Abstract
In this study Vibrio proteolyticus CW8T2 has been identified as a virulent pathogen for Artemia spp. Its infection route has been visualized with transmission electron microscopy. The pathogen affected microvilli and gut epithelial cells, disrupted epithelial cell junctions, and reached the body cavity, where it devastated cells and tissues. In vivo antagonism tests showed that preemptive colonization of the culture water with nine selected bacterial strains protected Artemia juveniles against the pathogenic effects. Two categories of the selected strains could be distinguished: (i) strains providing total protection, as no mortality occurred 2 days after the experimental infection with V. proteolyticus CW8T2, with strain LVS8 as a representative, and (ii) strains providing partial protection, as significant but not total mortality was observed, with strain LVS2 as a representative. The growth of V. proteolyticus CW8T2 in the culture medium was slowed down in the presence of strains LVS2 and LVS8, but growth suppression was distinctly higher with LVS8 than with LVS2. It was striking that the strains that gave only partial protection against the pathogen in the in vivo antagonism test showed also a restricted capability to colonize the Artemia compared to the strains providing total protection. The in vivo antagonism tests and the filtrate experiments showed that probably no extracellular bacterial compounds were involved in the protective action but that the living cells were required to protect Artemia against V. proteolyticus CW8T2.
Several alternative strategies for the use of antimicrobials in disease control have been proposed and have already been applied successfully in aquaculture, such as the use of vaccines (11), the use of immunostimulants for the enhancement of the nonspecific defense mechanisms of the host, and the use of probiotic bacteria (5). Considering their recent successes, these alternative approaches have been defined by the Food and Agriculture Organization of the United Nations (18) as major areas for further research in disease control in aquaculture.
Already in 1980 Yasuda and Taga (26) anticipated that bacteria would be found to be useful not only as food for cultured aquatic species but also as biological controllers of disease. Several well-documented studies on the use of probiotics as biological control agents in the farming of bivalve mollusks, crustaceans, and fish were recently published (6, 8; S. Rengpipat and S. Rukpratanporn, Book Abstr. Fifth Asian Fish. Forum, 1998).
The probiotic application of Aeromonas media A199 was found to prevent death of the oyster Crassostrea gigas larvae when they were challenged in vivo with the pathogen Vibrio tubiashii, although A. media A199 was not able to persist more than 4 days on the host (6). The administration of the probiotic strain to the larvae caused a spectacular decrease of the pathogen densities in the larvae compared to those in the larvae treated with V. tubiashii only.
Rengpipat and Rukpratanporn (Book Abstr. Fifth Asian Fish. Forum) reported the use of a Bacillus strain, S11, as a probiotic administered to larvae of the black tiger shrimp Penaeus monodon via enriched brine shrimp, Artemia spp. The P. monodon larvae fed with the Bacillus-fortified Artemia had significantly shorter development times and fewer disease problems than larvae reared without the Bacillus. After feeding for 100 days, P. monodon postlarvae were challenged with the pathogenic Vibrio harveyi D331 by immersion. Ten days later all the groups treated with Bacillus S11 had 100% survival, whereas the control group had only 26% survival.
Siderophore-producing Pseudomonas fluorescens has been successfully applied as a biological control agent; it limited the mortality of 40-g rainbow trout (Oncorynchus mykiss) experimentally infected with Vibrio anguillarum (8). Siderophores are low-molecular-mass compounds with a very high affinity for ferric iron, whose biosynthesis is iron-regulated (26). A correlation was found between the production of siderophores and the protective action of P. fluorescens, suggesting that competition for free iron is involved in the mode of action (8).
Juvenile and adult brine shrimp are used increasingly as suitable live diets for different aquaculture species (17). The intensive culture of the brine shrimp Artemia has always suffered from unpredictable results due to incidental crashes in individual production tanks (24). In previous research, manipulation of the microbiota by preemptive colonization of the culture water with selected bacterial strains has been shown to improve the culture performance of Artemia (23). It was demonstrated under monoxenic conditions that the selected bacterial strains improved the nutritional quality of the dry food. The aim of this study was to investigate whether these selected strains can also be active as biological control agents against bacterial infections. Experimental infections of Artemia were done with Vibrio proteolyticus CW8T2, which has previously been shown to cause mortality in monoxenic Artemia cultures (23). The infection route was determined by means of transmission electron microscope observations. In vivo antagonism tests were performed to see whether the selected bacterial strains are able to protect Artemia from the pathogenic actions of V. proteolyticus CW8T2. In addition, filtrate experiments were done to verify whether extracellular compounds were involved in the protective action.
MATERIALS AND METHODS
Bacterial strains.
The nine probiotic strains originate from well-performing Artemia cultures and were selected based on their positive effect on the monoxenic and xenic culture of Artemia juveniles, as described by Verschuere et al. (23). V. proteolyticus CW8T2 was isolated from artificial feed from a sea bass hatchery in Spain and was kindly provided by L. Verdonck of the Department of Microbiology, University of Ghent.
The nine selected strains were phenotypically identified based on their shape, size, motility, Gram stain reaction, catalase reaction, oxidase reaction, glucose metabolism, trimethylamine oxide (TMAO) reduction, and H2S production according to methods described previously (1). Some of these strains were further characterized by gas chromatography of fatty acid methyl esters (FAME) originating from the bacterial cell wall (21, 22) and/or with API20NE (BioMérieux, Marcy L'Etoile, France). The manufacturer's instructions were followed for the latter, except that the NaCl content of the Aux medium was increased to 1.5% and the reading was performed after 48 h of incubation at 28°C.
Axenic hatching and culture of Artemia.
In order to examine the action of added microbiota and avoid interference by other microorganisms, Artemia were disinfected with Merthiolate and axenically hatched as described by Verschuere et al. (23). Subsequently, 20 Artemia were transferred to sterile culture tubes containing 30 ml of autoclaved artificial seawater (Instant Ocean [33 g/liter]; Aquarium Systems, Sarrebourg, France). The culture tubes were kept at 28°C on a rotor. Gamma-irradiated (10 kGy) dry food was administered at a rate of 5 mg/day on days 0 and 1 after the transfer of the nauplii and 5.7 mg/day from day 2 onwards. Depending on the experiments, cultures were grown up to 3 or 5 days (see in vivo antagonism tests). The axenic condition of the control cultures was assessed regularly by inoculating 100 μl of the culture water on marine agar 2216 (MA) (Difco Laboratories, Detroit, Mich.). If any contamination occurred, the results of the experimental run were not accepted. Unless otherwise stated, this protocol was used whenever Artemia had to be cultured.
Pathogenicity of V. proteolyticus CW8T2 under conditions of feeding and starvation.
The first experiment was aimed at detecting the effect of several concentrations of V. proteolyticus CW8T2 on Artemia survival, in order to determine an appropriate range for further experimental infections. V. proteolyticus CW8T2 was grown overnight in marine broth, centrifuged at approximately 10,000 × g and resuspended in Nine Salts Solution (NSS) (12). Beforehand, the relationship between the optical density at 550 nm and the plate count was established by plating dilutions of a suspension with known values of optical density at 550 nm on marine agar plates. Twenty-four hours after the transfer of the nauplii to axenic culture tubes, V. proteolyticus CW8T2 was added at different concentrations (1 × 0, 1 × 102, 1 × 103, 1 × 104, 1 × 105, and 5 × 106 CFU/ml). The highest concentration used in these experiments corresponded to the highest concentrations of Vibrionaceae present in Artemia culture water as determined on TCBS Cholera medium in previous experiments (24). The culture conditions of the Artemia nauplii were similar to the description above. Forty-eight hours after the addition of V. proteolyticus CW8T2, the surviving Artemia nauplii were counted. The experiment was performed twice, with three replicates per treatment.
A similar experiment was performed under starvation conditions (no artificial food) in which V. proteolyticus CW8T2 was added at a concentration of 103 CFU/ml right after the transfer of the nauplii. The control treatment was axenic. As a control, a supplementary treatment was inoculated with strain LVS8 (5 × 106 CFU/ml). Survival was recorded after 24 and 48 h. The experiment was performed twice with three replicates each time.
Histological observation of infection process.
Artemia nauplii experimentally infected with V. proteolyticus CW8T2 (103 CFU/ml) 1 day after the transfer of the nauplii to the culture tubes were sampled after 1 day of infection for histological analysis. Axenically reared Artemia nauplii of the same age were also sampled. The Artemia nauplii were fixed in Karnovsky's fixative containing 2% (vol/vol) paraformaldehyde and 2.5% (vol/vol) glutaraldehyde in 0.2 M sodium cacodylate buffer, which was further diluted three times before use. After being rinsed in the same buffer the samples were postfixed in 2% (wt/vol) osmic tetroxide diluted in the same buffer. The samples were dehydrated in a graded concentration of ethanol and embedded in LX resin.
For light microscopy, semithin (approximately 2-μm) sections were made and they were stained with toluidine blue and viewed with a phase contrast microscope. For transmission electron microscopy, ultrathin sections (50 to 70 nm) were contrasted with uranyl acetate and lead citrate and viewed with a JEOL 100B electron microscope (JEOL Ltd., Tokyo, Japan).
In vivo antagonism test.
In vivo antagonism tests were done to examine the pathogenic action of V. proteolyticus CW8T2 in Artemia cultures preemptively colonized by each of the nine selected bacterial strains. Artemia nauplii were axenically hatched, transferred to the culture tubes, and maintained as described above. Immediately after the transfer of the nauplii, the culture water was inoculated with one of the selected bacterial strains at a calculated concentration of 5 × 106 cells/ml. Twenty-four hours later, the Artemia cultures were experimentally infected with V. proteolyticus CW8T2 administrated in the culture water at a concentration of either 102 or 103 CFU/ml. As control treatments Artemia nauplii were either (i) maintained under axenic conditions, (ii) infected only with V. proteolyticus CW8T2 (102 or 103 CFU/ml), or (iii) inoculated only with the selected bacterial strain (5 × 106 cells/ml). The survival of the Artemia nauplii was recorded 2 days after the experimental infection (3-day-old Artemia nauplii) by counting the number of living animals. For each strain at least two experimental runs were performed, with three or four replicates.
In order to determine the colonization capacity of the selected strains, Artemia nauplii were plated to enumerate the bacteria present inside and on the external surface of the shrimps. Therefore, 10 Artemia nauplii cultured in the presence of the selected strain were harvested 1 day after the transfer to the culture tubes, just before the experimental infection. They were put on a sterile 150-μm-pore-size nylon filter and rinsed twice with 10 ml of autoclaved NSS. The nylon filter was then placed aseptically in a sterile plastic bag containing 10 ml of sterile NSS. The sample was homogenized for 5 min with a stomacher blender (400SN; Seward Medical, London, United Kingdom). A serial 10-fold dilution of the suspension was made, and 100-μl volumes of the appropriate dilutions were spread on marine agar plates. The MA plates were incubated at 28°C, and counts were performed after 2 or 5 days, depending on the strain. The same was done with Artemia cultures inoculated immediately after the nauplii transfer with V. proteolyticus CW8T2 (at an initial concentration of 103 CFU/ml).
Based on the first results of the in vivo antagonism tests, two representative strains (LVS2 and LVS8) were selected for further experiments. Similar in vivo antagonism tests were performed, but the cultures were maintained up to 4 days after the experimental infection. The survival rate of the Artemia nauplii was determined after 2 and 4 days. Immediately after the infection and 1, 2, and 4 days later, samples of the culture water were serially diluted in NSS and were plated on TCBS Cholera medium (Oxoid, Unipath Ltd., United Kingdom) in order to determine the viable counts of V. proteolyticus CW8T2. Bacterial colony type allowed a clear distinction between LVS8 and V. proteolyticus CW8T2, as the pathogen develops characteristic green colonies on TCBS agar plates, while LVS8 has a bigger, yellow colony type. LVS2 is not able to grow on TCBS.
In vitro antagonism test.
The production of inhibitory compounds against V. proteolyticus CW8T2 was assessed in vitro using the double-layer method (4). Marine agar plates were spot-inoculated with 10 μl of an overnight marine broth culture of one of the nine strains and were incubated at 28°C for 48 h. After killing the macrocolonies with chloroform vapors, a soft marine agar overlay (marine broth plus 7.5 g of agar-agar/liter) just inoculated with an overnight broth culture of V. proteolyticus CW8T2 (1/100 dilution) was poured on top of it. The plates were then incubated for 24 h at 28°C, and the presence of a clear inhibition zone around the macrocolony indicated that inhibitory compounds had been produced by the macrocolony. Each strain was tested in triplicate, and autoinhibition of V. proteolyticus CW8T2 was examined.
A modified double-layer method was also applied. The procedure described above was used, but the overlay consisted of marine agar (not soft agar). Twenty-four hours after pouring, the overlay was spread with 100 μl of an overnight broth culture of V. proteolyticus CW8T2.
Filtrate experiments.
In order to determine whether extracellular components are involved in the protective action of LVS2 and LVS8, experimental infections were done in Artemia cultures grown in sterile filtrates of these strains. The filtrates were made by inoculating the selected strains at a concentration of 5 × 106 cells/ml in culture tubes containing 30 ml of autoclaved artificial seawater, but without Artemia. Dry food was added once at a dose of 7.14 mg/tube. The tubes were incubated for 2 days at 28°C. Subsequently the filtrates were prepared through filter sterilization (0.22 μm) of the bacterial suspensions. Tubes receiving only dry food were used to obtain a control filtrate. Another control consisted of untreated autoclaved artificial seawater. Furthermore, some of the culture tubes containing the filtrates of LVS2 and LVS8 were supplemented with 5 × 106 CFU/ml of the corresponding strain to check whether results similar to those of the in vivo antagonism tests would be obtained.
Twenty freshly hatched nauplii were transferred to culture tubes containing the different filtrates. After 1 day, V. proteolyticus CW8T2 was added (103 CFU/ml) and the survival was measured after 2 days, as in the in vivo antagonism test. The experiment was performed twice, with two to four replicates per treatment.
RESULTS
Identification of bacteria.
The nine bacterial strains were first identified based on several biochemical tests. These results are shown in Table 1. The strains that were tentatively identified as Vibrionaceae were further examined by FAME analysis and/or with API20NE. LVS3 and LVS9 were identified by FAME analysis as Aeromonas sp. and Vibrio alginolyticus, respectively. API20NE identified LVS3, LVS8, and LVS9 as Aeromonas hydrophila or Aeromonas caviae, V. alginolyticus, and V. alginolyticus, respectively, and confirmed the results obtained by FAME analysis.
TABLE 1.
Presumed identity of the nine selected bacterial strains, based on several biochemical tests
| Strain | Shapea | Size (μm) | Motility | Gram stain | Catalase | Oxidase | Glucose metabolismc | TMAO reduction | H2S production | Tentative identity |
|---|---|---|---|---|---|---|---|---|---|---|
| LVS1 | R | 5 | + | + | + | − | − | − | − | Kurthia |
| LVS2 | R | 5–6 | + | + | + | − | − | − | − | Kurthia |
| LVS3 | R | 3–4 | + | − | + | + | F | + | + | Vibrionaceae |
| LVS4 | R | 2–4 | − | − | + | + | − | − | − | Moraxella |
| LVS5 | R | 2–3 | + | − | + | + | − | − | − | Alcaligenes |
| LVS6 | R | 1–2 | + | + | + | − | − | − | − | Kurthia |
| LVS7 | R | NDb | − | + | + | − | O | ND | ND | Nocardia |
| LVS8 | R | 3–4 | + | − | + | − | F | + | + | Vibrionaceae |
| LVS9 | R | 2–3 | + | − | + | − | F | + | + | Vibrionaceae |
R, rod shaped.
ND, not determined.
−, inert; O, oxidative; F, fermentative.
Pathogenicity of V. proteolyticus CW8T2 under conditions of feeding and starvation.
Under conditions of feeding, all Artemia died within 48 h after the experimental infection, regardless of the applied concentration (from 1 × 102 to 5 × 106 CFU/ml), while less than 10% mortality was observed in the axenic control treatments.
As was observed visually in the tubes, mortality occurred faster at a higher concentration of V. proteolyticus CW8T2. Twenty-four hours after the experimental infection with 5 × 106 CFU/ml, the majority (approximately 90%) of Artemia nauplii died, while, compared to the axenic control, no clearly decreased density of Artemia in the tube was observed when 102 CFU of V. proteolyticus CW8T2 per ml had been added.
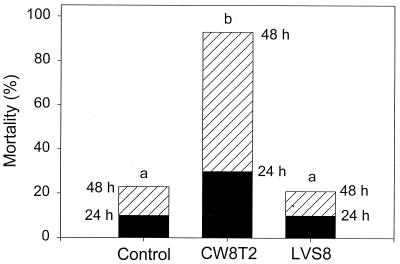
In Fig. 1 the survival data are shown for the experimental infection under conditions of starvation. Also under these conditions, V. proteolyticus CW8T2 added at a concentration of 103 CFU/ml caused high mortality among the Artemia nauplii (30% after 24 h and 93% after 48 h), while in the control treatment and in the treatment with LVS8, the mortality after 48 h was lower than 23%. Compared to that in the conditions of feeding mortality under conditions of starvation occurred less rapidly, as under conditions of feeding total mortality had always been recorded after 48 h. The statistical significance of these data was based on a Student t test performed on the Arcsinus-transformed survival data of the different treatments, with a significance level of 0.05.
FIG. 1.
Cumulative mortality of starved Artemia nauplii 24 and 48 h after administration of V. proteolyticus CW8T2 at a concentration of 103 CFU/ml and LVS8 at a concentration of 5 × 106 cells/ml. Different letters (above each bar) indicate significant differences in mortality after 48 h.
Histological observation of infection process.
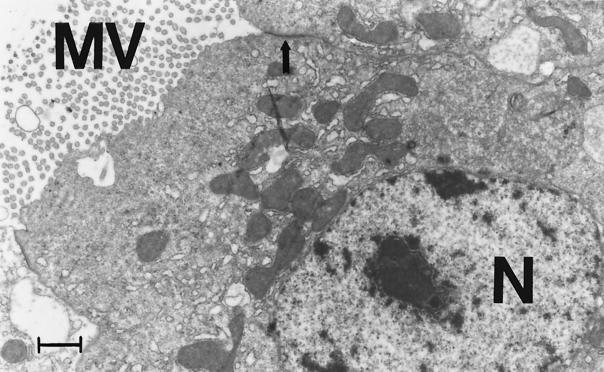
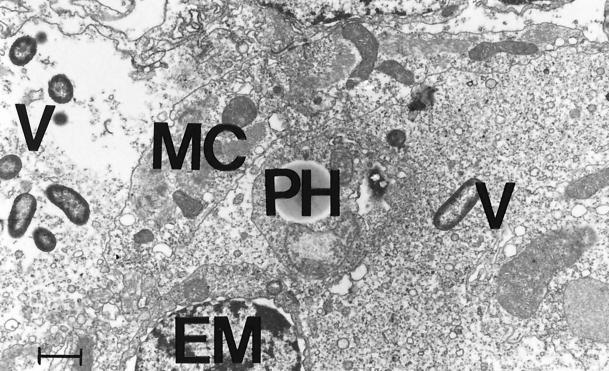
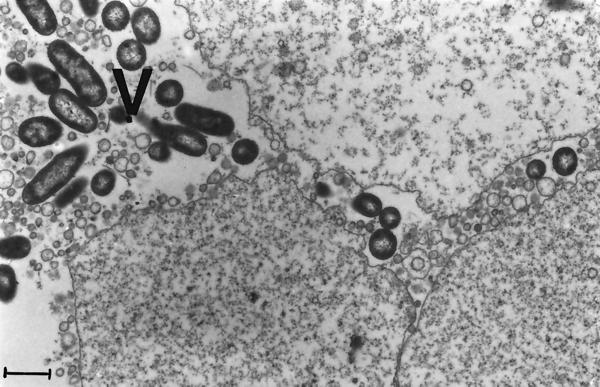
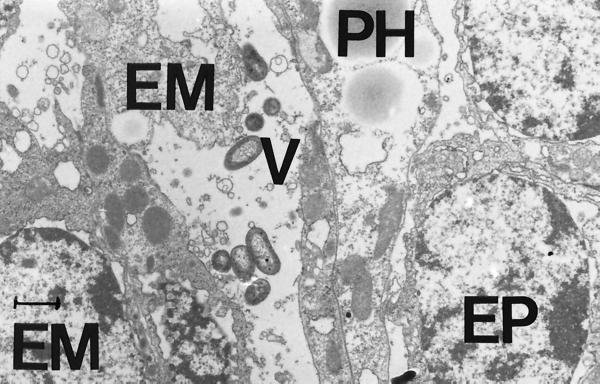
The process of infection of Artemia by V. proteolyticus CW8T2 on a histological level is illustrated in Fig. 2 to 5. In Fig. 2 epithelial cells from the anterior part of the gut of axenic Artemia are shown. The apical surface bordering the gut lumen shows short irregular microvilli and zonulae adherents between neighboring cells. Figure 3 shows a similar part of the gut of Artemia nauplii infected with V. proteolyticus CW8T2. The microvilli have disappeared, and the border of the epithelial cells seems to be liquefied. The cell junctions are affected, and the bacterial cells penetrate between the epithelial cells and force their way through the gut epithelium. Figures 4 and 5 show the body cavity in which the bacterial cells have penetrated and continue their devastating activity. One can see host cells that are not affected yet, such as muscle cells, fat-storing cells, and unidentified embryonic cells. Eventually these cells and whole tissues are also affected. In Fig. 5 one can see the bacterial cells surrounded by debris of destroyed host cells.
FIG. 2.
Epithelial cells of the anterior part of the gut of axenic Artemia. The apical cell surface is coated with short irregular microvilli (MV). Apical zonulae adherents link adjacent epithelial cells (arrow). N, nucleus. Bar = 1 μm. Magnification, ×9,000.
FIG. 5.
V. proteolyticus CW8T2 among cell debris in the body cavity of Artemia. PH, phagocytic storage cell; EM, unidentified embryonic cell; MC, muscle cell; V, bacterial cell. Bar = 1 μm. Magnification, ×9,000.
FIG. 3.
Electron micrograph demonstrating how V. proteolyticus CW8T2 causes lysis of the apical part of the epithelial cells of the anterior part of the gut. The apical zonulae adherents are destroyed, allowing the bacteria to penetrate between the epithelial cells. V, bacterial cells. Bar = 1 μm. Magnification, ×10,000.
FIG. 4.
V. proteolyticus CW8T2 in the body cavity of Artemia. PH, phagocytic storage cell; EP, epidermal cell; EM, unidentified embryonic cell; V, bacterial cell. Bar = 1 μm. Magnification, ×9,000.
When axenic and infected Artemia were viewed with light microscopy it was observed visually that the number of blood cells in the body cavity of infected Artemia nauplii was lowered to approximately one-fourth that found in axenic Artemia nauplii of the same age. Furthermore, no phagocytosis by the blood cells was observed.
In vivo antagonism test.
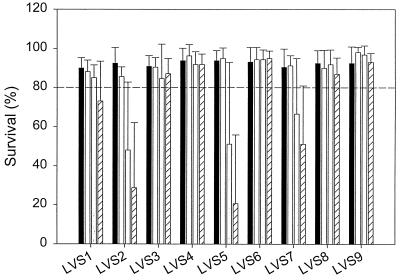
The results of the in vivo antagonism tests are shown in Fig. 6. The survival of the Artemia nauplii was recorded 2 days after the infection. The treatments shown in Fig. 6 are the axenic control treatment, the cultures inoculated with only the selected bacterial strains (5 × 106 cells/ml), and the cultures both inoculated with the selected strains (5 × 106 cells/ml) and infected with V. proteolyticus CW8T2 at a dose of 102 or 103 CFU/ml. In all Artemia cultures infected only with V. proteolyticus CW8T2 total mortality occurred within 2 days, regardless of the applied initial concentration of the pathogen (data not shown). The in vivo antagonism tests showed that all the selected strains were able to protect the Artemia from the pathogenic action of V. proteolyticus CW8T2, as the survival rate in the presence of each of these strains was always higher than that when only V. proteolyticus CW8T2 was added to the culture.
FIG. 6.
Survival of Artemia nauplii 48 h after infection with V. proteolyticus CW8T2 in culture water preemptively colonized with the nine selected bacterial strains (LVS1 to LVS9). Culture types are indicated by bar fill patterns, as follows: black, axenic Artemia culture (control); cross-hatched, Artemia culture preemptively colonized by the selected bacterial strain; white, Artemia culture preemptively colonized by the selected bacterial strain and infected with V. proteolyticus CW8T2 at a concentration of 102 CFU/ml; and striped, Artemia culture preemptively colonized by the selected bacterial strain and infected with V. proteolyticus CW8T2 at a concentration of 103 CFU/ml. Data are averages plus standard deviations (error bars).
Furthermore, based on Fig. 6, two categories of the selected strains can be distinguished. (i) In one category no increased mortality of the Artemia was observed following the infection, showing that these strains provided total protection against the pathogenic action of V. proteolyticus CW8T2 under the given experimental conditions, as the average rate of survival was higher than 80%. This category includes the strains LVS3, LVS4, LVS6, LVS8, and LVS9. (ii) A second category includes strains only providing a partial protection against V. proteolyticus CW8T2. Significant but not total mortality occurred with LVS1, LVS2, LVS5, and LVS7. The average survival rate was lower than 80% (except for that of LVS1 with the lowest concentration of the pathogen), and a decrease in survival was observed when the initial pathogen concentration was increased from 102 to 103 CFU/ml (Fig. 6).
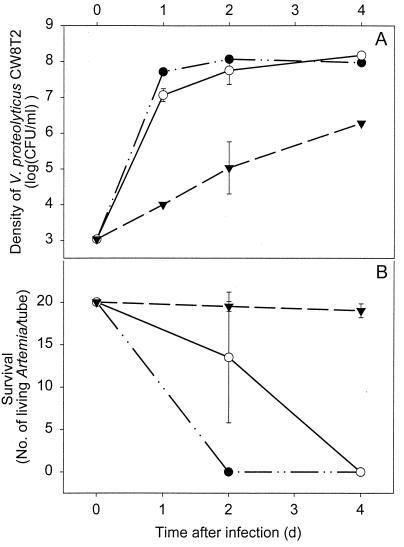
LVS2 and LVS8 were chosen as representatives of their categories. The in vivo antagonism test was repeated for these strains, but survival was now monitored up to 4 days after the infection and the viable counts of V. proteolyticus CW8T2 were determined in the culture water. These results are shown in Fig. 7. The protective action of LVS8 lasted at least till day 4 after the infection, while total mortality occurred after 4 days despite the preemptive colonization by LVS2 (Fig. 7B). The growth of V. proteolyticus CW8T2 in the culture medium was slowed down in the presence of both strains, but the growth suppression was distinctly higher with LVS8 than with LVS2 (Fig. 7A). The preemptive colonization of the culture medium with LVS8 reduced the viable counts of V. proteolyticus CW8T2 by factors of approximately 10,000, 1,000, and 100 in comparison with the control treatment when samples were taken 1, 2 and 4 days after the experimental infection, respectively.
FIG. 7.
Viable counts of V. proteolyticus CW8T2 in the culture water (A) and survival of Artemia up to 4 days after infection with V. proteolyticus CW8T2 (103 CFU/ml) (B) in the cultures preemptively colonized with LVS2 (○) and LVS8 (▾) and in axenic culture (●) Data are averages ± standard deviations (error bars). d, days.
Colonization capacity of selected bacterial strains and the pathogen.
The colonization of Artemia by the selected bacterial strains was quantified 24 h after the inoculation of the culture water. The results of the MA counts are given in Table 2. It is striking that the strains that gave only partial protection against the pathogen in the in vivo antagonism test (Fig. 6) also showed a restricted capability to colonize Artemia (from 2.23 to 2.954 log CFU/Artemia nauplius for LVS1, LVS2, LVS5, and LVS7). In comparison, the strains totally protecting the Artemia nauplii showed a higher colonization capacity (from 3.37 to 4.18 log CFU/Artemia nauplus for LVS3, LVS4, LVS6, LVS8 and LVS9).
TABLE 2.
Bacterial colonization of Artemiaa
| Strain | Bacterial colonization (log CFU/Artemia nauplius) |
|---|---|
| LVS1 | 2.954 ± 0.058 |
| LVS2 | 2.95 ± 0.33 |
| LVS3 | 3.54 ± 0.11 |
| LVS4 | 3.79 ± 0.12 |
| LVS5 | 2.80 ± 0.40 |
| LVS6 | 4.01 ± 0.59 |
| LVS7 | 2.23 ± 0.44 |
| LVS8 | 4.18 ± 0.31 |
| LVS9 | 3.37 ± 0.16 |
| Vibrio proteolyticus CW8T2 | 4.70 ± 0.13 |
Artemia nauplii were cultured in seawater preemptively colonized with one of the nine selected strains (5 × 106 cells/ml) or infected with V. proteolyticus CW8T2 (103 CFU/ml). Bacterial colonization was determined through MA plate counts (values are averages ± standard deviations; n ≥ 3).
The colonization capacity of V. proteolyticus CW8T2 was also quantified, but this strain was inoculated at 1 × 103 CFU/ml instead of 5 × 106 cells/ml. Despite the much lower number of bacteria added initially, the colonization of the Artemia with V. proteolyticus CW8T2 was the highest of all the strains tested. The data in Table 2 illustrate well the very high growth and colonization rate of this bacterium.
In vitro antagonism test.
The results of the double-layer in vitro antagonism tests were all negative, as no inhibition zone could be observed around the macrocolonies of the selected strains. Also, no autoinhibition of V. proteolyticus CW8T2 was observed. The same observations were made with the modified procedure.
Filtrate experiments.
Artemia was cultured in sterile filtrates of LVS2 and LVS8 to see whether extracellular compounds are involved in the protection of the animals against V. proteolyticus CW8T2. Two days after the experimental infection (103 CFU/ml) the survival in the different treatments was determined (Table 3). The filtrates of both LVS2 and LVS8 did not significantly increase the survival compared to the infected control filtrate and the infected control seawater, as virtually total mortality occurred in all those treatments. When living cells of LVS2 and LVS8 were added to their corresponding filtrates, results were similar to those obtained in the in vivo antagonism tests (Fig. 6): the living cells of LVS2 and LVS8 partially and totally protected Artemia against the pathogenic action of V. proteolyticus CW8T2, respectively.
TABLE 3.
Survival of Artemia nauplii infected with V. proteolyticusa
| Strain | Survival (%) |
|---|---|
| Control seawater | 1.3 ± 2.5 |
| Control filtrate | 1.3 ± 2.5 |
| LVS2 filtrate | 0 ± 0 |
| LVS2 filtrate plus LVS2 | 28 ± 27 |
| LVS8 filtrate | 0.6 ± 1.8 |
| LVS8 filtrate plus LVS8 | 98.0 ± 5.7 |
Artemia nauplii were infected with V. proteolyticus CW8T2 (103 CFU/ml), and survival rates were determined for sterile filtrates of the strains LVS2 and LVS8, with and without addition of the strain itself (5 × 106 cells/ml). Values are averages ± standard deviations (n = 4 to 8).
DISCUSSION
In this study V. proteolyticus CW8T2 has been identified as a virulent pathogen for Artemia (Fig. 1) and the infection process of V. proteolyticus CW8T2 has been visualized by transmission electron microscopy (Fig. 2 to 5). It was shown that preemptive colonization of the culture water with selected bacterial strains did protect, at least partially, the Artemia nauplii against the pathogenic effects of the strains (Fig. 6 and 7). The in vitro antagonism tests and the filtrate experiments showed that probably no extracellular compounds were involved in the protective action (Fig. 7 and Table 3).
Although V. proteolyticus CW8T2 demonstrated a high virulence under both feeding and starvation conditions, mortality occurred faster when Artemia nauplii were fed (Fig. 1). It is possible that under fed conditions V. proteolyticus CW8T2 developed faster due to the nutrient enrichment of the culture medium or that ingestion of the pathogen was increased due to adhesion to the food particles. Infection of fed Artemia nauplii has also been accomplished in previous experiments 4 days after hatching instead of 1 day after hatching. Despite the later development stages, all Artemia nauplii in those experiments also died within 48 h (data not shown), indicating that a better resistance towards infection with V. proteolyticus CW8T2 in the in vivo antagonism tests could not simply be assigned to a later developmental stage of Artemia due to the nutritional contribution of the selected strains.
The infection route of V. proteolyticus CW8T2 was monitored by electron microscopy (Fig. 2 to 5). It was observed that the bacteria penetrated through the gut epithelium and invaded the body cavity. The gut epithelium and the underlying cells and tissues were clearly affected by the devastating action of the pathogen, which probably eventually causes death of the organism. The observed decrease in blood cell numbers in infected Artemia compared to axenic ones could not be explained. No phagocytizing blood cells were found in the infected Artemia, contrary to the observations of G. G. Martin et al. (submitted for publication). It was also striking that the colonization capacity of V. proteolyticus CW8T2 was much higher than that of the selected strains, despite the much lower initial density (103 CFU/ml versus 5 × 106 cells/ml) (Table 2). This explosive growth capacity probably contributed to the virulence of the pathogen, causing a rapid death of infected Artemia.
Several pathogens for Artemia have been described so far (2, 10, 13, 14, 16). Overton and Bland (13) described extensively the infection process of Artemia by the fungus Haliphthoros milfordensis, from the attachment to the exoskeleton to the utilization of the host tissues, with final invasion of the gut by the fungus. Gunther and Catena (10) exposed Artemia nauplii to three species of Vibrio at a concentration of 108 cells/ml. Two of the three strains coated the shrimp's body in 2 to 8 h, as was shown on scanning electron micrographs of the nauplii, and completely inhibited swimming. Solangi et al. (16) observed also infestation of brine shrimp by a filamentous bacterium tentatively identified as Leucothrix mucor at the shrimp's exterior, causing a slow death of the Artemia. Tyson (19, 20) observed spirochetes inside the Artemia's body, but the infection route was not clarified and it was not clear whether this infection caused mortality or not. Similarly to this study, Grisez et al. (9) examined the infection route of V. anguillarum in turbot Scophthalmus maximus larvae and showed with immunohistochemistry that V. anguillarum was transported stepwise through the gut wall and subsequently by the blood to the different organs, eventually leading to septicemia and mortality.
The in vivo antagonism tests showed that all the selected strains were able to protect Artemia, at least partially, against the pathogenic action of V. proteolyticus CW8T2 (Fig. 6 and 7). LVS8 suppressed the growth of V. proteolyticus CW8T2 dramatically (Fig. 7A). The growth suppression of V. proteolyticus CW8T2 shown in Fig. 7A was clearly correlated with Artemia survival depicted in Fig. 7B, as survival after 2 days was the highest in the culture waters carrying the lowest density of V. proteolyticus CW8T2 and vice versa. Although the viable count of the pathogen still increased during the culture period, the survival of the Artemia cultured with LVS8 was apparently not affected, probably as a consequence of a lower exposure to the pathogen. Contrary to the observations of Gibson et al. (6), the densities of the pathogen did not decrease in the culture, but the proliferation of V. proteolyticus CW8T2 was slowed down. It is likely that eventually the concentration of V. proteolyticus CW8T2 will increase to a lethal concentration. Delay in mortality following a probiotic treatment was also observed by Gildberg and Mikkelsen (7). They supplemented a commercial dry feed with two strains of Carnobacterium divergens isolated from fish intestines and administered it during 3 weeks to Atlantic cod (Gadus morhua) fry. Twelve days after the infection with a virulent V. anguillarum a lower cumulative mortality was recorded, but 4 weeks after the infection the same cumulative mortality as in the control group was reached. Thus, the main effect of the probiotic treatment was a delay in mortality of infected cod fry. The authors argued, however, that this observation does not exclude the considerable importance of such methods under normal rearing conditions in the presence of moderate levels of opportunistic bacteria. Furthermore, total protection by LVS8 was demonstrated at least up to 4 days after the experimental infection (Fig. 7B). This time period can be qualified as considerable, as the culture period for Artemia juveniles in stagnant culture system is usually limited to 7 days (3).
No inhibition of V. proteolyticus CW8T2 was observed in the in vitro antagonism test (double-layer methods). Also the experimental infections of Artemia grown in the filtrates of LVS2 and LVS8 led to total mortality within 48 h (Table 3). One can conclude that no extracellular compounds such as antibiotics or siderophores are involved in the suppression of V. proteolyticus CW8T2 but that living cells are required. The observation that all the selected strains at least partially protected the Artemia after the experimental infection (Fig. 6) suggests a general mode of action, such as competition for chemicals, available energy, or adhesion sites. The correlation between the colonization potential and the protective ability of the selected strains is striking (Table 2 and Fig. 6, respectively). The lowest level of protection was observed for the strains with the lowest colonization capacity. This observation could support the hypothesis of competition for adhesion sites on or in the shrimps, but a higher colonization may also be a consequence of a more efficient use of resources like chemicals and available energy present in the ambient environment. The observed growth suppression in the culture medium (Fig. 7) and the fact that extracellular compounds do not seem to be involved in the protective action (Table 3) indicate that preemptive colonization allows the selected bacterial strains to compete efficiently for chemicals or available energy with the pathogen and to suppress its development.
It has been demonstrated in this study that preemptive colonization by the selected bacterial strains could prevent the proliferation not only of V. proteolyticus CW8T2 but probably also of other pathogens or opportunistic pathogens in the culture of Artemia juveniles. This shows that, apart from their nutritional contribution demonstrated by Verschuere et al. (23), the selected bacterial strains could also act as biological control agents of infections.
ACKNOWLEDGMENTS
This research was partly made possible by the project No. 3G006396 of the Fonds voor Wetenschappelijk Onderzoek (FWO).
We thank Lone Gram, Ria Vanhoudt, and Johan Vandenberghe for their help in the bacterial identification, Wim Mondelaers for the irradiation of the food, F. Roels for the use of the electron microscope, and Simone Van Hulle for her technical support.
REFERENCES
- 1.Barrow G I, Feltham R K A. Cowan and Steel's manual for identification of medical bacteria. 3rd ed. Cambridge, England: Cambridge University Press; 1993. [Google Scholar]
- 2.Criado-Fornelio A, Mialhe E, Constantin E, Grizel H. Experimental infection of Artemia sp. by Fusarium solani. Bull Eur Assoc Fish Pathol. 1989;9:35–37. [Google Scholar]
- 3.Dhont J, Lavens P. Tank production and use of ongrown Artemia. In: Lavens P, Sorgeloos P, editors. Manual on the production and use of live food for aquaculture. Food and Agricultural Organization Fisheries technical paper no. 361. Rome, Italy: Food and Agriculture Organization of the United Nations; 1996. pp. 164–195. [Google Scholar]
- 4.Dopazo C P, Lemos M L, Lodeiros C, Bolinches J, Barja J L, Toranzo A E. Inhibitory activity of antibiotic-producing marine bacteria against fish pathogens. J Appl Bacteriol. 1988;65:97–101. doi: 10.1111/j.1365-2672.1988.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 5.Garriques D, Arevalo G. An evaluation of the production and use of a live bacterial isolate to manipulate the microbial flora in the commercial production of Penaeus vannamei postlarvae in Ecuador. In: Browdy C L, Hopkins J S, editors. Swimming through troubled water. Proceedings of the Special Session on Shrimp Farming, Aquaculture '95. Baton Rouge, La: World Aquaculture Society; 1995. pp. 53–59. [Google Scholar]
- 6.Gibson L, Woodworth J, George A. Probiotic activity of Aeromonas media on the Pacific oyster, Crassostrea gigas, when challenged with Vibrio tubiashii. Aquaculture. 1998;169:111–120. [Google Scholar]
- 7.Gildberg A, Mikkelsen H. Effects of supplementing the feed of Atlantic cod (Gadus morhua) fry with lactic acid bacteria and immuno-stimulating peptides during a challenge trial with Vibrio anguillarum. Aquaculture. 1998;167:103–113. [Google Scholar]
- 8.Gram L, Melchiorsen J, Spanggaard B, Huber I, Nielsen T. Inhibition of Vibrio anguillarum by Pseudomonas fluorescens strain AH2—a possible probiotic treatment of fish. Appl Environ Microbiol. 1999;65:969–973. doi: 10.1128/aem.65.3.969-973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grisez L, Chair M, Sorgeloos P, Ollevier F. Mode of infection and spread of Vibrio anguillarum in turbot Scophthalmus maximus larvae after oral challenge through live feed. Dis Aquat Org. 1996;26:181–187. [Google Scholar]
- 10.Gunther D C, Catena A. The interaction of Vibrio with Artemia nauplii. In: Persoone G, Sorgeloos P, Roels O, Jaspers E, editors. The brine shrimp Artemia—ecology, culturing and use in aquaculture. Vol. 3. Wetteren, Belgium: Universa Press; 1980. pp. 213–221. [Google Scholar]
- 11.Lunestad B T. Notes of the workshop aquaculture/environment interactions: impacts on microbial ecology. Halifax, Nova Scotia, Canada: Canadian Aquaculture Society; 1998. Impact of farmed fish on the environment, presentation by representative of Norway; p. 53. [Google Scholar]
- 12.Olsson J C, Westerdahl A, Conway P L, Kjelleberg S. Intestinal colonization potential of turbot (Scophthalmus maximus)- and dab (Limanda limanda)-associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol. 1992;58:551–556. doi: 10.1128/aem.58.2.551-556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overton S V, Bland C E. Infection of Artemia salina by Haliphthoros milfordensis: a scanning and transmission electron microscope study. J Invertebr Pathol. 1981;37:249–257. [Google Scholar]
- 14.Puente M E, Vega-Villasanta F, Holguin G, Bashan Y. Susceptibility of the brine shrimp Artemia and its pathogen Vibrio parahaemolyticus to chlorine dioxide in contaminated seawater. J Appl Bacteriol. 1992;73:465–471. doi: 10.1111/j.1365-2672.1992.tb05006.x. [DOI] [PubMed] [Google Scholar]
- 15.Sakai M. Current research status of fish immunostimulants. Aquaculture. 1999;172:63–92. [Google Scholar]
- 16.Solangi M A, Overstreet R M, Gannam A L. A filamentous bacterium on the brine shrimp and its control. Gulf Res Rep. 1979;6:275–281. [Google Scholar]
- 17.Sorgeloos P, Coutteau P, Dhert P, Merchie G, Lavens P. Use of brine shrimp, Artemia spp., in larval crustacean nutrition: a review. Rev Fish Sci. 1998;6:55–68. [Google Scholar]
- 18.Subasinghe R. Fish health and quarantine. Review of the state of the world aquaculture. Food and Agriculture Organization Fisheries circular no. 886. Rome, Italy: Food and Agriculture Organization of the United Nations; 1997. pp. 45–49. [Google Scholar]
- 19.Tyson G E. Ultrastructure of a spirochete found in tissues of the brine shrimp, Artemia salina. Arch Microbiol. 1974;99:281–294. doi: 10.1007/BF00696243. [DOI] [PubMed] [Google Scholar]
- 20.Tyson G E. Phagocytosis and digestion of spirochetes by amoebocytes of infected brine shrimp. J Invertebr Pathol. 1975;26:105–111. doi: 10.1016/0022-2011(75)90175-5. [DOI] [PubMed] [Google Scholar]
- 21.Vandamme P, Vancanneyt M, Pot B, Mels L, Hoste B, Dewettinck D, Vlaes L, Van Den Borre C, Higgins R, Hommez J, Kersters K, Butzler J P, Goossens H. Polyphasic taxonomic study of the emended genus Arcobacter with Arcobacter butzleri comb. nov. and Acrobacter skirrowii sp. nov., an aerotolerant bacterium from veterinary specimens. Int J Syst Bacteriol. 1992;42:344–356. doi: 10.1099/00207713-42-3-344. [DOI] [PubMed] [Google Scholar]
- 22.Vandenberghe J, Verdonck L, Robles-Arozarena R, Rivera G, Bolland A, Balladares M, Gomez-Gil B, Calderon J, Sorgeloos P, Swings J. Vibrios associated with Litopenaeus vannamei larvae, postlarvae, broodstock, and hatchery probionts. Appl Environ Microbiol. 1999;65:2592–2597. doi: 10.1128/aem.65.6.2592-2597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verschuere L, Rombaut G, Huys G, Dhont J, Sorgeloos P, Verstraete W. Microbial control of the culture of Artemia juveniles through preemptive colonization by selected bacterial strains. Appl Environ Microbiol. 1999;65:2527–2533. doi: 10.1128/aem.65.6.2527-2533.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verschuere L, Dhont J, Sorgeloos P, Verstraete W. Monitoring biolog patterns and r/K-strategists in the intensive culture of Artemia juveniles. J Appl Microbiol. 1997;83:603–612. [Google Scholar]
- 25.Wooldridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda K, Taga N. A mass culture method for Artemia salina using bacteria as food. Mer. 1980;18:53–62. [Google Scholar]