Abstract
During submerged culture in the presence of glucose and glutamate, the filamentous fungus Monascus ruber produces water-soluble red pigments together with citrinin, a mycotoxin with nephrotoxic and hepatoxic effects on animals. Analysis of the 13C-pigment molecules from mycelia cultivated with [1-13C]-, [2-13C]-, or [1,2-13C]acetate by 13C nuclear magnetic resonance indicated that the biosynthesis of the red pigments used both the polyketide pathway, to generate the chromophore structure, and the fatty acid synthesis pathway, to produce a medium-chain fatty acid (octanoic acid) which was then bound to the chromophore by a trans-esterification reaction. Hence, to enhance pigment production, we tried to short-circuit the de novo synthesis of medium-chain fatty acids by adding them to the culture broth. Of fatty acids with carbon chains ranging from 6 to 18 carbon atoms, only octanoic acid showed a 30 to 50% stimulation of red pigment production, by a mechanism which, in contrast to expectation, did not involve its direct trans-esterification on the chromophore backbone. However, the medium- and long-chain fatty acids tested were readily assimilated by the fungus, and in the case of fatty acids ranging from 8 to 12 carbon atoms, 30 to 40% of their initial amount transiently accumulated in the growth medium in the form of the corresponding methylketone 1 carbon unit shorter. Very interestingly, these fatty acids or their corresponding methylketones caused a strong reduction in, or even a complete inhibition of, citrinin production by M. ruber when they were added to the medium. Several data indicated that this effect could be due to the degradation of the newly synthesized citrinin (or an intermediate in the citrinin pathway) by hydrogen peroxide resulting from peroxisome proliferation induced by medium-chain fatty acids or methylketones.
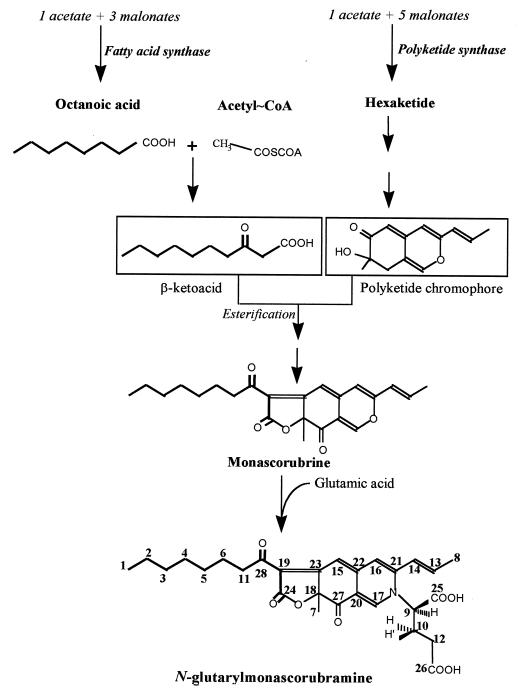
In filamentous fungi, many secondary metabolites with complex chemical structures are synthesized from the polyketide pathway (26, 29). These metabolites display a wide range of biological activities, including antibiotic, antifungal, immunosuppressive, and anticancer properties. In this respect, Monascus ruber is an interesting filamentous fungus which can excrete a broad spectrum of colored pigments that are routinely used in Asia as food additives. From previous works (12, 29), a scheme of the hypothetical routes for the biosynthesis of these various pigments in filamentous fungi is depicted in Fig. 1. The condensation of 1 mol of acetate with 5 mol of malonate leads to the formation of a hexaketide chromophore by the polyketide synthase. Then a medium-chain fatty acid such as octanoic acid, likely produced by the fatty acid biosynthetic pathway, is bound to the chromophore structure by a trans-esterification reaction to generate the orange pigment monascorubrin (or rubropunctatin upon trans-esterification with hexanoic acid). The reduction of the orange pigment gives rise to the yellow pigment ankaflavin from monascorubrin (or monascin from rubropunctatin), whereas red pigments (monascorubramine and rubropunctamine) are produced by amination of orange pigments with NH3 units (18). All these pigments remain essentially intracellular because of their high hydrophobicity. They are eventually excreted in the medium after reacting with an NH2 unit of amino acids (13, 32). For this reason, glutamate has been the most useful amino acid, since it can serve both as a carbon and as a nitrogen source (21, 22).
FIG. 1.
Scheme of the hypothetic metabolic routes leading to the final structure of the water-soluble red pigment N-glutarylmonascorubramine in M. ruber.
While these pigments are traditionally produced by solid-state fermentation on rice or bread crumbs, studies involving submerged fermentation have recently revealed that, together with pigment production, M. ruber excretes a mycotoxin, namely, citrinin (6), which has antibiotic properties against gram-positive bacteria. However, the nephrotoxic and hepatotoxic properties of this toxin (2, 4) compromise the use of red pigments as natural colorants for food technology. Therefore, biochemical and genetic studies should be undertaken to prevent the formation of citrinin while enhancing that of pigments. As a first step along this line, we recently demonstrated that the biosynthesis of citrinin originates from a tetraketide instead of a pentaketide as was found in Aspergillus terreus and Penicillium citrinum (14). Since pigments are produced from a hexaketide, this suggested the existence of a branch point at the tetraketide level which could account for a differential production of pigments and citrinin during the growth of M. ruber. However, the enzymes catalyzing the reactions at this junction have not been characterized yet.
Another method to potentially enhance pigment synthesis and eventually reduce that of citrinin came from the suggestion given above that the synthesis of pigments may arise from a combination of the polyketide and fatty acid synthase pathways (see Fig. 1). Therefore, it might be feasible to short-circuit the need for endogenous synthesis of these medium-chain fatty acids by adding them to the growth medium. We addressed this question by determining the fates of [1-13C]-, [2-13C]-, or [1,2-13C]acetate and [1-13C]octanoate during the biosynthesis of pigments using 13C nuclear magnetic resonance (NMR), and we investigated the effects of medium- and long-chain fatty acids on pigment and citrinin production. Our results showed that, contrary to expectations, the synthesis of pigments was barely affected whereas the production of citrinin was strongly inhibited, likely by a hydrogen peroxide-mediated degradation of the toxin due to fatty acid-induced peroxisome proliferation.
MATERIALS AND METHODS
Microorganism and growth conditions.
M. ruber ATCC 96218, characterized by its high level of pigment production, was purchased from the American Type Culture Collection. It was cultivated in a chemically defined medium which contained, per liter, 5 or 20 g of glucose, 5 g of monosodium glutamate (MSG), 5 g of K2HPO4, 5 g of KH2PO4, 0.1 g of CaCl2, 0.5 g of MgSO4 · 7H2O, 0.01 g of FeSO4 · 7H2O, 0.01 g of ZnSO4 · 7H2O, and 0.03 g of MnSO4 · H2O. The initial pH of the medium was adjusted to 6.5 with phosphoric acid. The stock culture was maintained on Difco potato dextrose agar (PDA) slants. Spores were prepared by growth on these slants for 10 days at 30°C, harvested, and washed with sterile water. A suspension of 108 spores was used to inoculate a 1-liter baffled Erlenmeyer flask containing 200 ml of medium. Culture was carried out for 2 days at 30°C and at 150 rpm. The pH of the cultures was not regulated, but the phosphate buffer ensured the stability of the pH during culturing. When required, fatty acids or methylketones were added to the culture medium at the indicated concentrations (see Fig. 2 and 3 and Tables 2 and 3). The fungal biomass was determined by gravimetric analysis after filtration of cell samples on preweighed nylon filters (diameter, 45 mm; pore size, 0.8 μm) and drying to a constant weight at 60°C under a partial vacuum (200 mm of Hg).
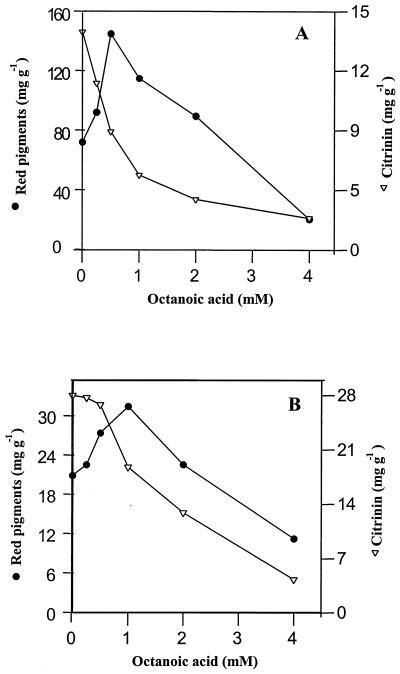
FIG. 2.
Effect of increasing the concentration of sodium octanoate on the specific production of water-soluble red pigments and citrinin. M. ruber was cultured in the presence of MSG at 5 g · liter−1 and glucose at 5 (A) or 20 (B) g · liter−1. Amounts of pigments, citrinin, and fungal biomass were determined after 120 h of culture.
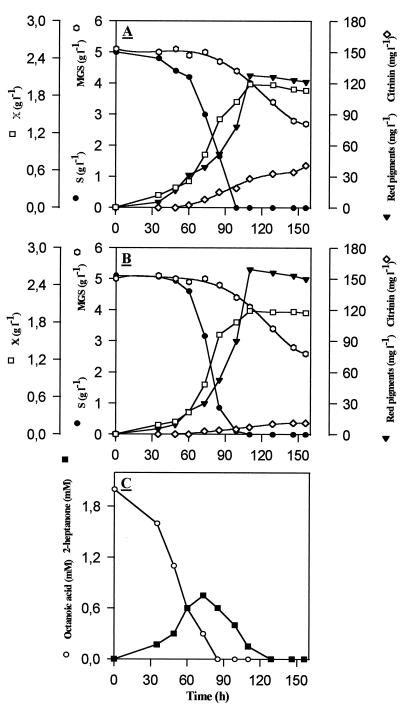
FIG. 3.
(A and B) Kinetics of red pigments and citrinin production during discontinuous culture of M. ruber in the absence (A) or in the presence (B) of 2 mM sodium octanoate. (C) Kinetics of octanoate consumption and production of 2-heptanone. X, biomass.
TABLE 2.
Effects of fatty acids of various carbon chain lengths on the specific production of red pigments and citrinin during submerged fermentation of M. ruber in the presence of glucose and MSGa
| Type of fatty acid addedb | Concn (mg/g of biomass) of:
|
|
|---|---|---|
| Red pigments | Citrinin | |
| Control | 54 | 14 |
| Hexanoic acid | 43.5 | 10 |
| Octanoic acid | 114 | 7.4 |
| Decanoic acid | 52.5 | 9.0 |
| Dodecanoic acid | 51 | 3.6 |
| Myristic acid | 54 | 14.6 |
| Stearic acid | 51 | 11.6 |
| Oleic acid | 55.5 | 13 |
Each at a concentration of 5 g · liter−1. Levels of citrinin and pigments were determined after 95 h of growth of M. ruber. The data reported are from one typical experiment which was repeated at least twice with consistent results (standard deviation, less than 10%).
At 1 mM.
TABLE 3.
Effects of methylketones on the specific production of red pigments and citrinin during submerged fermentation of M. ruber in the presence of glucose and MSGa
| Type of methylketone added (mM) | Concn (mg/g of biomass) of:
|
|
|---|---|---|
| Red pigments | Citrinin | |
| Control | 67 | 15 |
| 2-Pentanone (1.32) | 66 | 9 |
| 2-Heptanone (1.0) | 70.5 | 7 |
| 2-Nonanone (0.80) | 62.5 | 2 |
| 2-Tridecanone (0.57) | 54 | 0 |
Glucose and MSG were each present at a concentration of 5 g · liter−1. While levels of citrinin and pigments were determined throughout growth, only the maximal values are reported. For most of the experiments, the maximum was reached between 130 and 160 h of growth. The data reported are from one typical experiment which was repeated twice with consistent results (standard deviation, about 10%).
Labeling experiments and isolation of the labeled red pigments.
Pigments were labeled by 13C using 98% enriched [1-13C]-, [2-13C]-, or [1,2-13C]sodium acetate. An aqueous solution (1 ml) of [1-13C]- or [2-13C]sodium acetate (20 mg · ml−1) or [1,2-13C]sodium acetate (10 mg · ml−1) was added after 3, 4, 5, 6, and 7 days to 200 ml of culture. The fate of octanoate in fungal metabolism was determined using 1 ml of 90% enriched [1-13C]sodium octanoate (15 mg · ml−1) to 200 ml of culture, which was added after 3, 4, 5, 6, and 7 days to 200 ml of culture. Red pigments were isolated from the medium by filtration of the mycelium cultures on M 14 membranes (pore size, 0.8 μm; Tech-Sep, Bollene, France). The filtrate was lyophilized and extracted several times with water-saturated n-butanol. The organic phase was dried under anhydrous Na2SO4 and vacuum concentrated. The extract was applied to a chromatography column packed with silica gel (70/230 mesh) resuspended in chloroform. The column was first washed with a 90:10 (vol/vol) chloroform-methanol solution and with a 50:50 (vol/vol) chloroform-methanol elution which eluted the water-soluble pigments. The pigments were isolated from fractions obtained by preparative thin-layer chromatography on silica gel with chloroform-methanol-water (65:25:4, vol/vol/vol) as the solvent system. The water-soluble red pigment identified as a red band was scraped off the silica plates, solubilized in chloroform-methanol (1:3, vol/vol), and purified by high-pressure liquid chromatography (HPLC) on a C18 column using a separation gradient of water-methanol (80:20 to 0:100 [vol/vol]) over 30 min. The flow rate was 0.8 ml · min−1. The detector was a Waters Lambda Max spectrophotometer set at 480 nm. The purity of the product was verified by NMR spectroscopy. 13C and 1H NMR spectra were recorded on a Bruker ARX (400 Mhz) using CD3OD (99.8%) as a solvent. Spectra were referenced internally to the solvent for 13C NMR and to trimethylsilyl for 1H NMR.
Identification and quantitative determination of fatty acids and methylketones in fermentation broth.
Fatty acids and methylketones in fermentation broth were identified by mass spectrometry coupled to gas chromatography (MS-GC) (MS-MD 800, GC 8000 series; Fisons Instruments) using an OV100 column (15 mm by 0.2 μm). The injector was set at 180°C, with helium at 2.2 × 105 Pa as the carrier gas. The temperature cycle started at 65°C and rose with a slope of 15°C per min to reach 150°C, followed by a slope of 20°C per min until the temperature reached 250°C.
Fatty acids and methylketones were quantitatively determined as follows. Culture samples (5 ml) were harvested during growth and centrifuged at low speed (5 min at 4,500 × g). The pH of the supernatant was brought to about pH 3 by dropwise addition of 0.5 N H2SO4 to convert the fatty acids into their undissociated forms, and nonanoic acid (1 mM) was added to the suspension as an internal standard. The fatty acids were extracted three times with 10 ml of ether and were dried over Na2SO4, and 1 μl of sample was injected into a gas chromatograph (Thermo-Quest series 8000) equipped with a flame ionization detector and an integrator (Hewlett-Packard). The injection port temperature was set at 200°C, and the detector temperature was set at 220°C. A capillary column (Carbo Erba GC 8000) was used with N2 as the carrier gas. The temperature program started at 50°C, stayed at this level for 1 min, and then increased at a rate of 10°C/min to reach 200°C. Fatty acids and methylketones were identified and quantitated from chromatograms made with standards.
Other analytical procedures.
Glucose was measured using a YSI 200 autoanalyzer (YSI Inc., Yellow Springs, Ohio). Ethanol and acetic acid concentrations were determined by GC using a flame ionization detector and a Poraplot Q column (25 mm by 0.53 mm) at 190°C, and N2 at a flow rate of 30 liters · min−1. Glutamic acid in the medium was quantitated with an AminoQuant 1090 HPLC (Hewlett-Packard) after derivatization with ortho-phthalaldehyde in the presence of 3-mercaptopropionic acid, according to the procedure specified by the manufacturer. Levels of red pigments in the culture broth were determined spectrophotometrically by measuring the absorbance of culture filtrate at 480 nm (1 A480 unit corresponded to 15 mg of pigment · liter−1, and the average mass of the red pigment is 498 g · mol−1 [13]). Citrinin levels were determined by HPLC on a C18 column using the following linear separation gradient: water-methanol (80:20, vol/vol) to water-methanol (0:100, vol/vol) in 30 min. The flow rate was 0.8 ml/min. The detector used was a Waters spectrophotometer, and λmax was 260 nm.
Enzyme assays.
Isocitrate lyase (EC 4.1.3.1) activity was determined from cell extracts as described in reference 1. The activity was assayed at 37°C by monitoring the formation of glyoxylate phenylhydrazone at 324 nm. Assay mixtures of 1 ml contained 25 μmol of imidazole buffer (pH 6.8), 5 μmol of EDTA, 4 μmol of phenylhydrazine hydrochloride, and 2 μmol of dl-isocitrate.
RESULTS AND DISCUSSION
The fates of labeled acetate and octanoate in pigments and citrinin.
In order to verify the hypothesis that the formation of pigments arose from a combination of the polyketide and fatty acid synthesis pathways (12, 29), we performed 13C NMR experiments on purified red pigments produced by M. ruber cultivated in a glucose-glutamate medium in the presence of [1-13C]-, [2-13C]-, or [1,2-13C]sodium acetate. Quantification of the 13C enrichment in purified N-glutarylmonascorubramine indicated that C-2, -4, -6, -13, -17, -21, -22, -23, -24, -27, and -28 originated from C-1 of the acetate unit, whereas the methyl unit of acetate gave rise to the other carbons (C-1, -3, -5, -8, -11, -14, -15, -16, -18, -19, and -20), except for C-7, which arose from the endogenous C1 (most likely CO2) pools (Table 1). However, it was found that the relative enrichment factor whose definition was given in a previous report (14) (see Table 1) was higher for C-1, -2, -3, -4, -5, -6, and -11, i.e., those carbons corresponding to the octanoic unit bound to the chromophore (Table 1). This result conclusively showed that the production of pigments needs the participation of both the polyketide and fatty acid synthesis pathways. As both pathways reside in the cytoplasm (16, 25), and assuming that there is no discrimination in the metabolic fate of acetyl-coenzyme A (CoA) whether it derived from glucose metabolization or directly from the exogenous acetate, one can suggest that the higher enrichment of the 13C units in fatty acids is mainly due to the fact that acetyl-CoA is readily assimilated at the beginning of growth for synthesis of fatty acids, while it is taken up at a later stage in the polyketide pathway, as the latter is a minor and nonessential secondary metabolic pathway (12, 16).
TABLE 1.
Enrichment of carbon unit in purified N-glutarylmonascorubramine after cultivation of M. ruber in the presence of [1-13C]-, [2-13C]-, and [1,2-13C]acetate
| Carbon | Chemical shift (ppm) | Relative enrichmenta |
|---|---|---|
| 1 | 14.39 | 13.3 |
| 2 | 23.60 | 5.6 |
| 3 | 33.00 | 6.8 |
| 4 | 30.59 | 8.8 |
| 5 | 30.30 | 9.6 |
| 6 | 26.07 | 7.5 |
| 7 | 30.68 | cp∗ |
| 8 | 19.23 | 4.8 |
| 9 | 35.09 | og# |
| 10 | 30.80 | og# |
| 11 | 41.25 | 4.6 |
| 12 | 73.00 | og# |
| 13 | 141.44 | 3.7 |
| 14 | 123.82 | 4.7 |
| 15 | 98.95 | 3.7 |
| 16 | 119.46 | 4.2 |
| 17 | 141.44 | 4.7 |
| 18 | 86.98 | 5.4 |
| 19 | 101.78 | 6.3 |
| 20 | 11.59 | 5.9 |
| 21 | 153.33 | 3.6 |
| 22 | 153.44 | 3.5 |
| 23 | 173.47 | 5.6 |
| 24 | 173.87 | 5.4 |
| 25 | 174.06 | og# |
| 26 | 180.25 | og# |
| 27 | 196.74 | 3.2 |
| 28 | 198.60 | 4 |
Calculated as α IC-XE/IC-XN, where α is determined by IC-7N/IC-7E. C-X is the carbon number, C-7 is the reference carbon, IC-XN is the intensity of the NMR signal of carbon X naturally enriched, and IC-XE is the intensity of the NMR signal of carbon X enriched in the presence of [1,2-13C2]acetate. The calculation takes into account the experimental 13C NMR conditions. cp∗, C1 pool; og#, glutamate origin.
From these data, it was interesting to know whether octanoic acid, when added to the culture medium, could be directly incorporated in the formation of the pigments. To this end, the experiments were repeated by adding 2 mM [1-13C]sodium octanoate to the growth medium. Analysis of the purified pigments by 13C NMR showed that the specific enrichment of C-28 in N-glutarylmonascorubramine molecules (see Fig. 1) was the same whether cultures were carried out with or without [1-13C]octanoate, indicating that this fatty acid could not be directly incorporated into the chromophore structure of the pigments. This result was at variance with that obtained for the biosynthesis of aflatoxin by Aspergillus parasiticus, from which a direct incorporation of exogenous hexanoic acid on the chromophore has been reported (28). This discrepancy reinforces the suggestion that the utilization of exogenous fatty acids may not be the same in all filamentous fungi (3). In spite of this difference, we found that the assimilation of the exogenous octanoic acid during growth of M. ruber actually resulted in slightly enhanced pigment excretion but mainly in the inhibition of citrinin production. These findings were therefore investigated in further detail.
Effects of fatty acids and methylketones on the kinetics of pigment and citrinin production during submerged growth of M. ruber.
The effect of increasing the concentration of sodium octanoate added to the culture medium on the production of pigments and citrinin is shown in Fig. 2. It can be seen that the amounts of pigments excreted after 95 h of growth of M. ruber on a glucose-glutamate medium increased by about 2 times when the concentration of octanoic acid increased from 0 to 0.5 mM. Above this concentration, the stimulation of pigment production began to be less efficient, and the effect of octanoic acid eventually became inhibitory at concentrations higher than 2 mM. In contrast, the production of citrinin was strongly inhibited with increasing octanoate concentrations in the medium, and a fourfold decrease in toxin production was found with 2 mM octanoate. When the initial glucose concentration in the medium was increased from 5 to 20 g · liter−1, the production of pigment decreased fourfold, whereas that of citrinin increased twofold. This glucose concentration effect on red pigments and citrinin production has been reported previously (5), but the mechanism is still unexplained. However, the effects of exogenous octanoate on pigment and citrinin production were qualitatively similar, although they were less potent in the presence of glucose at 20 g · liter−1 in the growth medium than at 5. For both culture conditions, the presence of 4 mM octanoate resulted in a strong reduction in the production of both pigment and citrinin. This inhibitory effect was likely a consequence of the growth inhibition of M. ruber caused by the toxicity effect of a high concentration of fatty acids (15).
To analyze the effects of this fatty acid in more detail, we investigated the kinetics of pigment and citrinin production by M. ruber cultivated in the absence or presence of 2 mM octanoate with respect to glucose and glutamate consumption. Figure 3 shows that the rates of glucose and glutamate consumption and the synthesis of red pigment were not affected by the presence of 2 mM fatty acid during the first 90 h of growth, i.e., during the period of complete consumption of both glucose and octanoate in the medium. During this period of growth, two-thirds of the octanoate added to the medium was readily assimilated, likely by β-oxidation into acetyl-CoA via the activated acyl-CoA form, whereas the remaining third was converted into 2-heptanone by β-decarboxylation. The accumulation of this methylketone is in agreement with the suggestion that these flavored compounds are formed not only because they allow a rapid detoxification of fatty acids (15) but also because of an incomplete β-oxidation of medium-chain fatty acids due to the limiting availability of free CoA in the cell (3, 19). After 90 h of growth, the synthesis of pigments began to be slightly higher in M. ruber cultivated in the presence of octanoate, and this enhanced production coincided with a reassimilation of the methylketone (Fig. 3C) and reached a pigment content 30% higher than that obtained with control cultures. This increased pigment production may arise in part from additional acetyl-CoA units provided by the β-oxidation of 2-heptanone. With regard to citrinin production, it can be seen in Fig. 3 that the rate of citrinin production was threefold reduced in the presence of 2 mM octanoate.
We then wondered whether the effects of octanoate on pigment and citrinin production were specific to this fatty acid or could be induced by other fatty acids with shorter and longer carbon chains. The results of these experiments are shown in Table 2. Taking into account that most of the fatty acids started to exert an inhibitory effect on growth at concentrations above 2 mM (data not shown), all experiments were carried out by addition of only 1 mM fatty acid to the culture medium, and measurements were performed at different times during growth. Only maximal levels of both products obtained with each fatty acid are reported in Table 2. Under these conditions, only octanoic acid enhanced the final titer of red pigments twofold, whereas an inhibition of citrinin formation was observed with all fatty acids ranging from 8 to 12 carbon atoms, with dodecanoic acid giving the most potent effect. Fatty acids with longer carbon chains (C14, C16, and C18) were without effect, and hexanoic acid (C6) had only a minor effect on citrinin. It should be stressed that for all conditions tested, the fatty acid added to the medium was entirely consumed at the end of growth. As a proof of this assertion, we found that the biomass yield of M. ruber was 30% increased in the presence of 2 mM oleic acid (data not shown). Very interestingly, M. ruber cultures grown in the presence of shorter-chain fatty acids, including octanoate, decanoate, and dodecanoate were characterized by the production of heptanone, 2-nonanone, and 2-undecanone, as identified by MS-GC, whereas no methylketones were detected from the metabolization of fatty acids with longer carbon chains or of hexanoate. These results indicated that the assimilation of fatty acids does not necessarily follow the same route as was previously suggested (19) but varies depending on the chain length. Our data suggested that fatty acids with carbon chains longer than 14 carbons are likely assimilated by mitochondrial β-oxidation, whereas medium-chain fatty acids are either oxidized by peroxisomal β-oxidation or first converted to the corresponding methylketone 1 carbon atom shorter by a reaction resembling that described for n-alcane degradation (24, 27), after which the methylketones are β-oxidized by peroxisomes. This scenario is in agreement with a previous report showing that medium-chain fatty acids are readily converted into methylketones by Penicillium crustosom to reduce their toxicity (15). Therefore, we suspected that there could be a correlation between the type of methylketones derived from the fatty acids and the potency of their inhibition of citrinin. To investigate this correlation, we repeated the experiments by adding various methylketones directly to the growth medium, at concentrations such that the amounts of carbon (in moles) were identical (Table 3). The results clearly confirmed our expectation that the longer the carbon chain, the stronger the inhibition of citrinin production, with no effect on pigments. More interestingly, the most potent effect on citrinin production was found with 2-tridecanone, while myristic acid (C14) had no effect because it could not be converted into a methylketone by the fungus. This result also suggested a specificity of the β-decarboxylation reaction restricted to medium-chain fatty acids ranging from C8 to C12. This specificity of decarboxylation may be linked to the high toxicity of these medium-chain fatty acids as reported previously (15, 19).
Reduction of citrinin production by medium-chain fatty acids correlated with activation of peroxisomal activities.
At least two possibilities can be raised to account for the effect of fatty acids to reduce the titer of citrinin during submerged fermentation. The first possibility is that the presence of fatty acids specifically inhibits the biosynthesis of citrinin. This hypothesis seems unlikely because the fatty acid was readily assimilated by the mycelium and disappeared after 90 h of growth, while the inhibitory effect on citrinin production was maintained throughout growth. Therefore, one should assume a constant formation of an intermediate derived from fatty acid assimilation to account for this inhibition. Furthermore, because the synthesis of citrinin and the synthesis of pigments proceed from the same polyketide pathway up to the formation of a common tetraketide (14), this putative metabolite should inhibit only an enzyme specifically involved in citrinin synthesis downstream of this junction. A second possibility is that the titer of citrinin could be lowered through an immediate destruction of this molecule (or of an intermediate as it is produced). In favor of this suggestion, it is well known that citrinin, as well as other mycotoxins (ochratoxin), is highly sensitive to hydrogen peroxide (9). In A. parasiticus, the production of aflatoxin (a mycotoxin polyketide) was reported to be inversely correlated with peroxidase activity (8), and peroxisomal proliferation was likely to occur in P. crustosum as this microorganism became peroxidase positive (15) when it was cultivated in the presence of fatty acids with chain lengths ranging from 8 to 12 carbons. Hence, such a detoxification mechanism in M. ruber is quite plausible. We provided three arguments in favor of this hypothesis. Firstly, we confirmed that the incubation of 100 mg of citrinin and pigments · liter−1 in the presence of 0.05% hydrogen peroxide for 30 min at room temperature resulted in complete destruction of the toxin, while the pigments remained intact. Secondly, peroxisomes in yeast and fungi (7, 17, 27, 30) can be stimulated by fatty acids due to their detoxification by peroxisomal β-oxidation (10, 11, 23), and this stimulation is characterized by an increased activity of glyoxysomal and peroxisomal enzymes (31). Here, we found that the activity of isocitrate lyase increased from 5.33 to 30.50 mU/mg of protein in M. ruber cultivated in the presence of 2 mM octanoate, and a similar fivefold increase of this activity was obtained upon incubation with 1 mM 2-heptanone. An additional experimental finding which further argues in favor of a hydrogen peroxide-mediated destruction of citrinin (or an intermediate) was that the addition to M. ruber cultures of 1 mM clofibrate, a well-known stimulator of peroxisome proliferation in animals cells (20), completely prevented the production of citrinin, with no effect on growth or pigment synthesis (data not shown).
In summary, the effect of exogenous fatty acids in M. ruber was not to promote pigment production, since we demonstrated that the formation of these complex molecules required the de novo synthesis of a medium-chain fatty acid by the fatty acid synthase pathway. Contrary to expectation, the major effect of these fatty acids was to strongly reduce citrinin production through their action to stimulate the proliferation of microbodies and thereby the formation of hydrogen peroxide. Hence, the addition of a few milligrams of fatty acids, which have no other effect on growth, to submerged cultures of industrially relevant filamentous fungi could be considered an efficient and cheap technological method to prevent the production of various mycotoxins (aflatoxin, ochratoxin, and patulin).
ACKNOWLEDGMENTS
H. Hajjaj grateful acknowledges the financial support of INRA (Institut National de la Recherche Agronomique, France).
We thank N. D. Lindley for proofreading the manuscript and A. Reynes for the analysis of samples by MS-GC.
REFERENCES
- 1.Armitt S, McCullough W, Roberts C F. Analysis of acetate non-utilising (acu) mutants in Aspergillus nidulans. J Gen Microbiol. 1976;92:263–282. doi: 10.1099/00221287-92-2-263. [DOI] [PubMed] [Google Scholar]
- 2.Bagget J M, Berndt W O. Renal and hepatic glutathione concentrations in rats after treatment with hexachloro-1,3-butadiene and citrinin. Arch Toxicol. 1984;56:46–49. doi: 10.1007/BF00316352. [DOI] [PubMed] [Google Scholar]
- 3.Baltazar M F, Dickinson F M, Ratledge C. Oxidation of medium-chain acyl-CoA esters by extracts of Aspergillus niger: enzymology and characterization of intermediates by HPLC. Microbiology. 1998;145:271–278. doi: 10.1099/13500872-145-1-271. [DOI] [PubMed] [Google Scholar]
- 4.Bilgrami K S, Sinha S P, Jeswal P. Nephrotoxic and hepatoxic effects of citrinin in mice (Mus musculus) Proc Indian Natl Sci Acad B. 1988;54:35–37. [Google Scholar]
- 5.Blanc P J, Loret M O, Santerre A, Goma G. Production of citrinin by various species of Monascus. Biotechnol Lett. 1995;17:291–294. [Google Scholar]
- 6.Blanc P J, Laussac J P, Le Bars J, Le Bars P, Loret M O, Pareilleux A, Prome D, Prome J C, Santerre A L, Goma G. Characterization of monascidin A from Monascus as citrinin. Int J Food Microbiol. 1995;27:201–213. doi: 10.1016/0168-1605(94)00167-5. [DOI] [PubMed] [Google Scholar]
- 7.De Duve C, Baudhuin P. Peroxisomes (microbodies and related particles) Physiol Rev. 1966;46:323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- 8.Doyle M P, Marth E H. Peroxidase activity in mycelia of Aspergillus parasiticus that degrade aflatoxin. Eur J Appl Microbiol. 1979;7:211–217. [Google Scholar]
- 9.Fouler S G, Trivedi A B, Kitabatake N. Detoxification of citrinin and ochratoxin A by hydrogen peroxide. J AOAC Int. 1994;77:631–637. [PubMed] [Google Scholar]
- 10.Fukui S, Tanaka A. Peroxisomes of alkane- and methanol-grown yeasts. J Appl Biochem. 1979;1:171–201. [Google Scholar]
- 11.Greene R V, Gould J M. Fatty acyl-coenzyme A oxidase activity and H2O2 production in Phanerochaete chrysosporium mycelia. Biochem Biophys Res Commun. 1984;118:437–443. doi: 10.1016/0006-291x(84)91322-6. [DOI] [PubMed] [Google Scholar]
- 12.Hadfield J R, Holker J S E, Stanway D N. The biosynthesis of fungal metabolites. Part II. The β-oxo-lactone equivalents in rubropunctatin and monascorubrin. J Chem Soc Sect C. 1967;1967:751–755. [Google Scholar]
- 13.Hajjaj H, Klaebe A, Loret M O, Tzedakis T, Goma G, Blanc P J. Production and identification of N-glucosylrubropunctamine and N-glucosylmonascorubramine from Monascus ruber and the occurrence of electron donor-acceptor complexes in these red pigments. Appl Environ Microbiol. 1997;63:2671–2678. doi: 10.1128/aem.63.7.2671-2678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajjaj H, Klaebe A, Loret M O, Goma G, Blanc P J, François J. Biosynthetic pathway of citrinin in the filamentous fungus Monascus ruber as revealed by 13C nuclear magnetic resonance. Appl Environ Microbiol. 1999;65:311–314. doi: 10.1128/aem.65.1.311-314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatton P V, Kinderlerer J L. Toxicity of medium chain fatty acids to Penicillium crustosom thom and their detoxification to methyl ketones. J Appl Bacteriol. 1991;70:401–407. [Google Scholar]
- 16.Hopwood D A, Sherman D H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- 17.Kunau W H, Kionka C, Ledebur A, Mateblowski M, Moreno de la Garza M, Schultz-Borchard U, Thieringer R, Veenhuis M. Beta-oxidation systems in eukaryotic microorganisms. In: Fahimi H D, Sies H, editors. Peroxisomes in biology and medicine. Berlin, Germany: Springer-Verlag; 1987. pp. 128–140. [Google Scholar]
- 18.Kurono M, Nakanishi K, Shindo K, Tada M. Biosynthesis of monascorubrin and monascoflavin. Chem Pharm Bull. 1963;11:359–362. [Google Scholar]
- 19.Lawrence R C, Hawke J C. The oxidation of fatty acids by mycelium of Penicillium roquefortii. J Gen Microbiol. 1968;51:289–302. doi: 10.1099/00221287-51-2-289. [DOI] [PubMed] [Google Scholar]
- 20.Lazarow P B, de Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci USA. 1976;73:2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin T F, Yakushijin K, Büchi G H, Demain A L. Formation of water-soluble Monascus red pigments by biological and semi-synthetic processes. J Ind Microbiol. 1992;9:173–179. [Google Scholar]
- 22.Pastrana L, Blanc P J, Santerre A L, Loret M O, Goma G. Production of red pigments by Monascus ruber in synthetic media with a strictly controlled nitrogen source. Process Biochem. 1994;30:333–340. [Google Scholar]
- 23.Ratledge C, Evans C T. Lipids and their metabolism. In: Rose A H, Harrison J S, editors. The yeasts. Vol. 3. New York, N.Y: Academic Press; 1989. pp. 367–455. [Google Scholar]
- 24.Rehm H J, Reiff J. Mechanisms and occurrence of microbial oxidation of long chain alkanes. Adv Biochem Eng. 1981;19:175–215. [Google Scholar]
- 25.Robinson J A. Polyketide synthase complexes: their structure and function in antibiotic biosynthesis. Philos Trans Acad Soc Lond. 1991;332:107–114. doi: 10.1098/rstb.1991.0038. [DOI] [PubMed] [Google Scholar]
- 26.Simpson T J. Studies of polyketide chain-assembly processes, mycotoxins and phycotoxins. In: Steyn P S, Vleggaar R, editors. Mycotoxins and phycotoxins. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1986. pp. 85–96. [Google Scholar]
- 27.Tanaka A, Osumi M, Fukui S. Peroxisomes of alkane-grown yeast: fundamental and practical aspects. Ann N Y Acad Sci. 1982;386:183–186. doi: 10.1111/j.1749-6632.1982.tb21416.x. [DOI] [PubMed] [Google Scholar]
- 28.Townsend C A, Christensen S B, Trautwein K. Hexanoate as a strater unit in polyketide biosynthesis. J Am Chem Soc. 1984;106:3868–3869. [Google Scholar]
- 29.Turner W B. In fungal metabolites. London, United Kingdom: Academic Press; 1971. [Google Scholar]
- 30.Valenciano S, Lucas J R D, Pedrogosa A, Monistrol I F, Laborda F. Induction of beta-oxidation enzymes and microbody proliferation in Aspergillus nidulans. Arch Microbiol. 1996;166:336–341. doi: 10.1007/s002030050392. [DOI] [PubMed] [Google Scholar]
- 31.Valenciano S, Lucas J R D, Van der Klei I, Veenhuis M, Laborda F. Characterization of Aspergillus nidulans peroxisomes by immunoelectron microscopy. Arch Microbiol. 1998;170:370–376. doi: 10.1007/s002030050655. [DOI] [PubMed] [Google Scholar]
- 32.Wong H, Koehler P E. Production of red water-soluble Monascus pigments. J Food Sci. 1983;48:1200–1203. [Google Scholar]