Abstract
Background & aims
Several studies have shown conflicting results for the relationship between vitamin D deficiency and COVID-19 outcomes. Here, we aimed to evaluate whether plasma 25(OH)D levels predict mortality in adults admitted with COVID-19, considering potential confounders.
Methods
We conducted a retrospective cohort study that included 115 adults (age 62.1 ± 17.6 years, 65 males) admitted to a Brazilian public hospital for severely symptomatic COVID-19. Subjects were classified into two groups according to their plasma levels of 25(OH)D: sufficiency (≥50 nmol/L) and the deficiency (<50 nmol/L). The diagnosis of COVID-19 was performed using real-time polymerase chain reaction (qPCR). In addition, direct competitive chemiluminescence immunoassay assessed serum 25(OH)D levels.
Results
The all-cause 30-day mortality was 13.8% (95% CI: 6.5%–21%) in the group of patients with sufficient plasma 25(OH)D levels and 32.1% (95% CI: 14.8%–49.4%) among those with deficient plasma 25(OH)D levels. Cox regression showed that plasma 25(OH)D levels remained a significant predictor of mortality even after adjusting for the covariates sex, age, length of the delay between symptom onset and hospitalization, and disease severity (HR = 0.98, 95% CI: 0.96–1.00; p = 0.02).
Conclusion
Vitamin D deficiency predicts higher mortality risk in adults with COVID-19.
Keywords: COVID-19, SARS-CoV-2, Vitamin D, Mortality
1. Introduction
The disease caused by COVID-19 has a broad spectrum of severity, ranging from asymptomatic cases to death, usually attributed to interstitial pneumonia followed by acute respiratory distress syndrome (ARDS) [1]. However, subsequent studies have found that cardiovascular complications such as vascular dysfunction, thrombosis, myocardial insufficiency, and shock are also important causes of death [2].
The pathophysiology of COVID-19 includes viral invasion and replication in several tissues, such as respiratory and gastrointestinal epithelium and vascular endothelium. This is followed by a deregulated immune response, excessive inflammation and oxidation, cytokine storm, dysfunction of the renin-angiotensin system, and lack of endothelial integrity [2]. In addition, elevated serum levels of the pro-inflammatory cytokines IL-6 and TNF-α are associated with an increased likelihood of ARDS and death [3].
Vitamin D is a pluripotent steroid hormone that modulates innate and adaptive immune responses and acts on the integrity of the respiratory epithelium physical barrier [3,4]. In addition, vitamin D has an antiviral effect that can directly prevent viral replication [3,4].
There is strong evidence to indicate that vitamin D inhibits Th1 cell function and reduces the production of TNF-α, IL-2, IL-6, and IFN-β. In contrast, vitamin D enhances the action of Th2 cells and the production of anti-inflammatory cytokines, like IL 4, IL-5, and IL-10 [4,5]. This evidence led researchers to hypothesize that the excess innate immune response observed in patients with COVID-19 can be effectively modulated by vitamin D [[3], [4], [5]].
Observational studies have reported that serum 25-hydroxyvitamin D concentrations are inversely correlated with the severity of COVID-19 [[6], [7], [8]].
We aimed to evaluate whether plasma 25(OH)D levels predict mortality in adults with COVID-19, considering potential confounders. To this end, we examined subjects admitted to the hospital with different severity levels and followed them for 30 days. We hypothesized that plasma 25-hydroxyvitamin D levels could predict worse outcomes.
2. Materials & methods
We conducted a cohort study at the University Hospital of the Federal University of São Carlos (UFSCar), São Carlos, Brazil. Data were collected through in-person interviews of the study population between July and December 2020. Following the WHO guidelines, the baseline cohort consisted of a convenience sample of adults diagnosed with COVID-19. The exclusion criteria were pregnancy, breastfeeding, or current use of supplements containing vitamin D.
In addition, the study followed the guidelines of the Brazilian National Health Council (federal resolution 466/2012). The Institutional Research Ethics Committee approved all study participants’ procedures (30184220.8.0000.5504).
Subjects were assessed daily from hospital admission to hospital discharge or 30 days of hospitalization. The patients were treated with a standard protocol, which included antibiotics for concurrent bacterial pneumonia, enoxaparin for thromboembolism prevention, and dexamethasone in selected cases. At the time of the study, no specific treatment for COVID-19 had been approved in Brazil.
We obtained for each subject the following data at hospital admission: sociodemographic, clinical characteristics, chronic comorbidities (Charlson Comorbidity Index), and disease severity (baseline New Early Warning Score 2 - NEWS, and Sequential Organ Failure Assessment (SOFA). All patients received standard of care treatment for COVID-19 as the latest recommendations for managing the disease. Other specific treatments were also recorded, including dexamethasone, low-weight molecular heparin, and antibiotics.
SARS-CoV-2 infection was diagnosed using real-time polymerase chain reaction (qPCR), according to the guidelines set forth by the US Centers for Disease Control and Prevention (CDC). Plasma 25-hydroxyvitamin D levels were assessed just after the COVID-19 diagnosis, which coincided with the day of hospital admission in 40% of the patients. For the analysis, we used the LIAISON 25(OH)D Total Assay (DiaSorin, Saluggia, Italy) with functional sensitivity <10 nmol/L, 100% specificity, the dynamic range between 10 and 375 nmol/L, coefficient of variation within assay of 2.3, and inter-assay of 7.80. We used the Endocrine Society's Clinical Practice Guidelines to define vitamin D deficiency as plasma 25-hydroxyvitamin D concentrations below 50 nmol/L [9]. The primary outcome was the all-cause 30-day mortality rate.
Continuous data are presented as mean ± standard deviation or median [1st, third quartile], whereas categorical variables are presented as counts (percentages). Comparisons between groups were performed using the Wilcoxon–Mann–Whitney test for continuous variables and Pearson's Chi-squared test with Yates' continuity correction for categorical variables. The probability of survival was estimated by Kaplan–Meier analysis. The groups were compared using the log-rank test. The hazard risk (HR) and 95% confidence interval (CI) of mortality were estimated using Cox proportional hazards regression models. All variables included in the multivariate analysis were chosen based on previous studies. Statistical significance was assessed using a two-sided p-value of <0.05. All analyses were conducted using R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria) in R-Studio 1.3.1093 (RStudio Inc., Boston, USA).
3. Results
A total of 115 individuals were included in this study. Overall, the mean age was 62.1 ± 17.6 years, with a predominance of males (55.7%). The median Charlson Comorbidity Index (CCI) was 3 (1st – 3rd quartile, 1–5), and 17.4% of subjects had a high rate of comorbidities (CCI score > 5). At admission, the median NEWS-2 and SOFA scores were 4 [[3], [4], [5]] and 2 [2,3], respectively. The overall prevalence of vitamin D deficiency was 24.3% (95% confidence interval [95% CI], 17.4%–32.9%). The overall median time was 7 [[4], [5], [6], [7], [8], [9], [10]] days from symptom onset to hospital admission. A third (28.7%) of the subjects needed intensive care, and about a fifth (18.3%) died within 30 days of hospitalization. Supplementary Table 1 summarizes the study sample's baseline demographic and clinical characteristics.
The vitamin D status groups were somewhat similar for most variables, apart from age (p = 0.01) and SOFA score (p = 0.004). Supplementary Table 2 lists the characteristics of 30-day hospital outcomes and vitamin D status.
There was a trend towards a significant difference between the groups for the primary outcome (p = 0.05). The overall all-cause 30-day mortality was 18.3% (95% CI: 11.2%–25.3%) and occurred in 13.8% (95% CI: 6.5%–21%) of the patients with plasma 25-hydroxyvitamin D > 50 nmol/L and 32.1% (95% CI: 14.8%–49.4%) among those with plasma 25-hydroxyvitamin D < 50 nmol/L.
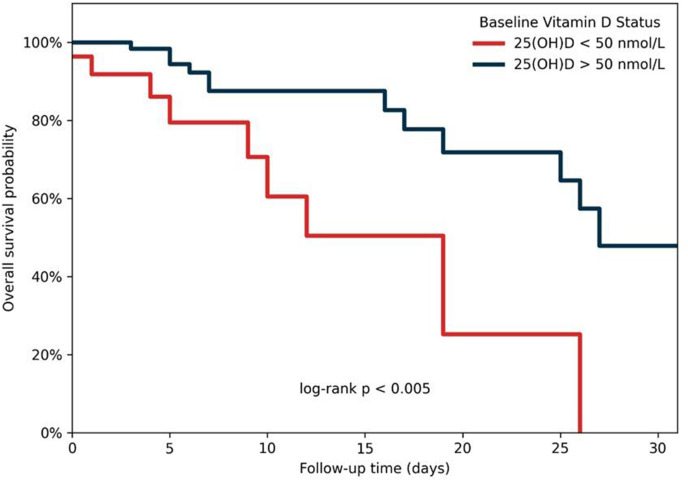
Fig 1 shows the Kaplan–Meier curves for survival probability according to vitamin D status. Subjects with plasma 25-hydroxyvitamin D levels <50 nmol/L presented a lower survival rate with faster time-to-event than those with plasma 25-hydroxyvitamin D > 50 nmol/L (log-rank test p < 0.005).
Fig. 1.
Distribution of 30-day mortality depending on vitamin D status.
The Cox regression model showed that the plasma 25-hydroxyvitamin D levels predict 30-day mortality among adults with COVID-19, even after adjusting for potential confounders such as age, sex, CCI, length (days) from symptom onset to hospital admission, SOFA score at hospital admission, and need for ICU admission during hospitalization (adjusted HR = 0.98, 95% CI: 0.96–1.00; p = 0.02) (see Supplementary Table 3).
4. Discussion
This cohort single-center study reports data on the possible association between 25-hydroxyvitamin D serum levels and the prognosis of in-hospital patients affected by SARS-CoV-2. Survival analysis showed that patients with vitamin D deficiency had a significantly higher mortality risk than those without vitamin D deficiency.
These data are consistent with the results of other observational studies. A systematic review published by Yisak et al. analyzed nine studies (1,005,042 participants) and observed a correlation between vitamin D status and COVID-19 prognosis in seven [6]. A meta-analysis aimed to assess whether low serum 25-hydroxyvitamin D levels are associated with severity and mortality related to COVID-19, including 14 studies comprising 999,179 participants. The authors concluded that low serum 25-hydroxyvitamin D levels were associated with COVID-19 severe presentation (OR = 1.90 [1.24–2.93], p = 0.003) and mortality (OR = 3.08 [1.35–7.00], p = 0.011) [7]. A new meta-analysis that analyzed seventy-two observational studies (1,976,099 participants) noted that vitamin D deficiency increased the odds of death due to COVID-19 (OR 2·07 [1·28–3·35], p = 0.003). However, this association was insignificant when studies with a high risk of bias or studies reporting unadjusted effect estimates were excluded [8].
The impact of vitamin D administration in patients with severe COVID-19 has been evaluated. A recent meta-analysis evaluated 2078 patients from nine randomized clinical trials (583 received vitamin D supplementation, while 1495 did not). Lower mortality was observed in the treated group (10.46%), compared to 25.81% in the non-treated group (OR 0.597 [0.318–1.121]; p = 0.109). The benefit of treatment was more significant in individuals who were admitted to the ICU (OR 0.326 [0.149–0.712]; p = 0.005) [10].
Our study had some limitations. First, the sample size was small. A larger sample could demonstrate a stronger association between serum 25-hydroxyvitamin D levels and COVID-19 severity. Second, there were no data available regarding the vitamin D status in these individuals before the research, not allowing us to state whether hypovitaminosis D is a cause or a consequence of severe COVID-19.
5. Conclusion
Despite these limitations, the results of our study show a higher risk of mortality in in-hospital COVID-19 patients with vitamin D deficiency, even after adjusting for potential confounders. In conclusion, vitamin D deficiency has emerged as an independent survival factor for COVID-19.
Funding statement
This work was supported by the São Paulo Research Foundation (FAPESP) grant #2020/06725-0.
Author contributions
Conceptualization, F.F.N. and H.P-J.; data curation, F.F.N., H.P-J. and S.S.S; formal analysis, F.F.N. and H.P-J.; funding acquisition, F.F.N.; investigation, F.F.N., H.P-J. and S.S.S; methodology, F.F.N., H.P-J., C.C.M.F, A.F.C. and A.A.J-J; resources, M.R.C., C.C.M.F, A.F.C. and A.A.J-J; software, H.P-J.; supervision, F.F.N. and H.P-J.; writing original draft, F.F.N. and H.P-J.; final approval: all authors.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
We are indebted to the staff and coworkers at University Hospital of the Federal University of São Carlos (UFSCar) and Brazilian Hospital Services Company (EBSERH).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnesp.2022.05.027.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Multimedia component 1
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee A., Ganguly U., Saha S., Chakrabarti S., Saini R.V., Rawal R.K., et al. Vitamin D and immuno-pathology of COVID-19: many interactions but uncertain therapeutic benefits. Expert Rev Anti Infect Ther. 2021;19(10):1245–1258. doi: 10.1080/14787210.2021.1905519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J Infect Public Health. 2020;13(10):1373–1380. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bui L., Zhu Z., Hawkins S., Cortez-Resendiz A., Bellon A. Vitamin D regulation of the immune system and its implications for COVID-19: a mini review. SAGE Open Med. 2021;9 doi: 10.1177/20503121211014073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yisak H., Ewunetei A., Kefale B., Mamuye M., Teshome F., Ambaw B., et al. Effects of vitamin D on COVID-19 infection and prognosis: a systematic review. Risk Manag Healthc Pol. 2021;14:31–38. doi: 10.2147/RMHP.S291584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbar M.R., Wibowo A., Pranata R., Setiabudiawan B. Low serum 25-hydroxyvitamin D (vitamin D) level is associated with susceptibility to COVID-19, severity, and mortality: a systematic review and meta-analysis. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.660420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dissanayake H.A., de Silva N.L., Sumanatilleke M., de Silva S.D.N., Gamage K.K.K., Dematapitiya C., et al. Prognostic and therapeutic role of vitamin D in COVID-19: systematic review and meta-analysis. J Clin Endocrinol Metab. 2021:dgab892. doi: 10.1210/clinem/dgab892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 10.Tentolouris N., Samakidou G., Eleftheriadou I., Tentolouris A., Jude E.B. The effect of vitamin D supplementation on mortality and Intensive Care Unit admission of COVID-19 patients. A systematic review, meta-analysis and meta-regression. Diabetes Metab Res Rev. 2021:e3517. doi: 10.1002/dmrr.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1