Abstract
BACKGROUND:
Patients with gestational diabetes mellitus are at increased risk for type 2 diabetes mellitus or glucose intolerance postpartum compared with those without diabetes mellitus.
OBJECTIVE:
We aimed to evaluate the association between early gestational diabetes mellitus and postpartum dysglycemia compared with gestational diabetes mellitus diagnosed by routine screening in a cohort of patients with obesity.
STUDY DESIGN:
This was a secondary analysis of a randomized controlled trial of patients with obesity and singleton, nonanomalous gestations that compared early gestational diabetes mellitus screening at 14 to 20 weeks of gestation with routine screening at 24 to 28 weeks of gestation. Patients were included in this analysis if they were diagnosed with gestational diabetes mellitus at the primary study site. The primary outcome was postpartum dysglycemia, defined as any abnormality on 2-hour oral glucose tolerance test 6 weeks postpartum or clinical diagnosis based on hyperglycemia requiring pharmacotherapy after delivery with deferred glucose tolerance test. Maternal characteristics and outcomes were compared in bivariable analysis, and logistic regression estimated the association between early gestational diabetes mellitus and postpartum dysglycemia.
RESULTS:
Of 119 patients included in this analysis, 30 were diagnosed by screening at <20 weeks of gestation and 89 at 24 to 28 weeks of gestation. Patients were overall similar in baseline characteristics. Patients with early gestational diabetes mellitus were more likely to have postpartum dysglycemia than those with gestational diabetes mellitus diagnosed with routine screening (36.7% vs 14.6%; odds ratio, 3.38; 95% confidence interval, 1.31–8.73). Most patients with early gestational diabetes mellitus who had postpartum dysglycemia were diagnosed clinically (n=7/11), whereas none of the patients with gestational diabetes mellitus established by routine testing were diagnosed with postpartum dysglycemia clinically. All (100%) patients with early gestational diabetes mellitus who completed a postpartum glucose tolerance test had dysglycemia compared with only 45% of patients with gestational diabetes mellitus diagnosed on routine screening. The proportion of patients who followed up for postpartum visits and the timing of follow-up were similar between groups. Postpartum glucose tolerance test completion was low but also similar between groups.
CONCLUSION:
Although postpartum glucose tolerance test completion is low, patients with gestational diabetes mellitus before 20 weeks of gestation, seem to be at higher risk for postpartum dysglycemia than those with gestational diabetes mellitus diagnosed at routine screening in a cohort of patients with obesity. Larger studies are needed to confirm these findings, but postpartum follow-up and diabetes mellitus testing may be even more important to improve long-term health in patients with early gestational diabetes mellitus.
Keywords: early gestational diabetes mellitus screening, gestational diabetes mellitus, postpartum dysglycemia
Introduction
Screening for gestational diabetes mellitus (GDM) is recommended for all pregnant patients, although the optimal method and timing for screening is controversial. The United States Preventive Services Task Force currently recommends screening for GDM in asymptomatic pregnant patients after 24 weeks’ gestation and does not recommend screening asymptomatic patients before this gestational age.1 The American College of Obstetricians and Gynecologists (ACOG) recommends screening patients who are overweight early (at the first prenatal visit) if they have additional risk factors for the development of GDM or undiagnosed type 2 diabetes mellitus, followed by routine screening for GDM if the initial screening is negative.2,3
GDM increases the risk of complications during pregnancy and also postpartum. Most recent data estimate that 15% to 60% of patients with GDM will develop overt type 2 diabetes mellitus within 5 to 15 years postpartum,1 with 70% of those diagnosed with GDM developing type 2 diabetes mellitus in the subsequent 28 years following pregnancy.3–5 For this reason, ACOG and the American Diabetes Association currently recommend that all patients diagnosed with GDM undergo screening for type 2 diabetes mellitus at a 4 to 12 week postpartum visit.3,6
It is unclear whether patients diagnosed with early GDM before 20 weeks of gestation have a higher risk of postpartum dysglycemia than patients diagnosed with GDM during routine screening. GDM diagnosed earlier in pregnancy (before the peak effect of pregnancy hormones that lead to insulin resistance) may represent lower baseline insulin sensitivity. If early GDM is a marker for underlying metabolic dysfunction, it could be associated with a higher risk of postpartum dysglycemia than that associated with GDM diagnosed later in pregnancy. Thus, we aimed to compare the risk of postpartum dysglycemia between a cohort of patients with obesity diagnosed with early GDM and those diagnosed at 24 to 28 weeks.
Materials and Methods
This was a secondary analysis of a randomized controlled trial conducted at the University of Alabama in pregnant patients with obesity (defined as body mass index [BMI] ≥30 kg/m2) that compared early screening for GDM (14–20 weeks of gestation) with routine screening (24–28 weeks of gestation). Institutional board review approval was obtained for the primary study. This secondary analysis was approved by the University of Alabama at Birmingham Institutional Review Board (IRB-121008004).
Details of the original trial have been published previously.7 Briefly, patients were eligible if their BMI at presentation was ≥30 kg/m2, if they carried a singleton, nonanomalous gestation, and presented for care at <20 weeks of gestation. Patients were excluded if they had previous cesarean delivery, preexisting diabetes mellitus, history of bariatric surgery, major medical complications, or chronic prednisone use. Participants were randomized to GDM screening at 14 to 20 weeks or at 24 to 28 weeks. Screening for GDM was accomplished with the 2-step method: a 50-g 1-hour glucose challenge test (GCT) was performed, followed by a 100-g, 3-hour glucose tolerance test if the result of the GCT resulted ≥135 mg/dL. Carpenter-Coustan criteria were used to diagnose GDM. If GDM was not diagnosed at 14 to 20 weeks, patients were rescreened at 24 to 28 weeks starting with the 50-g GCT. As part of the study protocol, all patients also underwent hemoglobin A1c testing at 14 to 20 weeks of gestation and again at 24 to 28 weeks of gestation.
If diagnosed with GDM, patients were managed in accordance with institutional guidelines. Patients performed home blood glucose monitoring with goals of fasting glucose <95 mg/dL and 2-hour postprandial glucose <120 mg/dL. Medications were initiated (glyburide, metformin, or insulin on the basis of provider preference) if more than half of reported blood glucose levels were above the goal. If medications were required, patients underwent weekly antenatal testing after 32 weeks. A more detailed discussion of limitations of this trial is available in the published manuscript.7
Patients were included in this secondary analysis if they were enrolled in the randomized controlled trial described previously, received prenatal care at our institution, and were diagnosed with GDM on the basis of early glucose tolerance test performed at 14 to 20 weeks of gestation or routine glucose tolerance test at 24 to 48 weeks of gestation.
Our primary outcome was postpartum dysglycemia established by 75-g 2-hour oral glucose tolerance test at 4 to 6 weeks after delivery or clinical diagnosis after delivery. Four to 6-week timing for testing was chosen because this is when patients are seen for postpartum exams and routinely complete glucose tolerance testing at our institution. Laboratory diagnosis of postpartum dysglycemia was defined as impaired fasting glucose (fasting glucose 100–125 mg/dL), impaired glucose tolerance (2-hour glucose 140–299 mg/dL), or overt type 2 diabetes mellitus (fasting glucose >125 mg/dL or 2-hour glucose >199 mg/dL). Clinical diagnosis of postpartum dysglycemia was defined as continued pharmacologic treatment required to maintain normal glucose levels after delivery with deferred postpartum glucose tolerance test. This diagnosis was made at the discretion of the primary obstetrical provider and not designated by any specific criteria in the parent trial.
Secondary outcomes included impaired fasting glucose, impaired glucose tolerance, and type 2 diabetes mellitus established by the 2-hour glucose tolerance test at the postpartum visit, return for a postpartum visit, and completion of the 2-hour glucose tolerance test.
Maternal baseline characteristics and outcomes were compared between those with early and routine GDM using the Student t test, Mann–Whitney U test, chi square, and Fisher exact test, as appropriate. The association between early GDM and postpartum dysglycemia was evaluated using logistic regression, and odds ratios with 95% confidence intervals were estimated. The significance level was set at P<.05, and all tests were 2-tailed.
All data were collected via extraction from Cerner-based electronic medical records. The primary outcome was abstracted by individual chart review by study authors (A.N.B. and M.L.C.). All statistical analyses were performed using STATA, version 16.1 (StataCorp, College Station, TX).
Results
Of the 913 patients included in the original trial, 119 (13%) were diagnosed with GDM at the primary site and included in this analysis (Figure 1). Patients who were diagnosed with early GDM were more likely to be non-Hispanic Black or non-Hispanic White, but otherwise were overall similar in baseline characteristics to those who were diagnosed with GDM by routine screening (Table 1). Specifically, they were similar with regard to age, early pregnancy BMI, insurance type, marital status, nulliparity, and presence of chronic hypertension. Patients who were diagnosed with early GDM were significantly more likely to use tobacco during pregnancy, but there was no difference in alcohol or illicit drug use (Table 1).
FIGURE 1:
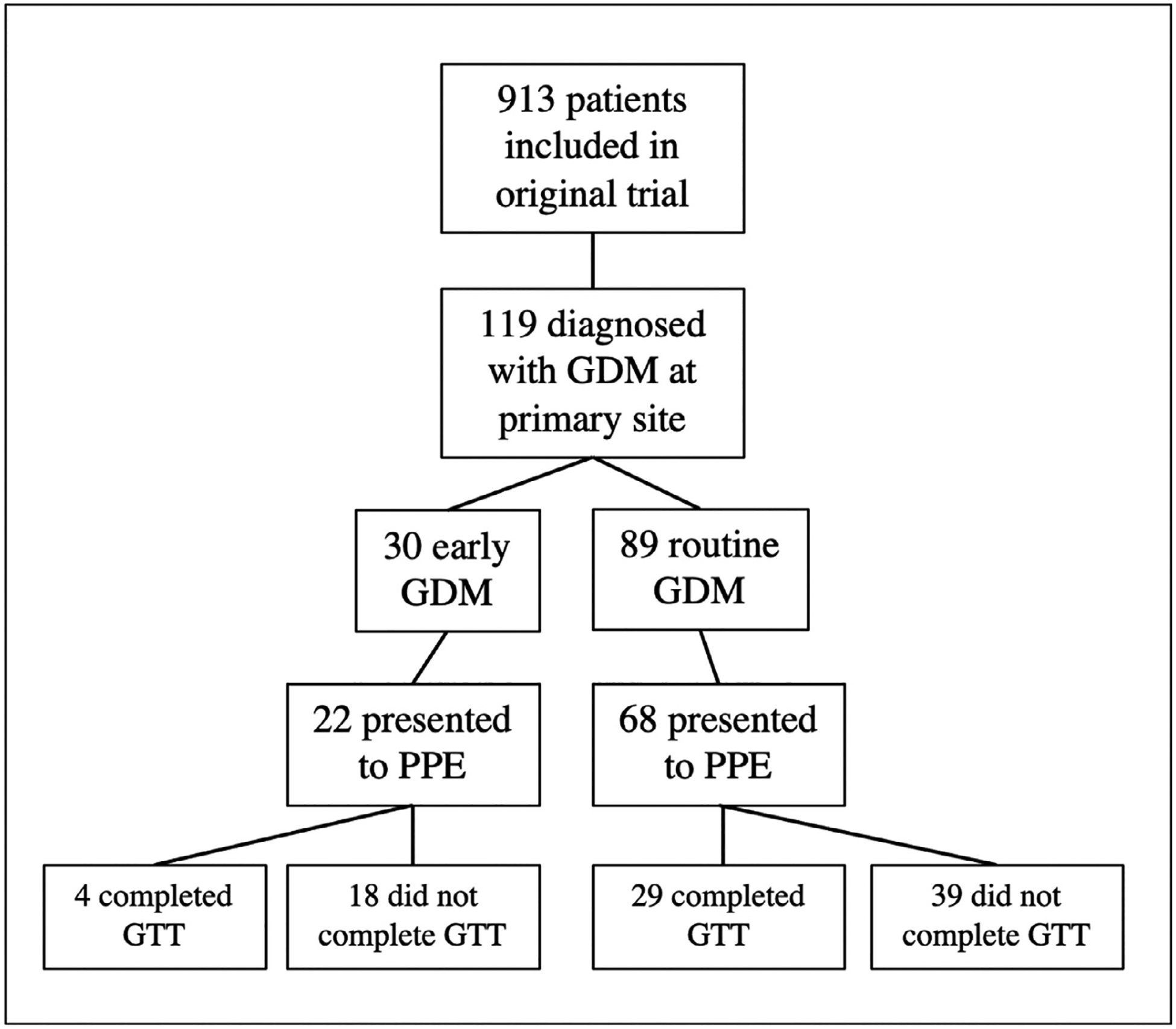
Flow diagram of study cohort,
GDM, gestational diabetes mellitus; GTT, glucose tolerance test; PPE, postpartum exam.
Champion. Early gestational diabetes mellitus and postpartum glucose intolerance. Am J Obstet Gynecol MFM 2022.
TABLE 1:
Maternal demographic and obstetrical characteristics by timing of gestational diabetes mellitus diagnosis
| Maternal age (y) | 30.2±5.8 | 28.9±5.9 | .29 |
| Race/ethnicity | |||
| Non-Hispanic White | 7 (23) | 6 (7) | .03 |
| Non-Hispanic Black | 16 (53) | 40 (45) | |
| Hispanic | 7 (23) | 40 (45) | |
| Other | 0 | 3 (3) | |
| BMI at randomization (kg/m2) | 35.6 (33.0–48.0) | 36.0 (32.8–39.6) | .49 |
| Insurance type | |||
| Private | 1 (3) | 1 (1) | .17 |
| Medicaid | 23 (77) | 55 (63) | |
| None | 6 (20) | 31 (34) | |
| Married | 10 (33) | 30 (34) | .97 |
| High school education or greater | 20 (69) | 54 (66) | .76 |
| Nulliparous | 14 (47) | 29 (33) | .17 |
| Tobacco use during pregnancy | 11 (37) | 7 (8) | <.001 |
| Alcohol use during pregnancy | 6 (20) | 6 (7) | .07 |
| Illicit drug use during pregnancy | 2 (7) | 5 (6) | >.99 |
| Chronic hypertensiona | 8 (27) | 10 (11) | .07 |
| Hemoglobin A1c (%) at 14–20 wk | 5.6 (5.1–6.1) | 5.5 (5.2–5.8) | .25 |
| Hemoglobin A1c (%) at 24–28 wk | 5.3 (4.9–5.8) | 5.7 (5.3–6.0) | .08 |
| GTT results at time of GDM diagnosis | |||
| Fasting glucose | 105 (97–121) | 101 (95–109) | .11 |
| 1 h glucose | 191 (186–213) | 194 (181–205) | .84 |
| 2 h glucose | 178 (162–198) | 169 (157–186) | .22 |
| 3 h glucose | 117 (90–143) | 140 (125–149) | .14 |
| GDM type | .33 | ||
| A1 | 15 (50) | 53 (60) | |
| A2 | 15 (50) | 35 (40) | |
BMI, body mass index; GDM, gestational diabetes mellitus; GTT, glucose tolerance test; HELLP, hemolysis, elevated liver enzymes and low platelets.
Pregnancy-induced hypertension includes gestational hypertension, preeclampsia, HELLP or eclampsia at time of hospital admission for delivery.
Champion. Early gestational diabetes mellitus and postpartum glucose intolerance. Am J Obstet Gynecol MFM 2022.
There was no difference in early hemoglobin A1c or third-trimester hemoglobin A1c between those who were diagnosed with GDM at 14 to 20 weeks or at 24 to 28 weeks (Table 1). In addition, the individual results of glucose tolerance tests at time of diagnosis were similar between groups. Patients with early GDM were as likely to be diagnosed with A1 or A2 GDM as those diagnosed with GDM during routine screening (Table 1).
Patients with early GDM were more likely to have postpartum dysglycemia than those with GDM diagnosed by routine screening (36.7% vs 14.6%; odds ratio, 3.38; 95% confidence interval, 1.31–8.73). Of the 11 patients with early GDM who had postpartum dysglycemia, 7 were diagnosed clinically and 4 were diagnosed by glucose tolerance testing. Those who were diagnosed clinically did not undergo a glucose tolerance test at the time of postpartum visit. However, 100% of patients with early GDM who completed a postpartum glucose tolerance test had dysglycemia compared with only 45% of patients who completed testing with GDM diagnosed on routine screening (Table 2). None of the patients with GDM diagnosed on routine screening were diagnosed with postpartum dysglycemia clinically. Neither the proportion of patients who returned for a postpartum visit (76% vs 73%) nor the timing of this visit (6.4 vs 6.0 weeks) differed between groups (Table 2). The proportion of patients who completed the recommended 2-hour glucose tolerance test was low but also similar between groups (13% vs 42%; P=.15).
TABLE 2:
Postpartum dysglycemia by timing of gestational diabetes mellitus diagnosis
| Postpartum dysglycemia | 11 (36.7) | 13 (14.6) | .01 |
| Clinical diagnosis | 7 | 0 | |
| Laboratory diagnosis | 4 | 13 | |
| Postpartum dysglycemia by 2 h GTT | 4/4 (100) | 13/29 (45) | |
| Impaired fasting glucosea | 3/4 (75) | 11/29 (38) | .10 |
| Impaired glucose toleranceb | 1/4 (25) | 5/29 (17) | |
| Type 2 diabetes mellitus c | 0/4 | 1/29 (4) | |
| Postpartum 2 h GTT results | |||
| Fasting glucose (mg/dL) | 104 (99–112) | 97 (92–110) | .62 |
| 2 h glucose (mg/dL) | 132 (100–144) | 115 (100–134) | .64 |
| Time from delivery to postpartum visit (wk) | 6.4 (6.0–7.6) | 6.0 (5.9–6.5) | .12 |
| Returned for PP visit | 22 (73) | 68 (76) | .74 |
| Completed 2 h GTTd | 4 (13) | 29 (42) | .15 |
| Breastfeeding | 19 (66) | 67 (75) | .30 |
GDM, gestational diabetes mellitus; GTT, glucose tolerance test; PP, postpartum.
Impaired fasting glucose defined as fasting glucose 100–125 mg/dL;
Impaired glucose tolerance defined as 2-hour glucose 140–199 mg/dL;
Overt type 2 diabetes mellitus defined as fasting glucose >125 mg/dL or 2 hour glucose >199 mg/dL;
Among those who were not already diagnosed with dysglycemia clinically.
Champion. Early gestational diabetes mellitus and postpartum glucose intolerance. Am J Obstet Gynecol MFM 2022.
Discussion
Principal findings
In a cohort of patients with obesity, those who were diagnosed with early GDM had more than 3-fold higher odds of postpartum dysglycemia than patients with GDM diagnosed at the time of routine 24 to 28-week screening. All patients diagnosed with early GDM who underwent a postpartum glucose tolerance test had evidence of postpartum dysglycemia, whereas only 45% of patients diagnosed with GDM on routine screening had postpartum dysglycemia. Of the patients with early GDM diagnosed with postpartum dysglycemia, 64% were diagnosed clinically and continued on pharmacotherapy immediately postpartum, whereas 36% were diagnosed with abnormal glucose tolerance test at the postpartum exam. Among patients with GDM diagnosed by routine screening, none were diagnosed with postpartum dysglycemia on the basis of clinical findings, and approximately half who underwent glucose tolerance testing had dysglycemia.
Results in the context of what is known
Our findings confirm and extend previously published data on the risk of postpartum dysglycemia with early GDM. An Australian study observed findings similar to ours, with patients with early GDM who were more likely to have postpartum dysglycemia than those with GDM diagnosed ≥24 weeks’ gestation.8 In this retrospective cohort study, the 3-month postpartum glucose tolerance test was normal in 85% of patients with GDM diagnosed ≥24 weeks compared with only 79% of patients with early GDM diagnosed <12 weeks and 71% of patients with early GDM diagnosed at 12 to 23 weeks (P<.05). There are 2 main differences in study design that should be noted. First, GDM was diagnosed using the Australasian Diabetes in Pregnancy Society diagnostic criteria,9 which uses a 1-step, 2-hour 75-g glucose tolerance test with thresholds of 126 mg/dL and 200 mg/dL for fasting and 2-hour glucose levels. The difference in criteria used for GDM diagnosis could have resulted in a greater proportion of patients with GDM and more mild cases in the Australian study compared with our analysis. Second, the patient populations were considerably different between our analysis and the Australian study. All of our patients had obesity, and most of our patients were Black or Hispanic with an average BMI of 36 kg/m2 in both groups, whereas patients included in the Australian study were primarily Anglo-Celtic and Southeast Asian people with an average BMI of <30 kg/m2. Both of these differences may explain the higher rate of postpartum glucose intolerance in patients with early GDM in our study compared with that of the Australian study (37% vs 28%). However, the fact that early GDM was associated with higher risk of postpartum glucose intolerance compared with GDM in both studies helps support the reliability of our findings.
A longer-term follow-up study conducted in Denmark10 offers insight into the risk of glucose intolerance up to 3 years postpartum in patients with early GDM compared with GDM diagnosed with routine screening. A 1-step 2-hour, 75-gram oral glucose tolerance test was also used for GDM diagnosis, and all patients diagnosed with GDM were recommended to follow-up annually for 3 years. Consistent with the findings in our study, patients with a diagnosis of GDM before 20 weeks of gestation were more likely to develop type 2 diabetes mellitus than those diagnosed after 20 weeks of gestation (36% vs 5%; P<.05).10 This study confirms the finding of increased glucose intolerance in patients with early GDM and provides additional information regarding the development of overt diabetes mellitus subsequent to delivery. These results highlight the importance of long-term follow-up, specifically in patients with early GDM because they seem to be at even higher risk for development of overt diabetes mellitus than those with GDM diagnosed with routine screening.
Clinical and research implications
Our results could be explained by either a more severe form of glucose intolerance present in patients with early GDM or could be related to underlying and undiagnosed pregestational diabetes mellitus in those diagnosed with early GDM. There is mounting evidence that early diagnosis of GDM may not improve perinatal outcomes; however, early diagnosis may help risk stratify patients who are at increased risk of persistent glucose intolerance after delivery. Future studies should evaluate long-term maternal health implications of GDM diagnosed early in pregnancy.
Strengths and limitations
The findings of our study should be interpreted within the context of the study design. This was a secondary analysis of a trial and hence we were limited to the testing strategies and methods of the parent trial. Our secondary analysis was limited by the low rate of postpartum glucose tolerance test completion, which is a consistent limitation in studies evaluating postpartum outcomes after GDM. Although it is possible that the clinical diagnosis of dysglycemia may have been biased given that obstetrical providers were not blinded to timing of GDM diagnosis, we found a significant difference in dysglycemia by glucose tolerance testing. Because of our study size, we were unable to adjust for potential confounding factors. Lastly, this cohort includes only patients with obesity and a predominantly non-Hispanic Black population with public insurance; thus, our findings may not be generalizable to all settings.
Our study has multiple strengths. This is a secondary analysis of a randomized controlled trial which adhered to standardized protocols for GDM screening and management. The primary outcome was reviewed individually by study authors to ensure accuracy. Lastly, this study evaluated postpartum dysglycemia as it relates to early diagnosis of GDM in a diverse, contemporary US population.
Conclusions
Overall, our study found that in a cohort of obese patients, those who are diagnosed with early GDM had a 3-fold increased risk of postpartum dysglycemia than those diagnosed with routine screening in the third trimester. Despite growing evidence that early GDM screening may not improve pregnancy outcomes; this diagnosis may help determine who is at the highest risk for developing type 2 diabetes mellitus later in life. Future studies are needed to confirm our findings and determine how to optimize long-term maternal health after early GDM.
AJOG MFM at a Glance.
Why was this study conducted?
Patients with gestational diabetes mellitus (GDM) have an increased risk of diabetes mellitus in the future. It is unclear whether patients diagnosed with early GDM before 20 weeks of gestation have a higher risk of postpartum dysglycemia than those diagnosed with routine screening at 24 to 28 weeks.
Key findings
Patients who have obesity and are diagnosed with GDM with early screening have an increased risk of postpartum dysglycemia than those diagnosed with routine screening.
What does this add to what is known?
Our study supports the findings of similar studies performed in Australia and Denmark but is the first to evaluate these outcomes in a diverse, contemporary US population. Overall, patients with early GDM may represent a subgroup of GDM patients who are at the highest risk for future type 2 diabetes mellitus and thus could benefit the most from lifestyle interventions after delivery.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development. A.N.B. was supported by the NICHD (grant number K23HD103875) during the study.
Footnotes
This work was presented as a poster at the 41st annual meeting for the Society for Maternal-Fetal Medicine, held virtually January 25–31, 2021.
All individuals who contributed to this work have met standard criteria for authorship. The authors report no conflict of interest.
There are no financial conflicts of interest to disclose.
References
- 1.Moyer VA. U.S. Preventive Services Task Force. Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:414–20. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl1):S81–90. [DOI] [PubMed] [Google Scholar]
- 3.ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol 2018;131:e49–64. [DOI] [PubMed] [Google Scholar]
- 4.England LJ, Dietz PM, Njoroge T, et al. Preventing type 2 diabetes: public health implications for women with a history of gestational diabetes mellitus. Am J Obstet Gynecol 2009;200:365. e1−8. [DOI] [PubMed] [Google Scholar]
- 5.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862–8. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43:S183–92. [DOI] [PubMed] [Google Scholar]
- 7.Harper LM, Jauk V, Longo S, Biggio JR, Szychowski JM, Tita AT. Early gestational diabetes screening in obese women: a randomized controlled trial. Am J Obstet Gynecol 2020;222:495. e1−8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweeting AN, Ross GP, Hyett J, et al. Gestational diabetes mellitus in early pregnancy: evidence for poor pregnancy outcomes despite treatment. Diabetes Care 2016;39:75–81. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman L, Nolan C, Wilson JD, Oats JJ, Simmons D. Gestational diabetes mellitus–management guidelines. The Australasian Diabetes in Pregnancy Society. Med J Aust 1998;169:93–7. [DOI] [PubMed] [Google Scholar]
- 10.Svare JA, Hansen BB, Mølsted-Pedersen L. Perinatal complications in women with gestational diabetes mellitus. Acta Obstet Gynecol Scand 2001;80:899–904. [DOI] [PubMed] [Google Scholar]


