Abstract
IS1203v is an insertion sequence which has been found in inactivated Shiga toxin 2 genes of Escherichia coli O157:H7. We analyzed the transpositional mechanism of IS1203v in order to investigate whether the Shiga toxin 2 genes inactivated by IS1203v could revert to the wild type. When the transposase activity of IS1203v was enhanced by artificial frameshifting, IS1203v was obviously excised from the Shiga toxin 2 gene in a circular form. The IS1203v circle consisted of the entire IS1203v, but an extra 3-bp sequence (ATC) intervened between the 5′ and 3′ ends of IS1203v. The extra 3-bp sequence was identical to a direct repeat which was probably generated upon insertion. Moreover, we detected the Shiga toxin 2 gene with a precise excision of IS1203v. In the wild-type situation, the transposition products of IS1203v could be observed by PCR amplification. These results show that IS1203v can transpose in a nonreplicative manner and that the Shiga toxin gene inactivated by this insertion sequence can revert to the wild type.
Certain strains of Escherichia coli are known to produce a family of related toxins, referred to as Shiga toxin 1 (Stx1) (also known as Verotoxin 1) and Shiga toxin 2 (Stx2) (also known as Verotoxin 2). Shiga toxin-producing E. coli (STEC), represented by serotype O157:H7, has been shown to be closely associated with sporadic and epidemic cases of hemorrhagic colitis, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura (6). STEC strains are roughly divided into three types with respect to the production of Shiga toxins, as follows: those producing both Stx1 and Stx2, those producing only Stx1, and those producing only Stx2. Generally, STEC possess the stx genes corresponding to the types of Shiga toxins that they produce.
We have recently reported novel stx2 genes from STEC strains of serotype O157:H7 (8). These stx2 genes were insertionally inactivated by a 1.3-kb insertion sequence (IS), designated IS1203v. IS1203v showed extremely high similarity to IS1203, identified 2 kb upstream from the stx1 gene of E. coli O111:H− (15). IS elements are a large group of bacterial, transposable DNA elements and cause various kinds of genome rearrangements, such as deletions, inversions, duplications, and replicon fusions, by their ability to transpose (2, 14). Several copies of sequences homologous to IS1203v were identified in pO157, a large plasmid of E. coli O157:H7 (1, 11). Plasmid pO157 has a composite structure containing several DNA segments from the F plasmid and the transmissible drug-resistant plasmid R100, which are flanked by IS elements, including the above IS1203v homologue. The homologue of the repE gene essential for autonomous replication of F plasmid (13) was disrupted by insertion of the IS1203v homologue in pO157. Therefore, the replication system driven by repE does not function and pO157 replicates using the R100 plasmid system. These findings suggest that IS elements are important in the evolution of pO157 and probably the O157:H7 genome. We think that analyses of the transpositional mechanism of IS1203v derived from STEC strains are interesting.
IS1203v is highly similar to IS629 (12) and IS3411 (5), as well as IS1203. They are classified into the IS3 family, the most widely spread group of insertion sequences (2, 3, 14, 19, 23). Their membership in the family is based on similarities in the terminal inverted repeats, the presence of two open reading frames (ORFs) that overlap in the −1 frame, and similarities in the putative amino acid sequence of the downstream ORF (2, 3, 7, 14). Another feature of the IS3 family is that the translational frameshifting event in the overlapping region of the two ORFs is involved in the production of a fusion protein that has the transposase activity (25).
In the present study, we cloned the stx2 gene including IS1203v (stx2::IS1203v) and analyzed the transpositional mechanism of IS1203v. The genetically frameshifted IS1203v was obviously excised from stx2::IS1203v in the circular form. We showed that the stx2 gene could be reverted to the wild type with a precise excision of IS1203v.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
E. coli O157:H7 (stx2::IS1203v) strains (8) were grown at 37°C for 16 h in Luria-Bertani (LB) broth (20). Recombinant strains were grown at 37°C for 16 h in 5 ml of LB broth containing 50 μg of ampicillin per ml. Subculture of the recombinant strain was performed by inoculation (0.2%) for five generations under the same culture conditions. Blue-and-white color screening was performed as described by Sambrook et al. (20).
DNA manipulation.
Restriction endonucleases and T4 DNA ligase were from Toyobo Co., Ltd. (Osaka, Japan). Transformation of E. coli cells was performed by the competent-cell method improved by Inoue et al. (4). Plasmid DNA was prepared from the culture of the recombinant strain using the alkaline lysis method (20), and it was electrophoresed in an agarose gel followed by staining with ethidium bromide. PCR was performed over a total of 30 cycles in a total volume of 50 μl containing template DNA, 0.2 μM concentrations of two primers, PCR buffer, a 0.2 mM concentration of each deoxynucleoside triphosphate, and 2.5 U of KOD DNA polymerase (Toyobo Co., Ltd.). The PCR cycles consisted of 94°C for 20 s, 65°C for 2 s, and 74°C for 1.5 min. The nucleotide sequences were determined by the dideoxy chain termination method (22) using the Sequencing PRO Autosequencer Core kit (Toyobo Co., Ltd.) and the ALFred DNA sequencer (Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer's instructions. All of the primers were synthesized using a DNA synthesizer (model 392; Applied Biosystems Inc.).
Construction of plasmids.
pNIS-STX2 is a plasmid carrying the full length of the stx2 gene including IS1203v (Fig. 1). Approximately 104 CFU of O157:H7 (stx2::IS1203v) strains (8) was used as the template in PCR amplification. The sequences of two primers containing the EcoRI site and specific for the 5′ and 3′ ends of the stx2 gene were 5′-CTGGAATTCGGCTTCTTCAGCCAAAAGGAACACCTGTATATG-3′ and 5′-GCAGAATTCGGCTTCACATACCACGAATCAGGTTATGCCTCA-3′, respectively. The amplified 2.6-kb fragment was digested with EcoRI and ligated into the EcoRI site of vector plasmid pUC18 (28).
FIG. 1.
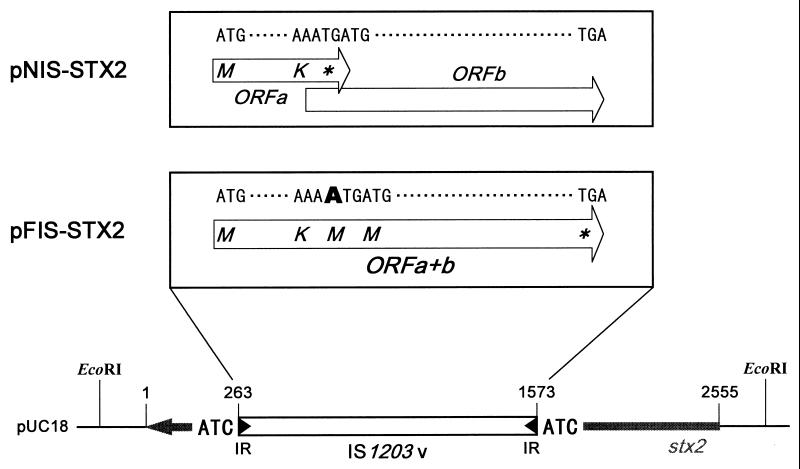
Structures of pNIS-STX2 and pFIS-STX2. IS1203v of pNIS-STX2 has two ORFs (open arrows), designated ORFa and ORFb, and that of pFIS-STX2 has a single ORF, designated ORFa+b. ORFa+b is constructed by a 1-bp insertion in the region where ORFa and ORFb overlap, and this inserted nucleotide is indicated in bold type. The amino acid translations of the ORFs are shown within the open arrows. The open box and the thick arrow of pFIS-STX2 indicate IS1203v and the stx2 gene, respectively. The direction of the stx2 gene is the opposite of that of ORFa+b of IS1203v. stx2::IS1203v is numbered from the first nucleotide (position 1) to the last nucleotide (position 2555) of the complementary strand of the stx2 gene. IS1203v extends from position 263 to position 1573. The 25-bp long inverted repeats (IRs) are indicated at both ends of IS1203v. The 3-bp sequences (ATC) shown on both sides of IS1203v are direct repeats probably generated upon insertion of this IS element. ∗, the stop codon.
pFIS-STX2 is a mutant of pNIS-STX2 and has a single ORF encoding the putative IS1203v transposase caused by a 1-bp insertion in the overlapping region of the two ORFs (Fig. 1). It was constructed by site-directed mutagenesis according to the method of Steffan et al. (26), using pNIS-STX2 and overlapping primers (5′-GGAGTTCGACCGCCTCTGGAAAAAAATGATGCCACTGCTGGATAAGCTGC-3′ and 5′-GCAGCTTATCCAGCAGTGGCATCATTTTTTTCCAGAGGCGGTCGAACTCC-3′).
pNIS-NC, containing a frameshift mutation of ORFa, was constructed by site-directed mutagenesis using pNIS-STX2 and overlapping primers (5′-ACTCGTTTTTCCCCCTGAAGTCCGTCAGCG-3′ and 5′-CGCTGACGGACTTCAGGGGGAAAAACGAGT-3′). With a deoxyribosylthymine inserted before the tenth codon of ORFa, the glutamic acid codon (GAA) was changed into the stop codon (TGA) and this reading frame was broken. The nucleotide sequences of the plasmids were confirmed, and there were no unexpected mutations introduced by PCR.
Analysis of excision of IS1203v.
DNA from the subculture of E. coli JM109(pFIS-STX2) was treated with BsiWI, which cleaves pFIS-STX2 at one site within IS1203v, and introduced by transformation into E. coli JM109. The sizes of the stx2 genes of the transformants were analyzed using the colony PCR method (21). For amplification of the stx2 gene, two specific primers (5′-GTAACGCCAGGGTTTTCCCAGTCACGAC-3′ and 5′-TTGTGAGCGGATAACAATTTCACACAGGAAAC-3′) were used. Those primers amplified the 2.7 kb of pFIS-STX2, including the full length of stx2::IS1203v. Nucleotide sequences of the stx2 genes which caused an amplicon of less than 2.7 kb were analyzed.
RESULTS AND DISCUSSION
Investigation of transposition of IS1203v.
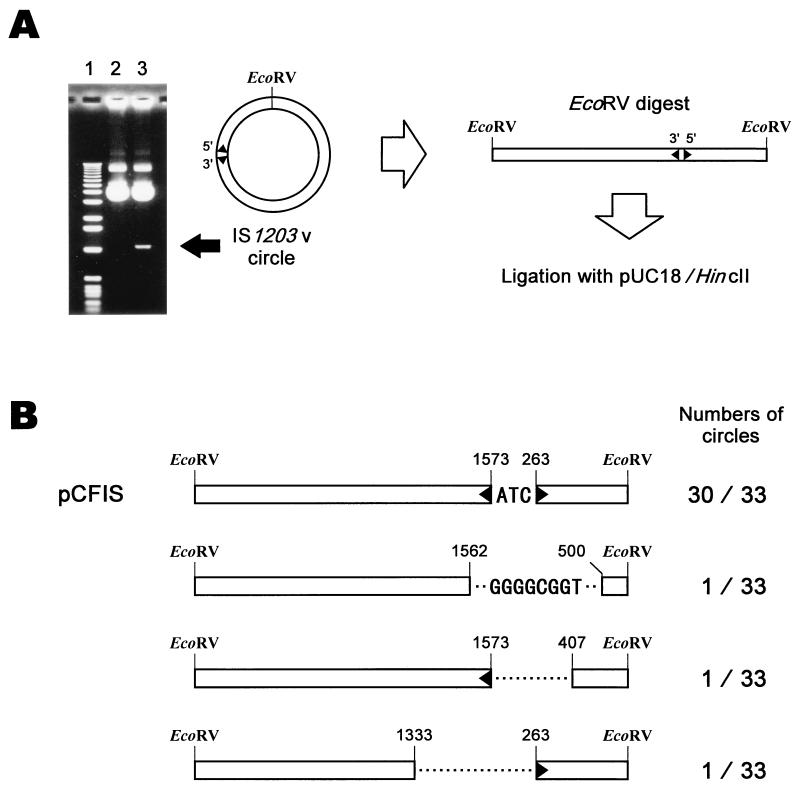
IS1203v consists of an AAAATGA sequence in the overlapping region of the two ORFs (8). The sequence is one of the frameshift signals, so-called “slippery” codons (2). The TGA sequence at the end of the heptanucleotide represents the stop codon of the first ORF, and the overlapping ATG is the start codon of the second reading frame (Fig. 1). A leftward shift of a lysyl tRNA within the slippery AAAA tetranucleotide brings the two ORFs into the same reading frame and overrides the stop codon of the first ORF. In order to enhance the transposase activity and analyze the transpositional mechanism of IS1203v, artificial frameshifting was performed by using site-directed mutagenesis in the slippery codons. The mutational IS1203v carrier, pFIS-STX2, was thus constructed. Growth of E. coli JM109(pFIS-STX2) was the same as that of E. coli JM109(pNIS-STX2) (data not shown). When the total DNA was prepared from the culture of E. coli JM109(pFIS-STX2) and electrophoresed, an extra DNA of approximately 1 kb was detected (Fig. 2A). As this extra DNA could be digested with EcoRV, which cleaves IS1203v at one site, it was ligated into the HincII site of the vector plasmid pUC18 and introduced by transformation into E. coli JM109. However, when the extra DNA was not digested, transformants possessing pUC18 with an insertion were not detected by blue-and-white color screening. The nucleotide sequences of the cloned extra DNA showed that the 5′ and 3′ ends of IS1203v were connected. The extra DNA must be a circular form of IS1203v, designated IS1203v circle, generated from the stx2::IS1203v in pFIS-STX2. Generation of IS circles has been observed with several IS elements (14, 17, 25).
FIG. 2.
Analysis of the IS1203v circle. (A) Cloning of the IS1203v circle. Lane 1, molecular weight marker (1-kb DNA ladder; Life Technologies, Rockville, Md.); lanes 2 and 3, results of agarose gel analysis of the DNA prepared from E. coli harboring pNIS-STX2 and pFIS-STX2, respectively. The IS1203v circle was isolated from the gel, linearized by digesting with EcoRV, which cleaves IS1203v at one site, and ligated into HincII-digested vector plasmid pUC18. Inverted repeats at the 5′ and 3′ ends of IS1203v are indicated by black triangles, as in Fig. 1. (B) Structures of the linearized IS1203v circles and numbers of the analyzed circles are represented. IS1203v is indicated and numbered in a way similar to that shown in Fig. 1. The sequences ATC and GGGGCGGT are one side of the direct repeat in the original plasmid pFIS-STX2.
Analysis of IS1203v circle.
E. coli JM109(pFIS-STX2) was cultured, and IS1203v circles were prepared and cloned into pUC18. The nucleotide sequences of 33 IS1203v circles from independent transformants were analyzed. Thirty of the 33 circles had the same sequences as IS1203v (Fig. 2B), and a 3-bp sequence (ATC) intervened between the 5′ and 3′ ends of IS1203v. This 3-bp sequence was identical to the direct repeat, which was probably generated by duplication of the target site in the stx2 gene upon insertion of IS1203v (8). The plasmid carrying the above-mentioned IS1203v circle was designated pCFIS. With respect to those the other three circles, the nucleotide sequences were different (Fig. 2B). Plasmids carrying these circles were about 250 bp smaller than pCFIS and consisted of a part of IS1203v that included neither the 5′ nor the 3′ end.
The IS1203v circle was not detected in E. coli JM109(pNIS-STX2) (Fig. 2A). The IS1203v transposase must be produced by −1 frameshifting between the two ORFs in the form of a fused protein of 404 amino acids, which generates the IS1203v circle. This is in agreement with reports for the other members of the IS3 family (14, 17, 25).
Analysis of the stx2 genes caused by transposition of IS1203v.
With a culture of E. coli JM109(pFIS-STX2), the IS1203v circle was generated at a level that could be readily detected by gel electrophoresis. On the other hand, the plasmids possessing the stx2 gene caused by excision of IS1203v could not be detected by gel electrophoresis. The plasmids generated by the excision could be detected as follows. When 0.2 μg of the DNA preparation from five generations of the subculture of E. coli JM109(pFIS-STX2) was treated with BsiWI, which cleaves pFIS-STX2 at one site within IS1203v, and introduced into E. coli JM109, 104 transformants were generated (Table 1). We analyzed the stx2 genes in 192 transformants by BsiWI digestion of colony PCR products. The amplicons for 128 of the 192 transformants were 2.7 kb, the same as those for E. coli JM109(pFIS-STX2), and the amplicons could be digested with BsiWI. This suggested that the 128 transformants possessed the plasmids without excision of IS1203v. With respect to the other 64 transformants, the amplicons were less than 2.7 kb and could not be digested with BsiWI. This suggested that the plasmids lacking IS1203v could be detected. Those 64 transformants appeared at the rate of 2.2 × 10−5 compared to the total number of transformants introduced by the same conditions without the BsiWI cleavage (Table 1). The number of detectable plasmids with excision of IS1203v was small, compared to a large number of the IS1203v circles. With respect to the plasmids cleaved by transposition of IS1203v, most of them were likely to be of the linear form because the 5′ and 3′ ends would not be ligated to each other. When analysis was performed as described above, except that a subculture of E. coli JM109(pNIS-STX2) was used, 5.0 × 103 transformants were generated (Table 1). When 192 transformants from this subculture were analyzed, since the amplicons were 2.7 kb and could be digested with BsiWI, all of them possessed plasmids without the excision of IS1203v.
TABLE 1.
Analysis of the stx2 gene by colony PCR
| Original plasmid | No. of transformants
|
% of transformants with excision of IS1203v (no. with excision/no. tested) | Apparent frequencya | |
|---|---|---|---|---|
| Total | BsiWI-digested DNA | |||
| pFIS-STX2 | 1.5 × 108 | 1.0 × 104 | 33 (64/192) | 2.2 × 10−5 |
| pNIS-STX2 | 1.2 × 108 | 5.0 × 103 | NDb (0/192) | <2.2 × 10−7 |
The apparent frequency was calculated as follows: number of transformants of BsiWI-digested DNA/total number of transformants × number of transformants with excision of IS1203v.
ND, not detected.
The frequency of excision was very low, compared with other transposons. For example, Perkins-Balding et al. showed the frequency of excision of IS492 as 0.95 (16). They cloned IS492 into the chloramphenicol resistance (Cmr) gene on plasmid pACYC184 and detected Cmr colonies. Though the result is clear, we consider it difficult to compare to our result, 2.2 × 10−5, because the plasmid systems are different.
The nucleotide sequences of the stx2 genes in the above 64 transformants were analyzed. The stx2 genes in 21 of the 64 transformants were identical to the wild type (10) (Fig. 3). Accordingly, it was demonstrated that the stx2::IS1203v could revert to the wild-type stx2 gene. The plasmid carrying the above stx2 gene as a transpositional product was designated pRSTX2. It was thought that a precise excision of IS1203v generated pRSTX2 and the IS1203v circle from the original pFIS-STX2. By nonreplicative transposition, which is one of the transpositional mechanisms of IS elements, they are excised from an original position of a donor and inserted into an acceptor. On the other hand, in the case of replicative transposition, IS elements are involved in both the donor and the acceptor (9). Generation of pRSTX2, i.e., excision of IS1203v, demonstrates that IS1203v transposes in a nonreplicative manner.
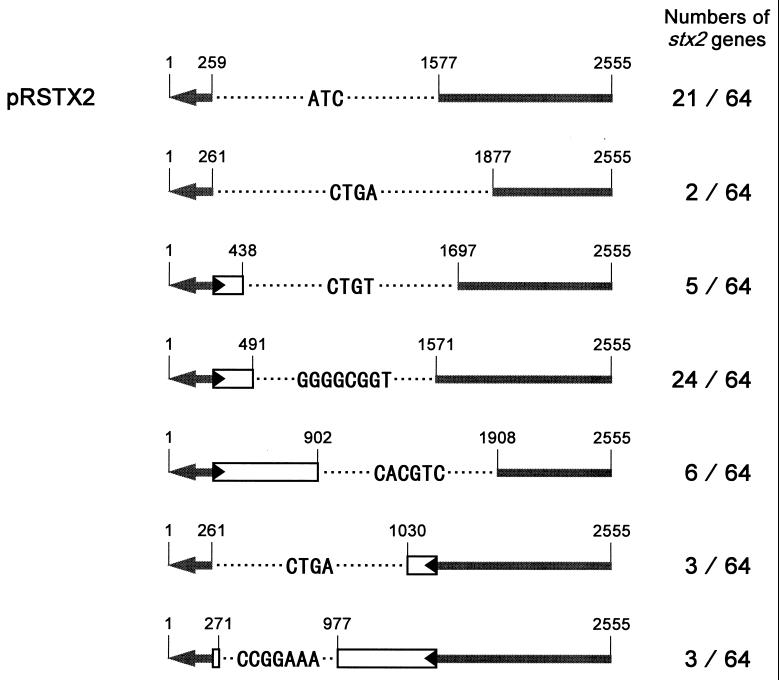
FIG. 3.
Analysis of the stx2 genes caused by transposition of IS1203v. Structures of the stx2 genes caused by transposition of IS1203v and numbers of the analyzed genes are represented. IS1203v and the stx2 genes are indicated and numbered in a way similar to that described in the legend to Fig. 1. The cleavage site of BsiWI is at position 955. The sequences ATC, CTGA, CTGT, GGGGCGGT, CACGTC, CTGA, and CCGGAAA are one side of the direct repeat in the original plasmid pFIS-STX2.
With respect to the other 43 transformants, there were six kinds of stx2 genes caused by excision of 1,611-, 1,254-, 1,071-, 999-, 764-, and 698-bp fragments including the part of IS1203v with the BsiWI site (Fig. 3).
All of the plasmids shown in Fig. 3 included only one side of the direct repeat in pFIS-STX2; i.e., the other side of the direct repeat and the regions between the two sides were excised, which suggested that transposition of IS1203v occurred at the direct repeat in pFIS-STX2. On the other hand, except for the pRSTX2 caused by the precise excision of IS1203v, obvious inverted repeats are not observed at either end of the excised fragments in those plasmids, although they are generally transposase recognition sites and contiguous to direct repeats. In the case of IS1203v, transposition at direct repeats may be one of the requirements to ligate both ends of the fragments cleaved by transposase. Further investigations are required to clarify these points and are now in progress.
Detection of transposition of IS1203v in the wild-type situation.
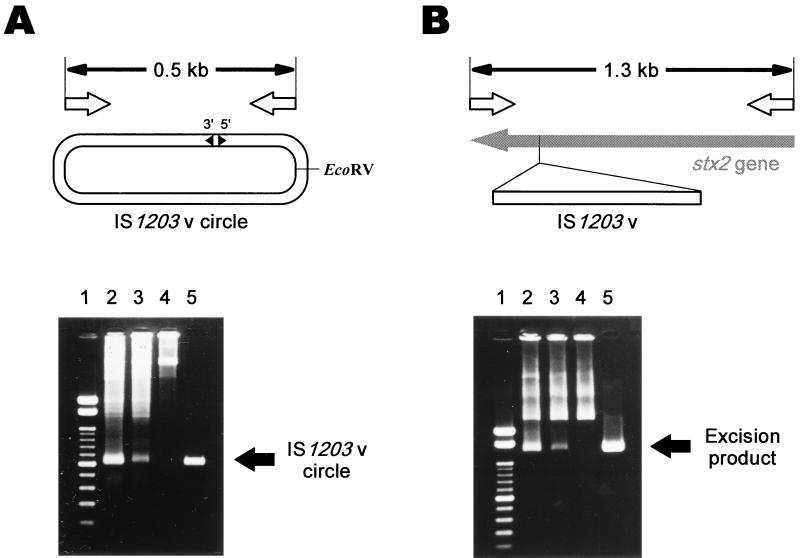
To determine whether the enhanced transposition of the frameshifted ORFs on the plasmid pFIS-STX2 resulted in suitable products, we tried to detect the transposition products caused by IS1203v in the wild-type situation. By PCR amplification using two specific primers for the circular form of IS1203v (5′-GCCGGATACATCGTGGGGTGGC-3′ and 5′-CCTCTTTCTCAGGGAGTTTAGTCTCC-3′), the IS1203v circle from pNIS-STX2 could be detected (Fig. 4A). The size of the amplicon was the same as that for pCFIS, as shown in Fig. 2B (0.5 kb). In the case of pNIS-NC, containing an inactive mutation in ORFa of IS1203v, the 0.5-kb amplicon was not apparent.
FIG. 4.
Detection of transposition of IS1203v in the wild-type situation. (A) Detection of the IS1203v circle using PCR with primers specific for the circular form of IS1203v. The open arrows indicate annealing sites of the primers. Lane 1, molecular weight marker (100-bp DNA ladder; Toyobo Co., Ltd.); lanes 2 to 5, results of agarose gel analysis of the amplicons for pFIS-STX2, pNIS-STX2, pNIS-NC, and pCFIS, respectively. (B) Detection of the excision product using PCR with primers specific for the 5′ and 3′ ends of the stx2 gene. The open arrows indicate annealing sites of the primers. Lane 1, molecular weight marker (100-bp DNA ladder; Toyobo Co., Ltd.); lanes 2 to 5, results of agarose gel analysis of the amplicons for the BsiWI digests of pFIS-STX2, pNIS-STX2, pNIS-NC, and pRSTX2, respectively.
The plasmid with excision of IS1203v from pNIS-STX2 could be detected as follows. When pNIS-STX2 was digested with BsiWI and PCR was performed using two primers specific for the 5′ and 3′ ends of the stx2 gene (5′-CTTCAGCCAAAAGGAACACCTGTATATG-3′ and 5′-CACATACCACGAATCAGGTTATGCCTCA-3′), a 1.3-kb amplicon the same size as that for pRSTX2 as shown in Fig. 3 was apparent (Fig. 4B). A precise excision of IS1203v was also detected in the wild-type situation. In the case of pNIS-NC, the 1.3-kb amplicon was not apparent.
In this study, we demonstrate that stx2::IS1203v can revert to a wild-type stx2 gene with the precise excision of IS1203v by nonreplicative transposition. This is caused by −1 frameshifting between the two overlapping ORFs in IS1203v that produces a fusion protein with transposase. The above frameshifting event is thought to occur at the translational level as previously observed for several IS elements (2, 14, 18, 24, 25, 27), and transposition of IS1203v is quite likely to occur spontaneously in O157:H7 and the other STEC strains in nature. Although the original O157:H7 (stx2::IS1203v) strains do not produce Stx2 (8), they must begin to produce Stx2 by the precise excision of IS1203v, as shown in this study. This may be an example of how the Stx productivity of STEC is regulated by an IS element, i.e., the insertional inactivation and the excisable reactivation of the stx gene. It is also a possibility that the above transposition occurred accidentally, as a result of genome rearrangements through the evolution of STEC caused by IS elements, as observed for pO157 (1, 11). As we showed in the case of E. coli JM109(pNIS-STX2), a cloned IS1203v without artificial frameshifting transposes with low frequency. Moreover, copy numbers of the stx gene on the O157:H7 genome are lower than those on the plasmid vector. Therefore, the frequency of transposition in natural STEC should be much lower than the 2.2 × 10−5 observed in this study. For natural STEC, the frequencies of transposition events which affect Stx productivity are very interesting.
REFERENCES
- 1.Burland V, Shao Y, Perna N T, Plunkett G, Sofia H J, Blattner F R. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Res. 1998;26:4196–4204. doi: 10.1093/nar/26.18.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandler M, Fayet O. Translational frameshifting in the control of transposition in bacteria. Mol Microbiol. 1993;7:497–503. doi: 10.1111/j.1365-2958.1993.tb01140.x. [DOI] [PubMed] [Google Scholar]
- 3.Fayet O, Ramond P, Polard P, Prère M F, Chandler M. Functional similarities between retroviruses and the IS3 family of bacterial insertion sequences? Mol Microbiol. 1990;4:1771–1777. doi: 10.1111/j.1365-2958.1990.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 4.Inoue H, Nojima H, Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96:23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 5.Ishiguro N, Sato G. Nucleotide sequence of insertion sequence IS3411, which flanks the citrate utilization determinant of transposon Tn3411. J Bacteriol. 1988;170:1902–1906. doi: 10.1128/jb.170.4.1902-1906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan E, Mack J P G, Katz R A, Kulkosky J, Skalka A M. Retroviral integrase domains: DNA binding and the recognition of LTR sequences. Nucleic Acids Res. 1991;19:851–860. doi: 10.1093/nar/19.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusumoto M, Nishiya Y, Kawamura Y, Shinagawa K. Identification of an insertion sequence, IS1203 variant, in a Shiga toxin 2 gene of Escherichia coli O157:H7. J Biosci Bioeng. 1999;87:93–96. doi: 10.1016/s1389-1723(99)80014-0. [DOI] [PubMed] [Google Scholar]
- 9.Lewin B. Genes V. Oxford, United Kingdom: Oxford University Press; 1994. [Google Scholar]
- 10.Lin Z, Yamasaki S, Kurazono H, Ohmura M, Karasawa T, Inoue T, Sakamoto S, Suginami T, Takeoka T, Taniguchi Y, Takeda Y. Cloning and sequencing of two new Verotoxin 2 variant genes of Escherichia coli isolated from cases of human and bovine diarrhea. Microbiol Immunol. 1993;37:451–459. doi: 10.1111/j.1348-0421.1993.tb03236.x. [DOI] [PubMed] [Google Scholar]
- 11.Makino K, Ishii K, Yasunaga T, Hattori M, Yokoyama K, Yutsudo C H, Kubota Y, Yamaichi Y, Iida T, Yamamoto K, Honda T, Han C-G, Ohtsubo E, Kasamatsu M, Hayashi T, Kuhara S, Shinagawa H. Complete nucleotide sequence of 93-kb and 3.3-kb plasmids of an enterohemorrhagic Escherichia coli O157:H7 derived from Sakai outbreak. DNA Res. 1998;5:1–9. doi: 10.1093/dnares/5.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Matsutani S, Ohtsubo E. Complete sequence of IS629. Nucleic Acids Res. 1990;18:1899. doi: 10.1093/nar/18.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori H, Kondo A, Ohshima A, Ogura T, Hiraga S. Structure and function of the F plasmid genes essential for partitioning. J Mol Biol. 1986;192:1–15. doi: 10.1016/0022-2836(86)90459-6. [DOI] [PubMed] [Google Scholar]
- 14.Ohtsubo E, Sekine Y. Bacterial insertion sequences. Curr Top Microbiol Immunol. 1996;204:1–26. doi: 10.1007/978-3-642-79795-8_1. [DOI] [PubMed] [Google Scholar]
- 15.Paton A W, Paton J C. Characterization of IS1203, an insertion sequence in Escherichia coli O111:H−. Gene. 1994;150:67–70. doi: 10.1016/0378-1119(94)90859-1. [DOI] [PubMed] [Google Scholar]
- 16.Perkins-Balding D, Duval-Valentin G, Glasgow A C. Excision of IS492 requires flanking target sequences and results in circle formation in Pseudoalteromonas atlantica. J Bacteriol. 1999;181:4937–4948. doi: 10.1128/jb.181.16.4937-4948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polard P, Prère M F, Fayet O, Chandler M. Transposase-induced excision and circularization of the bacterial insertion sequence IS911. EMBO J. 1992;11:5079–5090. doi: 10.1002/j.1460-2075.1992.tb05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polard P, Prère M F, Chandler M, Fayet O. Programmed translational frameshifting and initiation at an AUU codon in gene expression of bacterial insertion sequence IS911. J Mol Biol. 1991;222:465–477. doi: 10.1016/0022-2836(91)90490-w. [DOI] [PubMed] [Google Scholar]
- 19.Prère M F, Chandler M, Fayet O. Transposition in Shigella dysenteriae: isolation and analysis of IS911, a new member of the IS3 group of insertion sequences. J Bacteriol. 1990;172:4090–4099. doi: 10.1128/jb.172.7.4090-4099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Sandhu G S, Precup J W, Kline C. Rapid one-step characterization of recombinant vectors by direct analysis of transformed Escherichia coli colonies. BioTechniques. 1989;7:689–690. [PubMed] [Google Scholar]
- 22.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz E, Kröger M, Rak B. IS150: distribution, nucleotide sequence and phylogenetic relationships of a new E. coli insertion element. Nucleic Acids Res. 1988;16:6789–6802. doi: 10.1093/nar/16.14.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekine Y, Ohtsubo E. Frameshifting is required for production of the transposase encoded by insertion sequence 1. Proc Natl Acad Sci USA. 1989;86:4609–4613. doi: 10.1073/pnas.86.12.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekine Y, Esaki N, Ohtsubo E. Translational control in production of transposase and in transposition of insertion sequence IS3. J Mol Biol. 1994;235:1406–1420. doi: 10.1006/jmbi.1994.1097. [DOI] [PubMed] [Google Scholar]
- 26.Steffan N H, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 27.Vögele K, Schwartz E, Welz C, Schiltz E, Rak B. High-level ribosomal frameshifting directs the synthesis of IS150 gene products. Nucleic Acids Res. 1991;19:4377–4385. doi: 10.1093/nar/19.16.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]