean time in range increased from 69% at baseline (using a sensor-integrated pump with or without a predictive low glucose suspension feature, or a hybrid closed loop system) to 75% over 3 months during which the target set point was 100 mg/dL for half of the time period and 120 mg/dL for the other half21. When the set point was 100 mg/dL and active insulin time was 2 hours, mean time in range was 79%21. In a crossover trial of 59 individuals 7 to 80 years old with type 1 diabetes, mean time in range was 59% at baseline, 58% over four weeks using sensor-augmented pump therapy with predictive low glucose management, and 70% over four weeks using the AHCL system22. With different study designs and cohorts, other closed-loop trials have reported comparable results to those of the AHCL system in adolescents and/or young adults with type 1 diabetes. In a subgroup of 40 participants 14 to 24 years old in a study of the Control IQ system, time in range improved from 51% to 64% over six months.4 Tauschmann et al.23 reported a 14% increase in time in range over 12 weeks in a subgroup of 11 participants 13 to 21 years old. In a younger cohort of 6 to 13 years olds with type 1 diabetes, Breton et al reported an increase in time in range from 53% at baseline to 67% over 16 weeks with the Control IQ system (N=78) compared with 51% to 55% in a control group (N=23) using a continuous glucose monitor and insulin pump5.
This is the first trial to compare an experimental automated insulin delivery system to a commercially approved automated insulin delivery system. Strengths of this trial include a randomized, crossover design which provided inherent control of participant factors that can influence diabetes management, a high retention rate of study participants with all participants using the assigned system in each period, and a broad population of adolescents and young adults across a range of pre-study HbA1c levels and device use experience. Nevertheless, participants in a clinical trial may not be representative of the general population of individuals in this age group with type 1 diabetes. The main limitation is that each study period was only 3 months; thus we cannot determine if the observed benefit with the AHCL system would be sustained over a longer period of system use. Additionally, it cannot be assessed if additional benefit of the AHCL system could be achieved with more aggressive settings for both glucose target and active insulin time, such as starting or rapidly switching to a set point of 100 mg/dL and setting active insulin time to two hours, which was only in use for 24% of participants at the end of the AHCL arm.
In this crossover trial involving adolescents and young adults with type 1 diabetes, an age group in which diabetes self-management and glycaemic control are often suboptimal, daytime hyperglycaemia was reduced without increasing hypoglycaemia using the AHCL system compared with the 670G system. Long-term studies are needed to determine if advanced automated insulin delivery systems, like the AHCL system, will result in reduced complications and improved quality of life for a broad range of individuals by reducing hyperglycaemia, hypoglycaemia, and self-care burden.
Supplementary Material
Research in Context.
Evidence before this study
The objective of the study was to specifically compare the commercially-available Medtronic MiniMed™ 670G hybrid closed-loop system (670G) with a new investigational system the Medtronic advanced hybrid closed-loop system (AHCL). Preliminary unpublished data supported the safety of the AHCL system, suggesting that efficacy might be greater than that with the 670G system. The commercially-available 670G system was evaluated in a single-arm study that led to its regulatory approval and subsequently in several other single-arm studies, but data from a pivotal randomised trial has not been published. There was no evidence prior to this study directly comparing the 670G and AHCL systems.
Added Value of this Study
To our knowledge, this multinational study is the first randomised trial comparing two closed loop systems. In comparing the first-generation closed loop system to the next generation system, the study provided efficacy and safety data needed to evaluate the benefits and risks of the new system. The trial included adolescents and young adults with type 1 diabetes, the age group in which diabetes management and glycaemic control is the most challenging. Participants covered a wide range of screening HbA1c levels (7·0% to 10·9%, 53 mmol/mol to 96 mmol/mol) with 20% not using an insulin pump and 38% not using continuous glucose monitoring. The results showed that the AHCL system reduced hyperglycaemia and improved the percentage of time in the target range (70–180 mg/dL, 3·9–10·0 mmol/L) with no increase in hypoglycaemia.
Implications of All the Available Evidence
The results support the efficacy and safety of the new AHCL system, including the safety of automated correction boluses, compared with the existing 670G system. After the AHCL system receives regulatory approval, it can be expected to provide benefit to adolescents and young adults with type 1 diabetes across the broad range of eligibility criteria for the trial participants.
Acknowledgments
Funding/Support
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number C4DK10861. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Non-financial support: Medtronic MiniMed, Inc. provided the MiniMed 670G and AHCL systems, and infusion sets, used in the study.
Role of the Funder/Sponsor
The role of the Sponsor is specified for each of the following:
Design and conduct of the study: There was no involvement from the National Institute of Diabetes and Digestive and Kidney Diseases or Medtronic in the design and conduct of the study.
Collection, management, analysis, and interpretation of the data: There was no involvement from the National Institute of Diabetes and Digestive and Kidney Diseases or Medtronic in the collection, management, analysis, and interpretation of the data.
Preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication: The National Institute of Diabetes and Digestive and Kidney Diseases and Medtronic were not involved in the writing of the original manuscript draft submitted. The National Institute of Diabetes and Digestive and Kidney Diseases and Medtronic were sent the manuscript for review but any revisions made based on their comments were at the discretion of the authors and permission for submitting content to journal was not required. There was no approval of the National Institute of Diabetes and Digestive and Kidney Diseases and Medtronic required or obtained for manuscript submission.
Funding: National Institute of Diabetes and Digestive and Kidney Diseases UC4DK108611
Footnotes
Data Sharing Statement: Data will be made available on a public website (www.jaeb.org) at a later date.
Dr. Bergenstal had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The trial results were presented in part at the 80th Scientific Sessions (virtual) of the American Diabetes Association, June 12, 2020.
Study Group Members
Protocol Chairs
Protocol Chairs and Co-PI: Richard M. Bergenstal, MD and Moshe Phillip, MD
Clinical Sites
A listing of the FLAIR sites with participating principal investigator (PI), co-investigators (CI), sub-investigators (I), primary coordinator (PC), coordinators (C), study nurses (SN), research assistants (RA), project manager (PM), and administrative manager (AM) is included below. The number included in the randomized trial at each site is indicated in parenthesis after the sites’ name.
International Diabetes Center (23): Amy Criego (PI), Richard Bergenstal (PI), Anders Carlson (PI), Thomas Martens (I), Shannon Beasley (I), Mary Johnson (PM), Diane Whipple (PC), Jamie Hyatt (C), Alina Punel (C), Aimee Grieme (C), Lee Ann Thomas (RA), Amy LaFrance (AM), Caitlin Hasledalen (RA)
Joslin Diabetes Center (19): Lori Laffel (PI), Elvira Isganaitis (I), Emily Freiner (PC, I), Louise Ambler-Osborn (I), Hannah Desrochers (C), Christine Turcotte (C), Nisha Naik (C), Lindsay Roethke (C), Margaret Fisher (C)
Schneider Children’s Hospital (17): Revital Nimri (PI), Michal Nevo (I), Rachel Bello (I), Alona Hamou (PC), Orna Hermon (PC), Orit Horesh (SN), Galit Shiovitch Mantzuri (SN), Irit Drotz (SN), Nava Yehiel (SN), Rachel Naveh (C)
University of Florida (17) : Desmond Schatz (PI), Michael Haller (CI), Anastasia Albanese-O’Neill (CI), Eleni Sheehan (I), Julio Leey (I), Madison Smith (I), Laura Jacobsen (I), Janey Adams (PC), Jennifer Hosford (C), Loren Whyte (C)
University Medical Centre Ljubljana (16): Tadej Battelino (PI), Klemen Dovč (I), Nataša Bratina (I), Darja Šmigoc Schweiger (I), Urška Sever (C), Ana Gianini (C), Barbara Murn Berkopec (C), Brigita Mali (C)
Yale University (11): Stuart Weinzimer (PI), Kate Weyman (I), Lori Carria (PC), Melinda Zgorski (C)
Auf der Bult Centre for Children and Adolescents (10): Thomas Danne (PI), Torben Biester (PI), Thekla von dem Berge (I), Jantje Weiskorn (I), Olga Kordonouri (I), Sarah Biester (PC), Bärbel Aschemeier (C), Kerstin Remus (C), Nicole Pisarek (C)
Jaeb Center for Health Research Tampa, FL
Judy Sibayan, Roy Beck, Thomas Mouse, Julie Davis, Ryan Bailey, Amanda Hellmann, Nicole Reese, Peter Calhoun, Heidi Strayer, Nathan Cohen, Robert Henderson, Craig Kollman, Jennifer Kennedy, William Woodall, Israel Mahr
Quality of Life Investigator
Korey Hood, Stanford University
National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK)
Guillermo Arreaza-Rubin (Project Scientist), Thomas Eggerman (Program Officer), Neal Green (Project Manager)
Central Laboratory - University of Minnesota Advanced Research and Diagnostic Laboratory
Robert Janicek, Deanna Gabrielson
Data and Safety Monitoring Board (DSMB)
Steven H. Belle (Chair), Jessica Castle, Jennifer Green, Laurent Legault, Steven M. Willi, Carol Wysham, Thomas Eggerman (DSMB Executive Secretary for NIDDK)
Contributors
R. Bergenstal, R. Beck, and M. Phillip drafted manuscript. R. Bailey and P. Calhoun performed the statistical analysis. A. Criego, D. Schatz, S. Weinzimer, J. Sibayan, M. Johnson, A. Carlson, E. Isganaitis, R. Bello, A. Albanese-O’Neill, K. Weyman, K. Hood, R. Nimri, L. Laffel, T. Battelino, T. Danne, K. Dovc, and T. Biester reviewed and edited manuscript.
Declaration of Interests
Financial funding support: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (IUC4DK10861); grant provided to Jaeb Center for Health Research and paid to each of the investigator’s institutions.
Non-financial support: Medtronic Inc; provided study continuous glucose monitoring devices and sensors.
Additional financial disclosures are as follows:
R. Bergenstal reports grants and other from Abbott Diabetes Care, grants and other from Eli Lilly, outside the submitted work. R. Nimri reports grants from National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, non-financial support from Medtronic MiniMed, Inc during the conduct of the study; grants, personal fees and other from DreaMed Diabetes Ltd., grants and non-financial support from Medtronic MiniMed, Inc, non-financial support from Dexcom, non-financial support from Insulet, grants and personal fees from Eli Lilly, grants and personal fees from Novonordisk, outside the submitted work. R. Beck reports grants from National Institutes of Health, non-financial support from Medtronic during the conduct of the study; grants, non-financial support and study supplies from Dexcom, consulting fees paid to non-profit employer from Bigfoot Biomedical, outside the submitted work. A. Criego reports receiving grants from Dexcom, Medtronic, Insulet, Juvenile Diabetes Research Foundation, the National Institutes of Health, and Abbott and study supplies from Eli Lilly, outside the submitted work. L. Laffel reports grants from the National Institutes of Health during the conduct of the study; personal fees from Sanofi, NovoNordisk, Eli Lilly, Roche, Lifescan, Convatec, Insulogic, Laxmi, Boehringer Ingelheim, Johnson & Johnson, Dexcom, and Insulet, outside the submitted work. D. Schatz reports grants from the National Institutes of Health, during the conduct of the study; grants from the Leona M. and Harry B. Helmsley Charitable Trust, outside the submitted work. T. Battelino reports grants from the National Institutes of Health, during the conduct of the study; personal fees from Eli Lilly, Sanofi, NovoNordisk, Medtronic, Abbott, Dexcom, Astra Zeneca, Indigo, owning stock in DreaMed Ltd, grants from Zealand, Sanofi, NovoNordisk, INNODIA, and the Slovenian Research Agency, outside the submitted work. T. Danne reports owning stock in DreaMed Ltd. during the conduct of the study; grants and personal fees from AstraZeneca, Dexcom, Boehringer, NovoNordisk, Medtronic, Sanofi, Eli Lilly, and Insulet, outside the submitted work. S. Weinzimer reports grants from the National Institutes of Health, during the conduct of the study; personal fees from Medtronic, Insulet, Tandem, Eli Lilly, Sanofi, and Zealand, outside the submitted work. J. Sibayan reports no conflicts of interest. M. Johnson reports grants from the National Institutes of Health during the conduct of the study; grants from Abbott, Dexcom, Medtronic, Eli Lilly, NovoNordisk, NIH, Calibra, Insulet, Sanofi, the Leona M. and Harry B. Helmsley Charitable Trust, United Health, and the Juvenile Diabetes Research Foundation, outside the submitted work. R. Bailey reports no conflicts of interest. P. Calhoun reports personal fees from Dexcom outside the submitted work. A. Carlson reports grants from NovoNordisk, grants and speaker/consultancy fees from Medtronic, grants from Insulet, grants and consultancy fees from Sanofi, grants from Dexcom, grants from Abbott, grants and consultancy fees from Eli Lilly, grants from UnitedHealth, and grants from Companion Medical, outside the submitted work. E. Isganaitis reports no conflicts of interest. R. Bello reports grants from the National Institutes of Health, non-financial support from Medtronic Minimed, Inc, during the conduct of the study. A. Albanese-O’Neill reports grants from the National Institutes of Health, during the conduct of the study; grants from Helmsley Charitable Trust, grants from Dexcom Inc, non-profit employer has training contracts with Medtronic, Insulet, and Tandem, outside the submitted work. K. Dovc reports personal fees from Pfizer, Eli Lilly, and Sanofi Aventis, outside the submitted work. T. Biester reports grants and personal fees from Medtronic during the conduct of the study; personal fees from Yposmed, AstraZeneca, Roche, Dexcom, NovoNordisk, and Sanofi outside the submitted work. K. Weyman reports grants from National Institutes of Health during the conduct of the study. K. Hood reports grants from Dexcom, personal fees from Lifescan Diabetes Institute and Cercacor Inc, outside the submitted work. M. Phillip reports grants from the National Institutes of Health via Health Partners, during the conduct of the study; grants from Insulet, Dexcom, Medtronic, Roche, Eli Lilly, Lexicon, OPKO, and AstraZeneca, grants and personal fees from Novo Nordisk and Sanofi, grants, personal fees and owning stock from DreaMed Diabetes, personal fees from RSP Systems and Qulab Medical, grants and personal fees from Pfizer, grants and owning stock from Nutriteen Professional and NG Solutions, outside the submitted work. In addition, Dr. Phillip has a patent WO2010097796 issued to DreaMed Diabetes, a patent WO2011039741 issued to DraeMed Diabetes, and a patent WO2019077482 pending.
References
- 1.Martin CT, Criego AB, Carlson AL, Bergenstal RM. Advanced Technology in the Management of Diabetes: Which Comes First-Continuous Glucose Monitor or Insulin Pump? Curr Diab Rep 2019; 19(8): 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck RW, Bergenstal RM, Laffel LM, Pickup JC. Advances in technology for management of type 1 diabetes. Lancet 2019; 394(10205): 1265–73. [DOI] [PubMed] [Google Scholar]
- 3.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes. JAMA 2016; 316(13): 1407–8. [DOI] [PubMed] [Google Scholar]
- 4.Brown SA, Kovatchev BP, Raghinaru D, et al. Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N Engl J Med 2019; 381(18): 1707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breton MD, Kanapka LG, Beck RW, et al. A Randomized Trial of Closed-Loop Control in Children with Type 1 Diabetes. N Engl J Med 2020; 383: 836–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther 2017; 19(3): 155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isganaitis E, Raghinaru D, Ambler-Osborn L, et al. Closed-loop control (CLC) in teens and young adults improves glycemic control: Results from the International Diabetes Closed-Loop (iDCL) Trial. J Diabetes Sci Technol 2020; 14(2): A53. [Google Scholar]
- 8.Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes care 2010; 33(5): 1072–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nimri R, Muller I, Atlas E, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes care 2014; 37(11): 3025–32. [DOI] [PubMed] [Google Scholar]
- 10.Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013; 368(9): 824–33. [DOI] [PubMed] [Google Scholar]
- 11.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329(14): 977–86. [DOI] [PubMed] [Google Scholar]
- 12.Battelino T, Danne T, Bergenstal RM, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes care 2019; 42(8): 1593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polonsky WH, Fisher L, Hessler D, Edelman SV. Development of a New Measure for Assessing Glucose Monitoring Device-Related Treatment Satisfaction and Quality of Life. Diabetes Technol Ther 2015; 17(9): 657–63. [DOI] [PubMed] [Google Scholar]
- 14.Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with indepentdent statistics. Journal of Educational and Behavioral Statistics 2000; 25(1): 60–83. [Google Scholar]
- 15.Akturk HK, Giordano D, Champakanath A, Brackett S, Garg S, Snell-Bergeon J. Long-term real-life glycaemic outcomes with a hybrid closed-loop system compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes Obes Metab 2020; 22(4): 583–9. [DOI] [PubMed] [Google Scholar]
- 16.Berget C, Messer LH, Vigers T, et al. Six months of hybrid closed loop in the real-world: An evaluation of children and young adults using the 670G system. Pediatr Diabetes 2020; 21(2): 310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffus SH, Ta’ani ZA, Slaughter JC, Niswender KD, Gregory JM. Increased proportion of time in hybrid closed-loop “Auto Mode” is associated with improved glycaemic control for adolescent and young patients with adult type 1 diabetes using the MiniMed 670G insulin pump. Diabetes Obes Metab 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One Year Clinical Experience of the First Commercial Hybrid Closed-Loop System. Diabetes care 2019; 42(12): 2190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: Predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes 2020; 21(2): 319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of Time in Range as an Outcome Measure for Diabetes Clinical Trials. Diabetes care 2019; 42(3): 400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson A, Bode BW, Brazg RL, et al. Safety and glycemic outcomes of the MiniMed Advanced Hybrid Closed-Loop (AHCL) system in subjects with T1D. Diabetes 2020; 69(Suppl 1): 97-LB. [Google Scholar]
- 22.Collyns O, Meier R, Betts Z, et al. Improved glycemic outcomes with Medtronic Minimed Advanced Hybrid Closed-Loop delivery: Results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes 2020; 69(Suppl 1): 199-OR. [DOI] [PubMed] [Google Scholar]
- 23.Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018; 392(10155): 1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.