Abstract
Although abundant geochemical data indicate that anaerobic methane oxidation occurs in marine sediments, the linkage to specific microorganisms remains unclear. In order to examine processes of methane consumption and oxidation, sediment samples from mud volcanoes at two distinct sites on the Mediterranean Ridge were collected via the submersible Nautile. Geochemical data strongly indicate that methane is oxidized under anaerobic conditions, and compound-specific carbon isotope analyses indicate that this reaction is facilitated by a consortium of archaea and bacteria. Specifically, these methane-rich sediments contain high abundances of methanogen-specific biomarkers that are significantly depleted in 13C (δ13C values are as low as −95‰). Biomarkers inferred to derive from sulfate-reducing bacteria and other heterotrophic bacteria are similarly depleted. Consistent with previous work, such depletion can be explained by consumption of 13C-depleted methane by methanogens operating in reverse and as part a consortium of organisms in which sulfate serves as the terminal electron acceptor. Moreover, our results indicate that this process is widespread in Mediterranean mud volcanoes and in some localized settings is the predominant microbiological process.
Methane can have a stronger greenhouse effect than CO2, and recent work has highlighted its potential climatic impact on glacial timescales (24) and in relation to major geologic events (8, 12). Consequently, controls on methane production and consumption are important concerns in the evaluation of past and future climate change. In marine sediments, anaerobic methane oxidation could be the dominant pathway for methane consumption (2, 4, 6, 15, 25, 26), but the organisms involved have not been isolated and the mechanism remains controversial. Methanogenic archaea operating in reverse (9, 11, 14) or novel, previously uncharacterized archaea (13) have been proposed to play a vital role, but current evidence remains ambiguous.
Methane tends to be highly depleted in 13C, and organisms that consume methane either directly or indirectly via heterotrophic consumption of methanotroph biomass will be similarly depleted in 13C. For this study, we determined distributions and carbon isotope abundances of organic components in methane-rich mud volcano sediments of the Eastern Mediterranean Ridge. In particular, we examined the δ13C values of compounds derived from specific organisms (i.e., biomarkers) presumed to play a major role in methane oxidation, including methanogens, aerobic methanotrophs, sulfate-reducing bacteria, and anaerobic heterotrophic bacteria. From these results, we evaluate the controls on and microorganisms responsible for methane oxidation in these sediments and the conditions under which methane oxidation occurs.
MATERIALS AND METHODS
Samples.
Using a bordeaux core, samples were collected from surface sediments (upper 30 cm) of several mud volcano flows (mud breccias) in the Olimpi (Milano and Napoli mud volcanoes) and Anaximander (Amsterdam mud volcano) fields (10, 21, 38) using the submersible Nautile during the 1998 cruising of the R/V Nadir. Samples were then frozen at −20°C. Sampling via submersible provided a unique opportunity to obtain sediments from a variety of highly specific settings, including readily identifiable methane seeps, microbial mats, and brine pools (Table 1). In addition to these apparently “active” sites, we analyzed mud breccia profiles from Milano and Napoli mud volcanoes. In contrast to previous work with similar goals, this site-specific sampling allowed a detailed comparison of microbial processes in diverse settings.
TABLE 1.
Evidence for methane incorporation by methanogenic archaea in Mediterranean mud volcano sediments
| Volcano (field) | Deposit type | δ13C value (‰)
|
Abundance of archaeol (μg/g [dry wt] of sediment) | Most abundant compound (compound no.) | |
|---|---|---|---|---|---|
| PMI | Archaeol | ||||
| Napoli (Olimpi) | Seep | −64.8 | −76.2 | 18 | Archaeol (I) |
| Mud breccia | −52.0 | −40.6 | 1 | Archaeol (I)a | |
| Sediments below brine | −62.2 | −63.1 | 14 | Crocetane (VII) | |
| Microbial mat | −76.2 | −84.1 | 53 | Cholesterol (XVII) | |
| Milano (Olimpi) | Mud breccia (seep) | −66.9 | −81.1 | 8 | Unsaturated PMI (V)b |
| Mud breccia | NDc | −57.2 | 0.4 | C18 alcohol | |
| Amsterdam (Anaximander) | Mud breccia (seep) | −91.4 | −95.8 | 7 | Archaeol (I) |
Deepest sample (17 to 20 cm).
Sum of all unsaturated PMI compounds.
ND, not determined.
Extraction and separation.
Samples (8 to 25 g of sediment) were freeze-dried and extracted via sonication in a sequence of solvent mixtures with increasing dichloromethane/methanol ratios (0:1 three times, 1:1 three times, and 1:0 three times; solvent volume was ca. three times the sediment volume). For two samples (Amsterdam seep and Milano seep), 100 g of sediment was extracted for 24 h with a Soxhlet apparatus and a dichloromethane-methanol mixture (7.5:1 [vol/vol]). Elemental sulfur was removed from the total extracts by adding ca. 100 mg of activated copper and stirring the sample for 4 h.
Aliquots of total extracts (65%) were separated into acetone-soluble and insoluble fractions (16). The soluble component was further separated on an alumina column (activated for 2 h at 150°C; 3 to 30 g of alumina, depending on sample size) into apolar and polar fractions by using three times the column volume of hexane-dichloromethane (9:1 [vol/vol]) and methanol as the eluents, respectively. In the polar fraction, fatty acids were methylated by refluxing at 60°C for 5 min in BF3 (in methanol), and alcohols were converted to trimethylsilyl derivatives with 25 μl each of bis(trimethylsilyl)trifluoracetamide (BSTFA) and pyridine heated at 60°C for 30 min.
Analysis of biomarkers.
Apolar and polar fractions were analyzed by gas chromatography (GC) and GC-mass spectrometry (MS) for identification. GC-MS was conducted with a Hewlett-Packard 5890 gas chromatograph interfaced with a VG Autospec Ultima Q mass spectrometer operated at 70 eV with a mass range m/z of 50 to 800 and a cycle time of 1.8 s (resolution, 1,000). A fused-silica CP-Sil 5 capillary column (25 m by 0.32 mm, df = 0.12 μm) was used with helium as the carrier gas. Samples were injected at 70°C, and the temperature was programmed to increase at 20°C/min to 130°C and at 4°C/min to 320°C and held constant for 15 min. Compound identifications were based on mass spectra and retention times. The structures of sn-2- and sn-3-hydroxyarchaeol were confirmed by comparison to standards isolated from methanogens (K.-U. Hinrichs et al., unpublished data). Abundances of compounds were determined by GC with a flame ionization detector and the run conditions described above. Quantification was based on comparison of peak areas to standards [2,3-dimethyl-5-(1,1-d2-hexadecyl)thiophene] added to the sample after column chromatography.
Isotope ratio monitoring GC-MS.
Isotope ratio monitoring GC-MS was performed on a Finnigan Delta C device and used to determine compound-specific δ13C values. The GC conditions are the same as those used during GC-MS analyses. The δ13C value of bishomohopanoic acid was determined by measurement of a derivatized total lipid extract. δ13C values are expressed against virtual Pee Dee Belemnite (VPDB), have been corrected for the addition of carbon during derivatization, and have an error of less than ±1.0‰, unless otherwise noted (based on analytical accuracy and precision of measurements of coinjected standards—C20 and C24 perdeuterated n-alkanes—and considering the probable influence of coelution in some cases).
MPN counts.
Most probable number (MPN) counts were performed as follows. Surface sediment samples collected via the Nautile were suspended (1:10 [vol/vol]) in basal media (i.e., growth media without substrates added) for the functional groups of interest aboard the Nadir. For anaerobic organisms, the headspace of the suspension was kept anoxic by flushing with nitrogen gas. Following suspension, the inoculations were sonicated three times at 10 s (frequency, 41.7 kHz, 100 W) to detach microorganisms from the mineral matrix. After precipitation of the sediment, the supernatant was used in dilution series (up to a dilution of 108, 3, 5, or 8 replicates [see below]). Selective growth media and specific incubation conditions were used for methanogenic archaea (medium prepared according to reference 22, final volume of 10 ml in 18-ml Hungate tubes with H2/CO2 headspace, five replicates), sulfate reducers (medium prepared according to reference 35, final volume of 10 ml in 18-ml Hungate tubes with N2 headspace, five replicates), colorless sulfur bacteria (medium prepared according to reference 35, with a final volume of 200 μl in microtiter plates with air headspace, eight replicates) and methane-oxidizing bacteria (medium prepared according to reference 37, with a final volume of 20 ml in 120-ml butyl-rubber-stoppered crimp-seal vials with air headspace, three replicates). The dilution series were incubated for at least 10 weeks at in situ temperature (14°C), after which growth was evaluated. The presence of sulfide-oxidizing (colorless sulfur) bacteria was ascertained by color changes in response to pH variations, that of sulfate-reducing bacteria was ascertained by a measured increase in sulfide concentration, that of methanogens was ascertained by an increase in the concentration of methane, and that of aerobic methanotrophs was ascertained by a decrease in the concentration of methane.
RESULTS AND DISCUSSION
Geochemical background.
Mud volcanoes in the Mediterranean Sea appear to be caused by tectonic compression resulting in the extrusion of methane-rich sediments (mud breccias) (21, 38). The methane-rich character of extruded mud breccias has been established by high methane concentrations at depth (> ca. 1 m) in multiple cores from the Olimpi mud volcano area (10). It is unlikely that this methane is generated in the mud breccia after emplacement, because methane concentrations appear to decrease with increasing age of the mudflow.
There is considerable evidence that much of the extruded methane is oxidized by anaerobic processes. Indirect evidence for methane oxidation derives from the widespread occurrence of authigenic carbonate in mud breccia profiles and abundant 13C-depleted carbonate crusts at the sediment-water interface (many crusts have δ13C values below −20‰ [unpublished results of the MEDINAUT investigation])—presumably precipitated from inorganic carbon-rich waters generated during methane oxidation. Second, in multiple cores collected from Ocean Drilling Program sites 970 and 971 (10) (Milano and Napoli domes of the Olimpi field) and during the 1999 Medineth cruise (unpublished data from the Milano, Napoli, and Moscow domes of the Olimpi field and Amsterdam, Kula, and Kazan domes of the Anaximander field), methane concentrations increase with depth by as much as 4 orders of magnitude in the upper 1 m of the mud breccias. In most cases, this appears to be associated with the zone of sulfate reduction (7) and results in the generation of inorganic carbon and hydrogen sulfide. Such geochemical profiles are expected if methane is oxidized by an anaerobic consortium of methanogens and sulfate-reducing bacteria (9, 11, 14). It is highly unlikely that the methane is oxidized aerobically, because oxygen did not penetrate more than 2 cm into mud volcano sediments investigated during the MEDINAUT cruise.
Molecular biogeochemistry of active sites.
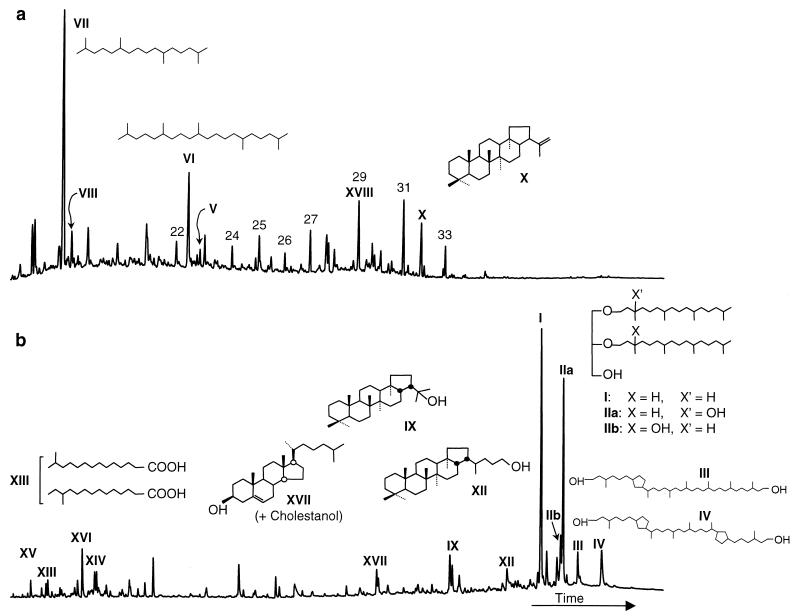
Mud volcano sediments contain abundant biomarkers diagnostic of archaea and, specifically, methanogens (Fig. 1). In a sample collected directly from a seep on the Napoli mud volcano (MN16CC1), the two most abundant compounds are diphytanylglycerol diether (archaeol, compound I), a lipid diagnostic of archaea (19), and sn-3-hydroxyarchaeol (compound IIa), with lesser amounts of sn-2-hydroxyarchaeol (compound IIb). Hydroxyarcheaol is found predominantly in methanogens (20), particularly in the orders Methanosarcinales and Methanococcales (3, 20, 32). Moreover, 2,6,10,15,19-pentamethylicosane (PMI; compound VI) (5) and PMI with one to five double bonds (denoted as a group by V), observed only in cultures of Methanosarcina mazei and Methanolobus bombayensis (30), were identified and are consistent with a predominance of methanogenic archaea in this sample. Other abundant compounds include crocetane (compound VII) (27) and crocetene (compound VIII), as well as biphytanediols (31) with zero, one (compound III), or two (compound IV) cyclopentane rings, all of which have been attributed to the archaea. These results differ from previous investigations of archaeal lipids in cold seeps (9, 13). This is the first report of a co-occurrence of irregular isoprenoids such as PMI and crocetane with archaeol and hydroxyarchaeol. It is also the first reported occurrence of biphytanediols in such settings. In total, unambiguous archaeal lipids comprise over 70% of the polar lipid fraction and over 60% of the total lipid extract.
FIG. 1.
Free lipids in Napoli cold seep sediments. Gas chromatograms showing the predominance of archaeal lipids in the apolar (a) and polar (b) fractions. Structures of key compounds are illustrated; the naturally occurring structures are shown, although the actual compounds analyzed in the polar fraction are trimethylsilyl ethers and methyl esters formed upon derivatization. The structures for compounds V and VIII are not shown, because the precise position of the double bonds has not been determined. In the apolar chromatogram, arabic numerals denote n-alkanes with the given chain length. In both fractions, roman numerals denote the following compounds: archaea—I, archaeol; IIa and IIb, sn-3- and sn-2-hydroxyarchaeol; III and IV, biphytanediols with one and two cyclopentane rings, respectively; V, PMI (diunsaturated); VI, PMI; VII, crocetane; VIII, crocetene; bacteria—IX, diplopterol; X, diploptene; XI, bishomohopanoic acid; XII, bishomohopanol; bacteria and eukaryotes (but likely derived from bacteria in this setting)—XIII, C15 (anteiso plus iso) fatty acid; XIV, C17 (anteiso plus iso) fatty acid; XV, C14 fatty acid; XVI, C16 fatty acid; eukaryotes—XVII, cholesterol plus cholestanol; XVIII, C29 n-alkane.
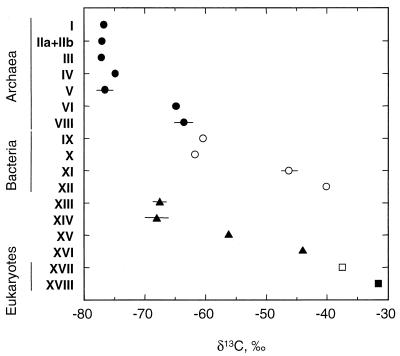
Such a predominance of methanogenic archaea in an environment characterized by a pronounced influx of methane from underlying sediments is both unexpected and enigmatic. Moreover, all biomarkers for methanogenic archaea in the Napoli seep sample have δ13C values below −65‰, significantly lower than those of other compounds in the same sample and profoundly depleted relative to photoautotrophic and some bacterial biomarkers (Fig. 2). This suggests that these methanogenic archaea assimilate rather than produce 13C-depleted methane, a process that has been previously proposed as reverse methanogenesis (14).
FIG. 2.
Contrasting carbon isotopic compositions (with lines indicating measurement error if greater than ± 1.0‰) of archaeal (solid circles), bacterial (open circles), and eukaryotic (squares) lipids in the extractable polar and apolar lipid fractions of the Napoli cold seep. Triangles denote compounds potentially derived from both bacteria and eukaryotes, but probably the former in these settings. Roman numerals denote the same structures as in Fig. 1.
In addition to archaea-derived compounds, a variety of bacterial biomarkers are also strongly depleted in 13C. δ13C values of iso- and anteiso-C15 and -C17 fatty acids (compounds XIII and XIV), compounds abundant in sulfate-reducing bacteria but present only in trace quantities in most chemolithotrophic and methanotrophic bacteria (18), are ca. −68‰, somewhat enriched relative to but within the range of values represented by archaea-derived biomarkers (Fig. 2). Low δ13C values are also observed for diplopterol (compound IX) and diploptene (compound X)—bacterial biomarkers that are not observed in sulfate-reducing bacteria, but which are present in many chemoorganotrophs (29).
Similar carbon isotopic relationships are present in other samples (Table 1). In a bacterial mat collected from beneath a seep-related brine pool on Napoli dome, compounds derived from archaea and bacteria dominate the extractable lipid fraction. Diplopterol and archaeol are among the most abundant compounds in the polar fraction, and PMI and crocetane are the most abundant compounds in the apolar fraction. The δ13C values of methanogen (archaeol, −85‰; PMI, −77‰), inferred sulfate reducer (anteiso-C15 fatty acid, −82‰), and inferred chemoorganotroph (diplopterol, −53‰) biomarkers are all low and comparable to those observed in the seep sample. The same compounds are also present in significant quantities and characterized by low δ13C values in samples collected from bulk brine pool sediments (MN16BB1), a carbonate crust on the Napoli (MN16BT4) mud volcano, and seeps on both the Milano (MN5CC1) and Amsterdam (MN13CC2) mud volcanoes.
MPN counts at active sites.
Table 2 shows the results of the enumeration of functional groups of microorganisms involved in the production and consumption of sulfide and methane in the sediments of cold seeps. Unfortunately, due to poor core recovery, insufficient sediment was collected to perform MPN counts on the Napoli seep sample. Also, because surface sediment samples were the focus of these initial MPN studies, the abundances of methanogenic archaea and sulfate-reducing bacteria, anaerobic organisms, are probably underestimated. Nonetheless, it appears that methanogenic archaea are present in relatively high numbers (between 102 and 104 organisms/cm3 of sediment) at active sites on the Milano (Olimpi region) and Amsterdam (Anaximander region) mud volcanoes. Significantly lower numbers were obtained for one site on the Amsterdam mud volcano and for sediments from beneath the brine pool on Napoli mud volcano. The numbers of sulfate-reducing bacteria are in the same order of magnitude as methanogenic archaea. Of the aerobic organisms, sulfide-oxidizing bacteria were detected on the Milano and Amsterdam mud volcanoes, with maximum numbers up to 2.4 × 104 organisms/cm3 sediment, and methane-oxidizing bacteria were not detected at any of the sites studied despite surface sediment sampling explicitly designed to ascertain the presence of such organisms.
TABLE 2.
MPN values for sulfate-reducing bacteria, sulfide-oxidizing bacteria, methanogenic archaea, and methane-oxidizing bacteria in sediments related to cold seeps at Mediterranean mud volcanos
| Volcano | Sample | MPN ofa:
|
|||
|---|---|---|---|---|---|
| Sulfate-reducing bacteria (5 replicates) | Sulfide-oxidizing bacteria (8 replicates) | Methanogenic archaea (5 replicates) | CH4-oxidizing bacteria (3 replicates) | ||
| Napoli | Brine | 4.0 × 101 (1.0 × 101–1.8 × 102) | NDb | 2.0 × 101 (0.4 × 101–5.0 × 101) | ND |
| Milano | Seep | 4.9 × 104 (1.6 × 104–1.6 × 105) | ND | 3.0 × 102 (1.0 × 102–7.0 × 102) | ND |
| Seepc | 1.4 × 104 (5.0 × 103–3.8 × 104) | 1.1 × 103 (5.0 × 102–2.5 × 103) | 9.1 × 104 (2.8 × 104–3.0 × 105) | ND | |
| Amsterdam | Seep | 0.9 × 101 (0.2 × 101–4.0 × 101) | ND | 9.0 × 101 (2.0 × 101–4.0 × 102) | ND |
| Seepc | 9.0 × 103 (2.0 × 103–3.9 × 104) | 2.4 × 104 (9.8 × 103–5.9 × 104) | 2.0 × 102 (4.0 × 101–5.0 × 102) | ND | |
Values represent the number of organisms per cubic centimeter of sediment, with 95% confidence limit shown in parentheses.
ND, not determined (no growth observed).
Sample for which lipid analyses were not performed.
Mud breccia profiles.
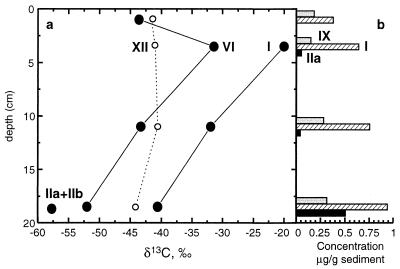
We also examined a depth profile (Fig. 3) collected from a mud breccia (MN16CC2) 1 m from the Napoli seep described above, but where the methane flux to surface sediments appears to be significantly lower. Because mud breccias are deposited as episodic events, depth profiles of compound distributions should not be interpreted as representing a stratigraphic record. Rather, they likely record the response of microorganisms to current geochemical conditions. Thus, these settings are ideal for studying the distribution and carbon isotopic compositions of compounds generated solely under anaerobic conditions. In the Napoli mud breccia profile, the abundances of archaeol, PMI, and hydroxyarchaeol increase with increasing depth. Similarly, in a mud breccia profile developed for the Milano mud volcano, archaeol and hydroxyarchaeol are absent in the upper 10 cm, but are present in a sample from a depth of 24 to 27 cm. In the deepest samples at both sites, the methanogen-derived biomarkers are depleted in 13C (Table 1), suggesting that these compounds are generated during methane consumption. Thus, in two distinct sites, methanotrophic activity is concentrated below the maximum depth of oxygen penetration (<2 cm). Additionally, in the Napoli profile, diplopterol and fatty acid concentrations also increase with depth, providing further evidence that these compounds are related to a consortium of prokaryotes that includes methanogens.
FIG. 3.
Napoli mud breccia depth profile. Profiles from core MN16CC2 show decreases in PMI (compound VI) and archaeol (compound I) δ13C values with depth (a) and concomitant increases in the abundances of archaeal compounds (VI, I, and, especially, IIa [sn-3-hydroxyarchaeol]) and diplopterol (compound IX [bacterial]) (b). The concentration of 13C-depleted lipids below the maximum depth of oxygen penetration (<2 cm) provides direct evidence for anaerobic methane oxidation.
The δ13C values of archaeol and PMI decrease significantly with depth in the Napoli mud breccia (Fig. 3). Assuming that archaea in shallower sediments also assimilate methane, the biomarker δ13C values indicate that methane δ13C values also decrease with depth. In other words, methane δ13C values increase as the distance from the methane source (underlying sediments) increases. Because methane oxidation results in 13C enrichment of the residual methane pool, the observed trend is expected if extensive anaerobic methane oxidation is occurring. The organisms responsible for anaerobic methane oxidation have yet to be isolated and studied, and the isotope effect of this process is unknown. Nonetheless, the isotope effect is almost certainly negative, and if we make the tenuous assumption that the values for anaerobic and aerobic methane oxidation are similar (ca. −9‰) (1), approximately 95% of the methane must be oxidized to explain the observed 30‰ shift. It is likely that carbon isotope fractionation during methanotrophy is highly variable (17), and the value of 95% should be considered only a first-order estimate. Nonetheless, it is probably a minimum estimate, because it ignores methane oxidation that occurred at depths greater than 20 cm. In fact, methane oxidation at greater depths is a likely explanation for the approximately 30‰ difference between archaeal lipid δ13C values in the lowermost mud breccia sample and the nearby seep. To explain a 60‰ change in methane δ13C values, at least 99% of the methane must be oxidized. Although these estimates are tentative, they strongly agree with methane profiles generated during the recent Medineth cruise. In contrast to archaeal lipids, bishomohopanol δ13C values do not change (and its abundance decreases with depth), confirming that this compound is derived neither directly nor indirectly from methanotrophic activity.
Interpretation.
It is unlikely that the low δ13C values of methanogen-derived biomarkers arise during methanogenic metabolism in these settings. Such 13C-depleted biomarker values can arise during methylotrophic methanogenesis utilizing organic substrates (34), but that would require extensive methane oxidation by either bacteria or other archaea to provide substrate carbon. (Exogenous organic carbon inputs appear to be small [36] and are not a likely source of substrate carbon.) Multiple lines of evidence indicate that aerobic methanotrophy by bacteria does not provide substrates for methanogenesis. Diagnostic fatty acids (23) and methylhopanoids (33, 39) of methanotrophic bacteria were not observed, and MPN analyses of multiple samples failed to produce any evidence for the presence of aerobic methanotrophs. Bishomohopanol (compound XII), a nonspecific bacterial biomarker, is present, but in a much lower concentration than archaeal lipids, and this compound has a significantly higher δ13C value than expected for a methanotroph (Fig. 2). The δ13C value for the hopanoid diplopterol would be consistent with an aerobic methanotroph source (Fig. 2), but in two mud breccia profiles, the abundance of this compound is greatest below the maximum depth of oxygen penetration, precluding an origin from aerobic bacterial methanotrophs. Finally, the geochemical evidence strongly suggests that methane is oxidized anaerobically. Thus, aerobic methanotrophic bacteria are present in either low abundances or absent, are probably not responsible for significant methane oxidation, and cannot supply substrates for methanogenesis.
Methanogens can also employ CO2 as a substrate; given the evidence for CO2 generation by methane oxidation, inorganic carbon could have very low δ13C values, and methanogens utilizing such carbon could likewise have low δ13C values. However, this explanation implicitly requires that some other organism is responsible for methane oxidation to generate such depleted inorganic carbon. Based on the above discussion, this organism is not likely an aerobic bacterial methanotroph. However, it has been proposed that a new order of obligately methanotrophic archaea distinct from methanogens exists in California methane seeps (13). Currently, there is no direct evidence that members of the newly discovered order of archaea in the California seeps are indeed the primary methane oxidizers at that site or that they are obligate methanotrophs. Nonetheless, it is possible that obligately methanotrophic archaea do exist; if such organisms are present in our samples, they could oxidize methane and generate CO2 through an as-yet-undetermined pathway. This depleted CO2 could then be assimilated by the methanogens and result in the observed 13C depletion of methanogen biomarkers.
Alternatively, it is possible that nominally methanogenic archaea operating in reverse (14) are responsible for the bulk of methane oxidation in these settings. The low δ13C values of such highly diagnostic methanogenic archaeal biomarkers as polyunsaturated PMI and hydroxyarchaeol are entirely consistent with this explanation. They are also consistent with the MPN counts, which clearly indicate that organisms capable of methanogenesis are present in these settings. Moreover, these findings sustain several previous investigations that strongly indicate that anaerobic methane oxidation is accomplished by a consortium of prokaryotes (9, 11, 14). Because of the abundance of methanogen biomarkers and the similarity of methanogen and less-specific archaeal biomarker δ13C values, we believe that reverse methanogenesis is a dominant pathway for methane oxidation in these settings. However, it is also possible that both facultative and obligate methane-oxidizing archaea are present in our samples, perhaps accounting for the variability in lipid distributions and MPN counts among different sites, and this will be discussed in future work.
Reverse methanogenesis as a reaction, whether employed by facultative or obligate methanotrophs, probably occurs in tandem with sulfate reduction (14). The latter reaction consumes H2 and allows methane oxidation to remain thermodynamically favorable under anoxic conditions (14). MPN analyses, very-low-sulfate pore water concentrations (7), geochemical profiles of both sulfate and HS− obtained during the Medineth investigation, and the presence of elemental sulfur in cores from throughout the sampling area (this work) clearly indicate that sulfate reducers are abundant and active in these sediments. Although it is unclear what would serve as the carbon substrate for such sulfate reducers, carbon ultimately derived from 13C-depleted methane is the most likely source in these settings. This provides an explanation for the low δ13C values observed for the biomarkers for sulfate-reducing bacteria, iso- and anteiso-C15 and -C17 fatty acids (Fig. 2).
Although some sulfate-reducing bacteria are facultatively autotrophic, sulfate reduction would be facilitated in these sediments by a supply of organic substrates. Chemoorganotrophic bacteria such as acetogens could consume methanogen biomass anaerobically and play an important role in the generation of organic substrates such as acetate or propionate. The presence of such organisms could explain the low δ13C values of diplopterol and diploptene. Hopanoid compounds have not yet been observed in anaerobic bacteria; nonetheless, the abundance profiles of these compounds in the mud breccia (Fig. 3) clearly indicate that these compounds are generated at depth and under anoxic conditions. The co-occurrence and predominance of 13C-depleted diplopterol, iso- and anteiso-C15 and -C17 fatty acids, and methanogen biomarkers in multiple seep samples, a brine sample, a carbonate crust, and a mat less than 0.5 cm thick suggest a close coupling of methanotrophy, sulfate reduction, and chemoorganotrophy. Ultimately, then, sulfate serves as the terminal electron acceptor for methane oxidation by a complex consortium of archaea and bacteria.
In addition to the several settings investigated on the Napoli mud volcano, similar patterns of 13C depletion in biomarkers for methanogens and bacteria were observed in samples collected from two sites on the Milano mud volcano, which like the Napoli volcano is part of the Olimpi field, and one site on the Amsterdam mud volcano, which is located nearly 500 km to the east in the Anaximander mountains. At all three sites, depleted methanogen biomarkers are present, and in two of the three sites, archaeal lipids are the most abundant compounds (Table 1). Thus, anaerobic methane oxidation by a consortium of prokaryotes is a widespread and important process in Mediterranean mud volcano sediments, and, in sites of highly localized methane release, methanogens appear to account for the vast majority of prokaryotic biomass.
The implications of these results are profound on both a local and global basis. In mud volcano settings, methanogens appear to play a significant but poorly understood role in regulating the methane flux into Mediterranean bottom waters. It appears that a significant portion—potentially the majority—of methane released from Mediterranean mud volcano sediments is consumed by these organisms. If so, a diffusive flux of methane released by increasing bottom water temperatures (28) and subsequent clathrate dissolution could be largely consumed anaerobically, mitigating its impact on bottom water redox states and methane flux to the atmosphere.
ACKNOWLEDGMENTS
We are indebted to the captain and crew of the R/V Nadir and the submersible Nautile. We are also grateful to the crew of the R/V Logachev and the Medineth scientific party. We are particularly grateful to S. K. Heijs, who assimilated and assisted in the interpretation of MPN counts. Two anonymous reviewers provided very useful comments on the manuscript.
We also thank The Research Council for Earth and Life Sciences (ALW) of the Netherlands Organization for Scientific Research (NWO) for their support of this work (grants NWO 750.199.01 and ALW 809.63.010).
Footnotes
This is NIOZ publication number 3439.
REFERENCES
- 1.Alperin M J, Reeburgh W S, Whiticar M J. Carbon and hydrogen isotope fractionation resulting from anaerobic methane oxidation. Global Biogeochem Cycles. 1988;2:279–288. [Google Scholar]
- 2.Blair N E, Aller R C. Anaerobic methane oxidation on the Amazon shelf. Geochim Cosmochim Acta. 1995;59:3707–3715. [Google Scholar]
- 3.Boone D R, Whitman W B, Rouvière P. Microbiology: diversity and taxonomy of methanogens. In: Ferry J G, editor. Methanogenesis: ecology, physiology, biochemistry, and genetics. New York, N.Y: Chapman and Hall; 1993. pp. 35–80. [Google Scholar]
- 4.Borowski W S, Paull C K, Ussler W., III Marine porewater sulfate profiles indicate in situ methane flux from underlying gas hydrate. Geology. 1996;24:655–658. [Google Scholar]
- 5.Brassell S C, Wardroper A M K, Thomson I D, Maxwell J R, Eglinton G. Specific acyclic isoprenoids as biological markers of methanogenic bacteria in marine sediments. Nature. 1981;290:693–696. doi: 10.1038/290693a0. [DOI] [PubMed] [Google Scholar]
- 6.Burns S J. Carbon isotopic evidence for coupled sulfate reduction-methane oxidation in Amazon Fan sediments. Geochim Cosmochim Acta. 1998;62:797–804. [Google Scholar]
- 7.de Lange G, Brumsack H-J. The occurrence of gas hydrates in Eastern Mediterranean mud dome structures as indicated by pore-water composition. In: Henriet J-P, Mienert J, editors. Gas hydrates: relevance to world margin stability and climate change. Special Publications 137. London, United Kingdom: Geological Society; 1998. pp. 167–175. [Google Scholar]
- 8.Dickens G R, O'Neil J R, Rea D K, Owen R M. Dissociation of oceanic methane hydrate as a cause of the carbon isotope excursion at the end of the Paleocene. Paleoceanography. 1995;10:965–971. [Google Scholar]
- 9.Elvert M, Suess E, Whiticar M J. Anaerobic methane oxidation associated with marine gas hydrates: superlight C-isotopes from saturated and unsaturated C20 and C25 irregular isoprenoids. Naturwissenschaften. 1999;86:295–300. [Google Scholar]
- 10.Emeis K-C, Robertson A H F, Richter C, et al. Proceedings of the ODP, Initial Reports, no. 160. College Station, Tex: Ocean Drilling Program; 1996. [Google Scholar]
- 11.Harder J. Anaerobic methane oxidation by bacteria employing 14C-methane uncontaminated with 14C-carbon monoxide. Mar Geol. 1997;137:13–23. [Google Scholar]
- 12.Henriet J-P, Mienert J, editors. Gas hydrates: relevance to world margin stability and climate change. Special Publications 137. London, United Kingdom: Geological Society; 1998. [Google Scholar]
- 13.Hinrichs K-U, Hayes J M, Sylva S P, Brewer P G, DeLong E F. Methane-consuming archaebacteria in marine sediments. Nature. 1999;398:802–805. doi: 10.1038/19751. [DOI] [PubMed] [Google Scholar]
- 14.Hoehler T M, Alperin M J, Albert D B, Martens C S. Field and laboratory studies of methane oxidation in an anoxic marine sediment: evidence for a methanogen-sulfate reducer consortium. Global Biogeochem Cycles. 1994;8:451–463. [Google Scholar]
- 15.Iverson N, Jørgensen B B. Anaerobic methane oxidation rates at the sulfate-methane transition in marine sediments from Kattegat and Skagerrak (Denmark) Limnol Oceanogr. 1985;30:944–955. [Google Scholar]
- 16.Jahnke L L, Summons R E, Dowling L M, Zahiralis K D. Identification of methanotrophic lipid biomarkers in cold-seep mussel gills: chemical and isotopic analysis. Appl Environ Microbiol. 1995;61:576–582. doi: 10.1128/aem.61.2.576-582.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahnke L L, Summons R E, Hope J M, Des Marais D J. Carbon isotopic fractionation in lipids from methanotrophic bacteria. II. The effects of physiology and environmental parameters on the biosynthesis and isotopic signatures of biomarkers. Geochim Cosmochim Acta. 1999;63:79–93. doi: 10.1016/s0016-7037(98)00270-1. [DOI] [PubMed] [Google Scholar]
- 18.Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koga Y, Nishihara M, Morii H, Akagawa-Matsushita M. Ether lipids of methanogenic bacteria: structures, comparative aspects, and biosyntheses. Microbiol Rev. 1993;57:164–182. doi: 10.1128/mr.57.1.164-182.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koga Y, Morii H, Akagawa-Matsushita M, Ohga M. Correlation of polar lipid composition with 16S rRNA phylogeny in methanogens. Further analysis of lipid component parts. Biosci Biotechnol Biochem. 1998;62:230–236. doi: 10.1271/bbb.62.230. [DOI] [PubMed] [Google Scholar]
- 21.Limonov A F, Woodside J M, Cita M B, Ivanov M K. The Mediterranean Ridge and related mud diapirism: a background. Mar Geol. 1996;132:7–20. [Google Scholar]
- 22.Ni S, Boone D R. Isolation and characterization of a dimethyl sulfide-degrading methanogen, Methanolobus siciliae HI350, from an oil well, characterization of M. siciliae T4/MT, and emendation of M. siciliae. Int J Syst Bacteriol. 1991;41:410–416. doi: 10.1099/00207713-41-3-410. [DOI] [PubMed] [Google Scholar]
- 23.Nichols P D, Smith G A, Antworth C P, Hanson R S, White D C. Phospholipid and lipopolysaccharide normal and hydroxy fatty acids as potential signatures for methane-oxidizing bacteria. FEMS Microbiol Ecol. 1992;31:327–335. [Google Scholar]
- 24.Petit J R, et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature. 1999;399:429–436. [Google Scholar]
- 25.Reeburgh W S. Anaerobic methane oxidation: rate depth distributions in Skan Bay sediments. Earth Planet Sci Lett. 1980;46:345–352. [Google Scholar]
- 26.Reeburgh W S. ‘Soft spots’ in the global methane budget. In: Lidstrom M E, Tabita F R, editors. Microbial growth on C1 compounds. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 334–342. [Google Scholar]
- 27.Robson S N, Rowland S J. Synthesis, chromatographic and spectral characterisation of 2,6,11,15-tetramethylhexadecane (crocetane) and 2,6,9,13-tetramethyltetradecane: reference acyclic isoprenoids for geochemical studies. Org Geochem. 1986;20:1093–1098. [Google Scholar]
- 28.Roether W, et al. Recent changes in Eastern Mediterranean deep waters. Science. 1996;271:333–335. [Google Scholar]
- 29.Rohmer M, Bouvier-Nave P, Ourisson G. Distribution of hopanoid triterpenes in prokaryotes. J Gen Microbiol. 1984;130:1137–1150. [Google Scholar]
- 30.Schouten S, van der Maarel M J E C, Huber R, Sinninghe Damsté J S. 2,6,10,15,19-Pentamethylicosenes in Methanolobus bombayensis, a marine methanogenic archaeon, and in Methanosarcina mazei. Org Geochem. 1997;26:409–414. [Google Scholar]
- 31.Schouten S, Hoefs M J L, Koopmans M P, Bosch H-J, Sinninghe Damsté J S. Structural characterization, occurrence, and fate of archaeal ether-bound acyclic and cyclic biphytanes and corresponding diols in sediments. Org Geochem. 1998;29:1305–1319. [Google Scholar]
- 32.Sprott G D, Dicaire C J, Choquet C G, Patel G B, Ekiel I. Hydroxydiether lipid structures in Methanosarcina spp. and Methanococcus voltae. Appl Environ Microbiol. 1993;59:912–914. doi: 10.1128/aem.59.3.912-914.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Summons R E, Jahnke L L. Identification of the methylhopanes in sediments and petroleum. Geochim Cosmochim Acta. 1990;54:247–251. doi: 10.1016/0016-7037(90)90212-4. [DOI] [PubMed] [Google Scholar]
- 34.Summons R E, Franzmann P D, Nichols P D. Carbon isotopic fractionation associated with methylotrophic methanogenesis. Org Geochem. 1998;28:465–476. [Google Scholar]
- 35.Visscher P T, Prins R A, Van Gemerden H. Rates of sulfate reduction and thiosulfate consumption in a marine microbial mat. FEMS Microbiol Ecol. 1992;86:283–294. [Google Scholar]
- 36.Wakefield S J, O'Sullivan G M. The inorganic geochemistry of a Mediterranean Ridge mud breccia. Mar Geol. 1996;132:203–214. [Google Scholar]
- 37.Whittenbury R, Phillips K S, Wilkinson J F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970;61:205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- 38.Woodside J M, Ivanov M K, Limonov A F . Shipboard Scientists of the Anaxiprobe Expeditions. Shallow gas and gas hydrates in the Anaximander Mountains region, eastern Mediterranean Sea. In: Henriet J-P, Mienert J, editors. Gas hydrates: relevance to world margin stability and climate change. Special Publications 137. London, United Kingdom: Geological Society; 1998. pp. 177–193. [Google Scholar]
- 39.Zundel M, Rohmer M. Prokaryotic triterpenoids. 1. 3β-Methylhopanoids from Acetobacter species and Methylococcus capsulatus. Eur J Biochem. 1985;150:23–27. doi: 10.1111/j.1432-1033.1985.tb08980.x. [DOI] [PubMed] [Google Scholar]