Abstract
Background
Peritoneal metastasis often occurs in patients with colorectal cancer peritoneal metastasis, and the prognosis is poor. A large body of evidence highlights the beneficial effects of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) on survival, but to date, there is little consensus on the optimal treatment strategy for patients with colorectal cancer peritoneal metastasis. The purpose of this study is to evaluate the impact of CRS + HIPEC on survival and provide reference for the treatment of patients with colorectal cancer peritoneal metastasis.
Methods
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The PubMed, Embase, Cochrane, Web of Knowledge, and ClinicalTrials.gov databases were screened from inception of the review to March 11, 2022. Ten studies were included in qualitative and quantitative analysis.
Results
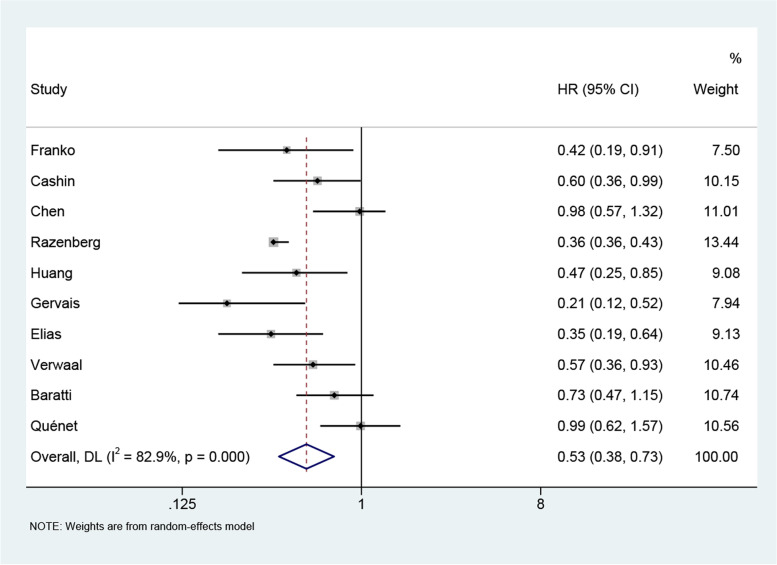
A total of 3200 patients were enrolled in the study, including 788 patients in the CRS and HIPEC groups and 2412 patients in the control group, of which 3 were randomized controlled trials and 7 were cohort studies. The 3 randomized controlled studies were of high quality, and the quality scores of the 7 cohort studies were all 7 or above, indicating high quality. The results showed that the OS of CRS + HIPEC group was higher than that of control group (HR: 0.53, 95% CI: 0.38–0.73; P < 0.00001, I2 = 82.9%); the heterogeneity of the studies was large. The subgroup analysis showed that the OS of CRS and HIPEC group was higher than that of PC group (HR: 0.37, 95% CI: 0.30–0.47; P = 0.215, I2 = 31%) and higher than that in CRS group (HR: 0.73, 95% CI: 0.49–1.07; P = 0.163, I2 = 44.8%); the heterogeneity of the studies was low. In the OPEN group, the OS of THE CRS and HIPEC groups was higher than that in the control group (HR: 0.51, 95% CI: 0.38–0.70; P = 0.353, I2 = 3.9%); OPEN group showed lower heterogeneity. The OS of 60–100-min group was higher than that in the control group (HR: 0.65, 95% CI: 0.49–0.88; P = 0.172, I2 = 37.4%); the heterogeneity of the studies was low. Sensitivity analysis showed that there was no significant difference in the results of the combined analysis after each study was deleted. The results of publication bias showed that the P-value of Egger and Begg tests was 0.078 > 0.05, indicating that there is no publication bias.
Conclusions
CRS + HIPEC can improve the survival rate of patients with colorectal cancer peritoneal metastasis
Keywords: Colorectal cancer, Peritoneal metastasis, Cytoreductive surgery, Hyperthermic intraperitoneal chemotherapy, Meta-analysis
Introduction
Colorectal cancer is responsible for close to 10% of cancer diagnoses and deaths throughout the world, with about 2 million new diagnoses per year [1]. Of these, between 20 and 25% of patients have advanced cancer, with the same numbers developing metastases after surgery [2]. Metastasis to the peritoneum and liver is common [3, 4]. Peritoneal metastases (PM) usually present with relatively nonspecific symptoms and are thus often only detected at advanced stages; thus, PM are associated with poor outcomes [5]. If untreated, such patients typically do not live longer than a year [6]. Systemic treatment for PM has limited success, often only increasing the median survival from 12 to 16 months [7]. In this context, cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been found to be successful for treating colorectal cancer accompanied by PM [8], and this combination, although initially developed for treating pseudomyxoma peritonei, is now accepted as a standard surgical treatment for all malignancies of the peritoneal surface regardless of their origin [9]. Patients have been found to respond well to this treatment, with median overall survival (OS) rates increasing to between 20 and 63 months and 5-year OS rates of 23–52% [10, 11]. Specific outcomes are associated with various factors representing the severity of the disease, including the peritoneal cancer index (PCI), the completeness of cytoreduction (CC), and tumor histopathology [12]. The success of CRS + HIPEC is dependent on the careful selection of suitable patients (e.g., PCI < 20), in whom the combined therapy has been reported to be better than the best current systemic chemotherapies [13]. However, the indications for CRS + HIPEC used in different centers vary considerably. Eastern Cooperative Oncology Group or World Health Organization indices > 2, together with the presence of critical comorbidities, such as severe cardiopulmonary or renal failure, usually represent contraindications for patient selection [14]. Age is also a factor, although there are no specific contraindications, and the presence of liver metastases complicates the issue. Several recent reports have indicated the effectiveness of liver metastasis resection in improving survival without causing additional morbidity [15, 16], although the optimal number of liver metastases influencing the effectiveness of CRS + HIPEC remains controversial [17]. However, there are limited data on the suitable treatment of patients with PM. Currently, the standard treatment is a combination of systemic and palliative therapy, and there is little consensus on the optimal treatment for these patients. Thus, the objective of the current systematic review and meta-analysis was to review and analyze studies on the use and effectiveness of CRS + HIPEC for treating patients with colorectal cancer and PM and to provide a reference for clinical practice.
Methods
Search strategy
This study conforms with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The protocol for this systematic review was registered on INPLASY (INPLASY202230093) and is available in full on inplasy.com (10.37766/inplasy2022.3.0093).
The PubMed, Embase, Cochrane, Web of Knowledge, and ClinicalTrials.gov databases were searched from inception to March 11, 2022. Articles in all languages were searched. The complete search terms used for PubMed were as follows: ((((((((((((Hyperthermic Intraperitoneal Chemotherapy [Title/Abstract]) OR (Chemotherapy, Hyperthermic Intraperitoneal[Title/Abstract])) OR (Intraperitoneal Chemotherapy, Hyperthermic[Title/Abstract])) OR (HIPEC[Title/Abstract])) OR (Hot Chemotherapy[Title/Abstract])) OR (Chemotherapy, Hot[Title/Abstract])) OR (Intraperitoneal Hyperthermic Chemotherapy[Title/Abstract])) OR (Chemotherapy, Intraperitoneal Hyperthermic[Title/Abstract])) OR (Chemotherapy, Intraperitoneal Hyperthermic [Title/Abstract])) OR (Intraperitoneal Hyperthermic Chemotherapies[Title/Abstract])) AND ((((((((((((((((Colorectal Neoplasms[Title/Abstract]) OR (Colorectal Neoplasm[Title/Abstract])) OR (Neoplasm, Colorectal[Title/Abstract])) OR (Neoplasms, Colorectal[Title/Abstract])) OR (Colorectal Tumors [Title/Abstract])) OR (Colorectal Tumor[Title/Abstract])) OR (Tumor, Colorectal[Title/Abstract])) OR (Tumors, Colorectal[Title/Abstract])) OR (Colorectal Cancer [Title/Abstract])) OR (Cancer, Colorectal[Title/Abstract])) OR (Cancers, Colorectal[Title/Abstract])) OR (Colorectal Cancers [Title/Abstract])) OR (Colorectal Carcinoma[Title/Abstract])) OR (Carcinoma, Colorectal[Title/Abstract])) OR (Carcinomas, Colorectal[Title/Abstract])) OR (Colorectal Carcinomas[Title/Abstract]))) AND ((cytoreductive surgery[Title/Abstract]) OR (CRS [Title/Abstract]))). All potentially eligible studies were considered, regardless of primary outcomes or language.
Inclusion criteria
A population (P), intervention (I), comparator (C), outcome (O), and study design (S) (PICOS) framework was used to describe the eligibility of studies. Specifically, the criteria below were included:
Population (P): patients with colorectal cancer with PM
Intervention (I): complete CRS + HIPEC
Comparison (C): patients undergoing surgery or any other systemic palliative therapy
Outcomes (O): patient survival outcomes
Study design (S): randomized controlled trials, case-control studies, or cohort studies
Exclusion criteria
Articles that did not contain survival data were excluded, as were studies investigating CRS + HIPEC in primary tumors other than colorectal cancer. Similarly, composite studies that included patients with colorectal cancer or other malignancies but did not report isolated results were considered ineligible.
Data extraction and quality assessment
The literature screening was conducted by two researchers (JL and ARW) independently, through reading the subject, selecting the standard subject, and subsequently reading the abstract and the full text. For randomized controlled studies, the two researchers cross-estimated the quality of the studies using the Jadad scale, including random allocation, randomized hiding, double-blind method setting, and exit and loss to follow-up (score out of 7 points: 1−3 for inferior quality and 4–7 points for good quality), while the evaluation of methodological quality used the method recommended by the Cochrane Review handbook. The Newcastle-Ottawa scale (NOS) was used for quality assessment of case-control and cohort studies; this includes eight items divided into three areas, namely, population selection, comparability, and exposure or outcome evaluation, using a scale of 0–9 points with scores above 5 rated as high quality [18]. Two researchers independently recorded the necessary information from the publications, including details of the first author, publication date, number of subjects, time of enrollment, type of study, treatment details of the control group, and the hazard ratios (HRs) for the experimental and control groups and their 95% confidence intervals (CIs). Any differences between the two researchers were decided by discussion with a third researcher (SQL).
Statistical analysis
The HR and 95% CI values in both groups were pooled and analyzed. If the HR and its 95% CI could not be extracted, data were extracted from survival curves using Engauge Digitizer software and converted. Inter-study heterogeneity was evaluated using the I2 statistic and Cochran’s Q test, with cutoff values of 25%, 50%, and 75% considered as low, moderate, and high, respectively [19]. Sensitivity analysis was performed in relation to the assessed effect sizes and heterogeneity of the studies. The risk of publication bias was assessed using funnel plots, with the asymmetry of the plot indicating potential bias; asymmetry was analyzed by Egger’s and Begg’s tests. Intercept significances were assessed using t-tests (P < 0.05).
Results
Features of the included studies
In all, 923 studies were initially identified. Duplicates between databases were removed, leaving 609 studies that were then screened in terms of titles and abstracts. A further 562 papers were subsequently excluded for not meeting the inclusion criteria, leaving 47 studies. Of these, a further 37 studies were excluded after examination of the full texts for the following reasons: (1) non-colorectal peritoneal metastases; (2) poor-quality studies; (3) not a survival study; and (4) case report. Finally, 10 studies [20–29] were included in the meta-analysis (Fig. 1). These included 3200 patients, with 788 patients in the CRS and HIPEC groups and 2412 patients in the control group. Three studies [22, 27, 29] were randomized controlled trials, and seven [20, 21, 23–26, 28] were cohort studies. The details of the included studies are summarized in Table 1.
Fig. 1.
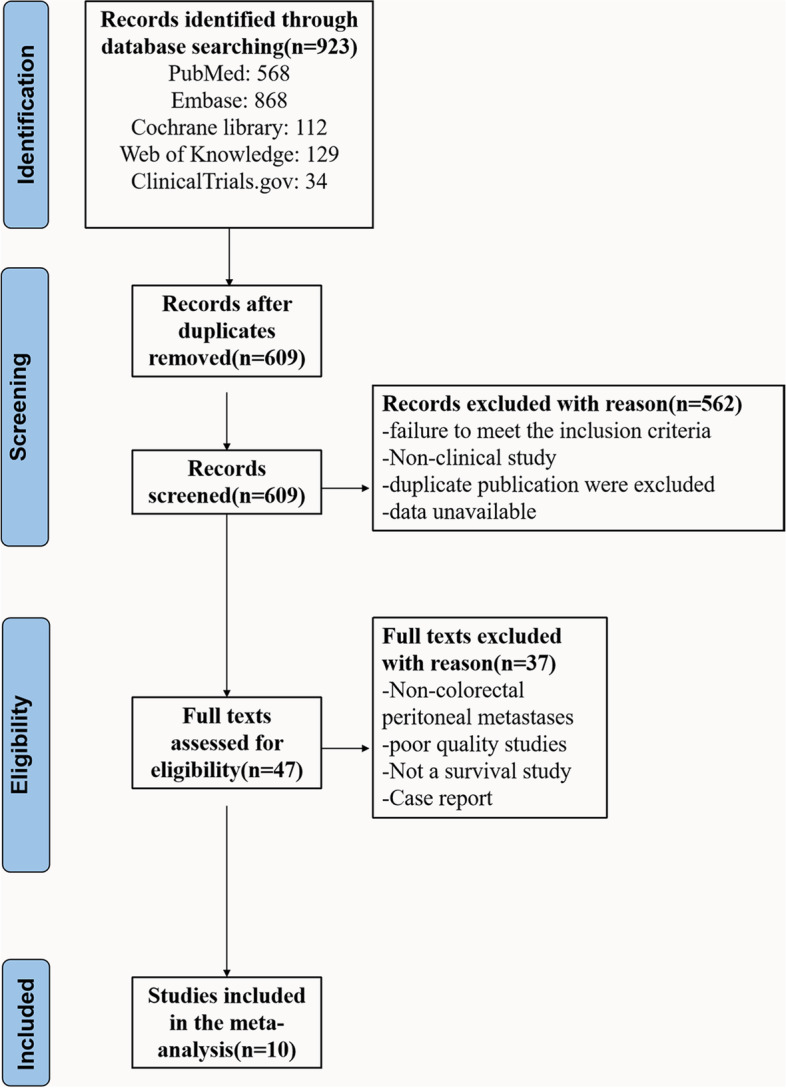
Flow chart of screening strategy for included studies
Table 1.
Main characteristics of all studies included in the meta-analysis
| Author | Year | Country | Enrollment | Type | HIPEC group (n) | Control group (n) | HIPEC characteristics | Control characteristics | HR 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Technique | Time (min) |
|||||||||
| Franko | 2010 | American | 2001–2007 | Cohort | 67 | 38 | Close | 100 | PC | 0.42 (0.19–0.91) |
| Cashin | 2012 | Sweden | 1996–2010 | Cohort | 69 | 57 | Open | 30 | CRS + SPIC | 0.60 (0.36–0.99) |
| Chen | 2020 | China | 2008–2016 | Randomized | 14 | 14 | Close | 90 | HIPEC + dCRS | 0.98 (0.57–1.32) |
| Razenberg | 2015 | Netherlands | 2005–2012 | Cohort | 297 | 1980 | NR | NR | PC | 0.36 (0.36–0.43) |
| Huang | 2014 | China | 2005–2013 | Cohort | 33 | 29 | Close | 90 | CRS | 0.47 (0.25–0.85) |
| Gervais | 2013 | Canada | 2004–2011 | Cohort | 25 | 15 | Close | 30 | PC | 0.21 (0.12–0.52) |
| Elias | 2009 | France | 1998–2003 | Cohort | 48 | 48 | Open | 30 | PC | 0.35 (0.19–0.64) |
| Verwaal | 2008 | Netherlands | 1998–2001 | Randomized | 54 | 51 | Open | 90 | PC | 0.57 (0.36–0.93) |
| Baratti | 2020 | Italy | 2012–2018 | Cohort | 48 | 48 | Close | 60 | CRS | 0.73 (0.47–1.15) |
| Quénet | 2021 | France | 2008–2014 | Randomized | 133 | 132 | NR | 30 | CRS | 0.99 (0.62–1.57) |
SPIC Sequential postoperative intraperitoneal chemotherapy, CRS Cytoreductive surgery, PC Palliative chemotherapy, dCRS Delayed cytoreductive surgery; open, the open Coliseum technique; close, the close Coliseum technique; NR, not reported
Quality assessment of the included studies
The Jadad scale was used to assess the quality of the randomized controlled trials, with scores between 1 and 3 indicating inferior quality and scores between 4 and 7 representing high quality. This evaluation showed that three studies were of high quality (Tables 2 and 3) The NOS, with scores between 5 and 9 indicating good quality, was used for the assessment of case-control and cohort studies and showed that the scores of all seven studies were above 7, indicative of high quality.
Table 2.
Quality assessment of included trials using the Jadad scale
| First author | Type | Random allocation | Randomized hiding | Double-blind method setting | Exit and loss to follow-up | Score |
|---|---|---|---|---|---|---|
| Chen | RCT | 2 | 2 | 2 | 0 | 6 |
| Verwaal | RCT | 1 | 1 | 1 | 1 | 4 |
| Quénet | RCT | 2 | 2 | 2 | 1 | 7 |
RCT Randomized controlled trial
Table 3.
Results of quality assessment using the Newcastle-Ottawa scale for cohort studies
| Study | Selection | Comparability | Outcome | Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | ||
| Franko | ● | ● | ● | ● | ● | ● | ● | ● | 8 |
| Cashin | ● | ● | ● | ● | ● | ● | ● | 7 | |
| Razenberg | ● | ● | ● | ● | ● | ● | ● | 7 | |
| Huang | ● | ● | ● | ● | ● | ● | ● | ● | 8 |
| Gervais | ● | ● | ● | ● | ● | ● | ● | 7 | |
| Elias | ● | ● | ● | ● | ● | ● | ● | ● | 8 |
| Baratti | ● | ● | ● | 1 | ● | ● | ● | ● | 8 |
Meta-analysis
It was found that the OS of the CRS + HIPEC group was higher than that of the control group (HR: 0.53, 95% CI: 0.38–0.73; P < 0.00001, I2 = 82.9%) (Fig. 2). Due to the large heterogeneity of the study, we then performed relevant subgroup analysis. This indicated that the OS of the CRS and HIPEC group was superior to that of the PC group (HR: 0.37, 95% CI: 0.30–0.47; P = 0.215, I2 = 31%) and higher than that of the CRS group (HR: 0.73, 95% CI: 0.49–1.07; P = 0.163, I2 = 44.8%) (Fig. 3A). The heterogeneity of the subgroups was low. We then divided the experimental groups into an OPEN group and a CLOSE group [30] according to the different HIPEC devices used. In the OPEN group, the OS rates of the CRS and HIPEC groups were higher than in the control group (HR: 0.51, 95% CI: 0.38–0.70; P = 0.353, I2 = 3.9%), while in the CLOSE group, the OS rates of the experimental group were higher (HR: 0.53, 95% CI: 0.32–0.87; P = 0.004, I2 = 73.7%). In addition, the OPEN group showed lower heterogeneity (Fig. 3B). After division into various subgroups based on the duration of HIPEC treatment, the 30-min group (HR: 0.48, 95% CI: 0.25 –0.90; P = 0.002, I2 = 80%) and the 60–100-min group (HR: 0.65, 95% CI: 0.49–0.88; P = 0.172, I2 = 37.4%) had longer OS than the control group, while the heterogeneity was lower in the 60–100-min group (Fig. 3C).
Fig. 2.
Meta-analysis of overall survival (OS) of patients with colorectal cancer peritoneal metastasis treated with CRS + HIPEC versus control group
Fig. 3.
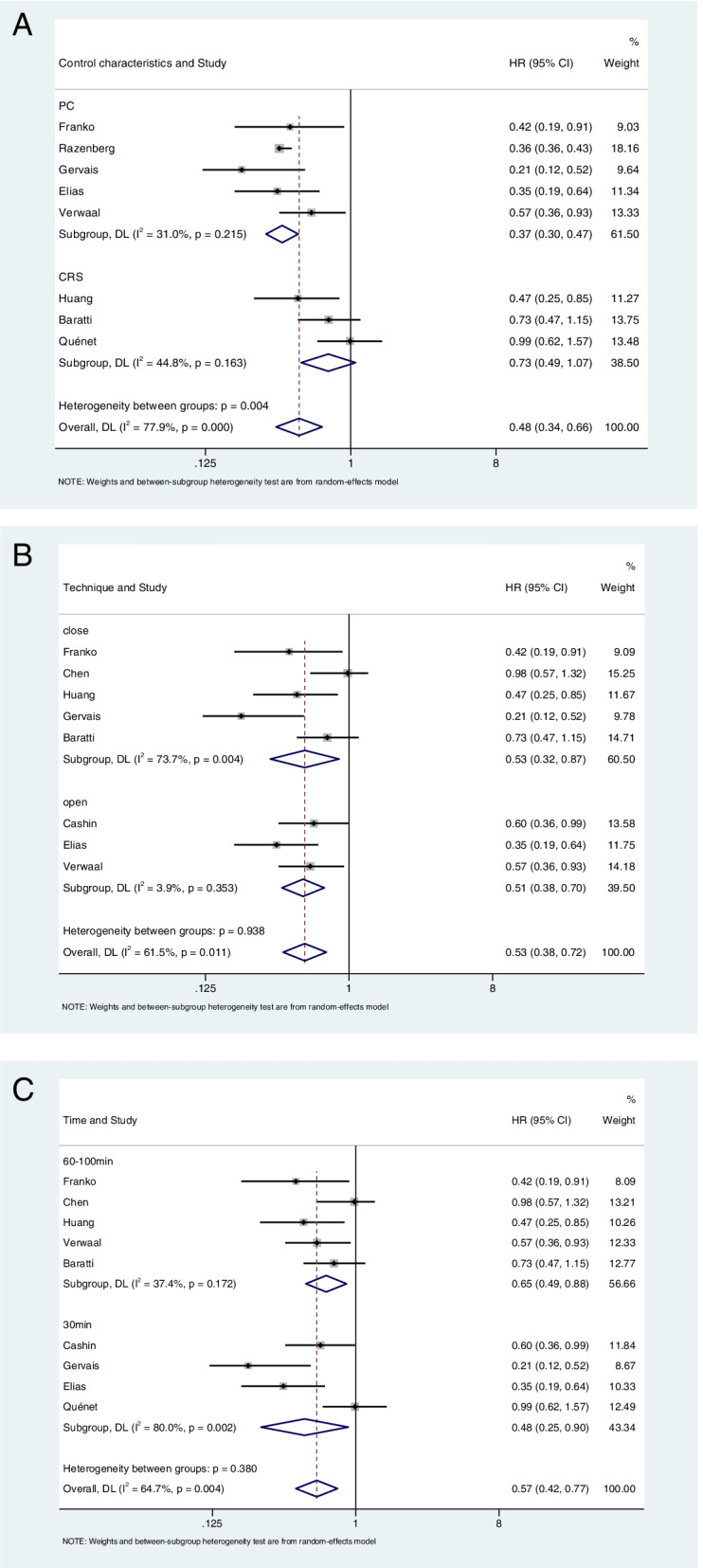
Subgroup analysis of colorectal cancer peritoneal metastasis treated with CRS + HIPEC and control group. A Subgroup analysis of different treatment regimens. B Subgroup analysis of different treatment devices. C Subgroup analysis of different HIPEC time. PC palliative chemotherapy, open the open Coliseum technique, close the close Coliseum technique
Assessment of publication bias
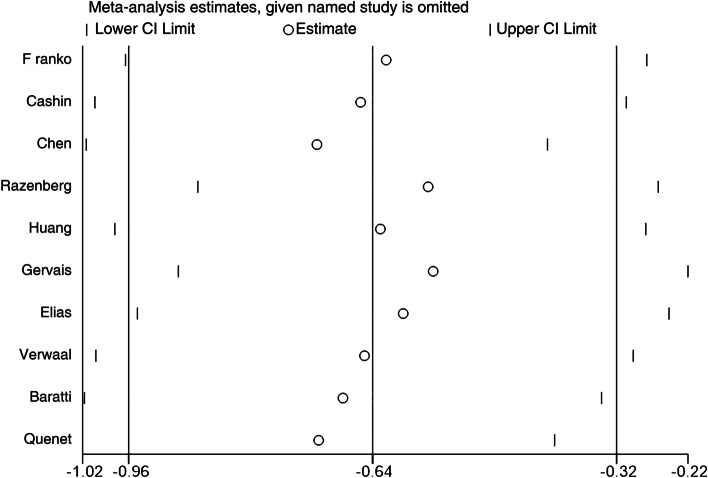
Sensitivity analysis indicated no significant differences in the results of the combined analysis after the deletion of individual studies, showing that the overall results were reliable (Fig. 4). Assessment of publication bias showed that the P-values of the Egger and Begg tests were 0.078 > 0.05. No obvious asymmetry was seen in the Begg funnel plot, indicating an absence of publication bias (Fig. 5).
Fig. 4.
Sensitivity analysis
Fig. 5.
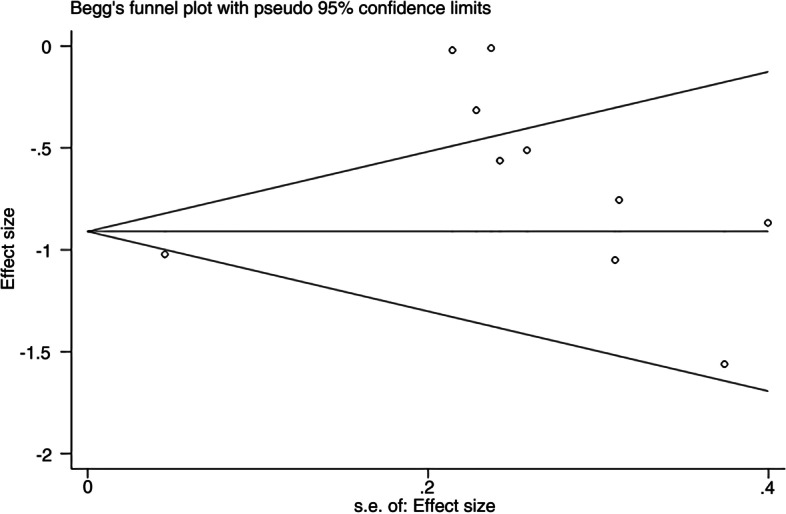
Begg funnel plot for publication bias test
Discussion
The objective of the current systematic review and meta-analysis was to investigate the outcomes of CRS + HIPEC in patients with colorectal cancer and PM. The analysis assessed outcomes in terms of OS, combining HR and 95% CI for the trial and control groups. It was found that the combined use of CRS + HIPEC was superior to both PC and CRS in extending the OS of these patients. These data offer evidence for the effectiveness of CRS + HIPEC for treating patients with colorectal cancer and PM, as well as laying a foundation for future prospective studies in these patients.
The reduction of tumor dimensions has long been recognized as critical in the response of the cancer to therapeutic intervention. CRS involves extensive peritoneal and visceral excision to remove all visible tumor foci, with the goal of minimizing tumor size [31]. Pharmacokinetics has shown that intraperitoneal drug administration is more effective than intravenous administration as the drugs are able to interact directly with the tumor cells while reducing the systemic levels and thus the potential adverse effects of the drugs. HIPEC permits the delivery of high drug concentrations, and their cytotoxicity to tumor cells is increased by hyperthermia [32]. This explains to some extent why the therapeutic effect of CRS and HIPEC is superior to other treatment regimens. Various factors have been found to affect the clinical efficacy of CRS + HIPEC. These include the PCI and CC, as well as the presence of serious adverse events, the status of lymph nodes, the use of systemic chemotherapeutic drugs, and peritoneal carcinomatosis, whether synchronous or metachronous. The Sugarbaker PCI score, ranging from 0 to 39, is the most commonly used PCI standard [33], with scores of 0–19 representing LPCI and those over 20, HPCI [34, 35]. Sugarbaker et al. [36] also reported 5-year OS rates of 50%, 20%, and 0% for patients with scores below 10, between 11 and 20, and over 20, respectively. In terms of CC scores, patients with CC0 experienced better survival outcomes than patients with scores between 1 and 3, with median OS values of 33.0 months and 10.0 months, respectively [37, 38]. However, to eliminate the tumor completely, extensive resection often involving a number of organs and regions of the abdomen is usually required. This may lead to increased blood and fluid loss, disruption of the hemodynamic balance, and an increased likelihood of serious adverse events [39]. In such cases, perioperative morbidity has been found to range between 14.8 and 57.0%, and mortality rates may increase to 12.0% [40]. Two multicenter studies by Glehen et al. [37] and Elias et al. [41] observed the perioperative mortality rates of 4% and 3%, respectively. An additional issue is that the CRS + HIPEC combination has an extended learning curve, which has negatively influenced the clinical popularity of the method [42, 43]. Several studies are currently investigating the factors affecting the posttreatment complications of CRS + HIPEC, aiming to reduce these as far as possible. Rotolo et al. [44] observed that the presence of low skeletal muscle mass at diagnosis influences the development of postoperative complications after CRS in patients with colorectal cancer and PM. Morgan et al. [45] reported that mutation of the RAS gene independently predicted early tumor recurrence after CRS + HIPEC, suggesting that this could be used for the identification of patients who may not benefit from the procedure.
Although we have demonstrated that CRS + HIPEC resulted in a better prognosis for patients with colorectal cancer and PM, the study still has limitations. First, only 10 studies were included, most of which were cohort studies with only three being randomized controlled trials [22, 27, 29]. In terms of subgroup analysis, only three studies compared CRS, and the conclusions drawn from these studies are thus based on limited evidence. Similarly, in the subgroup analysis based on the HIPEC device and treatment duration, although CRS + HIPEC showed better prognosis and lower heterogeneity in the OPEN and 60–100-min groups, the included studies were also limited, and the optimal CRS + HIPEC regimen was not further explored. In terms of publication bias, both the Begg and Egger tests have good sensitivity only when more than 20 studies are included [46], resulting in a low sensitivity result for publication bias. Secondly, when HR and 95% CI values were not provided in included studies, we extracted data through Engauge Digitizer software, which would inevitably lead to some error. Finally, we observed that the HR values of the two included high-quality randomized controlled trials [22, 29] were 0.98 (95% CI: 0.57–1.32) and 0.99 (95% CI: 0.62–1.57), respectively, which did not show satisfactory HR values. However, another randomized controlled trial [28] had an HR value of 0.57 (95% CI: 0.36–0.93). Thus, more analysis of randomized controlled trials is required in the future. All in all, the quality of the included studies was high, which provides evidence supporting the treatment of PM in patients with colorectal cancer by CRS + HIPEC, although further studies are required for verification.
Acknowledgements
Not applicable
Authors’ contributions
JL and SQL contributed to the conception, design, and modification of the study. ARW, XDC, YXZ, and HP extracted the data and organized the database search. JL and ARW performed the statistical analysis. SQL, XDC, YXZ, and HP drafted the manuscript. JL and SQL confirm the authenticity of all the raw data. All authors contributed to manuscript revision and read and approved the submitted version. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors read and approved the final manuscript.
Funding
None
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ji Li and An-Ran Wang contributed equally to this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Kuchel A, Ahern E, Collins S, et al. Trends in epidemiology, treatment and molecular testing of metastatic colorectal cancer in a real-world multi-institution cohort study. Asia Pac J Clin Oncol. 2021;17(1):84–93. doi: 10.1111/ajco.13420. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker PH. Colorectal cancer: prevention and management of metastatic disease. Biomed Res Int. 2014;2014:782890. doi: 10.1155/2014/782890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bijelic L, Ramos I, Goeré D. The landmark series: surgical treatment of colorectal cancer peritoneal metastases. Ann Surg Oncol. 2021;28(8):4140–4150. doi: 10.1245/s10434-021-10049-3. [DOI] [PubMed] [Google Scholar]
- 5.Goéré D, Sourrouille I, Gelli M, et al. Peritoneal metastases from colorectal cancer: treatment principles and perspectives. Surg Oncol Clin N Am. 2018;27(3):563–583. doi: 10.1016/j.soc.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Simkens GA, Razenberg LG, Lemmens VE, et al. Histological subtype and systemic metastases strongly influence treatment and survival in patients with synchronous colorectal peritoneal metastases. Eur J Surg Oncol. 2016;42(6):794–800. doi: 10.1016/j.ejso.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–1719. doi: 10.1016/S1470-2045(16)30500-9. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Hidalgo JM, Rodríguez-Ortiz L, Arjona-Sánchez Á, et al. Colorectal peritoneal metastases: optimal management review. World J Gastroenterol. 2019;25(27):3484–3502. doi: 10.3748/wjg.v25.i27.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klempner SJ, Ryan DP. HIPEC for colorectal peritoneal metastases. Lancet Oncol. 2021;22(2):162–164. doi: 10.1016/S1470-2045(20)30693-8. [DOI] [PubMed] [Google Scholar]
- 10.Hallam S, Tyler R, Price M, et al. Meta-analysis of prognostic factors for patients with colorectal peritoneal metastasis undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. BJS Open. 2019;3(5):585–594. doi: 10.1002/bjs5.50179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flood M, Narasimhan V, Waters P, et al. Survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: a systematic review and discussion of latest controversies. Surgeon. 2021;19(5):310–320. doi: 10.1016/j.surge.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Polderdijk MCE, Brouwer M, Haverkamp L, et al. Outcomes of Combined peritoneal and local treatment for patients with peritoneal and limited liver metastases of colorectal origin: a systematic review and meta-analysis. Ann Surg Oncol. 2022;29(3):1952–1962. doi: 10.1245/s10434-021-10925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yano H. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy for peritoneal metastasis from colorectal cancer. Clin Colon Rectal Surg. 2020;33(6):372–376. doi: 10.1055/s-0040-1714242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klaver CE, Groenen H, Morton DG, et al. Recommendations and consensus on the treatment of peritoneal metastases of colorectal origin: a systematic review of national and international guidelines. Colorectal Dis. 2017;19(3):224–236. doi: 10.1111/codi.13593. [DOI] [PubMed] [Google Scholar]
- 15.El-Nakeep S, Rashad N, Oweira H, et al. Intraperitoneal chemotherapy and cytoreductive surgery for peritoneal metastases coupled with curative treatment of colorectal liver metastases: an updated systematic review. Expert Rev Gastroenterol Hepatol. 2017;11(3):249–258. doi: 10.1080/17474124.2017.1284586. [DOI] [PubMed] [Google Scholar]
- 16.Elias D, Benizri E, Pocard M, et al. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. Eur J Surg Oncol. 2006;32(6):632–636. doi: 10.1016/j.ejso.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Bushati M, Rovers KP, Sommariva A, et al. The current practice of cytoreductive surgery and HIPEC for colorectal peritoneal metastases: results of a worldwide web-based survey of the Peritoneal Surface Oncology Group International (PSOGI) Eur J Surg Oncol. 2018;44(12):1942–1948. doi: 10.1016/j.ejso.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ., 3rd Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116(16):3756–3762. doi: 10.1002/cncr.25116. [DOI] [PubMed] [Google Scholar]
- 21.Cashin PH, Graf W, Nygren P, Mahteme H. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: prognosis and treatment of recurrences in a cohort study. Eur J Surg Oncol. 2012;38(6):509–515. doi: 10.1016/j.ejso.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Ba M, Chen C, Long H, et al. Cytoreductive surgery and HIPEC for malignant ascites from colorectal cancer - a randomized study. Medicine (Baltimore). 2020;99(33):e21546. doi: 10.1097/MD.0000000000021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Razenberg LG, van Gestel YR, Creemers GJ, et al. Trends in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of synchronous peritoneal carcinomatosis of colorectal origin in the Netherlands. Eur J Surg Oncol. 2015;41(4):466–471. doi: 10.1016/j.ejso.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Huang CQ, Yang XJ, Yu Y, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for patients with peritoneal carcinomatosis from colorectal cancer: a phase II study from a Chinese center. PLoS One. 2014;9(9):e108509. doi: 10.1371/journal.pone.0108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gervais MK, Dubé P, McConnell Y, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with oxaliplatin for peritoneal carcinomatosis arising from colorectal cancer. J Surg Oncol. 2013;108(7):438–443. doi: 10.1002/jso.23431. [DOI] [PubMed] [Google Scholar]
- 26.Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27(5):681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 27.Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 28.Baratti D, Kusamura S, Azmi N, et al. Colorectal peritoneal metastases treated by perioperative systemic chemotherapy and cytoreductive surgery with or without mitomycin c-based HIPEC: a comparative study using the Peritoneal Surface Disease Severity Score (PSDSS) Ann Surg Oncol. 2020;27(1):98–106. doi: 10.1245/s10434-019-07935-2. [DOI] [PubMed] [Google Scholar]
- 29.Quénet F, Elias D, Roca L, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256–266. doi: 10.1016/S1470-2045(20)30599-4. [DOI] [PubMed] [Google Scholar]
- 30.Pletcher E, Gleeson E, Labow D. Peritoneal cancers and hyperthermic intraperitoneal chemotherapy. Surg Clin North Am. 2020;100(3):589–613. doi: 10.1016/j.suc.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Cotte E, Passot G, Mohamed F, et al. Management of peritoneal carcinomatosis from colorectal cancer: current state of practice. Cancer J. 2009;15(3):243–248. doi: 10.1097/PPO.0b013e3181a58d6. [DOI] [PubMed] [Google Scholar]
- 32.Witkamp AJ, de Bree E, Van Goethem R, et al. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev. 2001;27(6):365–374. doi: 10.1053/ctrv.2001.0232. [DOI] [PubMed] [Google Scholar]
- 33.Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol. 2001;27(3):239–243. doi: 10.1053/ejso.2000.1038. [DOI] [PubMed] [Google Scholar]
- 34.Kuijpers AM, Mirck B, Aalbers AG, et al. Cytoreduction and HIPEC in the Netherlands: nationwide long-term outcome following the Dutch protocol. Ann Surg Oncol. 2013;20(13):4224–4230. doi: 10.1245/s10434-013-3145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavaliere F, De Simone M, Virzì S, et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol. 2011;37(2):148–154. doi: 10.1016/j.ejso.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Sugarbaker TA, Chang D, Koslowe P, et al. Patterns of spread of recurrent intraabdominal sarcoma. Cancer Treat Res. 1996;82:65–77. doi: 10.1007/978-1-4613-1247-5_5. [DOI] [PubMed] [Google Scholar]
- 37.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Yan TD, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for isolated colorectal peritoneal carcinomatosis: experimental therapy or standard of care? Ann Surg. 2008;248(5):829–835. doi: 10.1097/SLA.0b013e31818a15b5. [DOI] [PubMed] [Google Scholar]
- 39.da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203(6):878–886. doi: 10.1016/j.jamcollsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 40.Cao C, Yan TD, Black D, et al. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2009;16(8):2152–2165. doi: 10.1245/s10434-009-0487-4. [DOI] [PubMed] [Google Scholar]
- 41.Elias D, Gilly F, Boutitie F, et al.. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study [published correction appears in. J Clin Oncol. 2010;28(10):1808. J Clin Oncol. 2010;28(1):63-68. doi:10.1200/JCO.2009.23.9285 [DOI] [PubMed]
- 42.Smeenk RM, Verwaal VJ, Zoetmulder FA. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg. 2007;94(11):1408–1414. doi: 10.1002/bjs.5863. [DOI] [PubMed] [Google Scholar]
- 43.Chidambarasamy ES, Chia CS, Johnny Ong CA, et al. Effect of the learning curve of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) on the treatment of colorectal peritoneal metastasis. Asian J Surg. 2022;45(1):339–345. doi: 10.1016/j.asjsur.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Di Giorgio A, Rotolo S, Cintoni M, et al. The prognostic value of skeletal muscle index on clinical and survival outcomes after cytoreduction and HIPEC for peritoneal metastases from colorectal cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2022;48(3):649–656. doi: 10.1016/j.ejso.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Morgan Z, Chow BE, Strong EA, et al. RAS Mutation status confers prognostic relevance in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer. J Surg Res. 2019;240:130–135. doi: 10.1016/j.jss.2019.02.050. [DOI] [PubMed] [Google Scholar]
- 46.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.