Abstract
Aquatic hyphomycetes dominate leaf decomposition in streams, and their biomass is an important component in the diet of leaf-eating invertebrates. After 2 weeks of exposure in a first-order stream, maple leaf disks had low levels of fungal biomass and species diversity. Spore production by aquatic hyphomycetes also was low. Subsets of these disks were left in the stream for another 3 weeks or incubated in defined mineral solutions with one of three levels of nitrate and phosphate. Stream disks lost mass, increased ergosterol levels and spore production, and were colonized by additional fungal species. External N and P significantly stimulated mass loss, ergosterol accumulation, and spore production of laboratory disks. On disks incubated without added N and P, ergosterol levels declined while conidium production continued, suggesting conversion of existing hyphal biomass to propagules. In all other treatments, approximately equal amounts of newly synthesized biomass were invested in hyphae and conidia. Net yield (fungal biomass per leaf mass lost) varied between 1% (in the laboratory, without added N or P) and 31% (decay in stream). In most treatments, the three aquatic hyphomycete species that dominated spore production during the first 2 weeks in the stream also produced the largest numbers of conidia in the following 3 weeks. Principal-component analysis suggested two divergent trends from the initial fungal community established after 2 weeks in the stream. One culminated in the community of the second phase of stream exposure, and the other culminated in the laboratory treatment with the highest levels of N and P. The results suggest that fungal production in streams, and, by extension, production of invertebrates and higher tropic levels, is stimulated by inorganic N and P.
In a pioneering study of leaf decay in streams, fungi were shown to be more active than bacteria during the early stages, and fungal growth was often accompanied by an absolute increase in the nitrogen content of the substrate (21). These results imply that fungi acquire nitrogen from water flowing over the leaf surface. Increased nitrogen levels of decaying leaves make them more palatable and nutritious to stream invertebrates; fungi therefore act as an intermediate trophic level between autumn-shed leaves (the dominant source of food in most small streams) and leaf-eating invertebrates (4, 6, 8, 34, 41).
The fungi dominating leaf decomposition in streams are aquatic hyphomycetes, a phylogenetically heterogenous group (4, 40). When leaves are exposed in streams, fungal biomass (estimated by ergosterol levels) rapidly increases to a peak of up to 17% of total detrital mass (16, 19), and it may remain at this level for some time before gradually declining. In addition to increasing their biomass on the leaves, the fungi also release large numbers of conidia into the stream. Conidium production often is estimated by aerating stream-exposed leaves in the laboratory and collecting newly formed spores on filters (3). Up to 8 conidia day−1 μg of detrital mass−1, corresponding to approximately 5 mg g−1, has been reported (16). The contributions of the individual species to total spore production have been used to characterize fungal communities present during various stages of decay (3). Generally, more than 90% of all spores are produced by one to four species (6). There is some evidence of successional trends (9, 20). These changes may be in response to seasonal changes in temperature (32, 42), or rare species might arrive late during the decay of a substrate (2). More commonly, changes are due to shifts in the relative frequencies of species that appear early and persist during the leaf's decay (6), suggesting that each leaf receives an imprint when submersed in a stream that largely determines the subsequent development of the fungal community and, presumably, its contribution to leaf decay.
Fungal biomass, sporulation and enzymatic activities, and community structure are all influenced by leaf composition and by environmental factors such as water temperature and chemistry, which often fluctuate during extended periods of decay (3, 18, 30–32, 38, 42). The often-observed absolute increase in nitrogen levels of decaying leaves implies that fungi may acquire nitrogen and other inorganic nutrients from water flowing over the leaf surface. Not surprisingly, nitrogen and phosphorus have often been mentioned as potentially limiting factors of fungal activities.
Our objectives in this study were to investigate the relative impact of two factors on the fungal community developing on leaves: an early phase of stream exposure, which provides a fungal imprint, and a second, subsequent, laboratory phase, where stream-exposed leaves were incubated with various levels of nitrogen and phosphorus. We exposed leaves in a New Brunswick, Canada, stream for 2 weeks, which allows fungal colonization but is too short to result in large increases in biomass and spore production. In the laboratory, subsamples of these leaves were exposed to various levels of nitrogen and phosphorus. We hypothesize that production of fungal mycelia and conidia is strongly correlated with external nutrient supplies in the second phase. We expect little change in diversity and composition of the fungal community after the initial stream phase, regardless of subsequent nutrient regime. In addition to shedding light on factors shaping the fungal community, our study also provides information on fungal production in streams, which has implications for activity by invertebrates and higher trophic levels (4, 32).
MATERIALS AND METHODS
Study site.
The field experiment was conducted in Allen Creek (Wood Point, New Brunswick, Canada), a small stream (5) running through a mixed forest of maples (Acer rubrum L., A. saccharum Marsh., and Acer spicatum Lam.), birches (Betula alleghaniensis Britton and Betula papyrifera Marsh.), alder (Alnus rugosa [Du Roi] Spreng.), and conifers {Picea rubens Sarg., Picea glauca (Moench) Voss, Pinus strobus L., and Tsuga canadensis [L.] Carr.}. During a previous 12-month period, its pH varied between 4.5 and 6.9, and its conductance at 25°C varied between 20 and 30 μS (5). Stream water (SW) used in laboratory experiments was collected on 13 May 1997, filtered through Whatman no. 1 filter paper, autoclaved, and stored under sterile conditions until used.
Field experiment.
Leaves of Acer saccharum were collected in the fall of 1996, cut into 12-mm-diameter disks, and dried at room temperature. They were leached for 2 days under running tap water, dried again, and stored at room temperature. Sets of 10 preweighed disks were placed in litterbags (10 by 10 cm, 2-mm mesh size), which were attached to bricks placed in Allen Creek. The field study was initiated on 3 June 1997 by introducing 25 bricks, each with four bags. Each week, the contents of four randomly selected bags were used to determine fungal spore production; the contents of another four bags were used to estimate remaining mass and ergosterol content. On June 17, 30 additional bags were recovered for a laboratory study.
Water parameters.
On each sampling date, temperature and pH were measured with field instruments. Standard techniques were used to measure alkalinity (titration with HCl), nitrate (cadmium reduction), and orthophosphate (22).
Spore production.
Leaf disks recovered from the stream were aerated for 2 days at 18°C to induce spore formation (200 ml of autoclaved distilled water in a 250-ml flask). The entire volume with suspended conidia was filtered through an 8-μm-pore-size membrane filter (3). The filter was stained with cotton blue in lactophenol for 30 min at 40°C, and then the conidia trapped on the filter were counted and identified at magnifications between ×160 and ×1,000. Where possible, 200 to 300 conidia were identified to determine percentage distributions of the various species; in addition, the entire filter was scanned for rare taxa. The leaf material corresponding to the filters was dried and weighed, and results are expressed as conidia day−1 milligram of dry mass−1.
Ergosterol.
Ergosterol extraction and analysis methods were adapted from the work of Newell et al. (26). Freeze-dried leaf disks were refluxed in 85 ml of methanol plus 5 ml of 4% KOH (generally, 200 to 500 mg of leaf material; 30 min at 80°C). Sterols were partitioned into pentane (three successive washes). The pentane fractions were evaporated to dryness under an N2 stream, and the residue was redissolved in 1 ml of methanol and injected into a Gilson high-performance liquid chromatography system (Mandel, Guelph, Ontario, Canada). Ergosterol content was estimated with a UV detector at 282 nm (elution, 7.5 min).
Laboratory experiment.
The contents of each bag collected after 2 weeks of stream exposure was placed in a 250-ml Erlenmeyer flask containing 200 ml of a defined nutrient solution (three flasks per treatment). These flasks were aerated continuously for 20 days (approximately 1 ml of air min−1) at 18°C. Every second day, the solution was filtered through an 8-μm-pore-size membrane filter to collect any free conidia, which were counted and identified as described above. The filtrate was discarded, and the flasks were refilled with 200 ml of fresh nutrient solution. After 20 days, the remaining leaf disks were freeze-dried and weighed, and their ergosterol content was measured.
Nutrient solutions.
SW collected from Allen Creek on May 13 served as a control. It had a pH of 6.8, a nitrate content of 36 μg liter−1, an orthophosphate concentration of 31 μg liter−1, and an alkalinity of 10 mg of CaCO3 liter−1. All other solutions contained 15 mg of CaCl2 · 2H2O liter−1 in distilled water, and the pH was adjusted with 0.01 N NaOH or HCl to 6.8. In the 2 days between replacement of the solutions, the pH never changed by more than 0.3 U. Nitrate was added as KNO3 (levels N0, N1, and N2, 0, 50, and 500 μg of NO3 liter−1, respectively), and phosphate was added as NaH2PO4 (levels P0, P1, and P2, 0, 20, and 200 μg of PO4 liter−1, respectively). This gave a total of nine nitrate-phosphate combinations, characterized as N0P0 and N0P1, etc. All solutions were autoclaved before use.
Statistical analyses.
All analyses were done with SYSTAT 5.2.1 (SYSTAT, Evanston, Ill.) for Macintosh. To ensure normality for analysis of variance (ANOVA), we applied the arcsine transformation to percentage mass losses and the square root transformation for spore numbers. Mass loss, ergosterol concentrations, and conidium production from sets with defined N and P levels (0, 1, and 2) were analyzed with fully factorial ANOVA. For presenting the means and standard errors of the mean (SEM), nontransformed data were used. For cluster and principal-component analysis, we followed the guidelines described previously (45).
RESULTS
Both nitrate and phosphate levels in Allen Creek fluctuated considerably and exceeded the values of the previously collected SW used in the laboratory experiment (Table 1). The average alkalinities and pHs in Allen Creek (9.2 mg of CaCO3; pH 6.7) and SW (10 mg; pH 6.8) were similar.
TABLE 1.
Chemical parameters of Allen Creek during field experimenta
| Date | Temp (°C) | Value (mean ± SEM)
|
|||
|---|---|---|---|---|---|
| pH | Alkalinity (mg of CaCO3 liter−1) | NO3 concn (μg liter−1) | PO4 concn (μg liter−1) | ||
| June 3 | 8 | 7.0 ± 0.1 | 7.7 ± 0.2 | 12 ± 5 | 15 ± 5 |
| June 10 | 10.5 | 7.2 ± 0.1 | 6.5 ± 0.4 | 25 ± 3 | 78 ± 9 |
| June 17 | 11 | 6.9 ± 0.1 | 12 ± 0.4 | 37 ± 5 | 65 ± 8 |
| June 24 | 11 | 6.6 ± 0.1 | 9.9 ± 0.3 | 120 ± 9 | 32 ± 12 |
| July 1 | 15 | 6.0 ± 0.2 | 5.6 ± 0.1 | 89 ± 7 | 55 ± 10 |
| July 8 | 15.5 | 6.7 ± 0.1 | 13 ± 0.4 | 130 ± 11 | 42 ± 5 |
N = 4.
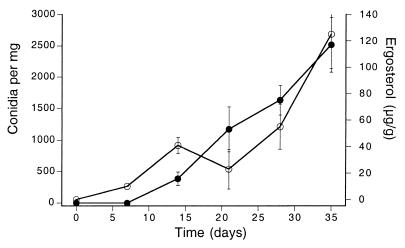
After 14 days in Allen Creek, maple leaf disks had lost 10% of their mass, the ergosterol content was 41 μg g−1, and spore production was 160 mg−1 day−1 (Fig. 1). Nonlinear curve-fitting of the remaining mass for the entire 5-week study gave an estimated intercept of 99.1% and a daily exponential decay rate of 0.0059 (R2 = 0.837). Both ergosterol content and spore production continued to increase and peaked after 5 weeks (data not shown). There was no obvious visual evidence of feeding by macroinvertebrates.
FIG. 1.
Maple leaf disks decaying in Allen Creek between 3 June and 8 July 1997. Ergosterol content (micrograms per gram of leaf mass) (●) and conidium release (conidia per day per milligram of leaf mass) (○). N = 4. Error bars indicate ± SEM.
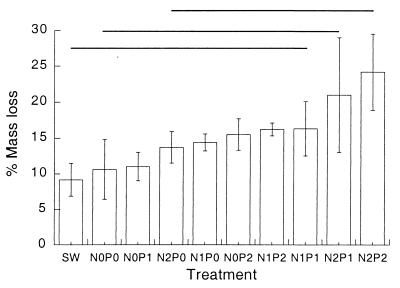
In the laboratory experiments, leaf mass losses, final ergosterol levels, and total spore production all increased significantly with both N and P (Table 2) (fully factorial ANOVA, P ≤ 0.05). N-P interactions were not significant. Mass losses during laboratory incubations are summarized in Fig. 2, with the mass after the 2 weeks in the stream set as 100%. Again, there is a clear relationship between external nutrients and mass loss, with the highest mass loss found with the highest levels of N and P. The corresponding mass loss for the leaves remaining in Allen Creek was 11%, similar to values in laboratory treatments with minimal nutrient additions.
TABLE 2.
Fully factorial ANOVA of leaf disks incubated in nutrient solutionsa
| Nutrient(s) | Mass loss
|
Ergosterol level
|
Conidium production
|
|||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| N | 7.6 | 0.004 | 42 | <0.001 | 33 | <0.001 |
| P | 4.7 | 0.023 | 9.0 | 0.002 | 3.9 | 0.04 |
| N-P | 1.2 | 0.34 | 1.4 | 0.29 | 2.6 | 0.11 |
Factors were N and P at levels 0, 1, and 2.
FIG. 2.
Mass loss of leaf disks, recovered after 14 days of exposure in Allen Creek and incubated for 20 days in nutrient solutions. Mass at start of 20 days was 100%. For comparison, identically treated leaf disks that continued to decay in Allen Creek lost 11% in the same period. SW, stream water collected on May 13; N, nitrate; P, phosphate; 0, 1, and 2, three nutrient levels. N = 3. Error bars indicate ± SEM. Lines indicate groups of no significant difference (Tukey's test, P ≤ 0.05).
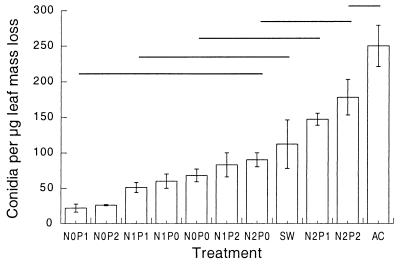
Conidia in laboratory cultures were harvested every second day. Assuming linear decay rates during the 20 days of the experiment, we calculated daily conidium production per milligram of remaining leaf mass. These values were used to estimate total numbers of conidia produced per unit mass lost (Fig. 3). The data for Allen Creek were estimated by linear extrapolation between the three data points. In laboratory experiments, conidium production clearly increased at higher nutrient levels. Estimated conidium production was highest in leaves remaining in Allen Creek, which had only moderate N and P levels (Table 1).
FIG. 3.
Conidia produced per unit of leaf mass loss over 20 days. Abbreviations are as described in the legend to Fig. 2. N = 3. Error bars indicate ± SEM. Lines indicate groups of no significant difference (Tukey's test, P ≤ 0.05). AC, Allen Creek.
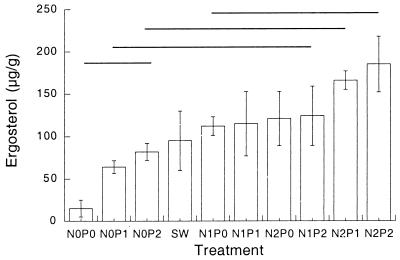
After 20 days of laboratory incubation, ergosterol content varied between 15 (N0P0) and 190 (N2P2) μg g of dry mass−1, again illustrating the positive connection between external nutrients and fungal activity (Fig. 4).
FIG. 4.
Ergosterol contents of leaf disks after 20 days of incubation in nutrient solutions. Abbreviations are as described in the legend to Fig. 2. N = 3. Error bars indicate ± SEM. Lines indicate groups of no significant difference (Tukey's test, P ≤ 0.05).
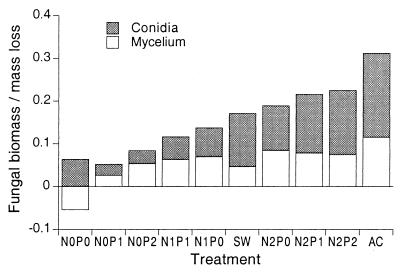
We calculated the amount of ergosterol produced per unit mass loss by subtracting the initial amount of ergosterol (41 μg g−1) and taking into account original and final masses. For N0P0, this value was negative, indicating that there was a net loss of mycelial biomass on disks incubated without nitrate and phosphate. Total ergosterol values were converted to fungal biomass, assuming an average content of 5.5 mg g−1 (17). This calculation gives mycelial mass produced per leaf mass loss (Fig. 5). Conidium production was similarly converted to fungal biomass (Fig. 5), using estimates of conidial volume and biomass described previously (7, 12). Generally, fungal investment in conidia exceeded investment in mycelium.
FIG. 5.
Combined fungal biomass produced per unit of leaf mass loss (grams per gram). Symbols are as described in the legend to Fig. 2. AC, Allen Creek.
During the initial 2 weeks of decomposition in Allen Creek, eight species were identified (Table 3). Three species (Anguillospora filiformis, Articulospora tetracladia, and Flagellospora curvula) dominated spore production. Even though their contributions fluctuated widely among the treatments, they remained dominant in all but one laboratory treatment (N2P0; Flagellospora curvula was replaced by Varicosporium elodeae), as well as during the further decomposition in Allen Creek. The total number of species in the 10 laboratory treatments varied between 9 (SW) and 15 (N2P2), but 15 species were found on disks decaying for another 3 weeks in Allen Creek (AC). The median number of species in the 10 laboratory treatments did not differ significantly (Kruskal-Wallis Statistic = 13.5; P = 0.095; based on three replicates per treatment). There was no significant correlation between number of species and mass loss during 20 days of laboratory incubation (R2 = 0.19, P = 0.20). We evaluated the data from Table 3 (average contributions of fungal species) with cluster analysis (Euclidean distances, single linkage), using absence and presence data as well as percentages of the various species. With both approaches, the communities from the last 3 weeks in Allen Creek and N2P2 treatment were clearly different from each other as well as from the remaining treatments (results not shown).
TABLE 3.
Percentage contributions of aquatic hyphomycete species to total spore productiona
| Species | % Contribution of species to total spore production
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Init (n = 8)b | AC (n = 15) | SW (n = 9) | N0P0 (n = 10) | N0P1 (n = 9) | N0P2 (n = 11) | N1P0 (n = 9) | N1P1 (n = 10) | N1P2 (n = 11) | N2P0 (n = 9) | N2P1 (n = 12) | N2P2 (n = 15) | |
| Anguillospora crassa | —c | — | — | — | — | 0.3 | 1.7 | |||||
| Anguillospora filiformis | 61 | 37 | 67 | 54 | 74 | 81 | 79 | 56 | 56 | 80 | 47 | 52 |
| Anguillospora longissima | 0.3 | 0.6 | 0.3 | 0.3 | 0.2 | — | 0.2 | 0.3 | — | — | 2.1 | 0.3 |
| Articulospora antipodea | 1.1 | — | — | 0.9 | — | — | — | — | — | |||
| Articulospora tetracladia | 30 | 36 | 31 | 24 | 21 | 16 | 14 | 31 | 34 | 17 | 24 | 7.2 |
| Casaresia sphagnorum | — | — | — | — | ||||||||
| Clavariopsis aquatica | 0.4 | — | — | 0.2 | ||||||||
| Clavatospora longibrachiata | 6.7 | 0.9 | 0.4 | — | — | 3.9 | 1.3 | 0.2 | 6.0 | 7.0 | ||
| Culicidospora aquatica | — | — | — | — | — | — | — | |||||
| Fontanospora breviramosa | — | — | — | — | ||||||||
| Dendrospora nana | — | |||||||||||
| Flagellospora curvula | 7.3 | 16 | 1.0 | 21 | 2.9 | 2.0 | 4.4 | 7.7 | 7.0 | 0.7 | 19 | 30 |
| Heliscus lugdunensis | 0.7 | — | — | — | — | |||||||
| Lemonniera aquatica | 0.1 | 0.1 | — | — | — | — | — | — | — | — | ||
| Lemonniera centrosphaera | — | 0.3 | — | — | — | 0.6 | — | |||||
| Mycocentrospora acerina | — | — | ||||||||||
| Tetracladium setigerum | 0.3 | |||||||||||
| Varicosporium elodeae | 2.6 | 0.3 | 0.9 | 1.2 | — | 2.2 | 1.0 | 1.2 | 0.8 | 1.5 | 1.4 | |
| Varicosporium giganteum | — | — | — | 0.3 | 0.1 | — | ||||||
Treatment symbols (e.g., N0P0) are as described in the legend to Fig. 2. Init, community during initial stream incubation; AC, community during subsequent 3 weeks in Allen Creek.
n = total number of species.
—, <1%.
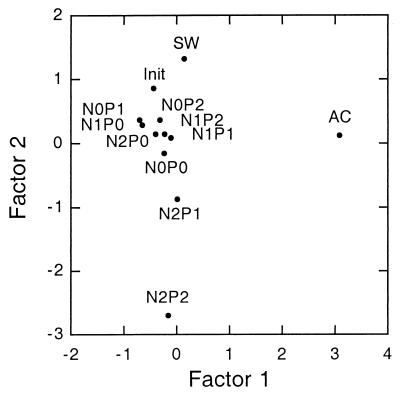
The first two factors of principal-component analysis (Fig. 6) accounted for 32 and 17%, respectively, of total variance. Most data points clustered relatively closely to Init (community of the first 2 weeks in Allen Creek). Since this was the starting point for all other communities, it is possible to identify two divergent trends: one from Init to N0P2, N1P2, or N1P1 and culminating in AC (community of the final 3 weeks in Allen Creek) and the second leading to N0P0 or N2P1 and N2P2.
FIG. 6.
Principal-component analysis of fungal communities during decay of leaf disks in Allen Creek (AC) or in nutrient solutions. Symbols are as described in the legend to Fig. 2. Factors 1 and 2 accounted for 32 and 17%, respectively, of the variance.
DISCUSSION
Fungal colonization of autumn-shed leaves in streams conditions them for invertebrate consumption (4, 34). In part, this mechanism is based on accumulation of fungal biomass, which can lead to absolute increases in nitrogen content of leaves (21, 41). Evidence for a similar increase in organic phosphorus is more ambiguous (11, 28). Adding nutrients to streams, to stimulate fungal activity, has produced mixed results: P increased leaf breakdown and microbial activity (13), but N did not (24, 39). The evidence is more clearcut when leaf decay rates in streams with naturally different nutrient regimes are contrasted (35, 38, 44). In general, higher nutrient levels (particularly nitrate) were correlated with faster decay rates, increased fungal biomass maxima on leaves (up to eightfold), and much higher sporulation rates (up to 80-fold). Equally convincing were experiments with two pure cultures growing on leaves, in which both decay and sporulation rates were stimulated by increasing amounts of either N or P (however, the nutrient not tested was provided at a very high level, 14 mg of NO3-N or 45 mg of PO4-P) (36). Biomass also increased, but the effect was not significant.
In these studies, nutrient regimes did not change during the experiment, and fungus-free leaves were used to begin. Our present study examined the reaction of an established fungal community to subsequent variations in N and P supply. Since freshly collected leaves were not sterilized before stream exposure, they probably carried epiphytic and endophytic fungi (27). However, these terrestrial fungi generally play a limited role in the stream environment, and their growth usually is negligible (6).
The initial fungal colonization of leaves in the stream was typical, since after an initial lag phase, ergosterol and spore production increased rapidly (Fig. 1). Leaves incubated in the stream for 2 weeks carried substantial inoculum, but the fungal community was far from its maximum development. For one control treatment, we compared the course of decomposition in Allen Creek with that in a laboratory treatment consisting of SW collected earlier from the same stream. Unfortunately, both N and P increased in the second phase of the stream experiment over values in SW. In addition, the temperature in Allen Creek was approximately 6°C lower than that in the laboratory experiments. These differences complicate comparisons between Allen Creek and SW. It is therefore not clear whether the lower spore production (Fig. 3) and ergosterol buildup (Fig. 4 and 5) in the SW treatment were due to limited nutrients, higher temperature, absence of new inoculum, or some combination of these and other factors.
As expected, treatments with defined nutrient concentrations clearly showed that increases in N or P significantly increased mass loss and fungal production (Table 2). When both were provided together, greater weight loss, sporulation, and ergosterol levels were observed. However, these effects were not significant, possibly due to high experimental variability. Earlier results suggested that N may be the primary inorganic nutrient limiting growth and sporulation of aquatic hyphomycetes (36, 38) and that any effect of P is conditional on sufficient supplies of N. Except for two transplant experiments (38), these earlier studies were initiated with fungus-free leaves, however, while we used leaves with an established fungal community. It is possible that during the initial colonization, the fungi accumulate more nutrients than necessary for their current metabolic needs. Such “luxury consumption” is well known from studies of phytoplankton (43).
In the second phase of the stream experiment (weeks 3 to 5), spore production increased by a factor of 16 and ergosterol content increased by a factor of 3. The laboratory experiments suggest that these increases require additional inputs of N and P (Fig. 3). At the highest level (N2P2), sporulation rates increased 32-fold, and ergosterol content increased 4.5-fold, over initial levels. The more pronounced effect on sporulation than on biomass buildup has been documented previously (29, 35–36, 38).
Sporulation continued at the initial level throughout the trial even without external nutrients (Fig. 3). Combined with the observation that the amount of ergosterol (in relative and absolute terms) (Fig. 4) declined, we think that existing mycelial contents were converted into conidia. A decline in mycelial cell contents during excessive sporulation has been reported (1), while declining N levels in stream-incubated leaves were attributed to release of conidia (35).
Total numbers of spores released during the experimental period divided by leaf mass lost again showed a clear correlation with added nutrients (Fig. 3). Surprisingly, the highest yield coefficient was found for the second phase of the stream experiment. This result may be an artifact, since we assumed that sporulation rates, as determined during a short laboratory period with fairly vigorous aeration, were representative of what happened in the stream between samples (total spore production was estimated by linear extrapolation between sample points). Since leaf disks in streams were enclosed in bags, which should lower exposure to water currents and therefore depress sporulation, values obtained from laboratory experiments probably overestimated actual spore production in the stream.
With the exception of N0P0, fungal biomass increased in all treatments. Again, there is a trend for increased growth with increased nutrient levels, particularly with N. Since increases in either N or P alone increased fungal growth, we hypothesize that during the initial 2 weeks in the stream, the fungal mycelia had accumulated unused N and P. The highest buildup of ergosterol was found in stream-incubated leaves, even though the estimated N and P levels in Allen Creek were well below those of the N2P2 treatment.
On average, 56% of total estimated production was used for spore formation (data from Fig. 5, N0P0 treatment excluded, since it had no net ergosterol production). Values above or close to 50% have been reported in several other articles (12, 14, 17, 23, 32–33). We combined absolute increase of leaf-associated mycelia and released conidia to estimate a fungal yield coefficient, defined as cumulative fungal production divided by loss of leaf mass (15). Provided that losses of fungal biomass (from death, invertebrate feeding, and sloughing off) are low, this approach is strongly correlated with estimates based on an instantaneous growth rate (37), which is measured by the incorporation of radiolabeled acetate into ergosterol (25). The fungal yield coefficient was 31% for stream-incubated leaves (Allen Creek); in laboratory-treated leaves, it varied between 1 (N0P0) and 23% (N2P2). In other stream studies, this value ranged between 11 and 15% (35) and 1.5 and 7.5% (44), and with pure cultures of aquatic hyphomycetes, it ranged from 15 to 23% (33). The terrestrial basidiomycete Mycena galopus growing on ash or birch leaf litter achieved a yield coefficient of 12 to 34% (15). While the results of the present study as a whole are comparable to those of other reports, the higher value for stream-incubated leaves is unusual and may again indicate an inflated estimate of spore production in Allen Creek.
While N and P clearly influenced decay rates, biomass accumulation, and spore production, the effect of these nutrients on diversity and composition of the fungal community is less obvious. Most of the species were present following the initial stream incubation, but additional species appeared in each treatment (Table 3). Since all solutions in the laboratory experiment were sterile, mycelia of these species must have colonized the leaves in the first 2 weeks of stream incubation. The subsequent nutrient regime presumably determined which of the mycelia were able to persist and sporulate. The number of species was highest in N2P2 (highest nutrient concentrations) and equaled the one on leaves incubated for another 3 weeks in the stream, suggesting a connection between nutrient supply and species diversity. However, the median number of species did not differ significantly among the laboratory treatments. This lack of difference, and the persistence of the dominant species regardless of treatment, underlines the importance of early arrival in determining the composition of the fungal community. Comparable conclusions were reached with transplant experiments of preinoculated leaves into different streams (4, 32). Nevertheless, multivariate analysis revealed some trends that may be associated with external factors (Fig. 6) since the greatest deviations from the initial community were found in the high-nutrient treatment (N2P2) and on leaves left in the stream (with moderate nutrients and the potential for new inoculum).
Fungal communities in this and earlier studies were characterized by spore production by their dominant members. However, the aquatic hyphomycete Tetrachaetum elegans consists of several, morphologically indistinguishable subpopulations, whose frequencies of occurrence vary with the leaf species colonized (10). If such variation among aquatic hyphomycetes is widespread, the fungal community may be considerably more dynamic than revealed by conventional taxonomic techniques, and our conclusion that the early phase of leaf colonization largely determines subsequent diversity and community organization may be premature. It does not change our conclusion that external supplies of N and P strongly stimulate fungal metabolism and growth, thereby accelerating the decay of leaves and their incorporation into higher trophic levels.
ACKNOWLEDGMENT
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- 1.Aimer R D. Ecology of aquatic hyphomycetes in New Zealand streams. Ph.D. thesis. Waikato, New Zealand: University of Waikato; 1989. [Google Scholar]
- 2.Bärlocher F. Leaf-eating invertebrates as competitors of aquatic hyphomycetes. Oecologia. 1980;47:303–306. doi: 10.1007/BF00398521. [DOI] [PubMed] [Google Scholar]
- 3.Bärlocher F. Conidium production from leaves and needles in four streams. Can J Bot. 1982;60:1487–1494. [Google Scholar]
- 4.Bärlocher F. The role of fungi in the nutrition of stream invertebrates. Bot J Linn Soc. 1985;91:83–94. [Google Scholar]
- 5.Bärlocher F. Aquatic hyphomycete spora in 10 streams of New Brunswick and Nova Scotia. Can J Bot. 1987;65:76–79. [Google Scholar]
- 6.Bärlocher F. Community organization. In: Bärlocher F, editor. The ecology of aquatic hyphomycetes. Berlin, Germany: Springer-Verlag; 1992. pp. 38–76. [Google Scholar]
- 7.Bärlocher F, Schweizer M. Effect of leaf size and decay rate on colonization by aquatic hyphomycetes. Oikos. 1983;41:205–210. [Google Scholar]
- 8.Chamier C-A. Cell-wall degrading enzymes of aquatic hyphomycetes: a review. Bot J Linn Soc. 1985;91:67–81. [Google Scholar]
- 9.Chamier C-A, Dixon P A. Pectinases in leaf degradation by aquatic hyphomycetes. I. The field study. Oecologia. 1982;52:109–115. doi: 10.1007/BF00349018. [DOI] [PubMed] [Google Scholar]
- 10.Charcosset J-Y, Gardes M. Infraspecific genetic diversity and substrate preference in the aquatic hyphomycete Tetrachaetum elegans. Mycol Res. 1999;103:736–742. [Google Scholar]
- 11.Chauvet E. Changes in the chemical composition of alder, poplar and willow leaves during decomposition in a river. Hydrobiologia. 1987;148:35–44. [Google Scholar]
- 12.Chauvet E, Suberkropp K. Temperature and sporulation of aquatic hyphomycetes. Appl Environ Microbiol. 1998;64:1522–1525. doi: 10.1128/aem.64.4.1522-1525.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elwood J W, Newbold J D, Trimble A F, Stark R W. The limiting role of phosphorus in a woodland stream ecosystem: effects of P enrichment on leaf decomposition and primary producers. Ecology. 1981;62:35–44. [Google Scholar]
- 14.Findlay S E G, Arsuffi T L. Microbial growth and detritus transformations during decomposition of leaf litter in a stream. Freshw Biol. 1989;21:261–269. [Google Scholar]
- 15.Frankland J C, Lindley D K, Swift M J. A comparison of two methods for the estimation of mycelial biomass in leaf litter. Soil Biol Biochem. 1978;10:323–333. [Google Scholar]
- 16.Gessner M O. Fungal biomass, production and sporulation associated with particulate organic matter in streams. Limnetica. 1997;13:33–44. [Google Scholar]
- 17.Gessner M O, Chauvet E. Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl Environ Microbiol. 1993;59:502–507. doi: 10.1128/aem.59.2.502-507.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gessner M O, Chauvet E. Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology. 1994;75:1807–1817. [Google Scholar]
- 19.Gessner M O, Chauvet E. Growth and production of aquatic hyphomycetes in decomposing leaf litter. Limnol Oceanogr. 1997;42:496–505. [Google Scholar]
- 20.Gessner M O, Thomas M, Jean-Louis A M, Chauvet E. Stable successional patterns of aquatic hyphomycetes on leaves decaying in a summer cool stream. Mycol Res. 1993;97:163–172. [Google Scholar]
- 21.Kaushik N K, Hynes H B N. The fate of the dead leaves that fall into streams. Arch Hydrobiol. 1971;68:465–515. [Google Scholar]
- 22.Lind O T. Handbook of common methods in limnology. St. Louis, Mo: Mosby; 1974. [Google Scholar]
- 23.Maharning A R, Bärlocher F. Growth and reproduction in aquatic hyphomycetes. Mycologia. 1996;88:80–88. [Google Scholar]
- 24.Newbold J D, Elwood J W, Schulze M S, Stark R W, Barmeier J C. Continuous ammonium enrichment of a woodland stream: uptake kinetics, leaf decomposition, and nitrification. Freshw Biol. 1983;13:193–204. [Google Scholar]
- 25.Newell S Y, Fallon R D. Toward a method for measuring instantaneous fungal growth rates in field samples. Ecology. 1991;72:1547–1559. [Google Scholar]
- 26.Newell S Y, Arsuffi T L, Fallon R D. Fundamental procedures for determining ergosterol content of decaying plant material by liquid chromatography. Appl Environ Microbiol. 1988;54:1876–1879. doi: 10.1128/aem.54.7.1876-1879.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrini O. Fungal endophytes of tree leaves. In: Andrews J H, Hirano S S, editors. Microbial ecology of leaves. Berlin, Germany: Springer-Verlag; 1992. pp. 179–197. [Google Scholar]
- 28.Raviraja N S, Sridhar K R, Bärlocher F. Breakdown of introduced and native leaves in two Indian streams. Int Rev Gesamten Hydrobiol. 1996;81:529–539. [Google Scholar]
- 29.Rosset J, Bärlocher F. Transplant experiments with aquatic hyphomycetes. Verh Int Verein Limnol. 1985;22:2786–2790. [Google Scholar]
- 30.Rosset J, Bärlocher F, Oertli J J. Decomposition of conifer needles and deciduous leaves in two Black Forest and two Swiss Jura streams. Int Rev Gesamten Hydrobiol. 1982;67:695–711. [Google Scholar]
- 31.Sridhar K R, Bärlocher F. Water chemistry and sporulation by aquatic hyphomycetes. Mycol Res. 1997;101:591–596. [Google Scholar]
- 32.Suberkropp K. Effect of temperature on seasonal occurrence of aquatic hyphomycetes. Trans Br Mycol Soc. 1984;82:53–62. [Google Scholar]
- 33.Suberkropp K. Relationships between growth and sporulation of aquatic hyphomycetes on decomposing leaf litter. Mycol Res. 1991;95:843–850. [Google Scholar]
- 34.Suberkropp K. Interactions with invertebrates. In: Bärlocher F, editor. The ecology of aquatic hyphomycetes. Berlin, Germany: Springer-Verlag; 1992. pp. 118–134. [Google Scholar]
- 35.Suberkropp K. The influence of nutrients on fungal growth, productivity, and sporulation during leaf breakdown in streams. Can J Bot. 1995;73(Suppl. 1):S1361–S1369. [Google Scholar]
- 36.Suberkropp K. Effects of dissolved nutrients on two aquatic hyphomycetes growing on leaf litter. Mycol Res. 1998;102:998–1002. [Google Scholar]
- 37.Suberkropp, K. Estimating production of litter-decomposing fungi in streams from rates of acetate incorporation into ergosterol. Verh. Internat. Verein. Limnol., in press.
- 38.Suberkropp K, Chauvet E. Regulation of leaf breakdown by fungi in streams: influences of water chemistry. Ecology. 1995;76:1433–1445. [Google Scholar]
- 39.Triska F J, Sedell J R. Decomposition of four species of leaf litter in response to nitrate manipulation. Ecology. 1976;57:783–792. [Google Scholar]
- 40.Webster J. Anamorph-teleomorph relationships. In: Bärlocher F, editor. The ecology of aquatic hyphomycetes. Berlin, Germany: Springer-Verlag; 1992. pp. 99–117. [Google Scholar]
- 41.Webster J R, Benfield E F. Vascular plant breakdown in freshwater ecosystems. Annu Rev Ecol Syst. 1986;17:567–594. [Google Scholar]
- 42.Webster J, Moran S T, Davey R A. Growth and sporulation of Tricladium chaetocladium and Lunulospora curvula in relation to temperature. Trans Br Mycol Soc. 1976;67:491–549. [Google Scholar]
- 43.Wetzel R G. Limnology. Philadelphia, Pa: Saunders; 1983. [Google Scholar]
- 44.Weyers H S, Suberkropp K. Fungal and bacterial production during the breakdown of yellow poplar leaves in 2 streams. J N Am Benthol Soc. 1996;15:408–420. [Google Scholar]
- 45.Wilkinson L, Blank G, Gruber C. Desktop analysis with SYSTAT. Upper Saddle River, N.J: Prentice Hall; 1996. [Google Scholar]