Abstract
Our understanding of the role of the parabrachial nucleus (PBN) has evolved as technology has advanced, in part due to cell-specific studies and complex behavioral assays. This is reflected in the heterogeneous neuronal populations within the PBN to the extended amygdala (EA) circuits which encompass the bed nucleus of the stria terminalis (BNST) and central amygdala (CeA) circuitry, as they differentially modulate aspects of behavior in response to diverse threat-like contexts necessary for survival. Here we review how the PBN→CeA and PBN→BNST pathways differentially modulate fear-like behavior, innate and conditioned, through unique changes in neurotransmission in response to stress-inducing contexts. Furthermore, we hypothesize how in specific instances the PBN→CeA and PBN→BNST circuits are redundant and in part intertwined with their respective reciprocal projections. By deconstructing the interoceptive and exteroceptive components of affect- and stress related behavioral paradigms, evidence suggests that the PBN→CeA circuit modulates innate response to physical stimuli and fear conditioning. Conversely, the PBN→BNST circuit modulates distress-like stress in unpredictable contexts. Thereby, the PBN provides a pathway for alarming interoceptive and exteroceptive stimuli to be processed and relayed to the EA to induce stress-relevant affect. Additionally, we provide a framework for future studies to detail the cell-type specific intricacies of PBN→EA circuits in mediating behavioral responses to threats, and the relevance of the PBN in drug-use as it relates to threat and negative reinforcement.
This article is part of the special Issue on ‘Neurocircuitry Modulating Drug and Alcohol Abuse’.
Keywords: Stress, Anxiety, Negative affect, Fear, Hyperkatifea
1. Introduction
Our understanding of the CNS aims to inform how humans perceive and respond to the world around them with an emphasis on the neuronal components that become dysregulated in disease states. As the field moves forward, we have begun to unravel the circuit intricacies modulating specific aspects of the naturalistic behaviors we investigate. The anatomical and functional data derived from preclinical models provide insight into the evolutionary role and thereby an understanding of brain region-specific functions across species. In the same way, the function that research attributes to the parabrachial nucleus (PBN) has evolved from its initial role as a feeding center to an alarm-like role in stress contexts. An alarm must process a variety of threats (Saper, 2016) and thus the following review will focus on how the circuitry stemming from the PBN modulates distinct aspects of this alarm-like role in stress-associated states. One of the major PBN afferent sources and efferent targets, the extended amygdala (EA) is heavily studied for its role in anxiety and fear. Thus, it is no surprise that an aspect of the function of the EA encompasses an “alarm” element. Below we review how the.
PBN→EA circuits process discrete but intertwined affective components of the PBN “alarm” system, particularly as they relate to behavioral responses to threats that are necessary for survival. Additionally, given that the EA modulates drug-associated negative states we explore how the PBN may modulate the EA during the negative reinforcement cycle of addiction.
2.1. Anatomical components of the PBN
The parabrachial nucleus (PBN) is a multisensory relay station that surrounds the superior cerebellar peduncles located in the dorsolateral pons (Chiang et al., 2019; Palmiter, 2018; Paxinos et al., 2012). The PBN conveys somatosensory signals from the spinal cord and cranial nerves. Nociceptive neurons in the PBN receive direct monosynaptic input from primary sensory neurons in the ipsilateral trigeminal ganglion (Rodriguez et al., 2017). This direct input from the sensory neurons underscores the importance of the PBN in receiving and conveying responses to external stimuli. The PBN is reciprocally connected to the nucleus tractus solitarius (NTS), the greater amygdala, insular cortex, and thalamus. The population of cells encompassing the efferent and afferent projections of the PBN are localized in a region-specific manner. In rodents, the cytoarchitecture of the PBN can be defined as 12 individual subnuclei (Chiang et al., 2019; Fulwiler and Saper, 1984). More broadly, the PBN is divided into the medial (mPBN) and lateral PBN (lPBN) differentiated by cell type, cell size, connectivity and neuronal cell markers as they are made of heterogenous and homogenous cell populations, respectively (Chiang et al., 2019). Compared to rodents, a similar cytoarchitecture and role has been described for the PBN in humans and nonhuman primates but with overall fewer subnuclei and absence of gustatory inputs (Chiang et al., 2019; Pritchard et al., 2000). This anatomical difference, hypothesized to have evolved over time, further emphasizes the complex nature of the PBN. While this species-specific difference is interesting and requires further investigation, as a whole the functional relevance of the overall PBN is maintained across species.
Cells in the PBN express various neuropeptides and neuromodulators, including tachykinin 1, cholecystokinin (CCK), enkephalin (Enk), oxytocin, vasopressin, tyrosine hydroxylase, neurotensin (NT), dynorphin (Dyn), prepronociceptin (PNOC), serotonin, and corticotrophin releasing hormone (Crh) (Fig. 1) (Block and Hoffman, 1987; Chiang et al., 2019; Palmiter, 2018). Cells in the PBN are also defined by expression of transcription factors such as Foxp2 or Lmx1b, or receptors including leptin receptor, neuropeptide Y receptor 1, or oxytocin receptor (Chiang et al., 2019; Palmiter, 2018). The main peptides expressed in PBN projections are calcitonin gene related peptide (CGRP) and pituitary adenylate cyclase-activating polypeptide (PACAP) and all neurons that project from the PBN to the EA express CGRP or PACAP (Qiao et al., 2019). On this basis, various studies and this review will use PACAP and CGRP manipulations in the EA to thereby interpret the role of the PBN→EA circuit. Two parallel circuits along the anterolateral pathway from projection neurons of the spinal cord to the PBN exist and are differentiated based on neuronal markers (Choi et al., 2020). One of the projections primarily synapses on CGRP + neurons in the PBN. Although it hasn’t been fully investigated, PACAP would be a potential marker for the other parallel anterolateral pathway. The expression of neuronal markers is distributed across specific nuclei of the PBN dependent on the function of the nuclei (Block and Hoffman, 1987). The complexity of the neuronal markers across the EA and the PBN will be simplified in this review, focusing solely on the neuropeptides involved in the reciprocal connections between the EA and PBN with an emphasis on PBN→EA and brief mention of EA– > PBN, described in Fig. 1.
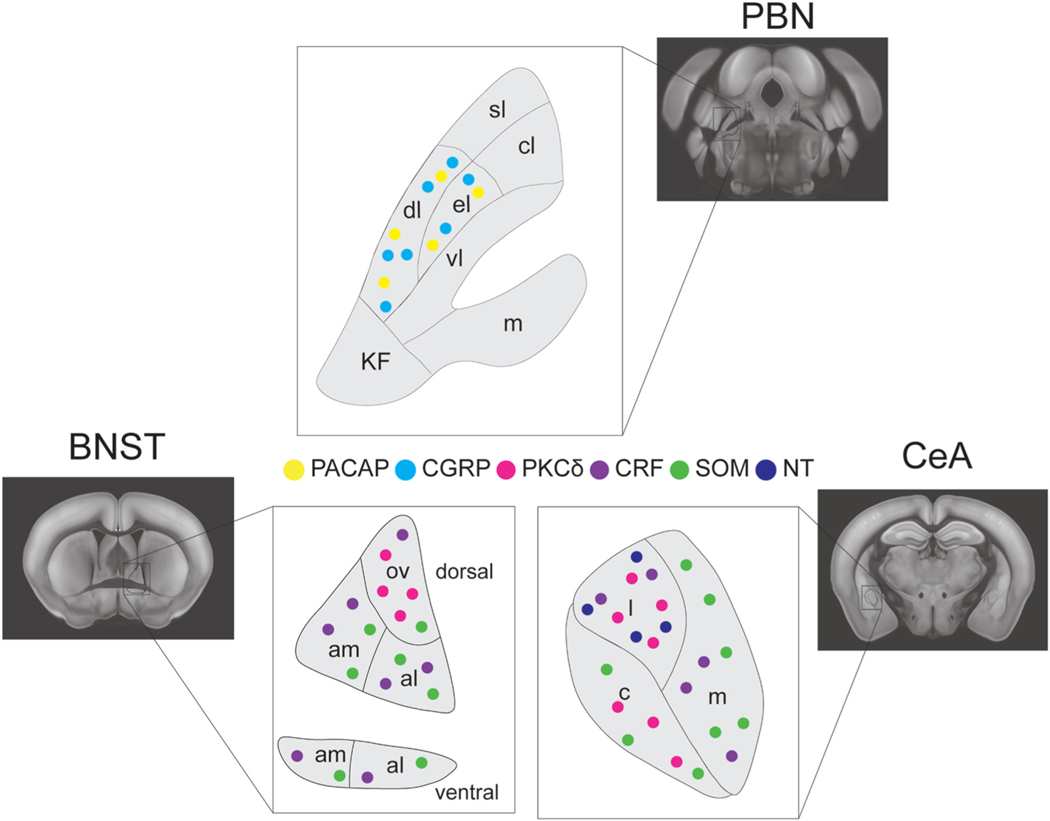
Fig. 1.
Distribution of cell types in the PBN and the EA. Representative image of the distribution of various cell markers across the CeA, BNST and PBN involved in the reciprocal connections of the PBN to the EA. Somatostatin (SOM), Protein Kinase C Delta (PKCd), Corticotrophin Releasing Factor (CRF), Pituitary Adenylate CyclaseActivating Polypeptide (PACAP), Calcitonin Gene Related Peptide (CGRP), Neurotensin (NT).
2.2. PBN to EA connectivity
Studies tracing afferent sources and efferent targets of the PBN demonstrate it is comprised of first order neurons organized in specific subregions of the PBN, reaching and including the EA (Chiang et al., 2019; Qiao et al., 2019; Sarhan et al., 2005; Tokita et al., 2010). The EA is a macrostructure encompassing the central amygdala (CeA), the nucleus accumbens shell (NAcS), and the bed nucleus of the stria terminalis (BNST) all of which receive connections from the PBN (Brog et al., 1993; Sarhan et al., 2005; Tokita et al., 2010). The PBN in turn receives dense reciprocal connections from the CeA and the BNST, which will be discussed throughout the review when relevant. While the synapses from the PBN to the CeA and BNST have been thoroughly studied and will be described below, the role of the projection from the PBN to the NAcS has yet to be fully investigated (Li et al., 2018). The majority of the neurons from the PBN projecting to the CeA and the BNST originate in the lateral PBN (lPBN; Tokita et al., 2010; Ye and Veinante, 2019). The glutamatergic inputs from the PBN into the BNST and the CeA form basket-like axosomatic synapses (Fig. 2) and thereby are anatomically positioned to rapidly modulate neurotransmission relative to dendritic synapses (Dobolyi et al., 2005; Flavin et al., 2014; Sarhan et al., 2005; Shimada et al., 1985) (Sarhan). CGRP terminals are present throughout the BNST and CeA but the axosomatic terminals are only visible in the dorsal BNST and lateral CeA (Dobolyi et al., 2005). In the BNST, these axosomatic terminals express vesicular glutamate transporter 2 (vGlut2) and CGRP (Dobolyi et al., 2005; Flavin et al., 2014). Human and rodent studies show that the CeA and the BNST are critical for responding to physical or perceived threats. This suggests substantial control by the PBN in the detection of threatening contexts and stimuli. The role of the PBN in modulating the extended amygdala will be explored in this review. Previous studies postulated the CeA and the BNST play distinct roles in response to different classes of stimuli, with the CeA primarily involved in the acute fear response while the BNST plays a role in anxiety as a long-term response (Walker and Davis, 2008). However, recent studies show that this original hypothesis was a simplified map of a more interconnected circuit (Kovner et al., 2019; Shackman and Fox, 2016), as both regions appear to overlap in their contribution to threat responses. This is in part due to the GABAergic neurons of the CeA and the BNST that project locally within each structure to form inhibitory microcircuits. Interestingly, anterograde tracing coupled with single axon reconstruction experiments revealed that PBN neurons synapse onto neurons in the BNST and the CeA simultaneously (Sarhan et al., 2005). It was shown that fibers from the PBN to the CeL also project to the BNST. Many of the axon reconstructions revealed fibers projecting to the CeA travel through the stria of the dBNST. The majority of the projections to the CeC, however, did not project onto the BNST. These findings support two delineated circuits from the PBN to the CeA, one that projects to the CeL through the dBNST and one that projects only to the CeC. The ability of the PBN to simultaneously modulate the CeA and the BNST further emphasizes the interconnectedness of the PBN and the EA and the potential for this circuit to modulate animal behavior. Additionally, control of these local circuits through reciprocal connections from the PBN to both regions is suggested to form a higher order circuit that culminates in the animal’s response to noxious stimuli.
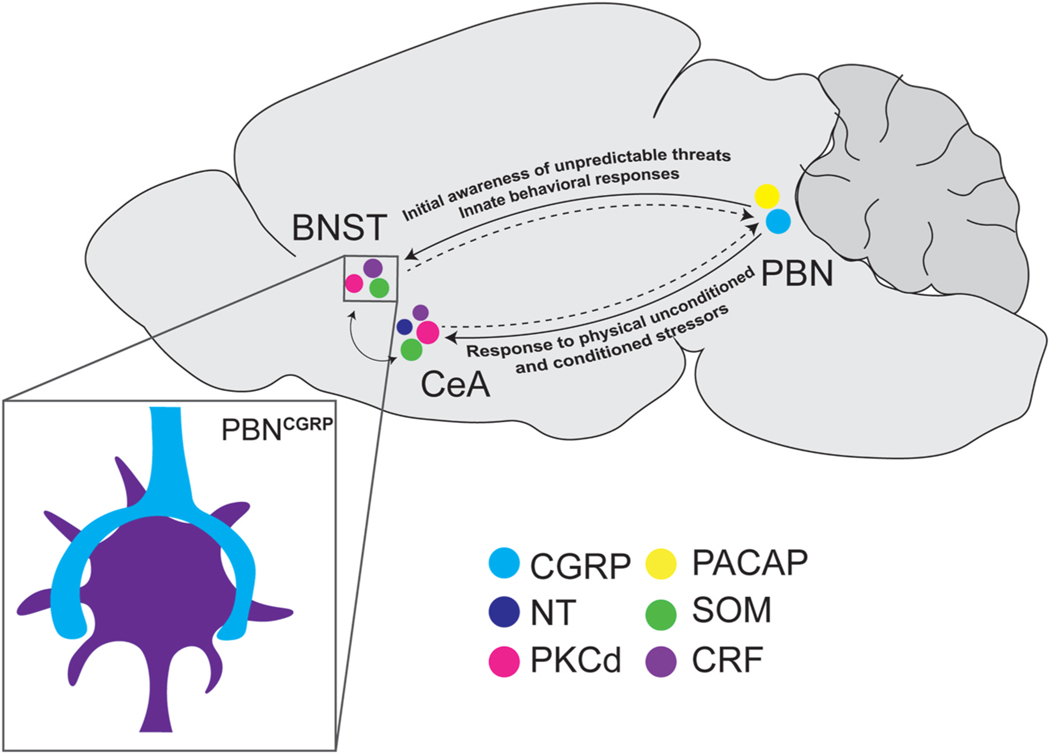
Fig. 2.
Reciprocal connections between the parabrachial nucleus and the extended amygdala. Representative image depicting dense basket-like axosomatic PBN projections (inset) onto the BNST and CeA. Hypothesized overall function of reciprocal connection described above projection. Somatostatin (SOM), Protein Kinase C Delta (PKCδ), Corticotrophin Releasing Factor (CRF), Pituitary Adenylate Cyclase-Activating. Polypeptide (PACAP), Calcitonin Gene Related Peptide (CGRP), Neurotensin (NT).
2.3. CeA
The CeA is subdivided into three main sections: the capsular (CeC), medial (CeM), and the lateral (CeL) (Fig. 1) (Kovner et al., 2019). The CeM is the major output region of the CeA that projects to downstream regions such as the brainstem.
The CeL projects locally to the CeM and to the BNST and regulates output from the CeM. The majority of the nociceptive PBN inputs into the CeA synapse onto neurons in the CeL and the CeC and respond strongly to noxious stimuli. This region of the CeA is often referred to as the “nociceptive amygdala” (Han et al., 2005; Li and Sheets, 2020; Neugebauer et al., 2020). The majority of the GABAergic neurons in the CeL and CeC form local inhibitory circuits. These interneurons express somatostatin (SOM), CRF or PKCδ and are often synapsed onto by neurons originating from the PBN (Neugebauer et al., 2020; Ye and Veinante, 2019). While a population of CRF and PKCδ neurons in the CeA express other neuropeptides including Dyn and Enk, respectively, these neuronal types are mostly non-overlapping and underlie fear learning, anxiety and feeding (Campos et al., 2016; Fadok et al., 2017; Li et al., 2013; Ye and Veinante, 2019). Similarly, PKCδ and SOM expressing neurons are primarily non-overlapping. CeC and the CeL are densely populated with PKCδ and SOM positive neurons, while CRF neurons are found primarily in the CeM and CeL (Cai et al., 2014). CRF neurons form local synapses onto both SOM and PKCδ neurons in the CeL but mainly receive input from SOM neurons (Fadok et al., 2017). CRF, PKCδ and SOM neurons in CeL are involved as input and output cells in the reciprocal local circuitry within the CeA and the connection of the CeA and innervate non-CRF cells in the BNST (Salmaso et al., 2001) BNST.
Using CGRP and CGRP-R expression and viral tracing as confirmation, it was found that inputs from the PBN project onto neurons expressing CRF, PKCδ, and SOM in the CeA (Li and Sheets, 2020; Neugebauer et al., 2020; Ye and Veinante, 2019). Anterograde tracing, optogenetics and immunofluorescence revealed CGRP expressing inputs from the PBN favor PKCδ expressing neurons in the CeL/CeC while a nonCGRP, potentially PACAP, expressing inputs from the lPBN favor SOM-positive neurons in the CeA (Li and Sheets, 2020; Ye and Veinante, 2019). These studies showing SOM-positive and PKCδ-positive cells as the input and output cells of the CeA, provide evidence that the differential innervation of CGRP and PACAP could bidirectionally regulate the CeA synaptic transmission. With approximately 50% of CRF neurons in the CeA receive inputs from the PBN determined by CGRP expression and optogenetic whole-cell recordings (Kozicz and Arimura, 2001; Li and Sheets, 2020). CGRP expression only accounting for 50% of the PBN innervation of the CeA suggests differential innervation of CRF cells by another neuropeptide, potentially through PACAP-expressing terminals from the PBN. PACAP containing fibers from the PBN in the CeC have been shown to regulate nociceptive-related responses in the CeA (Missig et al., 2014, 2017). These fibers mostly colocalize with CGRP containing fibers in the CeC but the role of each neuropeptide in nociceptive signaling is yet to be determined. Given PBN projections express PACAP, we hypothesize the non-CGRP expressing projections synapsing on SOM-positive neurons in the CeA are PACAP projections and may thereby provide a stronger input onto SOM positive neurons in the CeL/CeC. SOM and PKCδ neurons in the CeA often act in a bidirectional manner and are thought to have opposing roles in pain-related behaviors. PKCδ neurons in the CeA have been shown to modulate pronociceptive or increased pain states while SOM positive neurons are linked to decreased pain response or antinociceptive states (Li et al., 2013). The opposing roles of SOM and PKCδ positive neurons could result from their differential innervation by PACAP or CGRP expressing PBN projections, respectively. Overall PBN projections modulate CRF, PKCδ and SOM expressing neurons in the CeL/CeC and thus provide distinct control of the CeA.
2.4. BNST
The majority of neurons projecting from the PBN, specifically the lPBN, to the BNST culminate in the oval BNST (ovBNST) (Palmiter, 2018). The ovBNST is pivotal to the efferent and afferent projections of the BNST. The oval BNST modulates anxiety and assigns valence through the convergence of multiple neuromodulators including SOM, PKCδ, PACAP, CRF, dopamine, and GABA (Lebow and Chen, 2016). It is hypothesized that the BNST contains an inhibitory parallel circuit of SOM and PKCδ positive neurons similar to the CeL/CeC (Ye and Veinante, 2019). While PKCδ positive neurons in the dBNST project to the CeM, SOM positive neurons in the BNST are involved in more long-range projections including to the PBN and periaqueductal gray (PAG).
Using viral tracing and immunofluorescence, it has been shown that the PBN synapses onto PKCδ neurons in the BNST, with the majority of PKCδ neurons located in the ovBNST (Jaramillo et al., 2020; Ye and Veinante, 2019). PKCδ-positive neurons in the ovBNST receive the majority of the signal from the PBN through CGRP expressing cells originating in the lPBN (Ye and Veinante, 2019). Conversely, SOM positive neurons are dispersed across the BNST and comprise the majority of cells in the BNST that convey signals to the PBN (Ye and Veinante, 2019). CRF is expressed across the BNST with around 50% of cells innervated by the PBN (Fetterly et al., 2019; Kozicz and Arimura, 2001; Sink et al., 2013). CRF positive cells in the BNST are surrounded by CGRP and PACAP positive terminals suggesting multiple parallel pathways from the PBN synapsing onto the BNST (Kozicz and Arimura, 2001; Kozicz et al., 1997). Thus, PBN projections distinctly innervate PKCδ, CRF, and SOM expressing BNST neurons. Anterograde tracing revealed a non-CGRP input onto SOM and PKCδ positive neurons in the BNST suggesting similar PACAP innervation of SOM positive neurons but potential CGRP and PACAP innervation of PKCδ positive neurons in the BNST (Ye and Veinante, 2019). The various cell types expressed in each region of that are involved in PBN→EA circuits are described in Fig. 1. It is the plethora of different neuronal cell types across the BNST, CeA and the PBN that result in the bidirectional modulation of various emotional, psychological and physical states.
Overall, the PBN→EA circuit is ideally positioned to encompass an animal’s response to threat-inducing stimuli from the initial sensory stimuli to the resulting acute or chronic physical and emotional response. In this review, we focus on the incorporation of a PBN “alarm” response to nociceptive signals and the modulation of stress responses through its reciprocal connections to the EA. Fig. 2 describes the parallel projections from the PBN to the CeA and the BNST. Overall, the various cell populations and interconnectedness of the EA allows cells in the PBN to exert influence over the multitude of aspects that culminate in an animal’s response to noxious stimuli.
3. The PBN, interoceptive stimuli and appetitive behavior
The PBN is a component of orexigenic and anorexigenic circuitry in rodents (Campos et al., 2016; Palmiter, 2018), and thus, below we will highlight portions of these circuits as they relate to the EA. The inputs from the NTS to the PBN relay meal generated signals which include gustatory and gastric stimuli and thereby assist in conscious visceral perception (Saper, 2002). Adding to the generalization that the PBN is a part of a nutritional post-ingested feedback loop, is the multicomponent nature of the population of PBN gustatory neurons in rodents. A portion of PBN gustatory cells respond to both bitter and nociceptive orasensory stimuli (Li and Lemon, 2019) while others respond to nutritional information in food (de Araujo, 2009), including fluid intake (Ryan et al., 2017). Evidence suggests these PBN gustatory-responding neurons are predominately responsive to visceral information, as neuronal response to taste is decreased with gastric distention, independent of hedonic value (Baird et al., 2001). Furthermore, simultaneous stimulation of the vagal-receiving caudal NST and oral delivery NaCl potentiated neuronal activity, relative to individual stimulation (Hermann and Rogers, 1985) suggesting mechanistically sensitive changes in the presence of dual gastric- and gustatory stimuli. Thus, the nature of these orosensory-sensitive cells may be to detect potentially harmful gustatory stimuli while simultaneously prioritizing nutrients necessary for survival. Interestingly, PBN neurons respond to gustatory stimuli in rodents (Baez-Santiago et al., 2016; Li and Lemon, 2019; Sammons et al., 2016; Vincis and Fontanini, 2019) and evidence suggests these gustatory-responsive PBN neurons are absent in primates (Pritchard et al., 2000). This difference between species suggests studying the PBN may be more relevant to our understanding of the neuronal mechanisms underlying gastrointestinal stimuli. Therefore, through an evolutionary perspective, relevant to primates and appetitive behavior, we hypothesize the role of the PBN has morphed across species as a viscerally-related relay center.
The PBN can functionally regulate short-term food intake, in part through visceral detection. Rodents with lesions in the PBN do not compensate gastrointestinal removal, as they do not increase their consumption in their subsequent meal relative to their control counterparts (Zafra et al., 2016). The PBN→EA circuit differentially processes aspects of short-term consummatory behavior (potentially as a repetitive/backup circuit (Nagase et al., 2019). Optogenetic activation of PBN (CGRP) projections in the CeA immediately decreases intake of familiar food but manipulations in the PBN→BNST circuit have no effect (Carter et al., 2013; Jaramillo et al., 2020). Studies with cell-specific approaches using PKCδ as a neuronal cell marker, expressed in CeL(GABAergic) neurons, show PBN→CeL(PKCδ) are activated by the malaise-inducing agents LiCl and CCK (Cai et al., 2014). In the presence of anorexigenic stimuli chemogenetic inhibition of the PBN neurons projecting to the CeA pathway induces food intake (Carter et al., 2013). A possibility is that in response to anorexigenic visceral stimuli PBN projections recruit CeL(PKCδ) neurons to induce an immediate decrease in feeding. Overall PBN→CeA appears to contribute to the anorexigenic role of PBN in visceral-induced changes in behavior and the role of BNST in modulating food intake appears to not be directly mediated by PBN input (Wang et al., 2019).
There are also functional differences between reciprocal EA– > PBN circuits that demonstrate bidirectional and perhaps repetitive roles on feeding behavior, in part by cell-specificity. The role of CeA(NT)→PBN is suggested to be relevant in drinking as optogenetic stimulation of NT-expressing CeA projections in the PBN increased drinking of sweet fluids (Torruella-Suarez et al., 2020). However, optogenetic stimulation of prepronociceptin (PNOC)-expressing CeA projections in the PBN demonstrate no change in food consumption (Hardaway et al., 2019). Conversely, the BNST– > PBN projections contain both GABA and glutamatergic neurons (Luskin et al., 2021) that bidirectionally drive feeding behavior. In vivo studies show the BNST(vGAT) projections in the PBN respond during chow and sucrose consumption, the latter at a higher magnitude, and optogenetic circuit stimulation increases chow consumption (Luskin et al., 2021). In the same conditions, the BNST (vGlut)→PBN circuit is disengaged, as demonstrated by decreased in vivo activity, and functionally decreases consumption (Luskin et al., 2021). These studies demonstrate diverse reciprocal crosstalk within the circuits and emphasize complex neuropeptide specificity underlying the role of PBN and EA interactions in consumption. Furthermore, studies are needed to investigate the functional relationship between BNST and CeA efferents to the PBN as they relate to their separate roles in consummatory behavior.
Additionally, the PBN is recruited in states that require learned behavior to respond to ingested toxins by assessing the safety of novel food and changes in familiar food. Similar to unconditioned food-related stimuli, the PBN participates in conditioned aversive food consumption that is dependent on intragastric interoception (Reilly, 1999; Zafra et al., 2002, 2005). Early circuit studies demonstrate that contralateral block of the greater amygdala, including structures outside of the extended amygdala, prevents conditioned taste aversion (CTA) (Bielavska and Roldan, 1996) and follow-up studies show optogenetic stimulation of PBN→CeA or BNST pathways induce CTA of a novel food (Chen et al., 2018). Altogether, the PBN→CeA circuit mediates active learning of food-associated aversion while PBN→BNST comes on board following conditioning, thus suggesting a role for EA during and following food consumption. Together this suggests the overarching role of PBN circuitry in food/fluid related behavior is to detect the presence of a noxious interoceptive stimuli.
3. Reflex-like response to sensory and somatosensory stressors
In addition to visceral-related consumption, the PBN mediates immediate reflex-like behavior in response to a novel aversive exteroceptive stimulus. By using an aversive auditory stimulus, the innate startle response behavior can be measured in amplitude of the motion, and thereby an increase in startle serves as a measure of anxiety and fear. Using an acoustic startle response (ASR) paradigm, infusions of CGRP or PACAP peptides into the BNST dose dependently increase ASR and thus are anxiogenic (Seiglie et al., 2019; Sink et al., 2011). ASR is potentiated in paradigms using 2,3,5-trimethyl-3-thiazoline (TMT) odor, a component of fox urine and feces that induces fear in predator naïve rodents. Blocking the CGRP system, through CGRP receptor antagonist infusion into the BNST has anxiolytic effects as it blocks the TMT-induced potentiation of ASR (Sink et al., 2011). Circuit-specific data demonstrate optogenetic activation of lPBN(CGRP) projections into the ovBNST is sufficient to generate freezing behavior accompanied with tachycardia and hyperventilation, thereby demonstrating modulation of autonomic physiological responses during innate freezing behavior (Bowen et al., 2020). Interestingly the physiological effects and freezing behavior induced by optogenetic stimulation of lPBN(CGRP) projections are subregion specific in the CeA, as the caudal CeA increase heartrate and rostral CeA induce freezing behavior, respectively (Bowen et al., 2020). However, the freezing behavior induced by optogenetic PBN (CGRP) terminal activation in the CeA is gradual and not time-locked to stimulation (Han et al., 2015) and thus suggests the CeA contributes to but does not drive the behavior. Altogether the role of the PBN→EA circuit in innate startle is in part modulated by CGRP.
The PACAP system in the PBN→EA circuit is also recruited, albeit primarily in contexts in which stress-like stimuli potentiate ASR. Specifically, in a foot shock potentiated startle paradigm, an infusion of a PACAP receptor antagonists into the CeA or BNST blocks this potentiated startle amplitude (Seiglie et al., 2019). The role of PACAP in the PBN→BNST circuit is in part sex-specific, as PACAP infusion in the BNST enhances the increase of chronic variable stress (CVS) on ASR in males but not females (King et al., 2017). Additionally, the anxiogenic effect is in part long-lasting in a light-enhanced startle paradigm as PACAP infusion in the BNST increased baseline startle amplitude and persisted for seven days (Hammack et al., 2009). Furthermore, PACAP peptide and receptor transcript levels are upregulated in this paradigm suggesting behavioral and long-lasting molecular changes in PBN (PACAP)→BNST circuitry (Hammack et al., 2009). Thus, changes in neurotransmission within PBN(PACAP)→BNST may drive the stress-induced potentiated ASR. In vivo activity of the reciprocal circuits, BNST(vGAT)→PBN and BNST(vGlut)→PBN increases and decreases, respectively, following shock (Luskin et al., 2021). This suggests, albeit with cell specificity, the reciprocal circuit may be engaged and contribute to the processing of unconditioned aversive contexts. Overall, in regard to ASR, these data implicate a role for the CGRP and PACAP systems within the PBN→EA circuits, primarily the PBN→BNST pathway, in behavioral and physiological responses to a variety of unexpected stimuli under baseline and heightened stress conditions suggest that they contribute to the innate autonomic stress response.
4. Contextual assessment and affect
Unfamiliar contexts increase the probability of exposure to an unpredictable threat, thus historical paradigms measuring behavior in the open areas of the testing field provide a proxy for affective or anxiety-like state in the absence of stimuli. These paradigms suggest PBN→EA circuitry is engaged prior to the presence of aversive or noxious stimuli and subsequent associative learning, as infusion of PACAP into the BNST and in CeL decreased time spent in open areas of the open field and elevated plus maze (EPM) (Missig et al., 2014; Roman et al., 2014). Similarly, optogenetic stimulation of PBN(CGRP) projections in the BNST decreases open arm entries (Bowen et al., 2020) suggesting PBN→BNST circuit regulates affective state in threatening contexts. Furthermore, our recent study demonstrates a sexually dimorphic role for the PBN→BNST circuit in an open field assay, as chemogenetic activation of BNST neurons innervated by PBN terminals (BNSTPBN) increased latency to feed on a centrally-located pellet in the novelty suppressed feeding test (NSFT) in females and not males (Jaramillo et al., 2020). The differential effect of nonpeptide-specific manipulation of BNSTPBN neurons in our study and the appetitive component of NSFT suggests complex peptide and context interactions that may underlie increased anxiety-like behavior in females. Moreover, analysis of the dynamic nature of the PBN→BNST circuit during NSFT demonstrated temporal and behavioral specificity, as chemogenetic PBN(CGRP) activation delayed the initiation of feeding synchronized to potentiation of BNST transient activity in males and females (Jaramillo et al., 2020). Thus, different peptide systems within the PBN→BNST circuit may differentially modulate anxiety-like behavior across a variety of unpredictable threats. Interestingly activation of the reciprocal BNST– > PBN circuit bidirectionally modulates NSFT behavior with neurotransmitter specificity, as glutamatergic and GABAergic projections decrease and increase latency to feed, respectively, (Luskin et al., 2021). The effects of PBN→CeA manipulations on behavior on the EPM also suggest differences within the circuit, albeit relative to anatomy as PBN(CGRP) projection stimulation in the rostral CeA was anxiolytic and not caudal CeA manipulations (Bowen et al., 2020). Given the role of PBN→CeA on stress induced anxiety-like behavior it is likely that subsections of the CeA are selectively recruited by the PBN in specific contexts or are specifically regulated by the PBN(PACAP)→CeA circuit. The unpredictable threat induced by these open field contexts support the idea that PBN→EA is recruited by various factors and primed to respond in stress-inducing contexts.
The real-time place preference (RTPP) assay uses associative contextual learning by pairing manipulation of a circuit (e.g., photo-stimulation) in one context and lack thereof with another context, on separate days. On test day both contexts are open and time spent on each side is measured to thereby infer the preference for the interoceptive-state associated with the context of choice. By inducing an aversive or anxiety-like state, real-time place avoidance (RTPA) provides insight on the affect-driven mechanisms underlying PBN→EA activation. As expected, given PBN→EA manipulations are anxiogenic, optogenetic activation of lPBN projections in the BNST and CeA induce avoidance of the stimulation-paired side (Chiang et al., 2020). In the BNST, activation of PBN(CGRP) projections had no effect on RTPA, thus suggesting CGRP does not underlie avoidance behavior induced by the PBN→BNST circuit (Bowen et al., 2020) and may thus be mediated by other peptides released by PBN→BNST projections. Studies in the CeA suggest a subregion-specific role for the CGRP system, as optogenetic activation of PBN(CGRP) projections in the rostral and not caudal CeA was aversive (Bowen et al., 2020). The reciprocal EA– > PBN circuit also demonstrates peptide complexity as CeA(PNOC)→PBN circuit activation is rewarding (Hardaway et al., 2019) but activation of CeA(– > PBN is aversive in RTPP that is it regulated by glutamatergic and GABAergic balance within CeA- > PBN (Bowen et al., 2020). Relative to the studies demonstrating the PBN→EA circuits induced behavioral response to noxious stimuli, these data suggest the role of these pathways is initiated at the time of threat assessment and guided by affective states.
5. Fear-inducing exteroceptive stimuli
PBN→EA circuits also regulate stress-like behavior after the initial exposure to a noxious physical stimulus, as Pavlovian conditioning data demonstrate the PBN→EA circuits contribute to processing learned fear responses (Nagase et al., 2019). CGRP antagonist infusions into the greater amygdala decrease freezing behavior in response to an auditory conditioned stimulus (CS) associated with shock (Kocorowski and Helmstetter, 2001). Furthermore, an auditory CS previously paired with optogenetic activation of lPBN projections into the CeA is sufficient to induce freezing behavior (Han et al., 2015; Sato et al., 2015), demonstrating a functional role for lPBN→CeA in auditory conditioning. In this same manner, CGRP signaling in the BNST is implicated in context-associated shock learning, as infusion of a CGRP receptor antagonist into the BNST during consolidation and retrieval blocked freezing behavior, independent of the discrete light CS (Sink et al., 2013). With circuit specificity, PBN(CGRP) optogenetic terminal stimulation in ovBNST and rostral CeA had no effect on auditory-associated conditioning and retrieval (Bowen et al., 2020), further suggesting the role of PBN(CGRP)→BNST is independent of a conditioned cue. However, under the same conditions stimulation in the caudal CeA potentiated retrieval and thus implies the role of PBN(CGRP) in fear learning is subregion specific (Bowen et al., 2020). Additional data support a role for PBN→CeA, as conditioned threat behavior induces mechanistic changes within the PBN→CeA circuit. Specifically, indirect activation of lPBN projections (i.e., stimulation of pathway along the capsular division) to the CeC following fear conditioning and CS retrieval results in enhanced transmission mediated by presynaptic changes in vesicular release probability and postsynaptically by increased AMPA receptor function and slower NMDA receptor kinetics (Watabe et al., 2013). Molecular studies also suggest neuronal adaptations in the PACAP system within the EA. Engaging the system by intraventricular infusion of PACAP prior to the initial exposure to the foot shock (i.e., unconditioned stimulus) altered the consolidation of the threat memory and increased expression of Arc, a marker of synaptic plasticity, in the CeA and BNST (Meloni et al., 2019). Furthermore, the role of PBN→EA circuits in fear conditioning also encompasses the reciprocal BNST(vGAT)→PBN circuitry, as in vivo neuronal activity increases in BNST(vGAT) neurons that project to the PBN, during aversive shock (Luskin et al., 2021) and thus may provide feedback in response to PBN(PACAP). Altogether these studies demonstrate the PBN→EA circuit is a contributor to learned threat responses.
Similar to conditioned food-related aversion, the role of the PBN in learned behavioral response to physical noxious stimuli is in part recruited by somatosensory and sensory inputs (i.e., olfaction and hearing). The PBN is a part of the vestibular and olfactory circuitry (Di Lorenzo and Garcia, 1985; Grigson et al., 1998; Karimnamazi et al., 2002; Reilly et al., 1993) and may thereby drive conditioned behavior in assays that incorporate TMT-related olfactory agents or auditory stimuli. Given a portion of PBN neurons respond to individual and simultaneous gustatory and visceral stimuli, we hypothesize PBN neurons similarly respond to olfactory and auditory stimuli. That is PBN neurons may be heighted in response to multiple stimuli. Thus, the relevance of the sensory-related PBN neurons in primates may prioritize external stimuli that relay both somatosensory and sensory inputs to induce innate or conditioned fear response. Thus, the role of the PBN→EA circuits may be clinically relevant due to their contributions to maladaptive anxiety in response to non-threatening stimuli, as is evident in post-traumatic stress disorder (PTSD). For example, individuals with repetitive exposure to earthquakes demonstrate enhanced state anxiety (Honma et al., 2012) and demonstrate PACAP-related single nucleotide polymorphisms (SNP) in genes encoding PACAP and its receptor, PAC1 (Wang et al., 2013). Similarly, individuals with balance disorder often have comorbid anxiety and migraine (Balaban et al., 2011) the latter of which is treatable by CGRP treatments (Russo, 2019). Thus, the PBN may serve as a unique gateway of sensory information that leads to stress-relevant processing and consolidation in the EA.
6. Distress-like states
The literature implicate a functional role for the BNST and CeA in modulating changes in behavior sensitive to chronic stressors and distress-like states. In the BNST, a chronic variable stress (CVS) paradigm induces an increase in PACAP peptide and receptor mRNA expression (Roman et al., 2014). Physiologically, microinfusions of PACAP receptor antagonist into the BNST blocks CVS-induced increase in corticosterone levels and does not change levels induced by acute stress (Roman et al., 2014). Furthermore, PACAP receptor antagonist administration into the BNST selectively blunts time spent in open arms in EPM following CVS relative to nonstress controls (Roman et al., 2014). In the absence of CVS, PACAP or receptor agonist infusions into the BNST increased time spent in open areas, suggesting the PACAP system is engaged in the initial stress exposure (Roman et al., 2014). Additionally, a long-sustained stress modeled by a restraint stress paradigm implicates the PBN→BNST circuit, as chemogenetic inhibition of the PBN decreases stress-induced increases in BNST activity measured by cFOS, a marker of neuronal activity (Fetterly et al., 2019). The PBN→CeA circuit is also implicated restraint stress, as PACAP receptor expression was decreased and neurotransmission was dysregulated in the CeM (Varodayan et al., 2020). Specifically, PACAP-induced enhancement of CeM GABAergic tonic activity through presynaptic PAC1 receptor was blunted following a single restraint (Varodayan et al., 2020). However, following repeated restraint exposure PACAP-regulated enhancement on GABA transmission was no longer present (Varodayan et al., 2020), suggesting the stress-induced changes are transient in the CeA. Additionally, the PBN→CeA may be recruited to increase passive responses to physical stimuli, as a shock-probe fear assay, measuring exploration of a novel shock-inducing probe shows a PACAP infusion in the CeA decreased active (enhanced marble burying response) and increased passive (withdrawal and immobility) stress responses only when challenged by shock, with no differences in shock reactivity (Legradi et al., 2007). Altogether, the PACAP system in PBN→EA circuits is heavily implicated in stress-induced changes in behavioral responding and neuronal transmission (Dore et al., 2013; Hammack et al., 2010; Stroth et al., 2011) with the PBN→BNST primarily driving behavior in response to single and repeated unpredictable states of distress.
7. Hyperalgesia and inflammation-related states
Nerve ligation models of chronic deep (i.e., muscular and articular) tissue pain resulted in increased sensitivity to pain, hyperalgesia, parallel to potentiated PBN→CeA circuit neurotransmission. Interestingly the mechanisms underlying the potentiation of PBN→CeA circuit activity in states of hyperalgesia and inflammation are distinct and cell-specific. In a spinal nerve ligation model of neuropathic pain, PBN fiber track stimulation increased amplitude of spontaneous excitatory post synaptic currents (sEPSCs) in CeA neurons correlated with tactile- induced allodynia (pain response to non-painful stimuli), (Ikeda et al., 2007). Furthermore ligation-induced potentiation in PBN→CeA neurotransmission is independent of NMDA receptor function as a non-NMDA receptor antagonist, CNQX, blunted EPSCs evoked by PBN stimulation while a NMDA antagonist had no effect (Ikeda et al., 2007). Recent studies provide insight to the mechanisms underlying heighted PBN→CeA activity in a model of spared sciatic nerve injury by suggesting presynaptic dysregulation across heterogenous neuropeptide populations. Measuring paired pulse ratio (PPR) in subsections of the CeA during optogenetic activation of PBN terminals suggests a complex orchestrated bidirectional shift in neuronal excitability and activity of SOM ±and CRF ±neurons in the CeA (Li and Sheets, 2020). Specifically, CRF+and CRF- neurons in the CeL showed decreased release probability (Li and Sheets, 2020) in response to optogenetic stimulation of PBN glutamatergic terminals. Conversely the CeM(CRF+) system demonstrated increased release probability accompanied by a decrease in SOM neurons in response to optogenetic stimulation of PBN terminals in the CeM (Li and Sheets, 2020). The SOM system is differentially changed in CeC neurons, as synaptic efficacy is decreased in SOM + neurons and increased in SOM-neurons (Li and Sheets, 2020). Behavioral studies in a sciatic nerve ligation chronic constriction injury suggest decreasing PACAP activity can modulate the heighted PBN→CeA circuit activity, as a CeA infusion of PACAP antagonist blocks sensitivity, demonstrated by increased latency to paw withdrawal in response to thermal stimuli (Missig et al., 2017). Additionally, PACAP inhibition in the CeA was anxiolytic as it blocked the nerve ligation-induced decrease in center time in an open field (Missig et al., 2017), suggesting the analgesic effect may thereby decrease anxiety-like behavior. The reciprocal CeA– > PBN circuit may in part contribute to the heightened inhibitory neurotransmission within the PBN→CeA circuit, as a chronic constriction injury of the infraorbital nerve demonstrates decreased presynaptic efficacy (Raver et al., 2020). Thus, PBN→CeA and CeA– > PBN may bidirectionally modulate chronic pain, through heightened excitatory and inhibitory activity, respectively. Additionally, albeit through distinct mechanisms, acute persistent visceral pain in a zymosan-induced colitis model demonstrates increased sEPSC amplitude and frequency in CeA neurons following stimulation along the PBN projection track (Han and Neugebauer, 2004). This is in line with the heightened role of PBN→CeA neuronal transmission in response to noxious visceral stimuli and negative-affect, the latter independent of physical stimuli. Given heterogenous neuropeptide mechanisms mediate neuroplasticity in models of chronic pain, future research will inform the role of PBN→CeA circuit with peptide-specificity. It will be of specific interest to identify the mechanisms mediating decreased sensitivity in the PBN→CeA neurons as they have been historically ignored (Sugimura et al., 2016).
7.1. Studies targeting deep muscle and joint pain also demonstrate heightened
PBN→CeA neurotransmission, primarily mediated by CGRP and related molecular mechanisms. Nonspecific PBN track stimulation, in a model of acid-induced muscle pain, provided an overarching role for potentiated synaptic regulation of CeC neurons by pre and postsynaptic mechanisms, the latter being dependent on protein kinase C (PKC) and extracellular signal-regulated kinase (ERK) activation (Cheng et al., 2011). Similarly, PBN track stimulation in a model of arthritic pain causes increased spike frequency across time in CeA neurons, suggesting increased neuronal sensitivity (Neugebauer and Li, 2003). Follow-up studies in an arthritis pain model demonstrate CGRP receptor antagonists block potentiated miniature EPSCs (mEPSCs) in CeL/C neurons through postsynaptic mechanisms involving protein kinase A (PKA) signaling and NMDA currents (Han et al., 2005). Given the excitatory role of CGRP on NMDA-mediated transmission is dependent on PKA (Okutsu et al., 2017), the findings implicate recruitment of the CGRP system in arthritic-related pain. Moreover, blocking PBN-CeA CGRP activity through infusion of CGRP receptor antagonist into the CeL/C decreased the arthritis-induced increase in duration of ultrasonic vocalizations (USVs) (Han et al., 2005), suggesting inhibition of the CGRP system modulates pain-dysregulated synaptic transmission and negative affect. The molecular mechanisms underlying the behavioral effect may involve recruitment of PKA, as CGRP infusion in the CeL/C increases USVs and lowers the threshold for behavioral response to pain in a PKA-dependent manner under baseline conditions (Han et al., 2010). Thus, overall, the CGRP system is in part underlying the role of PBN→CeA circuit in hyperalgesia. This may be through mechanisms involving direct response to noxious stimuli in CeAPBN neurons, as they respond to noxious stimulation at the knee joint with half also responding to innocuous stimuli (Neugebauer and Li, 2002).
Additionally, transient acute cutaneous pain models using formalin injections demonstrate lasting effects on neurotransmission in the PBN (CGRP)→CeA circuit. Upper lip and intraplantar formalin injections increase EPSC amplitude and PPR in CeA neurons, suggesting presynaptic and postsynaptic-mediated plasticity in the PBN→CeA circuit (Miyazawa et al., 2018; Sugimura et al., 2016; Shinohara et al., 2017). In CGRP KO mice, the persistent formalin-induced changes in neurotransmission and decreased mechanical withdrawal threshold, as seen in the WT mice, were absent, suggesting CGRP signaling is critical for these changes (Shinohara et al., 2017). Interestingly the immediate formalin-induced increase in licking time in CGRP KO mice is time sensitive, as there is only a change from WT formalin 10 min post injection and then returns to baseline (Shinohara et al., 2017). Thus, the analgesic role of CGRP may be recruited immediately but may have more prominent long-lasting effects on transmission and behavior in models of chronic pain. An elegant tracing study shows neurons responding to nociceptive stimuli (formalin or capsaicin), respond more robustly when injected in the whisker relative to the hindpaw, suggesting the PBN is more responsive to craniofacial pain (Rodriguez et al., 2017). Moreover, the trigeminal neurons responding to the noxious facial stimuli directly project to the PBN and are relayed to the CeA and BNST (Rodriguez et al., 2017), demonstrating a direct connection to the craniofacial periphery. Formalin stimulates acute pain, recruiting CGRP in the PBN→CeA circuit correlated to negative affect and physical sensitivity in the area of stimulation, and thus may underlie the initiation of changes in chronic pain states.
Thermal stimulus-induced hyperalgesia states also increase PBN→CeA activity, albeit primarily through the PACAP system. Nonspecific PBN fiber tract stimulation, following one exposure to a thermal nociceptive stimulus, increases EPSCs in a population of CeA neurons, in part through AMPA-mediated changes that lasts for 3 days (Kissiwaa and Bagley, 2018). However, two exposures to thermal nociceptive stimuli were necessary for inducing hyperalgesia and prolonged the synaptic changes up to seven days (Kissiwaa and Bagley, 2018). The role of PACAP in pain states is supported by data showing PACAP peptide or receptor agonist infusion into the CeA increases thermal sensitivity (Missig et al., 2014), potentially through interactions with metabotropic glutamate receptor 1 (mGluR1) (Neugebauer et al., 2003), and NMDA receptors (Bird et al., 2005). The PACAP-induced decreased withdrawal latency is blocked by clathrin-mediated endocytosis inhibitors and a mitogen-activated ERK inhibitor (Missig et al., 2017). These studies suggest the PACAP system in the PBN→CeA circuit mediates immediate and prolonged hyperalgesia in part through increased circuit activity, mediated via presynaptic and postsynaptic mechanisms, in response to thermal stimuli. Given the changes in neurotransmission are prolonged with multiple exposures to noxious stimuli, this suggests the initial activation of the PBN→CeA primes the circuit for potential future recruitment. Activating reciprocal CeA– > PBN projections induces an analgesic response to acute formalin (Raver et al., 2020). Thus, the analgesic effect induced by the reciprocal CeA– > PBN circuit may occur in part by a potential feedback mechanism such that it prevents enhancement of PBN→CeA circuit activity present in hyperalgesia.
It is possible that CeA neurons, and in part those innervated by PBN, project to the PAG to induce analgesia in part through the opioid system (Oliveira and Prado, 2001; Xu et al., 2003). CeM neurons projecting into the PAG display altered excitability in complete Freund’s adjuvant (CFA) models of inflammatory pain (Li and Sheets, 2018). Specifically, PKCδ neurons in the CeM have been shown to inhibit CeA inputs onto the PAG. While further evidence is required, a PBN → CeA → PAG circuit could underlie the integration of sensory and emotional processing of pain. Furthermore, this CeA→PAG projection appears to be dysregulated in alcohol-dependent animals displaying hyperalgesia through reduced GABAergic input onto PAG neurons (Avegno et al., 2018). Thus, the upstream PBN→CeA circuit is a promising target to modulate hyperalgesia by intervening with PAG activity.
8. Autonomic survival
A population of CGRP-expressing PBN projections provide direct somatic innervation (Fig. 2) and thereby bypass projection-to-dendrite communication to rapidly and dynamically regulate EA neurons. The basket-like somatic innervation by PBN projections onto CeA and BNST neurons provides multiple release sites and thereby a single fiber can drive these neurons to meet the threshold for an action potential (i.e., suprathreshold stimulation) (Delaney et al., 2007; Flavin et al., 2014; Sarhan et al., 2005). Thus, the PBN is anatomically situated to initiate EA-driven behavior critical for survival. Interestingly the PBN→CeA circuit is modulated by external inputs as PBN projections are sensitive to heterosynaptic noradrenergic modulation, in part by alpha2a-adrenergic receptors (ARs) (Delaney et al., 2007). Specifically, alpha2a-AR agonists decrease CeA excitatory synaptic activity induced by PBN track stimulation, independently of calcium. This unconventional regulation by alpha2a-AR suggests the site of adrenergic-induced inhibition at PBN synapses is postsynaptic and does not involve a presynaptic decrease of release probability. Specifically, the functional role of alpha2a-AR on PBN neurotransmission requires action potential evoked presynaptic release of noradrenaline and is dependent on Gβγ subunits (Delaney et al., 2007). Thereby alpha2a-AR-induced inhibition at the PBN synapse in the CeA is due to a decrease in the number of active release sites (Delaney et al., 2007). In general, long-term depression (LTD) and long-term potentiation (LTP) at the PBN→CeA synapse are mediated by postsynaptic and presynaptic mechanisms, respectively (Lopez de Armentia and Sah, 2007). Thus alpha2a-ARs may contribute or override the aforementioned PBN→CeA synaptic adaptions that underlie stress-induced behavior. Similarly, alpha2a-AR are expressed axosomatically at the PBN→BNST synapse and inhibit excitatory transmission, as application of guanfacine, an alpha2a-AR partial agonist, suppresses optogenetically-induced PBN activity in BNST neurons (Flavin et al., 2014). Interestingly, PBN-induced inhibitory responses demonstrate excitatory and inhibitory response to alpha2aAR agonists (Flavin et al., 2014) demonstrating modulation by alpha2a-ARs in two distinct populations of PBN innervated BNST neurons. Given CGRP increases inhibitory transmission in the BNST, it is likely that (PBN)CGRP may drive the inhibitory responding BNST neurons sensitive to alpha2a-AR modulation (Gungor and Pare, 2014). These data suggest a role for alpha2a-ARs in filtering overall excitatory transmission by controlling excitatory/inhibitory balance. Thereby incoming noradrenaline inputs are positioned to fine tune the PBN→EA circuit in threat-related arousal.
The PBN is also highly implicated in respiratory circuitry (Chamberlin, 2004). Specifically, optogenetic silencing of PBN(CGRP) terminals in the CeA attenuates wakefulness in hypercapnia, a condition of excess carbon dioxide resulting in inadequate respiration (Kaur et al., 2017). Preclinical studies show amygdala stimulation near the CeA induces apnea which may contribute to in sudden unexpected death in epilepsy (SUDEP; Nobis et al., 2018) and seizure-related apnea correlates with the spread of seizure activity to the amygdala (Nobis et al., 2019), suggesting dysregulation is localized to the amygdala synapses. SUDEP-induced amygdala dysregulation may be in part through PBN→BNST circuit as ex vivo activity shows a hypoexcitable tone induced by excitatory-inhibitory synaptic imbalance in Dravet Syndrome mice, a model of early-onset epilepsy with increased risk for SUDEP (Yan et al., 2021). Specifically, relative to WT, dBNST neurons innervated by the PBN in Dravet Syndrome mice demonstrate decreased spontaneous inhibitory transmission and a higher resting membrane potential and thereby greater excitatory and decreased inhibitory neurotransmission at the PBN→dBNST synapse (Yan et al., 2021). The reciprocal BNST– > PBN circuit may also contribute to changes in breathing, as optogenetic activation of the BNST projections in the PBN, decreases breathing rate (Kim et al., 2013). Overall, this suggests reciprocal communication between the PBN→EA circuits in detecting changes in arousal with the reciprocal circuit inducing changes in respiration (Nobis et al., 2018, 2019).
9. Substances of abuse and the PBN alarm
The literature on substance use disorders demonstrates drug use is initially motivated by the rewarding effects of the drug and thereby leads to repeated intoxication and craving. The neuroadaptations induced by chronic drug use can lead to negative affective states and hypersensitivity to pain and aversion during drug withdrawal (i.e., hyperkatifea) leading to maladaptive response to stress (Koob, 2021). Thus the motivation to relapse is to alleviate these states (i.e., negative reinforcement) (Koob, 2021). Moreover, drug use becomes compulsive as it occurs despite negative consequences (e.g., foot-shock or quinine adulteration). It is well established that the EA circuits contribute to the negative aspect of addiction. We hypothesize that the role of the PBN in the induction of aversion and pain sensitivity is highly relevant in the context of drugs. Below we illustrate how the current literature suggests a potential role for the PBN→EA circuitry in drug related negative states.
9.1. Hyperkatifea
Drug withdrawal induces a negative emotional state which encompasses negative affect and increased intensity to stress stimuli termed hyperkatifea. The manifestation of hyperkatifea leads to increased motivation for drug taking, and thereby is hypothesized to be a leading cause of relapse (Koob, 2021). On this basis, we hypothesize PBN→EA circuits process interoceptive and exteroceptive stimuli that may in part contribute to the dysregulated affective state induced by abstinence from chronic drug use. Interestingly in a CTA paradigm, PBN-infused morphine can act as an aversive CS, suggesting the PBN functionally regulates aversive behavior in part through induction of discriminative stimulus effects (i.e., interoceptive effects) (Jaeger and van der Kooy, 1993). The data supporting a role for the PBN→EA on conditioned aversion together with the data demonstrating CTA is dysregulated in alcohol preferring bred rodents (Robinson et al., 2020) suggest the PBN→EA circuit may be dysregulated in models of alcohol-use. Moreover, withdrawal-induced increases in PBN, CeA, BNST, and NAcS cFOS in opioid dependent rats (Hamlin et al., 2001) suggest the PBN→EA circuit activity is heightened in withdrawal-induced negative states. Thus, the PBN may contribute to negative interoceptive states relevant to alcohol consumption.
Studies investigating the PBN in drug consumption reveal a complex role for neuropeptidergic systems in the PBN→EA circuits. Optogenetic activation of PBN(NT) terminals in the CeA increases drinking of alcohol and other reinforcing fluids (Torruella-Suarez et al., 2020). Conversely, PACAP infusion in the BNST decreases alcohol and not water self-administration in dependent rats (Ferragud et al., 2020), suggesting alcohol-specific fluid intake may primarily be modulated by the PBN→BNST circuit. Furthermore, two-bottle choice home cage drinking (2BC) increases CGRP expression in anterolateral, anteroventral-lateral and -medial sections of the BNST and decreases it in anteromedial BNST (Rossetti et al., 2019) of alcohol-preferring rats thereby potentially implicating the CGRP system in the PBN→BNST circuit. Given the projections in the PBN→EA circuits it is important to note that hyperalgesia exhibited by alcohol-dependent rats show a functional role of CeA projections to the PAG (Avegno et al., 2018). Given the proposed role of the PBN→CeA circuit in pain regulation it will be of interest to investigate if the CeA→PAG is recruited by the PBN.
9.2. Maladaptive response to threats
Building on the historical literature implicating the PBN→EA circuits in fear conditioning and stress, recent studies now add to this role in the context of stress-induced relapse. The PACAP system in the BNST exhibits cocaine-induced molecular changes with PACAP transcripts increasing in the BNST following a history of cocaine self-administration (Miles et al., 2018). Behavioral changes are also evident in the relapse portion of the addiction cycle, as PACAP inhibition in the BNST, via PACAP receptor antagonist, blocks foot shock-induced reinstatement (Miles et al., 2018). Furthermore, PACAP infusion into the BNST, in the absence of foot shock, is sufficient to induce reinstatement at levels comparable to foot shock (Miles et al., 2018). The role of PACAP in relapse also extends to alcohol as chronic intermittent ethanol vapor exposure (CIE) increases PACAP in the BNST and PACAP infusion into the BNST blocks alcohol drinking selectively in dependent rats (Ferragud et al., 2020).
Interestingly, PACAP expression does not change following CIE (Ferragud et al., 2020). This suggests a potential role for the PBN (PACAP)→BNST circuit throughout the stages of addiction. Moreover, PACAP signaling may serve as a target for blocking stress-induced reinstatement (Miles et al., 2019). Furthermore, given that a heightened response to nonthreatening stimuli is a characteristic of PTSD, and given the high prevalence for drug use in PTSD patients, these data potentially implicate the PBN→EA circuit in stress-induced drinking.
9.3. Visceral side effects
Drug use can require overriding the innate aversive qualities that accompany the physical route of administration (i.e., oral, intravenous, nasal administration). Visceral drug-induced alterations provide a basis for interpretation of the role of the PBN. Molecular evidence demonstrates experimenter administered alcohol via an intragastric injection increases cFOS relative to a LiCl-induced increase in the PBN suggests the initial alcohol exposure heightens PBN neuronal activity, and thus may induce a similar aversive interoceptive stimulus as LiCl (Chang et al., 1995; Thiele et al., 1996). Interestingly alcohol-induced context conditioning can induce context aversion or preference. That is a conditioned place paradigm, alcohol administration prior or post context exposure induces preference or aversion, respectively, in part through subregion-specific mechanistic changes in the BNST (Pati et al., 2019). Specifically, CPP increased and CPA decreased vBNST neuronal excitability (Pati et al., 2019). Given the aforementioned role of PBN→EA on aversion we hypothesize context-related conditioning assays are sensitive to PBN→EA manipulations and PBN→BNST circuit may process alcohol-induced visceral states and contexts.
The anorexigenic role of the PBN involves changes in opioid receptor signaling, a common target of sedative drugs of abuse. A side-effect of many drugs of abuse is hypophagia, which may in part be due to activation of μ-opioid receptors, as application of a μ-opioid receptor antagonist in the PBN decreases eating (Chaijale et al., 2008). Inhibition in the opioid system in the PBN may further be exacerbated by food restriction (Wolinsky et al., 1996) and may thereby be recruited in stress states related to food consumption (Nicklous and Simansky, 2003). Conversely, infusions of a μ-opioid agonist administered into the PBN can increase consumption of nutritional but not non-nutritional palatable food (Ward and Simansky, 2006; Wilson et al., 2003). Moreover, benzodiazepine agonists in the PBN increase intake of food with hedonic enhancement in spite of aversive taste, independent (Higgs and Cooper, 1996; Soderpalm and Berridge, 2000). This suggests that this system can be recruited to overcome hypophagia specific to nutrition.
9.4. Predisposing conditions
Alcohol-preferring rodent models provide a look into the genetic and molecular variants associated with increased susceptibility for alcohol use. Under baseline conditions, alcohol-preferring rats show differential levels of CGRP peptide and receptor expression in the CeA (Hwang et al., 1995) and not in the BNST (Rossetti et al., 2019) suggesting the CGRP system is compromised specifically in the CeA in baseline states of abuse-susceptible models. Interestingly, under alcohol conditions, comparison of alcohol-preferring vs non preferring rodents did not demonstrate changes in CGRP immunoreactivity when measured in the greater amygdala following chronic intermittent ethanol (CIE) (Ehlers et al., 1999). The absence of changes in CGRP immunoreactivity post alcohol exposure may suggest that changes in the CGRP system are rescued with alcohol intake or are region-specific.
Clinical data show single-nucleotide polymorphisms (SNPs) in CGRP and PACAP systems in some alcohol-drinking populations (Dragan et al., 2017; Guo et al., 2015; Kovanen et al., 2010). Specifically, sNPS of the PAC1 receptor gene are associated with problematic alcohol use in women aged 18–28 years (Dragan et al., 2017). Additionally sNP in the PACAP gene correlate with levels of consumption in social drinkers (Kovanen et al., 2010) and thereby suggest PACAP may associate with higher levels of alcohol drinking. The presence of sNPS in the CGRP gene are less prevalent in alcohol-drinking psoriasis patients relative to drinking controls (Guo et al., 2015) implicating the CGRP system may be compromised in select drinking populations. Overall, CGRP and PACAP interventions may be a viable treatment option to intervene with increased drinking and in abstinence to alleviate negative affect and thereby prevent relapse (Gargiulo et al., 2020).
10. Summary
When the PBN circuitry is parsed out a common theme in threat processing is evident across the EA, with the PBN→BNST circuit mediating initial awareness of unpredictable threats, and regulating innate behavioral responses and the PBN→CeA driving responses to physical unconditioned and conditioned stressors. PBN→BNST and PBN→CeA circuits share a similar number of anatomical projections that are distinctly modulated. In summary, the literature reviewed suggests the PBN detects visceral interoceptive and exteroceptive threats related to contexts and physical sensory stimuli. The PBN relays this information in part to the EA, adding an affective component to the behavioral response of a perceived threat. Thereby the PBN→EA circuits encompass a complex balance between the PBN→BNST and PBN→CeA to induce a state of distress in response to various threats.
Future studies are required to understand how the PBN→EA circuits directly interact and modulate each other. Interactions within the PBN→EA are likely, given the anatomical findings that PBN→CeA projections simultaneously innervate BNST neurons. However, dissecting the functional role of the polysynaptic PBN→EA projections will require technology that will specifically isolate these neurons and measure CeA and BNST independently. The reciprocal projections from CeA and BNST to the PBN were briefly discussed as much remains to be studied to conclude if the PBN→EA and EA→PBN interactions are bidirectional and serve a similar function. Studies including both sexes, the use of complex behavioral assays and advancements in measures of a dynamic neuronal activity will provide a clearer role for PBN→EA circuitry with cell- specificity and time sensitivity.
10.1. Concluding remarks
The paradigms measuring anxiety and innate fears (i.e.,unpredictable threats, exposure to pain) discussed in this review provide a simplistic view of the function of this alarm circuit in threat-like contexts relevant to complex human behavior. It is likely the PBN→ EA circuits have adapted across species to respond to new challenges. This may be evident in neuropsychiatric disorders that display dysregulated threat assessment and stress responses relevant to human contexts (e.g., PTSD, addiction, social anxiety). Much remains to be learned about the PBN→EA circuit including the PBN→NAcS projections that are to date overlooked. Furthermore the current evidence demonstrating neuronal heterogeneity in activity at the PBN→EA synapses that drive cell-type specific behavior will require further research at the microcircuit level. To this point the PBN→EA circuits provide a vast and promising area of study, as discerning the intricacies of this circuit will assist us in understanding how stress responses incorporate contextual awareness to drive affect and fear relevant to survival.
Acknowledgements
This work was supported by the National Institutes of Health NIAAA AA019455 (DGW), and other grants from NIDA DA042475 (DGW), and Howard Hughes Medical Institute Gilliam GT10823 (JAB).
Footnotes
Declaration of competing interest
None.
References
- Avegno EM, Lobell TD, Itoga CA, Baynes BB, Whitaker AM, Weera MM, Edwards S., Middleton JW, Gilpin NW, 2018. Central amygdala circuits mediate hyperalgesia in alcohol-dependent rats. J. Neurosci 38, 7761–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez-Santiago MA, Reid EE, Moran A., Maier JX, Marrero-Garcia Y., Katz DB, 2016. Dynamic taste responses of parabrachial pontine neurons in awake rats. J. Neurophysiol 115, 1314–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JP, Travers SP, Travers JB, 2001. Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am. J. Physiol. Regul. Integr. Comp. Physiol 281, R1581–R1593. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Jacob RG, Furman JM, 2011. Neurologic bases for comorbidity of balance disorders, anxiety disorders and migraine: neurotherapeutic implications. Expert Rev. Neurother 11, 379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielavska E., Roldan G., 1996. Ipsilateral connections between the gustatory cortex, amygdala and parabrachial nucleus are necessary for acquisition and retrieval of conditioned taste aversion in rats. Behav. Brain Res 81, 25–31. [DOI] [PubMed] [Google Scholar]
- Bird GC, Lash LL, Han JS, Zou X., Willis WD, Neugebauer V., 2005. Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurones. J Physiol 564, 907–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block CH, Hoffman GE, 1987. Neuropeptide and monoamine components of the parabrachial pontine complex. Peptides 8, 267–283. [DOI] [PubMed] [Google Scholar]
- Bowen AJ, Chen JY, Huang YW, Baertsch NA, Park S., Palmiter RD, 2020. Dissociable control of unconditioned responses and associative fear learning by parabrachial CGRP neurons. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A., Deutch AY, Zahm DS, 1993. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol 338, 255–278. [DOI] [PubMed] [Google Scholar]
- Cai H., Haubensak W., Anthony TE, Anderson DJ, 2014. Central amygdala PKCdelta (+) neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci 17, 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CA, Bowen AJ, Schwartz MW, Palmiter RD, 2016. Parabrachial CGRP neurons control meal termination. Cell Metabol. 23, 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD, 2013. Genetic identification of a neural circuit that suppresses appetite. Nature 503, 111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaijale NN, Aloyo VJ, Simansky KJ, 2008. A naloxonazine sensitive (mu1 receptor) mechanism in the parabrachial nucleus modulates eating. Brain Res. 1240, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, 2004. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir Physiol Neurobiol 143, 115–125. [DOI] [PubMed] [Google Scholar]
- Chang SL, Patel NA, Romero AA, 1995. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 679, 89–98. [DOI] [PubMed] [Google Scholar]
- Chen JY, Campos CA, Jarvie BC, Palmiter RD, 2018. Parabrachial CGRP neurons establish and sustain aversive taste memories. Neuron 100, 891–899 e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SJ, Chen CC, Yang HW, Chang YT, Bai SW, Chen CC, Yen CT, Min MY, 2011. Role of extracellular signal-regulated kinase in synaptic transmission and plasticity of a nociceptive input on capsular central amygdaloid neurons in normal and acid-induced muscle pain mice. J. Neurosci 31, 2258–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Bowen A., Schier LA, Tupone D., Uddin O., Heinricher MM, 2019. Parabrachial complex: a hub for pain and aversion. J. Neurosci 39, 8225–8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Nguyen EK, Canto-Bustos M., Papale AE, Oswald AM, Ross SE, 2020. Divergent neural pathways emanating from the lateral parabrachial nucleus mediate distinct components of the pain response. Neuron 106, 927–939 e925. [DOI] [PubMed] [Google Scholar]
- Choi S., Hachisuka J., Brett MA, Magee AR, Omori Y., Iqbal NU, Zhang D., DeLisle MM, Wolfson RL, Bai L., Santiago C., Gong S., Goulding M., Heintz N., Koerber HR, Ross SE, Ginty DD, 2020. Parallel ascending spinal pathways for affective touch and pain. Nature 587, 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, 2009. Gustatory and homeostatic functions of the rodent parabrachial nucleus. Ann. N. Y. Acad. Sci 1170, 383–391. [DOI] [PubMed] [Google Scholar]
- Delaney AJ, Crane JW, Sah P., 2007. Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron 56, 880–892. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Garcia J., 1985. Olfactory responses in the gustatory area of the parabrachial pons. Brain Res. Bull 15, 673–676. [DOI] [PubMed] [Google Scholar]
- Dobolyi A., Irwin S., Makara G., Usdin TB, Palkovits M., 2005. Calcitonin generelated peptide-containing pathways in the rat forebrain. J. Comp. Neurol 489, 92–119. [DOI] [PubMed] [Google Scholar]
- Dore R., Iemolo A., Smith KL, Wang X., Cottone P., Sabino V., 2013. CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology 38, 2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragan WL, Czerski PM, Dragan M., 2017. PAC1 receptor (ADCYAP1R1) genotype and problematic alcohol use in a sample of young women. Neuropsychiatric Dis. Treat 13, 1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Somes C., Li TK, Lumeng L., Hwang BH, Jimenez P., Mathe AA, 1999. Calcitonin gene-related peptide (CGRP) levels and alcohol. Int. J. Neuropsychopharmacol 2, 173–179. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Krabbe S., Markovic M., Courtin J., Xu C., Massi L., Botta P., Bylund K., Muller C., Kovacevic A., Tovote P., Luthi A., 2017. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100. [DOI] [PubMed] [Google Scholar]
- Ferragud A., Velazquez-Sanchez C., Minnig MA, Sabino V., Cottone P., 2020. Pituitary adenylate cyclase-activating polypeptide (PACAP) modulates dependence induced alcohol drinking and anxiety-like behavior in male rats. Neuropsychopharmacology 46 (3), 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterly TL, Basu A., Nabit BP, Awad E., Williford KM, Centanni SW, Matthews RT, Silberman Y., Winder DG, 2019. alpha2A-Adrenergic receptor activation decreases parabrachial nucleus excitatory drive onto BNST CRF neurons and reduces their activity in vivo. J. Neurosci 39, 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin SA, Matthews RT, Wang Q., Muly EC, Winder DG, 2014. alpha(2A) adrenergic receptors filter parabrachial inputs to the bed nucleus of the stria terminalis. J. Neurosci 34, 9319–9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB, 1984. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. Rev 7, 229–259. [DOI] [PubMed] [Google Scholar]
- Gargiulo AT, Curtis GR, Barson JR, 2020. Pleiotropic pituitary adenylate cyclase- activating polypeptide (PACAP): novel insights into the role of PACAP in eating and drug intake. Brain Res. 1729, 146626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Reilly S., Scalera G., Norgren R., 1998. The parabrachial nucleus is essential for acquisition of a conditioned odor aversion in rats. Behav. Neurosci 112, 1104–1113. [PubMed] [Google Scholar]
- Gungor NZ, Pare D., 2014. CGRP inhibits neurons of the bed nucleus of the stria terminalis: implications for the regulation of fear and anxiety. J. Neurosci 34, 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R., Li FF, Chen ML, Ya MZ, He HL, Li D., 2015. The role of CGRP and CALCA T-692C single-nucleotide polymorphism in psoriasis vulgaris. Pharmazie 70, 88–93. [PubMed] [Google Scholar]
- Hamlin A., Buller KM, Day TA, Osborne PB, 2001. Peripheral withdrawal recruits distinct central nuclei in morphine-dependent rats. Neuropharmacology 41, 574581. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cheung J., Rhodes KM, Schutz KC, Falls WA, Braas KM, May V., 2009. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 34, 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M., Grimmig B., Falls WA, Braas K., May V., 2010. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J. Mol. Neurosci 42, 327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Adwanikar H., Li Z., Ji G., Neugebauer V., 2010. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol. Pain 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Li W., Neugebauer V., 2005. Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior. J. Neurosci 25, 10717–10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Neugebauer V., 2004. Synaptic plasticity in the amygdala in a visceral pain model in rats. Neurosci. Lett 361, 254–257. [DOI] [PubMed] [Google Scholar]
- Han S., Soleiman MT, Soden ME, Zweifel LS, Palmiter RD, 2015. Elucidating an affective pain circuit that creates a threat memory. Cell 162, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Halladay LR, Mazzone CM, Pati D., Bloodgood DW, Kim M., Jensen J., DiBerto JF, Boyt KM, Shiddapur A., Erfani A., Hon OJ, Neira S., Stanhope CM, Sugam JA, Saddoris MP, Tipton G., McElligott Z., Jhou TC, Stuber GD, Bruchas MR, Bulik CM, Holmes A., Kash TL, 2019. Central amygdala prepronociceptin-expressing neurons mediate palatable food consumption and reward. Neuron 102, 1037–1052 e1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC, 1985. Convergence of vagal and gustatory afferent input within the parabrachial nucleus of the rat. J. Auton. Nerv. Syst 13, 1–17. [DOI] [PubMed] [Google Scholar]
- Higgs S., Cooper SJ, 1996. Hyperphagia induced by direct administration of midazolam into the parabrachial nucleus of the rat. Eur. J. Pharmacol 313, 1–9. [DOI] [PubMed] [Google Scholar]
- Honma M., Endo N., Osada Y., Kim Y., Kuriyama K., 2012. Disturbances in equilibrium function after major earthquake. Sci. Rep 2, 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BH, Kunkler PE, Lumeng L., Li TK, 1995. Calcitonin gene-related peptide (CGRP) content and CGRP receptor binding sites in discrete forebrain regions of alcohol-preferring vs. -nonpreferring rats, and high alcohol-drinking vs. low alcoholdrinking rats. Brain Res. 690, 249–253. [DOI] [PubMed] [Google Scholar]
- Ikeda R., Takahashi Y., Inoue K., Kato F., 2007. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain 127, 161–172. [DOI] [PubMed] [Google Scholar]
- Jaeger TV, van der Kooy D., 1993. Morphine acts in the parabrachial nucleus, a pontine viscerosensory relay, to produce discriminative stimulus effects. Psychopharmacology (Berl) 110, 76–84. [DOI] [PubMed] [Google Scholar]
- Jaramillo AA, Williford KM, Marshall C., Winder DG, Centanni SW, 2020. BNST transient activity associates with approach behavior in a stressful environment and is modulated by the parabrachial nucleus. Neurobiology of Stress 13, 100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimnamazi H., Travers SP, Travers JB, 2002. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res. 957, 193–206. [DOI] [PubMed] [Google Scholar]
- Kaur S., Wang JL, Ferrari L., Thankachan S., Kroeger D., Venner A., Lazarus M., Wellman A., Arrigoni E., Fuller PM, Saper CB, 2017. A genetically defined circuit for arousal from sleep during hypercapnia. Neuron 96, 1153–1167 e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A., Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M., Pak S., Mattis J., Lim BK, Malenka RC, Warden MR, Neve R., Tye KM, Deisseroth K., 2013. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SB, Lezak KR, O’Reilly M., Toufexis DJ, Falls WA, Braas K., May V., Hammack SE, 2017. The effects of prior stress on anxiety-like responding to intra- BNST pituitary adenylate cyclase activating polypeptide in male and female rats. Neuropsychopharmacology 42, 1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissiwaa SA, Bagley EE., 2018. Central sensitization of the spino- parabrachialamygdala pathway that outlasts a brief nociceptive stimulus. J Physiol 596 (18), 4457–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocorowski LH, Helmstetter FJ, 2001. Calcitonin gene-related peptide released within the amygdala is involved in Pavlovian auditory fear conditioning. Neurobiol. Learn. Mem 75, 149–163. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2021. Drug addiction: hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol. Rev 73, 163–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen L., Saarikoski ST, Haukka J., Pirkola S., Aromaa A., Lonnqvist J., Partonen T., 2010. Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol Alcohol 45, 303–311. [DOI] [PubMed] [Google Scholar]
- Kovner R., Oler JA, Kalin NH, 2019. Cortico-limbic interactions mediate adaptive and maladaptive responses relevant to psychopathology. Am. J. Psychiatr 176, 987999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T., Arimura A., 2001. Axon terminals containing CGRP-immunoreactivity form synapses with CRF- and Met-enkephalin-immunopositive neurons in the laterodorsal division of the bed nucleus of the stria terminalis in the rat. Brain Res. 893, 11–20. [DOI] [PubMed] [Google Scholar]
- Kozicz T., Vigh S., Arimura A., 1997. Axon terminals containing PACAP- and VIPimmunoreactivity form synapses with CRF-immunoreactive neurons in the dorsolateral division of the bed nucleus of the stria terminalis in the rat. Brain Res. 767, 109–119. [DOI] [PubMed] [Google Scholar]
- Lebow MA, Chen A., 2016. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatr 21, 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legradi G., Das M., Giunta B., Hirani K., Mitchell EA, Diamond DM, 2007. Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plast. 79102, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Penzo MA, Taniguchi H., Kopec CD, Huang ZJ, Li B., 2013. Experiencedependent modification of a central amygdala fear circuit. Nat. Neurosci 16, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Lemon CH, 2019. Mouse parabrachial neurons signal a relationship between bitter taste and nociceptive stimuli. J. Neurosci 39, 1631–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JN, Sheets PL, 2018. The central amygdala to periaqueductal gray pathway comprises intrinsically distinct neurons differentially affected in a model of inflammatory pain. J Physiol 596, 6289–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JN, Sheets PL, 2020. Spared nerve injury differentially alters parabrachial monosynaptic excitatory inputs to molecularly specific neurons in distinct subregions of the central amygdala. Pain 161, 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Chen Z., Fan G., Li A., Yuan J., Xu T., 2018. Cell-type-specific afferent innervation of the nucleus accumbens core and shell. Front. Neuroanat 12, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Armentia M., Sah P., 2007. Bidirectional synaptic plasticity at nociceptive afferents in the rat central amygdala. J Physiol 581, 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin AT, Bhatti DL, Mulvey B., Pedersen CE, Girven KS, Oden-Brunson H., Kimbell K., Blackburn T., Sawyer A., Gereau R.W.t., Dougherty JD, Bruchas MR, 2021. Extended amygdala-parabrachial circuits alter threat assessment and regulate feeding. Sci Adv 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Kaye KT, Venkataraman A., Carlezon WA Jr., 2019. PACAP increases Arc/Arg 3.1 expression within the extended amygdala after fear conditioning in rats. Neurobiol. Learn. Mem 157, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles OW, May V., Hammack SE, 2019. Pituitary adenylate cyclase-activating peptide (PACAP) signaling and the dark side of addiction. J. Mol. Neurosci 68, 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles OW, Thrailkill EA, Linden AK, May V., Bouton ME, Hammack SE, 2018. Pituitary adenylate cyclase-activating peptide in the bed nucleus of the stria Terminalis mediates stress-induced reinstatement of cocaine seeking in rats. Neuropsychopharmacology 43, 978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missig G., Mei L., Vizzard MA, Braas KM, Waschek JA, Ressler KJ, Hammack SE, May V., 2017. Parabrachial pituitary adenylate cyclase-activating Polypeptide activation of amygdala endosomal extracellular signal-regulated kinase signaling regulates the emotional component of pain. Biol. Psychiatr 81, 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missig G., Roman CW, Vizzard MA, Braas KM, Hammack SE, May V., 2014. Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain. Neuropharmacology 86, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa Y., Takahashi Y., Watabe AM, Kato F., 2018. Predominant synaptic potentiation and activation in the right central amygdala are independent of bilateral parabrachial activation in the hemilateral trigeminal inflammatory pain model of rats. Mol. Pain 14, 1744806918807102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase M., Mikami K., Watabe AM, 2019. Parabrachial-to-amygdala control of aversive learning. Current Opinion in Behavioral Sciences 26, 18–24. [Google Scholar]
- Neugebauer V., Li W., 2002. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J. Neurophysiol 87, 103112. [DOI] [PubMed] [Google Scholar]
- Neugebauer V., Li W., 2003. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J. Neurophysiol 89, 716–727. [DOI] [PubMed] [Google Scholar]
- Neugebauer V., Li W., Bird GC, Bhave G., Gereau R.W.t., 2003. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J. Neurosci 23, 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V., Mazzitelli M., Cragg B., Ji G., Navratilova E., Porreca F., 2020. Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology 170, 108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklous DM, Simansky KJ, 2003. Neuropeptide FF exerts pro- and anti-opioid actions in the parabrachial nucleus to modulate food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol 285, R1046–R1054. [DOI] [PubMed] [Google Scholar]
- Nobis WP, Gonzalez Otarula KA, Templer JW, Gerard EE, VanHaerents S., Lane G., Zhou G., Rosenow JM, Zelano C., Schuele S., 2019. The effect of seizure spread to the amygdala on respiration and onset of ictal central apnea. J. Neurosurg 132, 1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis WP, Schuele S., Templer JW, Zhou G., Lane G., Rosenow JM, Zelano C., 2018. Amygdala-stimulation-induced apnea is attention and nasal-breathing dependent. Ann. Neurol 83, 460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okutsu Y., Takahashi Y., Nagase M., Shinohara K., Ikeda R., Kato F., 2017. Potentiation of NMDA receptor-mediated synaptic transmission at the parabrachialcentral amygdala synapses by CGRP in mice. Mol Pain 13, 1744806917709201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MA, Prado WA, 2001. Role of PAG in the antinociception evoked from the medial or central amygdala in rats. Brain Res. Bull 54, 55–63. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, 2018. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 41, 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati D., Pina MM, Kash TL, 2019. Ethanol-induced conditioned place preference and aversion differentially alter plasticity in the bed nucleus of stria terminalis. Neuropsychopharmacology 44, 1843–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Xu-Feng H., Sengul G., Watson C., 2012. Organization of brainstem nuclei. The Human Nervous System 260–327. [Google Scholar]
- Pritchard TC, Hamilton RB, Norgren R., 2000. Projections of the parabrachial nucleus in the old world monkey. Exp. Neurol 165, 101–117. [DOI] [PubMed] [Google Scholar]
- Qiao Y., Zhang CK, Li ZH, Niu ZH, Li J., Li JL, 2019. Collateral projections from the lateral parabrachial nucleus to the central amygdaloid nucleus and the ventral tegmental area in the rat. Anat. Rec 302, 1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver C., Uddin O., Ji Y., Li Y., Cramer N., Jenne C., Morales M., Masri R., Keller A., 2020. An amygdalo-parabrachial pathway regulates pain perception and chronic pain. J. Neurosci 40, 3424–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S., 1999. The parabrachial nucleus and conditioned taste aversion. Brain Res. Bull 48, 239–254. [DOI] [PubMed] [Google Scholar]
- Reilly S., Grigson PS, Norgren R., 1993. Parabrachial nucleus lesions and conditioned taste aversion: evidence supporting an associative deficit. Behav. Neurosci 107, 1005–1017. [DOI] [PubMed] [Google Scholar]
- Robinson SL, Dornellas APS, Burnham NW, Houck CA, Luhn KL, Bendrath SC, Companion MA, Brewton HW, Thomas RD, Navarro M., Thiele TE, 2020. Distinct and Overlapping Patterns of Acute Ethanol-Induced C-Fos Activation in two inbred replicate lines of mice selected for drinking to high blood ethanol concentrations. Brain Sci. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E., Sakurai K., Xu J., Chen Y., Toda K., Zhao S., Han BX, Ryu D., Yin H., Liedtke W., Wang F., 2017. A craniofacial-specific monosynaptic circuit enables heightened affective pain. Nat. Neurosci 20, 1734–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman CW, Lezak KR, Hartsock MJ, Falls WA, Braas KM, Howard AB, Hammack SE, May V., 2014. PAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress. Psychoneuroendocrinology 47, 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti I., Zambusi L., Maccioni P., Sau R., Provini L., Castelli MP, Gonciarz K., Colombo G., Morara S., 2019. Predisposition to alcohol drinking and alcohol consumption alter expression of calcitonin gene-related peptide, neuropeptide Y, and microglia in bed nucleus of stria terminalis in a subnucleus-specific manner. Front. Cell. Neurosci 13, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo AF, 2019. CGRP-based migraine therapeutics: how might they work, why so safe, and what next? ACS Pharmacol Transl Sci 2, 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PJ, Ross SI, Campos CA, Derkach VA, Palmiter RD, 2017. Oxytocinreceptor-expressing neurons in the parabrachial nucleus regulate fluid intake. Nat. Neurosci 20, 1722–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons JD, Weiss MS, Victor JD, Di Lorenzo PM, 2016. Taste coding of complex naturalistic taste stimuli and traditional taste stimuli in the parabrachial pons of the awake, freely licking rat. J. Neurophysiol 116, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, 2002. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci 25, 433–469. [DOI] [PubMed] [Google Scholar]
- Saper CB, 2016. The house alarm. Cell Metabol. 23, 754–755. [DOI] [PubMed] [Google Scholar]
- Sarhan M., Freund-Mercier MJ, Veinante P., 2005. Branching patterns of parabrachial neurons projecting to the central extended amgydala: single axonal reconstructions. J. Comp. Neurol 491, 418–442. [DOI] [PubMed] [Google Scholar]
- Sato M., Ito M., Nagase M., Sugimura YK, Takahashi Y., Watabe AM, Kato F., 2015. The lateral parabrachial nucleus is actively involved in the acquisition of fear memory in mice. Mol. Brain 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiglie MP, Huang L., Cottone P., Sabino V., 2019. Role of the PACAP system of the extended amygdala in the acoustic startle response in rats. Neuropharmacology 160, 107761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, 2016. Contributions of the central extended amygdala to fear and anxiety. J. Neurosci 36, 8050–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S., Shiosaka S., Emson PC, Hillyard CJ, Girgis S., MacIntyre I., Tohyama M., 1985. Calcitonin gene-related peptidergic projection from the parabrachial area to the forebrain and diencephalon in the rat: an immunohistochemical analysis. Neuroscience 16, 607–616. [DOI] [PubMed] [Google Scholar]
- Shinohara K., Watabe AM, Nagase M., Okutsu Y., Takahashi Y., Kurihara H., Kato F., 2017. Essential role of endogenous calcitonin gene-related peptide in painassociated plasticity in the central amygdala. Eur. J. Neurosci 46, 2149–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Davis M., Walker DL, 2013. CGRP antagonist infused into the bed nucleus of the stria terminalis impairs the acquisition and expression of context but not discretely cued fear. Learn. Mem 20, 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Yang Y., Davis M., 2011. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J. Neurosci 31, 1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderpalm AH, Berridge KC, 2000. The hedonic impact and intake of food are increased by midazolam microinjection in the parabrachial nucleus. Brain Res. 877, 288297. [DOI] [PubMed] [Google Scholar]
- Stroth N., Holighaus Y., Ait-Ali D., Eiden LE, 2011. PACAP: a master regulator of neuroendocrine stress circuits and the cellular stress response. Ann. N. Y. Acad. Sci 1220, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura YK, Takahashi Y., Watabe AM, Kato F., 2016. Synaptic and network consequences of monosynaptic nociceptive inputs of parabrachial nucleus origin in the central amygdala. J. Neurophysiol 115, 2721–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Roitman MF, Bernstein IL, 1996. c-Fos induction in rat brainstem in response to ethanol- and lithium chloride-induced conditioned taste aversions. Alcohol Clin. Exp. Res 20, 1023–1028. [DOI] [PubMed] [Google Scholar]
- Tokita K., Inoue T., Boughter JD Jr., 2010. Subnuclear organization of parabrachial efferents to the thalamus, amygdala and lateral hypothalamus in C57BL/6J mice: a quantitative retrograde double labeling study. Neuroscience 171, 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torruella-Suarez ML, Vandenberg JR, Cogan ES, Tipton GJ, Teklezghi A., Dange K., Patel GK, McHenry JA, Hardaway JA, Kantak PA, Crowley NA, DiBerto JF, Faccidomo SP, Hodge CW, Stuber GD, McElligott ZA, 2020. Manipulations of central amygdala neurotensin neurons alter the consumption of ethanol and sweet fluids in mice. J. Neurosci 40, 632–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Minnig MA, Steinman MQ, Oleata CS, Riley MW, Sabino V., Roberto M., 2020. PACAP regulation of central amygdala GABAergic synapses is altered by restraint stress. Neuropharmacology 168, 107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincis R., Fontanini A., 2019. Central taste anatomy and physiology. Handb. Clin. Neurol 164, 187–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M., 2008. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct. Funct 213, 29–42. [DOI] [PubMed] [Google Scholar]
- Wang L., Cao C., Wang R., Qing Y., Zhang J., Zhang XY, 2013. PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD’s emotional numbing symptoms in Chinese earthquake survivors. J. Affect. Disord 150, 156–159. [DOI] [PubMed] [Google Scholar]
- Wang Y., Kim J., Schmit MB, Cho TS, Fang C., Cai H., 2019. A bed nucleus of stria terminalis microcircuit regulating inflammation-associated modulation of feeding. Nat. Commun 10, 2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward HG, Simansky KJ, 2006. Chronic prevention of mu-opioid receptor (MOR) Gprotein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology (Berl) 187, 435–446. [DOI] [PubMed] [Google Scholar]
- Watabe AM, Ochiai T., Nagase M., Takahashi Y., Sato M., Kato F., 2013. Synaptic potentiation in the nociceptive amygdala following fear learning in mice. Mol. Brain 6, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ, 2003. An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am. J. Physiol. Regul. Integr. Comp. Physiol 285, R1055–R1065. [DOI] [PubMed] [Google Scholar]
- Wolinsky TD, Carr KD, Hiller JM, Simon EJ, 1996. Chronic food restriction alters mu and kappa opioid receptor binding in the parabrachial nucleus of the rat: a quantitative autoradiographic study. Brain Res. 706, 333–336. [DOI] [PubMed] [Google Scholar]
- Xu W., Lundeberg T., Wang YT, Li Y., Yu LC, 2003. Antinociceptive effect of calcitonin gene-related peptide in the central nucleus of amygdala: activating opioid receptors through amygdala-periaqueductal gray pathway. Neuroscience 118, 10151022. [DOI] [PubMed] [Google Scholar]
- Yan WW, Xia M., Chiang J., Levitt A., Hawkins N., Kearney J., Swanson GT, Chetkovich D., Nobis WP, 2021. Enhanced synaptic transmission in the extended amygdala and altered excitability in an extended amygdala to brainstem circuit in a Dravet syndrome mouse model. eNeuro 8 (3), 0306–20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Veinante P., 2019. Cell-type specific parallel circuits in the bed nucleus of the stria terminalis and the central nucleus of the amygdala of the mouse. Brain Struct. Funct 224, 1067–1095. [DOI] [PubMed] [Google Scholar]
- Zafra MA, Aguera AD, Simon MJ, Molina F., Puerto A., 2016. Satiation and reintake after partial withdrawal of gastric food contents: a dissociation effect in external lateral parabrachial lesioned rats. Brain Res. Bull 127, 126–133. [DOI] [PubMed] [Google Scholar]
- Zafra MA, Simon MJ, Molina F., Puerto A., 2002. The role of the external lateral parabrachial subnucleus in flavor preferences induced by predigested food administered intragastrically. Brain Res. 950, 155–164. [DOI] [PubMed] [Google Scholar]
- Zafra MA, Simon MJ, Molina F., Puerto A., 2005. Lesions of the lateral parabrachial area block the aversive component and induced-flavor preference for the delayed intragastric administration of nutrients in rats: effects on subsequent food and water intake. Nutr. Neurosci 8, 297–307. [DOI] [PubMed] [Google Scholar]