Abstract
Conventional in vitro methods for biological evaluation of intra-arterial devices such as stents fail to accurately predict cytotoxicity and remodeling events. An ex vivo flow-tunable vascular bioreactor system (VesselBRx), comprising intra- and extra-luminal monitoring capabilities, addresses these limitations. VesselBRx mimics the in vivo physiological, hyperplastic, and cytocompatibility events of absorbable magnesium (Mg)-based stents in ex vivo stent-treated porcine and human coronary arteries, with in-situ and real-time monitoring of local stent degradation effects. Unlike conventional, static cell culture, the VesselBRx perfusion system eliminates unphysiologically high intracellular Mg2+ concentrations and localized O2 consumption resulting from stent degradation. Whereas static stented arteries exhibited only 20.1% cell viability and upregulated apoptosis, necrosis, metallic ion, and hypoxia-related gene signatures, stented arteries in VesselBRx showed almost identical cell viability to in vivo rabbit models (~94.0%). Hyperplastic intimal remodeling developed in unstented arteries subjected to low shear stress, but was inhibited by Mg-based stents in VesselBRx, similarly to in vivo. VesselBRx represents a critical advance from the current static culture standard of testing absorbable vascular implants.
Keywords: Vessel bioreactor, Magnesium stent, Local microenvironment, Hyperplasia, Cell apoptosis
1. Introduction
Stent therapy opens up blocked or narrowed vessels for a large variety of vascular diseases, most notably atherosclerosis and stenosis [1–3]. With the development of novel drug-eluting stents and absorbable metallic and polymer stents, conventional in vitro methods (i.e. ISO 10993 and ISO/TS 17137) fail to predict biological outcomes in animal studies and in clinical trials [4,5]. For more than two decades, static cell culture conditions have proven unsuitable for the measurement of release kinetics of drugs or bioactive degradation products, mass transfer, flow considerations, and diseased signaling cues that occur in vivo [6,7]. On the other hand, in vivo studies are importantly limited by the inability to sample intra-luminally and extra-luminally for concentrations of drugs or bioactive degradation products. Therefore, existing in vitro models are too simplistic and produce erroneous data that do not reflect in vivo experience, while in vivo methods are severely hampered by the inability to closely sample and monitor treated arteries over time. These limitations make the testing and optimization of novel drug-device combinations and novel absorbable stents extremely challenging, requiring many iterations in vivo and substantial trial and error before determining suitable absorbable stent materials, drug loading, drug dosage, and release kinetics.
Another critical issue is that conventional material-cell culture in vitro methods [8] cannot recapitulate the complexity of intimal hyperplasia for stent studies, and in vivo hyperplasia models require complicated treatments (e.g. balloon injury [9], high-fat/cholesterol diet [10], and Apolipoprotein E deficient and LDL-receptor knockout [11]) with long wait times, high expense, and potential ethical considerations [12, 13].
Creating an advanced extracorporeal model to mimic a diseased vessel is essential to study vascular stents and other vascular devices. In particular, native vascular structure as well as biological, biochemical, and biophysical microenvironment should be recapitulated to the degree feasible. Native vessels cultured ex vivo provide a three-dimensional (3-D) vascular structure [14], thereby allowing for the study of cellular and molecular responses to implanted stents [15,16]. Bioreactors are a promising way to culture live vessels ex vivo [17,18].
It is known that low shear stresses (<4 dyn/cm2) are prevalent at atherosclerosis-prone sites, and can stimulate an atherogenic phenotype [19]. Previous investigators have established early stage atherosclerotic culture models by varying low flow conditions on a cell culture plate [19] or a microfluidic cell chip [20]. Our bioreactor system takes advantage of this concept, using controlled flow-induced shear stress within arteries, to mimic cardiovascular stent therapy using pathologic (hyperplastic) and physiologic (healthy) vessels.
Absorbable magnesium(Mg)-based stents are an innovative vascular therapeutic solution in that they degrade over time with mild degradation products [21], thereby providing temporary mechanical support until healing is complete [22]. Clinical and preclinical trials have shown that Mg-based stents are beneficial for improving endothelialization and arterial recoil [23–25], while reducing neoatherosclerosis [26]. A critical issue in advancing the field of Mg-based implants is the inevitable cell death that occurs on the bare Mg-based surface in conventional, static cultures. No available experimental cell culture or vascular model can provide direct, quantitative data regarding the effect of Mg-stent degradation products on arterial cells [4], and currently existing experimental models provide data that directly contradicts results from animal studies and clinical trials [27]. Currently available in vitro cytotoxicity tests, such as those contained in the ISO 10993 series of standards, were designed without considering clearance of degradable ions from implanted bio-metals [28,29]. (Until the advent of the new standard ISO/TS 17137:2019 [30] for cardiovascular absorbable metallic implants, there was no known correlation between in vitro tests and in vivo results.)
To address this issue, we designed an ex vivo flow-tunable vascular bioreactor system (VesselBRx) with the normal and hyperplastic porcine coronary arteries, as well as atherosclerotic human coronary arteries for evaluation of the impacts of Mg-based stents and to measure local concentrations of degradation products. The VesselBRx is fitted with in-situ and real-time monitoring and provide direct, intra-luminal and extra-luminal measures of changes in metal ion concentrations and pH. Furthermore, analysis of treated arteries included gene expression-level analysis of the impacts of local degradation products, including Mg ion, change in pH and depletion of dissolved oxygen, on cellular phenotype and survival. Unlike statically cultured, stented arteries, the VesselBRx can support cells/arteries with a buffered and efficient mass transfer system, to avoid the local accumulation of Mg2+, and also combat tissue hypoxia and oxygen depletion by cell metabolism and by degrading Mg stent components. A correlation is shown between cellular responses to Mg-based stent degradation in ex vivo bioreactor culture and in vivo rabbit models, with very similar cell apoptotic rates within the vessel wall. Moreover, this bioreactor shows that Mg stents not only have favorable cytocompatibility, but also inhibit hyperplasia similarly ex vivo and in vivo.
2. Materials and methods
2.1. Harvest of porcine coronary arteries
Nine porcine hearts were harvested from 6-month-old Yucatan pigs weighing 50–60 kg. Pigs were anesthetized and heparinized (200 units/kg) before heart harvest. All procedures were performed according to a Yale University IACUC approved protocol. Porcine left and right coronary arteries were freshly and sterilely isolated in a biosafety cabinet. To protect endothelial cells (ECs), the arteries were kept in the VascuLife® VEGF-Mv medium containing vascular endothelial growth factor (Lifeline® Cell Technology) before bioreactor setup. The arteries with inner diameters of approximately 2.5 mm were selected and cut to a length of 4 cm. The fresh control native arteries were excised from the animals and then fixed immediately.
2.2. Bioreactor culture conditions
Both ends of the artery were sutured onto glass arms of the bioreactor, then the arms with the arteries were mounted on an autoclaved bioreactor chamber with a circulating system. The bioreactor system was filled with endothelial cell-smooth muscle cell (EC-SMC) medium consisting of Vasculife and Dulbecco’s Modified Eagle Medium (Gibco) (1:1) supplemented with 2.5% Fetal Bovine Serum (HyClone, GE Healthcare Life Sciences), 1% Gibco Antibiotic-Antimycotic (Life Technologies), and dextran (30 g/L, Sigma). This pseudo-physiological medium had a viscosity similar to that of whole blood. The original pH value of growth medium is 7.8±0.1.
To collect local medium in the vessel for in-situ and real-time monitoring of local pH and ion concentrations, a 22 g × 15 cm-long needle (Cadence Science) with an injection site was inserted through the chamber cap and into the vascular lumen. Three air filters (PURADISC 25 AS Disposable Filter Device, GE Healthcare Life Sciences) were connected to the tubes of the chamber cup. The bioreactor system was moved from the biosafety cabinet to the incubator (37 °C, 5% CO2). Sixty percent of the medium was changed every other day. The entire culture time was 7 days.
2.3. Bioreactor flow conditions and control
The bioreactor system consists of a blood vessel chamber, two glass arms, a variable-flow pump (EASY-LOAD II, MasterFlex), circulation tubing, a pressure transducer, pressure monitoring machine (ADInstruments), an injection site for medium exchange and local medium extraction, an inserted needle for local medium collection, and a 5% CO2 balanced air cell culture incubator (Fig. 1). Gas permeable platinum-cured silicone tubing was used for most of circulating loop. The circulated flow comes from the chamber to a 16# size tubing 35 cm in length, then through a 40 cm 16# size tubing (PharMed BPT, a wearproof material that is beneficial for rolling compaction on the pump head but is less gas permeable). The tube is then connected to a three-way connector inserted as a site for medium exchange, which is then attached to an 80 cm-length 14# size tubing for controlling the average cyclic pressure. A pressure transducer is connected next to the inflow arm, and the vessel is sutured on the two arms. The outflow of the vessel is connected to a 20 cm-length 14# size tubing for controlling flow resistance to ensure no negative pressure in the vessel. Culture medium is pumped through the vessel and feeds back into the vessel chamber macroenvironment (Fig. 1e arrows direction), meaning that the vessel exterior and lumen are cultured in the same circulating medium.
Fig. 1.
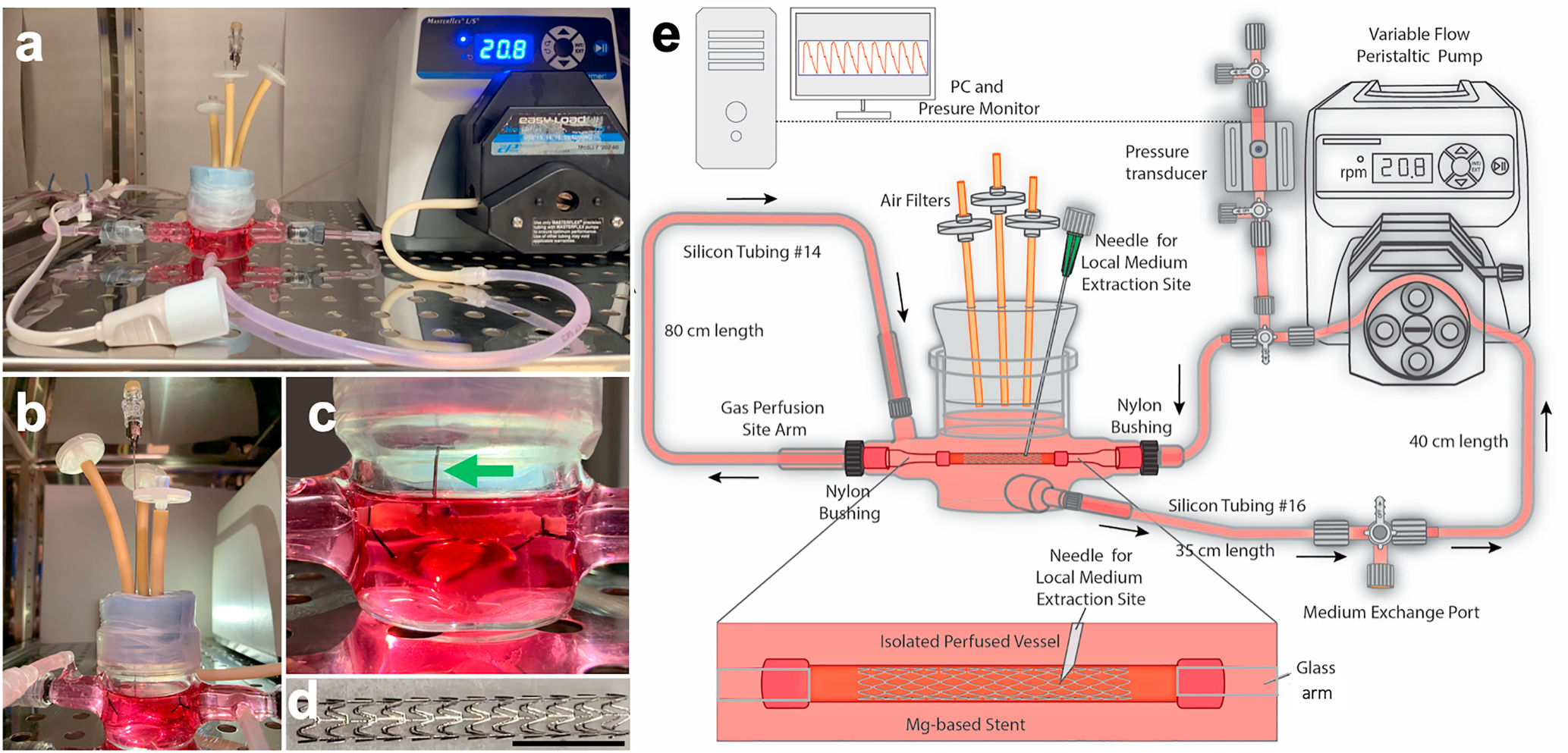
Dynamic flow stented (and non-stented) vessel culture bioreactor setup. a) Photo of entire dynamic bioreactor setup with bioreactor chamber, stented porcine coronary artery, variable-flow pump, circulation and perfusion tubing, bioreactor tubing, air filters, pressure transducer, local medium extraction site, and bulk medium exchange port. b) Close-up of bioreactor chamber, air filters, and local medium extraction site. c) Close-up of the stented artery and local injection needle (green arrow) inserted directly into the lumen of the vessel adjacent to the stent struts. d) Close-up of the MgZnMn stent, 1.5 cm in length, 175 μm in wall thickness, and 2.75 mm in expanded diameter. Scale bar = 1.0 cm. e) Schematic of all the components of the bioreactor setup.
For static culture, the vessel is contained within the same bioreactor, but is sutured onto a long needle rather than the two arms in order to make the vascular lumen stay fully in contact with the medium (Supplementary Fig. S1). The needle can also directly collect the local medium in the vessel. A stir bar with the slowest rotation provided some mixing and hence gas exchange in the medium.
Static (0 ml/min), low-flow (7.5 ml/min) and high-flow (18.3 ml/min) conditions were set up to create vascular wall shear stresses of 0, 2.8, and 6.8 dyn/cm2, labeled as Flow−, Flow+, and Flow++ respectively (Figs. 2–4). The calculations were based on Doriot’s equation [31] as given below.
| (1) |
where τ is shear stress, η is liquid viscosity (0.043 dyn s/cm2), Q (ml/min) is the flux of the solution, and D (2.75 mm) is the diameter of the vascular lumen. A pressure transducer (TruWave 3 cc/12in Pressure Monitoring Set, Edwards Lifesciences) was connected to the inflow glass arm to monitor real-time pressure of the vessel. The initial pressures of the low flow condition ranged from a minimum of 0 mmHg to a maximum of 25 mmHg, while the high flow condition had a range of 5–40 mmHg (Supplementary, Fig. S2). An average of 4.3% radial distention (strain) was applied to the vessels. Pulsatile radial stresses were applied to the vessels at ~35.0 beats per minute (BPM) and 4.3% radial distention (strain) under the low flow condition, and at ~83.5 BPM and 5.8% radial distention under the high flow condition. The number of biological replicates for the static, low-flow and high-flow bioreactor conditions was no less than five.
Fig. 2.
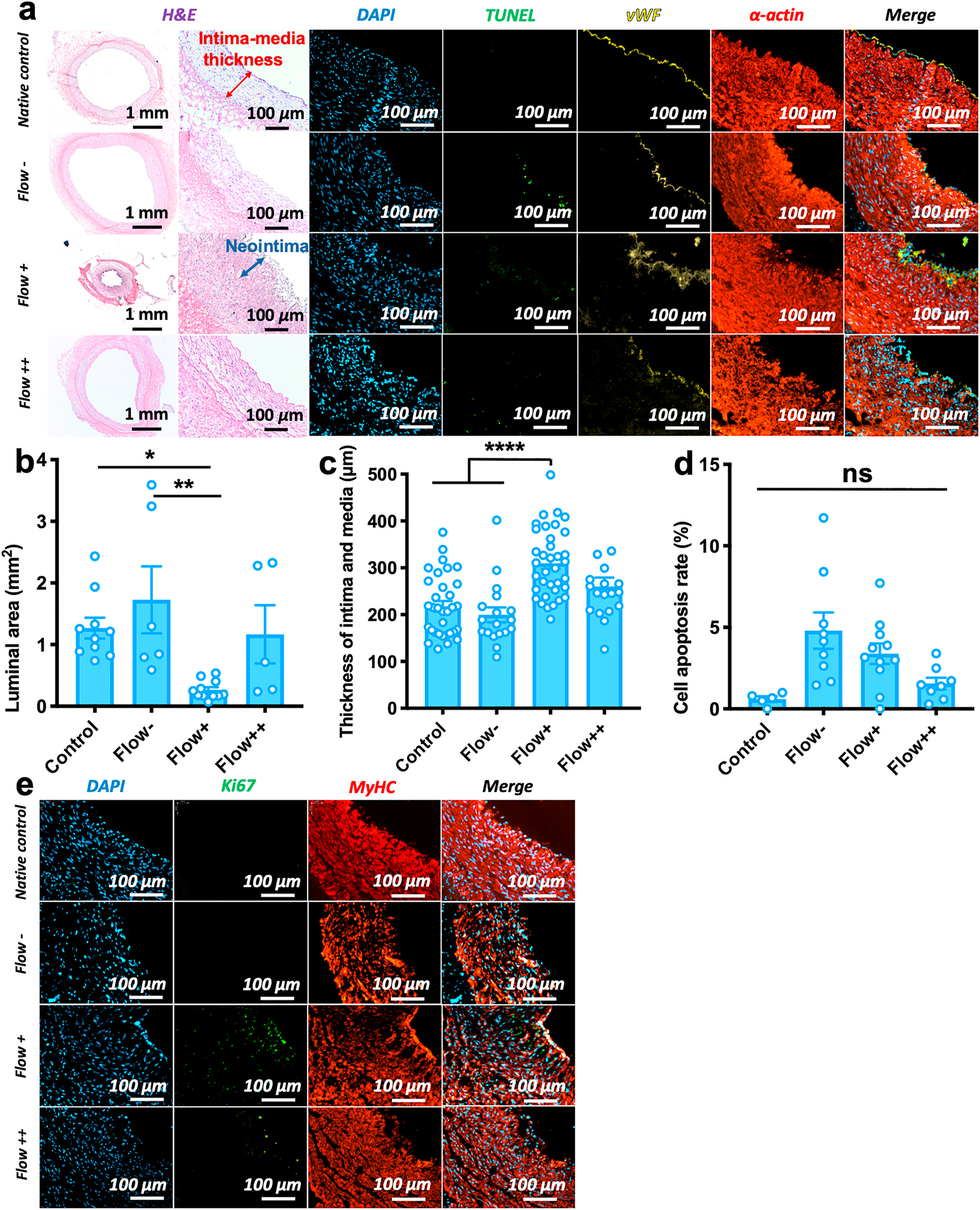
An ex vivo hyperplastic and physiologic vessel model. H&E and immunofluorescent stains for non-stented vessels in native control, static (Flow−, 0 dyn/cm2), low-flow (Flow+, 2.8 dyn/cm2), and high-flow (Flow++, 6.8 dyn/cm2) conditions. a) H&E and immunofluorescent stains (DAPI, TUNEL, vWF, smooth muscle α-actin) for non-stented vessels after 7 days of culture. Blue arrow shows the neointima in the low-flow artery. b) Luminal area of vessels in different culture conditions, indicating a dramatic loss of lumen area under the low-flow condition: Native control vs. Flow+ *p = 0.030; Flow-vs. Flow+ **p = 0.003; “ns” non-significant. Each data point represents an individual vessel/test. c) Thickness of intimal and medial layers in different culture conditions, showing a significantly thick intima and media layers formed under the low flow condition: Native control vs. Flow+, ****p < 0.0001; Flow-vs. Flow+, ****p < 0.0001. The thicknesses of intimal and medial layers are measured from H&E images as shown in the red arrow in panel (a). Three data point values are measured from each individual vessel/test. d) Low cell apoptosis rates according to the ratio of TUNEL and DAPI staining were non-significant in different culture conditions, p > 0.999. Each data point represents an individual vessel/test. For panels (b, c, d): data are mean ± S.E.M, statistically analyzed by one-way ANOVA, Tukey’s multiple comparisons test. e) Immunofluorescent stains (DAPI, Ki67, MyHC) for non-stented vessels after 7 days of culture, Ki67-positive and MyHC-negative stains show SMC proliferating under the low flow condition, while SMCs kept a contractile phenotype under the high flow condition.
Fig. 4.
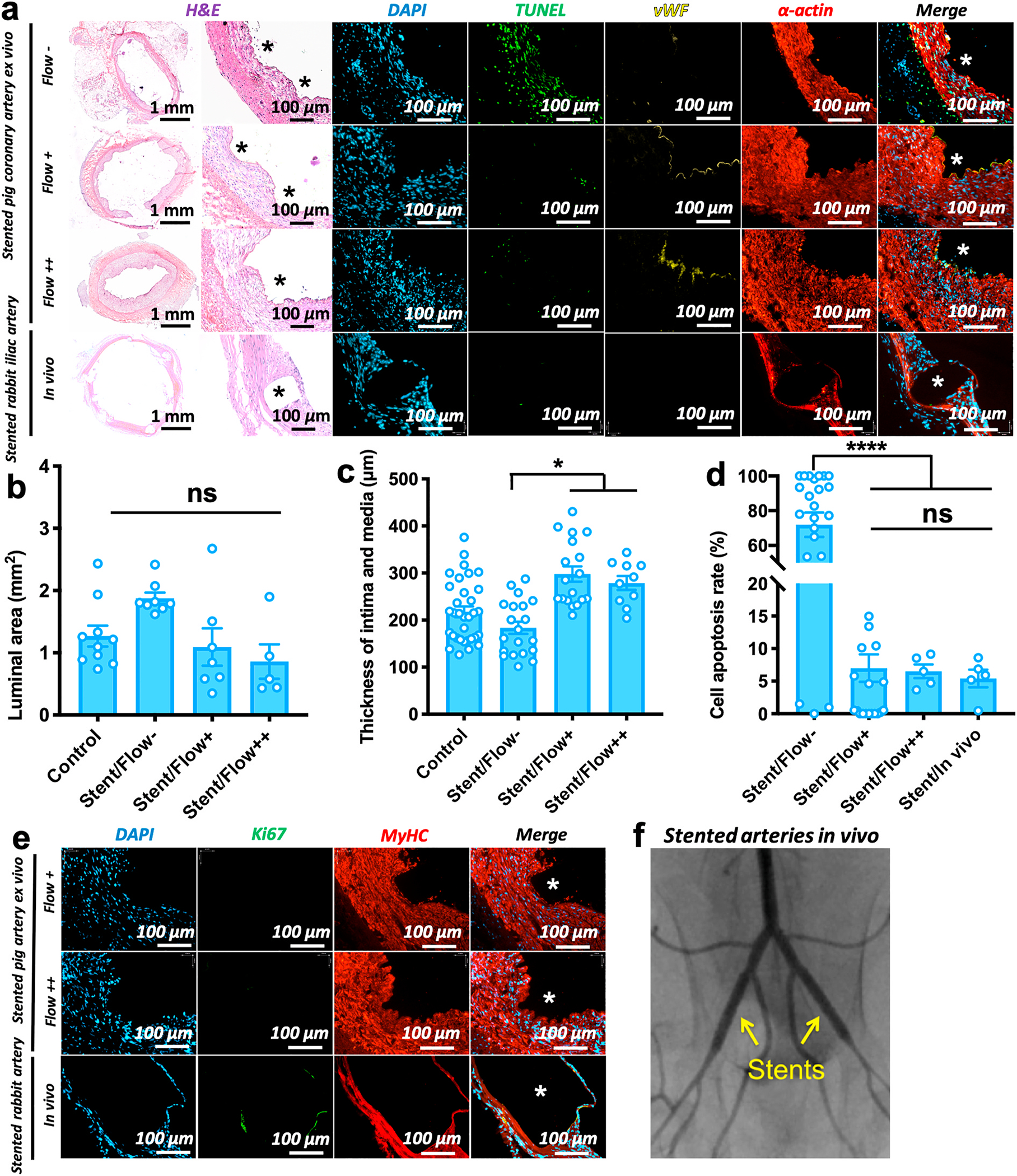
Absorbable Mg-based stent therapy in an ex vivo vessel bioreactor and an in vivo model. a) H&E and immunofluorescent stains (DAPI, TUNEL, vWF, α-actin) for MgZnMn stented vessels after 7 days of culture in the static (Flow−, 0 dyn/cm2), low flow (Flow+, 2.8 dyn/cm2), high flow (Flow++, 6.8 dyn/cm2) bioreactor conditions and in vivo implantation. White stars on the immunofluorescent images and black stars on H&E images show the original positions of stent struts (Supplementary, Fig. S5b). b) Luminal area of vessels in different culture conditions. Each data point represents an individual vessel/test. Control represents fresh native artery. c) Intima-media thickness in different culture conditions, showing significantly thin intima and media of the static stented vessels: Stent+/Flow-vs. Stent+/Flow+ *p = 0.036; Stent+/Flow-vs. Stent+/Flow++ *p = 0.020. Three data point values are measured from each individual vessel/test. d) Cell apoptosis rates according to the ratio of TUNEL and DAPI staining in different culture conditions, indicating a dramatically high cell apoptosis rate (~72%) in static stented vessels but good cell viability of the stented vessels in ex vivo flow and in vivo conditions: Stent+/Flow-vs. other condition, ****p < 0.0001. Each data point represents an individual vessel/test. Panela (b, c, d): mean ± S.E.M, statistically analyzed by one-way ANOVA, Tukey’s multiple comparisons test. e) Immunofluorescent stains (DAPI, Ki67, MyHC) for the stented vessels after 7 days of culture in ex vivo flow and in vivo conditions. All the SMCs are contractile (MyHC-positive stain) at each condition, and a few proliferating cells (Ki67-positive stain) only in vivo. f) Angiogram imaging confirming blood flow in the stented iliac arteries of rabbit at 7 days.
Unlike in vivo arteries, vessels cultured in bioreactors are isolated, without the constraints of surrounding tissue. Given the physiological EC and SMC phenotypes observed at 6.8 dyn/cm2 and the concern for vessel dilation and the associated reduction in cell viability at higher pressures and shear stresses [7,32], we do not recommend pursuing much higher shear stresses (eg. >15 dyn/cm2) [18,19].
2.4. Stented vessel culture
Mg-based stents were made with a MgZnMn alloy (1 wt% Zn, 0.2 wt % Mn) and had a length of 1.5 cm and wall thickness of 175 μm. The stents were provided by Guangzhou Nanchuang Everest Medical Technology Co., Ltd. (China). Stents were crimped onto 2.75 mm-diameter balloons, then sterilized with ethylene oxide. The stents were deployed into freshly isolated porcine coronary arteries using balloons with 8 atm inflation pressure, maintained for 60 s. The stented vessels were then mounted in perfusion bioreactors for 7 days as described above (Fig. 1a–d). The same flow conditions were applied to the stented vessels as the non-stented vessels (N ≥ 6 for each group). To monitor pH and Mg ion release into the medium of the bioreactor as a whole, as well as the medium immediately surrounding the stent, the bioreactor medium was collected both from both the entire system, and through a long needle inserted into the vascular lumen next to the stent struts (Fig. 1c).
2.5. Procurement of human coronary arteries
Atherosclerotic human coronary arteries were obtained from three heart transplant recipients having coronary heart disease. The excised (discarded) heart specimens from the organ transplant recipients were processed by the investigators within the operating room, to ensure precise anatomical selection and orientation of the coronary arteries from the discarded hearts. Research protocols were approved by the Institutional Review Boards of Yale University and New England Organ Bank. The arteries with inner diameters of approximately 2.5 mm were selected and cut to a length of 4 cm. As mentioned above, the atherosclerotic human coronary arteries with MgZnMn stents were cultured in the bioreactor under the static and flow (7.5 ml/min) conditions for 7 days (N = 3 for each group).
2.6. In vivo stent implantation
Six MgZnMn stents were implanted into the iliac arteries of three New Zealand White Rabbits (weight 3–3.5 kg, male, N = 3). The inner diameters of the iliac arteries were approximately 2.5 mm. The anesthetic, surgical, and post-operative care protocols were taken from National Institutes of Health “Guide and Care and Use of Laboratory Animals” and approved by the Animal Care and Use Committee of Southwest Jiaotong University. All procedures and handling were performed in a way to minimize discomfort to the animals. The region overlying the leg was prepared in sterile fashion. The left and right femoral arteries were surgically exposed. To counteract vasospasm, lidocaine (100 mg/mL) was applied topically onto the arteries. Heparin (100 units/kg) was administered systemically via the auricular vein. A 22 g syringe needle punched the left femoral artery, and a guide wire (0.014 inch) was inserted through this hole from the left femoral artery to the abdominal artery. Over the guide wire, a MgZnMn stent crimped onto a balloon catheter (diameter in 2.75 mm) and was advanced to the common iliac artery. A sterile polypropylene cling film was wrapped around the tip of the stented balloon catheter to overcome the friction between the stent and the vessel wall. The stent was deployed into the vessel while the polypropylene film was removed. The balloon was inflated for 60 s at 8 atm, with the diameter of the expanded balloon and stent approximately 20%–30% greater than the baseline arterial diameter. After stent deployment, the balloon catheter was removed. The femoral artery was sutured with an 8–0 monofilament suture. The incision was closed with a 2–0 monofilament suture at the end of the procedure. After surgery, rabbits were injected with penicillin (3 × 105U/kg/day) and orally medicated with warfarin (0.2 mg/kg/day) once a day for three consecutive days. Animals were euthanized at 7 days after stent implantation. The stented vessels were harvested for further analysis.
2.7. Histology and immunofluorescence
After 7-days of culture in the bioreactor or in vivo, segments of vessels were embedded into Tissue-Tek Optimal Cutting Temperature Compound (SAKURA), then cut into 5-μm slices in a cryostat (Avantik QS12). Unlike staining formalin-fixed, paraffin-embedded sections, cryotomy can minimize further Mg stent degradation during the staining process. The slices were stained with Hematoxylin-Eosin (H&E; Hematoxylin QS, VECTOR Laboratories; Eosin Y, Fischer Chemical). The lumen area of the vessels and the thickness of the intimal and medium layers were measured from H&E images taken with an Axioskop 2 plus light microscope (ZEISS) with AxioVision Rel. 4.6 software (Zeiss). All the vessel cross-sections were also analyzed by co-staining for terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL, In Situ Cell Death Detection Kit, POD, Roche) for cell apoptosis, von Willebrand factor (vWF; Dako, Cat. No. A0082, dilution 1:400) and CD31 (R&D System, Cat. No. YZU0119022, dilution 1:40) for endothelium, alpha smooth muscle actin (α-SMA, Dako, Cat. No. M0851, dilution 1:1000) for smooth muscle cells (SMCs) and 4′,6-diamidino-2-phenylindole (DAPI, Thermo Fisher Cat. No. 62247, dilution 1:000) for nuclei. The cell apoptosis rate was calculated as the percentage of TUNEL positive nuclei compared to DAPI stained nuclei using Image J 1.50i software (NIH). All the vessel cross-sections were also co-stained by Myosin Heavy Chain (MyHC, Sigma, Cat. No. M7786, dilution 1:200) for contractile SMCs and Ki67 (Abcam, Cat. No. ab15580, dilution 1:150) for proliferating cells. All the vessel cross-sections were stained with Hypoxia-inducible factor 1-alpha (HIF1-α, Santa Cruz, Cat. No. sc-13515, dilution 1:200) and DAPI, with a hypoxia chamber 12 h-culture vessel as a positive control and a native vessel as a negative control. The stented vessel cross-sections from rabbits were co-stained with CD45 (Novus Biologicals, Cat. No. NB100–77417SS, dilution 1:300) for leucocyte, α-SMA and CD31, and separately stained with CD68 (LifeSpan BioSciences, Cat. No. LS-C187543, dilution 1:100) for macrophage. All immunofluorescent images were taken with an Axiovert 200 M immunofluorescent microscope (ZEISS) with an HBO 100 microscope illuminator (ZEISS) and Hamamatsu Photonics Camera.
2.8. Solid degradation products analysis
The samples for visualization of Mg-based stent degradation products were prepared through polishing after mounting with epoxy resin (Epokwick® Epoxy resin, Buehler, USA). The samples were treated by sputter-coating, and the Mg-based stent degradation product layer and residual Mg strut material were observed by scanning electron microscopy (SEM, model S-4700, Hitachi) with backscattered electrons. The chemical compositions of the degradation products were determined using SEM combined with electron dispersive x-ray spectroscopy.
2.9. In-situ and real-time monitoring of degradation factors (Mg ion, pH, and dissolved O2)
To monitor medium pH and Mg ion concentration change during stent degradation, 200 μL from the bulk or microenvironmental medium near the stent was collected daily. pH values were measured using the Ross micro combination pH electrode (Thermo Fisher), and Mg ion concentrations were measured using the ELAN DRC-e inductively coupled plasma mass spectrometer (ICP-MS, PerkinElmer).
For intravascular Mg ion concentration measurement, the residual stents were removed from the tissues after 7-day culture in the static and flow conditions. To isolate cells, the tissues (~10 mg) were incubated with 3 mg/ml type II collagenase (Sigma-Aldrich) in 2 ml of DMEM with a 5/1 (w/v) ratio of tissue (mg) to enzyme solution (mL). After incubation at 37 °C for 30 min, the same volume of 1 mg/mL elastase (Worthington) solution was added to the solution containing the tissue and collagenase. The tissues were incubated for 2 h until complete digestion. After digestion, samples were centrifuged at 1200 RPM for 5 min, and cells were lysed using deionized water. (Cell density and cell volume was counted using a 10 μL volume immediately before cell lysis.) Lysed cell solutions were diluted 100 times with 1% HNO3 solution for Mg ion concentration (CICP) measurements with ICP-MS. The intracellular Mg ion concentrations were calculated by the equation CICP × 100/(cell density × single cell volume).
To measure dissolved O2, 30 mL of media was extracted at designated time points from the bioreactor. A Vernier Optical Dissolved Oxygen probe with a LabQuest 2.0 was then placed in the medium and allowed to equilibrate for 20 min.
2.10. RNA sequencing
All samples (Native control, Flow+, Flow++, Stent/Flow−, and Stent/Flow++) were acquired from the same culture conditions as mentioned above (N = 2 or 3). The one exception was the static stented conditions, which were cultured for only 3 days instead for the full 7 days with too high apoptotic rate and extremely low RNA quality. RNA extraction for sequencing was done using the RNeasy Mini kit with on-column DNase treatment (Qiagen). A one-cm length of cultured vessels was freeze dried in liquid nitrogen and homogenized. RNA quality (260/280 and 260/230 ratio) and concentration were then checked using NanoDrop (Thermo Fischer).
RNA sequencing libraries were prepared by the Yale Center for Genomics Analysis using the Pair End Sequencing Prep Kit, and sequenced using NovaSeq HiSeq (Ilumina) with sequencing read length of 100 base pairs (bp). Sequenced FASTQ samples were evaluated for overrepresented sequences using FASTQC and trimmed of poly(A) sequences using Trimmomatic v 0.36 (no overrepresented adapter sequences were found in any sample). Paired sequences, all with length longer than 30 bp, were then aligned with Rsubread (Bioconductor) with the default parameters. The pig transcriptome was aligned to the data using Ensembl Sscrofa11.1 (release 99).
For RNA sequencing analysis and heatmap clustering, features were counted using RSubread feature counts and genes were filtered to remove those with no counts across all conditions. A variance stabilizing transform was then performed on the dataset with the count matrix using the “vst” function to remove the dependence of data variance on the mean across the entire experiment [33]. DeSeq2 (Bioconductor) was then used to determine differentially expressed genes between the two different sample comparisons using the “results” function.
Fragments per Kilobase Million (FPKM) expression was extracted from the count matrices using the “assay” function and averaged across biological replicates for each culture condition. Clustered heatmaps were generated with normalized values across each gene, where the mean expression across a gene was subtracted from the expression level for each condition. For the supplemental heatmaps that combine 3 pairwise comparisons, DeSeq2 was used to conduct 3 comparisons separately then all significantly expressed genes (adjusted p < 0.05, log2 fold change >2 for native and engineered comparisons or log2 fold change >1 for between engineered comparisons) were clustered into a single heatmap in the same way as above, accounting for duplicates across comparisons. Ensembl Gene IDs were converted to gene symbols using biomaRt (Bioconductor).
RNA sequencing gene expression levels (log2 fold change) with an adjusted p-value < 0.05 between two groups were considered as differentially expressed and were investigated by Ingenuity pathway analysis (IPA) system (Qiagen). IPA was performed to identify cellular functions and canonical pathways that are most significantly different, using activation z-scores.
2.11. Statistical analysis
All quantitative results were obtained from at least three samples. The statistical significance of differences between groups was determined using one-way ANOVA, Tukey’s multiple comparisons test. Significance was established by a value of p < 0.05. Data are expressed as mean and standard error of mean (SEM). All the statistical analysis was conducted with Prism 8.3.1 software (GraphPad Software, Inc.).
3. Results
3.1. Ex vivo vessel bioreactor to build a hyperplastic diseased and physiologic vessel model
3.1.1. Ex vivo vessel bioreactor system
The bioreactor system consists of a blood vessel chamber, a variable-flow pump, circulation tubing, a pressure transducer, injection sites for medium exchange and local medium extraction, an inserted needle for local medium collection, and an incubator (Fig. 1). Culture medium was pumped through the vessel and feeds back into the vessel chamber macroenvironment, meaning the vessel exterior and lumen were cultured in the same circulating medium (Arrows in Fig. 1e shows flow direction.). Porcine coronary arteries were selected because of their similarity in terms of extracellular matrix architecture and mural cell alignment to human coronary arteries [34]. Endothelial cell-smooth muscle cell (EC-SMC) medium was rendered pseudo-physiological, by adding dextran which resulted in a viscosity similar to that of whole blood.
The wall shear stress of in vivo human coronary arteries was reported to be an average of 6.8 dyn/cm2 [31], while low shear stress (<4 dyn/cm2) is correlated with atherosclerosis-prone sites [19]. As such, static, low-flow and high-flow conditions were set up to create vascular wall shear stresses of 0, 2.8, and 6.8 dyn/cm2. These were labeled as Flow−, Flow+, and Flow++, respectively. Pulsatile radial stress was applied to the vessels to produce approximately 5% radial distention (strain) with each pulse, which roughly mimics the native distension of coronary arteries during the cardiac cycle [32]. Assembly of this bioreactor is facile, requiring only 15 min, which increases vessel viability and reduces contamination risk, unlike commercial vascular culture chambers [7] having complicated components and systems.
3.1.2. Impact of bioreactor-modulated flow on neointima formation in porcine arteries
High- and low-flow conditions in the ex vivo vascular bioreactor were designed to mimic physiological and hyperplastic vessel environments. Hematoxylin and eosin (H&E) stains revealed that the low-flow (Flow+) conditions resulted in formation of intimal hyperplasia in the porcine coronary arteries after 7-day culture (Fig. 2a). An 80% decrease in lumen area of cultured arteries (area = 0.26 ± 0.30 mm2) was observed under low-flow conditions, as compared to an area of 1.27 ± 0.36 mm2 in fresh controls (N = 10 for controls and Flow+, *p = 0.030, Fig. 2b). In contrast, in the high-flow (Flow++) conditions, luminal areas were similar to controls, at 1.17 ± 0.47 mm2 (N = 5 for Flow++, p > 0.99). Intima-media thickness of arteries in the low-flow conditions increased to 309.6 ± 12.0 μm, as compared to the native controls at 217.8 ± 11.4 μm (N = 10 for controls and Flow+, ****p < 0.0001, Fig. 2c). However, wall thickness in the high-flow and static conditions remained similar to that of the controls at 260.0 ± 19.1 μm (N = 5 for Flow++, p = 0.464) and 199.5 ± 15.9 μm (N = 6 for Flow−, p = 0.980), respectively. The thickening of the low-flow vessels corresponded to neointima formation with a thickness of 29.0 ± 15.3 μm, while no neointima formation was observed in the arteries subjected to high-flow and static conditions (Fig. 2a). Variations in pulsatile flow pressure of the arteries in bioreactors also showed that mean pressure increased under low-flow conditions over time (Supplementary, Fig. S2a), and there is an observed 2.5-fold increase in Δ P (Pmax-Pmin) from 0 to 7 days, accompanied by an 80% loss of lumen area (Fig. 2a). Conversely, mean pressure remained at stable values over time under high-flow conditions (Supplementary, Fig. S2b), and the pressure overall under high-flow conditions was higher than that under low-flow conditions due to its higher flow rate.
The arteries were stained with Ki-67 for proliferating nuclei. Ki67-positive cells were located in the neointima in low-flow (Flow+) conditions (Fig. 2e). These proliferating cells were comprised of ECs and SMCs, as indicated by co-staining with von Willebrand Factor (vWF, for ECs) and myosin heavy chain (MyHC, for SMCs) (Fig. 2a). In the Flow + conditions, ECs no longer formed a monolayer and instead extended into the lumen as multi-layered structures (Fig. 2a). There was also a decrease in the number of MyHC-positive SMCs near the lumen, perhaps indicating that local SMCs are proliferating and losing their contractile markers (Fig. 2e). These observations support the concept that a low-flow condition can provide a model for the development of neointimal hyperplasia. Moreover, VesselBRx cultured arteries can retain good cell viability and neointimal hyperplasia under the low flow culture for an even longer culture time at 14 days (Supplementary, Fig. S3).
Under high-flow conditions (Flow++), there were only a few proliferating ECs visible on the lumen of the arteries. ECs maintained a physiologic monolayer, and almost all SMCs retained a contractile phenotype, as shown by MyHC-positive and Ki67-negative stains in the arterial wall after 7-day culture (Fig. 2e). All culture conditions demonstrated minimal terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) staining, implying a low level of programmed cell death in the cultured arteries (Fig. 2d). Overall, the vessel structure of the high-flow condition was comparable to the native control, while the low-flow vessel structure and intimal hyperplasia formation were hallmarks of a diseased hyperplastic vessel model.
3.1.3. Impact of bioreactor-modulated flow on gene expression profiles of porcine arteries
Porcine arteries cultured for 7 days in the flow bioreactor and native controls were analyzed by bulk RNA sequencing (N = 2 each for Flow+, Flow++, and fresh native arteries). 440 genes (2.25% of the total number of 19,567 genes) were significantly different between fresh native arteries and cultured arteries (including low and high flow conditions), adjusted p < 0.05 (Supplementary, Fig. S4), demonstrating that the engineered system maintains the primary phenotypes of native arteries in vivo. However, Ingenuity Pathway Analysis (IPA) of these genes revealed critical differences between vessels cultured under low versus high flow conditions. Compared to gene expression in native arteries, the proliferation and cell invasion pathways for arteries cultured under low-flow (Flow+) conditions had activation z-scores > 2 (Fig. 3b and c). Atherosclerosis signaling pathways were also highly expressed in the low-flow cultured vessels, with a p-value of 2.22E-06 vs. native arteries. Conversely, these pathways were not activated under high-flow (Flow++) conditions. To further assess the differences between culture conditions, we identified genes differentially expressed (adjusted p < 0.05) between the low (Flow+) and high (Flow++) flow conditions and clustered them into a heatmap in Fig. 3a. Of note, genes downregulated in low-flow conditions included DUSP1 (a negative regulator of cell proliferation) [35] and other genes related to cell proliferation pathways (AVPR1A, CCN1, IL6) and RGS2 (a regulator of vascular smooth muscle relaxation) [36] (Fig. 3c green boxes). Thus, observations from histology and immunostaining – a loss of lumen area (Fig. 2b), increase in the intima-media thickness (Fig. 2c), neointima formation (Fig. 2a), and proliferation of SMCs (Fig. 2e) in low flow, compared to a more native-like vascular morphology and cellular phenotype in high flow – were consistent with the results of the bulk RNA-sequencing with pathway analysis (Fig. 3). These two vessel culture models – one (Flow++) more physiologic, and the other (Flow+) more hyperplastic - may provide variable and controllable platforms for further study of absorbable Mg-based stent therapy.
Fig. 3.
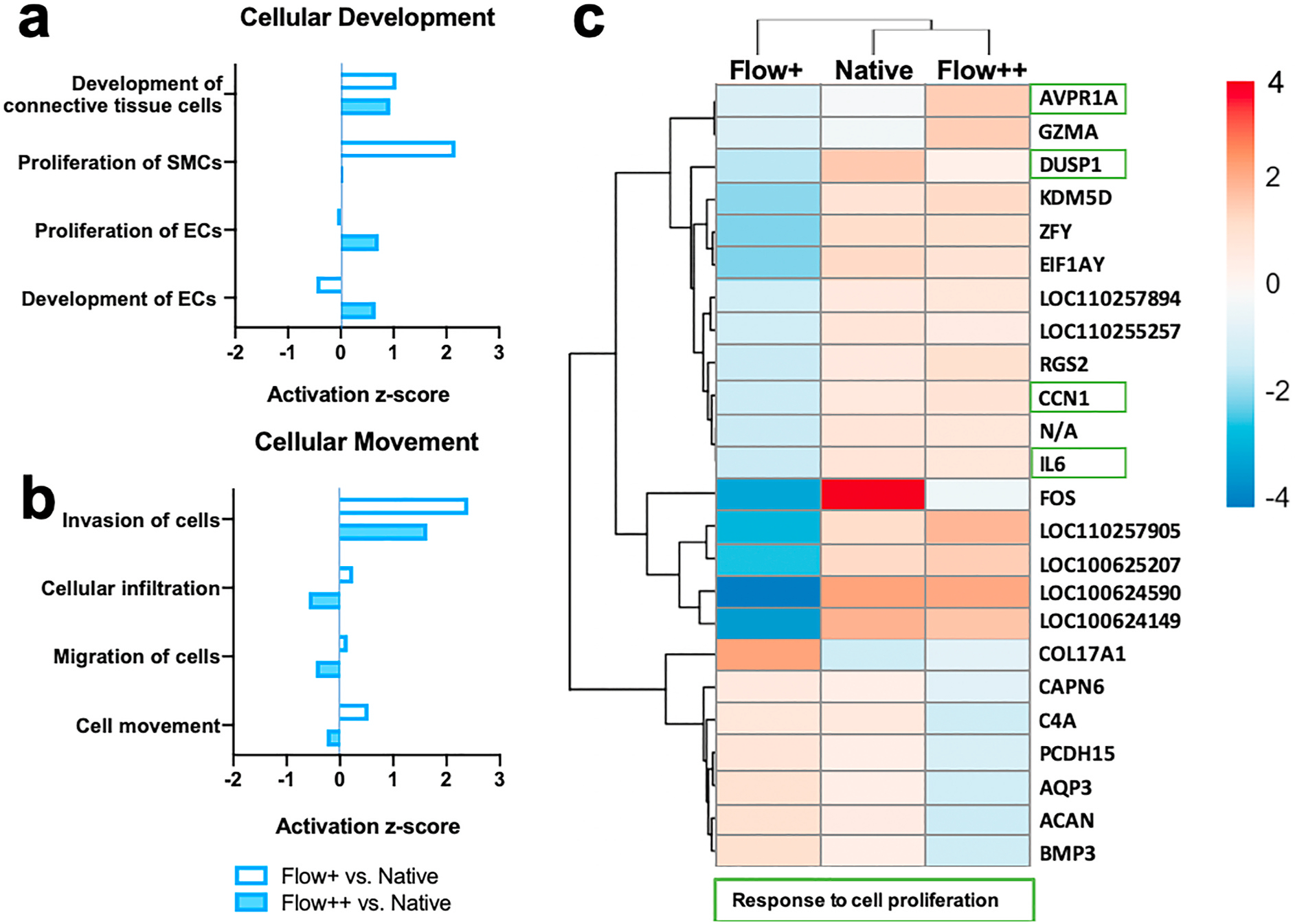
Bulk RNA Sequencing analysis of hyperplastic and physiologic vessel models under the low and high flow conditions respectively; N = 2 averaged per condition. a) and b) IPA activation z-score of cellular development and movement pathways under Flow + vs. native and Flow++ vs. native artery conditions, indicating SMC proliferation under the low flow condition. N = 2 for each condition, adjusted p < 0.05. c) Heatmap of significantly differentially expressed genes (adjusted p < 0.05, log2 fold change >1) between Flow+ and Flow++ conditions, with fragments per kilobase of transcript per million (FPKM) mapped reads normalized across each gene to show up-regulation (red) and down-regulation (blue) as compared to the other groups (see gradient). N/A is an unidentified gene. Genes pertaining to pathways of cell proliferation are indicated by green boxes.
3.2. Absorbable Mg-based stent therapy in ex vivo vessel bioreactor
After culturing native porcine arteries under varying flow conditions, we transitioned to study the impacts of Mg-based stents on vascular phenotype and cell survival. Mg-based (MgZnMn) stents were deployed into freshly isolated porcine and human coronary arteries using 2.75 mm-diameter balloons. Each stented vessel was then mounted into a bioreactor for 7 days (Fig. 1a–d). The same flow conditions, medium, and bioreactor setup were used as described above (N ≥ 6 for each group). To study the correlation between ex vivo and in vivo cellular responses, an in vivo experiment was carried out through the implantation of MgZnMn stents into the iliac arteries of New Zealand White Rabbits (N = 3) for 7 days.
3.2.1. Impact of luminal flow on cell survival in Mg-stented arteries – bioreactor and in vivo studies
The degradation process of Mg-stents was recorded for 7 days (Supplementary, Fig. S5a). The stented porcine arteries under flow (Flow+ and Flow++) conditions after 7-day culture showed very low rates of apoptosis by TUNEL stain (Fig. 4a). The fraction of apoptotic cells in stented arteries from the bioreactors was similar to that observed after 7 days of stenting in vivo in rabbit recipients: apoptosis rates of 6.50 ± 1.06% for high-flow; 7.00 ± 2.11% for low-flow; and 5.41 ± 1.36% for in vivo (Fig. 4d).
In contrast, in stented and statically-cultured vessels (Flow−), cell apoptosis increased dramatically to 71.93 ± 7.02% (Fig. 4b, N ≥ 5, ****p < 0.0001). TUNEL-positive nuclei were visualized in all three layers of the vessel wall: intima, media, and adventitia (Fig. 4a). Cell apoptosis was also manifested by the decreased thickness of the medial layer, as indicated by α-SMA staining, which was extremely thin in static-cultured vessels as compared to the controls and flow conditions (Fig. 4a). This may be caused by the decreased mass transfer of local pH changes, Mg ions, and localized oxygen depletion under static conditions. However, good cell viability can be achieved in the flow bioreactor conditions, which can therefore serve as a model for the physiological vessel-stent environment of Mg-based stent therapies.
Using human atherosclerotic coronary arteries that were obtained from heart transplant recipients with coronary heart disease, we repeated the bioreactor studies with MgZnMn stent deployment under both static and flow (2.8 dyn/cm2) conditions for 7 days (N = 3 for each group). The cells of the stented arteries showed high TUNEL positive staining and even lost nuclei under static conditions, but no TUNEL positive nuclei could be found under flow conditions (Supplementary, Fig. S6). Thus, the bioreactor can also provide good cell viability in response to Mg-based stents in atherosclerotic human coronary arteries.
3.2.2. Impact of Mg stent on neointima in cultured porcine arteries
After 7 days of Mg-stented culture in static, low-, and high-flow conditions, luminal area and intima-media layer thickness of porcine coronary arteries were measured based on H&E images. In low-flow conditions, which produce intimal hyperplasia in non-stented porcine arteries, the presence of the Mg stent inhibited the development of intimal hyperplasia, resulting in a near-normal luminal area and wall thickness (Fig. 4a). Luminal areas of stented arteries under low-flow conditions (1.09 ± 0.30 mm2 (Fig. 4b)), were significantly greater than those of non-stented arteries (0.26 ± 0.04 mm2 (Fig. 2b)), (N ≥ 6, *p = 0.0462). Conversely, there was no significant difference in luminal areas of Mg-stented arteries among static, low-, and high-flow conditions (Fig. 4b). Variations in pulsatile flow pressure of the arteries in bioreactors also show that mean pressure remained stable over time in low-flow and high-flow stented arteries (Supplementary, Fig. S2c,d).
Staining for vWF (Fig. 4a) showed that the endothelium remained intact after stent implantation with an EC monolayer, compared to the proliferative EC layers of non-stented vessels under the low-flow conditions (Fig. 2a). The presence of an implanted stent also decreased Ki67 fluorescence compared to non-stent conditions, further supporting the finding that stents inhibit the proliferation of SMCs (Figs. 2e and 4e). This was also observed in the MyHC stain through the entire SMC layer, for all stented vessels, regardless of low or high flow conditions (Fig. 4e). The opposite is seen in low-flow, non-stented conditions, where there was a low level of MyHC fluorescence observed in the neointima, instead replaced by Ki67+ proliferating cells (Fig. 2e). This may indicate that Mg-based stent therapy inhibits intimal hyperplasia in an ex vivo bioreactor vessel culture system and produces behavior similar to that of in vivo vessels [23,24].
3.2.3. Impact of bioreactor-modulated flow on gene expression profiles of Mg-stented arteries
Gene expression patterns from the stented vessels under the static (Stent/Flow-in 3-day culture, N = 2), high-flow (Stent/Flow++, in 7-day culture N = 3) conditions and fresh native arteries were compared using RNAseq. (Here, we utilized the 3-day culture for static stented vessels having a lower cell apoptosis rate of 13.9 ± 3.2%, due to the poor quality and quantity of RNA extracted from the highly apoptotic, stented vessels in 7-day static culture.) 456 genes (out of a total of 19,622 genes) were significantly different between fresh native arteries and stented arteries in the static and high-flow conditions, adjusted p < 0.05 (Supplementary, Fig. S7). In IPA analysis, the activation z-scores for necrosis and apoptosis pathways were more positive for static, stented vessels, but were negative for high-flow, stented vessels, as compared with fresh native arteries (Fig. 5a). The activation z-scores for cellular development pathways, including genes involved in proliferation of ECs and SMCs, are positive but low (less than 0.7) in both culture conditions (Fig. 5b and c).
Fig. 5.

Bulk RNA Sequencing analysis of stents impact on the vessels under the static and high flow conditions. a-c) IPA activation z-score of cell death and survival, cellular development and movement under Stent/Flow-vs. fresh native arteries, and Stent/Flow++ vs. fresh native arteries (N = 2 for Stent/Flow− and native arteries, N = 3 for Stent/Flow++, adjusted p < 0.05. d) Heatmap of significantly differentiated (adjusted p < 0.001, adjlog2 fold change >1) gene expression levels between Stent/Flow− and Stent/Flow++ conditions, normalized across each gene to show up-regulation (red) and down-regulation (blue) as compared to the other groups. Genes pertaining to pathways of apoptosis, cellular responses to metal ions, hypoxia and oxidative stress, cell growth, adhesion, migration, and differentiation are indicated by red, black, purple, and green boxes, respectively. e-h) Genes related to the regulation of specific pathways are extracted from the overall heatmap into smaller heatmaps, to provide insight into the differences between stented static (Stent/Flow−) and dynamic (Stent/Flow++) culture conditions. Particular genes were categorized by positive or negative regulation of relevant pathways.
To further assess the effects of static and flow conditions on stented arteries, we identified genes differentially expressed genes (adjusted p < 0.001) between the Stent/Flow− and Stent/Flow++ samples were extracted and clustered into a heatmap in Fig. 5d. Gene expression of stented arteries in flow conditions was similar to that of native controls. In static, stented culture, five genes related to negative regulation of apoptosis (WNT5A [37], SFRP1, MEGF10, CCN2, PTGS2) were downregulated as compared to controls and flow conditions (Fig. 5e). Five of the most highly upregulated genes were related to cellular responses to metal ions (MT1A, MT2A, ADH1C, MT1D, LOC100739663, Fig. 5f). Three downregulated genes related to cellular responses to hypoxia and oxidative stress (PTGS2, SFRP1, CCN2, Fig. 5g). This indicates that the accumulation of metallic ions in the cells and cellular responses to hypoxia were possible reasons for cell apoptosis in static, stented vessels. Additionally, the upregulation of MT1A, MT2A and GPC3 genes and the downregulation of SFRP1 and SFRP2 genes imply the inhibition of cell growth in static stented vessels (Fig. 5h).
Encouragingly, there was no upregulation of apoptosis-inducing genes in Stent/Flow++ conditions, as compared with the native arteries (Fig. 5b). Four upregulated genes (SFRP2, SHAS2, WNT5A, AREG) in Stent/Flow++ arteries related to cell migration and/or proliferation, compared with controls and static stented arteries (Fig. 5e). Interestingly, WNT5A, which is known to be involved in many regenerative and healing processes in arteries [38], particularly survival and proliferation of endothelial cells [39], was upregulated in Stent/Flow++ conditions. Additionally, the upregulation of SHAS2 expression in Stent/Flow++ vessels reflected the cellular response to fluid shear stress. This implies that Mg stent therapy may support a remodeling phenotype under flow conditions, at least as observed in the bioreactor system.
3.2.4. Ex vivo and in vivo correlation of Mg stent therapy
Evaluating the in vivo rabbit iliac artery model, an extra-thin layer of SMCs covered stent struts after 7 days, yet ECs were not visible by immunostaining (Fig. 4a). A few SMCs were Ki67-positive around the stent struts, and most of the SMCs expressed MyHC in a thin neointima (Fig. 4e). After two and four weeks of stent implantation, neointima grew along the stent struts (Supplementary, Fig. S8a and b). However, almost all the SMCs stained by MyHC still showed the contractile phenotype, with only a few Ki67-positive proliferating SMCs around the strut surface (Supplementary, Fig. S8c). ECs partially covered up the top of stent struts within 2 weeks, and the full endothelialization of the stents occurred at 4 weeks (Supplementary, Fig. S8b and S9, CD31 stain). Additionally, to study the inflammatory response, the stented arteries at the implantation time points of 1, 2, and 4 weeks were stained by CD45 and CD68. Only a few leucocytes were present at the interface between the struts and neointimal tissue (Supplementary, Fig. S9, CD45 staining). However, these leucocytes were not macrophages (CD68-negative stain). Overall, the MgZnMn stents showed reasonable control of intimal hyperplasia and very minimal inflammatory response up to 4-week implantation.
With regards to the stented arteries in flow ex vivo conditions, the intact EC layer and contractile SMCs were cultured very well, but there is the lack of cells on the luminal surface of the stents, as compared to a full coverage of cells on the stent struts in vivo. In terms of cell survival, apoptotic cells rarely appeared in the stented artery in vivo. The cell viability rate in vivo was not significantly different from that in the vascular bioreactor with flow, at nearly 95% (Fig. 4d and Supplementary, Fig. S8b).
3.3. In-situ and real-time monitoring of local environmental factors caused by Mg degradation in stented vessels
As Mg metal comes into contact with an aqueous environment, Mg reacts with H2O to form Mg2+ ions, hydroxide ion (OH− ) and hydrogen gas (H2) to release to the local aqueous environment and surrounding cells, and solid degradation products (Mg(OH)2 and calcium phosphate complex) on the surface of the stent structs (Supplementary, Fig. S10) [40–42]. The RNA sequencing findings also indicated some critical changes in gene expression related to cellular responses to metal ions and hypoxia. However, the factors in the local microenvironment are undetectable in the bulk, buffered medium. To better understand local microenvironmental changes, we measured pH and Mg concentration in the local medium immediately adjacent to the stented artery, and compared these values to the bulk medium measurements. Local medium adjacent to the stented artery was collected through a narrow-gauge needle that was inserted into the vascular lumen next to the stent struts (Fig. 1e, local medium extraction site). Intracellular Mg2+ concentration and cellular responses to hypoxia in the cultured arteries were also analyzed.
3.3.1. Cellular responses to Mg ion release from Mg stent degradation
Concentrations of Mg ions released into culture medium, and within the cultured vascular tissues, were quantified using an inductively coupled plasma mass spectrometer (ICP-MS) (Fig. 6a and b). The bulk and local medium samples had levels of Mg in a near-physiological range (Fig. 6b) for static and high-flow conditions, in the absence of any stenting. In contrast, Mg stenting had a marked effect on intracellular Mg2+ levels measured in cells of the arterial wall. Under high-flow conditions, local intracellular Mg2+ concentration was nearly identical to that in the native control (p = 0.99, N = 3), while in static conditions there was 6.27 times as much intracellular Mg ions as compared to controls (***p = 0.0002, N = 3 to 5, Fig. 6a). This is well beyond the tolerance of vascular cells [43] and thus Mg2+ accumulation in local cells likely contributes to cell death in static conditions (Fig. 4a,d).
Fig. 6.

Local environmental factors: pH, Mg ion, and O2. a) Intracellular Mg ion concentration of the stented vessels at 7-day culture under static (Flow−, 0 dyn/cm2) and high flow (Flow++, 6.8 dyn/cm2) conditions, normalized to fresh native vessels. Native control vs. Stent+/Flow− ***p = 0.0002; Stent+/Flow-vs. Stent+/Flow++ ***p = 0.0002, One-way ANOVA, Tukey’s multiple comparisons test. b) and c) Real-time monitoring of bulk and local Mg ion concentration (b) and pH values (c) and of medium in the static, and high flow conditions. The range between the two red lines is the normal level of blood Mg concentration. d) Dissolved O2 concentration of the bulk medium in the static and high flow conditions for 7 days. e) Hypoxia (red) staining with nuclei (DAPI, blue) for the stented vessels after 7-day culture under static and high flow conditions. White asterisks indicate vascular lumen. Native vessel as a negative control (Supplementary Fig. 11).
3.3.2. Cellular responses to pH changes from Mg stent degradation
Measured pH values (Fig. 6c dash lines) in the bulk medium were close to physiological levels in static (Flow−) and high-flow (Flow++) conditions, indicating that the overall environment of the culture system had some semblance to in vivo conditions. In contrast, local pH values collected from medium within the vessel lumen near the stent struts (Fig. 6c solid lines) were different from that of bulk medium: local pH in high-flow conditions (black) was slightly lower than that of static conditions (blue) for the first four days of culture. Encouragingly, the local pH values under high-flow conditions were nearly stable and were similar to fresh medium. Mass transfer in flow conditions prevented local accumulation of OH− ions, thus maintaining local pH near a physiological level. On the other hand, mass transfer was limited under static culture conditions, such that the maximum pH value under static conditions reached 8.4, which might lead to the low cell survival. Overall, pH change was not dramatical regardless of the static and flow conditions, due to pH buffer effect of culture medium.
3.3.3. Cellular responses to oxygen consumption of Mg stent degradation
Mg degradation is associated with a reduction of dissolved oxygen [44], but there has been a lack of studies on the effect of Mg degradation-induced hypoxia on cell survival. Thus, we measured the dissolved oxygen in the medium and cellular responses to oxidative stress. Dissolved O2 concentration in the bulk medium remained in a range of pO2 = 136–171 mmHg in the high-flow bioreactors with stents, which was higher than that in the static conditions (pO2 = 103–124 mmHg, Fig. 6d). Of note, the flow tubing of the bioreactor system was gas permeable, thereby contributing to a favorable level of dissolved O2 in the medium. While about 36% of cells expressed hypoxia-inducible factor-1 alpha (HIF1-α) under static conditions without stenting (Fig. 6e), the stented static vessels had 95% HIF1-α+ cells, which is consistent with the depletion of oxygen in the Mg-releasing environment. Hypoxia, as a regulator of apoptosis [45], can negatively affect cell survival. However, HIF1-α+ cells could not be found in high-flow conditions with and without the stents, implying superior oxygenation of vascular cells under flow conditions.
These data suggest that Mg ion accumulation and local hypoxia are the primary contributors to cell death in static, stented vessels, although elevated pH may also play a role. The ex vivo vascular bioreactor-modulated flow prevents the high intracellular Mg ion accumulation (Fig. 6a), O2 consumption (Fig. 6d and e) and pH changes (Fig. 6c) that occurred in conventional, static conditions. The VesselBRx provides a stable and physiological vascular environment for Mg-based stent studies, with cellular responses similar to those seen in stented arteries in vivo.
4. Discussion
We utilized a novel bioreactor model system, which provide physiological mass transfer and flow rates, and well as localized intra-luminal and extra-luminal sampled, to study the cellular responses to Mg-based stents. Superior to cell conventional culture approaches, the ex vivo vascular culture system can provide suitable mass transfer and an integrated 3-D vessel model with a highly interactive spatial microenvironment, including medium-stent, stent-cell, cell-cell and cell-medium interactions. It is known that ECs under low shear stress conditions can stimulate an early-stage atherosclerotic phenotype in microfluidic [20], and in simple 2-D cell culture models [19]. In VesselBRx, applying low shear stresses (2.8 dyn/cm2) causes the cultured arteries to develop hyperplastic characteristics within 7 days, while high shear stresses (6.8 dyn/cm2) maintain the physiologic wall thickness and mural cell proliferation rates. In the ex vivo bioreactor with low shear stress, Mg-based stent therapy inhibited intimal hyperplasia in a manner that simulated the results obtained with in vivo stenting [23–25].
Importantly, the VesselBRx helps to dispel a prior misconception regarding Mg-induced cytotoxicity that has been previously observed in conventional, static cell culture experiments. The high cell viability of 93.5% in the stented porcine arteries in the bioreactor, almost identical to the cell viability of 94.6% in the in vivo stented rabbit iliac arteries, shows that under physiological intra-luminal flow, Mg-stent degradation products are not highly toxic to vascular cells. To the best of our knowledge, there are no other in vitro approaches in the literature that accurately mimic the cell viability rate seen with in vivo experiments. Essentially, the VesselBRx is able to provide a buffered and efficient mass transfer system to not only avoid the accumulation of Mg degradation products, but also to supply fresh medium and oxygen to the cells of the vascular wall. Furthermore, the in-situ monitoring system is vital to capture the concentrations of local degradation factors near stent struts under physiological flow conditions. Because concentrations in the bulk medium are not representative of the local microenvironment around cells, having capacity for real-time and continuous local sampling of the stented vascular niche is an important tool to further the understanding of device-vessel interactions. The combination of exogenous factor control (eg. flow, shear stress, pressure, and oxygenation) and localized measurement capabilities allows us to better understand the mechanisms producing cell responses to Mg-based stent degradation, and, potentially, to other vascular therapeutics as well.
In static arterial culture, we provide strong evidence of local increases in intracellular Mg2+, local hypoxia, and changes in gene expression for proteins related to the handling of metal ions and cellular response to hypoxia. Such changes, not seen in VesselBRx likely contribute to the lack of correlation between prior, static in vitro findings and in vivo results. Interestingly, in the static bioreactor, there was ~6x as much intracellular Mg ions as compared to native artery controls, and compared to bioreactor flow conditions. This matches the recommendation in Wang et al. for a minimum 6 times to a maximal 10 times dilution of extracts for in vitro cytotoxicity test specified in ISO 10993–5 [4]. Moreover, many studies paid attention to Mg ion, H2, and pH, but the effect of Mg degradation-induced hypoxia on cell survival is neglected.
Many researchers are concerned with the potential cytotoxicity of Mg-based materials, based upon in vitro results using conventional, static culture methods (i.e. ISO 10993 Biological Evaluation of Medical Devices). Unfortunately, this concept has misinformed Mg compatibility studies for more than two decades. The ISO 10993 standard is designed for the testing of traditional non-degradable materials, which do not interact with culture medium. However, a newer generation of absorbable metals (eg. Mg, Zn, Fe, and their alloys) have active surfaces, and a dual-interaction between the degradable metals and culture environment. To improve the testing standard ISO/TS 17137, it seems important to use ex vivo vascular bioreactors and flow conditions for the study of absorbable metallic stent materials thereby achieving results that are more predictive of in vivo behavior.
The merits of VesselBRx as with compared to conventional in vitro and in vivo animal models are listed in Table 1. There is a strong correlation between ex vivo and in vivo results, particularly regarding cellular responses to Mg-based stent degradation, which is beneficial for improving the current standards for testing absorbable vascular implants. In addition to absorbable metallic stents, the ex vivo flow vascular bioreactor may also provide a superior platform for predicting cellular and tissue responses to drug-eluting stents, balloons, and other intravascular devices. The VesselBRx allows for high-fidelity testing of vascular implants in ex vivo human and animal tissues and can additionally minimize animal use by allowing multiple vessels from a single animal to be isolated and tested with different treatments under different conditions.
Table 1.
Merits and shortcomings of conventional in vitro, ex vivo flow vascular bioreactor, and in vivo animal models.
| Functions | Models |
||
|---|---|---|---|
| Conventional In vitro |
VesselBRx Ex vivo |
Animal models In vivo |
|
|
| |||
| Cytocompatibility | * False results | *** Very close to in vivo | *** Excellent |
| Cell phenotypes | * Difficult to keep in vivo cell phenotypes | *** Very close to in vivo | *** Excellent |
| Multiple types cells interactions | **Co-culture | *** Very close to in vivo | *** Excellent |
| Mechanism analysis | ** Basic | *** Advanced | *** Advanced |
| 3-D vascular structures | * No | *** Very close to in vivo | *** Excellent |
| Diseased models | * No | ** Easy to create models like hyperplasia | ** Hard to create diseased models |
| Monitoring | *** Easy | *** In-situ and real-time monitoring | * Very difficult for real-time monitoring |
| Accessibility | *** Very Easy | ** Fair | * Very difficult |
| Cost | *** Cheap | ** Moderate | * Expensive |
Excellent
Good
Poor.
5. Conclusion
To conclude, the VesselBRx can mimic hyperplasia formation due to disordered intra-luminal flow, and can simulate the arterial responses to Mg-based stents in vivo. The VesselBRx allows the tuning of intraluminal flow, pressure, and shear stress, while providing a means to sample local milieus in the artery for changes in stent degradation or drug products. The flow bioreactor provides sufficient mass transfer to resolve Mg degradation-induced issues of intracellular Mg2+ accumulation. We also show that, for the testing of Mg-based stents, the increased O2 consumption caused by Mg-stent degradation can render conventional static approaches non-representative. There is a strong correlation between ex vivo and in vivo results, particularly regarding cellular responses to Mg-based stent degradation, which is beneficial for improving the current standards for testing absorbable vascular implants. In principle, this approach can be applied to any vascular stent study, any drug screen for intimal hyperplasia treatment, or to the further study of the mechanisms underlying intimal hyperplasia formation. Furthermore, the VesselBRx has the potential to be used/tested for inducing hyperlipidemia, vascular calcification, and the subsequent development of atherosclerosis through the addition of specific growth factors and components in the culture medium.
Supplementary Material
Funding information
This work was funded by American Heart Association Postdoctoral Fellowship (17POST33661238) awarded to Dr. J. Wang, National Institutes of Health (NIH 1R01HL148819 and R01 HL127386) both to Prof. L.E. Niklason, and Young Scientists Fund of the National Natural Science Foundation of China (31600766) to Dr. J. Wang.
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Laura E. Niklason (L.E.N.) is a founder and shareholder in Humacyte, Inc, which is a regenerative medicine company. Humacyte produces engineered blood vessels from allogeneic smooth muscle cells for vascular surgery. L.E.N.’s spouse has equity in Humacyte, and L.E.N. serves on Humacyte’s Board of Directors. L.E.N. is an inventor on patents that are licensed to Humacyte and that produce royalties for L.E.N. L.E.N. has received an unrestricted research gift to support research in her laboratory at Yale. The other authors have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.biomaterials.2021.120911.
Data availability
The raw and processed data required to reproduce these findings are available from the corresponding author upon reasonable request. The RNA-seq data file generated during this study has been uploaded to GEO (GSE 174858).
References
- [1].Dzau VJ, Braun-Dullaeus RC, Sedding DG, Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies, Nat. Med. 8 (11) (2002) 1249–1256. [DOI] [PubMed] [Google Scholar]
- [2].Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R, The importance of the endothelium in atherothrombosis and coronary stenting, Nat. Rev. Cardiol. 9 (8) (2012) 439–453. [DOI] [PubMed] [Google Scholar]
- [3].Legrand V, Therapy insight: diabetes and drug-eluting stents, Nat. Clin. Pract. Cardiovasc. Med. 4 (3) (2007) 143–150. [DOI] [PubMed] [Google Scholar]
- [4].Wang J, Witte F, Xi T, Zheng Y, Yang K, Yang Y, Zhao D, Meng J, Li Y, Li W, Chan K, Qin L, Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials, Acta Biomater. 21 (2015) 237–249. [DOI] [PubMed] [Google Scholar]
- [5].Xi T, Gao R, Xu B, Chen L, Luo T, Liu J, Wei Y, Zhong S, In vitro and in vivo changes to PLGA/sirolimus coating on drug eluting stents, Biomaterials 31 (19) (2010) 5151–5158. [DOI] [PubMed] [Google Scholar]
- [6].Ma X, Oyamada S, Gao F, Wu T, Robich MP, Wu H, Wang X, Buchholz B, McCarthy S, Gu Z, Paclitaxel/sirolimus combination coated drug-eluting stent: in vitro and in vivo drug release studies, J. Pharmaceut. Biomed. Anal. 54 (4) (2011) 807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang J, Liu L, Wu Y, Maitz MF, Wang Z, Koo Y, Zhao A, Sankar J, Kong D, Huang N, Yun Y, Ex vivo blood vessel bioreactor for analysis of the biodegradation of magnesium stent models with and without vessel wall integration, Acta Biomater. 50 (2017) 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sanchez AHM, Luthringer BJ, Feyerabend F, Willumeit R, Mg and Mg alloys: how comparable are in vitro and in vivo corrosion rates? A review, Acta Biomater. 13 (2015) 16–31. [DOI] [PubMed] [Google Scholar]
- [9].Danenberg HD, Welt FG, Walker III M, Seifert P, Toegel GS, Edelman E, Systemic inflammation induced by lipopolysaccharide increases neointimal formation after balloon and stent injury in rabbits, Circulation 105 (24) (2002) 2917–2922. [DOI] [PubMed] [Google Scholar]
- [10].Henderson KK, Turk JR, Rush JW, Laughlin MH, Endothelial function in coronary arterioles from pigs with early-stage coronary disease induced by high-fat, high-cholesterol diet: effect of exercise, J. Appl. Physiol. 97 (3) (2004) 1159–1168. [DOI] [PubMed] [Google Scholar]
- [11].Veseli BE, Perrotta P, De Meyer GR, Roth L, Van der Donckt C, Martinet W, De Meyer GR, Animal models of atherosclerosis, Eur. J. Pharmacol. 816 (2017) 3–13. [DOI] [PubMed] [Google Scholar]
- [12].Byrne HM, Dissecting cancer through mathematics: from the cell to the animal model, Nat. Rev. Canc. 10 (3) (2010) 221–230. [DOI] [PubMed] [Google Scholar]
- [13].Porter DG, Ethical scores for animal experiments, Nature 356 (6365) (1992) 101–102. [DOI] [PubMed] [Google Scholar]
- [14].Vanerio N, Stijnen M, de Mol BAJM, Kock LM, An innovative ex vivo vascular bioreactor as comprehensive tool to study the behavior of native blood vessels under physiologically relevant conditions, J. Eng. Sci. Med. Diagn. Therap. 2 (4) (2019). [Google Scholar]
- [15].Yazdani SK, Berry JL, Development of an in vitro system to assess stent-induced smooth muscle cell proliferation: a feasibility study, J. Vasc. Intervent. Radiol. 20 (1) (2009) 101–106. [DOI] [PubMed] [Google Scholar]
- [16].Kamat N, Nguyen-Ehrenreich KL, Hsu SH, Ma AP, Sinn I, Coleman L, Tai J, Characterization of vascular injury responses to stent insertion in an ex-vivo arterial perfusion model, J. Vasc. Intervent. Radiol. 22 (2) (2011) 193–202. [DOI] [PubMed] [Google Scholar]
- [17].Kural MH, Wang J, Gui L, Yuan Y, Li G, Leiby KL, Quijano E, Tellides G, Saltzman WM, Niklason LE, Fas ligand and nitric oxide combination to control smooth muscle growth while sparing endothelium, Biomaterials 212 (2019) 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kural MH, Dai G, Niklason LE, Gui L, An ex vivo vessel injury model to study remodeling, Cell Transplant. 27 (9) (2018) 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Malek AM, Alper SL, Izumo S, Hemodynamic shear stress and its role in atherosclerosis, JAMA, J. Am. Med. Assoc. 282 (21) (1999) 2035–2042. [DOI] [PubMed] [Google Scholar]
- [20].Zheng W, Huang R, Jiang B, Zhao Y, Zhang W, Jiang X, An early-stage atherosclerosis research model based on microfluidics, Small 12 (15) (2016) 2022–2034. [DOI] [PubMed] [Google Scholar]
- [21].Jinnouchi H, Torii S, Sakamoto A, Kolodgie FD, Virmani R, Finn AV, Fully bioresorbable vascular scaffolds: lessons learned and future directions, Nat. Rev. Cardiol. 16 (5) (2019) 286–304. [DOI] [PubMed] [Google Scholar]
- [22].Ang HY, Huang YY, Lim ST, Wong P, Joner M, Foin N, Mechanical behavior of polymer-based vs. metallic-based bioresorbable stents, J. Thorac. Dis. 9 (Suppl 9) (2017) S923–S934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Haude M, Erbel R, Erne P, Verheye S, Degen H, Böse D, Vermeersch P, Wijnbergen I, Weissman N, Prati F, Waksman R, Koolen J, Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial, Lancet 381 (9869) (2013) 836–844. [DOI] [PubMed] [Google Scholar]
- [24].Haude M, Ince H, Abizaid A, Toelg R, Lemos PA, von Birgelen C, Christiansen EH, Wijns W, Neumann FJ, Kaiser C, Eeckhout E, Lim ST, Escaned J, Garcia-Garcia HM, Waksman R, Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial, Lancet 387 (10013) (2016) 31–39. [DOI] [PubMed] [Google Scholar]
- [25].Haude M, Ince H, Abizaid A, Toelg R, Lemos PA, von Birgelen C, Christiansen EH, Wijns W, Neumann FJ, Kaiser C, Eeckhout E, Lim ST, Escaned J, Onuma Y, Garcia-Garcia HM, Waksman R, Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial, Eur. Heart J. 37 (35) (2016) 2701–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nicol P, Bulin A, Castellanos MI, Stoeger M, Obermeier S, Fischer J, Baumgartner C, Steiger K, Joner M, Preclinical evaluation of a bioresorbable vascular scaffold (BRS) on the reduction of neoatherosclerosis, Eur. Heart J. 39 (2018), 528–528. [Google Scholar]
- [27].Erbel R, Di Mario C, Bartunek J, Bonnier J, de Bruyne B, Eberli FR, Erne P, Haude M, Heublein B, Horrigan M, Ilsley C, Böse D, Koolen J, Lüscher TF, Weissman N, Waksman R, Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial, Lancet 369 (9576) (2007) 1869–1875. [DOI] [PubMed] [Google Scholar]
- [28].Wu L, Luthringer BJ, Feyerabend F, Schilling AF, Willumeit R, Effects of extracellular magnesium on the differentiation and function of human osteoclasts, Acta Biomater. 10 (6) (2014) 2843–2854. [DOI] [PubMed] [Google Scholar]
- [29].Ahmad Agha N, Willumeit-Romer R, Laipple D, Luthringer B, Feyerabend F, The degradation interface of magnesium based alloys in direct contact with human primary osteoblast cells, PloS One 11 (6) (2016), e0157874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].ISO/TS 17137, 2019 Cardiovascular Implants and Extracorporeal Systems — Cardiovascular Absorbable Implants, 2019.
- [31].Doriot PA, Dorsaz PA, Dorsaz L, De Benedetti E, Chatelain P, Delafontaine P, In-vivo measurements of wall shear stress in human coronary arteries, Coron. Artery Dis. 11 (6) (2000) 495–502. [DOI] [PubMed] [Google Scholar]
- [32].Niklason L, Gao J, Abbott W, Hirschi K, Houser S, Marini R, Langer R, Functional arteries grown in vitro, Science 284 (5413) (1999) 489–493. [DOI] [PubMed] [Google Scholar]
- [33].Love MI, Huber W, Anders S, Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2, Genome Biol. 15 (12) (2014) 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS, Interferon-gamma elicits arteriosclerosis in the absence of leukocytes, Nature 403 (6766) (2000) 207–211. [DOI] [PubMed] [Google Scholar]
- [35].Shen J, Zhou S, Shi L, Liu X, Lin H, Yu H, DUSP1 inhibits cell proliferation, metastasis and invasion and angiogenesis in gallbladder cancer, Oncotarget 8 (7) (2017) 12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tang M, Wang G, Lu P, Karas RH, Aronovitz M, Heximer SP, Kaltenbronn KM, Blumer KJ, Siderovski DP, Zhu Y, Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure, Nat. Med. 9 (12) (2003) 1506–1512. [DOI] [PubMed] [Google Scholar]
- [37].Mill C, Monk BA, Williams H, Simmonds SJ, Jeremy JY, Johnson JL, George SJ, Wnt5a-induced Wnt1-inducible secreted protein-1 suppresses vascular smooth muscle cell apoptosis induced by oxidative stress, Arterioscler. Thromb. Vasc. Biol. 34 (11) (2014) 2449–2456. [DOI] [PubMed] [Google Scholar]
- [38].Shi Y-N, Zhu N, Liu C, Wu H-T, Gui Y, Liao D-F, Qin L, Wnt5a and its signaling pathway in angiogenesis, Clin. Chim. Acta 471 (2017) 263–269. [DOI] [PubMed] [Google Scholar]
- [39].Goodwin AM, Kitajewski J, D’Amore P, Wnt1 and Wnt5a affect endothelial proliferation and capillary length; Wnt2 does not, Growth Factors 25 (1) (2007) 25–32. [DOI] [PubMed] [Google Scholar]
- [40].Song GL, Atrens A, Understanding magnesium corrosion - a framework for improved alloy performance, Adv. Eng. Mater. 5 (12) (2003) 837–858. [Google Scholar]
- [41].Witte F, Hort N, Vogt C, Cohen S, Kainer KU, Willumeit R, Feyerabend F, Degradable biomaterials based on magnesium corrosion, Curr. Opin. Solid St M 12 (5–6) (2008) 63–72. [Google Scholar]
- [42].Wang G, Jiang W, Mo S, Xie L, Liao Q, Hu L, Ruan Q, Tang K, Mehrjou B, Liu M, Nonleaching antibacterial concept demonstrated by in situ construction of 2D nanoflakes on magnesium, Adv. Sci. 7 (1) (2020) 1902089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhao N, Zhu DH, Endothelial responses of magnesium and other alloying elements in magnesium-based stent materials, Metallomics 7 (1) (2015) 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Silva EL, Lamaka SV, Mei D, Zheludkevich ML, The reduction of dissolved oxygen during magnesium corrosion, ChemistryOpen 7 (8) (2018) 664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E, Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis, Nature 394 (6692) (1998) 485–490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw and processed data required to reproduce these findings are available from the corresponding author upon reasonable request. The RNA-seq data file generated during this study has been uploaded to GEO (GSE 174858).


