Abstract
Background
Glottic squamous cell carcinoma (GSCC) is the most prevalent type of laryngeal carcinoma. The value of prophylactic lymph node dissection (LND) in resected GSCC remains controversial. This study aims to quantitatively assess the probability of occult lymph node metastasis (LNM) for GSCC patients and devise individualized postoperative radiotherapy strategies.
Methods
A total of 1319 patients with GSCC were retrospectively analyzed.
Results
GSCC patients with T1‐T2 stages showed significantly lower LNM rate than those with T3‐T4 stages. For patients with T3‐T4 GSCC, multivariate logistic analyses indicated that three factors—maximum tumor diameter (MTD) of more than 2.0 cm, relatively low differentiation, and tumor invasive depth of no less than 1.0 cm—were independent risk factors for the existence of LNM. A predictive nomogram was established based on these factors. The accuracy and validity of our model were verified by 0.716 and remained at 0.717 after 1000 bootstrapping. The calibration curve was also plotted and showed a favorable agreement. The patients were stratified into two groups based on their individual LNM risk points. Possible LNM rates for low‐risk and high‐risk subgroups were 4.7% and 25.2%, respectively.
Conclusions
A new post‐operative strategy selection flow chart was established based on our newly created nomogram which can effectively predict the individualized possibility of occult LNM for GSCC patients. For clinical T3‐4N0 patients in the high‐risk subgroup, prophylactic dose post‐operative radiation therapy is recommended. However, for all those clinically diagnosed as T1‐2N0 stage, regular follow‐up is sufficient in view of the low occult LNM rate.
Level of Evidence: 2a
Keywords: Glottic squamous cell carcinoma, occult contralateral lymph node metastasis, postoperative adjuvant radiotherapy, risk prediction model, treatment choice
Short abstract
This study quantitatively assesses the probability of occult LNM for GSCC patients. Postoperative adjuvant therapy could adversely affect long‐term survival for some patients. This study devise individualized postoperative radiotherapy strategies.
1. INTRODUCTION
Laryngeal carcinoma is one of the most common malignancies of the head and neck region, most of which are squamous cell carcinoma. 1 Glottic squamous cell carcinoma (GSCC) accounts for around two‐thirds of all laryngeal carcinomas. GSCC is usually diagnosed at an early stage, owing to typical symptoms such as hoarseness caused by vocal cord invasion. 2 , 3 For early GSCC, single‐modality radiotherapy (RT) and organ preservation surgery, including laser surgery, are common treatment models in which both treatment options can provide favorable prognosis, with reported locoregional control rates ranging around 85%–100%. 4 , 5 , 6 , 7 Considering the paucity of lymphatic drainage in the glottic region, the rate of neck metastasis is extremely low for patients with T1‐T2 GSCC, 2 let alone the occult lymph node metastasis (LNM) in T1‐2N0 GSCC, so no prophylactic measures are typically needed for cervical lymph node regions for those patients. However, for patients with advanced T stages (T3‐T4) of laryngeal carcinoma, for whom laryngectomy still remains the standard treatment, 8 , 9 the neck involvement rate is high, and the reported incidence of occult cervical lymph node metastases in patients with cT3‐4N0 laryngeal carcinoma was no less than 20%. 10 , 11 In view of the characteristics of regional metastasis, prophylactic lymph node dissections (LNDs) are recommended for patients with cT3‐4N0 supraglottic and subglottic carcinoma in many clinical centers. In contrast, no consensus has been reached regarding the management of clinical N0 GSCC patients, and clear indications for neck management in patients with T3‐4N0 GSCC are still controversial.
The present study thus aims to quantitatively predict and stratify the risk of occult LNM in GSCC patients, especially in those with advanced T stages (T3 and T4), to decide the adjuvant treatment strategies postoperatively.
2. METHODS
2.1. Patient cohort
Retrospective analysis was performed on 1335 GSCC patients who underwent surgical treatment at Department of Otorhinolaryngology, Head and Neck Surgery at the Eye, Ear, Nose and Throat Hospital of Fudan University from 2005 to 2010. The following criteria were applied to exclude patients for our study: (1) patients with multiple primary tumors (N = 6); (2) patients with existing distant metastasis when diagnosed (N = 4); and (3) patients who had received any preoperative neoadjuvant treatments (N = 6). Patients with primary GSCC and received surgery as initial treatment were enrolled in this study. At last, a total of 1319 patients were enrolled in our study.
This study was approved by the Institutional Ethics Committee of the Eye & ENT Hospital of Fudan University. All patients enrolled gave informed consent to take part in the study.
2.2. Surgical treatments and data collection
The demographic and clinical characteristics of age, sex, history of smoking and alcohol drinking, history of hypertension and diabetes, TNM classification were documented (shown in Table 1). Postoperative data including tumor differentiation, maximum tumor diameter (MTD), and tumor invasive depth were also reviewed to create our retrospective database.
TABLE 1.
The clinicopathological characteristics of GSCC patients
| N = 1319 | % | |
|---|---|---|
| Age | ||
| ≥60 | 653 | 49.5 |
| <60 | 666 | 50.5 |
| Sex | ||
| Female | 33 | 2.5 |
| Male | 1286 | 97.5 |
| History of smoking | ||
| No | 390 | 29.6 |
| Yes | 929 | 70.4 |
| History of alcohol drinking | ||
| No | 699 | 53.0 |
| Yes | 620 | 47.0 |
| History of hypertension | ||
| No | 1087 | 82.4 |
| Yes | 232 | 17.6 |
| History of diabetes | ||
| No | 1261 | 95.6 |
| Yes | 58 | 4.4 |
| T stage | ||
| T1 | 370 | 28.1 |
| T2 | 555 | 42.1 |
| T3 | 300 | 22.7 |
| T4 | 94 | 7.1 |
| N stage | ||
| N0 | 1253 | 95.0 |
| N1 | 29 | 2.2 |
| N2 | 32 | 2.4 |
| N3 | 5 | 0.4 |
| AJCC Stage I–IV stratification | ||
| Stage I | 368 | 27.9 |
| Stage II | 541 | 41.0 |
| Stage III | 294 | 22.3 |
| Stage IV | 116 | 8.8 |
| Differentiation | ||
| High | 748 | 56.7 |
| Moderate and low | 571 | 43.3 |
All patients underwent primary tumor resection in our cohort. Organ‐preserving options, including partial laryngectomy and transoral laser microsurgery, were conducted for patients with T1‐T2 diseases. Both organ‐preserving surgery and total laryngectomy were performed for patients with T3 disease according to the preoperative evaluations of tumor extent. For patients with T4 stage GSCC, total laryngectomy was recommended as standard treatment and performed for most patients unless the patient declined. Cervical LND was performed for those with clinically detected neck involvement by preoperative imaging examination, or prophylactically conducted for those with high suspicion of LNM evaluated by clinicians. For those who received primary tumor resection only, positive LNM detected by postoperative follow‐up 6 months after initial surgery will be identified as having occult neck involvement at the time of initial treatment and will be categorized in the LNM group.
2.3. Statistical analysis
Chi‐square test was used for the comparison between categorical variable. Univariate and multivariate analyses were conducted to screen out the independent risk factors for occult cervical lymph node involvement. Variables with P‐values <.05 in the univariate analysis were enrolled into multivariate analysis. Risk‐scoring prediction models were created using R software (version 3.5.1; R Development Core Team). We conducted the receiver operating characteristic (ROC) and calculated the concordance index (C‐index) for assessing the discrimination of our newly established predictive model. The calibration curve was also built to evaluate the consensus degree of the model. A P‐value <.05 was considered statistically significant, and statistical analyses were conducted with SPSS 24.0 software (SPSS Inc., Chicago, IL, USA).
3. RESULTS
3.1. Demographics, clinical characteristics, and LNM of all GSCC patients
A total of 1319 patients with GSCC (1286 males and 33 females) were enrolled in our research. The mean age of all patients was 60.43 ± 10.05 years (ranging from 30 to 90). Nine hundred and twenty‐five (70.2%) tumors were Stage T1 or T2, and 394 (29.8%) tumors were stage T3 or T4. Further clinicopathological characteristics of the patent population are depicted in Table 1. Primary tumor resection, including partial and total hypopharyngectomy, was conducted for all patients.
Among the 925 patients with T1‐T2 stages, 30 (3.2%) patients received both primary tumor resection and cervical LND for preoperatively detected lymph node involvement before surgery and high suspicion of LNM evaluated by clinicians. Fifteen of the 30 patients conducted with LND were confirmed by postoperative pathology. For the other 895 stage T1‐2 patients who received primary tumor resection only, five were diagnosed as LNM by imaging and pathological examination during the postoperative follow‐up within 6 months from initial surgery. In total, only 20 (2.2%) of 925 patients with T1‐T2 stages were regarded as having cervical LNM at the time of initial diagnosis (shown in Figure 1).
FIGURE 1.
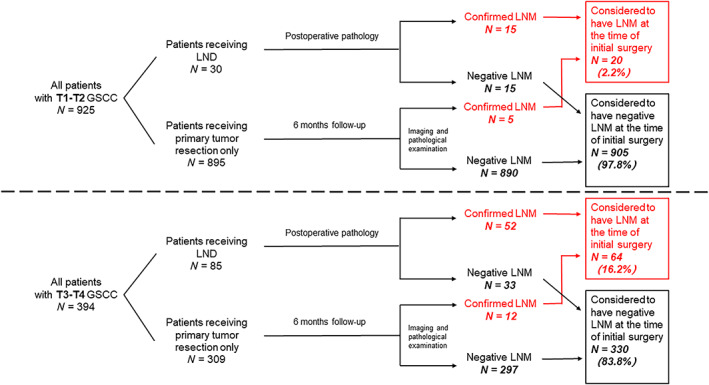
Flow diagram of case selection steps and follow‐up information for patients enrolled
Among the 394 GSCC patients with T3‐T4 stages, 85 (21.6%) patients received both primary tumor resection and cervical LND. Fifty‐two of the 85 patients receiving LND for preoperatively detected lymph node involvement before surgery and high suspicion of LNM by surgeons were confirmed by postoperative pathology. For the other patients (N = 309) who received primary tumor resection only, 12 were diagnosed as LNM by imaging and pathological examination during the postoperative follow‐up within 6 months from initial surgery. In total, 64 (16.2%) patients with T3‐T4 stages were defined as having cervical LNM at the time of initial diagnosis (shown in Figure 1).
The extremely low LNM rate (only 20 in 925) for patients with T1‐T2 stages indicated that occult LNM is exceedingly rare in those patients, so we focused on patients with T3‐T4 stages GSCC. No significant differences in the patient demographic data, including age, sex, history of smoking, alcohol drinking, hypertension, and diabetes, between the non‐LNM group and LNM group were observed. However, more T4 as well as low differentiation tumors were found in patients with positive cervical lymph node involvement (P‐value = .013 and .030, respectively). Patients in the LNM group also showed larger metastatic MTD and tumor invasive depth (P‐value = .000). Details were shown in Table 2.
TABLE 2.
The clinicopathological characteristics of T3‐T4 GSCC patients
| All patients | Non‐LNM group | LNM group | |||||
|---|---|---|---|---|---|---|---|
| n = 394 | % | n = 330 | % | n = 64 | % | P‐value | |
| Age | .429 | ||||||
| ≥60 | 210 | 53.3 | 173 | 52.4 | 37 | 57.8 | |
| <60 | 184 | 46.7 | 157 | 47.6 | 27 | 42.2 | |
| Sex | .451 | ||||||
| Female | 12 | 3.0 | 11 | 3.3 | 1 | 1.6 | |
| Male | 382 | 97.0 | 319 | 96.7 | 63 | 98.4 | |
| History of smoking | .311 | ||||||
| No | 113 | 28.7 | 98 | 29.7 | 15 | 23.4 | |
| Yes | 281 | 71.3 | 232 | 70.3 | 49 | 76.6 | |
| History of alcohol drinking | .840 | ||||||
| No | 220 | 55.8 | 185 | 56.1 | 35 | 54.7 | |
| Yes | 174 | 44.2 | 145 | 43.9 | 29 | 45.3 | |
| History of hypertension | .688 | ||||||
| No | 321 | 81.5 | 270 | 81.8 | 51 | 79.7 | |
| Yes | 73 | 18.5 | 60 | 18.2 | 13 | 20.3 | |
| History of diabetes | .877 | ||||||
| No | 374 | 94.9 | 313 | 94.8 | 61 | 95.3 | |
| Yes | 20 | 5.1 | 17 | 5.2 | 3 | 4.7 | |
| T stage | .013 | ||||||
| T3 | 300 | 76.1 | 259 | 78.5 | 41 | 64.1 | |
| T4 | 94 | 23.9 | 71 | 21.5 | 23 | 35.9 | |
| Differentiation | .030 | ||||||
| High | 178 | 45.2 | 157 | 47.6 | 21 | 32.8 | |
| Moderate and Low | 216 | 54.8 | 173 | 52.4 | 43 | 67.2 | |
| Maximum tumor diameter (MTD) | .000 | ||||||
| ≤2.0 cm | 130 | 33.0 | 123 | 37.3 | 7 | 10.9 | |
| >2.0 cm | 264 | 67.0 | 207 | 62.7 | 57 | 89.1 | |
| Tumor invasive depth | .000 | ||||||
| <1.0 cm | 123 | 31.2 | 118 | 35.8 | 5 | 7.8 | |
| ≥1.0 cm | 271 | 68.8 | 212 | 64.2 | 59 | 92.2 | |
3.2. Construction of the risk‐scoring model for predicting occult LNM in clinical T3‐4N0 patients
Demographic factors including the age and sex of patients, smoking and drinking history, hypertension and diabetes medical history, and clinicopathological characteristics including T stage, tumor differentiation, MTD, and tumor invasive depth were analyzed by the logistic univariate analysis. Factors with P‐values <.05 were incorporated into multivariate regression analysis for further screening. Finally, tumor differentiation, MTD, and tumor invasive depth were recognized as independent risk factors of LNM in PSSC patients with T3‐T4 stages (shown in Table 3). Then a nomogram incorporating the above‐mentioned three factors was constructed to assess the risk of occult cervical lymph node involvement in GSCC patients with T3‐4N0 stage (shown in Figure 2A).
TABLE 3.
Univariate and multivariate analyses for T3‐T4 GSCC patients
| Factors selected | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | |
| Age | .430 | |||
| ≥60 vs. <60 | 1.244 (0.724–2.136) | |||
| Sex | .461 | |||
| Female vs. male | 2.172 (0.276–17.128) | |||
| History of smoking | .312 | |||
| Yes vs. no | 1.380 (0.739–2.577) | |||
| History of alcohol drinking | .840 | |||
| Yes vs. no | 1.057 (0.617–1.810) | |||
| History of hypertension | .688 | |||
| Yes vs. no | 1.147 (0.587–2.242) | |||
| History of diabetes | .877 | |||
| Yes vs. no | 0.905 (0.257–3.185) | |||
| T stage | .015 | .602 | ||
| T4 vs. T3 | 2.046 (1.152–3.634) | 1.178 (0.635–2.185) | ||
| Differentiation | .032 | .025 | ||
| Low/moderate vs. high | 1.858 (1.056–3.269) | 1.951 (1.086–3.505) | ||
| Maximum tumor diameter (MTD) | .000 | .032 | ||
| >2.0 cm vs. ≤2.0 cm | 4.839 (2.139–10.943) | 2.649 (1.085–6.469) | ||
| Tumor invasive depth | .000 | .005 | ||
| ≥1.0 cm vs. <1.0 cm | 6.568 (2.565–16.818) | 4.267 (1.558–11.689) | ||
FIGURE 2.
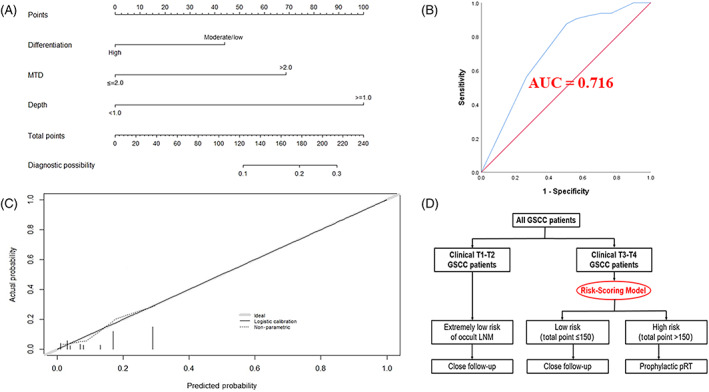
Construction, assessment, and validation of the predictive model. (A) The nomogram for predicting occult LNM risk in GSCC patients with T3‐T4 stages. (B) The ROC curve and AUC of the nomogram; ROC receiver operating characteristics. (C) The calibration curve of the nomogram for predicting occult LNM risk. Actual probability is plotted on the y‐axis, and nomogram predicted probability on the x‐axis. (D) Risk stratification and postoperative adjuvant radiotherapy strategy for GSCC patients
3.3. Evaluation and validation of the risk‐scoring model
The precision of our newly created predictive model was assessed in terms of the C‐index, and an internal validation was also conducted using 1000 bootstrap resamples to reconfirm the precision. The C‐index of our nomogram was found to be 0.716 (95% CI, 0.655–0.778) and remained at 0.717 (95% CI, 0.686–0.747) after 1000 bootstrapping, indicating satisfactory discrimination and accuracy of our model's prediction ability. The ROC curve was also exhibited in Figure 2B. Furthermore, the calibration plot was constructed and the actual and estimated probability of LNM were in fair agreement (shown in Figure 2C).
3.4. Novel risk stratification of occult LNM for GSCC patients with T3‐4N0 stage
Every variable that made up the nomogram had its corresponding risk points. The total risk points for each individual were calculated by summing up the LNM risk points of each variable based on our nomogram. In according to the distribution characteristic of the total risk points, the cutoff value was chosen to stratify GSCC patients with T3‐4N0 stage into two subgroups:
Patients with total LNM risk point of ≤150 (N = 172) were classified as low‐risk group.
Patients with total LNM risk point of >150 (N = 222) were classified as high‐risk group.
Chi‐square test showed a significant difference among the two subgroups in terms of cervical lymph node involvement (4.7% and 25.2% for low‐ and high‐risk group, respectively, P‐value =.000, shown in Table 4).
TABLE 4.
Postoperative risk stratification of T3‐T4 GSCC patients
| All T3‐T4 GSSC patients (N = 394) | |||
|---|---|---|---|
| Low risk (total point ≤150) | High risk (total point >150) | P‐value | |
| (N = 172, %) | (N = 222, %) | ||
| Negative LNM | 164 (95.3) | 166 (74.8) | .000 |
| Positive LNM | 8 (4.7) | 56 (25.2) | |
3.5. Analysis of contralateral LNM for GSCC patients
Fourteen (1.1%) patients were considered to have contralateral lymph node metastasis (cLNM) in our cohort. All those patients with cLNM were confirmed as having ipsilateral lymph node metastases, which meant no skip metastasis (positive contralateral lymph nodes with no ipsilateral lymph node involvement) was found in GSCC patients in our research. The cLNM rates for patients with T3‐T4 stages (3.0%, 12 in 394 patients) were extremely higher than those for patients with T1‐T2 stages (0.2%, only two in 925 patients, P‐value =.000). Among those with T3‐T4 stages, contralateral lymph node involvement was significantly more common in patients with T4 disease (6.3%, six in 95 patients) than in those with T3 disease (2.0%, six in 299 patients, P‐value =.033). Meanwhile, the cLNM rate for T3‐T4 patients with positive ipsilateral LNM was 18.8% (12 in 64 patients).
4. DISCUSSION
GSCC ranks first among the three types of carcinoma in the laryngeal region. In contrast to supraglottic carcinoma, which frequently presents with cervical lymph node involvement from early T stages 12 and the reported neck metastasis rate ranges from 20% to 33%, 13 , 14 the paucity of lymphatic drainage in the glottis region results in low rates of cervical involvement, with 0.4%–6.5% GSCC cases initially presenting with neck metastases from the literature. 12 , 13 , 15 , 16 In our research, 67 (5.1%) of 1319 patients [15 (1.6%) from the 925 patients who had T1‐T2 diseases and the other 52 (13.2%) from the 394 patients who had T3‐T4 diseases) had pathologically confirmed LNM. Meanwhile, 17 patients (5 from patients with T1‐T2 stages and 12 from patients with T3‐T4 stages) were diagnosed with LNM by imaging and pathological examination during the post‐operative follow‐up within 6 months from initial surgery and were identified as having occult neck involvement at the time of initial treatment and categorized as the LNM group. In total, 84 (6.4%) patients were defined as having cervical LNM at the time of initial diagnosis in our cohort. Considering that the presence of neck metastasis is predictive of a high recurrence rate and poor prognosis for patients with laryngeal carcinoma, the management of the neck region is key to the successful treatment of GSCC. 17
The preferred treatment options for patients with early stage GSCC include transoral endoscopic surgery and radical radiation therapy. Existing literature has compared the two approaches, 2 , 18 , 19 but there is little evidence definitively favoring one treatment, making both of the two approaches acceptable options. Regardless of the primary treatment option, prophylactic intervention of the clinically negative neck in patients with early stage GSCC is usually not recommended. 20 Pinilla et al. and Howell‐Burke et al. reported an occult neck involvement rate of 2.3% and 4.3% in clinical T2N0 GSCC patients, respectively 21 ; two other studies also found that none of the patients with early stage glottic carcinomas showed occult LNM in their series. 22 , 23 In our research, neck metastasis was proved to be present at initial diagnosis in 20 (2.2%) cases among all T1‐T2 GSCC patients. For the 895 patients with clinical T1‐2N0 stage who received primary tumor resection alone, positive LNM was only detected in five patients by follow‐up 6 months after initial surgery, with an occult LNM rate of just 0.6%. Thus, our study supports the concept that prophylactic intervention is unnecessary for patients with early GSCC.
The management of the N0 neck in patients with T3‐4 laryngeal squamous cell carcinoma still remains controversial. No specific indications for prophylactic neck dissection in those patients have been established. 8 Even though GSCC exhibits an extremely low neck metastasis rate compared to supraglottic carcinoma, 24 cervical lymph node recurrence still occurred in some patients, which raises the possibility of occult neck involvement at the time of initial diagnosis. Several studies have reported that the incidence of occult LNM in patients with cT3‐4N0 laryngeal cancer was no less than 20%. 8 , 10 , 11 Moreover, technological developments in radiation, such as intensity‐modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT), significantly decrease morbidity associated with prophylactic treatment of the neck. 25 , 26 , 27 Thus, selecting the right patients who are at high‐risk of developing occult LNM to avoid the potential neck recurrence seems to be a better strategy than a routinely performed prophylactic radiotherapy of the neck region or blindly conducted “wait and see” protocol for cT3‐4N0 GSCC patients. However, literature that quantitatively evaluated the risk of occult LNM among GSCC patients with advanced T stages remains scarce. In our research, three variables, including tumor differentiation, MTD, and tumor invasive depth, were confirmed to be independent risk factors for LNM for T3‐4 GSCC patients in our study, and a predictive model was established based on the above‐mentioned three variables to quantitatively measure the possibility of occult LNM in T3‐4 GSCC patients using a convenient method named nomogram, which is often applied for making a prediction of clinical disease diagnosis with no assistance of computer software. 28 Our newly created model was confirmed to be efficacious by both the C‐index and calibration curve. Considering that the three selected variables can be obtained not only by pathologic diagnosis after surgical resection, but also by tissue biopsy and imageological diagnosis before treatment, our predictive model can be applicable regardless of the primary treatment option (surgery or radical radiotherapy). Every patient can get a total risk score for quantifying the risk of occult LNM according to our model. Two subgroups [low (≤150, with LNM rate of 4.7%) and high‐risk (>150, with LNM rate of 25.2%) groups] were formed and confirmed to be rational (P < .001).
Finally, we established a detailed strategy selection flow chart for occult neck involvement risk assessment for GSCC patients with node‐negative necks. For patients with T1‐2 disease, considering the extremely low incidence rate of occult LNM, preventive interventions such as elective LND and prophylactic radiotherapy for neck regions are not recommended to avoid unnecessary postoperative complications and radiation‐related toxicity. Patients can be assessed using our nomogram. For those grouped into low‐risk groups, an observational strategy should be conducted. However, for those grouped into high‐risk subgroups, considering the high probability of neck involvement, a prophylactic dose of radiation for the ipsilateral neck region is recommended to minimize the complication risk brought by radiation on the premise of occult neck involvement control.
We also did research on the contralateral lymph node involvement rate for patients with GSCC. The results showed that cLNM was extremely rare in T1‐T2 GSCC patients, and the cLNM rates for T3 and T4 patients were 2.0% and 6.3%, respectively. In view of the low contralateral involvement rate, prophylactic treatment for contralateral neck is also unnecessary for patients with clinically negative contralateral neck results.
5. CONCLUSION
Our study is the first to quantitatively assess the risk of occult LNM in T3‐T4 GSCC patients. A treatment strategy selection flow chart is established for all GSCC patients with clinically negative neck involvement, which may aid in clinical decision‐making for those patients.
6. LIMITATIONS
Several limitations may still remain in our research. First of all, because of the retrospective nature, selection bias is inevitable. Second, our predictive model was validated by 1000 bootstrapping, which leads to the lack of external validation. Thirdly, all patients enrolled were all from one single medical center, and more data from multicenter prospective study are needed to confirm our results.
CONFLICT OF INTEREST
The authors report no conflicts of interest. The authors are responsible for the content and writing of the article.
Heng Y, Xu C, Lin H, et al. Management of clinically node‐negative glottic squamous cell carcinoma patients according to risk‐scoring model for occult lymph node metastases. Laryngoscope Investigative Otolaryngology. 2022;7(3):715-722. doi: 10.1002/lio2.762
Yu Heng and Chengzhi Xu are the co‐first authors.
Funding information The National Natural Science Foundation of China, Grant/Award Numbers: 81772878, 30801283, 30972691; The Science and Technology Commission of Shanghai Municipality, Grant/Award Number: 16411950100; The Science and Technology Innovation Project of Shanghai Shenkang Hospital Clinical Development Center, Grant/Award Number: SHDC12015114; The Shanghai Municipal Science and Technology Foundation, Grant/Award Number: 11JC1410802; The Shanghai Science and Technology Development Funds, Grant/Award Numbers: 09QA1401000, 10QA1405900, 14411961900; The Training Program of the Excellent Young Talents of Shanghai Municipal Health System, Grant/Award Numbers: XYQ2011055, XYQ2011015
Contributor Information
Chunping Wu, Email: wcpeent@163.com.
Lei Tao, Email: doctortaolei@163.com.
REFERENCES
- 1. Allegra E, Ferrise P, Trapasso S, et al. Early glottic cancer: role of MRI in the preoperative staging. Biomed Res Int. 2014;2014:890385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brady JS, Marchiano E, Kam D, Baredes S, Eloy JA, Park RC. Survival impact of initial therapy in patients with T1‐T2 glottic squamous cell carcinoma. Otolaryngol Head Neck Surg. 2016;155(2):257‐264. [DOI] [PubMed] [Google Scholar]
- 3. Abdurehim Y, Hua Z, Yasin Y, Xukurhan A, Imam I, Yuqin F. Transoral laser surgery versus radiotherapy: systematic review and meta‐analysis for treatment options of T1a glottic cancer. Head Neck. 2012;34(1):23‐33. [DOI] [PubMed] [Google Scholar]
- 4. Baird BJ, Sung CK, Beadle BM, Divi V. Treatment of early‐stage laryngeal cancer: a comparison of treatment options. Oral Oncol. 2018;87:8‐16. [DOI] [PubMed] [Google Scholar]
- 5. Hendriksma M, van Loon Y, Klop WMC, et al. Quality of life and voice outcome of patients treated with transoral CO2 laser microsurgery for early glottic carcinoma (T1‐T2): a 2‐year follow‐up study. Eur Arch Otorhinolaryngol. 2019;276(3):805‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shelan M, Anschuetz L, Schubert AD, et al. T1‐2 glottic cancer treated with radiotherapy and/or surgery. Strahlenther Onkol. 2017;193(12):995‐1004. [DOI] [PubMed] [Google Scholar]
- 7. Kayhan FT, Koc AK, Erdim I. Oncological outcomes of early glottic carcinoma treated with transoral robotic surgery. Auris Nasus Larynx. 2019;46(2):285‐293. [DOI] [PubMed] [Google Scholar]
- 8. Tsushima N, Hayashi R, Shinozaki T, Tomioka T, Okano W, Ikeda M. The role of elective neck dissection for cT4aN0 glottic squamous cell carcinoma. Jpn J Clin Oncol. 2019;49(6):525‐528. [DOI] [PubMed] [Google Scholar]
- 9. Francis E, Matar N, Khoueir N, Nassif C, Farah C, Haddad A. T4a laryngeal cancer survival: retrospective institutional analysis and systematic review. Laryngoscope. 2014;124(7):1618‐1623. [DOI] [PubMed] [Google Scholar]
- 10. Deganello A, Gitti G, Meccariello G, Parrinello G, Mannelli G, Gallo O. Effectiveness and pitfalls of elective neck dissection in N0 laryngeal cancer. Acta Otorhinolaryngol Ital. 2011;31(4):216‐221. [PMC free article] [PubMed] [Google Scholar]
- 11. Mnejja M, Hammami B, Bougacha L, et al. Occult lymph node metastasis in laryngeal squamous cell carcinoma: therapeutic and prognostic impact. Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127(5):173‐176. [DOI] [PubMed] [Google Scholar]
- 12. Raitiola H, Pukander J, Laippala P. Glottic and supraglottic laryngeal carcinoma: differences in epidemiology, clinical characteristics and prognosis. Acta Otolaryngol. 1999;119(7):847‐851. [DOI] [PubMed] [Google Scholar]
- 13. Raitiola H, Pukander J. Symptoms of laryngeal carcinoma and their prognostic significance. Acta Oncol. 2000;39(2):213‐216. [DOI] [PubMed] [Google Scholar]
- 14. Ma H, Lian M, Feng L, et al. Factors contributing to lymph node occult metastasis in supraglottic laryngeal carcinoma cT2–T4 N0M0 and metastasis predictive equation. Chin J Cancer Res. 2014;26(6):685‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansen O, Larsen S, Bastholt L, Godballe C, Jørgensen KE. Duration of symptoms: impact on outcome of radiotherapy in glottis cancer patients. Int J Radiat Oncol Biol Phys. 2005;61(3):789‐794. [DOI] [PubMed] [Google Scholar]
- 16. Tachibana T, Orita Y, Marunaka H, et al. Glottic cancer in patients without complaints of hoarseness. Head Neck. 2016;38 (Suppl 1):E316‐E320. [DOI] [PubMed] [Google Scholar]
- 17. Resnick JM, Uhlman D, Niehans GA, et al. Cervical lymph node status and survival in laryngeal carcinoma: prognostic factors. Ann Otol Rhinol Laryngol. 1995;104(9 Pt 1):685‐694. [DOI] [PubMed] [Google Scholar]
- 18. Ozturk K, Turhal G, Durusoy D, et al. Long‐term swallowing outcomes of radiotherapy and transoral laser microsurgery for T1 glottic cancer treatment. Clin Otolaryngol. 2021;46(2):340‐346. [DOI] [PubMed] [Google Scholar]
- 19. Warner L, Lee K, Homer JJ. Transoral laser microsurgery versus radiotherapy for T2 glottic squamous cell carcinoma: a systematic review of local control outcomes. Clin Otolaryngol. 2017;42(3):629‐636. [DOI] [PubMed] [Google Scholar]
- 20. Patel TR, Eggerstedt M, Toor J, et al. Occult lymph node metastasis in early‐stage glottic cancer in the National Cancer Database. Laryngoscope. 2021;131(4):E1139‐E1146. [DOI] [PubMed] [Google Scholar]
- 21. Pinilla M, González FM, López‐Cortijo C, et al. Management of N0 neck in laryngeal carcinoma. Impact on patient's survival. J Laryngol Otol. 2003;117(1):63‐66. [DOI] [PubMed] [Google Scholar]
- 22. Erdag TK, Guneri EA, Avincsal O, et al. Is elective neck dissection necessary for the surgical management of T2N0 glottic carcinoma? Auris Nasus Larynx. 2013;40(1):85‐88. [DOI] [PubMed] [Google Scholar]
- 23. Yang CY, Andersen PE, Everts EC, Cohen JI. Nodal disease in purely glottic carcinoma: is elective neck treatment worthwhile? Laryngoscope. 1998;108(7):1006‐1008. [DOI] [PubMed] [Google Scholar]
- 24. Tachibana T, Orita Y, Marunaka H, et al. Neck metastasis in patients with T1‐2 supraglottic cancer. Auris Nasus Larynx. 2018;45(3):540‐545. [DOI] [PubMed] [Google Scholar]
- 25. Wegner RE, Abel S, Bergin JJ, Colonias A. Intensity‐modulated radiation therapy in early stage squamous cell carcinoma of the larynx: treatment trends and outcomes. Radiat Oncol J. 2020;38(1):11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toledano I, Graff P, Serre A, et al. Intensity‐modulated radiotherapy in head and neck cancer: results of the prospective study GORTEC 2004‐03. Radiother Oncol. 2012;103(1):57‐62. [DOI] [PubMed] [Google Scholar]
- 27. Nutting CM, Morden JP, Harrington KJ, et al. PARSPORT trial management group. Parotid‐sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173‐e180. [DOI] [PMC free article] [PubMed] [Google Scholar]


