Abstract
Objective
Hemorrhage is the most common complication caused by transoral laryngopharyngeal surgery. It is believed that proper management of the superior laryngeal artery (SLA), the main feeding artery for the larynx and pharynx, may reduce intra‐ and postoperative hemorrhage incidence. The aim of this study was to illustrate the anatomy of the SLA via transoral endoscopic approach.
Methods
Fourteen sides of SLA from heads of seven fresh‐frozen and silicone‐injected cadavers were dissected. Transoral dissections were performed for the intra‐laryngeal segment of SLA, and transcervical dissections were performed to confirm the anatomical measurements.
Results
SLA had a slightly descending course from the origin to the larynx, and there was a major branch supplying the epiglottis, named pharyngo‐epiglottic artery (PEA). Parallel with the internal superior laryngeal nerve (ISLN), SLA passed through the thyrohyoid membrane and ended into the hypopharynx. The distance from SLA to the superior horn of thyroid cartilage (SHTC) was (9.11 ± 0.58)mm on the left and (9.01 ± 0.37)mm on the right; the distance from SLA to the inferior margin of the hyoid bone (IMHB) was (2.00 ± 0.11)mm on the left and (1.95 ± 0.08)mm on the right; the distance from SLA to ISLN was (5.98 ± 0.48)mm on the left and (5.78 ± 0.36)mm on the right. No significant difference was found between bilateral sides (p > 0.05). Moreover, the distance from SLA to superior margin of thyroid cartilage (SMTC) was (5.52 ± 0.24)mm on the left and (5.80 ± 0.15)mm on the right. A significant difference was also found between bilateral sides (p = 0.03), which might suggest the SLA is located further from the SMTC on the right side.
Conclusion
SHTC, SMTC, and IMHB could be regarded as anatomical landmarks to locate SLA when applying a transoral approach. Moreover, a complete understanding of the detailed anatomy of the superior laryngeal artery may improve the detection of hemostasis in transoral laryngeal or hypo‐pharyngeal surgery.
Keywords: anatomical landmark, anatomy, superior laryngeal artery, transoral approach
1. INTRODUCTION
Transoral surgery (TOS) includes transoral laser surgery (TOLS), transoral videolaryngoscopic surgery (TOVS), transoral robotic surgery (TORS), and transoral radiofrequency coblation surgery (TRS). 1 , 2 In 1975, Strong et al. 3 introduced the TOLS for the treatment of laryngeal carcinoma. Since then, TOS has been widely used to treat early, advanced, or recurrent laryngeal carcinoma, obtaining excellent outcomes and providing safety in terms of organ preservation and local control. 4 , 5 However, postoperative hemorrhage is the most common complication after TOS, with an incidence of 3.1%–7.5%. 6 , 7 , 8 The incidence of postoperative hemorrhage in the supra‐glottic laryngeal carcinoma has been reported to vary from 7% to 8.5%. 9 , 10 In some cases, severe bleeding may lead to patient's death. 6
The superior laryngeal artery (SLA) is the main feeding artery for the larynx and pharynx. It is believed that proper management of the SLA via transoral approach may reduce intra‐ and postoperative hemorrhage incidence. Yet, the transoral anatomy of SLA remains poorly understood. In this study, we used a transoral approach to further explain the detailed anatomy of SLA.
2. MATERIALS AND METHODS
2.1. Specimen preparation
This study was approved by the Institutional Review Board of Peking University First Hospital.
The anatomical dissections were conducted at the anatomy center in Peking University First Hospital. The specimens were collected from voluntary body donations to the Peking University First Hospital. In our study, we used 14 sides of SLA from heads of seven adult male fresh‐frozen and silicone‐injected cadavers with no history of trauma, surgery, and congenital malformation.
Transoral dissections were assisted by an endoscope (Karl Storz, Tuttlingen, Germany), suspension laryngoscope including the duck‐billed and Feyh‐Kastenbauer suspension laryngoscope, and microdissection instruments.
2.2. Anatomic dissection
Specimens were dissected using a transoral approach, and transcervical dissections were performed to confirm our anatomic measurements. In the transoral approach, the dissections were performed with the help of a suspension laryngoscope, using a superficial‐to‐deep method with endoscopic photo‐documentation at each layer, and carefully protecting the underlying structures. The distance was measured by a standard flexible surgical ruler.
2.3. Anatomic measurements
The following anatomic measurements were applied: (1) the course of SLA via transoral approach; (2) relationship with the superior horn of thyroid cartilage (SHTC), inferior margin of hyoid bone (IMHB), superior margin of thyroid cartilage (SMTC), and internal superior laryngeal nerve (ISLN); (3) branches of SLA; (4) variations of SLA origin.
3. RESULTS
3.1. The course of SLA
Since it branched from the superior thyroid artery, the SLA had a slightly descending course toward the SHTC and was separated by tiny branches feeding the constrictors on the way, after which it penetrated the thyrohyoid membrane. When reaching into the intra‐larynx, it was directed anteriorly along the superior border of the thyroid cartilage in the para‐glottic space until arriving at the pharyngo‐epiglottic fold, after which SLA separated into a major branch named as pharyngo‐epiglottic artery (PEA), adopting a slightly ascending course to feeding the epiglottis (Figure 1). The trunk of SLA had a posterior‐inferior direction, after which it branched into two main terminal branches, the anterior‐inferior branch and the posterior‐inferior branch. The terminal branches were mutually anastomosed and also anastomosed with the branches from the inferior laryngeal artery and cricothyroid artery.
FIGURE 1.

SLA and ISLN anatomy via transoral approach. AEF, aryepiglottic fold; HB, hyoid bone; ISLN, superior laryngeal nerve; PS, pyriform sinus; SLA, superior laryngeal artery; TC, thyroid cartilage
3.2. Relationship with the landmarks
The landmarks included the SHTC, IMHB, and SMTC (Figure 2). The distance from SLA to SHTC was (9.11 ± 0.58)mm on the left and (9.01 ± 0.37)mm on the right; the distance from SLA to IMHB was (2.00 ± 0.11)mm on the left and (1.95 ± 0.08)mm on the right; the distance from SLA to ISLN was (5.98 ± 0.48)mm on the left and (5.78 ± 0.36)mm on the right. No significant difference was found between bilateral sides (p > 0.05). In addition, the distance from SLA to SMTC was (5.52 ± 0.24)mm on the left and (5.80 ± 0.15)mm on the right; the difference between bilateral sides was also observed (p = 0.03) (Table 1).
FIGURE 2.

Diagram for the measurements of the superior laryngeal artery with the landmarks. EC, extension cord; HB, hyoid bone; ISLN, superior laryngeal nerve; SLA, superior laryngeal artery; TC, thyroid cartilage
TABLE 1.
Distance measurements between SLA with landmarks
| Landmarks | Left | Right | Max | Min | p |
|---|---|---|---|---|---|
| SHTC | 9.11 ± 0.58 | 9.01 ± 0.37 | 10.2 | 8.44 | 0.74 |
| IMHB | 2.00 ± 0.11 | 1.95 ± 0.08 | 2.12 | 1.83 | 0.45 |
| SMTC | 5.52 ± 0.24 | 5.80 ± 0.15 | 6.01 | 5.23 | 0.03* |
| ISLN | 5.97 ± 0.48 | 5.77 ± 0.36 | 6.82 | 5.13 | 0.43 |
Note: M ± Std, Max, Min unit: mm. Statistically significant differences between both sides: *p < 0.05.
Abbreviations: IMHB, inferior margin of hyoid bone; ISLN, superior laryngeal nerve; SHTC, superior horn of thyroid cartilage; SLA, superior laryngeal artery; SMHT, superior margin of thyroid cartilage.
3.3. Branches of the SLA
The major branch, described by Rusu et al. 11 as an anterior branch and we named as pharyngo‐epiglottic artery, penetrated the epiglottis at the lateral margin to supply the epiglottis (Figure 3).
FIGURE 3.

PEA pierced into the epiglottis at the lateral margin. E, epiglottis; LA, lingual artery, LA‐B, branch of lingual artery; PEA, pharyngo‐epiglottic artery; PS, pyriform sinus; TB, tongue base; TC, thyroid cartilage
3.4. Variations of SLA origin
The origin of SLA was not only the superior thyroid artery (STA) but also the lingual artery (LA) and external carotid artery (ECA) (Figure 4). There were two specimens that originated from the LA, both on the right sides, and one specimen that originated from LA on the right and ECA on the left. Though anatomical variations of SLA origin, the location in which SLA passed through the thyrohyoid membrane were relatively stable.
FIGURE 4.
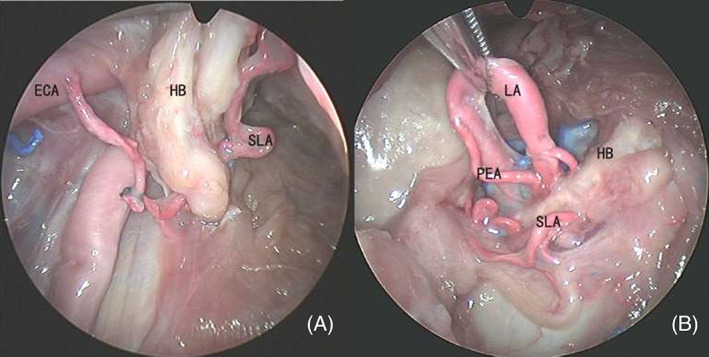
Variations of SLA. ECA, external carotid artery; HB, hyoid bone; LA, lingual artery; PEA, pharyngo‐epiglottic artery; SLA, superior laryngeal artery
4. DISCUSSION
In this study, we explored the anatomy of the SLA via a transoral endoscopic approach. The SLA can originate from different places. 12 Our data suggested that SLA originates from the superior thyroid artery but also the lingual artery and external carotid artery. Moreover, the location where SLA passes through the thyrohyoid membrane and the course of intra‐laryngeal segment were relatively stable.
The total laryngectomy in transoral robotic approach was first successfully performed at the Montefiore Medical Center in July of 2007. 13 Since then, it has been widely accepted in clinical work. Previous studies have suggested that hemostatic clip application to the bilateral superior laryngeal vascular pedicles is necessary for successful transoral surgery. 13 , 14 , 15 , 16 Our study showed that the SHTC, SMTC, and IMHB could be regarded as anatomical landmarks to locate SLA, thus providing a reference for the efficient hemostasis in transorally laryngeal or pharyngeal surgery. During the transoral surgery, we suggested that the assistant can move the laryngeal prominence; meanwhile, the operator can observe the movement of the thyroid cartilage through the suspension laryngoscope and then locate the SHTC. In this study, the distance from SLA to SMTC between bilateral sides was different (p = 0.03), suggesting that the SLA was located further to SMTC on the right side. However, further studies with a larger sample size should be performed to confirm these findings.
ISLN was paralleled with SLA, and the closest distance between the SLA and ISLN was observed around the SHTC. Consequently, there is a possibility to damage the ISLN during surgery when dealing with the SLA around the SHTC. In our study, we also found that the SLA was away from the ISLN when going through the pharyngo‐epiglottic fold, and the distance between the SLA and ISLN was wider when approaching the epiglottis. Thus, pharyngo‐epiglottic fold may be another landmark when dealing with SLA, especially when treating laryngeal carcinoma of the supra‐glottic type.
PEA is the major branch from SLA that supplies the tissue of epiglottis. For cases of epiglottic carcinoma, accurate homeostasis of PEA at the lateral margin of epiglottis may promote the smooth surgery procedure, shorten the operative time, and preserve the blood supply for the glottis.
Previous studies have reported on the aberrant superior laryngeal artery (ASLA). 10 , 11 , 17 Rusu et al. 11 discovered a branch of STA traversing the foramen thyroideum to supply the larynx, with an emerged frequency of 5%. In our study, no ASLA was observed, but it is still necessary to pay attention to the ASLA, as it may result in an alarming postoperative hemorrhage. 10
This study has a few limitations: (1) there are only seven cadaveric heads, and samples are too small, thus resulting in the bias of the statistics; (2) all cadavers in this study were adult males from China; (3) the cadaveric heads were transected at the seventh cervical vertebra‐first thoracic vertebra level, so the tension of the cervical vascular and muscle was lost, thus limiting the precision of measurements.
5. CONCLUSION
This study provided a comprehensive and detailed description of the endoscopic clinical anatomy of SLA. The SHTC, SMTC, and IMHB could be regarded as anatomical landmarks for locating the SLA via the transoral approach.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
ACKNOWLEDGMENT
We are deeply grateful to the cadaver donors without whom this work would not have been completed.
Jia J, Zhang J, Zeng Z, et al. Clinical anatomy of superior laryngeal artery via transoral approach. Laryngoscope Investigative Otolaryngology. 2022;7(3):702-706. doi: 10.1002/lio2.781
Funding information None.
REFERENCES
- 1. Okami K, Ebisumoto K, Sakai A, et al. Transoral en bloc resection of superficial laryngeal and pharyngeal cancers. Head Neck. 2013;35(8):1162‐1167. [DOI] [PubMed] [Google Scholar]
- 2. Carney AS, Timms MS, Marnane CN, Krishnan S, Rees G, Mirza S. Radiofrequency coblation for the resection of head and neck malignancies. Otolaryngol Head Neck Surg. 2008;138(1):81‐85. [DOI] [PubMed] [Google Scholar]
- 3. Strong MS. Laser excision of carcinoma of the larynx. Laryngoscope. 1975;85(8):1286‐1289. [DOI] [PubMed] [Google Scholar]
- 4. Canis M, Ihler F, Martin A, Matthias C, Steiner W. Transoral laser microsurgery for T1a glottic cancer: review of 404 cases. Head Neck. 2015;37(6):889‐895. [DOI] [PubMed] [Google Scholar]
- 5. Weiss BG, Bertlich M, Canis M, Ihler F. Transoral laser microsurgery or total laryngectomy for recurrent squamous cell carcinoma of the larynx: retrospective analysis of 199 cases. Head Neck. 2017;39(6):1166‐1176. [DOI] [PubMed] [Google Scholar]
- 6. Stokes W, Ramadan J, Lawson G, Ferris FL, Holsinger FC, Turner T. Bleeding complications after transoral robotic surgery: a meta‐analysis and systematic review. Laryngoscope. 2021;131(1):95‐105. 10.1002/lary.28580 [DOI] [PubMed] [Google Scholar]
- 7. Chia SH, Gross ND, Richmon JD. Surgeon experience and complications with transoral robotic surgery (TORS). Otolaryngol Head Neck Surg. 2013;149(6):885‐892. [DOI] [PubMed] [Google Scholar]
- 8. Asher SA, White HN, Kejner AE, Rosenthal EL, Carroll WR, Magnuson JS. Hemorrhage after transoral robotic‐assisted surgery. Otolaryngol Head Neck Surg. 2013;149(1):112‐117. [DOI] [PubMed] [Google Scholar]
- 9. Vilaseca‐Gonzalez I, Bernal‐Sprekelsen M, Blanch‐Alejandro JL, Moragas‐Lluis M. Complications in transoral CO2 laser surgery for carcinoma of the larynx and hypopharynx. Head Neck. 2003;25(5):382‐388. [DOI] [PubMed] [Google Scholar]
- 10. Weingarten TN, Bojanic K, Scavonetto F, Sprung J. Management of delayed hemorrhage after partial vocal cord cordectomy. J Clin Anesth. 2013;25(8):666‐668. [DOI] [PubMed] [Google Scholar]
- 11. Rusu MC, Nimigean V, Banu MA, Cergan R, Niculescu V. The morphology and topography of the superior laryngeal artery. Surg Radiol Anat. 2007;29(8):653‐660. [DOI] [PubMed] [Google Scholar]
- 12. Vázquez T, Cobiella R, Maranillo E, et al. Anatomical variations of the superior thyroid and superior laryngeal arteries. Head & Neck. 2009;31:(8):1078‐1085. 10.1002/hed.21077 [DOI] [PubMed] [Google Scholar]
- 13. Smith RV, Schiff BA, Sarta C, Hans S, Brasnu D. Transoral robotic total laryngectomy. Laryngoscope. 2013;123(3):678‐682. [DOI] [PubMed] [Google Scholar]
- 14. Lawson G, Mendelsohn AH, Van Der Vorst S, Bachy V, Remacle M. Transoral robotic surgery total laryngectomy. Laryngoscope. 2013;123(1):193‐196. [DOI] [PubMed] [Google Scholar]
- 15. Genden EM, Desai S, Sung CK. Transoral robotic surgery for the management of head and neck cancer: A preliminary experience. Head & Neck. 2009;31(3):283‐289. 10.1002/hed.20972 [DOI] [PubMed] [Google Scholar]
- 16. Dowthwaite S, Nichols AC, Yoo J, et al. Transoral robotic total laryngectomy: report of 3 cases. Head Neck. 2013;35(11):E338‐E342. [DOI] [PubMed] [Google Scholar]
- 17. Anthony JP, Argenta P, Trabulsy PP, Lin RY, Mathes SJ. The arterial anatomy of larynx transplantation: microsurgical revascularization of the larynx. Clin Anat. 1996;9(3):155‐159. [DOI] [PubMed] [Google Scholar]


