Abstract
Background
Computer‐assisted navigated piezoelectric surgery (CANPS) is a surgical technique that combines the surgical navigation with a piezoelectric device. This association multiplies the advantages of both technologies, taking the best of each one providing a synergistic association.
Objective
To describe and assess the indications, advantages, disadvantages, and complications of this association of surgical techniques.
Methods
CANPS was used in 32 patients. The clinical diagnosis was facial trauma, tumors, orthognathic surgeries, temporomandibular joint ankylosis, pathology of the frontal sinus, and alveolar distraction. Nineteen patients were men and 13 were women. Planning software iPlan 3.05 of Brainlab, and Elements of Brainlab were used for planning and the Kolibri and Kurve of Brainlab for surgical navigation. The piezoelectric device used was a “Vercelotti” type in all patients.
Results
CAPNS could be performed successfully in all cases without complications and reduced the surgeon's uncertainty during the osteotomies. There is continuous control of the position of the surgical instrument. The use of the navigated piezoelectric device allowed the surgeon's uncertainty to be reduced during the performance of the osteotomies in depth, in poorly visible areas, with little access or reduced visibility. It also increases the safety of bone resections near important anatomical structures.
Conclusions
CANPS combines the advantages of piezoelectric surgery and navigation. CANPS affords real‐time control of the position of the cutting tip and allows semiburied approaches. CANPS allows surgery to be precise, safer, and minimally invasive.
Keywords: computer‐assisted surgery, piezoelectric device, piezoelectric surgery, simulation‐guided navigation, surgical navigation, virtual surgery
1. INTRODUCTION
With the development of modern digital imaging technology, computer‐assisted surgery (CAS), virtual surgery (VS), and surgical navigation (SN) play a growing role in the diagnosis and treatment of multiple pathologies of oral and maxillofacial surgery in the fields of traumatology, oncology, reconstructive surgery, temporomandibular joint surgery, orthognathic surgery, removal of foreign bodies, fibrous dysplasia, skull base surgery, implantology, and osteogenic distraction techniques. 1 , 2 , 3 , 4
Besides, piezosurgery (PS) has become a great improvement in oral and maxillofacial surgery. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 In 2000, Vercellotti applied the PS and developed an angulated thin and tapered cutting saw tip that is now widely used.
PS has many advantages such as prevention or reduction of soft tissue injury, less blood loss, excellent visualization of the surgical field, allows making minor, and less invasive approaches due to less bleeding and better visibility of the surgical field; and it is associated with less postoperative pain. 10 , 11 , 14 , 15 , 16 , 17 , 18 This property makes it especially useful for access to difficult and limited areas and is also beneficial for the use of endoscopic techniques. 8
The association of PS with surgical navigation, called navigated piezosurgery (NPS), computer‐assisted piezoelectric surgery (CAPS) or computer‐assisted navigated piezoelectric surgery (CANPS), allows not only sequential or indirect navigation but also direct and continuous navigation with a piezoelectric device. CANPS consists of applying the computer planning and surgical navigation to a piezoelectric device in such a way that the active handpiece of the piezoelectric device should become “navigable.”
In 2015, Bianchi described the CAPS technique. This allowed him to control the position of the active piezo handpiece on the navigator screen. Bianchi used CAPS in 18 patients for orthognathic surgery, craniofacial procedures, orthodontic surgery corticotomies, and oncological cases. 11
In 2019, Robiony used the piezo‐navigated approach as an evolution of their original piezo‐surgical external technique in percutaneous osteotomies in rhinoplasty. 14
According to the literature review, there are few publications of this new technique, which opens up new possibilities for improvement and development in oral and maxillofacial surgery (Table 1).
TABLE 1.
Published previous and present references of CANPS in English‐language literature
| Author | Year | Number of patients | Pathology | Procedure | Navigation system | |
|---|---|---|---|---|---|---|
| 1 | Bianchi | 2015 | 18 |
Orthognathic surgery Craniofacial surgery Orthodontic Oncology |
Le Fort I osteotomy SARME Corticotomies Check the limit of lesion Control resection margins |
Stryker |
| 2 | Newman | 2018 | ? | TMJ ankylosis | Lateral gap arthroplasty |
BrainLab Stryker |
| 3 | Robiony | 2019 | ? | Rhinoplasty | Nasal osteotomies | Medtronic |
| 4 | Dean | Present | 32 |
Intraosseous hemangioma Oncology Fibrous dysplasia Frontal sinus fractures Trauma sequelae TMJ ankylosis Orthognathic surgery Condylar hyperplasia |
Orbitotomies Maxillectomies Maxillary recontouring OAWFS Removal of the frontal sinus mucosa Middle third osteotomies Nasal, maxillary and Orbital osteotomies Lateral gap arthroplasty SARME Proportional condylectomy Resection of the Inferior border of the mandible |
BrainLab |
Abbreviations: OAWFS, osteotomy of the anterior wall of the frontal sinus; SARME, surgically assisted rapid maxillary expansion; TMJ, temporomandibular joint.
The purpose of this study is to show the clinical applications and indications of CANPS and to evaluate the advantages, disadvantages, possible errors, and complications of this new surgical technique.
2. MATERIALS AND METHODS
This is a study of a case series of 32 patients treated using CANPS from January 2013 to July 2021. All patients provided informed written consent to participate prior to enrollment in the study. The study was conducted under the tenets of the WMA Declaration of Helsinki in the context of ethical principles for medical research involving human subjects and was approved by the local institutional review board of our institution (Act. Number 280, Ref. 4009).
The planning softwares used were the iPlan 3.05 and Elements of Brainlab, the navigation systems used were Kolibri and Kurve infrared navigation systems of Brainlab, and the piezoelectric devices were always Vercelotti type, with an angulated thin and tapered cutting saw tip. Table 2 describes the demographic and pathologic data of the patients.
TABLE 2.
Demographic and pathologic features of the patients
| Total CANPS (N = 32) (%) | |
|---|---|
| Gender | |
| Male | 19 (59.4%) |
| Female | 13 (40.6%) |
| Age (years) | |
| Mean (range) | 35 (15–73) |
| Disease characteristics | |
| Facial fractures | 7 |
| Condylar hyperplasia | 4 |
| Orthognathic surgery | 4 |
| Maxillary/mandibular tumors | 4 |
| Intraosseous hemangioma | 3 |
| Temporomandibular joint ankylosis | 3 |
| Fibrous dysplasia | 3 |
| Recurrent inflammatory pathology frontal sinus | 2 |
| Alveolar distraction | 1 |
| Osteoma | 1 |
The steps of the procedure are as follows:
Importation of DICOM (digital imaging and communication in medicine) data to the planning software from the PACs of the Hospital.
A VS with a treatment planning was done.
The preoperative patient CT data and treatment planning were imported into the computer navigation system.
Registration of the patient in the operating room. To identify the position of the patient's head, a skull post with the dynamic reference frame is fixed to the patient's skull. Patient registration was performed either using the surface laser or by unequivocal bone points.
Registration and calibration of the cutting tip of the piezoelectric device. The piezoelectric handpiece was registered by anchoring the three reflecting spheres tracking tool to the handpiece of the piezo and linking it to the navigator with a calibration matrix (Figure 1). The more distal point of the cutting tip of the saw is registered with the help of a calibration matrix (Figure 2).
The control of the precision of the cutting tip of the piezoelectric device is performed before starting the surgery and periodically during the surgery. Measurement accuracy was checked by placing the end of the calibrated cutting tip of the piezoelectric device on unequivocal anatomical points. The surgery was performed with a precision of 1 mm. In case of a deviation superior to that, the piezo device was again registered and recalibrated.
Perform live piezoelectric navigation and view real‐time results on the screen of the navigation workstation. Brainlab navigation software displays the tip of the instrument as a yellow cross‐centered or a yellow pointer.
Postoperative accuracy is checked by superimposing the postoperative CT on the preoperative CT containing the surgical plan. Two‐dimensional measurements were made in the axial, sagittal, and coronal planes.
We describe the workflow of some representative cases.
FIGURE 1.
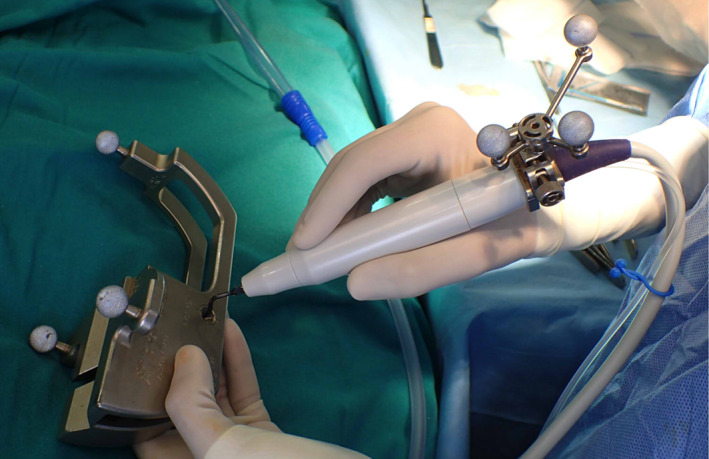
The piezoelectric device is tracked by a clamped dynamic reference frame with three spheres using the calibrating matrix
FIGURE 2.
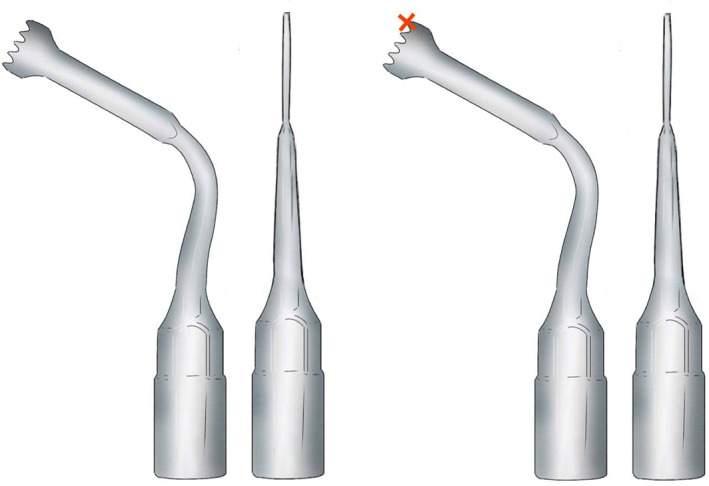
The cutting tip used for CANPS. Only one point of the instrument (in this case the distal part) is available for surgical navigation (red cross)
2.1. Case 1
In block resection and immediate prosthesis reconstruction was planned in this 55‐year‐old woman with an intraosseous hemangioma of the right supraorbital rim. The tumor shape was outlined and then segmented using the planning software. The object “tumor resection” were converted into STL files and sent to Materialize (Materialize, Leuven, Belgium; www.materialise.com), who built a custom‐made PEEK prosthesis, and custom‐made resection guides for the external cuts. Then, the surgical plan, the resection guide, and prosthesis were converted into STL files and were imported into the iPlan 3.05 software. The .STL files of the plan were superimposed onto patient‐specific CT scan data. The osteotomy was performed with CANPS and with the aid of the custom made surgical guide over the bone surface of the frontal bone (Figure 3). Then in‐depth, only with the aid of the CANPS, to reproduce a real‐time osteotomy line of resection of the tumor created in the virtual plan (Figure 4). Accuracy is postoperatively checked by superimposing the postoperative CT on the surgical plan (Figure 5).
FIGURE 3.
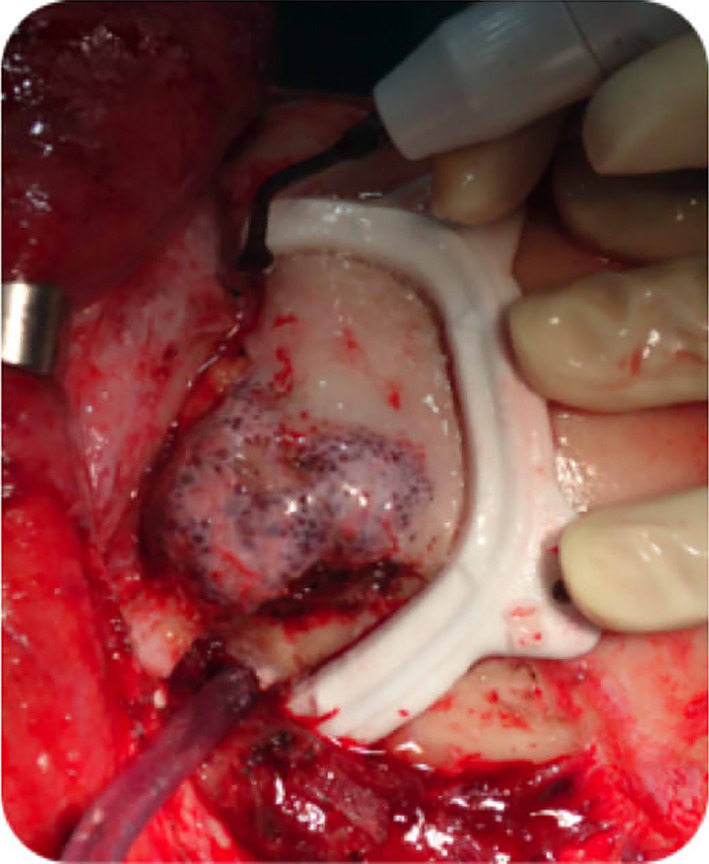
Intraosseous hemangioma of the right supraorbital rim, frontal bone, and orbital roof. The tumor and the planned resection and the surgical guide
FIGURE 4.
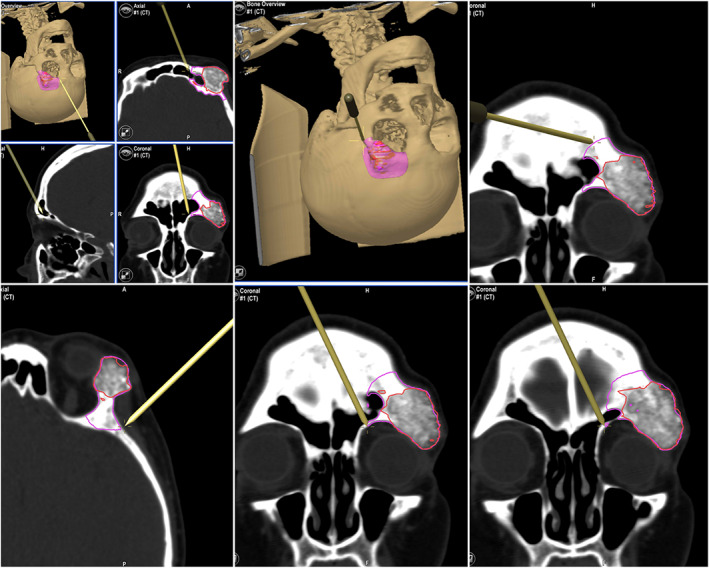
CANPS controls the resection in depth, on the orbital roof and the lateral orbital wall. On the screen of the Brainlab navigator, the tip of the instrument is marked during navigation in yellow
FIGURE 5.
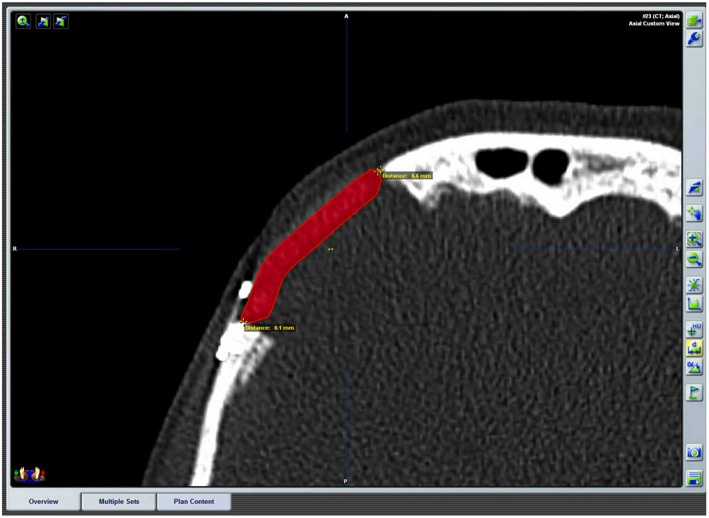
Postoperative CT with the planned prosthesis superimposed. The measurements of the distances between the plan and the result are shown. Accuracy is postoperatively checked by superimposing the postoperative CT on the surgical plan
2.2. Case 2
A total hemimaxillectomy was planned to resect a primary T4a adenoid cystic carcinoma of the maxilla in a 75‐year‐old man. The surgical plan was performed with the iPlan 3.05. Image fusion was accomplished to determine the extension of the tumor by superimposing MRI and PET‐TC over the CT. The tumor shape was outlined and the appropriate surgical margin was automatically created after segmentation using the tool “enlarge.” The plan was imported to the navigator to proceed with de CANPS and to assure adequate margins for the tumor resection (Figure 6).
FIGURE 6.
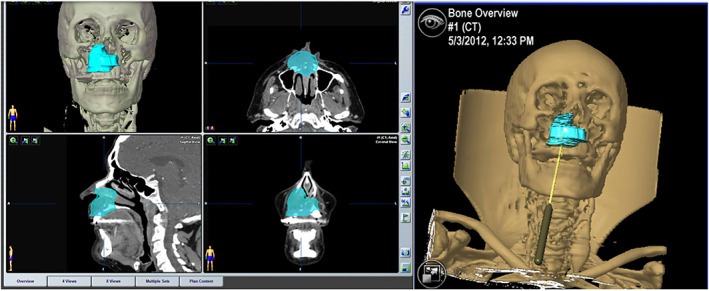
Surgical plan with the iPlan 3.05, CMF Brainlab, and resection of the tumor‐assisted with CANPS
3. RESULTS
CAPNS was successfully performed in all cases. There were no complications related to navigated piezosurgery.
It was possible to follow the osteotomy lines planned during the three‐dimensional VS, thus maximizing the precision of surgery, and increasing safety. In oncologic patients, the surgeon has been able to check the tumor resection margins on the bony surface and the palatal mucosa as well as in deep inner bone structures according to the previous virtual planning. The surgery could be performed according to the surgical plan with a precision of 1 mm.
In TMJ ankylosis, CANPS has been used to maintain a safe distance from the middle cranial fossa, the anterior border of the bony external auditory canal, and the vessels deep to the ankylosed block.
The use of the navigated piezoelectric device allowed the surgeon's uncertainty to be reduced during the performance of the osteotomies in depth in depth, in poorly visible areas, with little access, or reduced visibility.
4. DISCUSSION
CANPS is a new surgical technique that combines the simultaneous use of SN and a piezoelectric device. In contrast to classical sequential navigation, where navigation and surgical work cannot be simultaneously performed, CANPS allows real‐time navigation, being the piezoelectric instrument itself the active handpiece. CANPS allows a substantial advance in navigated surgery by synergistically adding the advantages of both techniques (Figure 7).
FIGURE 7.
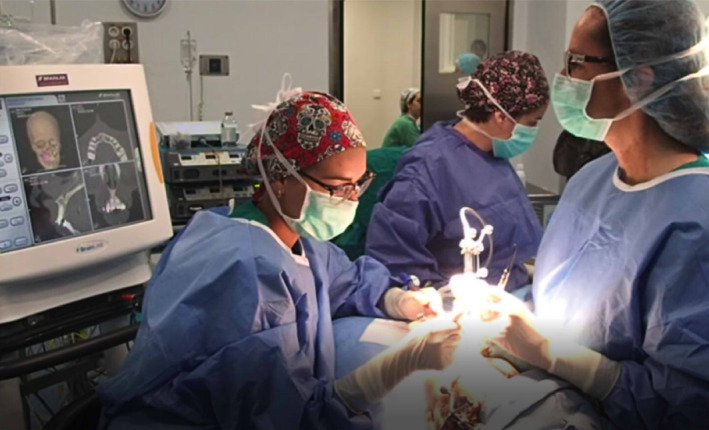
Intraoperative real time navigation
Piezo Surgery reduces the damage to adjacent soft tissues. 11 , 14 This has led to the development of minimally invasive techniques with buried or semi‐buried approaches. 6 , 7 , 11 , 14 CANPS allows the surgeon to know the precise anatomical position of the tip by reference to CT data. This ensures safety and precise surgery, even in deep regions without direct vision. 11
With regard to surgical navigation, three different moments/types can be considered: a “first” or “anatomical” navigation, which allows the surgeon to check anatomical structures; a “second” or “working” navigation, which is used to accomplish the surgical plan, by assisting the surgeon in delineating the resection margins of a tumor, or the preplanned osteotomies; and a “third” or “checking” navigation, which is used to verify the reconstruction, or the new position of bone fragments. The surgical plan acts as a virtual template to check the accuracy of the treatment results. Some authors also call this third navigation, “simulation‐guided navigation” (SGN). 11 CANPS is a “working” navigation.
According to the way of working with the instruments, two types of navigation are considered: “indirect” or “sequential” navigation, in which a navigation probe is used from time to time throughout the surgical procedure; and a “direct” or “live” or “continuous,” or “real‐time” navigation, in which the operating instrument has been registered, and acts as a navigation probe thus allowing to navigate nonstop during the surgery. CANPS is a “direct” navigation.
CANPS allows in frontal sinus fractures to design the osteotomy on the outer cortex. It also allows checking the progression of the piezo tip in‐depth to control and avoid injury to meninges.
CANPS can be applied in post‐traumatic middle third deformities guiding osteotomies in‐depth and in areas where it is not possible to place surgical guides because of their limited accessibility.
A cutting guide can be used for resection of the outer face of the bone of intraosseous hemangiomas, 19 but it is not possible to use it for the control of the resection of deep areas of the bone. We use CANPS during the resection of facial intraosseous hemangiomas to control these hidden osteotomies.
In 2015, Bianchi first described the use of CANPS to minimize the incision and to directly control the teeth roots in the surgically assisted rapid maxillary expansion (SARME). 11 , 20 In our cases of SARME, CANPS helped us to avoid lesion to the dental roots, and in the sagittal osteotomy, to avoid lesion to the roots or to the palatal mucosa and minimizing the incision of the mucosa allowing a semi‐buried approach. The association of piezosurgery with navigation allows not only to do a small incision but also to do a direct and live control of the tip during the surgery.
We have also used CANPS for an accurate and controlled resection of the lower mandibular border in hemimandibular hyperplasia with continuous monitoring of the position of the inferior alveolar nerve.
SN plays an important and growing role in management of craniofacial tumors. 21 , 22 , 23 CANPS provides a guide in‐depth and along the palate, on the navigator screen to perform tumor resections according to the resection margins planned in the preoperative plan.
CANPS allows performing a real‐time checking of the progression of the resection of the fibrous dysplasia, thus avoiding the surgeon's subjectivity. Gui et al. use real‐time instrument‐based navigation using another type of instrument (drills, saws, and chisels) to manage the bone remodeling of craniofacial fibrous dysplasia patients. 24
SN and CANPS can play an important role in guiding the osteotomy in‐depth in condylar hyperplasia. 25 Furthermore, incisions and soft tissue retraction necessary can be minimized. Yu et al. applied the computer‐assisted planning and intraoperative navigation for the treatment of a condylar osteochondroma. 26
CANPS can be made on the mandible under intermaxillary fixation using a dental splint. Another method to use CANPS on the mandible is to attach a mini tripod dynamic reference frame to the mandible. Thereby, it is possible to navigate the mandible as an independent bone and it is possible to open and close the mouth during mandible surgical navigation. 27
Surgical navigation has been used in temporomandibular ankylosis surgery, thus achieving a more safety and extensive removal of the ankylosed bone toward the skull base ensuring a safety distance. 9 , 13 , 20 , 28 , 29 Gui et al. in 2014 reported that navigation‐guided lateral gap arthroplasty showed great benefits for accuracy and safety in this potentially complicated procedure. 30 Newman also used CANPS to allow for “real‐time” navigation during osteotomy in ankylosis. 29 CANPS has helped us to perform the arthroplasty gap in temporomandibular joint ankylosis surgery as planned and avoiding lesions to important anatomical structures.
CANPS is useful in distraction osteogenesis to perform a continuous control of the osteotomy and the position of the inferior alveolar nerve.
CANPS combines the advantages of piezosurgery and surgical navigation with a continuous control of the cutting tip of the instrument, thus allowing for precise and safe surgery, even in deep regions. CANPS increases the information and visibility of the surgeon without the need of having a direct vision of the surgical field. Surgery is performed with less blood loss and excellent visualization of the surgical field. It minimizes the extension of soft tissue approaches (decreases the size of cutaneous and mucous incisions), allowing semi‐buried and minimally invasive approaches. The association of these two new technologies produces a synergy of their advantages separately.
Drawbacks of the technique include the need for an appropriate CT scan, the long learning curve (30–40 h), an initially high investment cost, it is preoperatively time‐consuming, the size of the equipment (navigator) may be bulky, and the light line between the navigation system lamp and the handpiece and the reference frame must be kept clear while navigating. Also, some surgeons may have a concern about piezosurgery speed.
Potential errors of the technique can come from the possible inadvertent mobilization of the reflective spheres attached to the device. Whenever a new piezo insert must be used, the device must be re‐registered. Another drawback of the technique is that to check the position of the piezoelectric instrument you have to look up from the surgical field and look at the screen of the navigator. Probably the future use of augmented reality with surgical goggles will allow simultaneous viewing of the surgical plan on the CT and the surgical field.
Other calibrated surgical instruments such as saws or burrs can also be used, but vibrations of the handpiece, and the danger of taking the eyes away from the surgical field to watch a monitor make such instruments inappropriate. Furthermore, the problem with navigating with a saw is that the active tip of the instrument is not a single point but has a more or less wide path, so it is not suitable for direct navigation. The linear vibrations of the micro‐saw tip of the piezoelectric device are practically imperceptible, and the surgeon can safely pause to look at a screen. 11
CANPS is a new surgical technique resulting from applying surgical navigation to a piezoelectric device. This association multiplies the advantages of both technologies, taking the best of each one providing a synergistic association. An accurate and safe surgery is achieved with “live” information and control of the position of the cutting tip of the piezoelectric instrument. It also opens a path to increasingly smaller, buried or semi‐buried approaches.
CONFLICT OF INTEREST
The first author has received speakers’ fees from the BrainLab Company.
Dean A, Heredero‐Jung S, Solivera J, Sanjuan A, Alamillos‐Granados FJ. Computer‐assisted and navigated piezoelectric surgery: A new technology to improve precision and surgical safety in craniomaxillofacial surgery. Laryngoscope Investigative Otolaryngology. 2022;7(3):684‐691. doi: 10.1002/lio2.786
REFERENCES
- 1. Schramm A, Suárez‐Cunqueiro MM, Barth EL, et al. Computer‐assisted navigation in craniomaxillofacial tumors. J Craniofac Surg. 2008;19:1067‐1074. [DOI] [PubMed] [Google Scholar]
- 2. Schramm A, Suarez‐Cunqueiro MM, Rücker M, et al. Computer‐assisted therapy in orbital and mid‐facial reconstructions. Int J Med Robotics Comput Assist Surg. 2009;5:111‐124. [DOI] [PubMed] [Google Scholar]
- 3. Bell RB. Computer planning and intraoperative navigation in cranio‐maxillofacial surgery. Oral Maxillofac Surg Clin North Am. 2010;22:135‐156. [DOI] [PubMed] [Google Scholar]
- 4. Yu H, Shen SG, Wang X, Zhang L, Zhang S. The indication and application of computer‐assisted navigation in oral and maxillofacial surgery‐Shanghai's experience based on 104 cases. J Craniomaxillofac Surg. 2013;41:770‐774. [DOI] [PubMed] [Google Scholar]
- 5. Vercellotti T. Piezoelectric surgery in implantology: a case report—a new piezoelectric ridge expansion technique. Int J Periodontics Restorative Dent. 2000;20:358‐365. [PubMed] [Google Scholar]
- 6. Robiony M, Polini F, Costa F, Vercellotti T, Politi M. Piezoelectric bone cutting in multipiece maxillary osteotomies. J Oral Maxillofac Surg. 2004;62:759‐761. [DOI] [PubMed] [Google Scholar]
- 7. Robiony M, Toro C, Costa F, Sembronio S, Polini F, Politi M. Piezosurgery: a new method for osteotomies in rhinoplasty. J Craniofac Surg. 2007;18:1098‐1100. [DOI] [PubMed] [Google Scholar]
- 8. Robiony M, Costa F, Politti M. Ultrasound endoscopic bone cutting for rapid maxillary expansion. J Oral Maxillofac Surg. 2014;72:980‐990. [DOI] [PubMed] [Google Scholar]
- 9. Jose A, Nagori SA, Virkhare A, Bhatt K, Bhutia O, Roychoudhury A. Piezoelectric osteoarthrectomy for management of ankylosis of the temporomandibular joint. Br J Oral Maxillofac Surg. 2014;52:624‐628. [DOI] [PubMed] [Google Scholar]
- 10. Spinelli G, Lazzeri D, Conti M, Agostini T, Mannelli G. Comparison of piezosurgery and traditional saw bimaxillary orthognathic surgery. J Craniomaxillofac Surg. 2014;42:1211‐1220. [DOI] [PubMed] [Google Scholar]
- 11. Bianchi A, Badiali G, Piersanti L, Marchetti C. Computer‐assisted piezoelectric surgery: a navigated approach toward performance of craniomaxillofacial osteotomies. J Craniofac Surg. 2015;26:867‐872. [DOI] [PubMed] [Google Scholar]
- 12. Spalthoff S, Zimmerer R, Dittmann O, et al. Piezoelectric surgery and navigation: a safe approach for complex cases of eagle syndrome. Int J Oral Maxillofac Surg. 2016;45:1261‐1267. [DOI] [PubMed] [Google Scholar]
- 13. Spinelli G, Valente D, Mannelli G, Raffaini M, Arcuri F. Surgical management of ankyloses of the temporomandibular joint by a piezoelectric device. J Craniomaxillofac Surg. 2017;45:441‐448. [DOI] [PubMed] [Google Scholar]
- 14. Robiony M, Lazzarotto A, Nocini R, Costa F, Sembronio S, Franz L. Piezosurgery: ten years´ experience of percutaneous osteotomies in rhinoplasty. J Oral Maxillofac Surg. 2019;77:1237‐1244. [DOI] [PubMed] [Google Scholar]
- 15. Bertossi D, Lucchese A, Albanese M, et al. Piezosurgary versus conventional osteotomy in orthognathic surgery: a paradigm shift in treatment. J Craniofac Surg. 2013;24:1763‐1766. [DOI] [PubMed] [Google Scholar]
- 16. Mouraret S, Houschyar KS, Hunter DJ, et al. Cell viability after osteotomy and bone harvesting: comparison of piezoelectric surgery and conventional bur. Int J Oral Maxillofac Surg. 2014;43:966‐971. [DOI] [PubMed] [Google Scholar]
- 17. AlAsseri N, Swennen G. Minimally invasive orthognathic surgery: a systematic review. Int J Oral Maxillofac Surg. 2018;47:1299‐1310. [DOI] [PubMed] [Google Scholar]
- 18. Hernández‐Alfaro F, Mareque‐Bueno J, Díaz A, Pagés CM. Minimally invasive surgically assisted rapid palatal expansion with limited approach under sedation: a report of 283 consecutive cases. J Oral Maxillofac Surg. 2010;68:2154‐2158. [DOI] [PubMed] [Google Scholar]
- 19. Powers DB, Fisher E, Erdmann D. Zygomatic Intraosseous hemangioma: case report and literature review. Craniomaxillofac Trauma Reconstr. 2017;10:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He Y, Huang T, Zhang Y, An J, He L. Application of a computer‐assisted surgical navigation system in temporomandibular joint ankylosis surgery: a retrospective study. Int J Oral Maxillofac Surg. 2017;46:189‐197. [DOI] [PubMed] [Google Scholar]
- 21. Rana M, Essig H, Eckardt AM, et al. Advances and innovations in computer‐assisted head and neck oncologic surgery. J Craniofac Surg. 2012;23:272‐278. [DOI] [PubMed] [Google Scholar]
- 22. Heiland M, Habermann CR, Schmelzle R. Indications and limitations of intraoperative navigation in maxillofacial surgery. J Oral Maxillofac Surg. 2004;62:1059‐1063. [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Sun G, Lu M, Hu Q. Surgical management of maxillofacial fibrous dysplasia under navigational guidance. Br J Oral Maxillofac Surg. 2015;53:336‐341. [DOI] [PubMed] [Google Scholar]
- 24. Gui H, Zhang S, Shen SG, Wang X, Bautista JS, Voss PJ. Real‐time‐guided recontouring in the management of craniofacial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:680‐685. [DOI] [PubMed] [Google Scholar]
- 25. Sembronio S, Tel A, Costa F, Robiony M. An updated protocol for the treatment of condylar hyperplasia: computer‐guided proportional condylectomy. J Oral Maxillofac Surg. 2019;77:1457‐1465. [DOI] [PubMed] [Google Scholar]
- 26. Yu HB, Li B, Zhang L, Shen SG, Wang XD. Computer‐assisted surgical planning and intraoperative navigation in the treatment of condylar osteochondroma. Int J Oral Maxillofac Surg. 2015;44:113‐118. [DOI] [PubMed] [Google Scholar]
- 27. Sukegawa S, Kanno T, Furuki Y. Application of computer‐assisted navigation systems in oral and maxillofacial surgery. Jpn Dent Sci Rev. 2018;54:139‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmelzeisen R, Gellrich NC, Schramm A, Schön R, Otten JE. Navigation‐guided resection of temporomandibular joint ankylosis promotes safety in skull base surgery. J Oral Maxillofac Surg. 2002;60:1275‐1283. [DOI] [PubMed] [Google Scholar]
- 29. Newman MF, Lee DG, Lecholop MK. Protocol for single‐stage bilateral temporomandibular joint replacement using intraoperative navigation in patients with ankylosis. J Oral Maxillofac Surg. 2018;76:1418‐1423. [DOI] [PubMed] [Google Scholar]
- 30. Gui H, Wu J, Shen SG, Bautista JS, Voss PJ, Zhang S. Navigation‐guided lateral gap arthroplasty as the treatment of temporomandibular joint ankylosis. J Oral Maxillofac Surg. 2014;72:128‐138. [DOI] [PubMed] [Google Scholar]


