Abstract
Azadirachta indica (A. Juss), also known as the neem tree, has been used for millennia as a traditional remedy for a multitude of human ailments. Also recognized around the world as a broad-spectrum pesticide and fertilizer, neem has applications in agriculture and beyond. Currently, the extensive antimicrobial activities of A. indica are being explored through research in the fields of dentistry, food safety, bacteriology, mycology, virology, and parasitology. Herein, some of the most recent studies that demonstrate the potential of neem as a previously untapped source of novel therapeutics are summarized as they relate to the aforementioned research topics. Additionally, the capacity of neem extracts and compounds to act against drug-resistant and biofilm-forming organisms, both of which represent large groups of pathogens for which there are limited treatment options, are highlighted. Updated information on the phytochemistry and safety of neem-derived products are discussed as well. Although there is a growing body of exciting evidence that supports the use of A. indica as an antimicrobial, additional studies are clearly needed to determine the specific mechanisms of action, clinical efficacy, and in vivo safety of neem as a treatment for human pathogens of interest. Moreover, the various ongoing studies and the diverse properties of neem discussed herein may serve as a guide for the discovery of new antimicrobials that may exist in other herbal panaceas across the globe.
Keywords: neem (Azadirachta indica A. Juss), antibacterial, antiviral, antifungal, antiparasitic, phytochemicals, natural products, antibiofilm
Medicinal Plants as Sources for Novel Antimicrobial Agents
The need to expand the available pharmaceutical repertoire is underlined by several recent reports, including the 2019 Antibiotic Resistance Threat Report by the Centers for Disease Control and Prevention; this document states that in the United States alone, more than 2.8 million antibiotic-resistant infections and more than 35,000 related deaths occur each year (CDC, 2019). These fatal infections are most frequently caused by the 18 species of bacteria and fungi listed as current urgent, serious, or concerning human health threats (CDCE, 2019). Additionally, on a global scale, infectious diseases cause approximately 20% of all deaths each year and are the leading cause of death of children under 5 years old (Martens and Demain, 2017). Many in the medical field agree that devastating statistics like these are a consequence of entering the “post-antibiotic era,” a time in which the efficacies of antibiotics and other antimicrobials are unreliable (Wang et al., 2020; Streicher, 2021).
Despite the continuous popularity of herbal medicine across the globe, traditional antibiotics have previously overshadowed the exploration of plant-based products as therapeutics. However, due to the growing need for new antimicrobial agents, many scientists have now expanded their searches to include novel plant and other environmental sources. Indeed, mainstream medicine is increasingly receptive to the use of plant-derived drugs, especially those to which antimicrobial resistance is more difficult or unlikely to develop. Notably, 26% of all new approved drugs and 33% of all new small-molecule approved drugs between 1981 and 2014 were botanical drugs, unaltered natural products, or derivatives thereof (Newman and Cragg, 2016). This abundance underscores the vast, untapped potential of plants around the world to yield desperately needed novel drugs. In fact, only around 6% of the ∼300,000 species of higher plants have been pharmacologically investigated (Cragg and Newman, 2013). However, recent reviews by Khameneh et al. (2019) and Chassagne et al. (2020) have highlighted the increasingly evident antibacterial properties of various plant species and phytochemicals. Furthermore, there is an increasing amount of evidence that suggests that phytochemicals may be used in conjunction with current antimicrobials to obtain synergistic effects (Aiyegoro and Okoh, 2009; Borges et al., 2016; Barbieri et al., 2017; Ayaz et al., 2019). For example, an early study by Ahmad and Aqil (2007) found that crude extracts of multiple plant species showed in vitro synergistic activity with existing antibiotics when used against two multidrug-resistant enteric bacterial species (Ahmad and Aqil, 2007). Thus, the combination of phytochemicals and antibiotics may help to combat resistance to conventional monotherapies for many diseases. As evidence for the use of natural products to treat human disease continues to accumulate, it will become increasingly important to perform in-depth safety studies on the identified extracts, compounds, and their derivatives. So far, there is a general consensus that natural products, as compared to synthetic drugs, have relatively low toxicity to mammals and have less harmful effects on nontarget beneficial organisms (Brahmachari, 2004), which is yet another appealing aspect of utilizing plant species for the identification of effective pharmaceuticals.
The Importance of Azadirachta indica (Neem) as a Medicinal Plant
Azadirachta indica (A. Juss), commonly known as the neem tree, is a tropical evergreen tree that is native to the Indian subcontinent (Noorul Aneesa, 2016). For thousands of years, neem has been recognized for its wide array of beneficial properties, including those in agriculture for pest control and in traditional medicine for various common human ailments. A. indica originally provoked world-wide interest due to its capacity as a non-toxic infection-control agent for use in farming (Govindachari, 1992). Indeed, one of the most abundant compounds found within the neem plant, azadirachtin, is an increasingly common biopesticide (Chaudhary et al., 2017; Pasquoto-Stigliani et al., 2017; Kilani-Morakchi et al., 2021). However, various parts of the neem tree have been used for millennia in traditional Indian medicine for their claimed antipyretic, antacid, antiparasitic, antibacterial, antiviral, antidiabetic, contraceptive, antidermatitic, anticancer, anti-inflammatory, antioxidant, antifungal, dental, and other healing and protective properties (Govindachari, 1992; Alzohairy, 2016). Almost every part of A. indica (e.g., the stem, bark, roots, leaves, gum, seeds, fruits, flowers, etc.) have been used as house-hold remedies for human illnesses. Moreover, millions of people globally use neem twigs as a source of chewing sticks for dental hygiene (Brahmachari, 2004; Gupta et al., 2017). More recently, the neem tree has gained attention from modern medicine and infectious disease researchers as a potential source for new antimicrobials, in addition to the applications of A. indica in the fields of oncology, dentistry, dermatology, and endocrinology, among others; for reviews on some of these individual topics, see (Lakshmi et al., 2015; Patel et al., 2016; Aumeeruddy and Mahomoodally, 2021; Iman et al., 2021; Patil et al., 2021; Singh et al., 2021; Yarmohammadi et al., 2021).
Subsequent sections of this review seek to provide an overview of the most recent scientific findings that support the consideration of neem extracts and phytochemicals as antimicrobial agents. Specifically, the potential for neem and neem-related products to target pathogens that are resistant to first-line antibiotics, bacterial species that affect oral health and/or form difficult to eradicate biofilms, fungal infections that threaten food sources, and viral infections that have major impacts on human health are highlighted. Research in these fields is supported by a worldwide interest in neem products that stretches from ancient medicinal practices to an abundance of publications that highlight A. indica as a plant with modern pharmacological attributes. Apart from the activities highlighted herein, we would like to point the reader to the expert review by Saleem et al. (2018) that covers the most recent evidence supporting neem as a treatment for specific human ailments and known mechanisms of action of neem components; therein, the specific protective qualities of A. indica and related clinical trials are thoroughly reported.
Phytochemistry of Azadirachta indica
Although nearly every part of neem has been used for traditional medicinal purposes in India, the most widely available A. indica product on the market today is neem oil (Sir and Chopra, 1994). The country of India alone produces hundreds of thousands of tons of neem oil annually (National Research Council, 1992) and a byproduct of neem oil production includes neem cake, which is abundantly used in agriculture around the world at ∼600 pounds per acre of farmland (Reddy, 2020). Neem oil is considered a vegetable oil that is cold pressed from the fruits and seeds of neem (Noorul Aneesa, 2016). However, neem oil can be further processed into various types of extracts, via different solvents, that are then used for subsequent preclinical and clinical studies.
Although various solvents can be implemented to extract different active components from plant products, most of the compounds that are thought to be responsible for the biological activities of neem can be found in the extracts that are typically used in laboratories (e.g., water, ethanol, methanol, chloroform, and ether) (Cowan, 1999). In recently published literature, methanol and ethanol extracts are those that are most commonly used for antimicrobial testing. The general biological activities of the tested neem oil extracts have been attributed to the presence of many secondary plant metabolites, which include classes of compounds such as isoprenoids (e.g., terpenoids containing limonoid structures) and non-isoprenoids (e.g., tannins) (Saleem et al., 2018).
The neem tree contains hundreds of compounds (i.e., phytochemicals), many of which have been found to be bioactive and to have diverse utility on their own. Out of the more than 300 unique compounds have been identified within the neem tree, some of the more abundant phytochemicals (e.g., azadirachtin, gedunin, and nimbolide) have already been defined as potential drugs with a wide range of biological activities (Saleem et al., 2018; Braga et al., 2020; Nagini et al., 2021). The compounds that have been most thoroughly investigated for their individual antimicrobial properties thus far are limonoids, which are compounds that typically consist of four, six-membered rings and one five-membered aromatic ring (i.e., a furanolactone core) and make up one-third of the phytochemicals derived from the neem tree (Roy and Saraf, 2006; Gupta et al., 2017). This class of compounds has also been explored for its abundance of antioxidant activities [reviewed in (Tundis et al., 2014; Gualdani et al., 2016; Sarkar et al., 2021)] and includes nimbolide, nimbin, and nimbidin (triterpenoids), azadirachtin, and gedunin. Previously established in vitro activities of these compounds and others isolated from neem seed oil have been reviewed and range from anti-inflammatory and antiulcer to spermicidal and anti-psoriasis (Brahmachari, 2004). Two recent comprehensive reviews by Saleem et al. (2018) and Gupta et al. (2017) have expertly covered the extensive phytochemistry of neem.
Antimicrobial Testing and Safety of Azadirachta indica
To test the antimicrobial activities of neem oil extracts and phytochemicals, in vitro methods such as broth dilution, disc or agar diffusion, and agar overlay assays are commonly used to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of each treatment. A few in vivo models have been implemented to more accurately reflect human infection and disease testing; these models include intraperitoneal or intravenous injection, or oral or gastric administration of neem oil-related drugs in mice, rats, guinea pigs, and rabbits. These published animal studies indicate that the acute toxicity level of neem greatly depends on the plant component and solvent used to make the extract, as well as on the treatment route and species used in the model; for a comprehensive review on this topic, see (Braga et al., 2021). As an example, oral administration of an ethanolic neem leaf extract less than 2000 mg/kg body weight did not cause mortality in mice (Kanagasanthosh et al., 2015). Conversely, the ethanolic extract of neem stem bark given to rats at 50–200 mg/kg altered the biochemical markers of toxicity and may have consequential effects on organ function (Ashafa et al., 2012). In contrast, one small human study showed that the lyophilized powder of an aqueous neem bark extract given at doses of 30–60 mg twice daily for 10 weeks had therapeutic potential in adults for controlling gastric hypersecretion and gastroesophageal and gastroduodenal ulcers; there were no obvious effects on blood parameters indicative of organ toxicity (Bandyopadhyay et al., 2004). Also, after 1 year of external exposure to 1% neem oil, 156 adults and 110 children did not experience any major adverse effects (Brahmachari, 2004). Of note, Arsene et al. (2021) showed that the Galleria mellonella (wax moth larva) model is a reliable method to assess the acute in vivo toxicity of medicinal plants, in which ethanolic extracts of neem leaves and seeds had higher levels of toxicity than aqueous extracts of the same materials. Although the available in vivo data will need to be further developed before neem oil extracts and phytochemicals are applied in a clinical setting, the United States Environmental Protection Agency has stated that cold-pressed neem oil should have “no unreasonable adverse effects to the US population and the environment” (Agency U.S E.P, 2012). For neem-derived products, non-aqueous extracts are generally the most toxic, while unprocessed materials and pure phytochemicals from the neem tree have relatively low toxicities (Boeke et al., 2004). Given the currently available information summarized here, an important goal of future antimicrobial testing of neem oil and its products should be the standardization of extracts and administration methods. Additional more consistent studies on this topic may allow researchers to draw more detailed conclusions about the potential use of A. indica in a clinical setting.
Antibacterial Evidence
The rising rates of antibiotic resistance for bacterial pathogens has led to the need for novel therapeutics; thus, much of the recent work on the antimicrobial potential of neem has focused on the antibacterial properties of the plant. This area of research is supported by the traditional use of neem products for dental hygiene and the successful applications of neem in the food industry. In addition to standard antibiotic resistance, the ability of pathogenic bacterial species to form biofilms has led to an increased interest in describing how these communities contribute to heightened tolerance to antibacterial substances. Although the importance of biofilm-associated infections to human disease is well-recognized, few novel solutions that effectively eliminate biofilms have been developed thus far. However, encouraging data suggest that neem is consistently more effective at prohibiting bacterial growth and at targeting biofilm-grown cells than many other herbal extracts and is, therefore, worth pursuing as a source for drug discovery (Noor, 2011). For a more comprehensive list of the antibacterial properties of the neem tree described in the following sections, see Supplementary Table S1.
Azadirachta indica and Dentistry
As mentioned above, the use of neem twigs as dental cleaning sticks is commonplace in many countries to which A. indica is a native species (Gupta et al., 2017). This traditional use has translated to a growing number of studies that have tested neem products for their ability to improve dental hygiene and to prevent or treat oral diseases. Typically, these studies aim to identify an application for neem extracts as a mouthwash alternative, root canal irrigant, toothpaste, etc. Previously, the antimicrobial activity of A. indica against common endodontic pathogens, such as Enterococcus faecalis, Staphylococcus aureus, Streptococcus mutans, and Candida albicans, has been well-established (Mistry et al., 2014; Barua et al., 2017; Singh et al., 2020). For example, a decade ago, it was shown that at 7.5%, aqueous neem leaf extract was able to inhibit the growth of E. faecalis, S. mutans, and C. albicans and that the MIC of an ethanolic neem leaf extract was 1.88%, 7.5%, and 3.75% against these three important dental pathogens, respectively (Nayak Aarati et al., 2011). More recently, another group was able to determine that a methanolic extract of A. indica showed considerable antimicrobial activity against a three-week-old polymicrobial dental biofilm grown on extracted human teeth consisting of S. mutans, E. faecalis, S. aureus and C. albicans (Mistry et al., 2015). The results from two small human studies indicate that a neem-based toothpaste or gel can reduce the levels of S. mutans in the mouth and that the gel formulation can reduce plaque and gingivitis to the same degree as a chlorhexidine gel control (Nimbulkar et al., 2020; Selvaraj et al., 2020). Additional biologically relevant studies may be able to provide even more evidence for the use of neem in dentistry in combination with, or as an alternative to, antimicrobials already used in the field. Of note, several studies have already suggested that neem extracts have similar levels of activity as chlorhexidine or hypochlorite (typical components of oral washes) against plaque, gingivitis, and pain in vivo (Jalaluddin et al., 2017; Hosny et al., 2021) and against biofilm-forming bacteria (e.g., Streptococcus viridans, Porphyromonas gingivalis, and S. aureus) and C. albicans in vitro or ex vivo (Joy Sinha et al., 2015; Anand et al., 2016; Kankariya et al., 2016; Heyman et al., 2017; Andonissamy et al., 2019; Bansal et al., 2019; Tasanarong et al., 2021). Another human pathogen that causes plaque and other biofilm-related diseases in the body, E. faecalis, is also just as susceptible to various neem extracts as it is to chlorhexidine in vitro (Chandrappa et al., 2015; Mustafa, 2016; Bhardwaj et al., 2017; Joy Sinha et al., 2017).
The relevance of neem as an antimicrobial in dentistry is, so far, the most researched area and has led to many conclusions about A. indica extracts as compared to those from other plants used in traditional medicine. In fact, some studies suggest that neem has greater antibacterial activity than Commiphora myrrha (myrrh), Acacia tree (e.g., catechu), Cinnamomum verum (cinnamon), Salvadora persica (miswak), Syzygium aromaticum (clove), Zingiber officinale (ginger), Allium sativum (garlic) and Curcuma longa (tumeric) extracts against some species of bacteria and cultured dental caries (Kanth et al., 2016; Jagannathan et al., 2020; Arora et al., 2021). This being said, it is important to note that certain bacterial species (e.g., S. mutans and E. faecalis) appear to be more susceptible to extracts from other plants in some studies (Jain et al., 2015; Dedhia et al., 2018; Kalita et al., 2019; Panchal et al., 2020). While this does not diminish the antimicrobial potential of A. indica, it does underline the importance of thoroughly taking advantage of the wide variety of antimicrobial plants and compounds that are at the disposal of modern medicine.
The Use of Azadirachta indica in the Food Industry
Originally introduced around the world as a potent pesticide and fertilizer for use in agriculture (Govindachari, 1992; Nicoletti et al., 2012; Chaudhary et al., 2017), neem has been recognized in more recent years as a safe and effective broad-spectrum antimicrobial with uses throughout the food industry that range from food production and storage to packaging and human consumption. During meat production, the presence of several species of bacteria can affect the quality and safety of the product, including Campylobacter, Lactobacillus, and Carnobacterium spp. Neem cake extract, which is a waste product from neem seed oil production, has antibacterial activities against all of these potentially pathogenic species (Del Serrone et al., 2015a). Additionally, Ravva and Korn (2015) found that neem leaf and bark supplements were able to successfully eliminate Escherichia coli O157:H7 from cultured cow manure; because this E. coli strain was isolated from an apple juice outbreak of O157:H7, these results may have broad applications on farms where crops and orchards frequently exist in close proximity to cattle (Ravva and Korn, 2015). In the production of another source of protein for human consumption, specifically in shrimp aquaculture, antibiotic-resistant Vibrio parahaemolyticus can compromise both shrimp and human health. A thorough study on the potential use of neem in this industry included in vitro and in vivo assays that showed that aqueous neem extract had a MIC against V. parahaemolyticus of 62.5 mg/ml and was able to significantly increase survival of shrimp by 76% as compared to the untreated control (Morales-Covarrubias et al., 2016).
One of the next steps in the production of pathogen-free human food is storage and/or packaging. In the last couple of years, several groups have shown that food preservation films that are made from polyethylene or sustainable materials such as seaweed can be manufactured to incorporate neem leaf extracts, neem oil, and other plant-based products (e.g., turmeric and curcumin) (Ahmed et al., 2022). The resulting composite films are shelf-stable, block ultraviolet light, and have increased antifungal and antibacterial activities against C. albicans and a wide range of Gram-negative and Gram-positive organisms, including E. coli, S. aureus, Pseudomonas aeruginosa, and Bacillus subtilis (Sunthar et al., 2020; Uthaya Kumar et al., 2020; Oyekanmi et al., 2021; Subbuvel and Kavan, 2021). Furthermore, the ability of A. indica to prevent activity of food-spoiling fungi is evident by several recent reports that outline the following: 1) the ability of neem oil to prevent the growth of the grape product-spoiling species Aspergillus carbonarius and to inhibit the production of mycotoxin by strains of this fungus (Rodrigues et al., 2019), 2) the ability of neem leaves to prevent the production of aflatoxins by Aspergillus parasiticus during long-term storage of rice, wheat, and maize (Sultana et al., 2015), 3) the ability of neem seed methanol and ethanol extracts to inhibit Aspergillus flavus and A. parasiticus by 10% in the context of maize storage (An et al., 2019), 4) the ability of multiple neem seed, bark, and leaf extracts to inhibit the growth of three major potato-spoiling fungi, Aspergillus niger, Fusarium oxyporium, and Pythium spp., by 72–100% (Ezeonu et al., 2019), and 5) the ability of aqueous neem leaf extract to inhibit growth of A. niger and A. parasiticus, as well as to detoxify aflatoxin B1 and ochratoxin A in vivo (Hamad et al., 2021). These diverse antifungal properties of neem are highlighted in Supplementary Table S1. Altogether, these food-related studies should encourage further investigation of the utility of A. indica-derived products throughout the food industry; these products may serve as a sustainable antimicrobial alternative that can potentially improve food security via improved stable long-term storage, as well as improve human health through the elimination of foodborne pathogens.
Additional Antibacterial Activities of Neem
In several other areas of antibacterial discovery, A. indica has been shown to be effective against many important human pathogens. Overall, a large number of studies have been published in the last decade on this topic, especially as they relate to the ever-growing number of antibiotic-resistant organisms. In this vein, several bacterial species that cause wound infections are found on the long list of antibiotic resistant threats, including S. aureus and P. aeruginosa (CDC, 2019). Several groups have tested the antibacterial activity of neem against these species. For example, Garg et al. (2015) demonstrated that methanol and chloroform neem extracts performed better than other plant extracts and better than several different antibiotics against both S. aureus and P. aeruginosa (Garg et al., 2015). More specifically, it has also been determined that the MIC of limonoid compounds that were isolated from neem seeds are between 32 μg/ml and 128 μg/ml against P. aeruginosa and another opportunistic skin pathogen, Staphylococcus epidermidis (Lu et al., 2019). As a translational approach to this area of research, several studies have demonstrated that an aqueous neem leaf extract that was used to make alginate fibers for wound dressings (Hussain et al., 2017), a nanofibrous mat embedded with neem leaf extract (Ali et al., 2019), and a topical gel containing a methanolic neem extract all inhibit S. aureus growth (Raju and Jose, 2019). In the case of a polyesteramide synthesized from neem oil to produce a nanofibrous mat, the incorporation of A. indica into this wound treatment method resulted in increased tissue regeneration in rats, as compared to the control commercial cream (Killi et al., 2019). These results potentially have broad applications for many areas of medicine in which the risk of antibiotic resistant skin/wound infections is high.
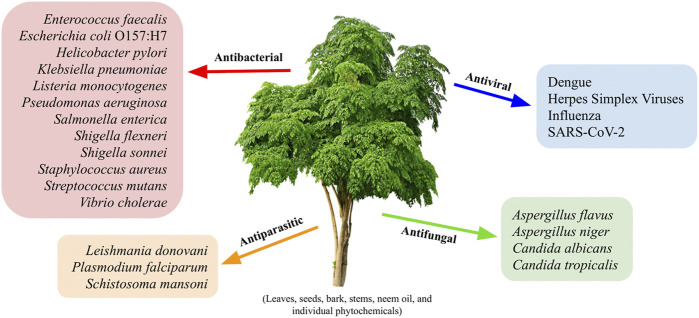
Another large group of infectious bacteria that cause morbidity and mortality all over the world are the gastrointestinal pathogens, which include foodborne and diarrhea-causing organisms. Relatedly, some of the traditional uses of neem are antidiarrheal, antacid, and antiulcer; this has led to a large body of research that has investigated the antibacterial properties of neem products against pathogens such as Salmonella spp., Shigella spp., E. coli, Listeria monocytogenes, and Bacillus cereus, as shown in Figure 1. To summarize, the Salmonella spp. and Shigella spp. that have been tested, which includes more than a dozen multidrug-resistant isolates from patients suffering from typhoid fever complications, are susceptible to seed, bark, and leaf extracts of neem from either ethanol, methanol, or acetone extraction; in some cases, the activity of neem extract was also found to be greater than that of gentamycin, erythromycin, and other plants used in traditional medicine (Mahfuzul Hoque et al., 2007; Susmitha et al., 2013; Tesso et al., 2015; Melese et al., 2016; Al Akeel et al., 2017; Panchal et al., 2020; Essuman et al., 2021). Similarly, dried leaf, seed, and bark neem extracts in any of the three previously mentioned solvents have significant antibacterial activity against E. coli, with the methanolic extract of neem seeds demonstrating the greatest level of activity (Susmitha et al., 2013; Sharma and Nupur, 2014; Melese et al., 2016). Additionally, neem oil was as effective as ciprofloxacin against 48 tested isolates of E. coli, 14 of which were diarrheagenic strains (Del Serrone et al., 2015b). Given that there are more than a quarter of a million cases of E. coli each year in the United States alone, associated world-wide morbidity is a huge issue for this pathogen (CDCE, 2019). Indeed, resistant E. coli infections were found to be responsible for nearly a quarter of all disability-adjusted life years caused by resistant bacterial infections in the European Union in 2015 (Cassini et al., 2019). On the chronic disease spectrum of gastrointestinal illnesses, Helicobacter pylori is a pathogen that colonizes approximately 50% of the human population and causes ulcers in millions of people each year; it also causes stomach cancer in ∼1% of people who are infected (Kuipers, 1999; Moodley et al., 2012; Bray et al., 2018). Recently, two studies found that neem oil extract and a neem-associated phytochemical, nimbolide, have potent in vitro bactericidal activity against H. pylori in liquid cultures and in biofilms (Blum et al., 2019; Wylie et al., 2021). Similarly, another group determined that an ethanolic neem leaf extract has activity against this species as well (Saxena et al., 2021). Overall, studies like those described above provide strong evidence that novel plant-derived treatments, like neem oil extracts and/or the phytochemicals contained therein, may be able to reduce the burden of pervasive organisms like E. coli and H. pylori.
FIGURE 1.
Representative antimicrobial targets of the neem tree, Azadirachta indica. Virtually all parts of the neem tree (leaves, seeds, bark, and stems), neem oil, and individual neem-associated phytochemicals have been shown to possess antibacterial, antiviral, antiparasitic, and/or antifungal activities. Some of the pathogens that have been studied and shown to be susceptible to A. indica-associated compounds are listed in their respective categories; the length of the provided list indicates the relative amount of published information on each of the four topics. A more exhaustive list of the pathogens that are susceptible to neem-derived products is available in Supplementary Table S1.
Finally, there have been many studies published on the antibacterial properties of neem against a variety of diverse human pathogens. The overwhelming conclusion from the majority of these investigations is that many elements of A. indica (e.g., seeds, bark, leaves, etc.) produce extracts that have moderate to significant levels of antimicrobial activity against several pathogens: S. aureus, E. coli, E. faecalis, P. aeruginosa, Salmonella typhi, Streptococcus agalactiae, Shigella boydii, B. subtilis, Klebsiella pneumoniae, and Candida tropicalis, as listed in Figure 1 (Dahiya and Purkayastha, 2012; Melese et al., 2016; Al Saiqali et al., 2018; Ibrahim and Kebede, 2020). Moreover, in side-by-side comparisons A. indica-based components often show superiority to other plants; a few exceptions include aqueous garlic extract and ethanolic green tea extract that were each shown to work better than neem extracts to kill Bacillus anthracis or S. aureus and E. coli, respectively (Zihadi et al., 2019; Kaur et al., 2021). Overall, studies support the notion that A. indica is an omnipotent plant that possesses antimicrobial activity against many bacterial pathogens. Future studies should include more standardized research approaches to test the potential of neem-derived products and individual compounds against other microbes both in vitro and in vivo; a priority should also be placed on determining the mechanisms of action of these products in order to fully understand the ideal clinical significance of A. indica.
Neem and Biofilm-Forming Pathogens
Biofilms, or communities of bacteria composed of biofilm-associated cells and extracellular polymeric substance (EPS) components (e.g., proteins, polysaccharides, and extracellular DNA), are notably recalcitrant to outside stressors, including those from the immune system and from therapeutics (Bae and Jeon, 2013; Yonezawa et al., 2019; de Vor et al., 2020). Depending on the species of biofilm-associated bacteria, mechanisms of tolerance include slow diffusion/limited penetration of the drug, metabolic heterogeneity and decreased growth rates among the cells within the biofilm, and the formation of persister cells (Mah and O'Toole, 2001; Stewart and Costerton, 2001; Attaran et al., 2017). Backed by thorough research, plants such as A. indica may provide the tools needed to treat pervasive biofilm infections. To this end, there is evidence that natural products may be an ideal source of quorum sensing inhibitors, efflux pump inhibitors, and metal chelators, which are all potentially powerful antibiofilm agents (Borges et al., 2016). Additionally, synergistic interactions between the many constituents found within plant extracts may provide a benefit over a single isolated ingredient; this may explain the efficacy of lower doses of herbal products like neem oil as compared to individual compounds (Aiyegoro and Okoh, 2009).
Significant biofilm-associated human infections are caused by species such as S. aureus, E. faecalis, and P. aeruginosa (Vestby et al., 2020). There is already evidence that A. indica has activity against biofilm-forming strains of some of these pathogens. For example, a neem leaf ethanolic extract was found to inhibit S. aureus and methicillin-resistant S. aureus (MRSA) biofilm adherence at 62.5 and 125 μg/ml, respectively (Quelemes et al., 2015). More recently, another group found that a petroleum ether neem extract had a MIC and MBC of 125 and 500 μg/ml, respectively, against a strain of MRSA (S et al., 2020). In the same in vitro study, the addition of 1 mg/ml of the neem extract resulted in a 68.9% reduction in MRSA biofilm; 2 mg/ml of the same extract resulted in a 83.8% reduction (S et al., 2020). Additionally, Guchhait et al. found that ripe neem seed extracts had antibiofilm activity against S. aureus and Vibrio cholerae. The minimum biofilm inhibitory concentrations (MBIC) and minimum biofilm eradication concentrations (MBEC) for this extract were 100 and 300 μg/ml, respectively, against S. aureus and 300 and 500 μg/ml, respectively, against V. cholerae (Guchhait et al., 2022). Furthermore, using a mouse model of V. cholerae infection, Thakurta et al. showed that administration of methanolic neem leaf extract at 100–1800 mg/kg body weight reduced intestinal fluid secretion by 27.7%–77.9% and doses ≥300 mg/kg inhibited Vibrio-induced hemorrhage in the murine intestine without signs of toxicity (Thakurta et al., 2007). Of note, individual neem-associated phytochemicals, such as catechin, may have greater potential for biofilm eradication, persister cell damage, disruption of EPS structural components, and prevention of quorum sensing (Lahiri et al., 2021). In addition, a methanolic neem oil extract and nimbolide both killed cells within in vitro biofilms produced by the Gram-negative carcinogenic pathogen, H. pylori (Wylie et al., 2021). Overall, evidence indicates that neem has great potential to be used as a therapeutic for resistant bacterial infections. However, future research that utilizes animal models will be crucial to determine whether neem-derived products fit in with established antibiotic regimens and/or work alone to eradicate biofilms in vivo.
Antiviral Evidence
Although most recent studies have investigated the antibacterial and antifungal potential of A. indica, some work has also explored the antiviral activities of neem; this topic has previously been reviewed in (Dhawan, 2012). To date, most publications have centered around human immunodeficiency virus (HIV), as well as the herpes, Dengue, and influenza viruses; however, recent reports have also included SARS-CoV-2, which is responsible for the COVID-19 pandemic. Moreover, a few groups have successfully experimented with the use of neem products against other viruses, such as Japanese encephalitis virus (Dwivedi et al., 2021b), hepatitis C (Ashfaq et al., 2016), and coxsackie virus (Younus et al., 2016). The antiviral studies have primarily focused on the ability of individual neem-associated phytochemicals to block critical processes of the viral life cycle, including cell entry and replication. Intriguingly, this means that the obtained results may describe a mechanism of action as well as identify a distinct drug candidate that can be directly modified for pharmaceutical development. Finally and importantly, as an alternative method to control specific classes of viral or parasitic diseases, many researchers have demonstrated the ability of A. indica derivatives to deter and/or to negatively affect the many species of insect vectors that transmit these pathogens [reviewed in (Benelli et al., 2017a)] (Soni and Prakash, 2014; Abiy et al., 2015; Benelli et al., 2015; Maheswaran and Ignacimuthu, 2015; Poopathi et al., 2015; Chandramohan et al., 2016; Murugan et al., 2016; Yerbanga et al., 2016; Benelli et al., 2017b; Kaura et al., 2019; Paramo et al., 2020; Rasool et al., 2020; Ejeta et al., 2021; Zatelli et al., 2022).
Neem and HIV
Human immunodeficiency virus, or HIV, is arguably one of the most devastating modern human pathogens. Since its discovery in the early 1980s, there have been over one million new HIV infections each year; hundreds of thousands of people still die from the subsequent acquired immunodeficiency syndrome (AIDS) annually (Barre-Sinoussi et al., 1983; Gallo et al., 1984; Levy et al., 1984; UNAIDS, 2022). Although antiretroviral therapy (ART) is well-established and is successful at diminishing viral load and preventing disease progression, ART drugs are expensive, and require life-long treatment that is not without side effects (CDC, 2022). To this end, natural products, such as those obtained from A. indica, that are traditionally used for HIV-associated infections (Nagata et al., 2011; Anywar et al., 2020), have been explored for their abilities to protect the CD4+ T cell population that is vulnerable during HIV infection, to reduce persistent immune activation during ART, and to decrease the toxicity of ART drugs. For instance, small trials have concluded that neem leaf extract given daily is safe and effective at improving CD4+ T cell counts in HIV patients (Udeinya et al., 2004; Mbah et al., 2007). Furthermore, when A. indica and Senna siamea leaf extracts were given in combination with ART, this HIV patient group had improved T cell numbers and fewer markers of hepatic and renal toxicity than the group that was given ART alone (Goni Hamad et al., 2021). To address the possibility of T cell exhaustion in HIV-infected patients, Olwenyi et al. (2021) performed an in vitro study with peripheral blood cells isolated from infected and uninfected individuals; following exposure to enterotoxin, the lymphocytic response indicated that A. indica extract, but not the extracts from two other plants, was able to down-regulate CD4+ T cell activation in a concentration-dependent manner without affecting general T cell-specific functions. Overall, these results support the idea that neem has immunomodulatory abilities that can be exploited to increase the efficacies of certain treatments and to improve the condition of chronically infected patients, such as those with HIV.
Herpes and Sulfonoquinovosyldiacylglyceride From Azadirachta indica
Herpes simplex viruses (HSV) most commonly cause oral and genital infections, with an estimated half a billion people living with HSV type 2 and nearly four billion people with HSV type 1 in any given year (James et al., 2020). Because there is no cure for HSV infections and they can recur many times throughout a person’s life, broad searches for novel antiviral medications are warranted in order to identify agents that may reduce the morbidity associated with these infections; these searches include phytochemicals isolated from medicinal plant such as A. indica. For example, the glycolipid sulfonoquinovosyldiacylglyceride (SQDG) that has been isolated from neem leaves was shown to have potent antiviral activity against HSV-1 and -2; the half maximal effective concentrations (EC50) were 9.1 and 8.5 μg/ml, respectively. The same study also found that HSV-infected, SQDG-treated macrophages produced significantly less proinflammatory cytokines than untreated controls (Bharitkar et al., 2014). It has been suggested that the antiviral and anti-inflammatory properties of SQDG may indicate that A. indica contains other phytochemicals with therapeutic potential against viruses (Shanmugam et al., 2020). Concurrently, it was found that two polysaccharides isolated from neem leaves, along with their sulfated derivates, are able to inhibit HSV-1 nucleic acid synthesis at concentrations that were not cytotoxic (Faccin-Galhardi et al., 2019). Additionally, an aqueous neem bark extract was able to block HSV-1 glycoprotein binding to and virus entry into target cells in vitro (Tiwari et al., 2010). The preliminary data reported here indicate that products derived from A. indica can act at several steps of the viral life cycle to prevent herpes infections.
Azadirachta indica Components Against Dengue Proteins
Several groups have demonstrated that computational screening methods can be successfully used to identify novel inhibitors of viruses that infect hundreds of millions of people globally each year, including vector-borne diseases such as Dengue (CDC, 2021). In the context of Dengue virus (DENV), 49 different bioflavonoids that are present in the neem tree were virtually screened for binding to the DENV serine protease, NS2B-NS3pro. Subsequent in vitro assays with promising candidates revealed that kaempferol 3-O-β-rutinoside and epicatechin were able to inhibit DENV-2 infectivity by 77.7% and 66.2%, respectively, without significant cell toxicity (Dwivedi et al., 2021a). Similarly, it was previously shown that three members of another important class of neem phytochemicals, the terpenoids, were able to bind to NS2B-NS3pro with high affinity in silico; this binding ability was subsequently confirmed in vitro (Dwivedi et al., 2016). Nimbin, one of the more common triterpenoids isolated from neem leaf extracts, was shown to be effective against the envelope protein of all four types of DENV in silico (Lavanya et al., 2015). Overall, the ability of neem-derived phytochemicals to block the activities of both the protease and envelope proteins of DENV, and potentially other viruses, further suggest that A. indica may be a novel source for antiviral drugs (Shanmugam et al., 2020).
Influenza and Neem Phytochemicals
Flu leads to an estimated 290,000-650,000 deaths annually (WHO, 2020). Due to these consistently high levels of associated morbidity and mortality around the world, influenza is one of the most intensely researched viruses. Though new vaccines for influenza are constantly in development, the burden of flu could be additionally reduced by the introduction of an antiviral that is effective at all stages of disease. Recent evidence suggests that A. indica may represent a robust source of novel drugs against viruses such as influenza. To summarize, molecular docking experiments identified a total of four neem phytochemicals that interact with conserved residues of either the nucleoprotein or the non-structural (NS1) protein of influenza. Though requiring further testing, this may indicate the ability to act as a universal drug against the flu virus (Ahmad et al., 2015; Ahmad et al., 2016).
Azadirachta indica and SARS-CoV-2
Taking the lives of more than five million people over the last 2 years, SARS-CoV-2 and the associated disease, COVID-19, continue to be significant threats to public health for which there are few treatment options (WHO, 2021b). Consequently, many researchers have successfully screened chemical libraries for viral inhibitors that act against SARS-CoV-2 (Garg et al., 2020; Vardhan and Sahoo, 2020; Baildya et al., 2021; Borkotoky and Banerjee, 2021; Gogoi et al., 2021; Nallusamy et al., 2021; Navabshan et al., 2021; Senapati et al., 2021; Ogidigo et al., 2022; Vardhan and Sahoo, 2022); detailed reviews on the potential for medicinal plants to be used against SARS-CoV-2 are available (Thota et al., 2020; Adithya et al., 2021). Phytochemicals and plant-derived products like those from A. indica are sometimes included in these chemical libraries. For example, in silico binding simulations by Parida et al. (2020) identified nimolicinol as a compound with strong affinity for both the main protease and the spike protein of SARS-CoV-2; similar docking studies found that nimbocinol binds to the papain-like protease of SARS-CoV-2 with higher affinity than remdesivir, indicating its potential to hinder viral replication (Balkrishna et al., 2021). Although these types of studies are high throughput and can detect candidates for further investigation, virtual studies must typically be followed by in vitro experiments before an antiviral can have clinical relevance. Despite this, given the devastation associated with the ongoing pandemic, several clinical trials are in progress to test neem as a component of mouthwashes, nasal sprays, and capsules for suspected, confirmed, or hospitalized COVID-19 patients, or for prophylaxis (Khan et al., 2020; Farhan Raza Khan, 2021; Nesari et al., 2021). Additionally, one animal study has been completed in which coronavirus-infected mice were treated orally or intranasally with neem bark extract; overall, the therapy was able to prevent systemic injury and pathologic effects of the virus both in vivo and in vitro using multiple model systems (Sarkar et al., 2022). Advocating for additional trials of neem against SARS-CoV-2, Eze et al. (2022) made connections between some of the known mechanisms of A. indica products against other illnesses and the potential efficacy of neem against the virus that causes COVID-19. For additional information about the pharmacological implications for the use of medicinal plants against respiratory infections, see the recent review by Timalsina et al. (2021). All in all, the antiviral action of A. indica against a variety of viruses that cause human disease is evident (Supplementary Table S1). Moreover, outside of HIV, HSV, DENV, influenza, and SARS-CoV-2, there is a strong indication that the aforementioned phytochemical screening methods may be used to identify novel inhibitors of other viral life cycles.
Antiparasitic Evidence
Although the area of research regarding A. indica as a potential therapeutic against parasites is relatively underdeveloped, most of the investigations that have been published so far have focused on Plasmodium, the human pathogen of global importance that causes malaria; more than half a million deaths attributed to malaria are estimated to occur each year (WHO, 2021c). While there are effective prophylactics and therapeutics approved for this disease, these resources are not readily available in all parts of the world. In these instances, there is the possibility that natural products may be more easily acquired, distributed, and accepted; neem products could be part of the solution. Recently, a comprehensive review on the antiplasmodial activities of African medicinal plants was published and found that neem extracts consistently performed well across experiments (Tajbakhsh et al., 2021). Studies using both in vitro and in vivo models indicate that aqueous, methanolic, or ethanolic extracts of neem stems, leaves, or bark all have significant antimalarial activity against a variety of Plasmodium falciparum and Plasmodium berghei strains (Benoit et al., 1996; MacKinnon et al., 1997; El-Tahir et al., 1999; Gathirwa et al., 2011; BC et al., 2013; Tepongning et al., 2018). These publications suggest that A. indica and other medicinal plants are active against malaria; however, only one clinical trial with an African plant species has been conducted so far (Benoit-Vical et al., 2003). Despite the lack of controlled human studies, the malaria mouse model has been used to demonstrate that neem bark and seed extracts are active against Plasmodium infection both alone and as part of a polyherbal mixture; in the case of the bark extract, this was indicated by parasite suppression or decreased erythrocyte infection (Habluetzel et al., 2019; Alaribe et al., 2021). The gestational malaria mouse model has also been used to show that administration of A. indica leaves improves the overall health of P. falciparum-infected dams, including reduced parasitemia, increased platelet counts, lower levels of preeclampsia biomarkers, and increased birth weight of pups (Amadi et al., 2021). Additionally, the limonoid deacetylnimbin, which is found within neem seed extracts, is able to interfere with the early sporogony stages of P. bergehi; this finding suggests that certain neem-associated phytochemicals may have the ability to limit Plasmodium transmissibility, warranting further investigation in other models and clinical trials (Tapanelli et al., 2016). Another commonly studied phytochemical of A. indica, azadirachtin, was shown to bind to Gephyrin E almost as well as artesunate during in silico analyses, which indicates that, upon further study, azadirachtin may be an effective treatment for cerebral malaria (Okoh et al., 2021). Providing additional convincing data for the investigation of neem products as therapeutics, Somsak et al. (2015) used the mouse model of Plasmodium infection to show that an aqueous neem leaf extract was able to reduce the blood markers of malaria-induced renal injury to normal levels without being toxic to the animals. Taken together, the available in vitro and in vivo data suggest that plants used in traditional medicine (e.g., neem) should be further explored in clinical trials for their antimalarial capacity.
Throughout tropical and subtropical areas around the world, the neglected tropical protozoan leishmania causes an estimated 700,000 to 1 million infections each year and is highly fatal if left untreated; visceral leishmaniasis is the most severe form of associated disease (WHO, 2021a). Leishmaniasis can be treated, but there is not currently a drug available that eliminates the leishmania parasite from the body; thus, the patient is susceptible to relapse if immunosuppression occurs (WHO, 2021a). Although studies on this topic are limited, there is some evidence that plant extracts can kill leishmania parasites, including an ethyl acetate fraction (EAF) of neem leaf extract (Dayakar et al., 2015). When treated with this EAF, promastigotes underwent an apoptosis-like death and intracellular amastigotes were also killed in vitro and in vivo (Dayakar et al., 2015). With this evidence, more in vivo and translational research on the antileishmanial activity of neem is merited.
Finally, the last antimicrobial activity of A. indica that has been recently explored is that against parasitic worms (Figure 1). As the most widespread neglected tropical diseases globally, the burden of schistosomiasis and soil-transmitted helminth infections could undoubtedly be reduced with the addition of new and accessible plant-derived treatments or prophylactics to the already available drug regimens (Molyneux et al., 2017). Although still a minor area of research, the antischistosomal and anthelminthic properties of neem suggest that natural products can be potent inhibitors of larger eukaryotic pathogens as well. Indeed, the same neem leaf extract that was effective against S. aureus and MRSA biofilms also caused severe tegument morphology changes, significant reduction of motor activity, or death of Schistosoma mansoni worms in vitro (Quelemes et al., 2015). For helminth related studies, an in vivo experiment showed that neem leaf powder used at 500 mg/kg body weight worked as well as 5 mg/kg fenbendazole to treat cows with bovine Strongyloides infections (Jamra et al., 2015); Strongyloides is a major hurdle to profitable farming in tropical and subtropical regions and can have a substantial economic impact (Jamra et al., 2015). Similarly, problematic helminths for the poultry industry are Raillietina spp., which are parasitic tapeworms. These organisms were found to be severely paralyzed, damaged, or killed by short exposures to SQDG, a glycolipid from neem extracts that also has antiviral activity (Ash et al., 2017). Although not yet tested on human helminth infections, neem-derived products appear to have strong potential against animal pathogens, thus supporting the need for a deeper investigation of the overall antiparasitic activity of A. indica.
The Future of Neem as an Antimicrobial
As summarized in this review, a variety of extracts and phytochemicals from the Indian evergreen tree, A. indica, have significant antimicrobial activity against a multitude of pathogens that affect human health. The dental application of neem appears to be one of the most researched areas, with the general antibacterial properties of neem not far behind. Intriguingly, there are several recent studies that suggest that neem may have broad applications in the food industry, aside from its traditional uses in agriculture for pest-control and fertilizer. Some of the least-developed areas, however, are the antiviral and antiparasitic activities of neem-derived products. Overall, there is sufficient evidence to warrant further investigation into these properties of A. indica; undoubtedly, in vivo models will be crucial to understanding the clinical relevance of neem against all of the microorganisms mentioned here and those that have yet to be tested.
Of note in recent years, there are several groups who have incorporated neem into novel materials and technologies that have broad implications for human health. Specifically, green-synthesized copper or silver nanoparticles and hydrogels, nanocellulose films, chitosan-copper oxide biopolymers, and hydroxyapatite have all been constructed to include neem extracts and have substantial antimicrobial activity, including against multidrug-resistant bacterial species (Nagaraj and Samiappan, 2019; Revathi and Thambidurai, 2019; Algebaly et al., 2020; Asghar and Asghar, 2020; Lakkim et al., 2020; Sharma and Bhardwaj, 2020; Ulaeto et al., 2020; Chinnasamy et al., 2021; Ghazali et al., 2022; Lan Chi et al., 2022). Both in vitro and in vivo data suggest that these composite materials represent a growing industry of creative antimicrobial technologies that have the potential to revolutionize infectious disease treatments and biomedical science as a whole.
In addition to the many recently published manuscripts that indicate that A. indica-derived substances have broad-spectrum antimicrobial properties, it is worth noting that dozens of patents are filed each year that mention neem-based products. Indeed, a simple query of the United States Patent and Trademark Office’s online Patent Public Search tool (USPTO, 2022) using the terms “neem and antimicrobial” yields over 400 results since 2015. This available list of patents helps to demonstrate the limitless applications of the antimicrobial properties of neem. While too many exist to cover in detail here, some notable examples include very diverse applications. For example, medical gloves (Wong et al., 2022) and a polymeric yarn for use in hygienic textiles (Mandawewala and Mandawewala, 2021) have both been enriched with neem derivates and shown to have beneficial antimicrobial properties. Furthermore, in line with the reported antimicrobial uses of neem in the fields of dentistry, dermatology, and agriculture, many of the patented neem-containing products fall into these categories as well; patents have been awarded for neem-containing dental rinses and composites (Tatch, 2021; Ramana, 2022), for neem and/or other plant extract-containing topical treatments for mild skin disorders (Balaraman, 2015), and for neem-based pest control formulas (Mazariegos, 2016). Clearly, the investigation of neem as an antimicrobial is an area of research that is constantly expanding and is generating valuable products that may improve human health.
In order to develop realistic A. indica-based treatment regimens that could be used in humans, there are clearly many intriguing areas for future investigation. Undoubtably, future experiments will need to elucidate the mechanisms of action of neem and the associated phytochemicals. Given the available data summarized in this review, some of the most promising areas of investigation moving forward appear to be 1) the application of individual neem phytochemicals and derivatives thereof as antimicrobial agents alone or used in combination with existing treatments, 2) additional pre-clinical and clinical studies to determine the toxicity and effective in vivo dosing of specific phytochemicals as compared to parent neem extracts, and 3) the inclusion of hundreds of available medicinal plant products, extracts, and phytochemicals in screens for potential inhibitors of emerging and resistant infectious diseases. It is important to note that to gain maximal utility from these areas of research, close attention should be paid to the types of extracts (including both the particular part of the plant and the solvent) that have already been tested against which organisms. Some level of standardization should be considered so that comparisons can be made and patterns can be recognized across multiple studies. This may become easier when the antimicrobial activities of more individual phytochemicals are determined. En masse, A. indica represents a novel source of antimicrobials that may be used to combat drug resistance and emerging threats to human health. Furthermore, the research that has been done on the neem tree can be used as a guide to encourage the investigation of other traditionally used natural products for their utility as modern pharmaceuticals.
Acknowledgments
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Uniformed Services University or the Department of Defense (DoD). We would like to acknowledge Jeannette Whitmire for critical review of the manuscript.
Author Contributions
MW and DM both contributed to conceptualization of the review. MW performed the investigation and wrote the original draft. MW and DM contributed to manuscript revision and both authors read and approved the final version.
Funding
Research in the Merrell lab is supported by funding from the NIH and the United States DoD.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.891535/full#supplementary-material
Abbreviations
AIDS, acquired immunodeficiency syndrome; DENV, Dengue virus; EAF, ethyl acetate fraction; EC50, half maximal effective concentration; EPS, extracellular polymeric substance; HIV, human immunodeficiency virus; HSV, herpes simplex virus; MBC, minimum bactericidal concentration; MBEC, minimum biofilm eradication concentration; MBIC, minimum biofilm inhibitory concentration; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; SQDG, sulfonoquinovosyldiacylglyceride.
References
- Abiy E., Gebre-Michael T., Balkew M., Medhin G. (2015). Repellent Efficacy of DEET, MyggA, Neem (Azedirachta indica) Oil and Chinaberry (Melia azedarach) Oil against Anopheles arabiensis, the Principal Malaria Vector in Ethiopia. Malar. J. 14, 187. 10.1186/s12936-015-0705-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adithya J., Nair B., Aishwarya T. S., Nath L. R. (2021). The Plausible Role of Indian Traditional Medicine in Combating Corona Virus (SARS-CoV 2): A Mini-Review. Curr. Pharm. Biotechnol. 22 (7), 906–919. 10.2174/1389201021666200807111359 [DOI] [PubMed] [Google Scholar]
- Ahmad A., Ahad A., Rao A. Q., Husnain T. (2015). Molecular Docking Based Screening of Neem-Derived Compounds with the NS1 Protein of Influenza Virus. Bioinformation 11 (7), 359–365. 10.6026/97320630011359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Javed M. R., Rao A. Q., Husnain T. (2016). Designing and Screening of Universal Drug from Neem (Azadirachta indica) and Standard Drug Chemicals against Influenza Virus Nucleoprotein. BMC Complement. Altern. Med. 16 (1), 519. 10.1186/s12906-016-1469-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad I., Aqil F. (2007). In Vitro efficacy of Bioactive Extracts of 15 Medicinal Plants against ESbetaL-Producing Multidrug-Resistant Enteric Bacteria. Microbiol. Res. 162 (3), 264–275. 10.1016/j.micres.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Ahmed W., Azmat R., Khojah E., Ahmed R., Qayyum A., Shah A. N., et al. (2022). The Development of a Green Innovative Bioactive Film for Industrial Application as a New Emerging Technology to Protect the Quality of Fruits. Molecules 27 (2), 486. 10.3390/molecules27020486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Akeel R., Mateen A., Janardhan K., Gupta V. C. (2017). Analysis of Anti-bacterial and Anti Oxidative Activity of Azadirachta indica Bark Using Various Solvents Extracts. Saudi J. Biol. Sci. 24 (1), 11–14. 10.1016/j.sjbs.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Saiqali M., Tangutur A. D., Banoth C., Bhukya B. (2018). Antimicrobial and Anticancer Potential of Low Molecular Weight Polypeptides Extracted and Characterized from Leaves of Azadirachta indica . Int. J. Biol. Macromol. 114, 906–921. 10.1016/j.ijbiomac.2018.03.169 [DOI] [PubMed] [Google Scholar]
- Aiyegoro A., Okoh A. I. (2009). Use of Bioactive Plant Products in Combination with Standard Antibiotics: Implications in Antimicrobial Chemotherapy. J. Med. Plants Res. 3 (13), 1147–1152. [Google Scholar]
- Alaribe S. C., Oladipupo A. R., Uche G. C., Onumba M. U., Ota D., Awodele O., et al. (2021). Suppressive, Curative, and Prophylactic Potentials of an Antimalarial Polyherbal Mixture and its Individual Components in Plasmodium Berghei-Infected Mice. J. Ethnopharmacol. 277, 114105. 10.1016/j.jep.2021.114105 [DOI] [PubMed] [Google Scholar]
- Algebaly A. S., Mohammed A. E., Abutaha N., Elobeid M. M. (2020). Biogenic Synthesis of Silver Nanoparticles: Antibacterial and Cytotoxic Potential. Saudi J. Biol. Sci. 27 (5), 1340–1351. 10.1016/j.sjbs.2019.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A., Shahid M. A., Hossain M. D., Islam M. N. (2019). Antibacterial Bi-layered Polyvinyl Alcohol (PVA)-chitosan Blend Nanofibrous Mat Loaded with Azadirachta indica (Neem) Extract. Int. J. Biol. Macromol. 138, 13–20. 10.1016/j.ijbiomac.2019.07.015 [DOI] [PubMed] [Google Scholar]
- Alzohairy M. A. (2016). Therapeutics Role of Azadirachta indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment. Evid. Based Complement. Altern. Med. 2016, 7382506. 10.1155/2016/7382506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadi P. U., Agomuo E. N., Ukaga C. N., Njoku U. C., Amadi J. A., Nwaekpe C. G. (2021). Preclinical Trial of Traditional Plant Remedies for the Treatment of Complications of Gestational Malaria. Med. (Basel) 8 (12). 10.3390/medicines8120079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An A., Je A., Cb U., Mn I. (2019). Identification and Control of Specific Aflatoxin-Producing Fungi in Stored Maize Seeds in Awka Using Azadirachta indica (Neem) and garcinia Kola Seeds. Pak J. Pharm. Sci. 32 (4), 1679–1686. [PubMed] [Google Scholar]
- Anand P. J., Athira S., Chandramohan S., Ranjith K., Raj V. V., Manjula V. D. (2016). Comparison of Efficacy of Herbal Disinfectants with Chlorhexidine Mouthwash on Decontamination of Toothbrushes: An Experimental Trial. J. Int. Soc. Prev. Community Dent. 6 (1), 22–27. 10.4103/2231-0762.175406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andonissamy L., Karthigeyan S., Ali S. A., Felix J. W. (2019). Effect of Chemical Denture Disinfectants and Tree Extracts on Biofilm-Forming Staphylococcus aureus and Viridans Streptococcus Species Isolated from Complete Denture. J. Contemp. Dent. Pract. 20 (11), 1307–1314. [PubMed] [Google Scholar]
- Anywar G., Kakudidi E., Byamukama R., Mukonzo J., Schubert A., Oryem-Origa H. (2020). Indigenous Traditional Knowledge of Medicinal Plants Used by Herbalists in Treating Opportunistic Infections Among People Living with HIV/AIDS in Uganda. J. Ethnopharmacol. 246, 112205. 10.1016/j.jep.2019.112205 [DOI] [PubMed] [Google Scholar]
- Arora S., Saquib S. A., Algarni Y. A., Kader M. A., Ahmad I., Alshahrani M. Y., et al. (2021). Synergistic Effect of Plant Extracts on Endodontic Pathogens Isolated from Teeth with Root Canal Treatment Failure: An In Vitro Study. Antibiot. (Basel) 10 (5). 10.3390/antibiotics10050552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsene M. M. J., Viktorovna P. I., Davares A. K. L. (2021). Galleria mellonella (Greater Wax Moth) as an Eco-Friendly In Vivo Approach for the Assessment of the Acute Toxicity of Medicinal Plants: Application to Some Plants from Cameroon. Open Vet. J. 11 (4), 651–661. 10.5455/OVJ.2021.v11.i4.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar M. A., Asghar M. A. (2020). Green Synthesized and Characterized Copper Nanoparticles Using Various New Plants Extracts Aggravate Microbial Cell Membrane Damage after Interaction with Lipopolysaccharide. Int. J. Biol. Macromol. 160, 1168–1176. 10.1016/j.ijbiomac.2020.05.198 [DOI] [PubMed] [Google Scholar]
- Ash A., Bharitkar Y. P., Murmu S., Hazra A., Ravichandiran V., Kar P. K., et al. (2017). Ultrastructural Changes in Raillietina (Platyhelminthes: Cestoda), Exposed to Sulfonoquinovosyldiacylglyceride (SQDG), Isolated from Neem (Azadirachta indica). Nat. Prod. Res. 31 (20), 2445–2449. 10.1080/14786419.2017.1305383 [DOI] [PubMed] [Google Scholar]
- Ashafa A. O., Orekoya L. O., Yakubu M. T. (2012). Toxicity Profile of Ethanolic Extract of Azadirachta indica Stem Bark in Male Wistar Rats. Asian Pac J. Trop. Biomed. 2 (10), 811–817. 10.1016/S2221-1691(12)60234-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfaq U. A., Jalil A., Ul Qamar M. T. (2016). Antiviral Phytochemicals Identification from Azadirachta indica Leaves against HCV NS3 Protease: an In Silico Approach. Nat. Prod. Res. 30 (16), 1866–1869. 10.1080/14786419.2015.1075527 [DOI] [PubMed] [Google Scholar]
- Attaran B., Falsafi T., Ghorbanmehr N. (2017). Effect of Biofilm Formation by Clinical Isolates of Helicobacter pylori on the Efflux-Mediated Resistance to Commonly Used Antibiotics. World J. Gastroenterol. 23 (7), 1163–1170. 10.3748/wjg.v23.i7.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumeeruddy M. Z., Mahomoodally M. F. (2021). Ethnomedicinal Plants for the Management of Diabetes Worldwide: A Systematic Review. Curr. Med. Chem. 28 (23), 4670–4693. 10.2174/0929867328666210121123037 [DOI] [PubMed] [Google Scholar]
- Ayaz M., Ullah F., Sadiq A., Ullah F., Ovais M., Ahmed J., et al. (2019). Synergistic Interactions of Phytochemicals with Antimicrobial Agents: Potential Strategy to Counteract Drug Resistance. Chem. Biol. Interact. 308, 294–303. 10.1016/j.cbi.2019.05.050 [DOI] [PubMed] [Google Scholar]
- Agency, U.S.E.P. (2012). in Cold Pressed Neem Oil PC Code 025006. Editor B.A P. P. D. (NW Washington DC: Office of Pesticide Programs; ). [Google Scholar]
- Bae J., Jeon B. (2013). Increased Emergence of Fluoroquinolone-Resistant Campylobacter jejuni in Biofilm. Antimicrob. Agents Chemother. 57 (10), 5195–5196. 10.1128/AAC.00995-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baildya N., Khan A. A., Ghosh N. N., Dutta T., Chattopadhyay A. P. (2021). Screening of Potential Drug from Azadiractha indica (Neem) Extracts for SARS-CoV-2: An Insight from Molecular Docking and MD-simulation Studies. J. Mol. Struct. 1227, 129390. 10.1016/j.molstruc.2020.129390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaraman B. (2015). Compositions and Methods for Their Dermatological Use. 14/646049. [Google Scholar]
- Balkrishna A., Mittal R., Arya V. (2021). Computational Evidences of Phytochemical Mediated Disruption of PLpro Driven Replication of SARS-CoV-2: A Therapeutic Approach against COVID-19. Curr. Pharm. Biotechnol. 22 (10), 1350–1359. 10.2174/1389201021999201110204116 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay U., Biswas K., Sengupta A., Moitra P., Dutta P., Sarkar D., et al. (2004). Clinical Studies on the Effect of Neem (Azadirachta indica) Bark Extract on Gastric Secretion and Gastroduodenal Ulcer. Life Sci. 75 (24), 2867–2878. 10.1016/j.lfs.2004.04.050 [DOI] [PubMed] [Google Scholar]
- Bansal V., Gupta M., Bhaduri T., Shaikh S. A., Sayed F. R., Bansal V., et al. (2019). Assessment of Antimicrobial Effectiveness of Neem and Clove Extract against Streptococcus Mutans and Candida Albicans: An In Vitro Study. Niger. Med. J. 60 (6), 285–289. 10.4103/nmj.NMJ_20_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri R., Coppo E., Marchese A., Daglia M., Sobarzo-Sánchez E., Nabavi S. F., et al. (2017). Phytochemicals for Human Disease: An Update on Plant-Derived Compounds Antibacterial Activity. Microbiol. Res. 196, 44–68. 10.1016/j.micres.2016.12.003 [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., et al. (1983). Isolation of a T-Lymphotropic Retrovirus from a Patient at Risk for Acquired Immune Deficiency Syndrome (AIDS). Science 220 (4599), 868–871. 10.1126/science.6189183 [DOI] [PubMed] [Google Scholar]
- Barua D. R., Basavanna J. M., Varghese R. K. (2017). Efficacy of Neem Extract and Three Antimicrobial Agents Incorporated into Tissue Conditioner in Inhibiting the Growth of C. albicans and S. mutans . J. Clin. Diagn Res. 11 (5), ZC97–ZC101. 10.7860/JCDR/2017/23784.9950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bc A.-O., Aj N., Salisu I., Hajia Mairo I., E O., E A., et al. (2013). Antimalarial Effect of Neem Leaf and Neem Stem Bark Extracts on Plasmodium berghei Infected in the Pathology and Treatment of Malaria. Int. J. Res. Biochem. Biophysics 3 (1), 7–14. [Google Scholar]
- Benelli G., Bedini S., Cosci F., Toniolo C., Conti B., Nicoletti M. (2015). Larvicidal and Ovideterrent Properties of Neem Oil and Fractions against the Filariasis Vector Aedes albopictus (Diptera: Culicidae): a Bioactivity Survey across Production Sites. Parasitol. Res. 114 (1), 227–236. 10.1007/s00436-014-4183-3 [DOI] [PubMed] [Google Scholar]
- Benelli G., Canale A., Toniolo C., Higuchi A., Murugan K., Pavela R., et al. (2017a). Neem (Azadirachta indica): towards the Ideal Insecticide? Nat. Prod. Res. 31 (4), 369–386. 10.1080/14786419.2016.1214834 [DOI] [PubMed] [Google Scholar]
- Benelli G., Chandramohan B., Murugan K., Madhiyazhagan P., Kovendan K., Panneerselvam C., et al. (2017b). Neem Cake as a Promising Larvicide and Adulticide against the Rural Malaria Vector Anopheles culicifacies (Diptera: Culicidae): a HPTLC Fingerprinting Approach. Nat. Prod. Res. 31 (10), 1185–1190. 10.1080/14786419.2016.1222390 [DOI] [PubMed] [Google Scholar]
- Benoit F., Valentin A., Pelissier Y., Diafouka F., Marion C., Kone-Bamba D., et al. (1996). In Vitro antimalarial Activity of Vegetal Extracts Used in West African Traditional Medicine. Am. J. Trop. Med. Hyg. 54 (1), 67–71. 10.4269/ajtmh.1996.54.67 [DOI] [PubMed] [Google Scholar]
- Benoit-Vical F., Valentin A., Da B., Dakuyo Z., Descamps L., Mallié M. (2003). N'Dribala (Cochlospermum Planchonii) versus Chloroquine for Treatment of Uncomplicated Plasmodium Falciparum Malaria. J. Ethnopharmacol. 89 (1), 111–114. 10.1016/s0378-8741(03)00277-0 [DOI] [PubMed] [Google Scholar]
- Bhardwaj A., Srivastava N., Rana V., Adlakha V. K., Asthana A. K. (2017). How Efficacious Are Neem, Tulsi, Guduchi Extracts and Chlorhexidine as Intracanal Disinfectants? A Comparative Ex Vivo Study. Ayu 38 (1-2), 70–75. 10.4103/ayu.AYU_72_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharitkar Y. P., Bathini S., Ojha D., Ghosh S., Mukherjee H., Kuotsu K., et al. (2014). Antibacterial and Antiviral Evaluation of Sulfonoquinovosyldiacylglyceride: a Glycolipid Isolated from Azadirachta indica Leaves. Lett. Appl. Microbiol. 58 (2), 184–189. 10.1111/lam.12174 [DOI] [PubMed] [Google Scholar]
- Blum F. C., Singh J., Merrell D. S. (2019). In Vitro activity of Neem (Azadirachta indica) Oil Extract against Helicobacter pylori . J. Ethnopharmacol. 232, 236–243. 10.1016/j.jep.2018.12.025 [DOI] [PubMed] [Google Scholar]
- Boeke S. J., Boersma M. G., Alink G. M., van Loon J. J., van Huis A., Dicke M., et al. (2004). Safety Evaluation of Neem (Azadirachta indica) Derived Pesticides. J. Ethnopharmacol. 94 (1), 25–41. 10.1016/j.jep.2004.05.011 [DOI] [PubMed] [Google Scholar]
- Borges A., Abreu A. C., Dias C., Saavedra M. J., Borges F., Simões M. (2016). New Perspectives on the Use of Phytochemicals as an Emergent Strategy to Control Bacterial Infections Including Biofilms. Molecules 21 (7). 10.3390/molecules21070877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkotoky S., Banerjee M. (2021). A Computational Prediction of SARS-CoV-2 Structural Protein Inhibitors from Azadirachta indica (Neem). J. Biomol. Struct. Dyn. 39 (11), 4111–4121. 10.1080/07391102.2020.1774419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga T. M., Rocha L., Chung T. Y., Oliveira R. F., Pinho C., Oliveira A. I., et al. (2021). Azadirachta indica A. Juss. In Vivo Toxicity-An Updated Review. Molecules 26 (2). 10.3390/molecules26020252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga T. M., Rocha L., Chung T. Y., Oliveira R. F., Pinho C., Oliveira A. I., et al. (2020). Biological Activities of Gedunin-A Limonoid from the Meliaceae Family. Molecules 25 (3). 10.3390/molecules25030493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari G. (2004). Neem--an Omnipotent Plant: a Retrospection. Chembiochem 5 (4), 408–421. 10.1002/cbic.200300749 [DOI] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 68 (6), 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Cassini A., Högberg L. D., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G. S., et al. (2019). Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: a Population-Level Modelling Analysis. Lancet Infect. Dis. 19 (1), 56–66. 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2021). About Dengue: What You Need to Know cdc.Gov: Centers for Disease Control and Prevention. Available: cdc.gov/dengue/about/index.html [Google Scholar]
- CDC (2019). Antibiotic Resistance Threats in the United States, 2019. Available: www.cdc.gov/DrugResistance/Biggest-Threats.html . [Google Scholar]
- CDC E (2019). E. coli (Escherichia coli) Questions & Answers [Online]. U.S. Department of Health & Human Services. Available: https://www.cdc.gov/ecoli/general/index.html#:∼:text=How%20common%20are%20STEC%20infections,O157%20STEC%20cause%20the%20rest . [Google Scholar]
- CDC (2022). HIV Treatment cdc.Gov: Centers for Disease Control and Prevention. Available: cdc.gov/hiv/basics/livingwithhiv/treatment.html [Google Scholar]
- Chandramohan B., Murugan K., Panneerselvam C., Madhiyazhagan P., Chandirasekar R., Dinesh D., et al. (2016). Characterization and Mosquitocidal Potential of Neem Cake-Synthesized Silver Nanoparticles: Genotoxicity and Impact on Predation Efficiency of Mosquito Natural Enemies. Parasitol. Res. 115 (3), 1015–1025. 10.1007/s00436-015-4829-9 [DOI] [PubMed] [Google Scholar]
- Chandrappa P. M., Dupper A., Tripathi P., Arroju R., Sharma P., Sulochana K. (2015). Antimicrobial Activity of Herbal Medicines (Tulsi Extract, Neem Extract) and Chlorhexidine against Enterococcus faecalis in Endodontics: An In Vitro Study. J. Int. Soc. Prev. Community Dent. 5 (Suppl. 2), S89–S92. 10.4103/2231-0762.172952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassagne F., Samarakoon T., Porras G., Lyles J. T., Dettweiler M., Marquez L., et al. (2020). A Systematic Review of Plants with Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 11, 586548. 10.3389/fphar.2020.586548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary S., Kanwar R. K., Sehgal A., Cahill D. M., Barrow C. J., Sehgal R., et al. (2017). Progress on Azadirachta indica Based Biopesticides in Replacing Synthetic Toxic Pesticides. Front. Plant Sci. 8, 610. 10.3389/fpls.2017.00610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnasamy G., Chandrasekharan S., Koh T. W., Bhatnagar S. (2021). Synthesis, Characterization, Antibacterial and Wound Healing Efficacy of Silver Nanoparticles from Azadirachta indica . Front. Microbiol. 12, 611560. 10.3389/fmicb.2021.611560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan M. M. (1999). Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 12 (4), 564–582. 10.1128/CMR.12.4.564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg G. M., Newman D. J. (2013). Natural Products: a Continuing Source of Novel Drug Leads. Biochim. Biophys. Acta 1830 (6), 3670–3695. 10.1016/j.bbagen.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya P., Dahiya P., Purkayastha S. (2012). Phytochemical Screening and Antimicrobial Activity of Some Medicinal Plants against Multi-Drug Resistant Bacteria from Clinical Isolates. Indian J. Pharm. Sci. 74 (5), 443–450. 10.4103/0250-474X.108420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayakar A., Chandrasekaran S., Veronica J., Sundar S., Maurya R. (2015). In Vitro and In Vivo Evaluation of Anti-leishmanial and Immunomodulatory Activity of Neem Leaf Extract in Leishmania Donovani Infection. Exp. Parasitol. 153, 45–54. 10.1016/j.exppara.2015.02.011 [DOI] [PubMed] [Google Scholar]
- de Vor L., Rooijakkers S. H. M., van Strijp J. A. G. (2020). Staphylococci Evade the Innate Immune Response by Disarming Neutrophils and Forming Biofilms. FEBS Lett. 594 (16), 2556–2569. 10.1002/1873-3468.13767 [DOI] [PubMed] [Google Scholar]
- Dedhia J., Mukharjee E., Luke A. M., Mathew S., Pawar A. M. (2018). Efficacy of Andrographis Paniculata Compared to Azadirachta indica, Curcuma longa, and Sodium Hypochlorite when Used as Root Canal Irrigants against Candida Albicans and Staphylococcus aureus: An In Vitro Antimicrobial Study. J. Conserv. Dent. 21 (6), 642–645. 10.4103/JCD.JCD_118_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Serrone P., Failla S., Nicoletti M. (2015a). Natural Control of Bacteria Affecting Meat Quality by a Neem (Azadirachta indica A. Juss) Cake Extract. Nat. Prod. Res. 29 (10), 985–987. 10.1080/14786419.2014.964708 [DOI] [PubMed] [Google Scholar]
- Del Serrone P., Toniolo C., Nicoletti M. (2015b). Neem (Azadirachta indica A. Juss) Oil to Tackle Enteropathogenic Escherichia coli . Biomed. Res. Int. 2015, 343610. 10.1155/2015/343610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan B. N. (2012). Anti-Viral Activity of Indian Plants. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 82 (1), 209–224. 10.1007/s40011-011-0016-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi V. D., Bharadwaj S., Afroz S., Khan N., Ansari M. A., Yadava U., et al. (2021a). Anti-dengue Infectivity Evaluation of Bioflavonoid from Azadirachta indica by Dengue Virus Serine Protease Inhibition. J. Biomol. Struct. Dyn. 39 (4), 1417–1430. 10.1080/07391102.2020.1734485 [DOI] [PubMed] [Google Scholar]
- Dwivedi V. D., Singh A., El-Kafraway S. A., Alandijany T. A., Faizo A. A., Bajrai L. H., et al. (2021b). Mechanistic Insights into the Japanese Encephalitis Virus RNA Dependent RNA Polymerase Protein Inhibition by Bioflavonoids from Azadirachta indica . Sci. Rep. 11 (1), 18125. 10.1038/s41598-021-96917-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi V. D., Tripathi I. P., Mishra S. K. (2016). In Silico evaluation of Inhibitory Potential of Triterpenoids from Azadirachta indica against Therapeutic Target of Dengue Virus, NS2B-NS3 Protease. J. Vector Borne Dis. 53 (2), 156–161. [PubMed] [Google Scholar]
- Ejeta D., Asme A., Asefa A. (2021). Insecticidal Effect of Ethnobotanical Plant Extracts against Anopheles arabiensis under Laboratory Conditions. Malar. J. 20 (1), 466. 10.1186/s12936-021-04004-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tahir A., Satti G. M., Khalid S. A. (1999). Antiplasmodial Activity of Selected Sudanese Medicinal Plants with Emphasis on Acacia Nilotica . Phytother. Res. 13 (6), 474–478. [DOI] [PubMed] [Google Scholar]
- Essuman E. K., Boakye A. A., Tettey C. O., Hunkpe G., Kortei N. K., Kwansa-Bentum H., et al. (2021). Evaluation of the Antidiarrheal and Antioxidant Effects of Some Chewing Sticks Commonly Used for Oral Hygiene in Ghana. Evid. Based Complement. Altern. Med. 2021, 7270250. 10.1155/2021/7270250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze M. O., Ejike C. E. C. C., Ifeonu P., Udeinya I. J., Udenigwe C. C., Uzoegwu P. N. (2022). Anti-COVID-19 Potential of Azadirachta indica (Neem) Leaf Extract. Sci. Afr. 16, e01184. 10.1016/j.sciaf.2022.e01184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeonu C. S., Tatah V. S., Imo C., Mamma E., Mayel M. H., Kukoyi A. J., et al. (2019). Inhibitory Effect of Aqueous and Ethanolic Extracts of Neem Parts on Fungal Rot Disease of Solanum tuberosum . Pak J. Biol. Sci. 22 (5), 206–213. 10.3923/pjbs.2019.206.213 [DOI] [PubMed] [Google Scholar]
- Faccin-Galhardi L. C., Ray S., Lopes N., Ali I., Espada S. F., Dos Santos J. P., et al. (2019). Assessment of Antiherpetic Activity of Nonsulfated and Sulfated Polysaccharides from Azadirachta indica . Int. J. Biol. Macromol. 137, 54–61. 10.1016/j.ijbiomac.2019.06.129 [DOI] [PubMed] [Google Scholar]
- Farhan Raza Khan B. (2021). A Double Blind, Randomized Controlled Pilot Trial of Gargling Agents in Reducing Intraoral Viral Load Among Laboratory Confirmed COVID-19 Patients: GARGLES STUDY [Online]. MS, FCPS, Aga Khan University (Identifier NCT04341688). Available: https://clinicaltrials.gov/ct2/show/NCT04341688 . [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., et al. (1984). Frequent Detection and Isolation of Cytopathic Retroviruses (HTLV-III) from Patients with AIDS and at Risk for AIDS. Science 224 (4648), 500–503. 10.1126/science.6200936 [DOI] [PubMed] [Google Scholar]
- Garg R., Perveen S., Gupta B., Bajpai V. K. (2015). Evaluation of Antibacterial Activity of Annona Squamosa, Psidium Guajava and Azadirachta indica against Pathogenic Bacterial Cultures. [Google Scholar]
- Garg S., Anand A., Lamba Y., Roy A. (2020). Molecular Docking Analysis of Selected Phytochemicals against SARS-CoV-2 Mpro Receptor. Vegetos 33 (4), 1–16. 10.1007/s42535-020-00162-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathirwa J. W., Rukunga G. M., Mwitari P. G., Mwikwabe N. M., Kimani C. W., Muthaura C. N., et al. (2011). Traditional Herbal Antimalarial Therapy in Kilifi District, Kenya. J. Ethnopharmacol. 134 (2), 434–442. 10.1016/j.jep.2010.12.043 [DOI] [PubMed] [Google Scholar]
- Ghazali S. Z., Mohamed Noor N. R., Mustaffa K. M. F. (2022). Anti-plasmodial Activity of Aqueous Neem Leaf Extract Mediated Green Synthesis-Based Silver Nitrate Nanoparticles. Prep. Biochem. Biotechnol. 52 (1), 99–107. 10.1080/10826068.2021.1913602 [DOI] [PubMed] [Google Scholar]
- Gogoi B., Chowdhury P., Goswami N., Gogoi N., Naiya T., Chetia P., et al. (2021). Identification of Potential Plant-Based Inhibitor against Viral Proteases of SARS-CoV-2 through Molecular Docking, MM-PBSA Binding Energy Calculations and Molecular Dynamics Simulation. Mol. Divers 25 (3), 1963–1977. 10.1007/s11030-021-10211-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni Hamadama O., Leonel Javeres M. N., Nyemb N., Mba Fabrice M., Manuela Elsa P. T. (2021). Effect of Azadirachta indica and Senna siamea Decoction on CD4+ and CD8+ Level, Toxicological, and Antioxidant Profile in HIV/AIDS Positive Persons. J. Toxicol. 2021, 5594505. 10.1155/2021/5594505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindachari T. R. (1992). Chemical and Biological Investigations on Azadirachta indica (The Neem Tree). Curr. Sci. 63 (3), 117–122. [Google Scholar]
- Gualdani R., Cavalluzzi M. M., Lentini G., Habtemariam S. (2016). The Chemistry and Pharmacology of Citrus Limonoids. Molecules 21 (11). 10.3390/molecules21111530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guchhait K. C., Manna T., Barai M., Karmakar M., Nandi S. K., Jana D., et al. (2022). Antibiofilm and Anticancer Activities of Unripe and Ripe Azadirachta indica (Neem) Seed Extracts. BMC Complement. Med. Ther. 22 (1), 42. 10.1186/s12906-022-03513-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. C., Prasad S., Tyagi A. K., Kunnumakkara A. B., Aggarwal B. B. (2017). Neem (Azadirachta indica): An Indian Traditional Panacea with Modern Molecular Basis. Phytomedicine 34, 14–20. 10.1016/j.phymed.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Habluetzel A., Pinto B., Tapanelli S., Nkouangang J., Saviozzi M., Chianese G., et al. (2019). Effects of Azadirachta indica Seed Kernel Extracts on Early Erythrocytic Schizogony of Plasmodium Berghei and Pro-inflammatory Response in Inbred Mice. Malar. J. 18 (1), 35. 10.1186/s12936-019-2671-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad G. M., Mohdaly A. A. A., El-Nogoumy B. A., Ramadan M. F., Hassan S. A., Zeitoun A. M. (2021). Detoxification of Aflatoxin B1 and Ochratoxin A Using Salvia farinacea and Azadirachta indica Water Extract and Application in Meat Products. Appl. Biochem. Biotechnol. 193 (10), 3098–3120. 10.1007/s12010-021-03581-1 [DOI] [PubMed] [Google Scholar]
- Heyman L., Houri-Haddad Y., Heyman S. N., Ginsburg I., Gleitman Y., Feuerstein O. (2017). Combined Antioxidant Effects of Neem Extract, Bacteria, Red Blood Cells and Lysozyme: Possible Relation to Periodontal Disease. BMC Complement. Altern. Med. 17 (1), 399. 10.1186/s12906-017-1900-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosny N. S., El Khodary S. A., El Boghdadi R. M., Shaker O. G. (2021). Effect of Neem (Azadirachta indica) versus 2.5% Sodium Hypochlorite as Root Canal Irrigants on the Intensity of Post-operative Pain and the Amount of Endotoxins in Mandibular Molars with Necrotic Pulps: a Randomized Controlled Trial. Int. Endod. J. 54 (9), 1434–1447. 10.1111/iej.13532 [DOI] [PubMed] [Google Scholar]
- Hussain F., Khurshid M. F., Masood R., Ibrahim W. (2017). Developing Antimicrobial Calcium Alginate Fibres from Neem and Papaya Leaves Extract. J. Wound Care 26 (12), 778–783. 10.12968/jowc.2017.26.12.778 [DOI] [PubMed] [Google Scholar]
- Ibrahim N., Kebede A. (2020). In Vitro antibacterial Activities of Methanol and Aqueous Leave Extracts of Selected Medicinal Plants against Human Pathogenic Bacteria. Saudi J. Biol. Sci. 27 (9), 2261–2268. 10.1016/j.sjbs.2020.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iman M., Taheri M., Bahari Z. (2021). The Anti-cancer Properties of Neem (Azadirachta indica) through its Antioxidant Activity in the Liver; its Pharmaceutics and Toxic Dosage Forms; a Literature Review. J. Complement. Integr. Med. 10.1515/jcim-2021-0009 [DOI] [PubMed] [Google Scholar]
- Jagannathan J., Nagar P., Kaniappan A. S., Raveendran A., Shekhar S. (2020). Comparison of Antimicrobial Efficacy of Natural Extracts as a Disinfectant for Removable Orthodontic Appliances: An Ex Vivo Study. Int. J. Clin. Pediatr. Dent. 13 (6), 640–643. 10.5005/jp-journals-10005-1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain I., Jain P., Bisht D., Sharma A., Srivastava B., Gupta N. (2015). Use of Traditional Indian Plants in the Inhibition of Caries-Causing Bacteria--Streptococcus Mutans . Braz Dent. J. 26 (2), 110–115. 10.1590/0103-6440201300102 [DOI] [PubMed] [Google Scholar]
- Jalaluddin M., Rajasekaran U. B., Paul S., Dhanya R. S., Sudeep C. B., Adarsh V. J. (2017). Comparative Evaluation of Neem Mouthwash on Plaque and Gingivitis: A Double-Blind Crossover Study. J. Contemp. Dent. Pract. 18 (7), 567–571. 10.5005/jp-journals-10024-2085 [DOI] [PubMed] [Google Scholar]
- James C., Harfouche M., Welton N. J., Turner K. M., Abu-Raddad L. J., Gottlieb S. L., et al. (20202016). Herpes Simplex Virus: Global Infection Prevalence and Incidence Estimates, 2016. Bull. World Health Organ 98 (5), 315–329. 10.2471/BLT.19.237149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamra N., Das G., Singh P., Haque M. (2015). Anthelmintic Efficacy of Crude Neem (Azadirachta indica) Leaf Powder against Bovine Strongylosis. J. Parasit. Dis. 39 (4), 786–788. 10.1007/s12639-014-0423-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy Sinha D., D S Nandha K., Jaiswal N., Vasudeva A., Prabha Tyagi S., Pratap Singh U. (2017). Antibacterial Effect of Azadirachta indica (Neem) or Curcuma Longa (Turmeric) against Enterococcus faecalis Compared with that of 5% Sodium Hypochlorite or 2% Chlorhexidine In Vitro . Bull. Tokyo Dent. Coll. 58 (2), 103–109. 10.2209/tdcpublication.2015-0029 [DOI] [PubMed] [Google Scholar]
- Joy Sinha D., Garg P., Verma A., Malik V., Maccune E. R., Vasudeva A. (2015). Dentinal Tubule Disinfection with Propolis & Two Extracts of Azadirachta indica against Candida albicans Biofilm Formed on Tooth Substrate. Open Dent. J. 9, 369–374. 10.2174/1874210601509010369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalita C., Raja D., Saikia A., Saikia A. K. (2019). Antibacterial Property of Azadirachta indica, Ocimum sanctum, and Vitex negundo against Oral Microbes. J. Conserv. Dent. 22 (6), 602–606. 10.4103/JCD.JCD_268_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagasanthosh K., Shanmugapriyan S., Kavirajan V. (2015). Evaluation of Acute Toxicity, Anti-inflammatory Activity and Phytochemical Screening of Ethanolic Extract of Azadirachta indica Leaves. Int. J. Res. Dev. Pharm. Life Sci. 4 (5), 1737–1742. [Google Scholar]
- Kankariya A. R., Patel A. R., Kunte S. S. (2016). The Effect of Different Concentrations of Water Soluble Azadirachtin (Neem Metabolite) on Streptococcus Mutans Compared with Chlorhexidine. J. Indian Soc. Pedod. Prev. Dent. 34 (2), 105–110. 10.4103/0970-4388.180394 [DOI] [PubMed] [Google Scholar]
- Kanth M. R., Prakash A. R., Sreenath G., Reddy V. S., Huldah S. (2016). Efficacy of Specific Plant Products on Microorganisms Causing Dental Caries. J. Clin. Diagn Res. 10 (12), ZM01–ZM03. 10.7860/JCDR/2016/19772.9025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R., Tiwari A., Manish M., Maurya I. K., Bhatnagar R., Singh S. (2021). Common Garlic (Allium Sativum L.) Has Potent Anti-Bacillus Anthracis Activity. J. Ethnopharmacol. 264, 113230. 10.1016/j.jep.2020.113230 [DOI] [PubMed] [Google Scholar]
- Kaura T., Mewara A., Zaman K., Sharma A., Agrawal S. K., Thakur V., et al. (2019). Utilizing Larvicidal and Pupicidal Efficacy of Eucalyptus and Neem Oil against Aedes Mosquito: An Approach for Mosquito Control. Trop. Parasitol. 9 (1), 12–17. 10.4103/tp.TP_35_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khameneh B., Iranshahy M., Soheili V., Fazly Bazzaz B. S. (2019). Review on Plant Antimicrobials: a Mechanistic Viewpoint. Antimicrob. Resist Infect. Control 8, 118. 10.1186/s13756-019-0559-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F. R., Kazmi S. M. R., Iqbal N. T., Iqbal J., Ali S. T., Abbas S. A. (2020). A Quadruple Blind, Randomised Controlled Trial of Gargling Agents in Reducing Intraoral Viral Load Among Hospitalised COVID-19 Patients: A Structured Summary of a Study Protocol for a Randomised Controlled Trial. Trials 21 (1), 785. 10.1186/s13063-020-04634-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilani-Morakchi S., Morakchi-Goudjil H., Sifi K. (2021). Azadirachtin-Based Insecticide: Overview, Risk Assessments, and Future Directions. Front. Agron. 3. 10.3389/fagro.2021.676208 [DOI] [Google Scholar]
- Killi N., Pawar A. T., Gundloori R. V. (2019). Polyesteramide of Neem Oil and its Blends as an Active Nanomaterial for Tissue Regeneration. ACS Appl. Bio Mater 2 (8), 3341–3351. 10.1021/acsabm.9b00354 [DOI] [PubMed] [Google Scholar]
- Kuipers E. J. (1999). Review Article: Exploring the Link between Helicobacter pylori and Gastric Cancer. Aliment. Pharmacol. Ther. 13 (Suppl. 1), 3–11. 10.1046/j.1365-2036.1999.00002.x [DOI] [PubMed] [Google Scholar]
- Lahiri D., Nag M., Dutta B., Mukherjee I., Ghosh S., Dey A., et al. (2021). Catechin as the Most Efficient Bioactive Compound from Azadirachta indica with Antibiofilm and Anti-Quorum Sensing Activities against Dental Biofilm: an In Vitro and In Silico Study. Appl. Biochem. Biotechnol. 193 (6), 1617–1630. 10.1007/s12010-021-03511-1 [DOI] [PubMed] [Google Scholar]
- Lakkim V., Reddy M. C., Pallavali R. R., Reddy K. R., Reddy C. V., Inamuddin, et al. (2020). Green Synthesis of Silver Nanoparticles and Evaluation of Their Antibacterial Activity against Multidrug-Resistant Bacteria and Wound Healing Efficacy Using a Murine Model. Antibiot. (Basel) 9 (12). 10.3390/antibiotics9120902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi T., Krishnan V., Rajendran R., Madhusudhanan N. (2015). Azadirachta indica: A Herbal Panacea in Dentistry - an Update. Pharmacogn. Rev. 9 (17), 41–44. 10.4103/0973-7847.156337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Chi N. T., Narayanan M., Chinnathambi A., Govindasamy C., Subramani B., Brindhadevi K., et al. (2022). Fabrication, Characterization, Anti-inflammatory, and Anti-diabetic Activity of Silver Nanoparticles Synthesized from Azadirachta indica Kernel Aqueous Extract. Environ. Res. 208, 112684. 10.1016/j.envres.2022.112684 [DOI] [PubMed] [Google Scholar]
- Lavanya P., Ramaiah S., Anbarasu A. (2015). Computational Analysis Reveal Inhibitory Action of Nimbin against Dengue Viral Envelope Protein. Virusdisease 26 (4), 243–254. 10.1007/s13337-015-0280-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. (1984). Isolation of Lymphocytopathic Retroviruses from San Francisco Patients with AIDS. Science 225 (4664), 840–842. 10.1126/science.6206563 [DOI] [PubMed] [Google Scholar]
- Lu X. F., Lin P. C., Zi J. C., Fan X. N. (2019). Limonoids from Seeds of Azadirachta indica and Their Antibacterial Activity. Zhongguo Zhong Yao Za Zhi 44 (22), 4864–4873. 10.19540/j.cnki.cjcmm.20190813.202 [DOI] [PubMed] [Google Scholar]
- MacKinnon S., Durst T., Arnason J. T., Angerhofer C., Pezzuto J., Sanchez-Vindas P. E., et al. (1997). Antimalarial Activity of Tropical Meliaceae Extracts and Gedunin Derivatives. J. Nat. Prod. 60 (4), 336–341. 10.1021/np9605394 [DOI] [PubMed] [Google Scholar]
- Mah T. F., O'Toole G. A. (2001). Mechanisms of Biofilm Resistance to Antimicrobial Agents. Trends Microbiol. 9 (1), 34–39. 10.1016/s0966-842x(00)01913-2 [DOI] [PubMed] [Google Scholar]
- Maheswaran R., Ignacimuthu S. (2015). A Novel Biopesticide PONNEEM to Control Human Vector Mosquitoes Anopheles stephensi L. And Culex quinquefasciatus Say. Environ. Sci. Pollut. Res. Int. 22 (17), 13153–13166. 10.1007/s11356-015-4586-4 [DOI] [PubMed] [Google Scholar]
- Mahfuzul Hoque M. D., Bari M. L., Inatsu Y., Juneja V. K., Kawamoto S. (2007). Antibacterial Activity of Guava (Psidium Guajava L.) and Neem (Azadirachta indica A. Juss.) Extracts against Foodborne Pathogens and Spoilage Bacteria. Foodborne Pathog. Dis. 4 (4), 481–488. 10.1089/fpd.2007.0040 [DOI] [PubMed] [Google Scholar]
- Mandawewala A. R., Mandawewala K. A. (2021). POLYMERIC YARN, COMPOSITION AND METHOD. 17/148618. [Google Scholar]
- Martens E., Demain A. L. (2017). The Antibiotic Resistance Crisis, with a Focus on the United States. J. Antibiot. (Tokyo) 70 (5), 520–526. 10.1038/ja.2017.30 [DOI] [PubMed] [Google Scholar]
- Mazariegos L. A. (2016). Pest Control Formulation of Neem and Beauveria Bassiana and Methods of Making and Using Same. 14/494199. [Google Scholar]
- Mbah A. U., Udeinya I. J., Shu E. N., Chijioke C. P., Nubila T., Udeinya F., et al. (2007). Fractionated Neem Leaf Extract Is Safe and Increases CD4+ Cell Levels in HIV/AIDS Patients. Am. J. Ther. 14 (4), 369–374. 10.1097/MJT.0b013e3180a72199 [DOI] [PubMed] [Google Scholar]
- Melese S., Kumar S., Nair P., Sharmila B. (2016). Preliminary Phytochemical Analysis and In Vitro Antibacterial Activity of Bark and Seeds of Ethiopian Neem (Azadirachta indica A. Juss). WORLD J. Pharm. Pharm. Sci. 5 (4), 1714–1723. [Google Scholar]
- Mistry K. S., Sanghvi Z., Parmar G., Shah S., Pushpalatha K. (2015). Antibacterial Efficacy of Azadirachta indica, Mimusops elengi and 2% CHX on Multispecies Dentinal Biofilm. J. Conserv. Dent. 18 (6), 461–466. 10.4103/0972-0707.168810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry K. S., Sanghvi Z., Parmar G., Shah S. (2014). The Antimicrobial Activity of Azadirachta indica, Mimusops elengi, Tinospora cardifolia, Ocimum sanctum and 2% Chlorhexidine Gluconate on Common Endodontic Pathogens: An In Vitro Study. Eur. J. Dent. 8 (2), 172–177. 10.4103/1305-7456.130591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux D. H., Savioli L., Engels D. (2017). Neglected Tropical Diseases: Progress towards Addressing the Chronic Pandemic. Lancet 389 (10066), 312–325. 10.1016/S0140-6736(16)30171-4 [DOI] [PubMed] [Google Scholar]
- Moodley Y., Linz B., Bond R. P., Nieuwoudt M., Soodyall H., Schlebusch C. M., et al. (2012). Age of the Association between Helicobacter pylori and Man. PLoS Pathog. 8 (5), e1002693. 10.1371/journal.ppat.1002693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Covarrubias M. S., García-Aguilar N., Bolan-Mejía M. D., Puello-Cruz A. C. (2016). Evaluation of Medicinal Plants and Colloidal Silver Efficiency against Vibrio Parahaemolyticus Infection in Litopenaeus Vannamei Cultured at Low Salinity. Dis. Aquat. Organ 122 (1), 57–65. 10.3354/dao03060 [DOI] [PubMed] [Google Scholar]
- Murugan K., Panneerselvam C., Samidoss C. M., Madhiyazhagan P., Suresh U., Roni M., et al. (2016). In Vivo and In Vitro Effectiveness of Azadirachta indica-Synthesized Silver Nanocrystals against Plasmodium Berghei and Plasmodium Falciparum, and Their Potential against Malaria Mosquitoes. Res. Vet. Sci. 106, 14–22. 10.1016/j.rvsc.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Mustafa M. (2016). Antibacterial Efficacy of Neem (Azadirachta indica) Extract against Enterococcus faecalis: An In Vitro Study. J. Contemp. Dent. Pract. 17 (10), 791–794. 10.5005/jp-journals-10024-1932 [DOI] [PubMed] [Google Scholar]
- Nagaraj A., Samiappan S. (2019). Presentation of Antibacterial and Therapeutic Anti-inflammatory Potentials to Hydroxyapatite via Biomimetic with Azadirachta indica: An In Vitro Anti-inflammatory Assessment in Contradiction of LPS-Induced Stress in RAW 264.7 Cells. Front. Microbiol. 10, 1757. 10.3389/fmicb.2019.01757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata J. M., Jew A. R., Kimeu J. M., Salmen C. R., Bukusi E. A., Cohen C. R. (2011). Medical Pluralism on Mfangano Island: Use of Medicinal Plants Among Persons Living with HIV/AIDS in Suba District, Kenya. J. Ethnopharmacol. 135 (2), 501–509. 10.1016/j.jep.2011.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagini S., Nivetha R., Palrasu M., Mishra R. (2021). Nimbolide, a Neem Limonoid, Is a Promising Candidate for the Anticancer Drug Arsenal. J. Med. Chem. 64 (7), 3560–3577. 10.1021/acs.jmedchem.0c02239 [DOI] [PubMed] [Google Scholar]
- Nallusamy S., Mannu J., Ravikumar C., Angamuthu K., Nathan B., Nachimuthu K., et al. (2021). Exploring Phytochemicals of Traditional Medicinal Plants Exhibiting Inhibitory Activity against Main Protease, Spike Glycoprotein, RNA-dependent RNA Polymerase and Non-structural Proteins of SARS-CoV-2 through Virtual Screening. Front. Pharmacol. 12, 667704. 10.3389/fphar.2021.667704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (1992). Neem: A Tree for Solving Global Problems. Washington (DC): The National Academies Press. 10.17226/1924 [DOI] [PubMed] [Google Scholar]
- Navabshan I., Sakthivel B., Pandiyan R., Antoniraj M. G., Dharmaraj S., Ashokkumar V., et al. (2021). Computational Lock and Key and Dynamic Trajectory Analysis of Natural Biophors against COVID-19 Spike Protein to Identify Effective Lead Molecules. Mol. Biotechnol. 63 (10), 898–908. 10.1007/s12033-021-00358-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak Aarati N. R. N., Soumya G. B., Kishore B., Mithun K. (2011). Evaluation of Antibacterial and Anticandidial Efficacy of Aqueous and Alcoholic Extract of Neem (Azadirachta indica) an In Vitro Study. Int. J. Res. Ayurveda Pharm. 2 (1), 230–235. [Google Scholar]
- Nesari T. M., Bhardwaj A., ShriKrishna R., Ruknuddin G., Ghildiyal S., Das A., et al. (2021). Neem (Azadirachta indica A. Juss) Capsules for Prophylaxis of COVID-19 Infection: A Pilot, Double-Blind, Randomized Controlled Trial. Altern. Ther. Health Med. 27 (S1), 196–203. [PubMed] [Google Scholar]
- Newman D. J., Cragg G. M. (2016). Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 79 (3), 629–661. 10.1021/acs.jnatprod.5b01055 [DOI] [PubMed] [Google Scholar]
- Nicoletti M., Maccioni O., Coccioletti T., Mariani S., Vitali F. (2012). “Neem Tree (Azadirachta indica A. Juss) as Source of Bioinsectides,” in Insecticides - Advances In Integrated Pest Management. IntechOpen). 10.5772/28786 [DOI] [Google Scholar]
- Nimbulkar G., Garacha V., Shetty V., Bhor K., Srivastava K. C., Shrivastava D., et al. (2020). Microbiological and Clinical Evaluation of Neem Gel and Chlorhexidine Gel on Dental Plaque and Gingivitis in 20-30 Years Old Adults: A Randomized Parallel-Armed, Double-Blinded Controlled Trial. J. Pharm. Bioallied Sci. 12 (Suppl. 1), S345–S351. 10.4103/jpbs.JPBS_101_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor A. A. (2011). Dose Response Curve of Plants Extracts against the Human Pathogens. Gomal Univ. J. Res. 27 (2). [Google Scholar]
- Noorul Aneesa G. (2016). Beneficial Effects of Neem Oil-An Updated Review. J. Pharm. Sci. Res. 8 (8), 756–758. [Google Scholar]
- Ogidigo J. O., Iwuchukwu E. A., Ibeji C. U., Okpalefe O., Soliman M. E. S. (2022). Natural Phyto, Compounds as Possible Noncovalent Inhibitors against SARS-CoV2 Protease: Computational Approach. J. Biomol. Struct. Dyn. 40 (5), 2284–2301. 10.1080/07391102.2020.1837681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoh M. P., Singla R. K., Madu C., Soremekun O., Adejoh J., Alli L. A., et al. (2021). Phytomedicine in Disease Management: In-Silico Analysis of the Binding Affinity of Artesunate and Azadirachtin for Malaria Treatment. Front. Pharmacol. 12, 751032. 10.3389/fphar.2021.751032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olwenyi O. A., Asingura B., Naluyima P., Anywar G. U., Nalunga J., Nakabuye M., et al. (2021). In-vitro Immunomodulatory Activity of Azadirachta indica A.Juss. Ethanol: Water Mixture against HIV Associated Chronic CD4+ T-Cell Activation/Exhaustion. BMC Complement. Med. Ther. 21 (1), 114. 10.1186/s12906-021-03288-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyekanmi A. A., Kumar U. S. U., H. P. S. A. K., Olaiya N. G., Amirul A. A., Rahman A. A., et al. (2021). Functional Properties of Antimicrobial Neem Leaves Extract Based Macroalgae Biofilms for Potential Use as Active Dry Packaging Applications. Polymers 13 (10), 1664. 10.3390/polym13101664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal V., Gurunathan D., Muralidharan N. P. (2020). Comparison of Antibacterial Efficacy of Cinnamon Extract, Neem Extract as Irrigant and Sodium Hypochlorite against Enterococcus Fecalis: An In Vitro Study. Indian J. Dent. Res. 31 (1), 124–128. 10.4103/ijdr.IJDR_177_18 [DOI] [PubMed] [Google Scholar]
- Páramo M. E. R., Falvo M., García J., Lastra C. C. L. (2020). Compatibility between Leptolegnia chapmanii and Diflubenzuron and Neem Oil for the Control of Aedes aegypti . Rev. Argent. Microbiol. 52 (3), 240–244. 10.1016/j.ram.2019.10.001 [DOI] [PubMed] [Google Scholar]
- Parida P. K., Paul D., Chakravorty D. (2020). The Natural Way Forward: Molecular Dynamics Simulation Analysis of Phytochemicals from Indian Medicinal Plants as Potential Inhibitors of SARS-CoV-2 Targets. Phytother. Res. 34 (12), 3420–3433. 10.1002/ptr.6868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquoto-Stigliani T., Campos E. V. R., Oliveira J. L., Silva C. M. G., Bilesky-José N., Guilger M., et al. (2017). Nanocapsules Containing Neem (Azadirachta indica) Oil: Development, Characterization, and Toxicity Evaluation. Sci. Rep. 7 (1), 5929. 10.1038/s41598-017-06092-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. M., Nagulapalli Venkata K. C., Bhattacharyya P., Sethi G., Bishayee A. (2016). Potential of Neem (Azadirachta indica L.) for Prevention and Treatment of Oncologic Diseases. Semin. Cancer Biol. 40-41, 100–115. 10.1016/j.semcancer.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Patil S. M., Shirahatti P. S., Ramu R. (2021). Azadirachta indica A. Juss (Neem) against Diabetes Mellitus: a Critical Review on its Phytochemistry, Pharmacology, and Toxicology. J. Pharm. Pharmacol. 10.1093/jpp/rgab098 [DOI] [PubMed] [Google Scholar]
- Poopathi S., De Britto L. J., Praba V. L., Mani C., Praveen M. (2015). Synthesis of Silver Nanoparticles from Azadirachta indica-A Most Effective Method for Mosquito Control. Environ. Sci. Pollut. Res. Int. 22 (4), 2956–2963. 10.1007/s11356-014-3560-x [DOI] [PubMed] [Google Scholar]
- Quelemes P. V., Perfeito M. L., Guimarães M. A., dos Santos R. C., Lima D. F., Nascimento C., et al. (2015). Effect of Neem (Azadirachta indica A. Juss) Leaf Extract on Resistant Staphylococcus aureus Biofilm Formation and Schistosoma Mansoni Worms. J. Ethnopharmacol. 175, 287–294. 10.1016/j.jep.2015.09.026 [DOI] [PubMed] [Google Scholar]
- Raju D., Jose J. (2019). Development and Evaluation of Novel Topical Gel of Neem Extract for the Treatment of Bacterial Infections. J. Cosmet. Dermatol 18 (6), 1776–1783. 10.1111/jocd.12965 [DOI] [PubMed] [Google Scholar]
- Ramana V. (2022). Dental Enamel Compositions with Anti-inflammatory Agents for Animals, 17–523091. [Google Scholar]
- Rasool S., Raza M. A., Manzoor F., Kanwal Z., Riaz S., Iqbal M. J., et al. (2020). Biosynthesis, Characterization and Anti-dengue Vector Activity of Silver Nanoparticles Prepared from Azadirachta indica and Citrullus colocynthis . R. Soc. Open Sci. 7 (9), 200540. 10.1098/rsos.200540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravva S. V., Korn A. (2015). Effect of Neem (Azadirachta indica) on the Survival of Escherichia coli O157:H7 in Dairy Manure. Int. J. Environ. Res. Public Health 12 (7), 7794–7803. 10.3390/ijerph120707794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. (2020). Neem Cake Fertilizer, Uses, Application, Benefits [Online]. Agrifarming. Available: https://www.agrifarming.in/neem-cake-fertilizer-uses-application-benefits .
- Revathi T., Thambidurai S. (2019). Cytotoxic, Antioxidant and Antibacterial Activities of Copper Oxide Incorporated Chitosan-Neem Seed Biocomposites. Int. J. Biol. Macromol. 139, 867–878. 10.1016/j.ijbiomac.2019.07.214 [DOI] [PubMed] [Google Scholar]
- Rodrigues M. P., Astoreca A. L., Oliveira Á. A., Salvato L. A., Biscoto G. L., Keller L. A. M., et al. (2019). In Vitro Activity of Neem (Azadirachta indica) Oil on Growth and Ochratoxin A Production by Aspergillus carbonarius Isolates. Toxins (Basel) 11 (10). 10.3390/toxins11100579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Saraf S. (2006). Limonoids: Overview of Significant Bioactive Triterpenes Distributed in Plants Kingdom. Biol. Pharm. Bull. 29 (2), 191–201. 10.1248/bpb.29.191 [DOI] [PubMed] [Google Scholar]
- S L. P., A U., S J G. F. (2020). Investigation on the Biofilm Eradication Potential of Selected Medicinal Plants against Methicillin-Resistant Staphylococcus aureus . Biotechnol. Rep. (Amst) 28, e00523. 10.1016/j.btre.2020.e00523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem S., Muhammad G., Hussain M. A., Bukhari S. N. A. (2018). A Comprehensive Review of Phytochemical Profile, Bioactives for Pharmaceuticals, and Pharmacological Attributes of Azadirachta indica . Phytother. Res. 32 (7), 1241–1272. 10.1002/ptr.6076 [DOI] [PubMed] [Google Scholar]
- Sarkar L., Oko L., Gupta S., Bubak A. N., Das B., Gupta P., et al. (2022). Azadirachta indica A. Juss Bark Extract and its Nimbin Isomers Restrict β-coronaviral Infection and Replication. Virology 569, 13–28. 10.1016/j.virol.2022.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Singh R. P., Bhattacharya G. (2021). Exploring the Role of Azadirachta indica (Neem) and its Active Compounds in the Regulation of Biological Pathways: an Update on Molecular Approach. 3 Biotech. 11 (4), 178. 10.1007/s13205-021-02745-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A., Arivaradarajan P., Arivaradarajan P., Mukhopadhyay A. K., Nandi S. P. (2021). Bactericidal Effect of Neem (Azadirachta indica) Leaf Extract on Helicobacter pylori . Jeb 42, 1591–1597. 10.22438/jeb/42/6/mrn-2070 [DOI] [Google Scholar]
- Selvaraj K., Bharath N., Natarajan R., Dinesh S., Murugesan S., Selvaraj S. (2020). Comparative Evaluation of Antimicrobial Efficacy of Toothpastes Containing Probiotic and Neem as Primary Ingredient on Salivary Streptococcus mutans in Melmaruvathur Population: An In Vivo Study. J. Pharm. Bioallied Sci. 12 (Suppl. 1), S595–S600. 10.4103/jpbs.JPBS_209_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senapati S., Banerjee P., Bhagavatula S., Kushwaha P. P., Kumar S. (2021). Contributions of Human ACE2 and TMPRSS2 in Determining Host-Pathogen Interaction of COVID-19. J. Genet. 100. 10.1007/s12041-021-01262-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam A., Ramakrishnan C., Velmurugan D., Gromiha M. M. (2020). Identification of Potential Inhibitors for Targets Involved in Dengue Fever. Curr. Top. Med. Chem. 20 (19), 1742–1760. 10.2174/1568026620666200618123026 [DOI] [PubMed] [Google Scholar]
- Sharma C., Bhardwaj N. K. (2020). Fabrication of Natural-Origin Antibacterial Nanocellulose Films Using Bio-Extracts for Potential Use in Biomedical Industry. Int. J. Biol. Macromol. 145, 914–925. 10.1016/j.ijbiomac.2019.09.182 [DOI] [PubMed] [Google Scholar]
- Sharma D. D., Nupur S. S. (2014). Comparative Study of Different Parts of Azadirachta indica (Neem) Plant on the Basis of Antibacterial Activity, Phytochemical Screening and its Effect on Rat Pc-12 (Pheochromocytoma) Cell Line. Int. J. Biotechnol. Humanit. Sports Allied Fields 2 (7), 144–154. [Google Scholar]
- Singh M., Sharma D., Kumar D., Singh G., Swami G., Rathore M. S. (2020). Formulation, Development, and Evaluation of Herbal Effervescent Mouthwash Tablet Containing Azadirachta indica (Neem) and Curcumin for the Maintenance of Oral Hygiene. Recent Pat. Drug Deliv. Formul. 14 (2), 145–161. 10.2174/1872211314666200820142509 [DOI] [PubMed] [Google Scholar]
- Singh V., Roy M., Garg N., Kumar A., Arora S., Malik D. S. (2021). An Insight into the Dermatological Applications of Neem: A Review on Traditional and Modern Aspect. Recent Adv. Antiinfect Drug Discov. 16 (2), 94–121. 10.2174/2772434416666210604105251 [DOI] [PubMed] [Google Scholar]
- Sir C., Chopra R. N. (1994). Indigenous Drugs of India. Academic Publishers. [Google Scholar]
- Somsak V., Chachiyo S., Jaihan U., Nakinchat S. (2015). Protective Effect of Aqueous Crude Extract of Neem (Azadirachta indica) Leaves on Plasmodium Berghei-Induced Renal Damage in Mice. J. Trop. Med. 2015, 961205. 10.1155/2015/961205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni N., Prakash S. (2014). Silver Nanoparticles: a Possibility for Malarial and Filarial Vector Control Technology. Parasitol. Res. 113 (11), 4015–4022. 10.1007/s00436-014-4069-4 [DOI] [PubMed] [Google Scholar]
- Stewart P. S., Costerton J. W. (2001). Antibiotic Resistance of Bacteria in Biofilms. Lancet 358 (9276), 135–138. 10.1016/s0140-6736(01)05321-1 [DOI] [PubMed] [Google Scholar]
- Streicher L. M. (2021). Exploring the Future of Infectious Disease Treatment in a Post-antibiotic Era: A Comparative Review of Alternative Therapeutics. J. Glob. Antimicrob. Resist 24, 285–295. 10.1016/j.jgar.2020.12.025 [DOI] [PubMed] [Google Scholar]
- Subbuvel M., Kavan P. (2022). Preparation and Characterization of Polylactic Acid/fenugreek Essential Oil/curcumin Composite Films for Food Packaging Applications. Int. J. Biol. Macromol. 194, 470–483. 10.1016/j.ijbiomac.2021.11.090 [DOI] [PubMed] [Google Scholar]
- Sultana B., Naseer R., Nigam P. (2015). Utilization of Agro-Wastes to Inhibit Aflatoxins Synthesis by Aspergillus parasiticus: A Biotreatment of Three Cereals for Safe Long-Term Storage. Bioresour. Technol. 197, 443–450. 10.1016/j.biortech.2015.08.113 [DOI] [PubMed] [Google Scholar]
- Sunthar T. P. M., Marin E., Boschetto F., Zanocco M., Sunahara H., Ramful R., et al. (2020). Antibacterial and Antifungal Properties of Composite Polyethylene Materials Reinforced with Neem and Turmeric. Antibiot. (Basel) 9 (12). 10.3390/antibiotics9120857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susmitha S., Vidyamol K. K., Ranganayaki P., Vijayaragavan R. (2013). Phytochemical Extraction and Antimicrobial Properties of Azadirachta indica (Neem). Glob. J. Pharmacol. 7 (3), 316–320. [Google Scholar]
- Tajbakhsh E., Kwenti T. E., Kheyri P., Nezaratizade S., Lindsay D. S., Khamesipour F. (2021). Antiplasmodial, Antimalarial Activities and Toxicity of African Medicinal Plants: a Systematic Review of Literature. Malar. J. 20 (1), 349. 10.1186/s12936-021-03866-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapanelli S., Chianese G., Lucantoni L., Yerbanga R. S., Habluetzel A., Taglialatela-Scafati O. (2016). Transmission Blocking Effects of Neem (Azadirachta indica) Seed Kernel Limonoids on Plasmodium Berghei Early Sporogonic Development. Fitoterapia 114, 122–126. 10.1016/j.fitote.2016.09.008 [DOI] [PubMed] [Google Scholar]
- Tasanarong T., Patntirapong S., Aupaphong V. (2021). The Inhibitory Effect of a Novel Neem Paste against Cariogenic Bacteria. J. Clin. Exp. Dent. 13 (11), e1083–e1088. 10.4317/jced.58781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatch W. (2021). Compositions and Methods for Promoting and Maintaining Oral Health. U. S. Pat. Appl. 17, 282430. [Google Scholar]
- Tepongning R. N., Mbah J. N., Avoulou F. L., Jerme M. M., Ndanga E. K., Fekam F. B. (2018). Hydroethanolic Extracts of Erigeron Floribundus and Azadirachta indica Reduced Plasmodium Berghei Parasitemia in Balb/c Mice. Evid. Based Complement. Altern. Med. 2018, 5156710. 10.1155/2018/5156710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesso H., Nisha A. K., Kumsa K. (2015). Antibacterial Activity and Phytochemical Screening of Some Important Medicinal Plants against Human Diarrheal Pathogens in Adama City, Ethiopia. Int. J. Microbiol. Immunol. Res. 3 (3), 029–035. [Google Scholar]
- Thakurta P., Bhowmik P., Mukherjee S., Hajra T. K., Patra A., Bag P. K. (2007). Antibacterial, Antisecretory and Antihemorrhagic Activity of Azadirachta indica Used to Treat Cholera and Diarrhea in India. J. Ethnopharmacol. 111 (3), 607–612. 10.1016/j.jep.2007.01.022 [DOI] [PubMed] [Google Scholar]
- Thota S. M., Balan V., Sivaramakrishnan V. (2020). Natural Products as Home-Based Prophylactic and Symptom Management Agents in the Setting of COVID-19. Phytother. Res. 34 (12), 3148–3167. 10.1002/ptr.6794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timalsina D., Pokhrel K. P., Bhusal D. (2021). Pharmacologic Activities of Plant-Derived Natural Products on Respiratory Diseases and Inflammations. Biomed. Res. Int. 2021, 1636816. 10.1155/2021/1636816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V., Darmani N. A., Yue B. Y., Shukla D. (2010). In Vitro antiviral Activity of Neem (Azardirachta indica L.) Bark Extract against Herpes Simplex Virus Type-1 Infection. Phytother. Res. 24 (8), 1132–1140. 10.1002/ptr.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tundis R., Loizzo M. R., Menichini F. (2014). An Overview on Chemical Aspects and Potential Health Benefits of Limonoids and Their Derivatives. Crit. Rev. Food Sci. Nutr. 54 (2), 225–250. 10.1080/10408398.2011.581400 [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Mbah A. U., Chijioke C. P., Shu E. N. (2004). An Antimalarial Extract from Neem Leaves Is Antiretroviral. Trans. R. Soc. Trop. Med. Hyg. 98 (7), 435–437. 10.1016/j.trstmh.2003.10.016 [DOI] [PubMed] [Google Scholar]
- Ulaeto S. B., Mathew G. M., Pancrecious J. K., Nair J. B., Rajan T. P. D., Maiti K. K., et al. (2020). Biogenic Ag Nanoparticles from Neem Extract: Their Structural Evaluation and Antimicrobial Effects against Pseudomonas nitroreducens and Aspergillus unguis (NII 08123). ACS Biomater. Sci. Eng. 6 (1), 235–245. 10.1021/acsbiomaterials.9b01257 [DOI] [PubMed] [Google Scholar]
- UNAIDS (2022). Global HIV & AIDS Statistics - Fact Sheet [Online]. unaids.Org: UNAIDS. Available at: unaids.org/en/resources/fact-sheet .
- USPTO (2022). Patent Public Search [Online]. United States Patent and Trademark Office. Available: https://ppubs.uspto.gov/pubwebapp/ .
- Uthaya Kumar U. S., Abdulmadjid S. N., Olaiya N. G., Amirul A. A., Rizal S., Rahman A. A., et al. (2020). Extracted Compounds from Neem Leaves as Antimicrobial Agent on the Physico-Chemical Properties of Seaweed-Based Biopolymer Films. Polym. (Basel) 12 (5). 10.3390/polym12051119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhan S., Sahoo S. K. (2020). In Silico ADMET and Molecular Docking Study on Searching Potential Inhibitors from Limonoids and Triterpenoids for COVID-19. Comput. Biol. Med. 124, 103936. 10.1016/j.compbiomed.2020.103936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhan S., Sahoo S. K. (2022). Virtual Screening by Targeting Proteolytic Sites of Furin and TMPRSS2 to Propose Potential Compounds Obstructing the Entry of SARS-CoV-2 Virus into Human Host Cells. J. Tradit. Complement. Med. 12 (1), 6–15. 10.1016/j.jtcme.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestby L. K., Grønseth T., Simm R., Nesse L. L. (2020). Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiot. (Basel) 9 (2). 10.3390/antibiotics9020059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. H., Hsieh Y. H., Powers Z. M., Kao C. Y. (2020). Defeating Antibiotic-Resistant Bacteria: Exploring Alternative Therapies for a Post-Antibiotic Era. Int. J. Mol. Sci. 21 (3). 10.3390/ijms21031061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2020). Burden of Disease. Available: https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/burden-of-disease .
- Who (2021a). Leishmaniasis [Online]. World Health Organization. Available: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis .
- Who (2021b). WHO Coronavirus (COVID-19) Dashboard [Online]. World Health Organization. Available: https://covid19.who.int/ .
- Who (2021c). World Malaria Report. Geneva: Global Malaria Programme. [Google Scholar]
- Wong C. B., Mohamad N., Chandrasakaran P., Selvadurai A., Subramaniam R. (2022). Antimicrobial Elastomeric Article, 17–483140. [Google Scholar]
- Wylie M. R., Windham I. H., Blum F. C., Wu H., Merrell D. S. (2022). In Vitro antibacterial Activity of Nimbolide against Helicobacter pylori . J. Ethnopharmacol. 285, 114828. 10.1016/j.jep.2021.114828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmohammadi F., Mehri S., Najafi N., Salar Amoli S., Hosseinzadeh H. (2021). The Protective Effect of Azadirachta indica (Neem) against Metabolic Syndrome: A Review. Iran. J. Basic Med. Sci. 24 (3), 280–292. 10.22038/ijbms.2021.48965.11218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerbanga R. S., Rayaisse J. B., Vantaux A., Salou E., Mouline K., Hien F., et al. (2016). Neemazal ® as a Possible Alternative Control Tool for Malaria and African Trypanosomiasis? Parasit. Vectors 9, 263. 10.1186/s13071-016-1538-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa H., Osaki T., Hojo F., Kamiya S. (2019). Effect of Helicobacter pylori Biofilm Formation on Susceptibility to Amoxicillin, Metronidazole and Clarithromycin. Microb. Pathog. 132, 100–108. 10.1016/j.micpath.2019.04.030 [DOI] [PubMed] [Google Scholar]
- Younus I., Siddiq A., Ishaq H., Anwer L., Badar S., Ashraf M. (2016). Evaluation of Antiviral Activity of Plant Extracts against Foot and Mouth Disease Virus In Vitro . Pak J. Pharm. Sci. 29 (4), 1263–1268. [PubMed] [Google Scholar]
- Zatelli A., Fondati A., Maroli M., Canine Leishmaniosis Working G. (2022). The Knowns and Unknowns of the Efficacy of Neem Oil (Azadirachta indica) Used as a Preventative Measure against Leishmania Sand Fly Vectors (Phlebotomus Genus). Prev. Vet. Med. 202, 105618. 10.1016/j.prevetmed.2022.105618 [DOI] [PubMed] [Google Scholar]
- Zihadi M. A. H., Rahman M., Talukder S., Hasan M. M., Nahar S., Sikder M. H. (2019). Antibacterial Efficacy of Ethanolic Extract of Camellia Sinensis and Azadirachta indica Leaves on Methicillin-Resistant Staphylococcus aureus and Shiga-Toxigenic Escherichia coli . J. Adv. Vet. Anim. Res. 6 (2), 247–252. 10.5455/javar.2019.f340 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.